Impact of ZnO Addition on Er3+ Near-Infrared Emission, the Formation of Ag Nanoparticles, and the Crystallization of Sodium Fluorophosphate Glass
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seddon, A.B.; Tang, Z.; Furniss, D.; Sujecki, S.; Benson, T.M. Progress in rare-earth-doped mid-infrared fiber lasers. Opt. Express 2010, 18, 26704–26719. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, D.; Peng, Y.; Lu, Y.; Chen, X.; Li, X.; Ji, Z. A review on nanostructured glass ceramics for promising application in optical thermometry. J. Alloys Compd. 2018, 763, 34–48. [Google Scholar] [CrossRef]
- de A. Florêncio, L.; Gómez-Malagón, L.A.; Lima, B.C.; Gomes, A.S.L.; Garcia, J.A.M.; Kassab, L.R.P. Efficiency enhancement in solar cells using photon down-conversion in Tb/Yb-doped tellurite glass. Sol. Energy Mater. Sol. Cells 2016, 157, 468–475. [Google Scholar] [CrossRef]
- Tünnermann, A.; Schreiber, T.; Limpert, J. Fiber lasers and amplifiers: An ultrafast performance evolution. Appl. Opt. 2010, 49, F71–F78. [Google Scholar] [CrossRef]
- Click, C.A.; Brow, R.K.; Alam, T.M. Properties and structure of cesium phosphate glasses. J. Non Cryst. Solids 2002, 311, 294–303. [Google Scholar] [CrossRef]
- Campbell, J.H. Recent advances in phosphate laser glasses for high-power applications. In Proceedings of the SPIE; A Critical Review; Inorganic Optical Materials: Denver, CO, USA, 8 August 1996; Volume 10286, pp. 1028602:1–1028602:37. [Google Scholar]
- Hwang, B.C.; Jiang, S.; Luo, T.; Watson, J.; Honkanen, S.; Hu, Y.; Smektala, F.; Lucas, J.; Peyghambarian, N. Erbium-doped phosphate glass fibre amplifiers with gain per unit length of 2.1 dB/cm. Electron. Lett. 1999, 35, 1007–1009. [Google Scholar] [CrossRef]
- Veasey, D.L.; Funk, D.S.; Peters, P.M.; Sanford, N.A.; Obarski, G.E.; Fontaine, N.; Young, M.; Peskin, A.P.; Liu, W.-C.; Houde-Walter, S.N.; et al. Yb/Er-codoped and Yb-doped waveguide lasers in phosphate glass. J. Non Cryst. Solids 2000, 263–264, 369–381. [Google Scholar] [CrossRef]
- Francini, R.; Giovenale, F.; Grassano, U.M.; Laporta, P.; Taccheo, S. Spectroscopy of Er and Er–Yb-doped phosphate glasses. Opt. Mater. 2000, 13, 417–425. [Google Scholar] [CrossRef]
- Dousti, M.R.; Sahar, M.R.; Ghoshal, S.K.; Amjad, R.J.; Arifin, R. Up-conversion enhancement in Er3+-Ag co-doped zinc tellurite glass: Effect of heat treatment. J. Non Cryst. Solids 2012, 358, 2939–2942. [Google Scholar] [CrossRef]
- Epifani, M.; Giannini, C.; Tapfer, L.; Vasanelli, L. Sol–Gel Synthesis and Characterization of Ag and Au Nanoparticles in SiO2, TiO2, and ZrO2 Thin Films. J. Am. Ceram. Soc. 2000, 83, 2385–2393. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Li, H.; Wang, X. Red shift of plasmon resonance frequency due to the interacting Ag nanoparticles embedded in single crystal SiO2 by implantation. Appl. Phys. Lett. 1998, 72, 1823–1825. [Google Scholar] [CrossRef]
- Dubiel, M.; Schneider, R.; Hofmeister, H.; Schicke, K.-D.; Pivin, J.C. Formation of argentic clusters and small Ag nanoparticles in soda-lime silicate glass. Eur. Phys. J. D 2007, 43, 291–294. [Google Scholar] [CrossRef]
- Marchi, G.D.; Caccavale, F.; Gonella, F.; Mattei, G.; Mazzoldi, P.; Battaglin, G.; Quaranta, A. Silver nanoclusters formation in ion-exchanged waveguides by annealing in hydrogen atmosphere. Appl. Phys. A 1996, 63, 403–407. [Google Scholar] [CrossRef]
- de Almeida, R.; da Silva, D.M.; Kassab, L.R.P.; de Araújo, C.B. Eu3+ luminescence in tellurite glasses with gold nanostructures. Opt. Commun. 2008, 281, 108–112. [Google Scholar] [CrossRef]
- Hayakawa, T.; Tamil Selvan, S.; Nogami, M. Enhanced fluorescence from Eu3+ owing to surface plasma oscillation of silver particles in glass. J. Non Cryst. Solids 1999, 259, 16–22. [Google Scholar] [CrossRef]
- Kassab, L.R.P.; de Araújo, C.B.; Kobayashi, R.A.; de Almeida Pinto, R.; da Silva, D.M. Influence of silver nanoparticles in the luminescence efficiency of Pr3+-doped tellurite glasses. J. Appl. Phys. 2007, 102, 103515:1–103515:4. [Google Scholar] [CrossRef]
- Naranjo, L.P.; de Araújo, C.B.; Malta, O.L.; Cruz, P.A.S.; Kassab, L.R.P. Enhancement of Pr3+ luminescence in PbO–GeO2 glasses containing silver nanoparticles. Appl. Phys. Lett. 2005, 87, 241914:1–241914:3. [Google Scholar] [CrossRef]
- Hamanaka, Y.; Nakamura, A.; Omi, S.; Del Fatti, N.; Vallée, F.; Flytzanis, C. Ultrafast response of nonlinear refractive index of silver nanocrystals embedded in glass. Appl. Phys. Lett. 1999, 75, 1712–1714. [Google Scholar] [CrossRef]
- Fares, H.; Elhouichet, H.; Gelloz, B.; Férid, M. Silver nanoparticles enhanced luminescence properties of Er3+ doped tellurite glasses: Effect of heat treatment. J. Appl. Phys. 2014, 116, 123504:1–123504:10. [Google Scholar] [CrossRef]
- Clara Gonçalves, M.; Santos, L.F.; Almeida, R.M. Rare-earth-doped transparent glass ceramics. C. R. Chim. 2002, 5, 845–854. [Google Scholar] [CrossRef]
- Isogai, M.; Veber, A.; Cicconi, M.R.; Hayakawa, T.; De Ligny, D. Devitrification Behavior of Sol-Gel Derived ZrO2-SiO2 Rare-Earth Doped Glasses: Correlation between Structural and Optical Properties. Ceramics 2018, 1, 22. [Google Scholar] [CrossRef]
- Dejneka, M.J. Transparent Oxyfluoride Glass Ceramics. MRS Bull. 1998, 23, 57–62. [Google Scholar] [CrossRef]
- Tarafder, A.; Molla, A.R.; Mukhopadhyay, S.; Karmakar, B. Fabrication and enhanced photoluminescence properties of Sm3+-doped ZnO–Al2O3–B2O3–SiO2 glass derived willemite glass–ceramic nanocomposites. Opt. Mater. 2014, 36, 1463–1470. [Google Scholar] [CrossRef]
- Ferrari, M.; Righini, G.C. Glass-Ceramic Materials for Guided-Wave Optics. Int. J. Appl. Glass Sci. 2015, 6, 240–248. [Google Scholar] [CrossRef]
- Berberan-Santos, M.N.; Bodunov, E.N.; Valeur, B. Mathematical functions for the analysis of luminescence decays with underlying distributions 1. Kohlrausch decay function (stretched exponential). Chem. Phys. 2005, 315, 171–182. [Google Scholar] [CrossRef]
- Veber, A.; Cicconi, M.R.; Puri, A.; de Ligny, D. Optical Properties and Bismuth Redox in Bi-Doped High-Silica Al–Si Glasses. J. Phys. Chem. C 2018, 122, 19777–19792. [Google Scholar] [CrossRef]
- Konidakis, I.; Varsamis, C.-P.E.; Kamitsos, E.I.; Möncke, D.; Ehrt, D. Structure and Properties of Mixed Strontium−Manganese Metaphosphate Glasses. J. Phys. Chem. C 2010, 114, 9125–9138. [Google Scholar] [CrossRef]
- Gao, H.; Tan, T.; Wang, D. Effect of composition on the release kinetics of phosphate controlled release glasses in aqueous medium. J. Control. Release 2004, 96, 21–28. [Google Scholar] [CrossRef]
- Abou, E.N.; Chrzanowski, W.; Pickup, D.M.; O’Dell, L.A.; Mordan, N.J.; Newport, R.J.; Smith, M.E.; Knowles, J.C. Structure and properties of strontium-doped phosphate-based glasses. J. R. Soc. Interface 2009, 6, 435–446. [Google Scholar] [CrossRef]
- Cui, S.; Massera, J.; Lastusaari, M.; Hupa, L.; Petit, L. Novel oxyfluorophosphate glasses and glass-ceramics. J. Non Cryst. Solids 2016, 445–446, 40–44. [Google Scholar] [CrossRef]
- Ilieva, D.; Jivov, B.; Bogachev, G.; Petkov, C.; Penkov, I.; Dimitriev, Y. Infrared and Raman spectra of Ga2O3–P2O5 glasses. J. Non Cryst. Solids 2001, 283, 195–202. [Google Scholar] [CrossRef]
- Brow, R.K. Review: The structure of simple phosphate glasses. J. Non Cryst. Solids 2000, 263–264, 1–28. [Google Scholar] [CrossRef]
- Karakassides, M.A.; Saranti, A.; Koutselas, I. Preparation and structural study of binary phosphate glasses with high calcium and/or magnesium content. J. Non Cryst. Solids 2004, 347, 69–79. [Google Scholar] [CrossRef]
- Velli, L.L.; Varsamis, C.P.E.; Kamitsos, E.I.; Möncke, D.; Ehrt, D. Structural investigation of metaphosphate glasses. Phys. Chem. Glass 2005, 46, 178–181. [Google Scholar]
- Sancho-Parramon, J.; Janicki, V.; Dubček, P.; Karlušić, M.; Gracin, D.; Jakšić, M.; Bernstorff, S.; Meljanac, D.; Juraić, K. Optical and structural properties of silver nanoparticles in glass matrix formed by thermal annealing of field assisted film dissolution. Opt. Mater. 2010, 32, 510–514. [Google Scholar] [CrossRef][Green Version]
- Gangopadhyay, P.; Magudapathy, P.; Kesavamoorthy, R.; Panigrahi, B.K.; Nair, K.G.M.; Satyam, P.V. Growth of silver nanoclusters embedded in soda glass matrix. Chem. Phys. Lett. 2004, 388, 416–421. [Google Scholar] [CrossRef][Green Version]
- Siegel, G.H. Ultraviolet spectra of silicate glasses: A review of some experimental evidence. J. Non Cryst. Solids 1974, 13, 372–398. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials, 1st ed.Springer: Berlin/Heidelberg, Germany, 1994; ISBN 978-3-540-58019-5. [Google Scholar]
- Abramchuk, M.; Lebedev, O.I.; Hellman, O.; Bahrami, F.; Mordvinova, N.E.; Krizan, J.W.; Metz, K.R.; Broido, D.; Tafti, F. Crystal Chemistry and Phonon Heat Capacity in Quaternary Honeycomb Delafossites: Cu[Li1/3Sn2/3]O2 and Cu[Na1/3Sn2/3]O2. Inorg. Chem. 2018, 57, 12709–12717. [Google Scholar] [CrossRef]
- Hosogi, Y.; Kato, H.; Kudo, A. Visible light response of AgLi1/3M2/3O2 (M = Ti and Sn) synthesized from layered Li2MO3 using molten AgNO3. J. Mater. Chem. 2008, 18, 647–653. [Google Scholar] [CrossRef]
- Lopez-Iscoa, P.; Petit, L.; Massera, J.; Janner, D.; Boetti, N.G.; Pugliese, D.; Fiorilli, S.; Novara, C.; Giorgis, F.; Milanese, D. Effect of the addition of Al2O3, TiO2 and ZnO on the thermal, structural and luminescence properties of Er3+-doped phosphate glasses. J. Non Cryst. Solids 2017, 460, 161–168. [Google Scholar] [CrossRef]
- Goldner, P.; Schaudel, B.; Prassas, M.; Auzel, F. Influence of the host structure and doping precursors on rare earth clustering in phosphate glasses analysed by co-operative luminescence. J. Lumin. 2000, 87–89, 688–690. [Google Scholar] [CrossRef]
- Ponader, C.W.; Brown, G.E. Rare earth elements in silicate glassmelt systems: I. Effects of composition on the coordination environments of La, Gd, and Yb. Geochim. Cosmochim. Acta 1989, 53, 2893–2903. [Google Scholar] [CrossRef]
- Marcus, M.A.; Polman, A. Local structure around Er in silica and sodium silicate glasses. J. Non Cryst. Solids 1991, 136, 260–265. [Google Scholar] [CrossRef]
- Nommeots-Nomm, A.; Boetti, N.G.; Salminen, T.; Massera, J.; Hokka, M.; Petit, L. Luminescence of Er3+ doped oxyfluoride phosphate glasses and glass-ceramics. J. Alloys Compd. 2018, 751, 224–230. [Google Scholar] [CrossRef]
- Rolli, R.; Montagna, M.; Chaussedent, S.; Monteil, A.; Tikhomirov, V.K.; Ferrari, M. Erbium-doped tellurite glasses with high quantum efficiency and broadband stimulated emission cross section at 1.5 μm. Opt. Mater. 2003, 21, 743–748. [Google Scholar] [CrossRef]
- Yang, J.; Dai, S.; Wen, L.; Dai, N.; Hu, L.; Jiang, Z. Mixed heavy metal effect on emission properties of Er3+-doped borosilicate glasses. Chin. Opt. Lett. 2003, 1, 294–295. [Google Scholar]
- Yan, Y.; Faber, A.J.; de Waal, H. Luminescence quenching by OH groups in highly Er-doped phosphate glasses. J. Non Cryst. Solids 1995, 181, 283–290. [Google Scholar] [CrossRef]
- Shestakov, M.V.; Meledina, M.; Turner, S.; Tikhomirov, V.K.; Verellen, N.; Rodríguez, V.D.; Velázquez, J.J.; Van Tendeloo, G.; Moshchalkov, V.V. The size and structure of Ag particles responsible for surface plasmon effects and luminescence in Ag homogeneously doped bulk glass. J. Appl. Phys. 2013, 114, 073102:1–073102:5. [Google Scholar] [CrossRef]
- Stoica, M.; Patzig, C.; Bocker, C.; Wisniewski, W.; Kracker, M.; Höche, T.; Rüssel, C. Structural evolution of CaF2 nanoparticles during the photoinduced crystallization of a Na2O–K2O–CaO–CaF2–Al2O3–ZnO–SiO2 glass. J. Mater. Sci. 2017, 52, 13390–13401. [Google Scholar] [CrossRef]
- Soltani, I.; Hraiech, S.; Horchani-Naifer, K.; Massera, J.; Petit, L.; Férid, M. Thermal, structural and optical properties of Er3+ doped phosphate glasses containing silver nanoparticles. J. Non Cryst. Solids 2016, 438, 67–73. [Google Scholar] [CrossRef]
- Pontuschka, W.M.; Giehl, J.M.; Miranda, A.R.; Da Costa, Z.M.; Alencar, A.M. Effect of the Al2O3 addition on the formation of silver nanoparticles in heat treated soda-lime silicate glasses. J. Non Cryst. Solids 2016, 453, 74–83. [Google Scholar] [CrossRef]
- Geddes, C.D.; Lakowicz, J.R. Metal-Enhanced Fluorescence. J. Fluoresc. 2002, 12, 121–129. [Google Scholar] [CrossRef]
- Fukushima, M.; Managaki, N.; Fujii, M.; Yanagi, H.; Hayashi, S. Enhancement of 1.54-μm emission from Er-doped sol-gel SiO2 films by Au nanoparticles doping. J. Appl. Phys. 2005, 98, 024316:1–024316:4. [Google Scholar] [CrossRef]
- Fares, H.; Elhouichet, H.; Gelloz, B.; Férid, M. Surface plasmon resonance induced Er3+ photoluminescence enhancement in tellurite glass. J. Appl. Phys. 2015, 117, 193102:1–193102:11. [Google Scholar] [CrossRef]
- Gestraud, C.; Glorieux, B.; Massera, J.; Petit, L.; Fargues, A.; Dussauze, M.; Cardinal, T.; Hupa, L. Effect of partial crystallization on the structural and Er3+ luminescence properties of phosphate-based glasses. Opt. Mater. 2017, 64, 230–238. [Google Scholar] [CrossRef]
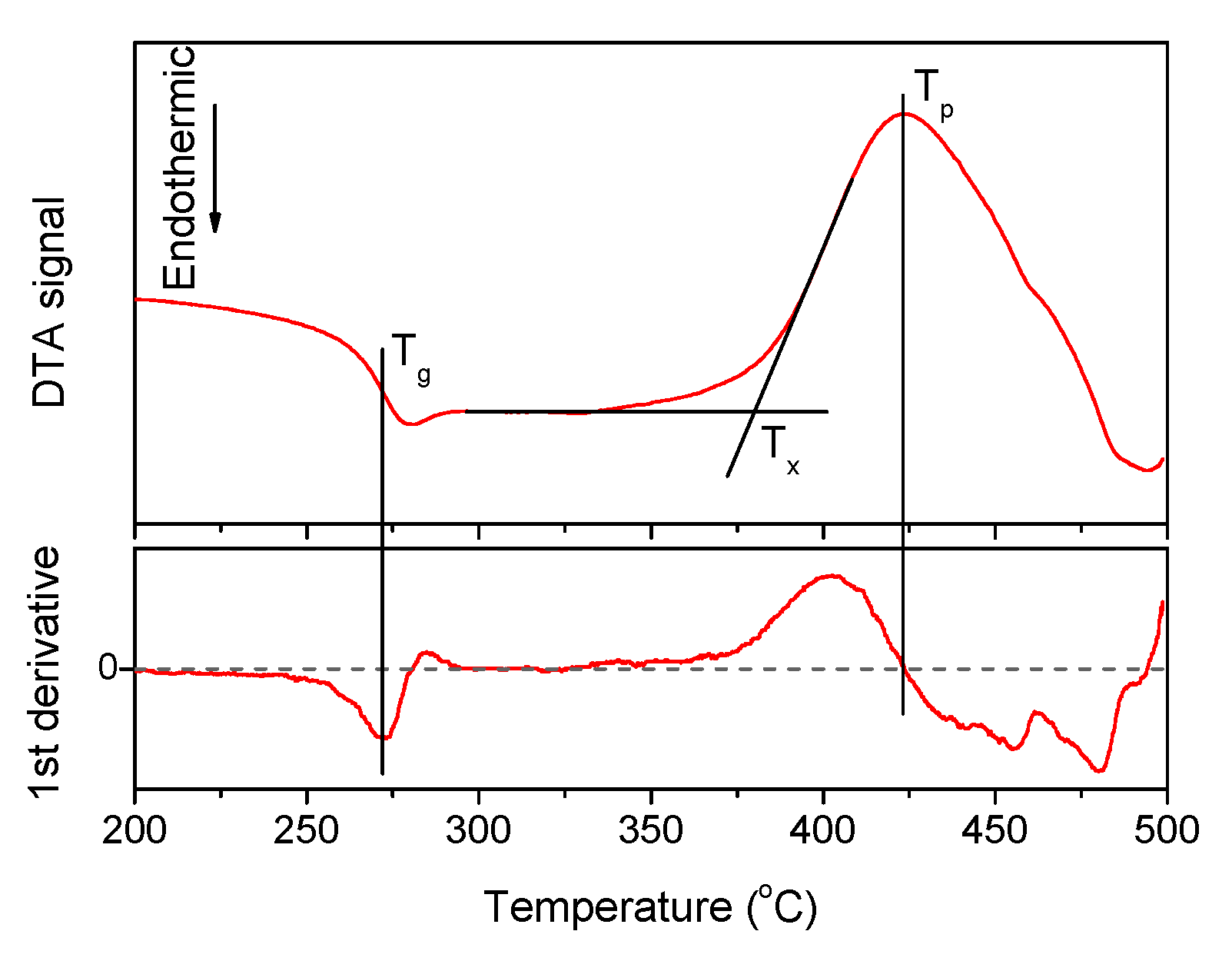
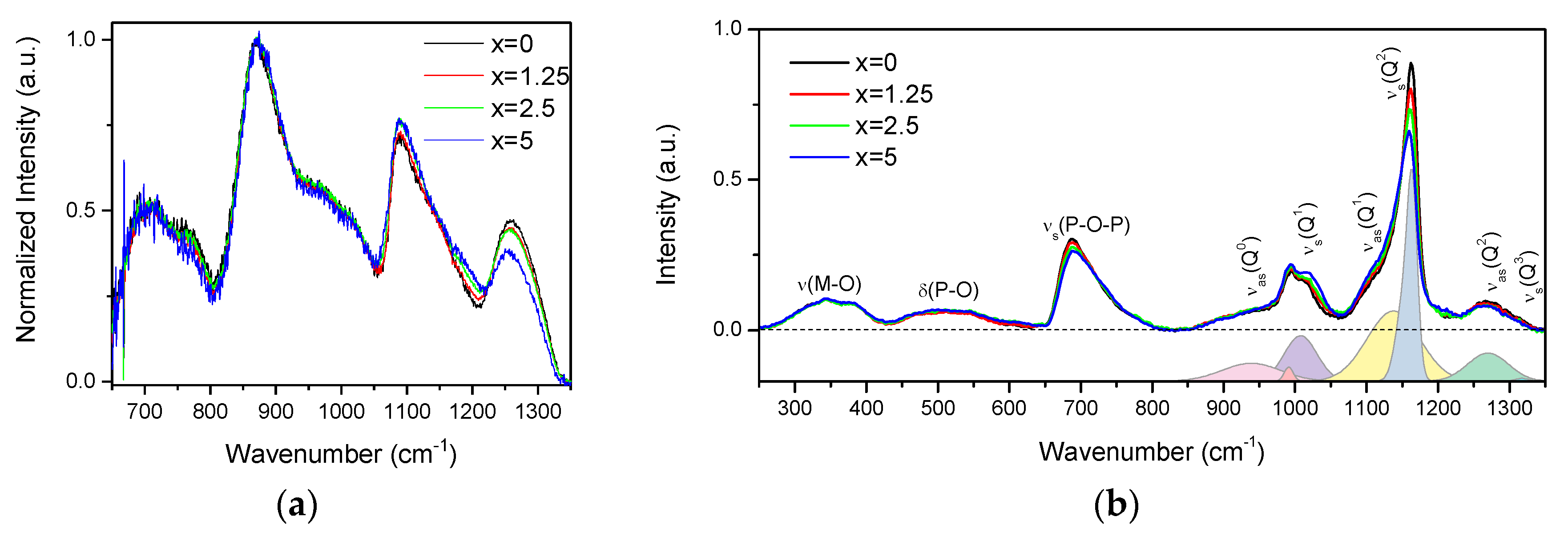
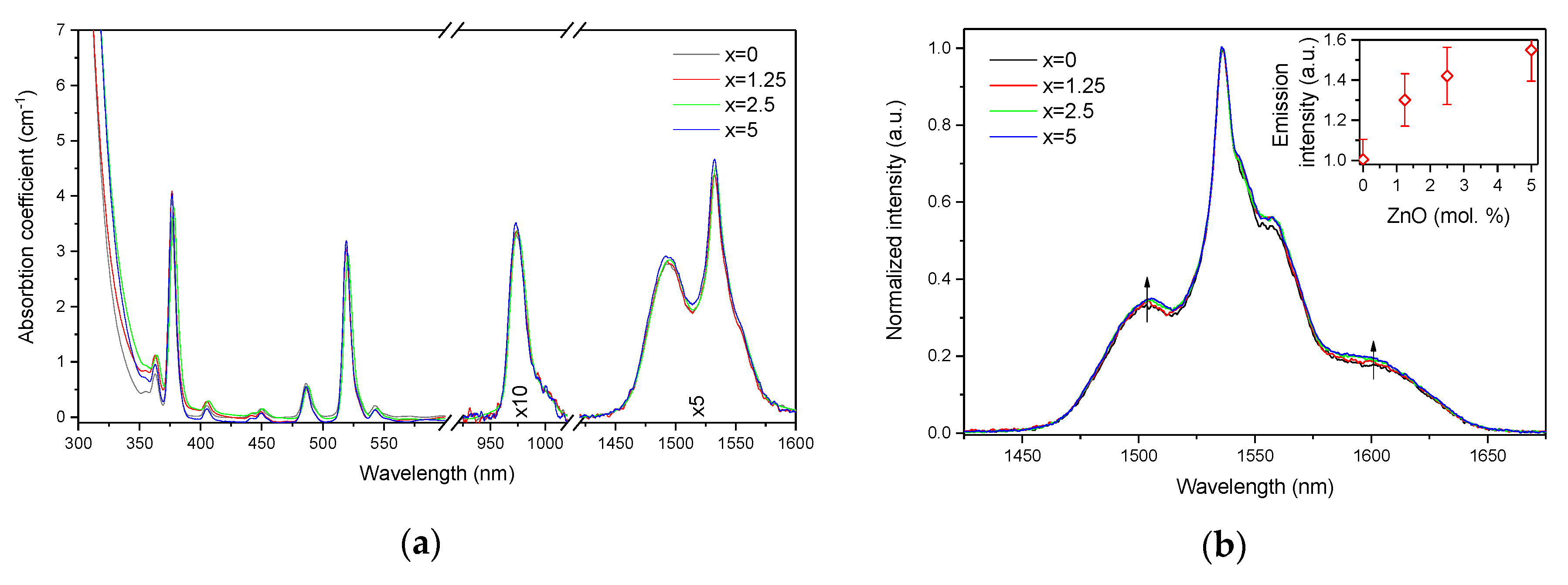
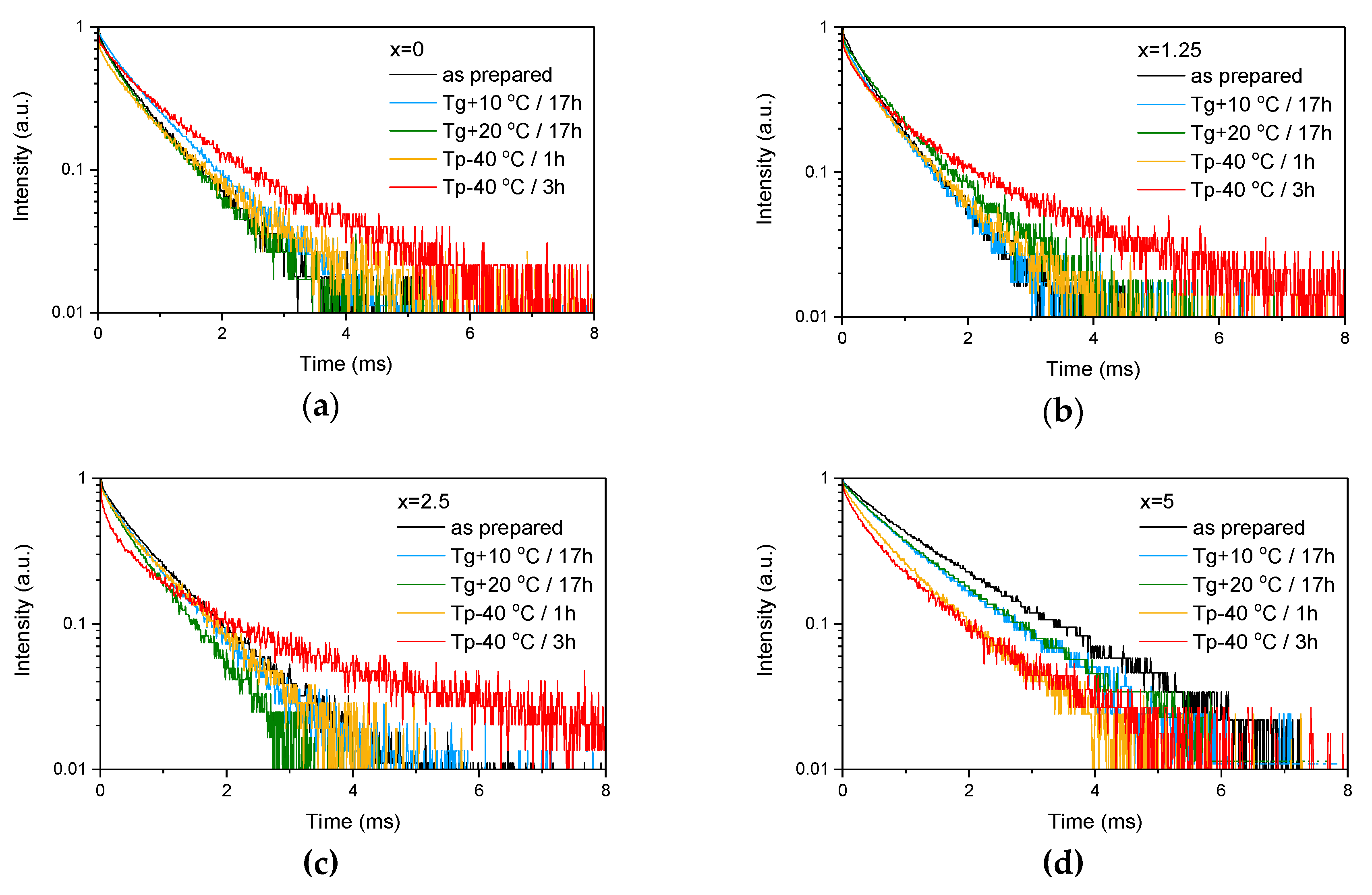
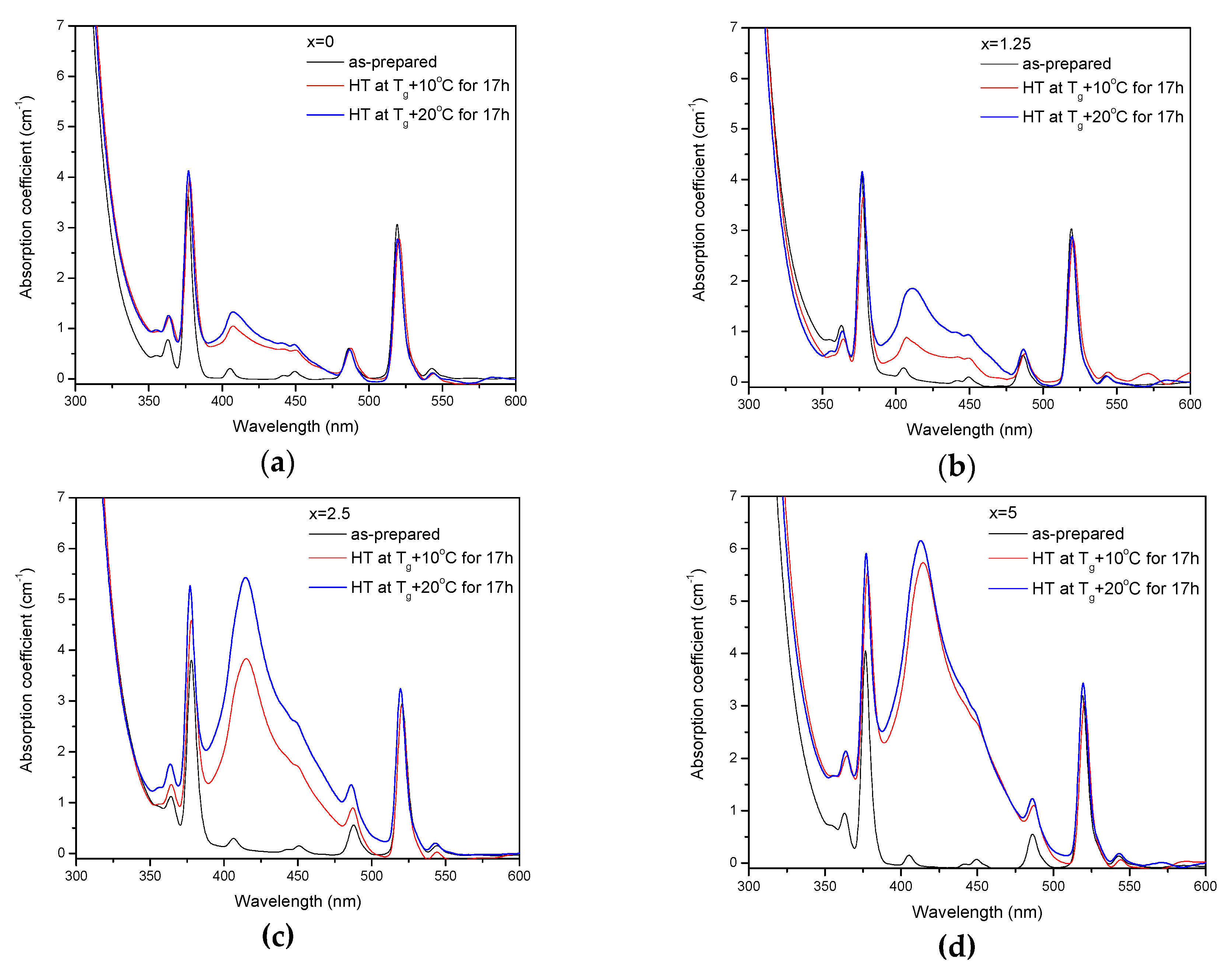
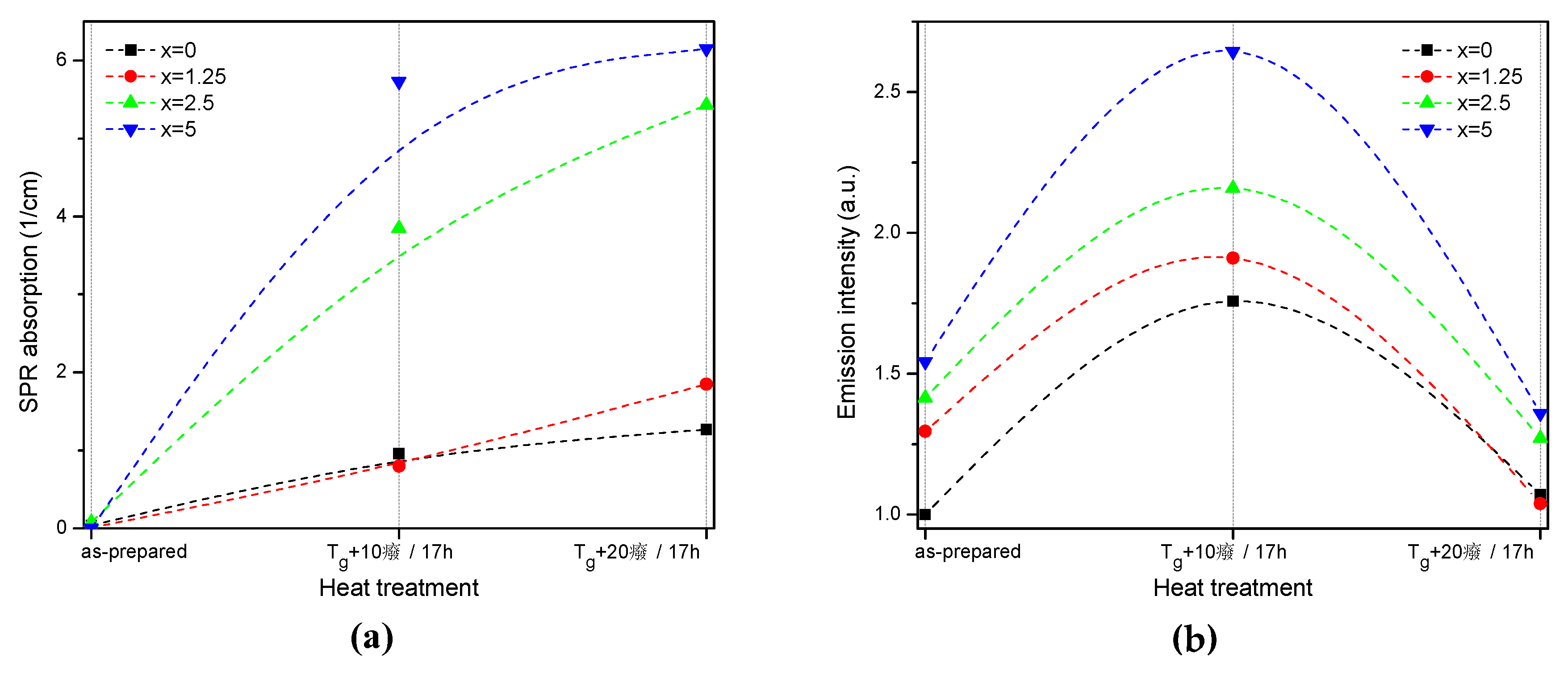
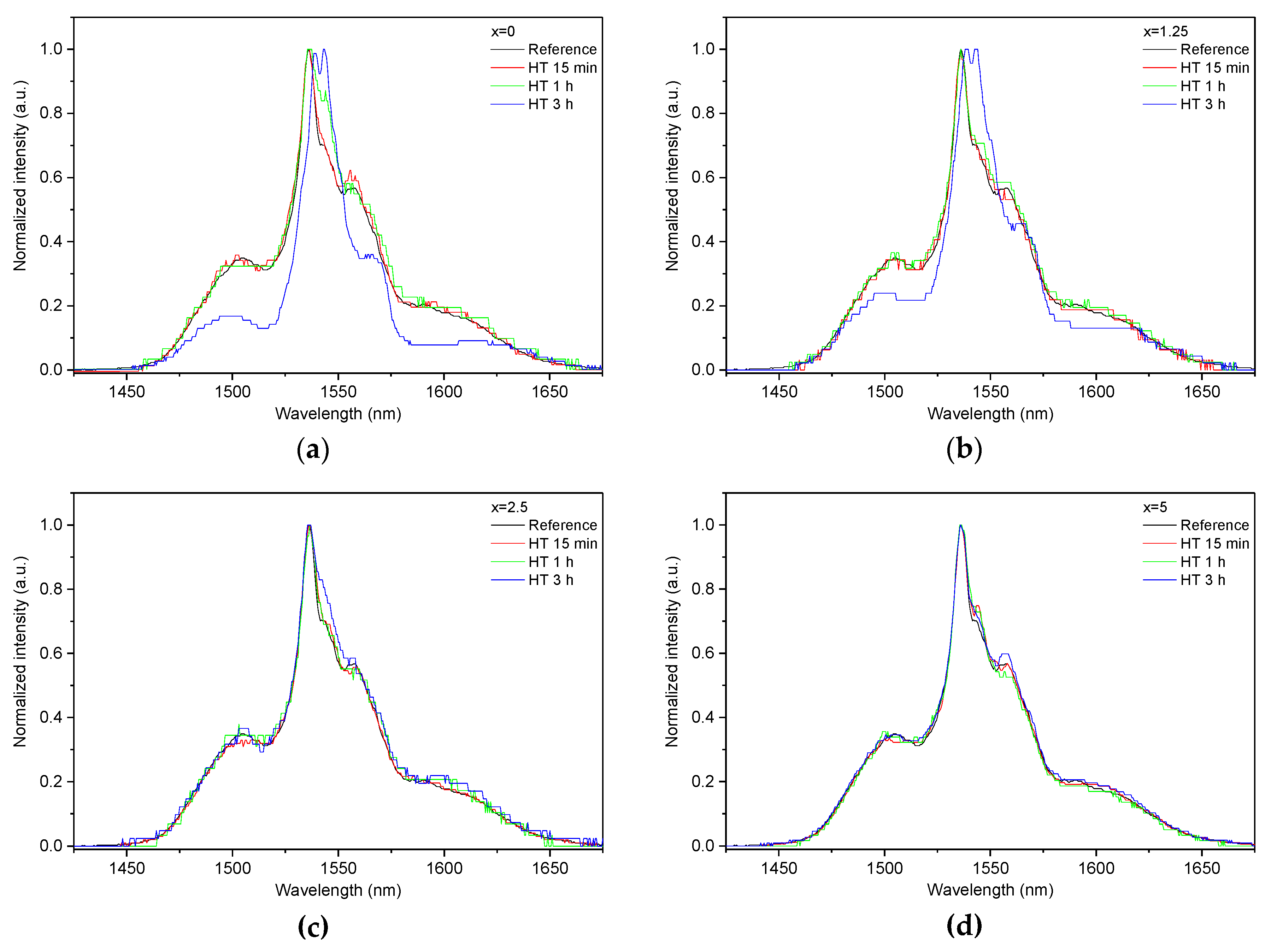
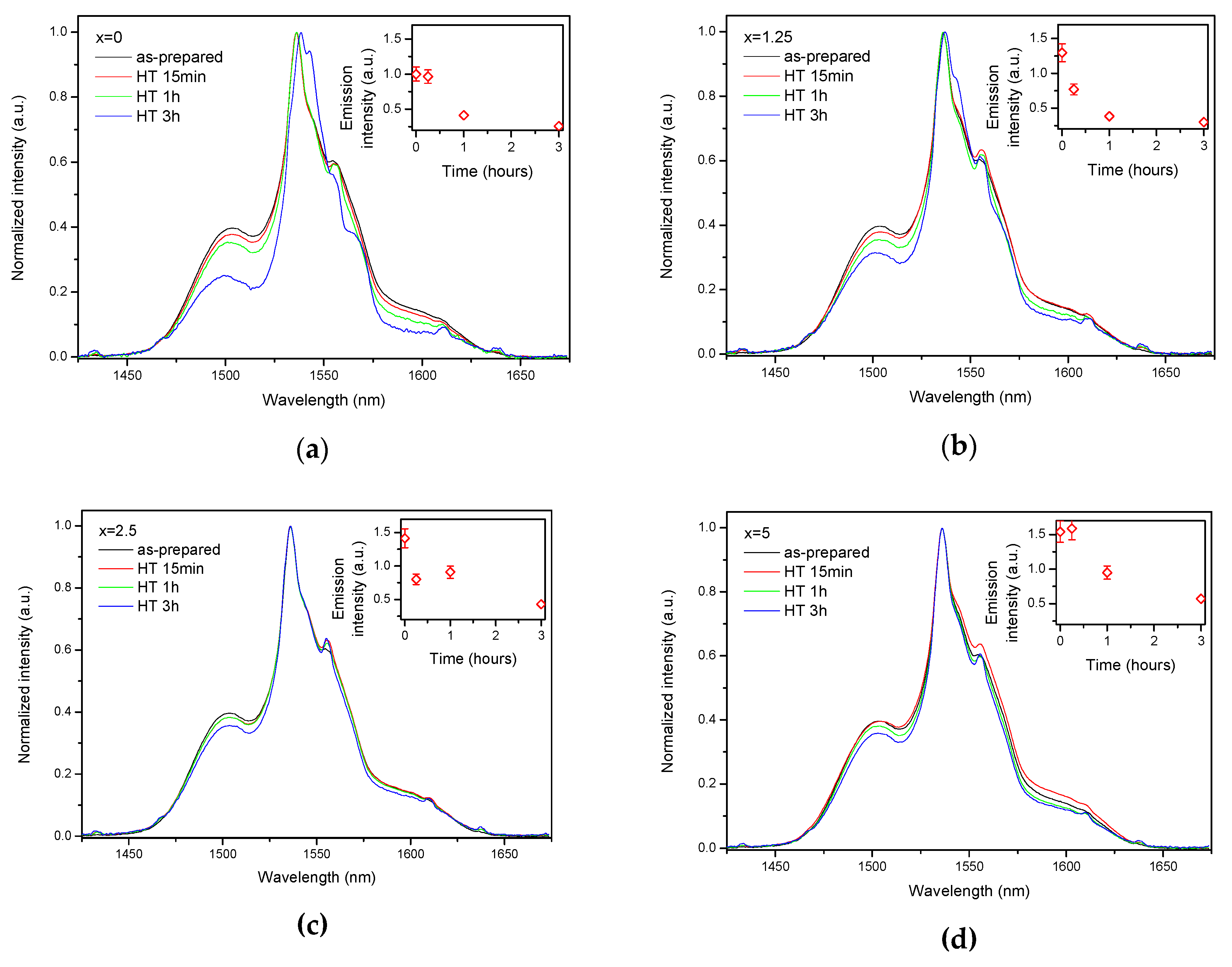
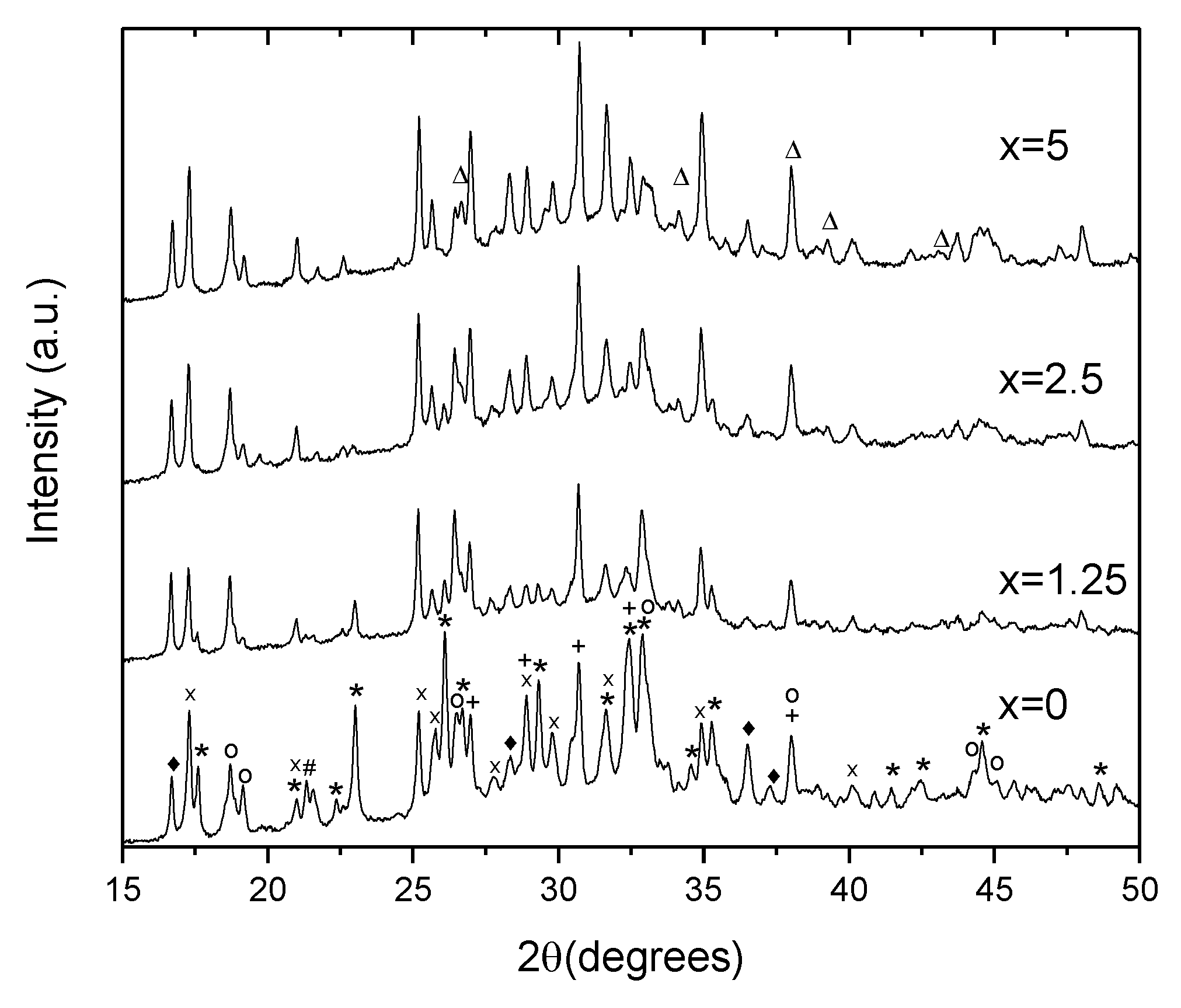
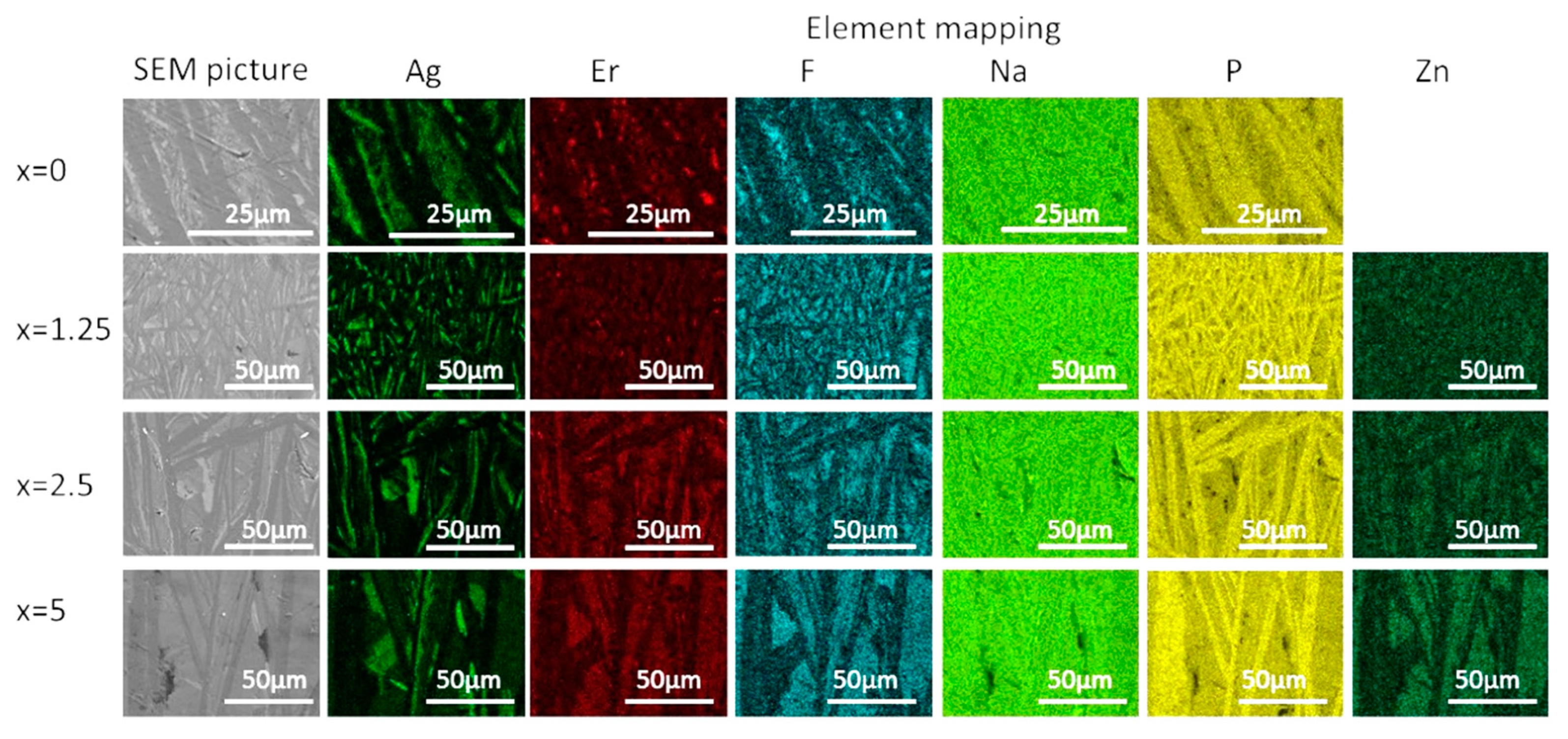
| x | ρ ± 0.02 (g/cm3) | Tg ± 3 (°C) | Tx ± 3 (°C) | Tp ± 3 (°C) | ΔT ± 6 (°C) | α 0.98 μm ± 0.03 (cm−1) | α 1.53 μm ± 0.06 (cm−1) | Er3+ (1019 ions/cm3) | σAbs 0.98 μm ± 0.6 (10−21 cm2) | σAbs 1.53 μm ± 1 (10−21 cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2.67 | 276 | 378 | 401 | 102 | 0.329 | 0.875 | 5.15 | 6.38 | 17.0 |
| 1.25 | 2.70 | 273 | 383 | 423 | 110 | 0.336 | 0.876 | 5.21 | 6.45 | 16.8 |
| 2.5 | 2.70 | 275 | 390 | 434 | 115 | 0.336 | 0.903 | 5.21 | 6.45 | 17.3 |
| 5 | 2.73 | 277 | 388 | 446 | 111 | 0.352 | 0.932 | 5.27 | 6.68 | 17.7 |
| x | as-prepared | Heat Treated for 17 h at | Heat Treated at (Tg + 20 °C) for 17 h and then at (Tp − 40 °C) for | ||
|---|---|---|---|---|---|
| (Tg + 10 °C) | (Tg + 20 °C) | 1 h | 3 h | ||
| 0 | 0.88 | 0.98 | 0.90 | 1.14 | 1.57 |
| 1.25 | 0.80 | 0.80 | 0.99 | 0.91 | 1.49 |
| 2.5 | 0.99 | 0.95 | 0.82 | 1.04 | 1.79 |
| 5 | 1.58 | 1.34 | 1.39 | 1.15 | 1.32 |
| x | as-prepared | Heat Treated for 17 h at | Heat treated at (Tg + 20 °C) for 17 h and then at (Tp − 40 °C) for | ||
|---|---|---|---|---|---|
| τ (ms)/β | (Tg + 10 °C) | (Tg + 20 °C) | 1 h | 3 h | |
| τ (ms)/β | τ (ms)/β | τ (ms)/β | τ (ms)/β | ||
| 0 | 0.55/0.74 | 0.69/0.80 | 0.56/0.76 | 0.53/0.65 | 0.80/0.70 |
| 1.25 | 0.5/0.76 | 0.52/0.76 | 0.61/0.75 | 0.46/0.67 | 0.46/0.54 |
| 2.5 | 0.66/0.77 | 0.66/0.81 | 0.57/0.80 | 0.63/0.74 | 0.3/0.44 |
| 5 | 1.26/0.84 | 1.01/0.83 | 1.06/0.83 | 0.71/0.76 | 0.56/0.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuusela, L.; Veber, A.; Boetti, N.G.; Petit, L. Impact of ZnO Addition on Er3+ Near-Infrared Emission, the Formation of Ag Nanoparticles, and the Crystallization of Sodium Fluorophosphate Glass. Materials 2020, 13, 527. https://doi.org/10.3390/ma13030527
Kuusela L, Veber A, Boetti NG, Petit L. Impact of ZnO Addition on Er3+ Near-Infrared Emission, the Formation of Ag Nanoparticles, and the Crystallization of Sodium Fluorophosphate Glass. Materials. 2020; 13(3):527. https://doi.org/10.3390/ma13030527
Chicago/Turabian StyleKuusela, Luukas, Alexander Veber, Nadia G. Boetti, and Laeticia Petit. 2020. "Impact of ZnO Addition on Er3+ Near-Infrared Emission, the Formation of Ag Nanoparticles, and the Crystallization of Sodium Fluorophosphate Glass" Materials 13, no. 3: 527. https://doi.org/10.3390/ma13030527
APA StyleKuusela, L., Veber, A., Boetti, N. G., & Petit, L. (2020). Impact of ZnO Addition on Er3+ Near-Infrared Emission, the Formation of Ag Nanoparticles, and the Crystallization of Sodium Fluorophosphate Glass. Materials, 13(3), 527. https://doi.org/10.3390/ma13030527







