High-Temperature Oxidation Behavior of Fe–1Cr–0.2Si Steel
Abstract
1. Introduction
2. Experimental
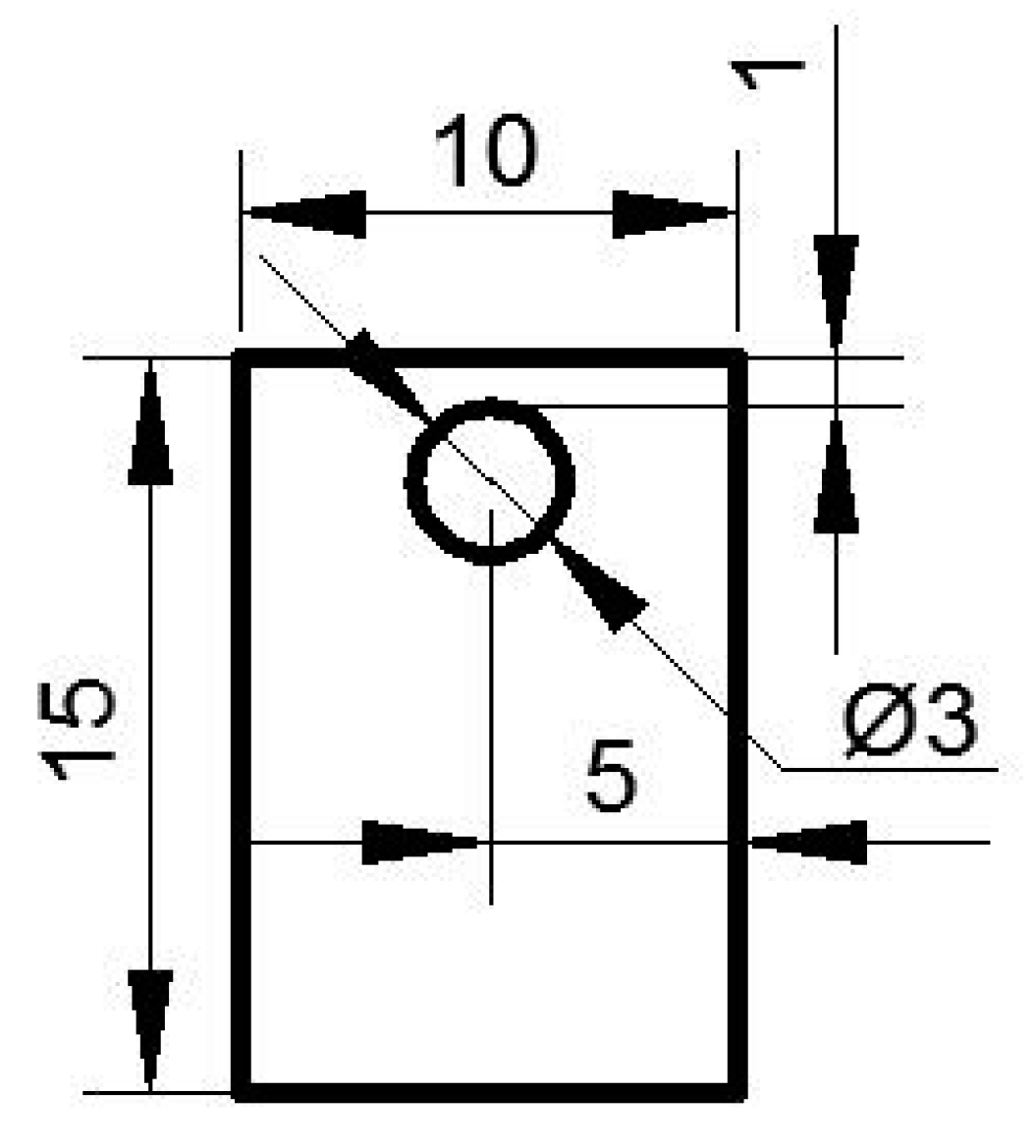
2.1. Oxidation Kinetics Experiment
2.2. Tube Furnace Oxidation Experiment
3. Results
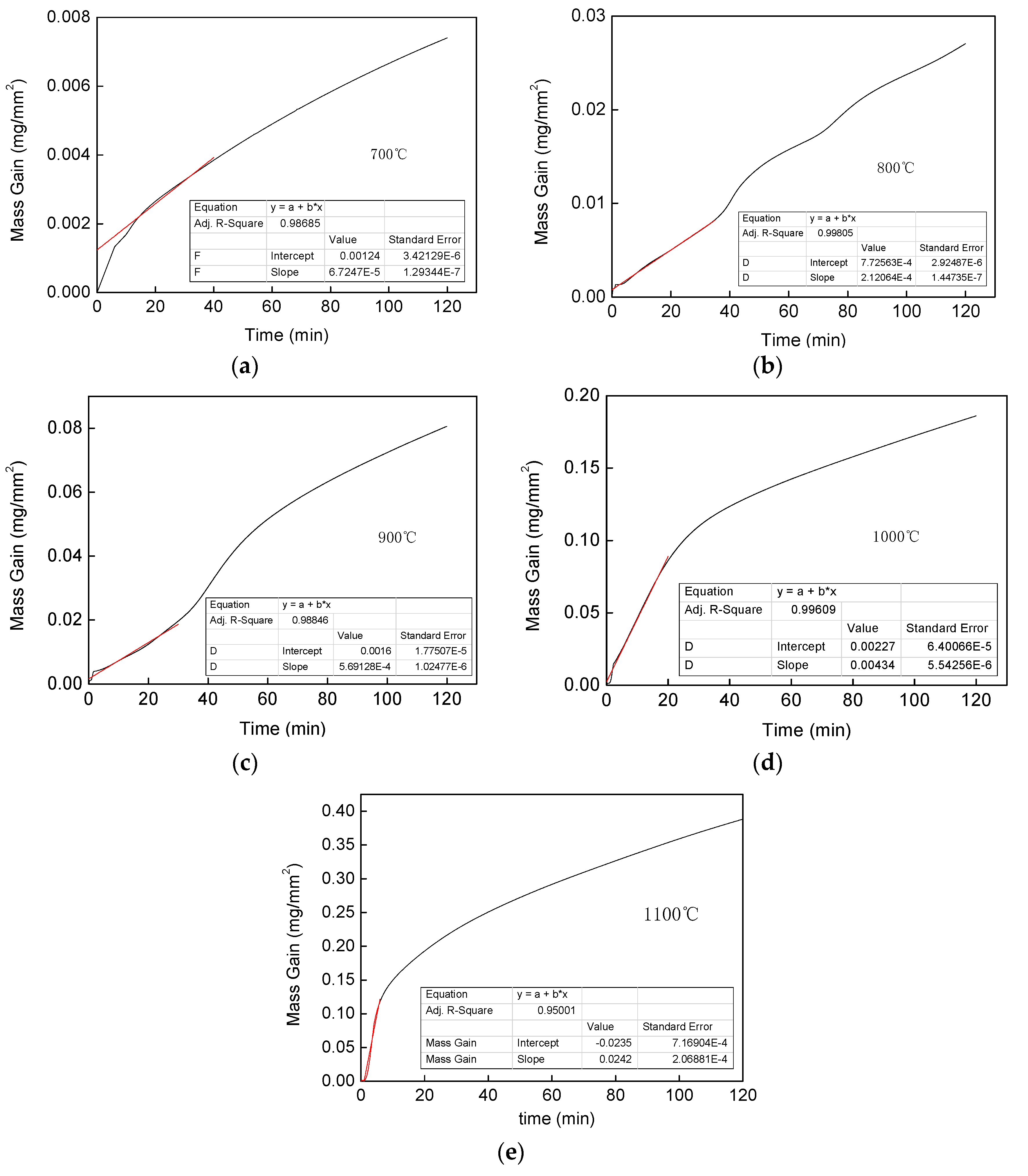
3.1. Oxidation Kinetics
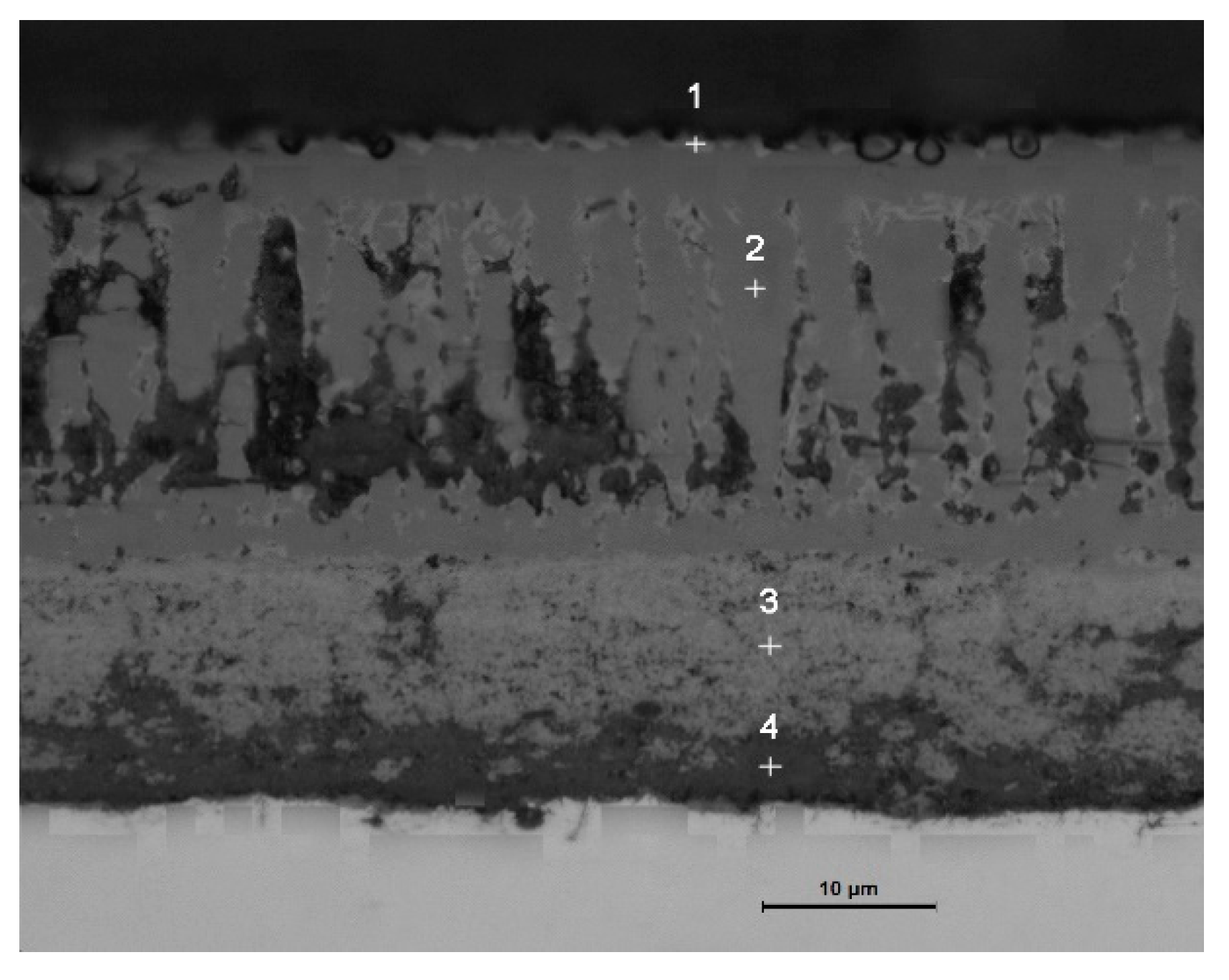
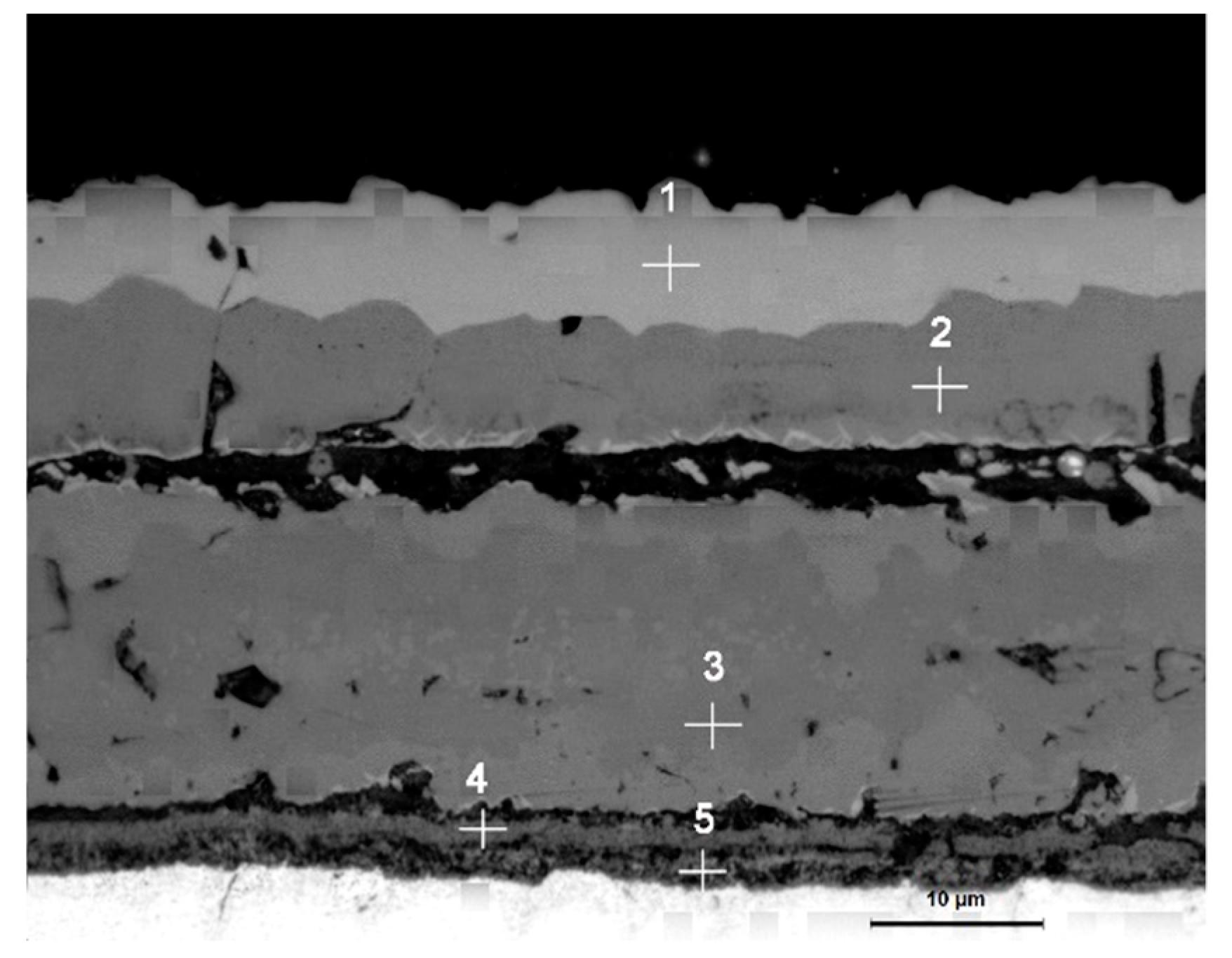
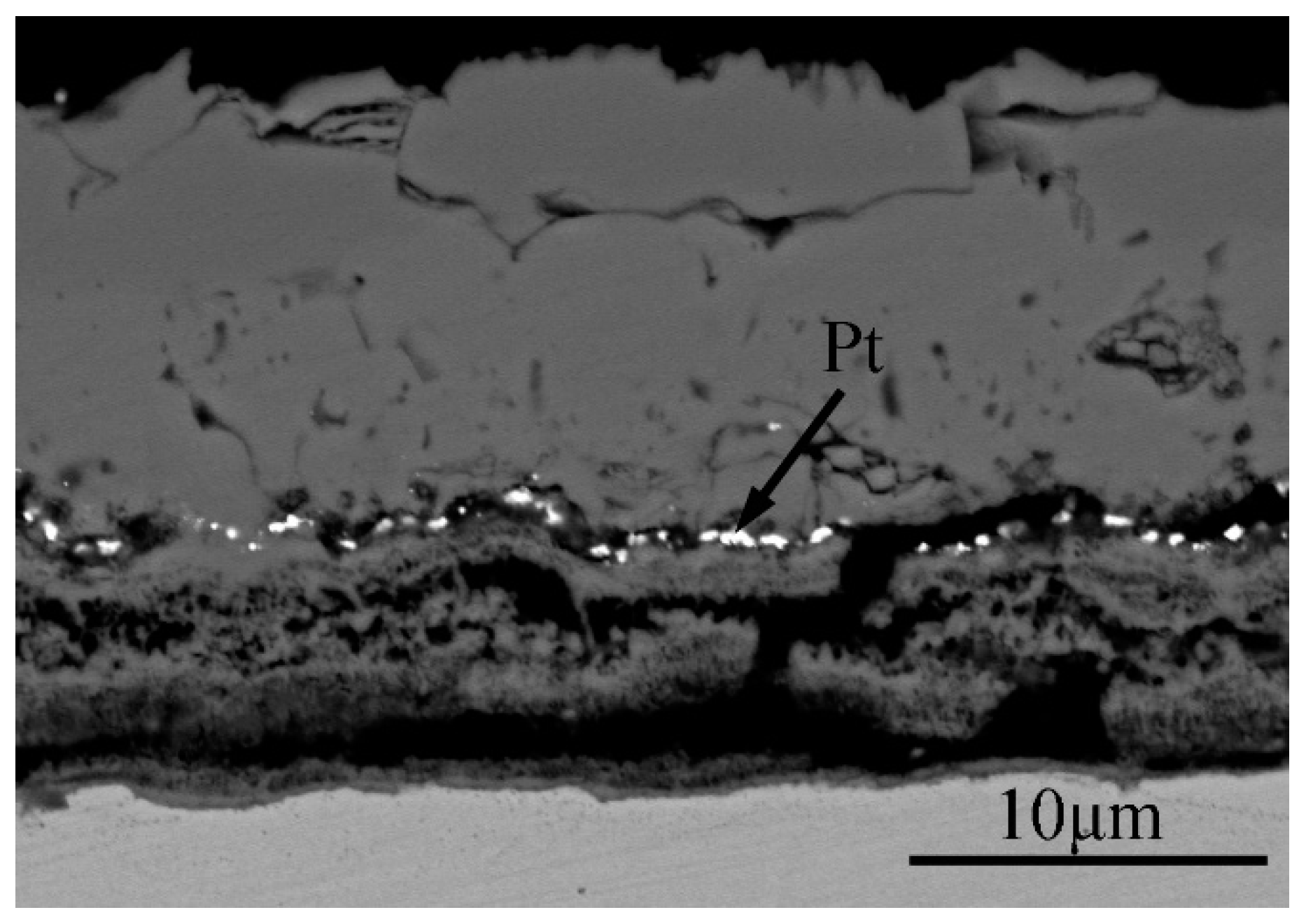
3.2. Cross-Sectional Morphology of the Oxide Scale on Tube Furnace Oxidation
4. Discussion
4.1. Oxidation Kinetic Model
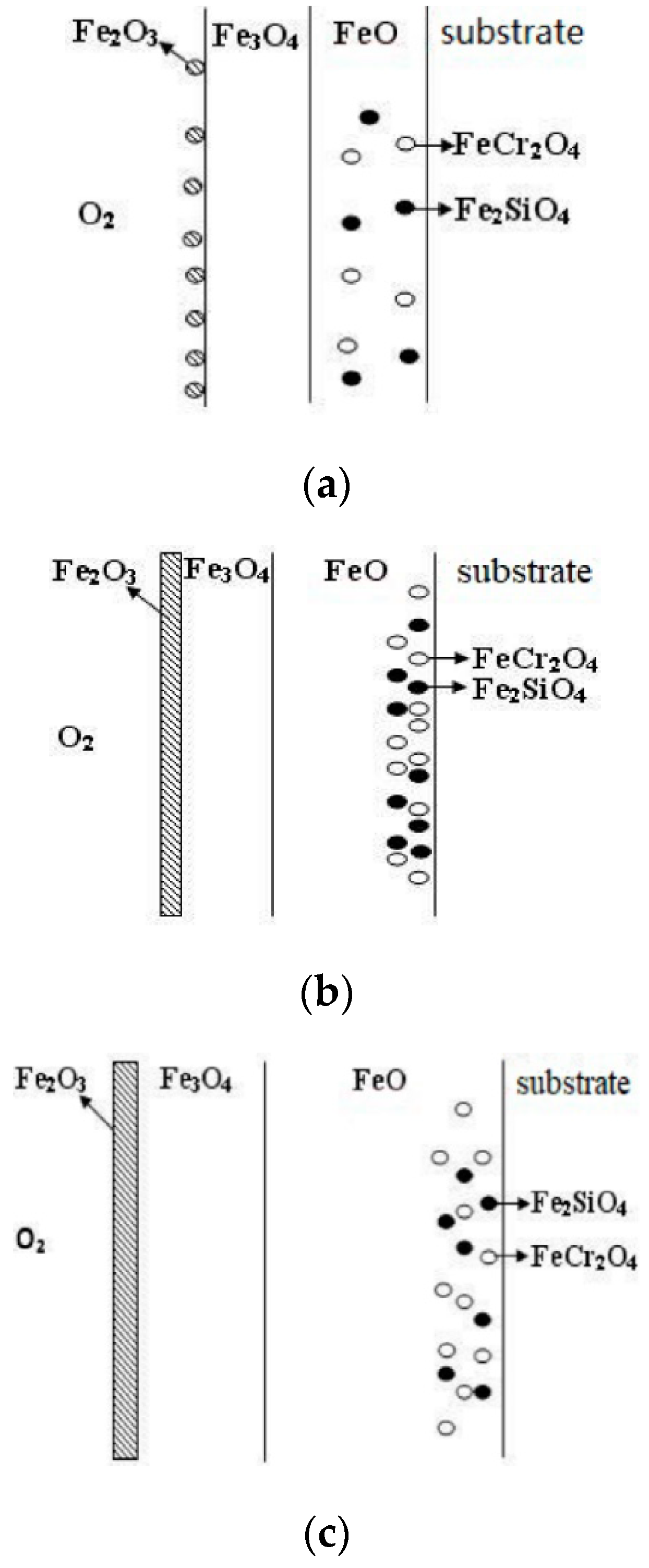
4.2. Oxidation Reaction
5. Conclusions
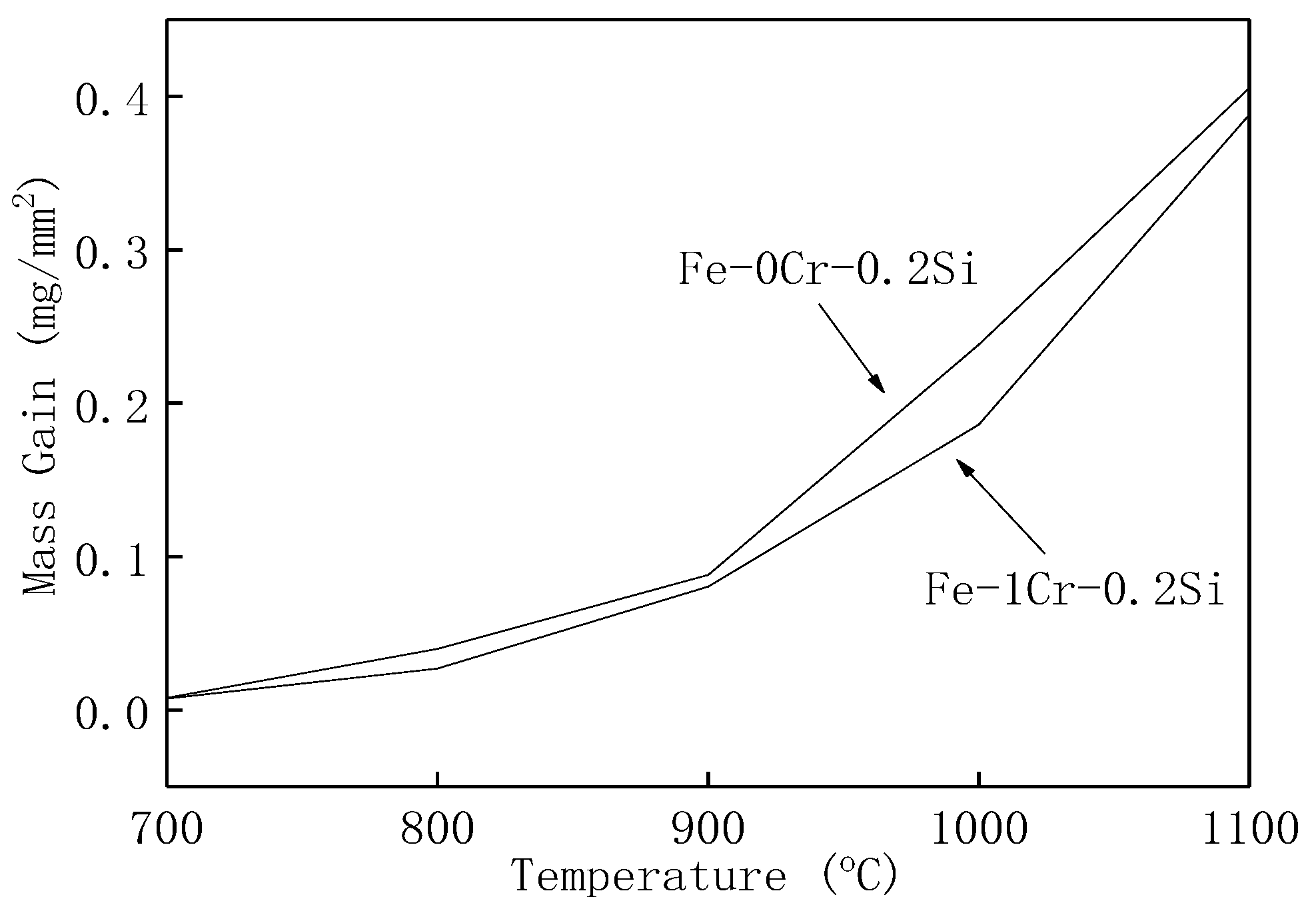
- After 120 min of oxidation at the same temperature, the oxidation weight gain per unit area of the Fe–1Cr–0.2Si steel is more significant than that of Fe–0.2Si steel, and the activation energy of Fe–1Cr–0.2Si steel is slightly larger than that of Fe–0.2Si steel. Since the steel used in this research has less chromium content, its oxidation resistance is not significantly improved.
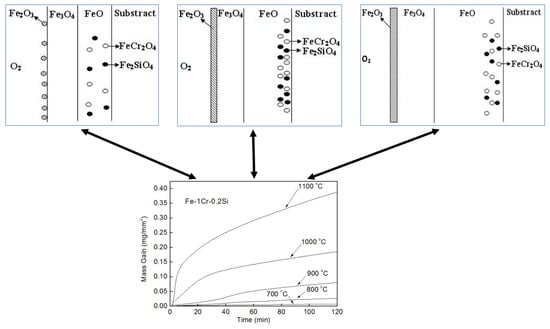
- The temperature is an essential factor affecting the formation of oxidation products in the high-temperature oxidation process of Fe–1Cr–0.2Si steel. The time for the linearity of the oxidation phase is shortened with an increase in the oxidation temperature. When the oxidation temperature exceeds 900 °C, the value of WTransition decreases, and the oxidation rule changes.
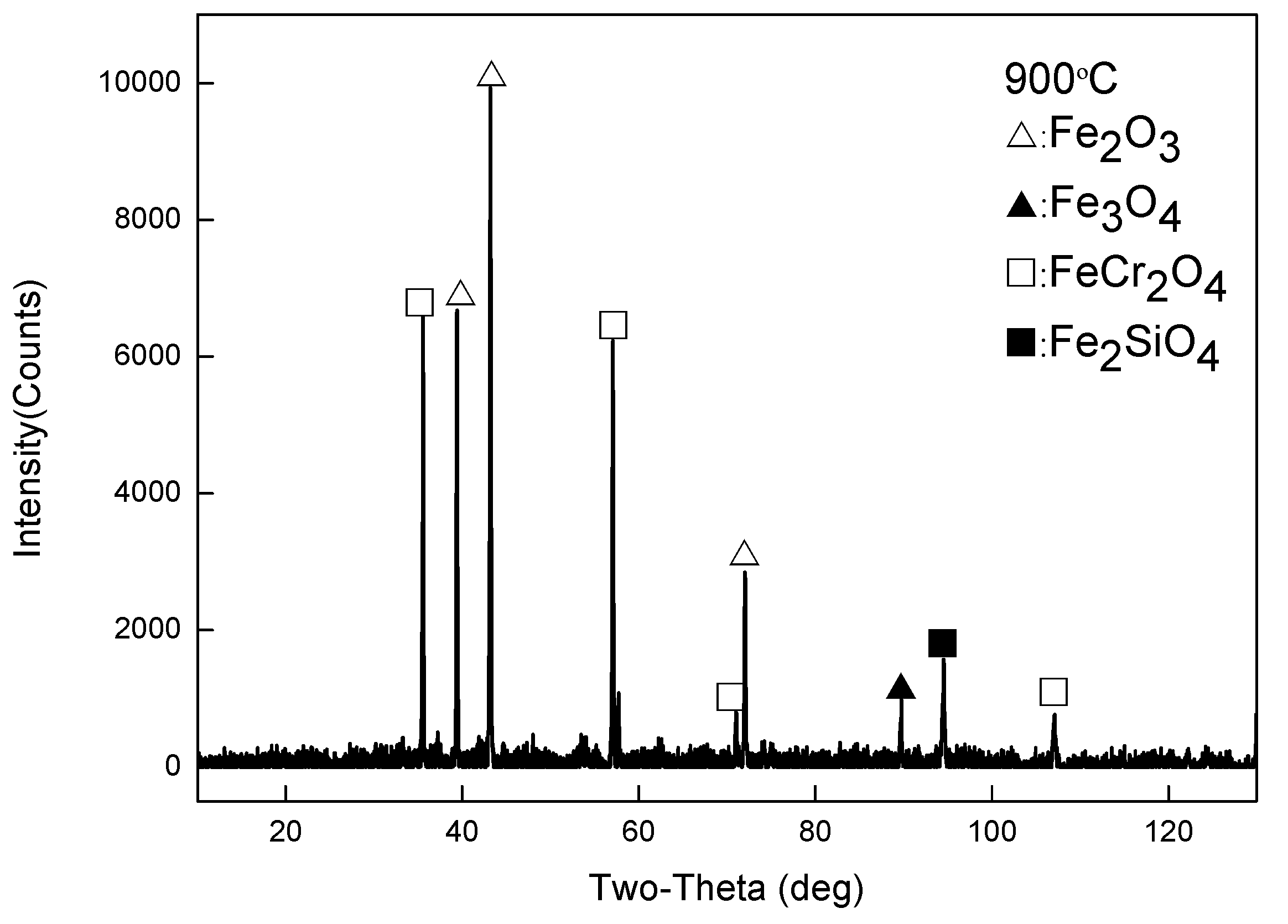
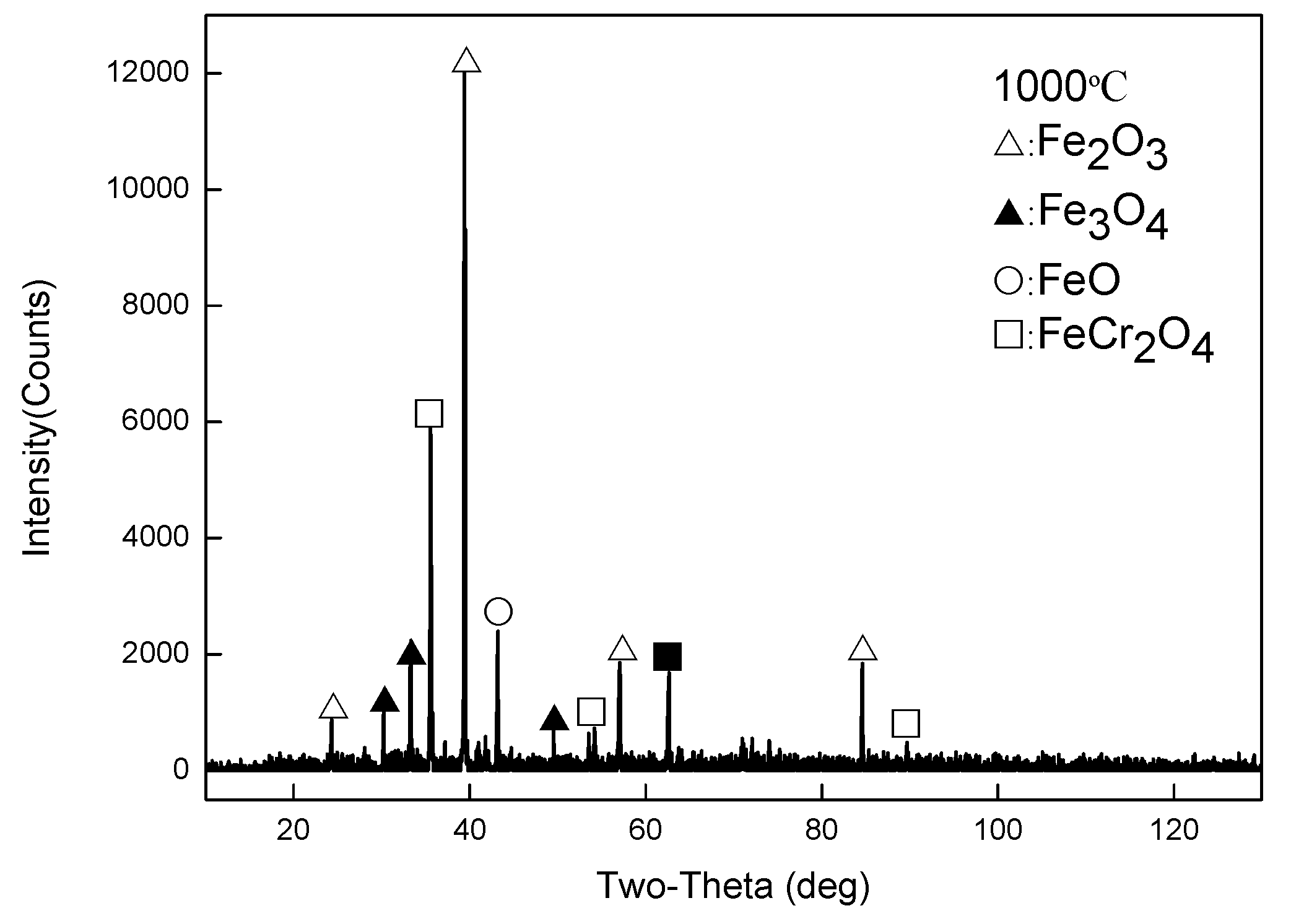
- When Fe–1Cr–0.2Si steel was oxidized at 900 °C, only a separate Fe3O4 layer in the iron oxide scale was noted, and no independent FeO layer was found out. Cr and Si elements enrich near the side of the substrate. At 1000 °C and 1100 °C, separate Fe2O3, Fe3O4, and FeO layers appear. The enrichment zone of Cr and Si also appears near the side of the substrate, and the degree of enrichment is more substantial than at 900 °C.
- The Cr and Si elements were enriched near the substrate side in the form of FeCr2O4 and Fe2SiO4, respectively, and no composite containing both Cr and Si was found.
- In the initial stage of oxidation, oxides such as SiO2, Cr2O3, Fe2O3, Fe3O4, and FeO were first formed. As the oxidation continued, the island-like Cr2O3 and SiO2 were surrounded by FeO, and then FeCr2O4 and Fe2SiO4 were formed by the solid-phase reaction, and the transition from internal oxidation to external oxidation was also completed.
Author Contributions
Funding
Conflicts of Interest
References
- Galerie, A.; Henry, S.; Wouters, Y.; Mermoux, M.; Petit, J.-P.; Antoni, L. Mechanisms of chromia scale failure during the course of 15–18Cr ferritic stainless steel oxidation in water vapour. Mater. High Temp. 2017, 21, 105–112. [Google Scholar]
- Adachi, T.; Meier, G.H. Oxidation of iron-silicon alloys. Oxid. Met. 1987, 27, 347–366. [Google Scholar] [CrossRef]
- Taniguchi, S.; Yamamoto, K.; Megumi, D.; Shibata, T. Characteristics of scale/substrate interface area of Si-containing low-carbon steels at high temperatures. Mater. Sci. Eng. A 2001, 308, 250–257. [Google Scholar] [CrossRef]
- Yang, Y.L.; Yang, C.H.; Lin, S.N.; Chen, C.-H.; Tsai, W.-T. Effects of Si and its content on the scale formation on hot-rolled steel strips. Mater. Chem. Phys. 2008, 112, 566–571. [Google Scholar] [CrossRef]
- Juricic, C.; Pinto, H.; Cardinali, D.; Klaus, M.; Genzel, C.; Pyzalla, A.R. Evolution of microstructure and internal stresses in multi-phase oxide scales grown on (110) surfaces of iron single crystals at 650 °C. Oxid. Met. 2009, 73, 115–138. [Google Scholar] [CrossRef]
- Westg, D.; Birosca, S.; Higginson, R.L. Phase determination and microstructure of oxide scales formed. J. Microsc. 2005, 217, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Y.; Yuen, W.Y.D. Oxide-scale structures formed on commercial hot-rolled steel strip and their formation mechanisms. Oxid. Met. 2001, 56, 89–118. [Google Scholar] [CrossRef]
- Juricic, C.; Pinto, H.; Cardinali, D.; Genzel, C.; Pyzalla, A.R. Effect of substrate grain size on the growth, texture and internal stresses of iron oxide scales forming at 450 °C. Oxid. Met. 2010, 73, 15–41. [Google Scholar] [CrossRef]
- Kahveci, A.I.; Welsch, G.E. Oxidation of Fe-3 Wt.% Cr alloy. Oxid. metals 1986, 26, 213–230. [Google Scholar] [CrossRef]
- Li, X.S.; Baek, S.I.; Oh, C.S.; Kim, S.-J.; Kim, Y.-W. Dew-point controlled oxidation of Fe–C–Mn–Al–Si–Cu transformation induced plasticity-aided steels. Scr. Mater. 2008, 59, 290–293. [Google Scholar] [CrossRef]
- Chen, R.Y.; Yuen, W.Y.D. A study of the scale structure of hot-rolled steel strip by simulated coiling and cooling. Oxid. Metals 2000, 53, 539–560. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Żurek, J.; Hänsel, M. Effect of water vapor on high-temperature oxidation of Fe-Cr alloys. JOM J. Miner. Metals Mater. Soc. 2009, 61, 44–50. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Xu, J.J.; Zhao, G.Q.; Guan, Y.J.; Li, M.S. Oxidation characteristic of ferritic-martensitic steel T91 in water- vapour atmosphere. Chin. J. Mater. Res. 2008, 22, 599–605. [Google Scholar]
- Ishitsuka, T.; Inoue, Y.; Ogawa, H. Effect of silicon on the steam oxidation resistance of a 9%Cr heat resistant steel. Oxid. Metals 2004, 61, 125–142. [Google Scholar] [CrossRef]
- Xu, L.N.; Wang, B.; Zhu, J.Y.; Zheng, Z.Y. Effect of Cr content on the corrosion performance of low-Cr alloy steel in a CO2 environment. Appl. Surf. Sci. 2016, 379, 39–42. [Google Scholar] [CrossRef]
- Bauer, R.; Baccalaro, M.; Jeurgens, L.P.; Pohl, M.; Mittemeijer, E.J. Oxidation behavior of Fe–25Cr–20Ni–2.8Si during isothermal oxidation at 1286 K; life-time prediction. Oxid. Metals 2008, 69, 265–281. [Google Scholar] [CrossRef]
- Neil, B.; Gerald, H.M.; Frederick, S.P. Introduction to the High-Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: London, UK, 2006; pp. 23–25. [Google Scholar]
- Chun, C.M.; Ramanarayanant, A. Corrosion resistance of a high-silicon alloy in metal-dusting environments. Oxid. Metals 2007, 67, 215–225. [Google Scholar] [CrossRef]
- Xia, W.C.; Zheng, Y.J.; Dong, B.W.; Zhao, J.W.; Monaghan, B.J.; Longbottom, R.J.; Jiang, L.Z. Characteristics of oxide scale formed on ferritic stainless steels in simulated reheating atmosphere. Surf. Coat. Technol. 2014, 258, 257–262. [Google Scholar]
- Pettersson, R.; Liu, L.; Soud, J. Cyclic oxidation performance of silicon-alloyed stainless steels in dry and moist air. Corros. Eng. Sci. Technol. 2005, 40, 211–219. [Google Scholar] [CrossRef]
- Wen, W.; Capolungo, L.; Patra, A.; Tomé, C.N. A physics-based crystallographic modeling framework for describing the thermal creep behavior of Fe-Cr alloys. Metall. Mater. Trans. 2017, 48, 2603–2617. [Google Scholar] [CrossRef]
- Bamba, G.; Donnadieu, P.; Wouters, Y.; Galerie, A. Evidence of Silica Layer at the Interface Between Ferrite and the Chromium Oxide Scale in Oxidized Fe-Cr-Si Alloys. In Proceedings of the EMC 2008 14th European Microscopy Congress, Aachen, Germany, 1–5 September 2008; Springer: Heidelberg, Germany, 2008; pp. 415–416. [Google Scholar]
- Chen, R.Y.; Yuen, W.Y.D. Review of the high-temperature oxidation of iron and carbon steels in air or oxygen. Oxid. Metals 2003, 59, 433–468. [Google Scholar] [CrossRef]
- Kiyoshi, K.; Ryoko, W.; Tomoharu, I. High-temperature oxidation behavior and scale morphology of si-containing steels. ISIJ Int. 2007, 47, 1329–1334. [Google Scholar]
- Dubiel, S.M.; Żukrowski, J. Kinetics of phase separation, border of miscibility gap in Fe–Cr and limit of Cr solubility in iron at 832 K. Mater. Charact. 2019, 158, 109937. [Google Scholar] [CrossRef]
- Chen, R.Y.; Yuen, W.Y.D. Short-time oxidation behavior of low-carbon, low-silicon steel in air at 850–1180 °C I: Oxidation Kinetics. Oxid. Metals 2008, 70, 39–68. [Google Scholar] [CrossRef]
- NevioBalo, S.; Yakuphanoglu, F. The effects of Cr on isothermal oxidation behavior of Fe-30Mn-6Si alloy. Thermochim. Acta 2013, 560, 43–46. [Google Scholar]
- Chen, R.Y.; Yuen, W.Y.D. Short-time oxidation behavior of low-carbon, low-silicon steel in air at 850–1180 °C II: Linear to parabolic transition determined using existing gas-phase transport and solid-phase diffusion theories. Oxid. Metals 2009, 73, 353–373. [Google Scholar] [CrossRef]
- Yuen, W.Y.D.; Chen, R.Y. Short-time oxidation behavior of low-carbon, low-silicon steel in air at 850–1180 °C III: Mixed linear-and-Parabolic to parabolic transition determined using local mass-transport theories. Oxid. Metals 2010, 74, 255–274. [Google Scholar] [CrossRef]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals; Elsevier: Amsterdam, The Netherlands, 2008; pp. 187–190. [Google Scholar]
- Michihisa, F.; Shigeru, M.; Shigenari, H.; Narita, T. Effect of water vapor on the oxidation behavior of Fe-1.5Si in air at 1073 and 1273K. Oxid. Metals 2001, 55, 401–411. [Google Scholar]
- Li, T.P. High Temperature Oxidation of Metal and hot Corrosion; Beijing Chemical Industry Press: Beijing, China, 2003; pp. 168–171. [Google Scholar]
- Issartel, C.; Buscailu, H.; Wang, Y.; Rolland, R.; Vilasi, M.; Aranda, L. Water vapour effect on ferritic 4509 steel oxidation between 800 and 1000 °C. Oxid. Metals 2011, 76, 127–134. [Google Scholar] [CrossRef]
- Chattopadhyay, A.L.; Chanda, T. Role of silicon on oxide morphology and pickling behaviour of automotive steels. Scr. Mater. 2008, 58, 882–891. [Google Scholar] [CrossRef]
- Lucia, S.; Pablo, R.C.; Yvan, H.; Rafael, C. Oxidation of ultra low carbon and silicon bearing steels. Corros. Sci. 2010, 52, 2044–2058. [Google Scholar]
- Peng, X.; Yan, J.; Zhou, Y.; Wang, F. Effect of grain refinement on the resistance of 304 stainless steel to breakaway oxidation in wet air. Acta Mater. 2005, 53, 5079–5097. [Google Scholar] [CrossRef]
- Asteman, H.; Svensson, J.E.; Johansson, L.G. Evidence for chromium evaporation influencing the oxidation of 304L: The effect of temperature and flow rate. Oxid. Metals 2002, 57, 193–201. [Google Scholar] [CrossRef]
- Xu, L.N.; Guo, S.Q.; Chang, W.; Chen, T.H.; Hu, L.H.; Lu, M.X. Corrosion of Cr bearing low alloy pipeline steel in CO2 environment at static and flowing conditions. Appl. Surf. Sci. 2013, 270, 395–407. [Google Scholar] [CrossRef]






| Sample | C | Cr | Si | P | Mn | S |
|---|---|---|---|---|---|---|
| Fe–1Cr–0.2Si | 0.09 | 1.05 | 0.22 | 0.22 | 0.47 | 0.012 |
| Point Position | O | Fe | Si | Cr |
|---|---|---|---|---|
| 1 | 58.26 | 41.74 | - | - |
| 2 | 56.69 | 43.31 | - | - |
| 3 | 55.13 | 35.89 | 3.42 | 5.57 |
| 4 | 60.33 | 26.40 | 5.12 | 8.14 |
| Point Position | O | Fe | Si | Cr |
|---|---|---|---|---|
| 1 | 58.26 | 41.74 | - | - |
| 2 | 56.69 | 43.31 | - | - |
| 3 | 52.13 | 47.87 | - | - |
| 4 | 55.13 | 35.89 | 3.42 | 5.57 |
| 5 | 60.33 | 26.40 | 5.12 | 8.14 |
| Temperature | Secondary Electron Image | Cr | Si |
|---|---|---|---|
| 900 °C | 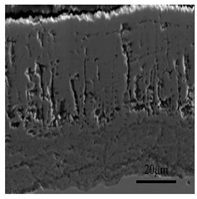 | 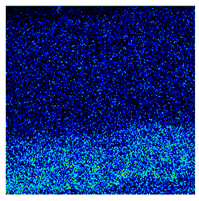 | 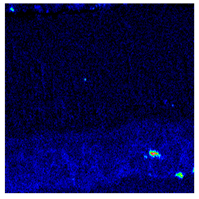 |
| 1000 °C | 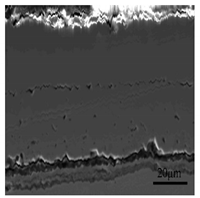 | 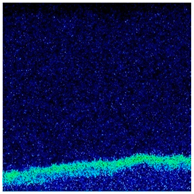 | 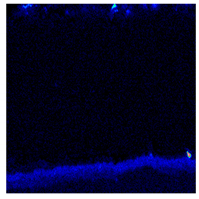 |
| 1100 °C | 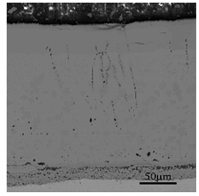 | 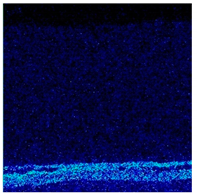 |  |
| Temperature (°C) | Kp (mg2mm−4s−1) | |
|---|---|---|
| Fe–1Cr–0.2Si | Fe–0.2Si | |
| 700 | 7.75 × 10−9 | 9.38 × 10−9 |
| 800 | 1.09 × 10−7 | 2.54 × 10−7 |
| 900 | 1.02 × 10−6 | 1.18 × 10−6 |
| 1000 | 4.72 × 10−6 | 7.69 × 10−6 |
| 1100 | 1.96 × 10−5 | 2.42 × 10−5 |
| Temperature (°C) | kl (mg.mm−2.min−1) |
|---|---|
| 700 | 6.7247 × 10−5 |
| 800 | 2.12064 × 10−4 |
| 900 | 5.69128 × 10−4 |
| 1000 | 4.34 × 10−3 |
| 1100 | 24.2 × 10−3 |
| Temperature (°C) | WTransition (mg·mm−2) |
|---|---|
| 700 | 0.003457189 |
| 800 | 0.015404453 |
| 900 | 0.053911422 |
| 1000 | 0.032651959 |
| 1100 | 0.024373388 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, M.; Sun, B.; Wang, H. High-Temperature Oxidation Behavior of Fe–1Cr–0.2Si Steel. Materials 2020, 13, 509. https://doi.org/10.3390/ma13030509
Hao M, Sun B, Wang H. High-Temperature Oxidation Behavior of Fe–1Cr–0.2Si Steel. Materials. 2020; 13(3):509. https://doi.org/10.3390/ma13030509
Chicago/Turabian StyleHao, Mingxin, Bin Sun, and Hao Wang. 2020. "High-Temperature Oxidation Behavior of Fe–1Cr–0.2Si Steel" Materials 13, no. 3: 509. https://doi.org/10.3390/ma13030509
APA StyleHao, M., Sun, B., & Wang, H. (2020). High-Temperature Oxidation Behavior of Fe–1Cr–0.2Si Steel. Materials, 13(3), 509. https://doi.org/10.3390/ma13030509