Impact of Pyrolysis Temperature on the Properties of Eucalyptus Wood-Derived Biochar
Abstract
1. Introduction
2. Material and Methods
2.1. Biochar Production
2.2. Biochar Characterization
2.2.1. Elemental and Energy Dispersion X-ray Fluorescence Spectrometry Analyses
2.2.2. Ash and Volatile Matter (VM) Contents
2.2.3. pH Value and Electrical Conductivity (EC)
2.2.4. Specific Surface Area (SSA) and Morphology
2.2.5. Chemical Analysis
2.3. Data Analysis
3. Results and Discussion
3.1. Effects of Pyrolysis Temperature on Yield, VM Content, Ash Content, pH Value, EC, and Inorganic Minerals from Biochar Produced
3.2. Elemental Analysis
3.3. SSA, TPV, Surface Morphology, Crystalline Phase, and Functional Groups
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ronsse, F.; Van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2012, 5, 104–115. [Google Scholar] [CrossRef]
- Lin, Q.; Xu, X.; Wang, L.; Chen, Q.; Fang, J.; Shen, X.; Lou, L.; Tian, G. The speciation, leachability and bioaccessibility of Cu and Zn in animal manure-derived biochar: Effect of feedstock and pyrolysis temperature. Front. Environ. Sci. Eng. 2017, 11, 5. [Google Scholar] [CrossRef]
- Kookana, R.; Sarmah, A.; Van Zwieten, L.; Krull, E.; Singh, B. Biochar Application to Soil. Adv. Agron. 2011, 112, 103–143. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Manna, S.; Singh, N. Biochars mediated degradation, leaching and bioavailability of pyrazosulfuron-ethyl in a sandy loam soil. Geoderma 2019, 334, 63–71. [Google Scholar] [CrossRef]
- IPEA. Instituto de Pesquisas Aplicadas Diagnóstico dos Resíduos Orgânicos do Setor Agrossilvopastoril e Agroindústrias Associadas: Relatório de Pesquisa. Governo Federal. Brasília. 2012. Available online: https://www.ipea.gov.br/portal/images/stories/PDFs/relatoriopesquisa/120917_relatorio_residuos_organicos.pdf (accessed on 12 October 2019).
- IBÁ Indústria Brasileira de Árvores (Brazilian Tree Industry). Annual Report 2017; IBÁ: São Paulo, Brazil, 2017; Available online: http://iba.org/images/shared/Biblioteca/IBA_RelatorioAnual2017.pdf (accessed on 12 October 2019).
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A Synthesis of Its Agronomic Impact beyond Carbon Sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A Review of Non-Soil Biochar Applications. Materials 2020, 13, 261. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Thermogravimetric, thermochemical, and infrared spectral characterization of feedstocks and biochar derived at different pyrolysis temperatures. Waste Manag. 2018, 78, 198–207. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Li, Z.; Feng, R.; Zhang, Y. Characterization of corncob-derived biochar and pyrolysis kinetics in comparison with corn stalk and sawdust. Bioresour. Technol. 2014, 170, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass-Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Huff, M.D.; Kumar, S.; Lee, J.W. Comparative analysis of pinewood, peanut shell, and bamboo biomass derived biochars produced via hydrothermal conversion and pyrolysis. J. Environ. Manag. 2014, 146, 303–308. [Google Scholar] [CrossRef]
- Jones, D.; Edwards-Jones, G.; Murphy, D. Biochar mediated alterations in herbicide breakdown and leaching in soil. Soil Biol. Biochem. 2011, 43, 804–813. [Google Scholar] [CrossRef]
- Heidari, A.; Khaki, E.; Younesi, H.; Lu, H.R. Evaluation of fast and slow pyrolysis methods for bio-oil and activated carbon production from eucalyptus wastes using a life cycle assessment approach. J. Clean. Prod. 2019, 241, 118394. [Google Scholar] [CrossRef]
- Júnior, A.F.D.; Pirola, L.P.; Takeshita, S.; Lana, A.Q.; Brito, J.O.; De Andrade, A.M. Higroscopicity of charcoal produced in different temperatures. CERNE 2016, 22, 423–430. [Google Scholar] [CrossRef]
- Standards ASTM International. Ash in the Analysis Sample of Coal and Coke from Coal. D3174-12; Standards ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Standards ASTM International. Volatile Matter in the Analysis Sample of Coal and Coke. D3175; Standards ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ISO 10390. Soil Quality–Determination of pH; ISO (International Standards Organization): Chiswick, UK, 2005. [Google Scholar]
- BGK. Methodenbuch zur Analyse von Kompost; Bundesgütegemeinschaft Kompost e.V. (Federal Compost Quality Assurance Organisation FCQAO): Stuttgart, Germany, 1994. [Google Scholar]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Li, S.; Barreto, V.; Li, R.; Chen, G.; Hsieh, Y.P. Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2018, 133, 136–146. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Zhu, L. Transformation, Morphology, and Dissolution of Silicon and Carbon in Rice Straw-Derived Biochars under Different Pyrolytic Temperatures. Environ. Sci. Technol. 2014, 48, 3411–3419. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, T.; Huang, H.; Zhao, D.; Kobayashi, N.; Chen, Y. Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. J. Anal. Appl. Pyrolysis 2015, 112, 284–289. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, M.; Fan, X.; Song, J.; Peng, P.; Li, K.; Jia, W.; Song, H. Influence of pyrolysis temperature and feedstock on carbon fractions of biochar produced from pyrolysis of rice straw, pine wood, pig manure and sewage sludge. Chemosphere 2019, 218, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management. Science, Technology and Implementation; Routledge: London, UK, 2015. [Google Scholar]
- Zhao, R.; Coles, N.; Kong, Z.; Wu, J. Effects of aged and fresh biochars on soil acidity under different incubation conditions. Soil Tillage Res. 2015, 146, 133–138. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Álvarez, M.L.; De Figueiredo, C.C.; Méndez, A.; Gascó, G. Effect of Biochar and Hydrochar on Forms of Aluminium in an Acidic Soil. Appl. Sci. 2020, 10, 7843. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Nieto, A.; Méndez, A.; Askeland, M.P.J.; Gascó, G. Biochar from Biosolids Pyrolysis: A Review. Int. J. Environ. Res. Public Health 2018, 15, 956. [Google Scholar] [CrossRef]
- Bouqbis, L.; Daoud, S.; Koyro, H.W.; Kammann, C.I.; Ainlhout, F.Z.; Harrouni, M.C. Phytotoxic effects of argan shell biochar on salad and barley germination. Agric. Nat. Resour. 2017, 51, 247–252. [Google Scholar] [CrossRef]
- Olszyk, D.M.; Shiroyama, T.; Novak, J.M.; Johnson, M.G. A rapid-test for screening biochar effects on seed germination. Commun. Soil Sci. Plant Anal. 2018, 49, 2025–2041. [Google Scholar] [CrossRef]
- Mumme, J.; Getz, J.; Prasad, M.; Lüder, U.; Kern, J.; Mašek, O.; Buss, W. Toxicity screening of biochar-mineral composites using germination tests. Chemosphere 2018, 207, 91–100. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Moghaddam, M.; Lakzian, A. Alleviating negative effects of salinity stress in summer savory (Satureja hortensis L.) by biochar application. Acta Physiol. Plant. 2019, 41, 98. [Google Scholar] [CrossRef]
- Rezaie, N.; Razzaghi, F.; Sepaskhah, A.R. Different Levels of Irrigation Water Salinity and Biochar Influence on Faba Bean Yield, Water Productivity, and Ions Uptake. Commun. Soil Sci. Plant Anal. 2019, 50, 611–626. [Google Scholar] [CrossRef]
- Usman, A.R.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- A Spokas, K. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Huang, H.-J.; Yang, T.; Lai, F.-Y.; Wu, G.-Q. Co-pyrolysis of sewage sludge and sawdust/rice straw for the production of biochar. J. Anal. Appl. Pyrolysis 2017, 125, 61–68. [Google Scholar] [CrossRef]
- Comparison of European Biochar Certificate Version 4. 8 and IBI Biochar Standards Version 2.0. March 2012; pp. 1–5. Available online: https://www.biochar-international.org/wp-content/uploads/2018/04/IBI-EBC_comparison_Oct2014.pdf (accessed on 21 December 2020).
- Zhang, J.; Liu, J.; Liu, R. Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S. Interaction between biomass and different rank coals during co-pyrolysis. Renew. Energy 2010, 35, 288–292. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Neves, D.S.F.; Thunman, H.; Matos, M.A.A.; Tarelho, L.A.D.C.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Wang, X.; Zhang, S.; Chen, H. Biomass-based pyrolytic polygeneration system on cotton stalk pyrolysis: Influence of temperature. Bioresour. Technol. 2012, 107, 411–418. [Google Scholar] [CrossRef]
- Essandoh, M.; Wolgemuth, D.; Pittman, C.U.; Mohan, D.; Mlsna, T.E. Phenoxy herbicide removal from aqueous solutions using fast pyrolysis switchgrass biochar. Chemosphere 2017, 174, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, M.; Kunwar, B.; Pittman, C.U.; Mohan, D.; Mlsna, T.E. Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem. Eng. J. 2015, 265, 219–227. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, Z.; So, C.-L.; Hse, C.-Y. Rapid prediction of wood crystallinity in Pinus elliotii plantation wood by near-infrared spectroscopy. J. Wood Sci. 2007, 53, 449–453. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef]
- Paris, O.; Zollfrank, C.; Zickler, G.A. Decomposition and carbonisation of wood biopolymers—A microstructural study of softwood pyrolysis. Carbon 2005, 43, 53–66. [Google Scholar] [CrossRef]
- Tushar, M.S.H.K.; Mahinpey, N.; Khan, A.; Ibrahim, H.; Kumar, P.; Idem, R. Production, characterization and reactivity studies of chars produced by the isothermal pyrolysis of flax straw. Biomass-Bioenergy 2012, 37, 97–105. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of Pyrolysis Temperature on Biochar Property and Function as a Heavy Metal Sorbent in Soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional Adsorption and Partition of Nonpolar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Adel, A.M.; El–Wahab, Z.H.A.; Ibrahim, A.A.; Al–Shemy, M.T. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. Acid catalyzed hydrolysis. Bioresour. Technol. 2010, 101, 4446–4455. [Google Scholar] [CrossRef]
- Marx, S.; Chiyanzu, I.; Piyo, N. Influence of reaction atmosphere and solvent on biochar yield and characteristics. Bioresour. Technol. 2014, 164, 177–183. [Google Scholar] [CrossRef]
- Yang, F.; Gao, Y.; Sun, L.; Zhang, S.; Li, J.; Zhang, Y. Effective sorption of atrazine by biochar colloids and residues derived from different pyrolysis temperatures. Environ. Sci. Pollut. Res. 2018, 25, 18528–18539. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hoffman-Krull, K.; Bidwell, A.L.; DeLuca, T.H. Locally produced wood biochar increases nutrient retention and availability in agricultural soils of the San Juan Islands, USA. Agric. Ecosyst. Environ. 2016, 233, 43–54. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Sci. Total Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef] [PubMed]


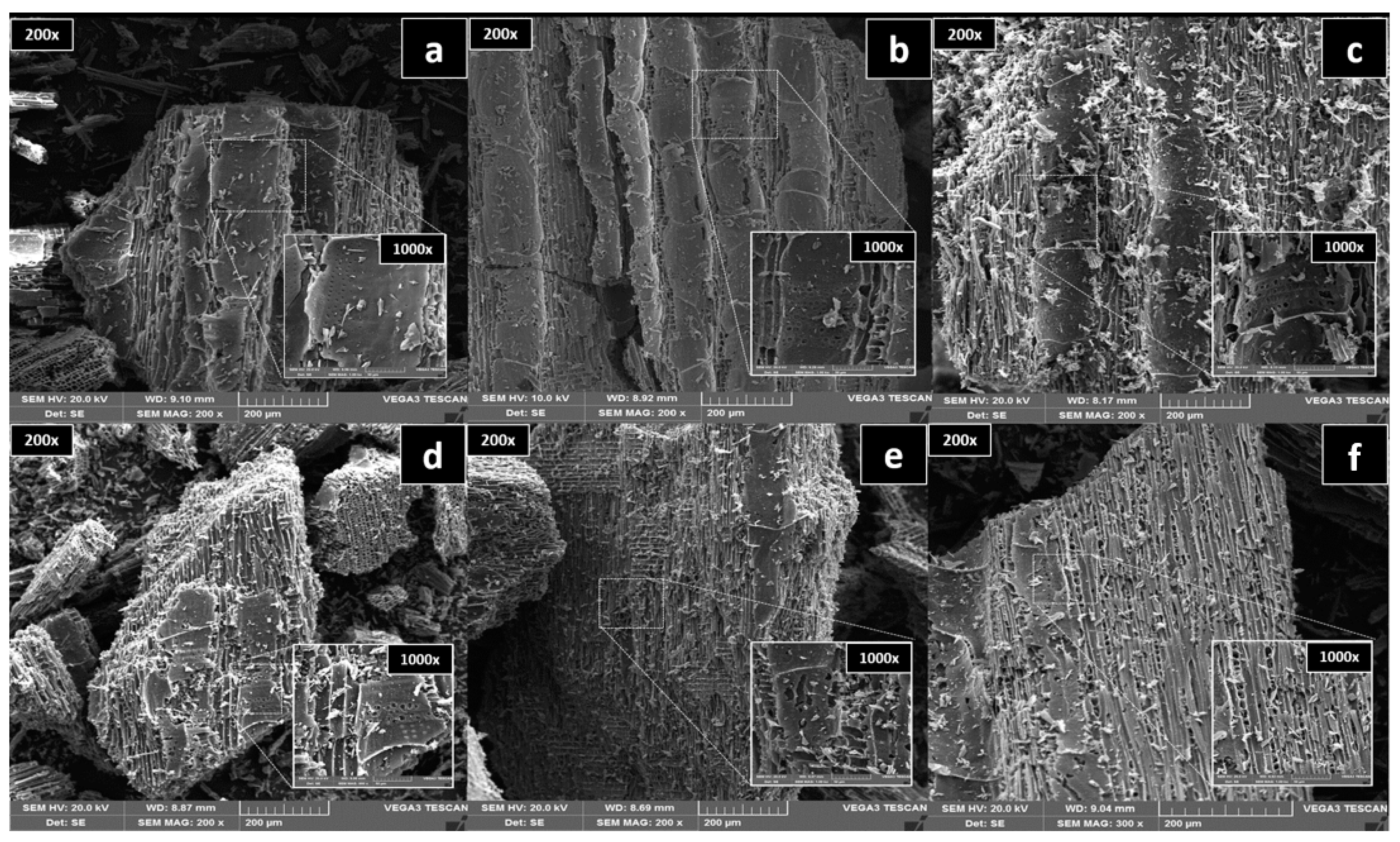
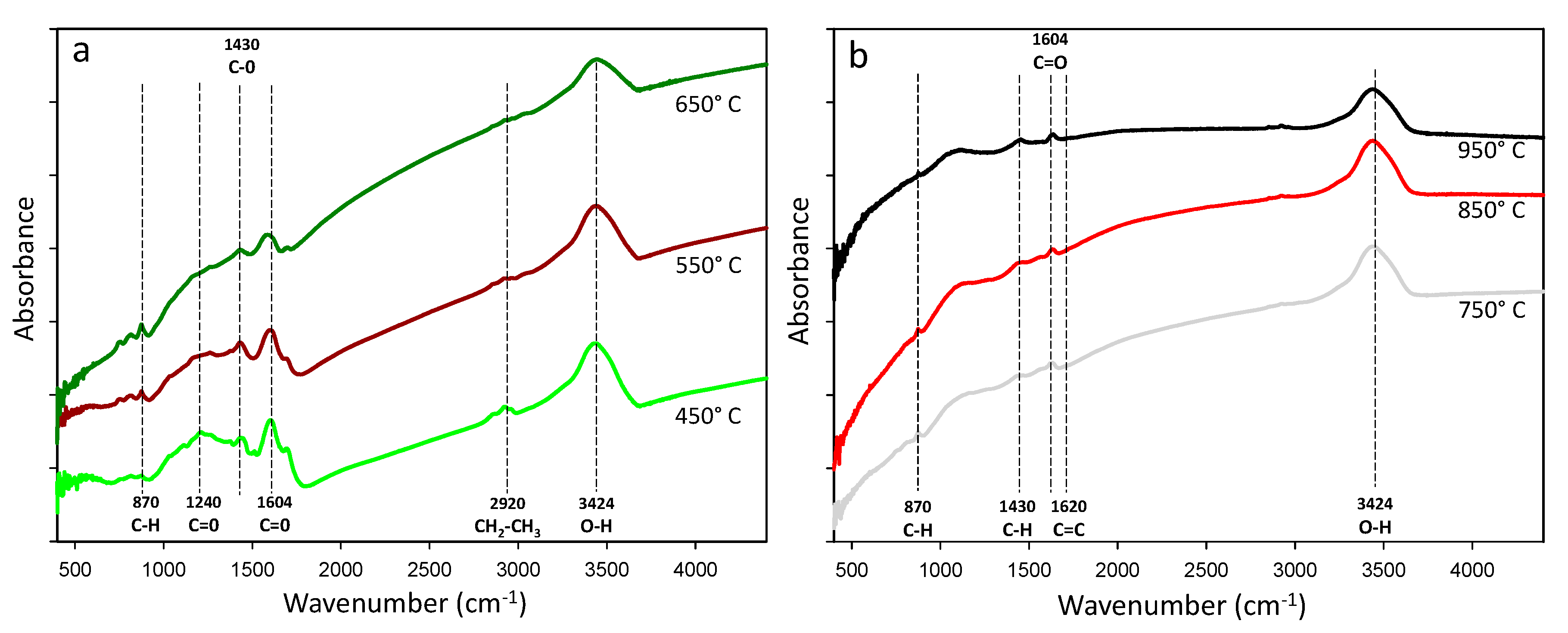
| Temperature (°C) | Yield (%) | Ash (%) | (VM) (%) | pH (CaCl2) | EC µS/cm |
|---|---|---|---|---|---|
| 450 | 42.76 ± 0.54 | 0.60 ± 0.001 | 30.32 ± 1.09 | 5.3 ± 0.070 | 123.3 ± 1.414 |
| 550 | 38.00 ± 0.44 | 0.74 ± 0.012 | 16.49 ± 0.20 | 6.7 ± 0.142 | 97.4 ± 2.121 |
| 650 | 34.69 ± 0.79 | 0.63 ± 0.009 | 11.92 ± 0.50 | 9.7 ± 0.260 | 134.3 ± 1.697 |
| 750 | 34.27 ± 0.44 | 0.84 ± 0.002 | 5.52 ± 0.67 | 9.8 ± 0.035 | 164.6 ± 1.272 |
| 850 | 32.44 ± 0.37 | 0.82 ± 0.012 | 3.43 ± 0.65 | 9.1 ± 0.106 | 161.6 ± 1.187 |
| 950 | 33.47 ± 0.61 | 1.06 ± 0.031 | 3.63 ± 0.74 | 9.2 ± 0.084 | 273.1 ± 1.626 |
| Correlation | −0.90 * | 0.89 * | −0.90 * | 0.83 * | 0.85 * |
| Temperature (°C) | K (%) | Ca (%) | P (%) | Fe (%) | S (%) | Mn (%) | Zn (%) | Cr (%) | Si (%) |
|---|---|---|---|---|---|---|---|---|---|
| 450 | 0.182 ± 0.001 | 0.085 ± 0.002 | 0.016 ± 0.002 | 0.001 ± 0.0003 | 0.014 ± 0.001 | 0.002 ± 0.0002 | |||
| 550 | 0.140 ± 0.007 | 0.104 ± 0.001 | 0.016 ± 0.024 | 0.003 ± 0.0003 | 0.013 ± 0.001 | 0.003 ± 0.0001 | 0.045 ± 0.0007 | ||
| 650 | 0.164 ± 0.001 | 0.115 ± 0.004 | 0.023 ± 0.001 | 0.006 ± 0.0002 | 0.016 ± 0.0007 | 0.003 ± 0.0001 | 0.0002 ± 0.00006 | 0.002 ± 0.0001 | |
| 750 | 0.210 ± 0.003 | 0.130 ± 0.002 | 0.017 ± 0.001 | 0.006 ± 0.0007 | 0.010 ± 0.0007 | 0.003 ± 0.0002 | |||
| 850 | 0.210 ± 0.004 | 0.104 ± 0.002 | 0.025 ± 0.0003 | 0.004 ± 0.0001 | 0.016 ± 0.0007 | 0.002 ± 0.0002 | |||
| 950 | 0.286 ± 0.011 | 0.176 ± 0.004 | 0.031 ± 0.002 | 0.004 ± 0.0001 | 0.013 ± 0.0014 | 0.003 ± 0.0002 | |||
| Correlation | 0.82 * | 0.82 * | 0.84 * | 0.51 | −0.05 | 0.21 |
| Temperature (°C) | Elemental Analysis | SSA and TPV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C (%) | H (%) | O (%) | N (%) | H/C | O/C | (O + N)/C | SSA (m2 g−1) | TPV (mm3 g−1) | |
| 450 | 74.96 ± 0.07 | 3.76 ± 0.38 | 19.41 ± 0.69 | 1.25 ± 0.21 | 0.050 ± 0.005 | 0.25 ± 0.009 | 0.27 ± 0.006 | ||
| 550 | 79.25 ± 0.24 | 1.00 ± 0.14 | 18.01 ± 0.78 | 1.49 ± 0.68 | 0.012 ± 0.001 | 0.22 ± 0.01 | 0.25 ± 0.001 | ||
| 650 | 87.06 ± 0.68 | 1.60 ± 0.41 | 10.70 ± 0.27 | 0.00 | 0.018 ± 0.004 | 0.12 ± 0.004 | 0.12 ± 0.004 | 410.48 ± 0.82 | 0.05 ± 0.002 |
| 750 | 87.74 ± 0.91 | 0.87 ± 0.10 | 9.47 ± 0.81 | 1.06 ± 0.007 | 0.019 ± 0.001 | 0.11 ± 0.01 | 0.12 ± 0.01 | 402.51 ± 0.91 | 0.07 ± 0.003 |
| 850 | 88.01 ± 1.03 | 0.89 ± 0.24 | 8.99 ± 0.25 | 1.27 ± 0.51 | 0.010 ± 0.002 | 0.10 ± 0.004 | 0.14 ± 0.01 | 362.90 ± 1.41 | 0.08 ± 0.003 |
| 950 | 86.90 ± 0.13 | 0.90 ± 0.04 | 10.57 ± 0.11 | 1.34 ± 0.183 | 0.002 ± 0.0005 | 0.12 ± 0.001 | 0.17 ± 0.01 | 224.43 ± 2.90 | 0.02 ± 0.002 |
| Correlation | 0.84 * | 0.72 | 0.84 * | 0.08 | −0.80 | −0.85 * | −0.81 | −0.89 * | −0.34 |
| FT-IR Peak (cm−1) | Pyrolysis Temperature (°C) | Vibration | References | |||||
|---|---|---|---|---|---|---|---|---|
| 450 | 550 | 650 | 750 | 850 | 950 | |||
| 3.424 | Strong | Medium | Weak | Medium | Medium | Weak | O–H Alcohol or phenol | [56] |
| 2.920 | Weak | Weak | - | - | - | - | CH2–CH3 Aliphatic | [52] |
| 1.638 | Weak | Weak | Medium | Strong | Strong | Strong | C=C | [52] |
| 1.604 | Medium | Medium | Weak | - | - | - | C=O/C=C Aromatics, ketones | [55] |
| 1.430 | Strong | Strong | Weak | Weak | Weak | Weak | C–H carboxymethyl-cellulose | [57] |
| 870 | Weak | Weak | Strong | Weak | Weak | Weak | C–H in polycyclic aromatic | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves Fernandes, B.C.; Ferreira Mendes, K.; Dias Júnior, A.F.; da Silva Caldeira, V.P.; da Silva Teófilo, T.M.; Severo Silva, T.; Mendonça, V.; de Freitas Souza, M.; Valadão Silva, D. Impact of Pyrolysis Temperature on the Properties of Eucalyptus Wood-Derived Biochar. Materials 2020, 13, 5841. https://doi.org/10.3390/ma13245841
Chaves Fernandes BC, Ferreira Mendes K, Dias Júnior AF, da Silva Caldeira VP, da Silva Teófilo TM, Severo Silva T, Mendonça V, de Freitas Souza M, Valadão Silva D. Impact of Pyrolysis Temperature on the Properties of Eucalyptus Wood-Derived Biochar. Materials. 2020; 13(24):5841. https://doi.org/10.3390/ma13245841
Chicago/Turabian StyleChaves Fernandes, Bruno Caio, Kassio Ferreira Mendes, Ananias Francisco Dias Júnior, Vinícius Patrício da Silva Caldeira, Taliane Maria da Silva Teófilo, Tatiane Severo Silva, Vander Mendonça, Matheus de Freitas Souza, and Daniel Valadão Silva. 2020. "Impact of Pyrolysis Temperature on the Properties of Eucalyptus Wood-Derived Biochar" Materials 13, no. 24: 5841. https://doi.org/10.3390/ma13245841
APA StyleChaves Fernandes, B. C., Ferreira Mendes, K., Dias Júnior, A. F., da Silva Caldeira, V. P., da Silva Teófilo, T. M., Severo Silva, T., Mendonça, V., de Freitas Souza, M., & Valadão Silva, D. (2020). Impact of Pyrolysis Temperature on the Properties of Eucalyptus Wood-Derived Biochar. Materials, 13(24), 5841. https://doi.org/10.3390/ma13245841






