Pre-Treatment of Furniture Waste for Smokeless Charcoal Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
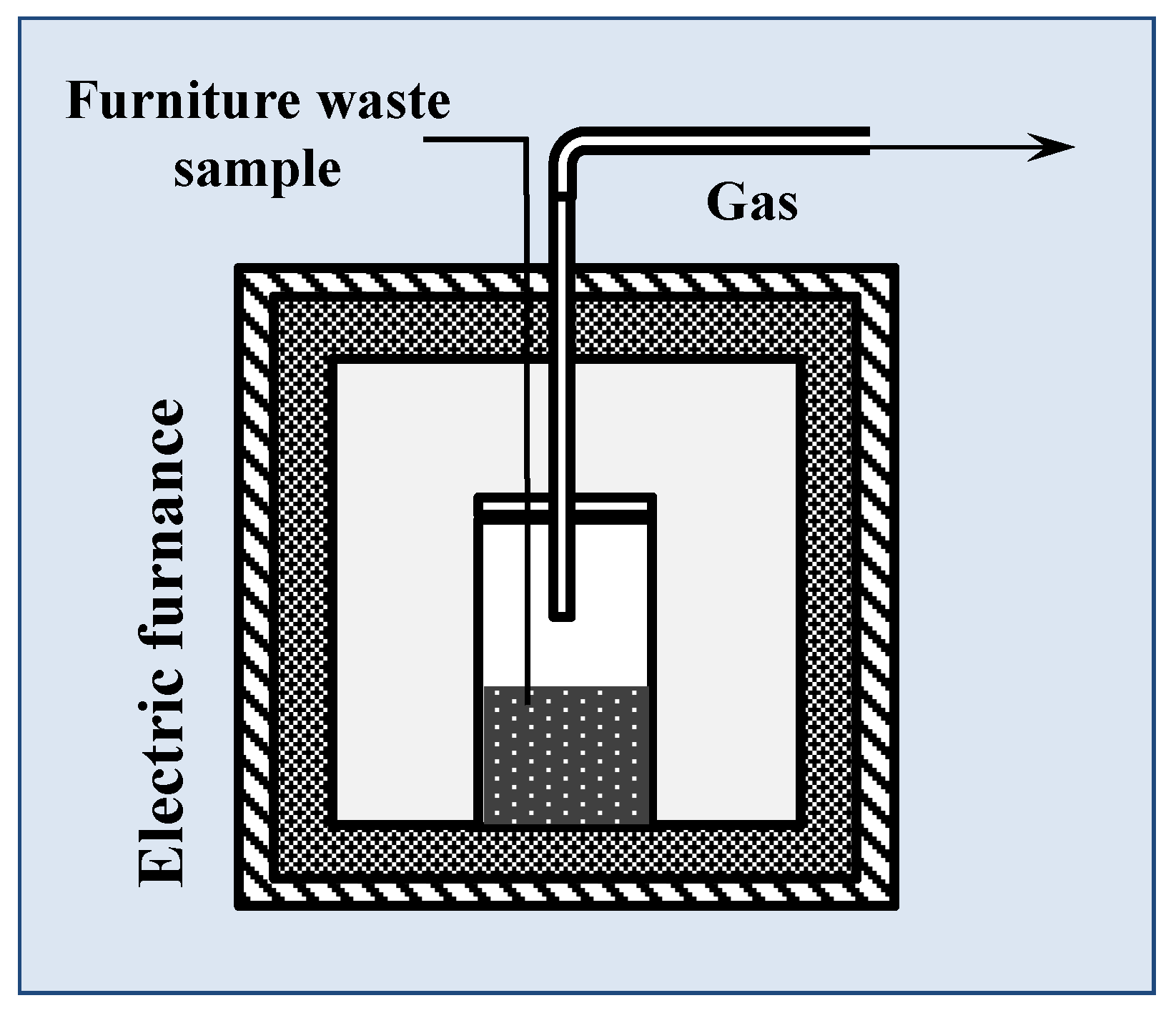
2.2. Pyrolysis Process in Laboratory and in Microgram Scale
2.3. Characterization of Samples
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency CO2 Emissions from Fuel Combustion 201 -Highlights. Available online: https://iea.blob.core.windows.net/assets/eb3b2e8d-28e0-47fd-a8ba-160f7ed42bc3/CO2_Emissions_from_Fuel_Combustion_2019_Highlights.pdf (accessed on 6 April 2020).
- OECD Statistics Emissions of Air Pollutants. Available online: https://stats.oecd.org/Index.aspx?DataSetCode=AIR_EMISSIONS (accessed on 29 April 2020).
- Matuszek, K.; Hrycko, P.; Stelmach, S.; Sobolewski, A. Carbonaceous smokeless fuel and modern small-scale boilers limiting the residential emission. Part 1. General aspects. Przem. Chem. 2016, 95, 223–227. [Google Scholar] [CrossRef]
- Matuszek, K.; Hrycko, P.; Stelmach, S.; Sobolewski, A. Carbonaceous smokeless fuel and modern small-scale boilers limiting the residential emission. Part 2. Experimental tests of a new carbonaceous smokeless fuel. Przem. Chem. 2016, 95. [Google Scholar] [CrossRef]
- Niesler, M.; Stecko, J.; Gierad, D.; Stelmach, S.; Nowak, M. Ocena możliwości wykorzystania “błękitnego węgla” jako zamiennika części koksiku w procesie spiekania rud żelaza. The assessment of the possibility of the “blue coal” utilization in the iron ore sintering process. Pr. Inst. Metal. Zelaza 2017, 69, 31–43. [Google Scholar]
- Eurostat-Data Explorer, Sawnwood and Panels. Available online: https://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do (accessed on 26 May 2020).
- Eurostat-Data Explorer, Generation of Waste by Waste Category, Hazardousness and NACE Rev. 2 Activity. Available online: https://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do (accessed on 26 May 2020).
- Arditi, S.; Forrest, A.; Hilton, M.; Ballinger, A.; Whittaker, D. Circular Economy Opportunities in the Furniture Sector; EEB: Bruxelles, Belgium, 2017. [Google Scholar]
- Dąbrowska-Salwin, M.; Raczkowska, D.; Świętochowski, A. Physical properties of wastes from furniture industry for energy purposes. Agron. Res. 2017, 15, 388–394. [Google Scholar]
- Januszewicz, K.; Kazimierski, P.; Klein, M.; Kardaś, D.; Łuczak, J. Activated carbon produced by pyrolysis of waste wood and straw for potential wastewater adsorption. Materials 2020, 13, 2047. [Google Scholar] [CrossRef]
- Moreno, A.I.; Font, R.; Conesa, J.A. Physical and chemical evaluation of furniture waste briquettes. Waste Manag. 2016, 49, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Nyemba, W.R.; Hondo, A.; Mbohwa, C.; Madiye, L. Unlocking economic value and sustainable furniture manufacturing through recycling and reuse of sawdust. Procedia Manuf. 2018, 21, 510–517. [Google Scholar] [CrossRef]
- Abjaghou, H.; Bourret, J.; Tessier-Doyen, N.; Fassier, M.; Bruneaux, M.A.; Lacanilao, A.; Quint, V.; Smith, A.; Smith, D.S.; Peyratout, C. Incorporation of wooden furniture wastes in fired clay bricks for improved thermal insulation: A feasability study. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef]
- Pringle, A.M.; Rudnicki, M.; Pearce, J.M. Wood furniture waste-based recycled 3-D printing filament. For. Prod. J. 2018, 68, 86–95. [Google Scholar] [CrossRef]
- Stamm, A.J.; Harris, E.E. Chemical Processing of Wood; Chemical Publishing Company: New York, NY, USA, 1953. [Google Scholar]
- Chembukulam, S.K.; Dandge, A.S.; Kovllur, N.L.; Seshaglri, R.K.; Valdyeswaran, R. Smokeless fuel from carbonized sawdust. Ind. Eng. Chem. Prod. Res. Dev. 1981, 20, 714–719. [Google Scholar] [CrossRef]
- Sciazko, M.; Kubica, K.; Rzepa, S. The smokeless fuel-properties and testing methodology. Fuel Process. Technol. 1993, 36, 123–128. [Google Scholar] [CrossRef]
- Arayici, S. Coal pyrolysis and coal briquetting for production of smokeless fuel. J. Environ. Sci. Health A 1996, 31, 1899–1907. [Google Scholar] [CrossRef]
- Dattaiah, B.; Agrawal, D. Shaped smokeless domestic fuel from char fines from wardha valley coals. Chem. Age India 1969, 20, 521–525. [Google Scholar]
- Gaines, A.F.; Pitt, G.J. Smokeless fuels from high-volatile coals. II. The smoke emission of low-temperature chars prepared from high-volatile coals. Int. J. Air Pollut. 1959, 1, 266–275. [Google Scholar] [PubMed]
- Adams, W.N.; Gaines, A.F.; Gregory, D.H.; Pitt, G.J. Smoke emission of low-temperature chars. Nature 1959, 183, 33. [Google Scholar] [CrossRef]
- Nishino, H.; Takeda, S. Studies on hot briquetting of char obtained by fluidized heat treatment of fine coal. J. Fuel Soc. Japan 1969, 48, 226–234. [Google Scholar] [CrossRef]
- Cengizler, H.; Kemal, M. Formcoke production from char fines of hard brown coals by air curing. Miner. Process. Extr. Metall. 2013, 115, 132–138. [Google Scholar] [CrossRef]
- Blesa, M.J.; Miranda, J.L.; Moliner, R.; Izquierdo, M.T.; Palacios, J.M. Low-temperature co-pyrolysis of a low-rank coal and biomass to prepare smokeless fuel briquettes. J. Anal. Appl. Pyrolysis 2003, 70. [Google Scholar] [CrossRef]
- Blesa, M.J.; Miranda, J.L.; Izquierdo, M.T.; Moliner, R. Curing time effect on mechanical strength of smokeless fuel briquettes. Fuel Process. Technol. 2003, 80, 155–167. [Google Scholar] [CrossRef]
- Blesa, M.J.; Miranda, J.L.; Izquierdo, M.T.; Moliner, R. Curing temperature effect on mechanical strength of smokeless fuel briquettes prepared with molasses. Fuel 2003, 82, 943–947. [Google Scholar] [CrossRef]
- Blesa, M.J.; Miranda, J.L.; Moliner, R.; Izquierdo, M.T. Curing temperature effect on smokeless fuel briquettes prepared with molasses and H3PO4. Fuel 2003, 82, 1669–1673. [Google Scholar] [CrossRef]
- Moliner, R.; Lázaro, M.J.; Suelves, I.; Blesa, M.J. Valorization of selected biomass and wastes by co-pyrolysis with coal. Int. J. Power Energy Syst. 2004, 24, 186–193. [Google Scholar]
- Blesa, M.J.; Fierro, V.; Miranda, J.L.; Moliner, R.; Palacios, J.M. Effect of the pyrolysis process on the physicochemical and mechanical properties of smokeless fuel briquettes. Fuel Process. Technol. 2001, 74, 1–17. [Google Scholar] [CrossRef]
- Bičáková, O.; Straka, P. Co-pyrolysis of waste tire/coal mixtures for smokeless fuel, maltenes and hydrogen-rich gas production. Energy Convers. Manag. 2016, 116, 203–213. [Google Scholar] [CrossRef]
- Tongcumpou, C.; Usapein, P.; Tuntiwiwattanapun, N. Complete utilization of wet spent coffee grounds waste as a novel feedstock for antioxidant, biodiesel, and bio-char production. Ind. Crop. Prod. 2019, 138. [Google Scholar] [CrossRef]
- Orge, R.F.; McHenry, M.P.; Quiros, E.N. Waste rice husk continuous carbonizers for carbon sequestration and energy in rural Philippine Regions. In Agriculture Management for Climate Change; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2015; pp. 109–122. ISBN 9781634830515. [Google Scholar]
- Nwabue, F.I.; Unah, U.; Itumoh, E.J. Production and characterization of smokeless bio-coal briquettes incorporating plastic waste materials. Environ. Technol. Innov. 2017, 8, 233–245. [Google Scholar] [CrossRef]
- Zhou, J.; Duan, H.; Li, S.; Liu, Y. Preparation and combustion properties of inflammable carbon from poplar carbon. Trans. Chin. Soc. Agric. Eng. 2010, 26, 257–261. [Google Scholar] [CrossRef]
- Tippayawong, K.Y.; Panyakom, S.; Suriyanarakorn, C.; Wiratkasem, K.; Tippayawong, N. Supply chain analysis of smokeless charcoal from maize residues. Energy Rep. 2020, 6, 60–66. [Google Scholar] [CrossRef]
- Beckman, J.A.; Laman, J.R. Destructive Distillation of used Tires. In Proceedings of the National Industrial Solid Waste Management Conference, Framingham, Mass, 12–13 May 1970; pp. 388–392. [Google Scholar]
- Uttamaprakrom, W.; Vitidsant, T. Production of smokeless briquette charcoals from wet cake waste of ethanol industry. Eng. J. 2012, 16, 5–17. [Google Scholar] [CrossRef]
- Ryms, M.; Januszewicz, K.; Kazimierski, P.; Łuczak, J.; Klugmann-Radziemska, E.; Lewandowski, W.M. Post-Pyrolytic Carbon as a Phase Change Materials (PCMs) carrier for application in building materials. Materials 2020, 13, 1268. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Yang, Y.; Chiang, K.; Burke, N. Porous carbon-supported catalysts for energy and environmental applications: A short review. Earth Sci. Resour. Eng. 2011, 178, 197–205. [Google Scholar] [CrossRef]
- Liew, R.K.; Chong, M.Y.; Osazuwa, O.U.; Nam, W.L.; Phang, X.Y.; Su, M.H.; Cheng, C.K.; Chong, C.T.; Lam, S.S. Production of activated carbon as catalyst support by microwave pyrolysis of palm kernel shell: A comparative study of chemical versus physical activation. Res. Chem. Intermed. 2018, 44, 3849–3865. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]


| Type | Pine Sawdust | Chipboard | Medium-Density Fiberboard | Oriented Strand Board |
|---|---|---|---|---|
| Raw Material |  | 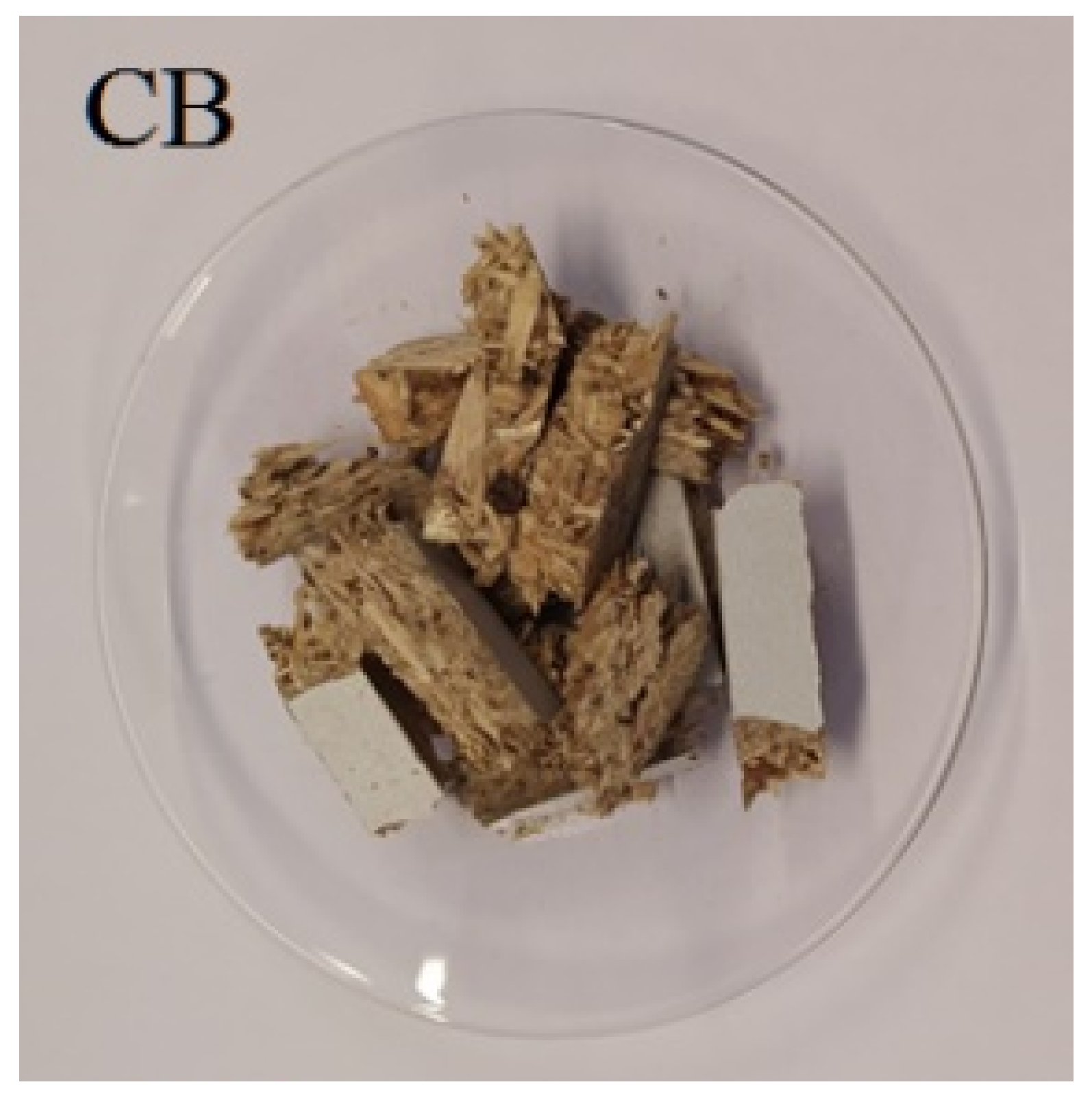 |  |  |
| Char | 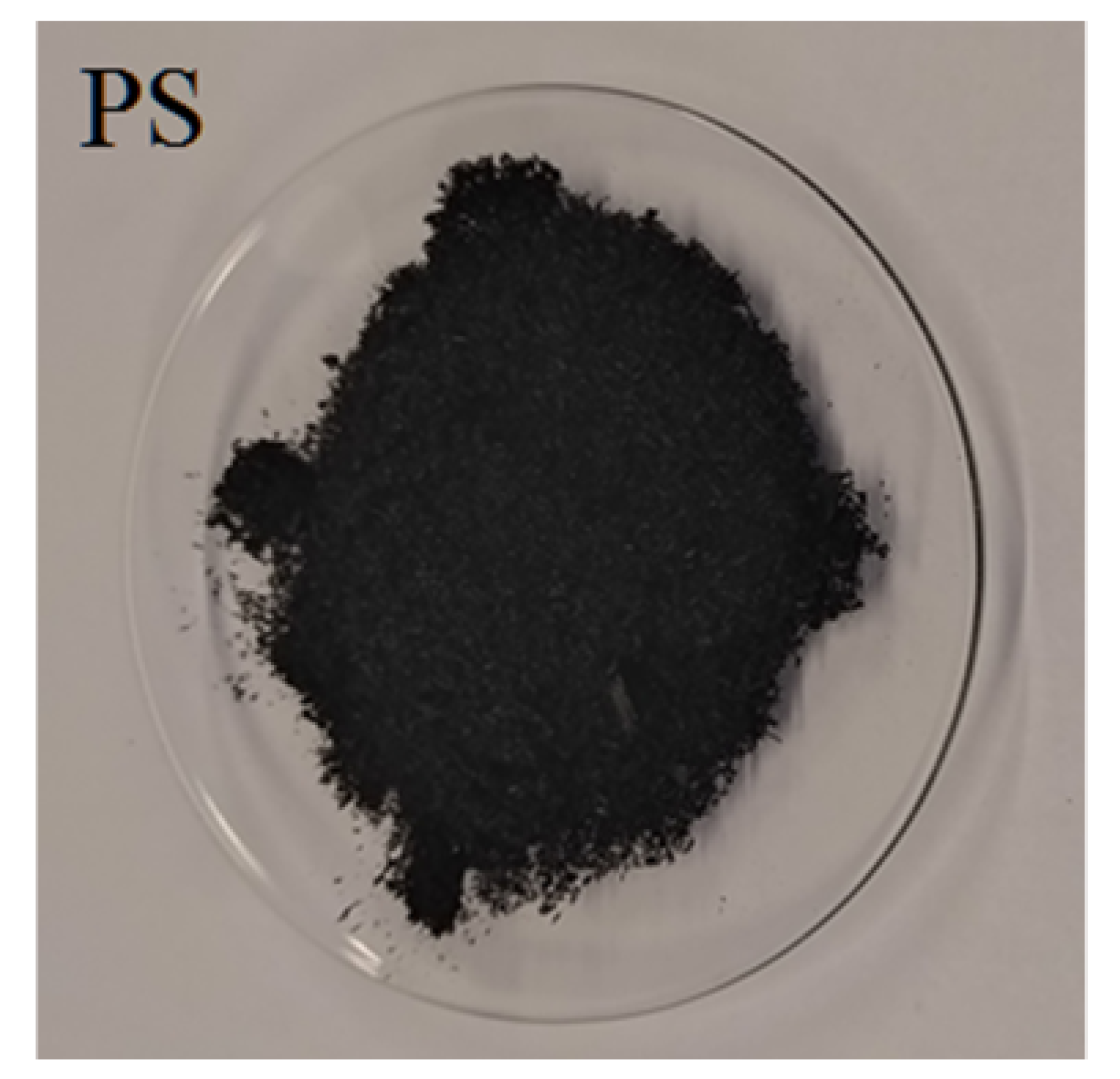 | 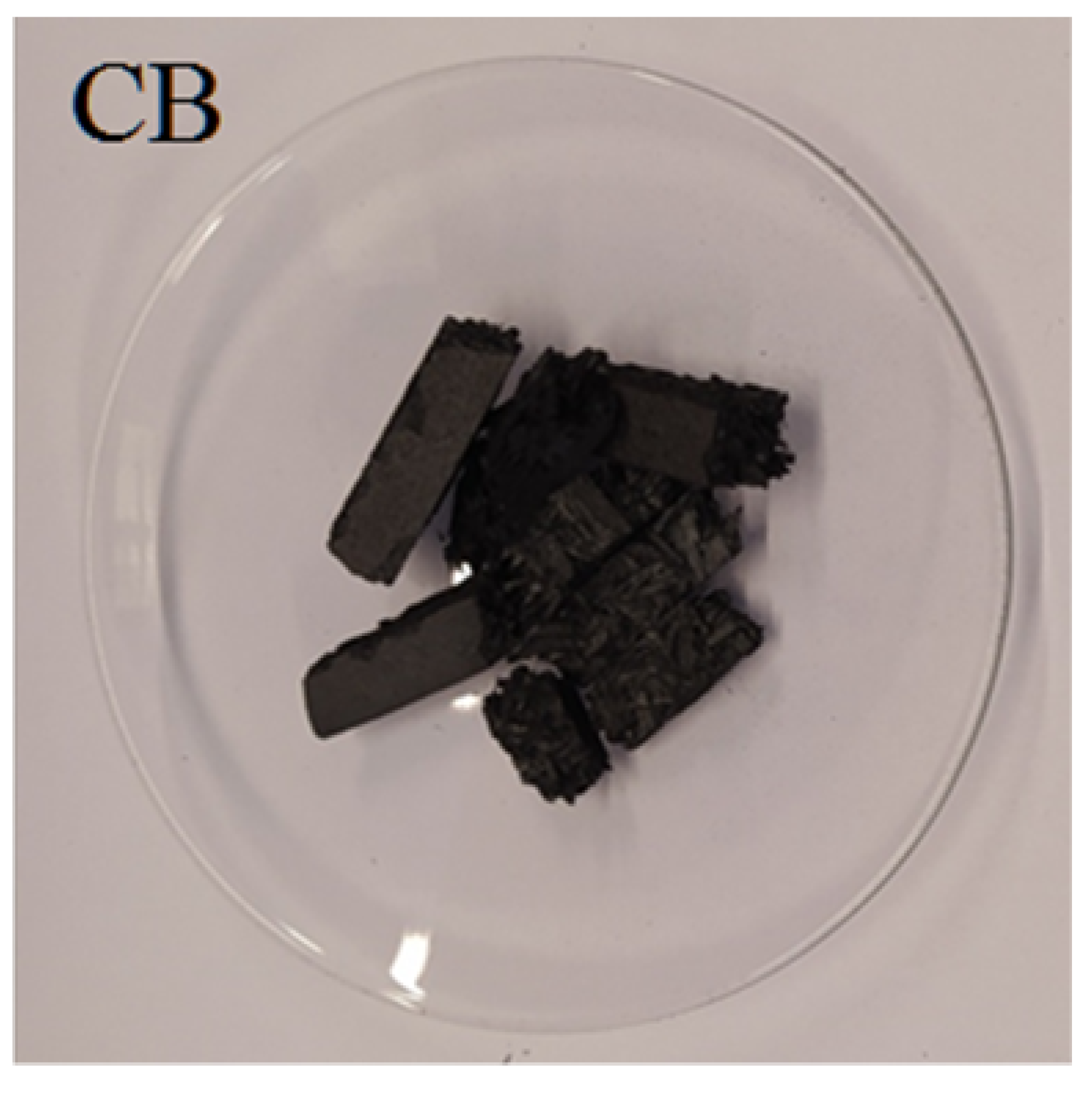 |  |  |
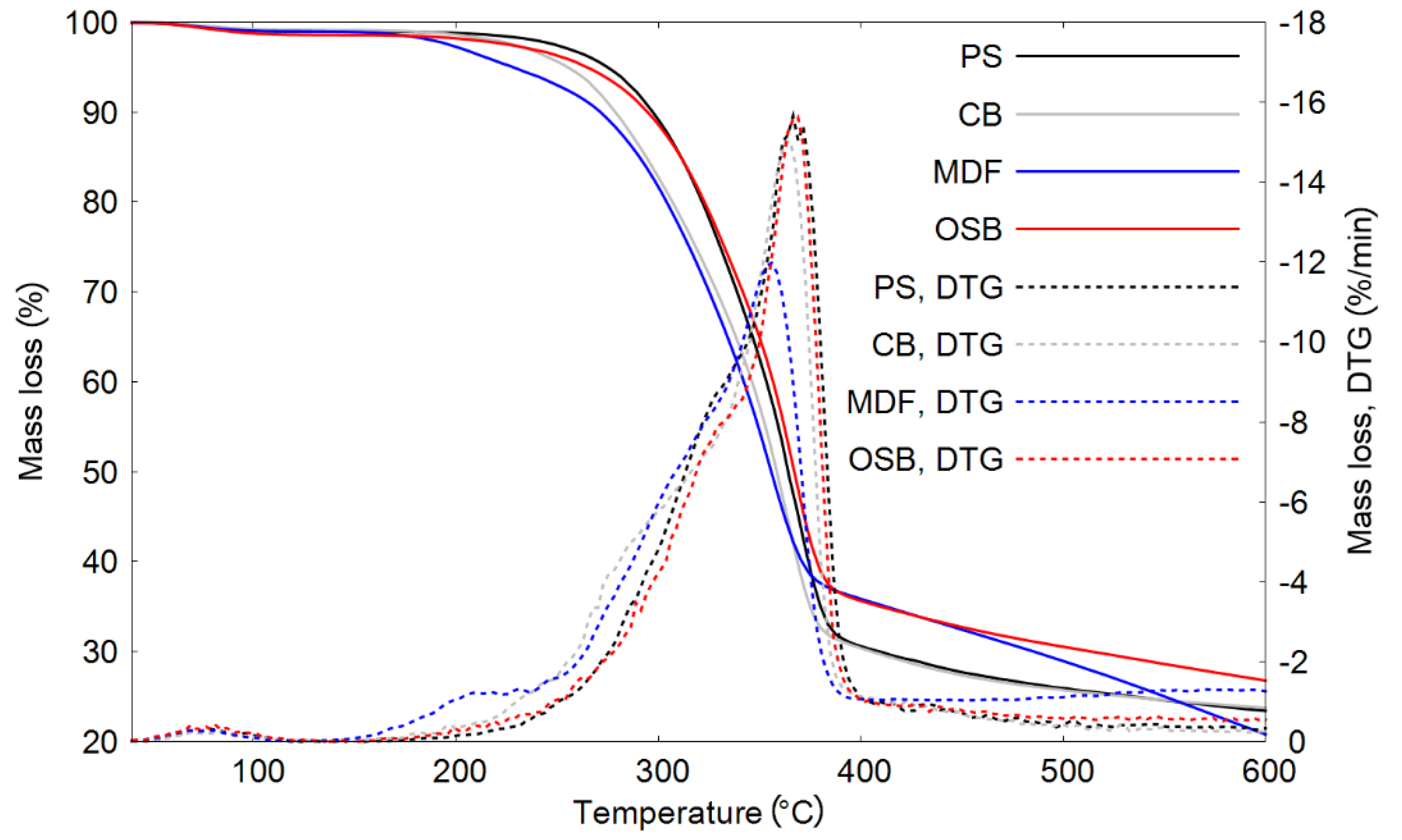
| Raw Material | Decomposition Temperature (°C) | DTG Maxima (°C) | Solid Residue (%) | |||
|---|---|---|---|---|---|---|
| T5% | T10% | T50% | Tmax | % of Mass/°C | T600 | |
| PS | 274 | 297 | 365 | 367 | 1.00 | 23.4 |
| CB | 255 | 278 | 358 | 365 | 0.94 | 23.7 |
| MDF | 226 | 271 | 355 | 356 | 0.77 | 20.8 |
| OSB | 263 | 293 | 365 | 369 | 0.97 | 26.7 |
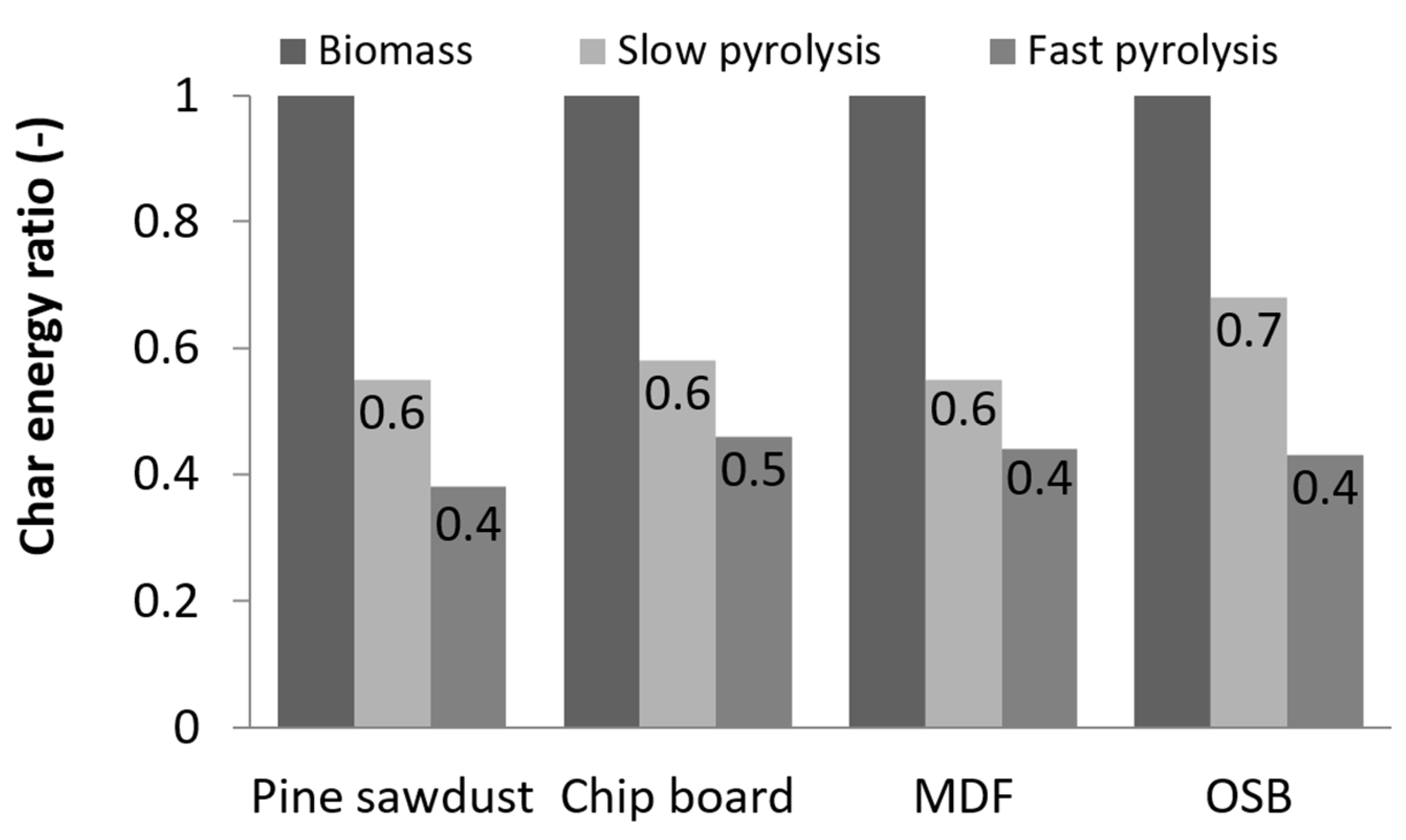
| Raw Materials | Slow Pyrolysis (15 °C/min) | Fast Pyrolysis (100 °C/min) |
|---|---|---|
| PS | 29.6% | 19.6% |
| CB | 31.8% | 25.1% |
| MDF | 31.4% | 24.4% |
| OSB | 37.0% | 23.3% |
| Carbon Content (%) | Hydrogen Content (%) | Nitrogen Content (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomass type | Biomass | Biochar (SP) | Biochar (FP) | Biomass | Biochar (SP) | Biochar (FP) | Biomass | Biochar (SP) | Biochar (FP) |
| PS | 48.8 | 76.9 | 86.4 | 6.1 | 4.4 | 3.2 | 0.6 | 1.0 | 1.4 |
| CB | 47.5 | 74.5 | 80.4 | 5.9 | 4.0 | 2.9 | 3.4 | 4.9 | 4.2 |
| MDF | 48.5 | 74.5 | 80.8 | 5.9 | 4.0 | 2.8 | 3.8 | 4.3 | 4.4 |
| OSB | 49.4 | 77.2 | 83.5 | 5.8 | 4.0 | 2.8 | 1.4 | 2.8 | 3.1 |
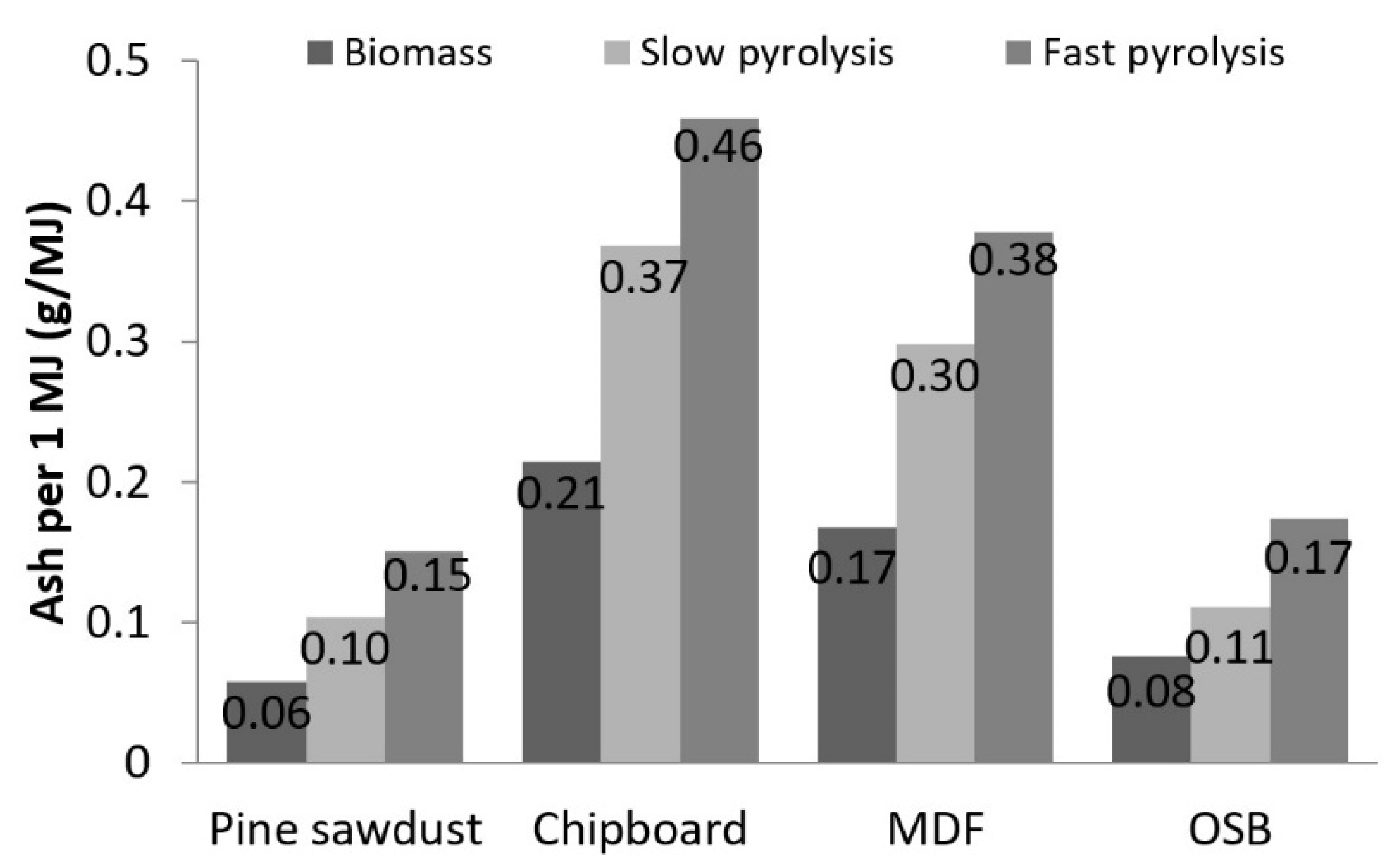
| HHV (MJ/kg) | |||
|---|---|---|---|
| Biomass Type | Pyrolysis Type | ||
| SP | FP | ||
| raw material | char | char | |
| PS | 17.41 | 29.90 | 33.14 |
| CB | 17.03 | 28.55 | 29.85 |
| MDF | 17.53 | 28.40 | 30.02 |
| OSB | 17.38 | 29.56 | 31.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierski, P.; Hercel, P.; Januszewicz, K.; Kardaś, D. Pre-Treatment of Furniture Waste for Smokeless Charcoal Production. Materials 2020, 13, 3188. https://doi.org/10.3390/ma13143188
Kazimierski P, Hercel P, Januszewicz K, Kardaś D. Pre-Treatment of Furniture Waste for Smokeless Charcoal Production. Materials. 2020; 13(14):3188. https://doi.org/10.3390/ma13143188
Chicago/Turabian StyleKazimierski, Paweł, Paulina Hercel, Katarzyna Januszewicz, and Dariusz Kardaś. 2020. "Pre-Treatment of Furniture Waste for Smokeless Charcoal Production" Materials 13, no. 14: 3188. https://doi.org/10.3390/ma13143188
APA StyleKazimierski, P., Hercel, P., Januszewicz, K., & Kardaś, D. (2020). Pre-Treatment of Furniture Waste for Smokeless Charcoal Production. Materials, 13(14), 3188. https://doi.org/10.3390/ma13143188







