Analysis of the Fire Behavior of Polymers (PP, PA 6 and PE-LD) and Their Improvement Using Various Flame Retardants
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
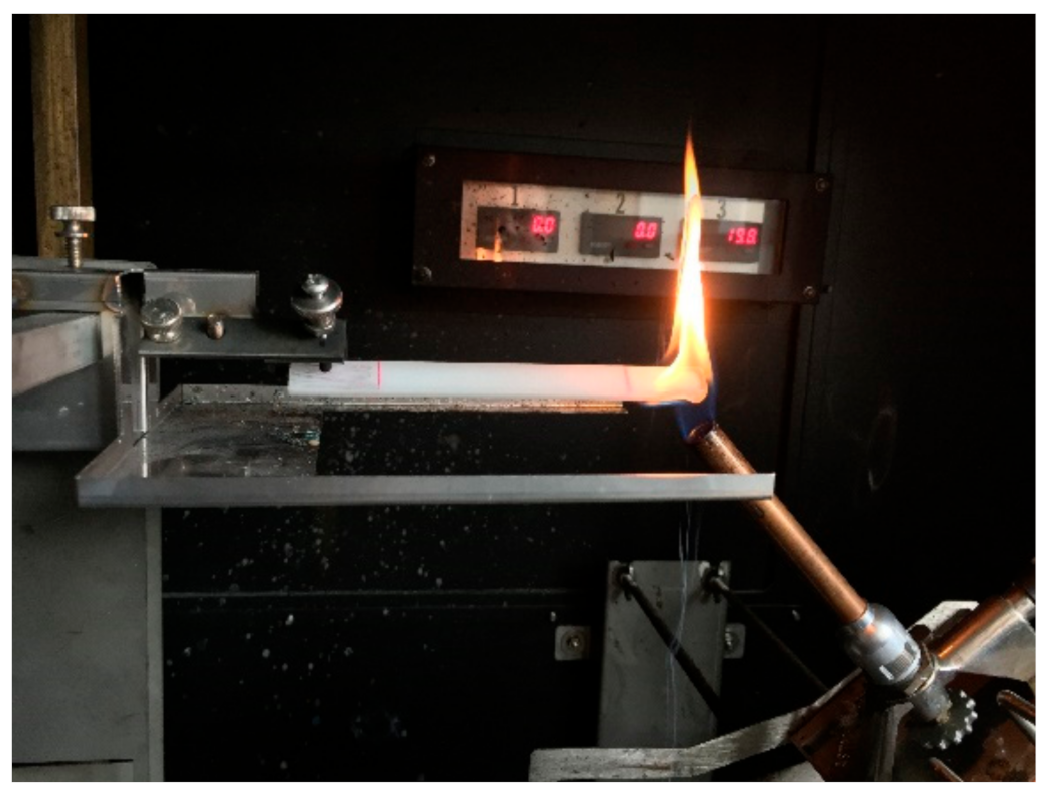
2.2. Determination of the Fire Behavior
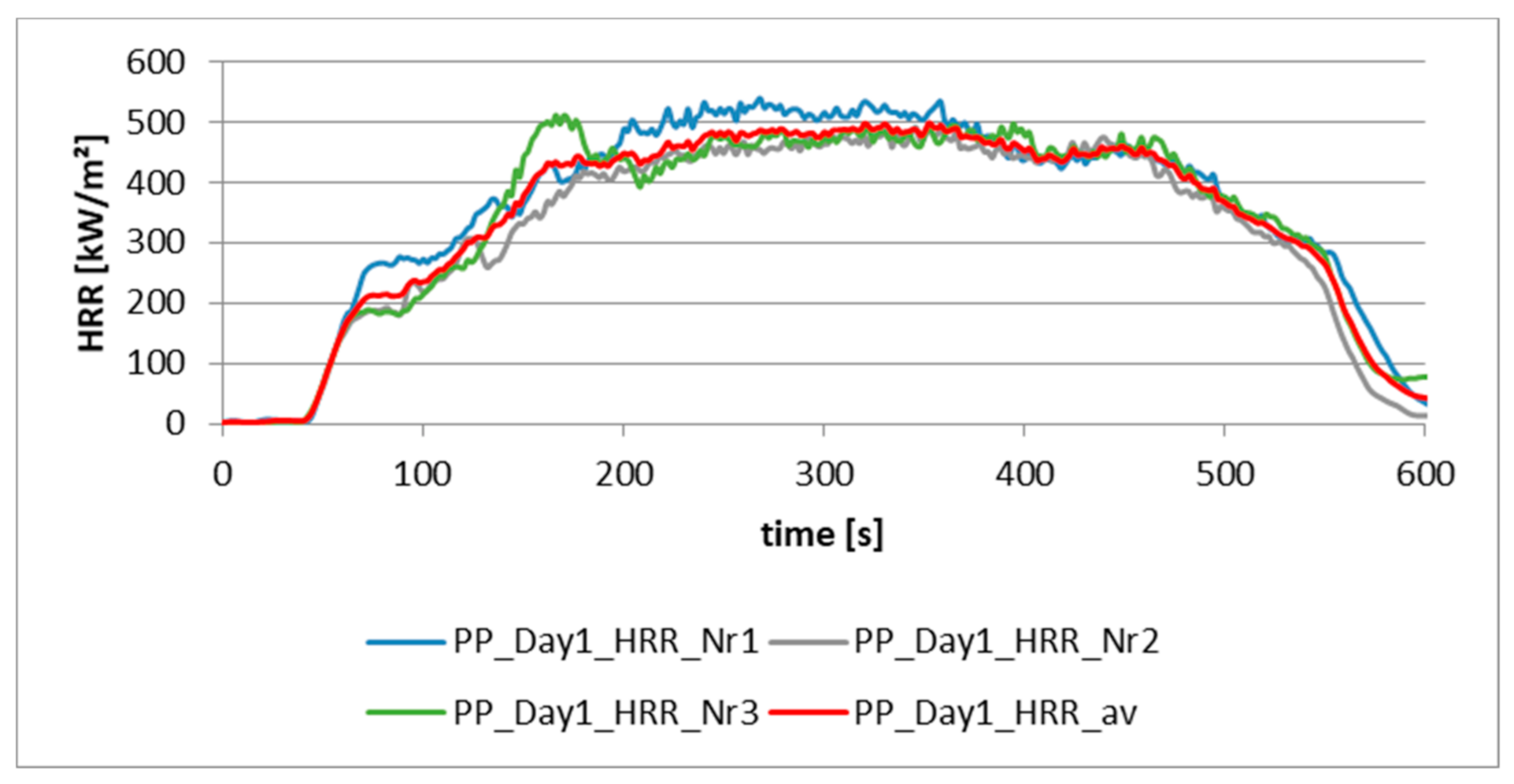
2.3. Precision of Fire Behavior Tests
2.4. Differential Scanning Calorimetry
3. Results and Discussion
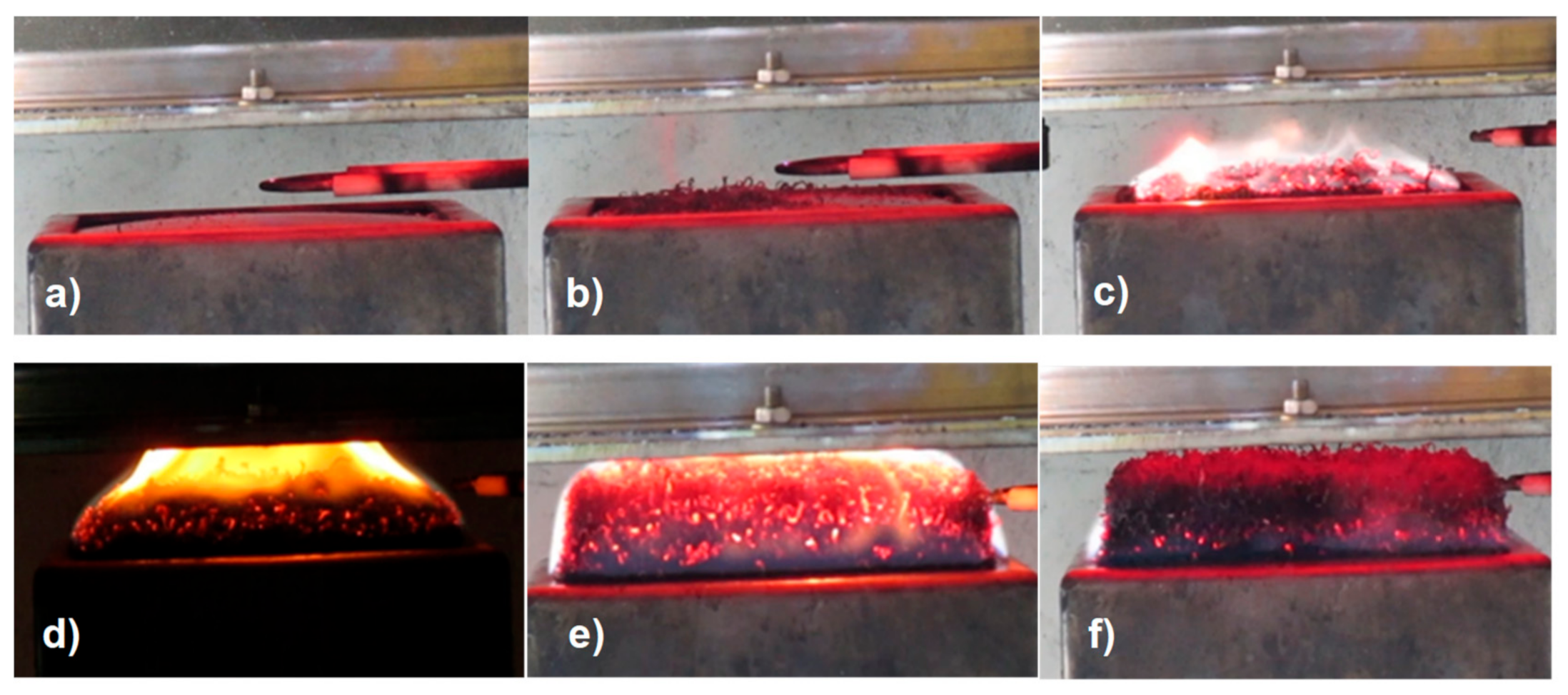
3.1. Burning Behavior of Polymeric Materials without Flame Retardant
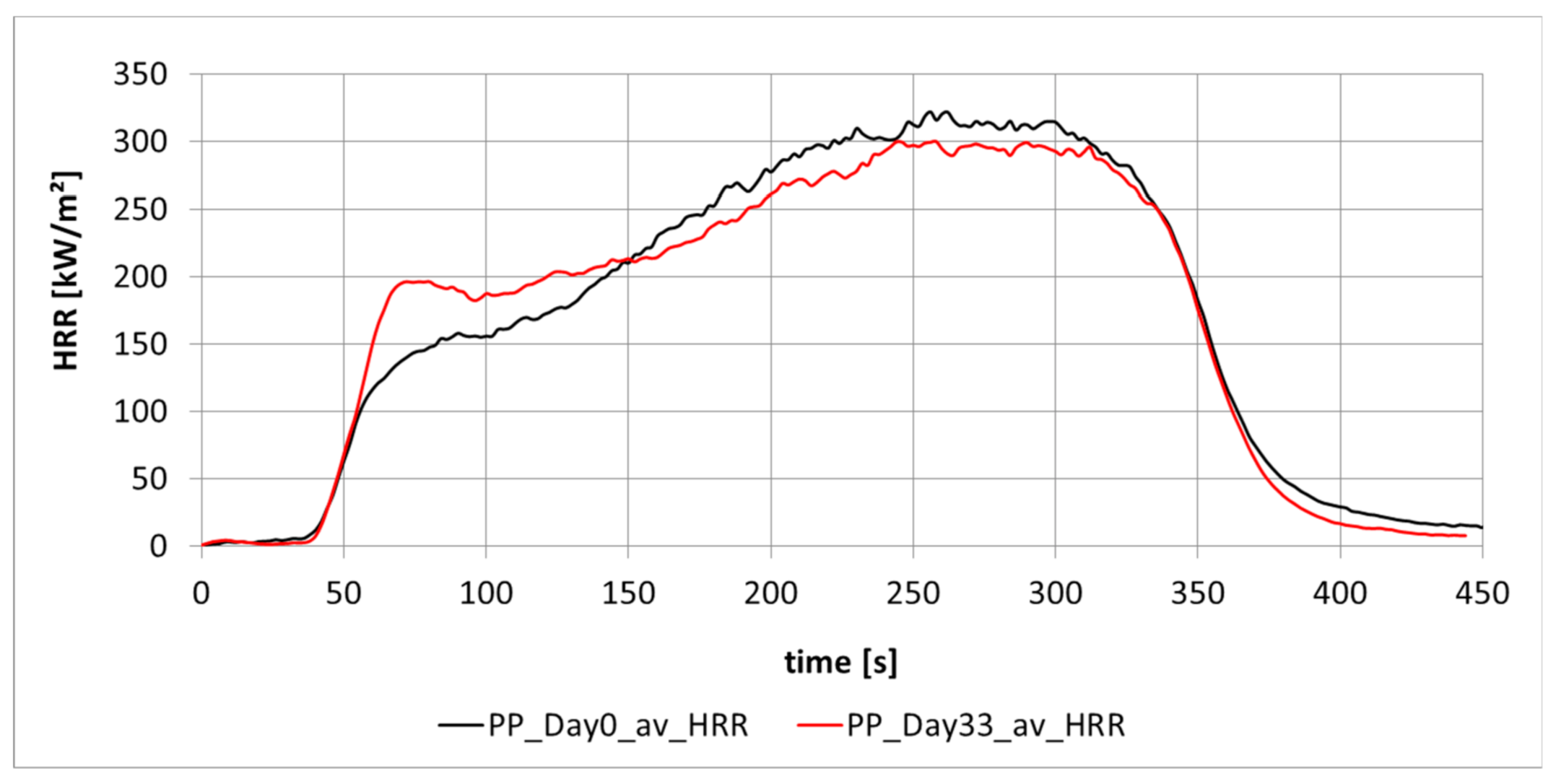
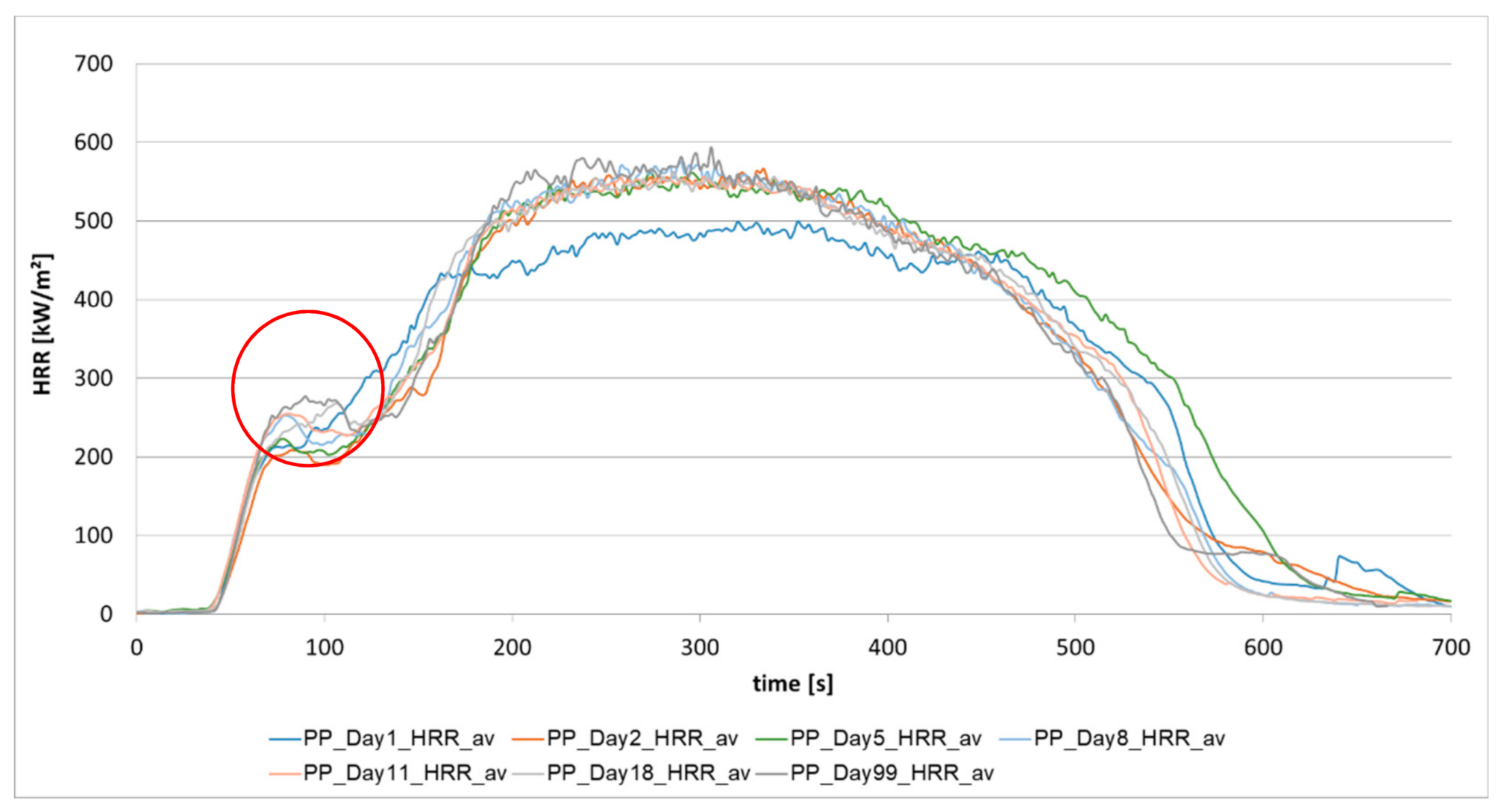
3.1.1. Aging of Polypropylene (PP)
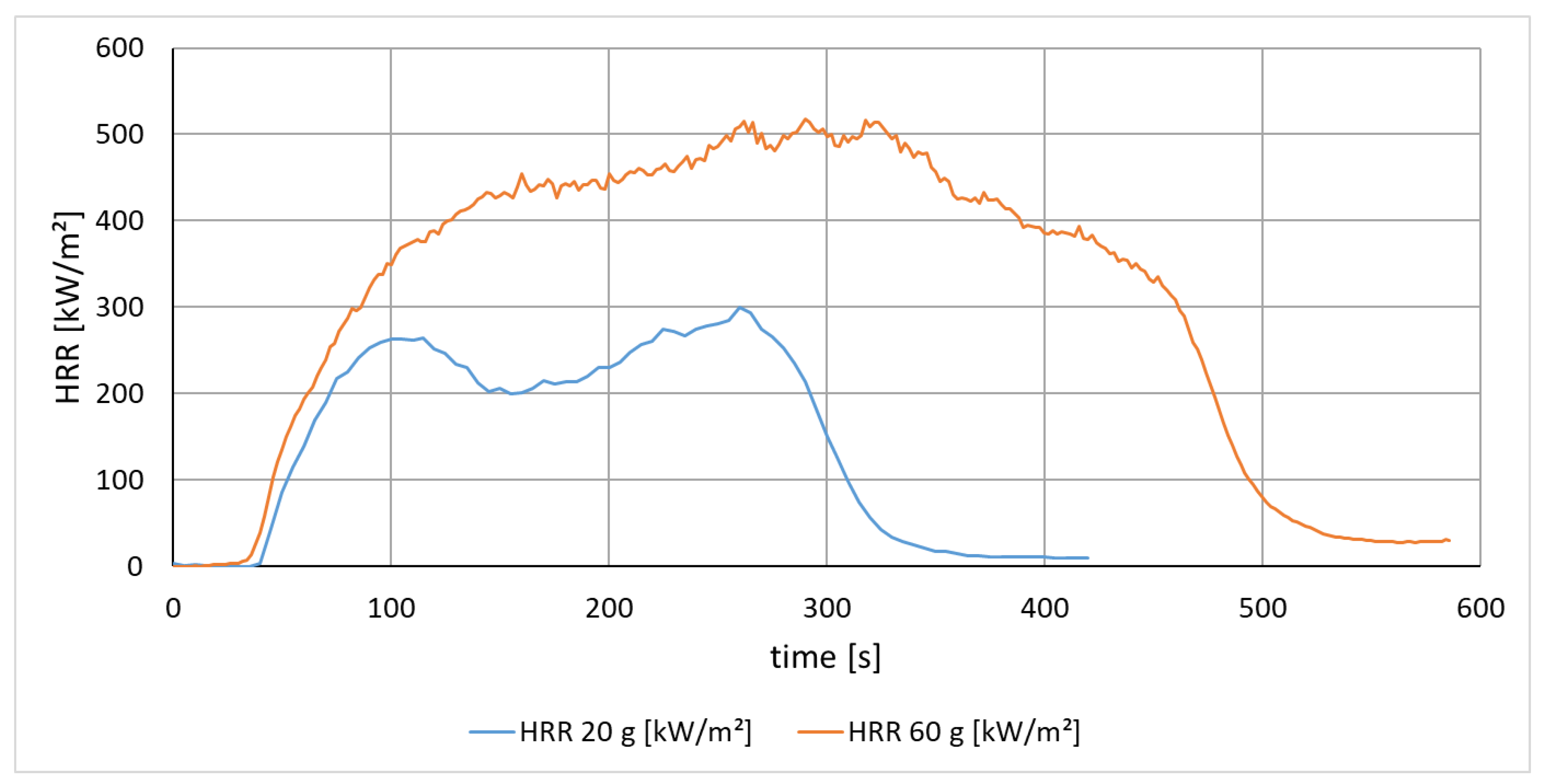
3.1.2. Effect of Mass on the Heat Release of Polyamide (PA 6)
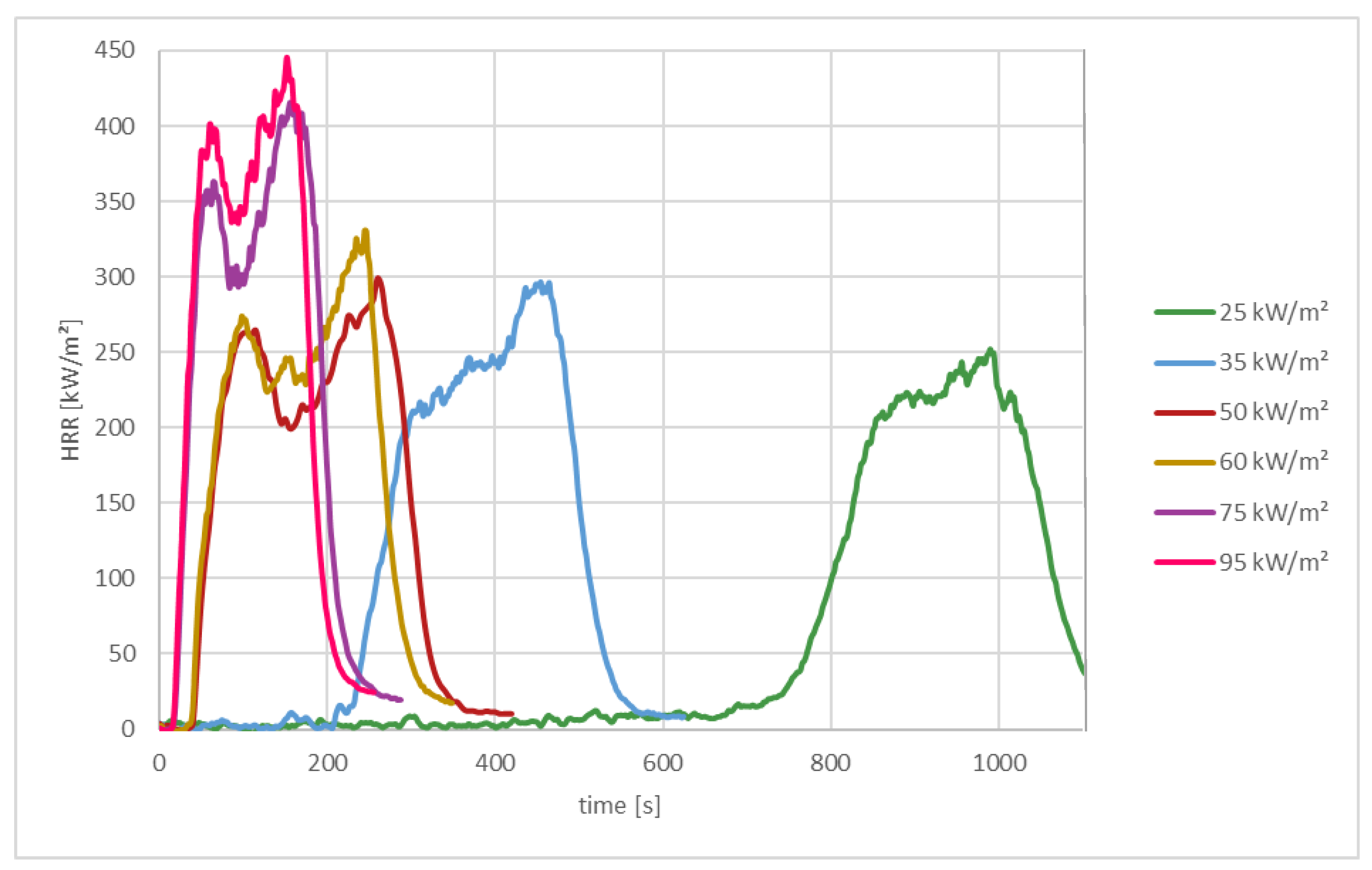
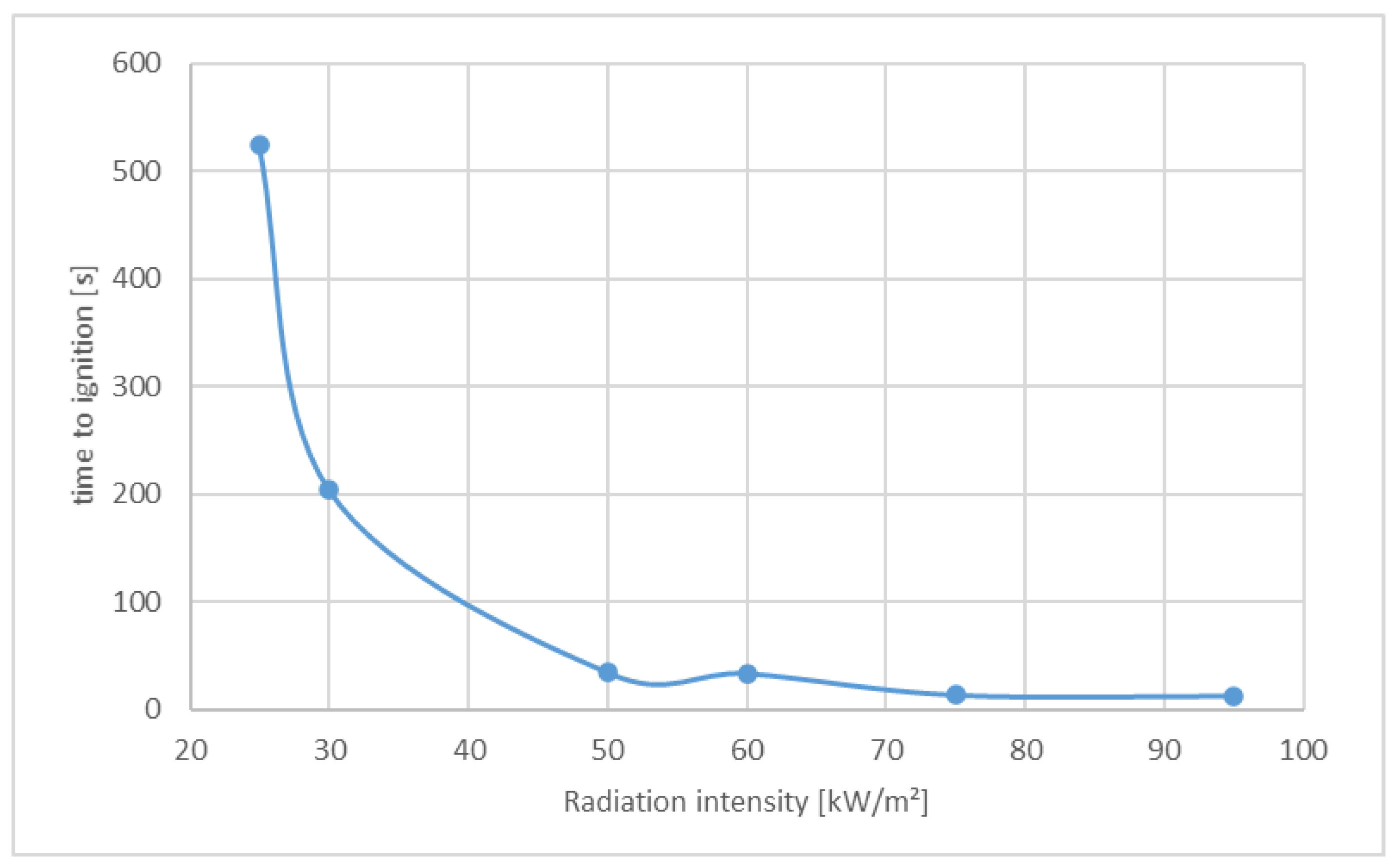
3.1.3. Effects of Different Radiation Intensities on the Heat Release Rate of Polyamide
- tig—time to ignition [s]
- Tig—ignition temperature [K]
- Tambient—ambient temperature [K]
- Radiation intensity [kW/m2]
- C(kρc)—Constant dependent on the material properties
- k—thermal conductivity [W/mK]
- ρ—density [kg/m3]
- c—heat capacity [J/K]
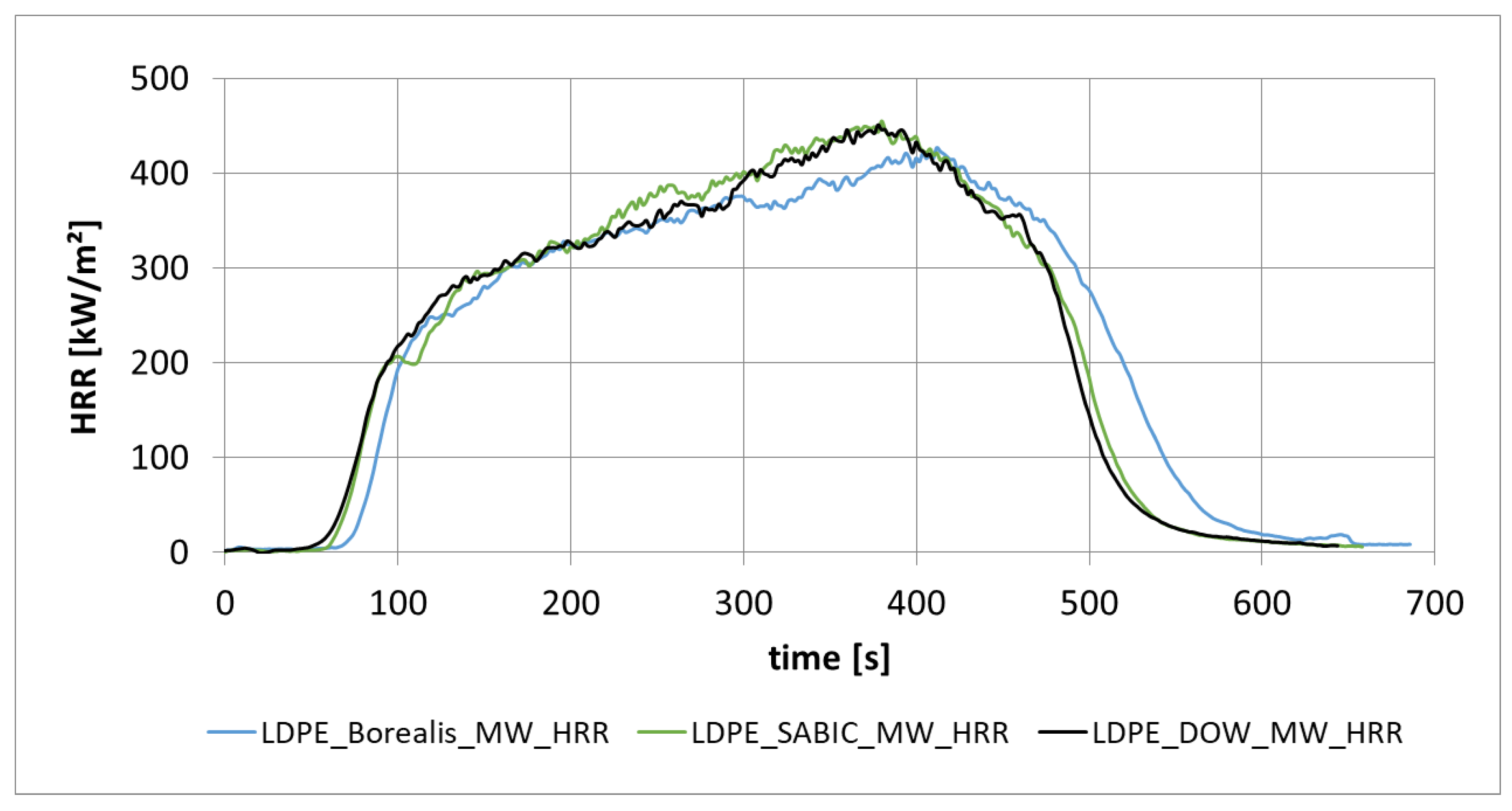
3.1.4. Heat Release Rate from Different Low-Density Polyethylene (PE-LD) from Different Manufacturers (Borealis, Sabic, DOW)
3.2. Flaming and Irradiation of Polypropylene (PP) with Various Flame Retardants
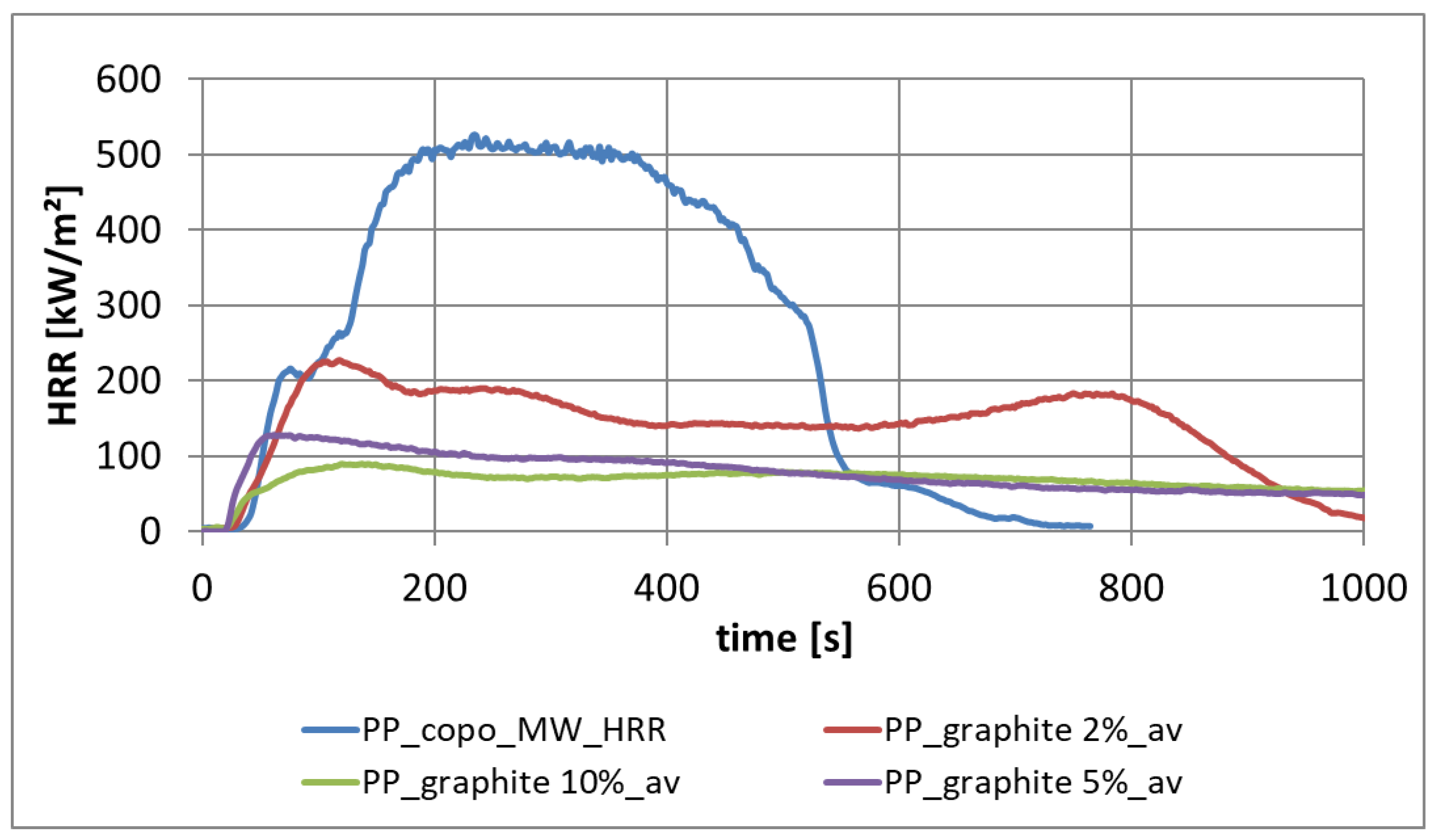
3.2.1. Heat Release from PP-Copo with Different Filling Contents of Expandable Graphite
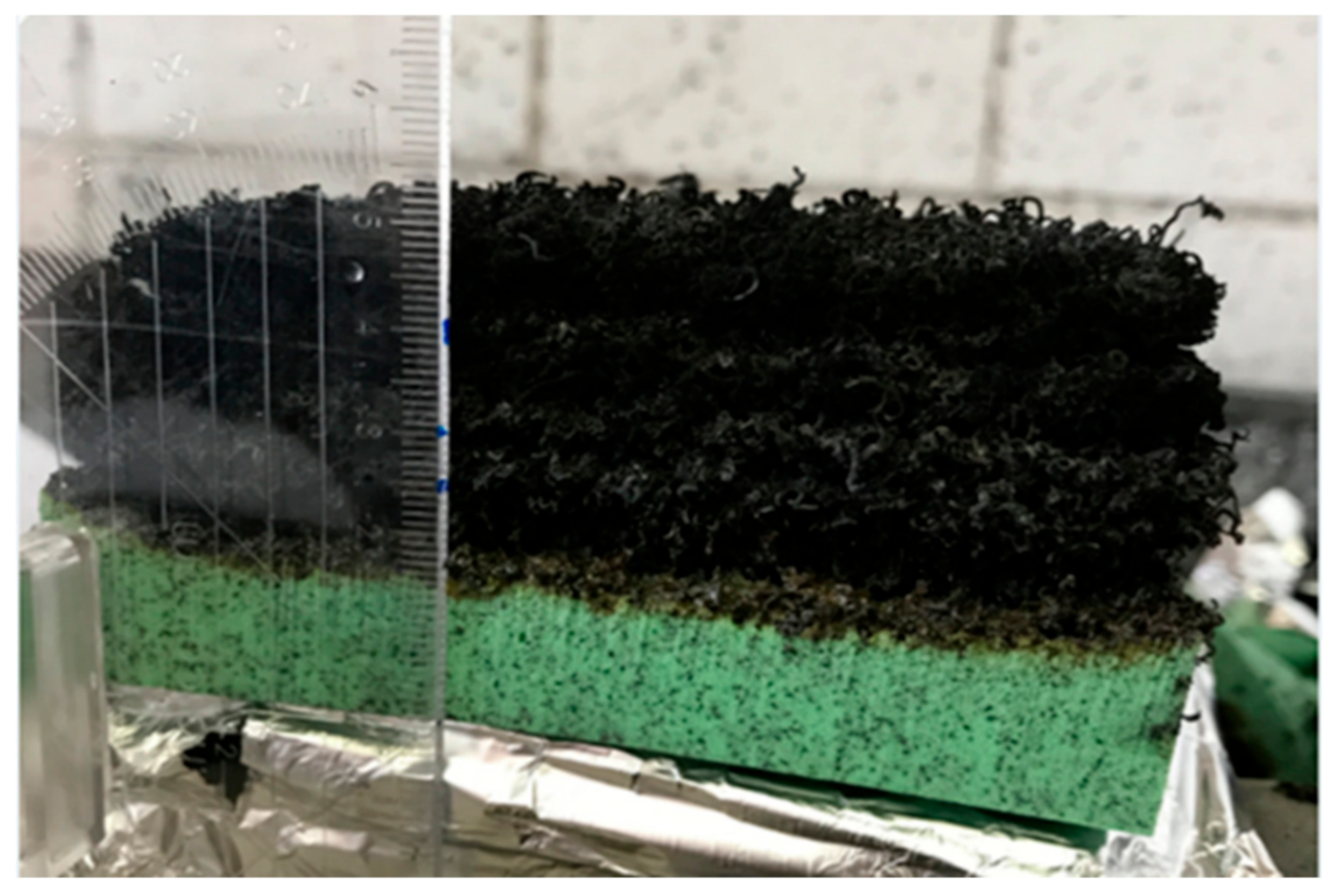
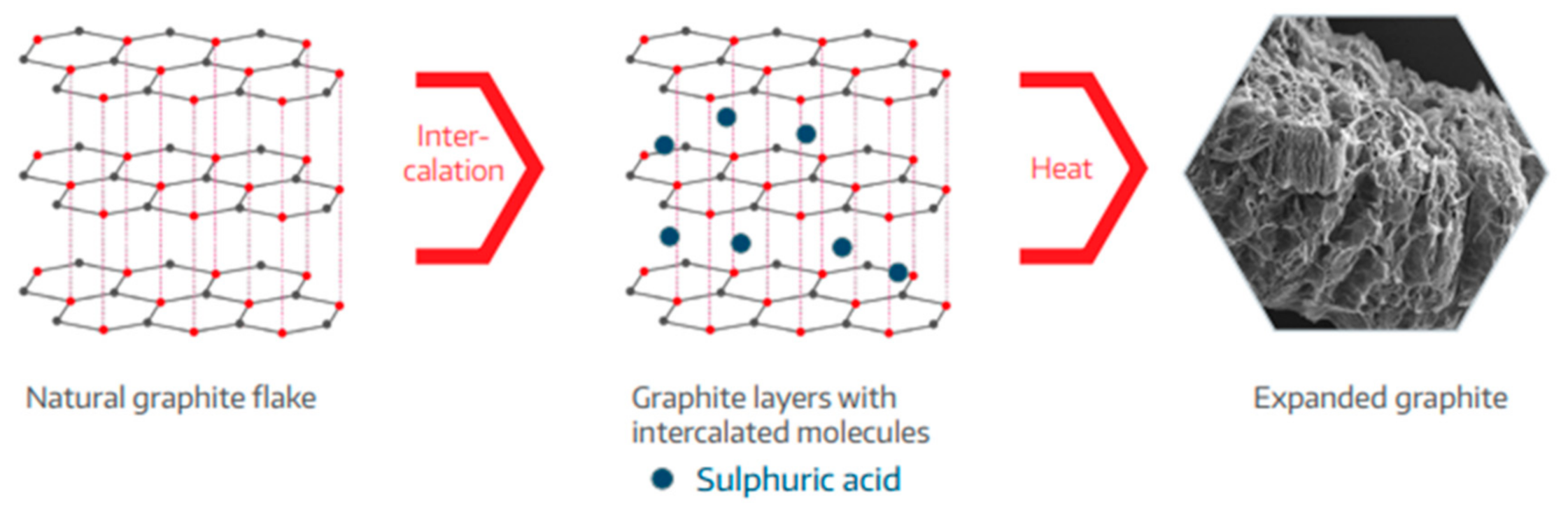
3.2.2. Effect of Expandable Graphite as a Flame Retardant
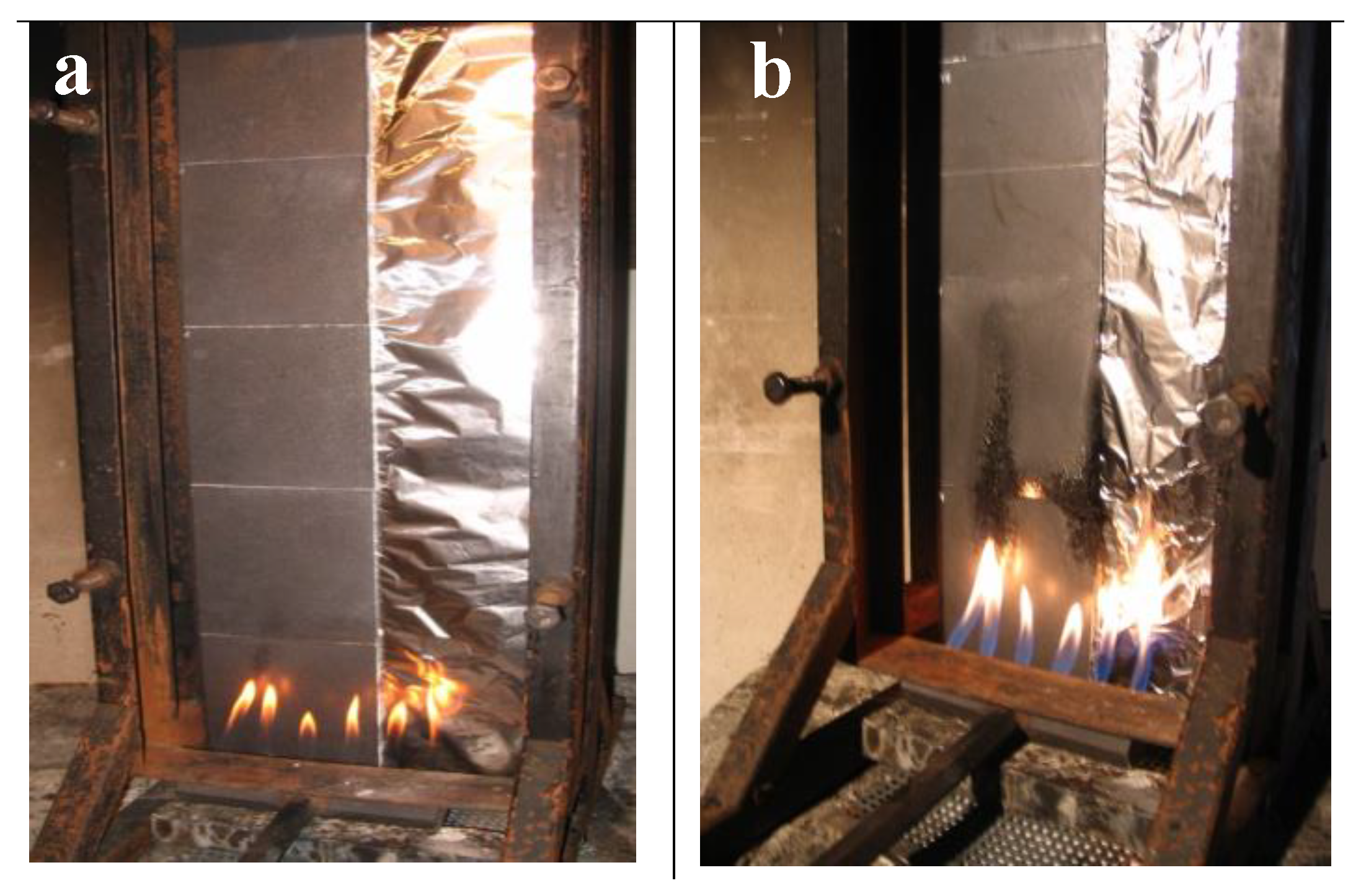
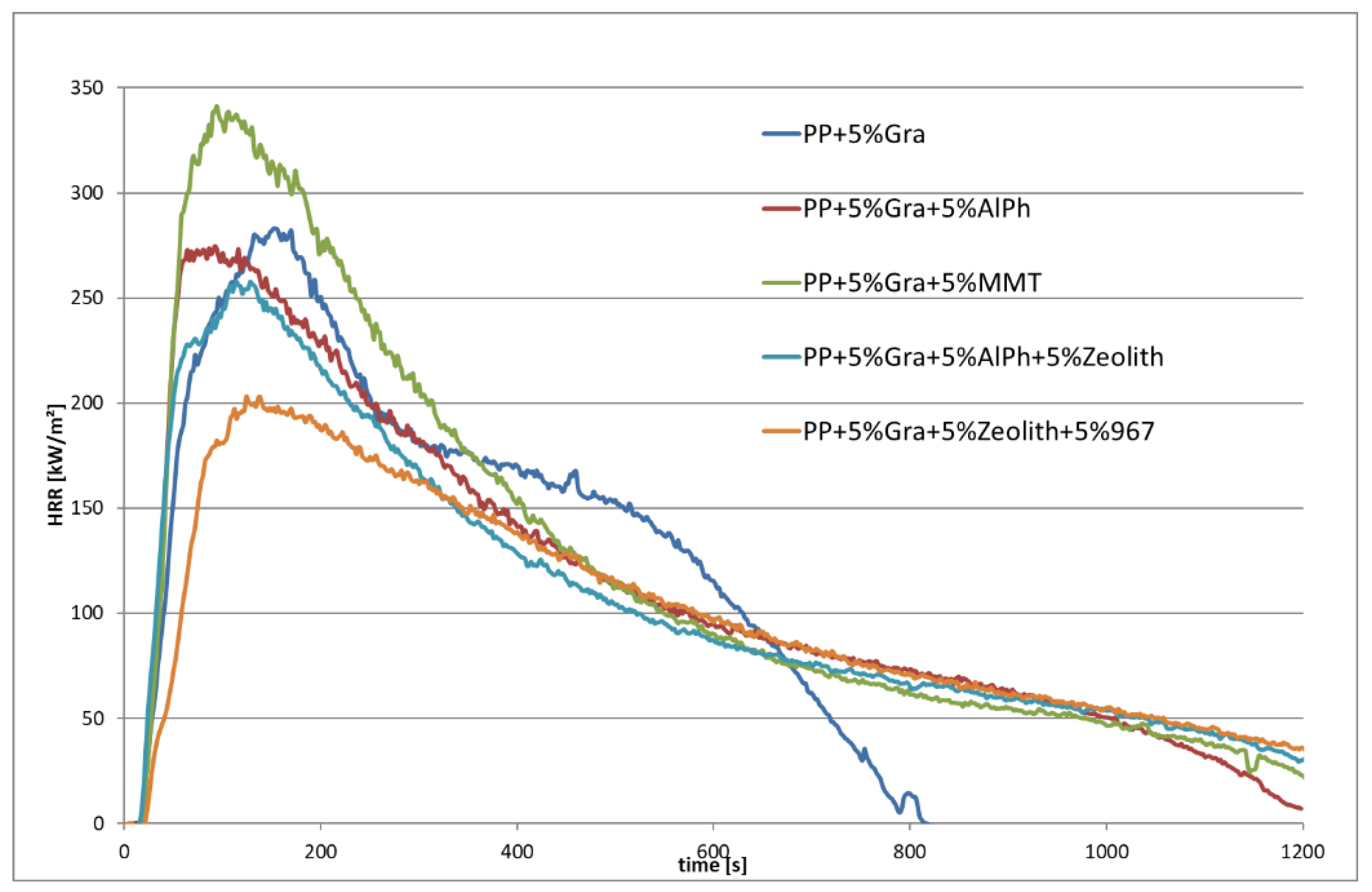
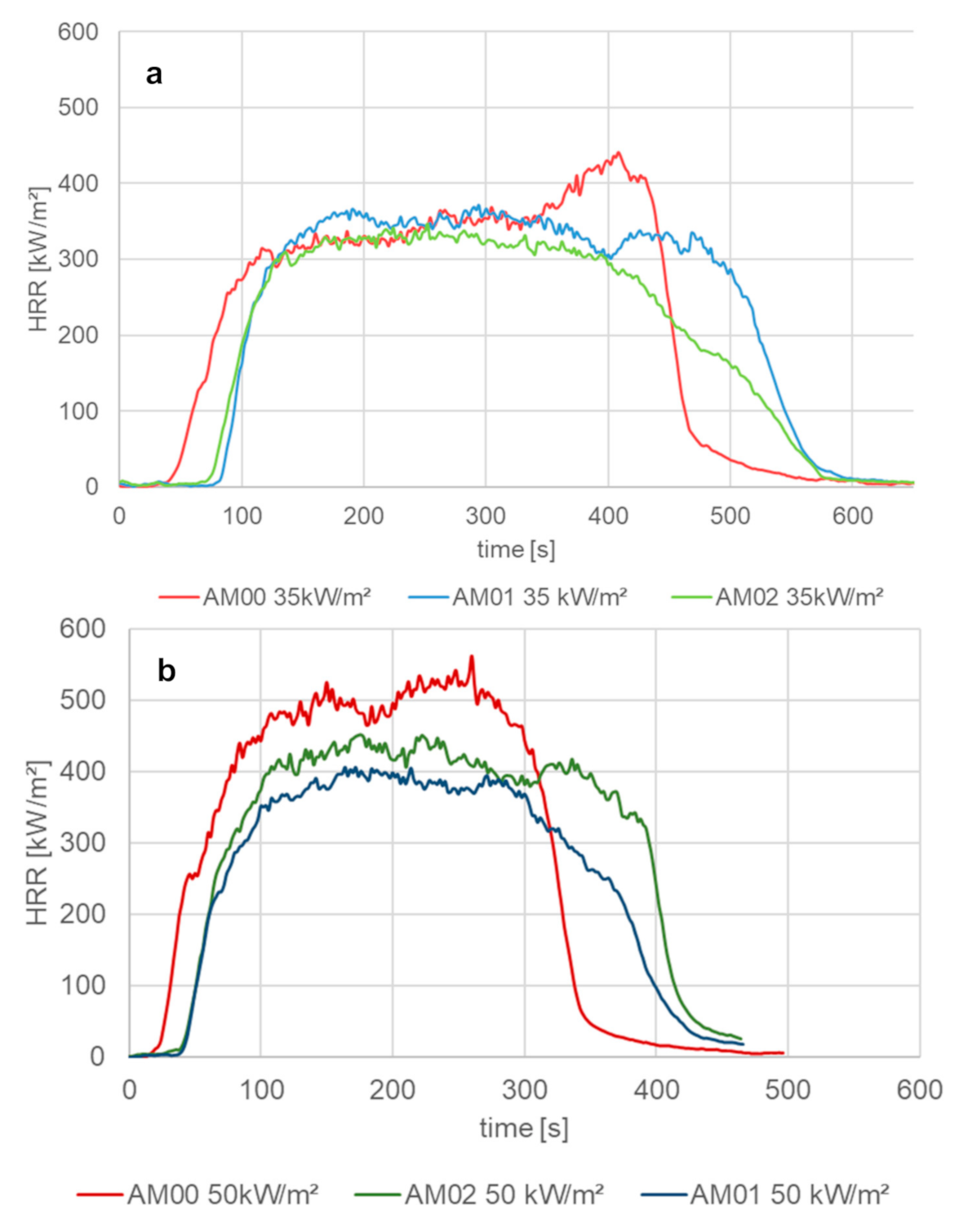
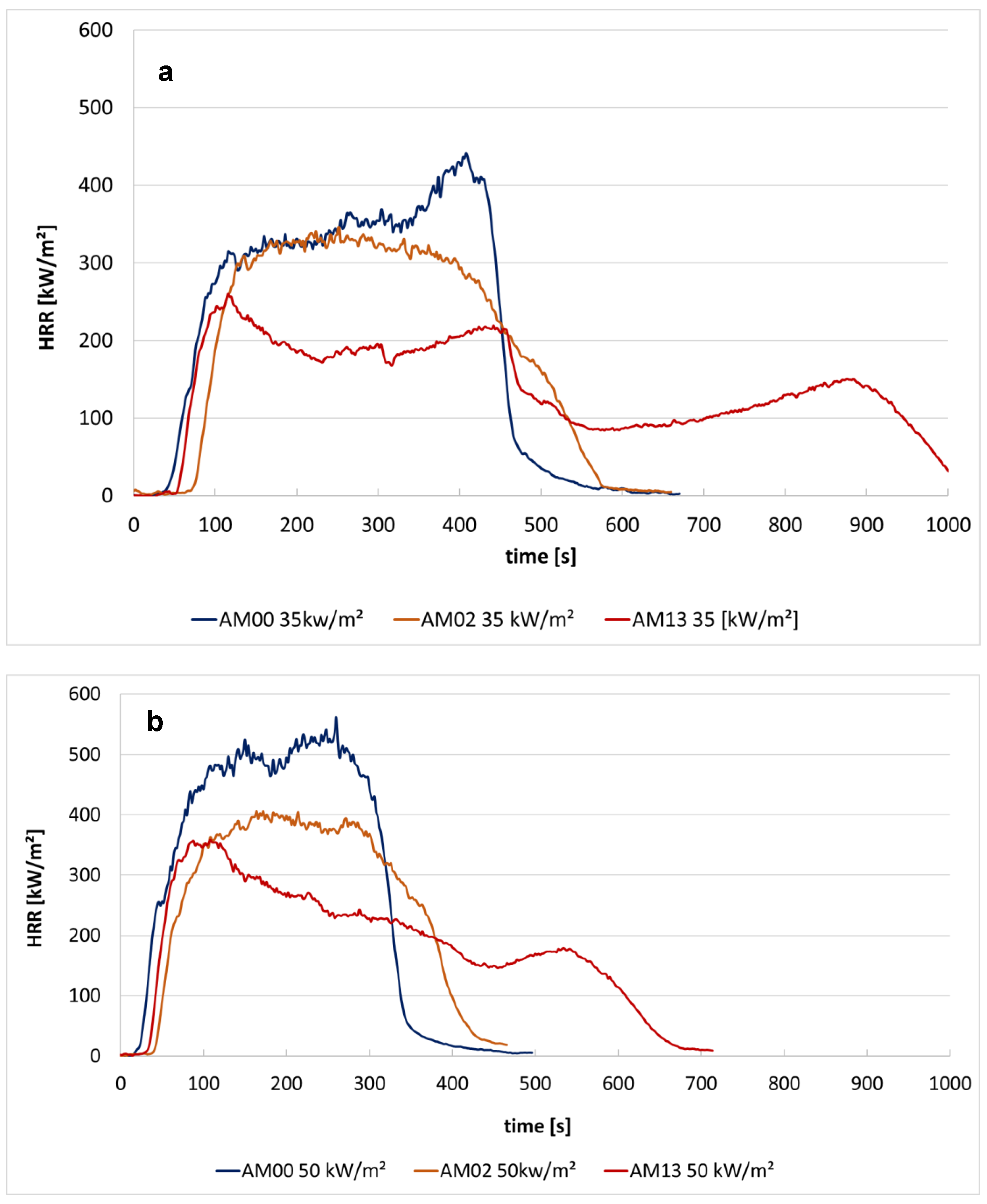
3.2.3. Heat Release Rate and Burning Behavior (Schlyter Test) of PP with Various Flame Retardants
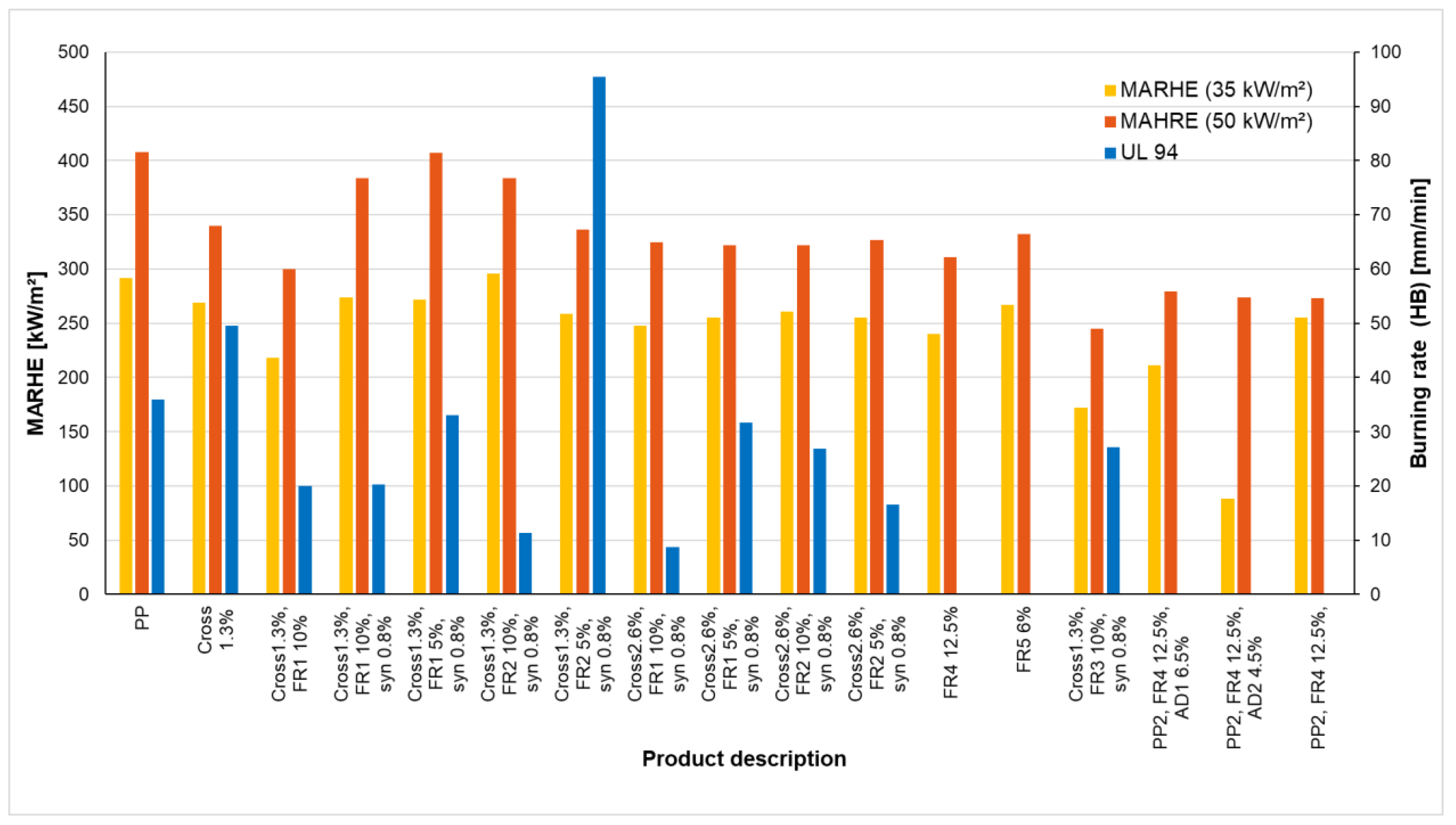
3.2.4. Change in the Heat Release rate of PP Sheets Due to Various Additivation of the Test Samples and Results of the UL 94-HB Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ISO 5660-1. Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). 2015. Available online: shop.austrian-standards.at/action/de/public/details/661613/ISO_5660-1_A1_2019_08_15 (accessed on 15 December 2020).
- Gahleitner, M.; Fiebig, J.; Wolfschwenger, J.; Dreiling, G.; Paulik, C. Post-Crystallization and Physical Aging of Polypropylene: Material and Processing Effects. J. Macromol. Sci. Part B 2002, 41, 833–849. [Google Scholar] [CrossRef]
- Fiebig, J.; Gahleitner, M.; Paulik, C.; Wolfschwenger, J. Ageing of polypropylene: Processes and consequences. Polym. Test. 1999, 18, 257–266. [Google Scholar] [CrossRef]
- Troitzsch, J. Plastics Flamability Handbook; Carl Hanser Verlag: Munich, Germany, 2004. [Google Scholar]
- Hohenwarter, D. Experience Gained of Fire Tests According EN 45 545-2 and DIN 5510-2 for Seats; Fire Safety in Railway Systems 2015; Bahntechnik aktuell Band 54; Interdisziplinärer Forschungsverbund ifv Bahntechnik e.V.: Berlin, Germany, 2015; pp. 23–38. [Google Scholar]
- Hohenwarter, D.; Fischer, C.; Berger, M. Influence of 3D-Printing on the Flammability Properties of Railway Applications Using Polycarbonate (PC) and Polylactic acid (PLA), Problemy Kolejnictwa; Railway Reports; Issue 187; Railway Research Institute: Warszawa, Poland, 2020; pp. 99–107. [Google Scholar]
- Zweifel, H.; Maier, R.D.; Schiller, M. Plastics Additives Handbook; Carl Hanser Verlag: Munich, Germany, 2009. [Google Scholar]
- Qin, Z.; Li, D.; Li, Q.; Yang, R. Effect of nano-aluminum hydroxide on mechanical properties, flame retardancy and combustion behavior of intumescent flame retarded polypropylene. Mater. Des. 2016, 89, 988–995. [Google Scholar] [CrossRef]
- Liang, J.Z.; Feng, J.Q.; Tsui, C.P.; Tang, C.Y.; Liu, D.F.; Zhang, S.D.; Huang, W.F. Mechanical properties and flame-retardant of PP/MRP/Mg(OH)2/Al(OH)3 composites. Compos. Part B Eng. 2015, 71, 74–81. [Google Scholar] [CrossRef]
- Lau, S.; Atakan, B. Isothermal pyrolysis investigation of aluminum diethylphosphinate mixed as a flame retardant additive into ultra-high molecular weight polyethylene. Combust. Flame 2020, 222, 272–284. [Google Scholar] [CrossRef]
- Vaari, J.; Paajanen, A. Evaluation of the reactive molecular dynamics method for Research on flame retardants: ATH-filled polyethylene. Comput. Mater. Sci. 2018, 153, 103–112. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Synergistic effects of organically modified montmorillonite on the flame-retardant and smoke suppression properties of transparent intumescent fire-retardant coatings. Prog. Org. Coat. 2018, 122, 107–118. [Google Scholar] [CrossRef]
- Batistella, M.; Sonnier, R.; Otazaghine, B.; Petter, C.; Lopez-Cuesta, J.-M. Interactions between kaolinite and phosphinate-based flame retardant in Polyamide 6. Appl. Clay Sci. 2018, 157, 248–256. [Google Scholar] [CrossRef]
- Khanal, S.; Lu, Y.; Ahmed, S.; Ali, M.; Xu, S. Synergistic effect of zeolite 4A on thermal, mechanical and flame retardant properties of intumescent flame retardant HDPE composites. Polym. Test. 2020, 81, 106177. [Google Scholar] [CrossRef]
- Lu, C.; Gao, X.-P.; Yao, D.-H.; Cao, C.-L.; Luo, Y.-J. Improving flame retardancy of linear low-density polyethylene/nylon 6 blends via controlling localization of clay and intumescent flame-retardant. Polym. Degrad. Stab. 2018, 153, 75–87. [Google Scholar] [CrossRef]
- Ramani, A.; Dahoe, A.E. On flame retardancy in polycaprolactam composites by aluminium diethylphosphinate and melamine polyphosphate in conjunction with organically modified montmorillonite nanoclay. Polym. Degrad. Stab. 2014, 105, 1–11. [Google Scholar] [CrossRef]
- Sonnier, R.; Caro, A.; Dumazert, L.; Longerey, M.; Otazaghine, B. Influence of radiation-crosslinking on flame retarded polymer materials—How crosslinking disrupts the barrier effect. Radiat. Phys. Chem. 2015, 106, 278–288. [Google Scholar] [CrossRef]
- Yuan, B.; Fan, A.; Yang, M.; Chen, X.; Hu, Y.; Bao, C.; Jiang, S.; Niu, Y.; Zhang, Y.; He, S.; et al. The effects of graphene on the flammability and fire behavior of intumescent flame retardant polypropylene composites at different flame scenarios. Polym. Degrad. Stab. 2017, 143, 42–56. [Google Scholar] [CrossRef]
- Uhl, F.M.; Yao, Q.; Hiroyoshi, N.; Maniasc, E.; Wilkiea, C.A. Expandable graphite/polyamide-6 nanocomposites. Polym. Degrad. Stab. 2005, 89, 70–84. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, Q.; Li, J.; Tao, K.; Xue, L.; Yan, Q. Synergistic effect between expandable graphite and ammonium polyphosphate on flame retarded polylactide. Polym. Degrad. Stab. 2011, 96, 183–189. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, L.; Lv, J. Effects of Expandable Graphite and Modified Ammonium Polyphosphate on the Flame-Retardant and Mechanical Properties of Wood Flour-Polypropylene Composites. Polym. Polym. Compos. 2013, 21, 449–456. [Google Scholar] [CrossRef]
- UL 94, Standard for Safety Tests for Flammability of Plastic Materials for Parts in Devices and Appliances, 5th ed.; Title Page Reprint 2013; Underwriters Laboratories: Northbrook, IL, USA, 1996.
- EN 60695-11-10: Fire Hazard Testing—Part 11-10: Test Flames-50 W Horizontal and Vertical Flame Test Methods (IEC 60695-11-10: 1999 +A1:2003). Available online: shop.austrian-standards.at/action/de/public/details/552333/OEVE_OENORM_EN_60695-11-10_AC_2015_11_01 (accessed on 15 December 2020).
- ÖNORM A 3800-1, Burning Behaviour of Materials Excluding Building Products—Part 1: Requirements, Tests and Evaluation; Austrian Standards Institute: Vienna, Austria, 2005; Available online: shop.austrian-standards.at/action/de/public/details/206070/OENORM_A_3800-1_2005_11_01 (accessed on 15 December 2020).
- DIN 4102-1, Fire Behaviour of Building Materials and Elements, Part 1: Classification of Building Materials, Requirements and Testing; Deutsches Institut für Normung: Berlin, Germany, 1998; Available online: shop.austrian-standards.at/action/de/public/details/111997/DIN_4102-1_1998_05 (accessed on 15 December 2020).
- ÖNORM EN 13501-1, Fire Classification of Construction Products and Building Elements—Part 1: Classification Using Data from Reaction to Fire Tests; Austrian Standards Institute: Vienna, Austria, 2019; Available online: shop.austrian-standards.at/action/de/public/details/669992/OENORM_EN_13501-1_2020_01_15 (accessed on 15 December 2020).
- Morgan, A.B.; Bundy, M. Cone calorimeter analysis of UL-94 V-rated plastics. Fire Mater. 2007, 31, 257–283. [Google Scholar] [CrossRef]
- Certifer, Eurocomparison 2018 Report, Interlaboratory Comparison on Railway Fire Behaviour Tests. Available online: www.certifer.fr/en/agrement-feu-fume-en (accessed on 15 December 2020).
- DRRR, Deutsches Referenzbüro für Ringversuche und Referenzmaterialien, Bericht DRRR-Ringversuche zu Eignungsprüfung, RVEP 180231 Brennverhalten UL 94 HB, RVEP 180232 Brennverhalten UL 94 V. 2018. Available online: www.DRRR.de (accessed on 15 December 2020).
- ÖNORM EN ISO 11357-3, Plastics—Differential Scanning Calorimetry (DSC)—Part 3: Determination of Temperature and Enthalpy of Melting and Crystallization; Austrian Standards Institute: Vienna, Austria, 2018.
- Ehrenstein, G.W.; Riedel, G.; Trawiel, P. Thermal Analysis of Plastics—Theory and Practice; Carl Hanser Verlag: Munich, Germany, 2004. [Google Scholar]
- ÖNORM EN ISO 291, Plastics—Standard Atmospheres for Conditioning and Testing; Austrian Standards Institute: Vienna, Austria, 2008; Available online: shop.austrian-standards.at/action/de/public/details/298136/OENORM_EN_ISO_291_2008_07_01 (accessed on 15 December 2020).
- Quintiere, J.G. Principles of Fire Behavior, Delmar, USA, DELMAR Cengage Learning. 1998. Available online: www.cengage.com/delmar (accessed on 15 December 2020).
- Graphit Kropfmühl GmbH: EXPANDED GRAPHITE. Available online: www.gk-graphite.com/fileadmin/user_upload/Leaflet_Expanded_Graphite.pdf (accessed on 10 February 2020).
- Wilkie, C.A.; Morgan, A.B. Fire Retardancy of Polymeric Materials, 2nd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Tao, S.; Wei, S.; Yulan, Y. Characterization of Expanded Graphite Microstructure and Fabrication of Composite Phase-Change Material for Energy Storage. J. Mater. Civ. Eng. 2015, 27, 04014156. [Google Scholar] [CrossRef]
- Mattausch, H. Development of Halogen-Free Flame Retardant Polypropylene Compounds for Pipe Application; Polymer Processing at Montanuniversität: Leoben, Austria, 2015. [Google Scholar]
- Mattausch, H.; Laske, S.; Đuretek, I.; Kreith, J.; Maier, G.; Holzer, C. Investigation of the influence of processing conditions on the thermal, rheological and mechanical behavior of polypropylene nanocomposites. Polym. Eng. Sci. 2013, 53, 1001–1010. [Google Scholar] [CrossRef]
- Mattausch, H.; Laske, S.; Hohenwarter, D.; Holzer, C. Influence of mineral fillers on the flame retardancy of expanded graphite/polypropylene materials. Proceedings of ANTEC 2014, Las Vegas, NV, USA, 28–30 April 2014. [Google Scholar]
- Mattausch, H.; Laske, S.; Hohenwarter, D.; Holzer, C. The effect of mineral fillers on the rheological, mechanical and thermal properties of halogen-free flame-retardant polypropylene/expandable graphite compounds. In Proceedings of the PPS-30: The 30th International Conference of the Polymer Processing Society–Conference Papers; AIP Publishing: Melville, NY, USA, 2015. [Google Scholar]
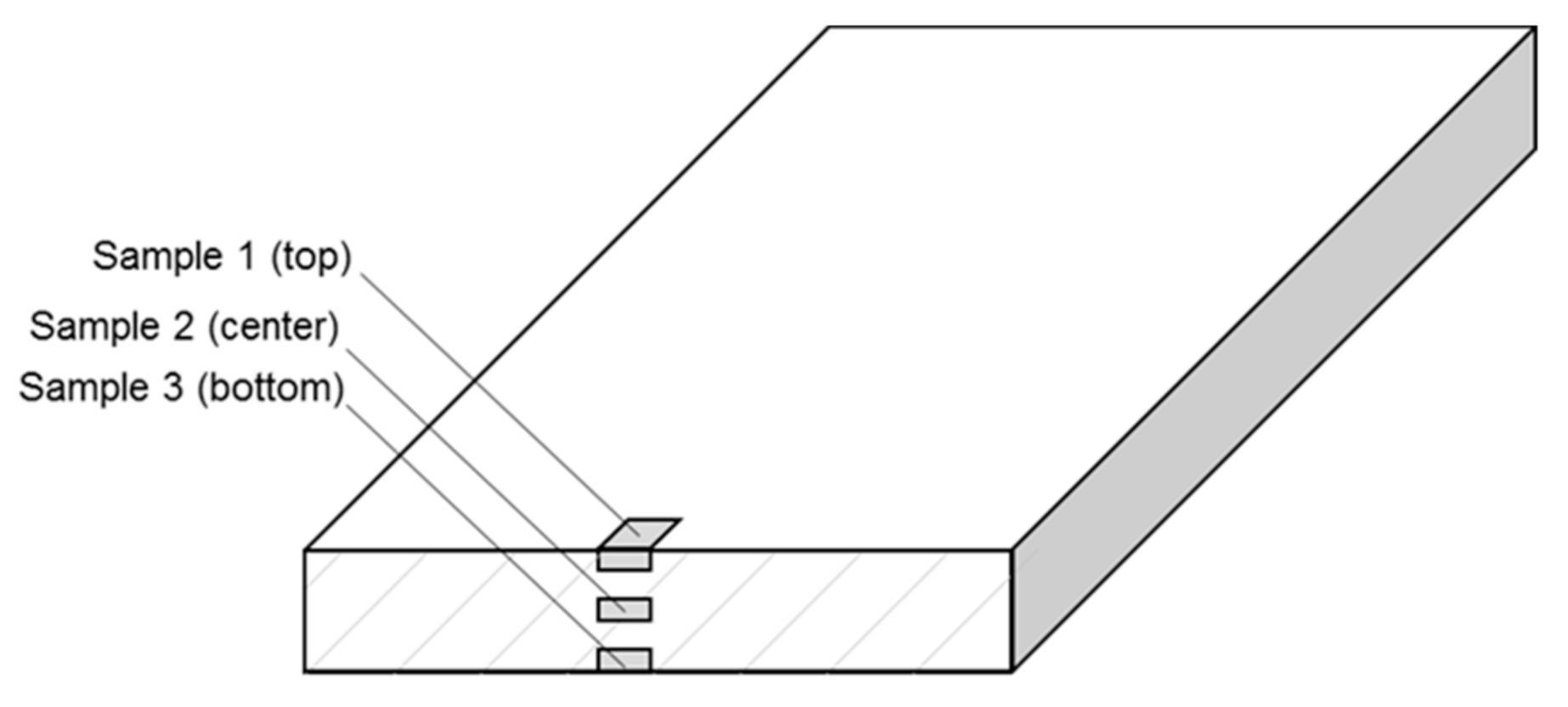





| Material | Producer | Polymer Type | Grade | Additional Information |
|---|---|---|---|---|
| PP | Borealis | PP Copolymer | BB412E | medium molecular weight block copolymer |
| PA | BASF | PA 6 | Ultramid B27E | low viscosity general-purpose, extrusion grade |
| PE-LD | Borealis | PE low density | FT5230 | unmodified for film extrusion |
| PE-LD | Dow | PE low density | 300E/302E | unmodified for blow film extrusion |
| PE-LD | Sabic | PE low density | 2601 × 1 | ultra melt strength grade with slip and antiblocking agents for foam applications |
| Gra | AMG Mining | - | ES350F5 | Expandable graphite, D80 300 µm |
| Zeolite | Paltentaler Minerals | - | 100_15 | D50 15 μm |
| MMT | Rockwood Clay Additives | - | Nanofil 5 | Montmorillonite, D50 10 μm |
| MgOH | Ankerpoort N.V. | - | Securoc B9 | Magnesium hydroxide, D50 2.6 µm |
| Silane | Sigma Aldrich | - | 3-(trimethoxysilyl) propyl methacrylate | - |
| Peroxide | Sigma Aldrich | - | Dicumyl peroxide | - |
| AlPh | Italmatch Chemicals SpA | - | Phoslite B85AX | Aluminium phosphinate |
| Perlit | Montanuniversitaet Leoben, Chair of Mineral Processing | - | - | Expanded perlite, D50 0.525 mm, bublon process |
| 967 | Thor | - | AFLAMMIT PPN 967 | Multi-component blend based on ammonium polyphosphate |
| NOR | BASF | - | NOR116 | monomeric N-alkoxy hindered amine (triazine derivative) |
| NP | Adeka Palmarole | - | ADK STAB FP-2100JC | nitrogen/phosphorus-based, halogen-free flame retardant |
| Storing Time [Days] | Storing Temperature [°C] | Degree of Crystallinity [%] |
|---|---|---|
| 1 | 23 | 34 |
| 5 | 8 | 35 |
| 7 | 23 | 34 |
| 5 | 80 | 35 |
| Parameter | Sample Mass [g] | |
|---|---|---|
| 20 | 60 | |
| HRR Peak [kW/m2] | 299 | 530 |
| MARHE [kW/m2] | 197 | 375 |
| EHC [MJ/kg] | 29 | 77 |
| Time of Ignition [s] | 34 | 32 |
| Burning Time [s] | 266 | 552 |
| Radiation Intensity [kW/m2] | 25 | 35 | 50 | 60 | 75 | 95 |
| HRR Peak [kW/m2] | 252 | 297 | 299 | 330 | 415 | 445 |
| MAHRE [kW/m2] | 57 | 116 | 197 | 205 | 291 | 313 |
| EHC [MJ/kg] | 32 | 31 | 29 | 30 | 29 | 30 |
| Time to Ignition tig [s] | 524 | 204 | 34 | 33 | 13 | 12 |
| Burning Time [s] | 626 | 326 | 266 | 348 | 256 | 218 |
| Radiation Int. 35 kW/m2 | Radiation Int. 50 kW/m2 | UL94 HB | ||||
|---|---|---|---|---|---|---|
| Sample | Weight Percentages of Additives [%] | HRRp | MARHE | HRRp | MARHE | mm/min |
| AM 00 | PP1 100% (native) | 441 | 292 | 562 | 408 | 35.9 |
| AM 01 | PP1 + Cross 1.3% | 371 | 269 | 451 | 340 | 49.6 |
| AM 02 | PP1 + Cross 1.3% + FR1 10% | 346 | 218 | 406 | 300 | 20 |
| AM 03 | PP1 + Cross 1.3% + FR1 10% + Syn 0.8% | 398 | 274 | 593 | 384 | 20.3 |
| AM 04 | PP1 + Cross 1.3% + FR1 5% + Syn 0.8% | 377 | 272 | 605 | 407 | 33 |
| AM 05 | PP1 +Cross 1.3% + FR2 10% + Syn 0.8% | 435 | 296 | 589 | 384 | 11.4 |
| AM 06 | PP1 + Cross 1.3% + FR2 5% + Syn 0.8% | 399 | 259 | 534 | 336 | 95.5 |
| AM 07 | PP1 + Cross 2.6% + FR1 10% + Syn 0.8% | 375 | 248 | 478 | 325 | 8.7 |
| AM 08 | PP1 + Cross 2.6% + FR1 5% + Syn 0.8% | 379 | 255 | 443 | 322 | 31.7 |
| AM 09 | PP1 + Cross 2.6% + FR2 10% + Syn 0.8% | 374 | 261 | 443 | 322 | 26.8 |
| AM 10 | PP1 + Cross 2.6% + FR2 5% + Syn 0.8% | 346 | 255 | 415 | 327 | 16.5 |
| AM 11 | PP1 + FR4 12.5% | 393 | 240 | 483 | 311 | 0 |
| AM 12 | PP1 + FR5 6% | 536 | 267 | 584 | 332 | 0 |
| AM 13 | PP1 + Cross 1.3% + FR3 10% + Syn 0.8% | 261 | 172 | 358 | 245 | 27.2 |
| RO 01 | PP2 + FR4 12.5% + Ad1 6.5% | 334 | 211 | 387 | 279 | – |
| RO 02 | PP2 + FR4 12.5% + Ad2 4.5% | 136 | 88 | 414 | 274 | – |
| RO 03 | PP2 + FR4 12.5% | 381 | 255 | 435 | 273 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohenwarter, D.; Mattausch, H.; Fischer, C.; Berger, M.; Haar, B. Analysis of the Fire Behavior of Polymers (PP, PA 6 and PE-LD) and Their Improvement Using Various Flame Retardants. Materials 2020, 13, 5756. https://doi.org/10.3390/ma13245756
Hohenwarter D, Mattausch H, Fischer C, Berger M, Haar B. Analysis of the Fire Behavior of Polymers (PP, PA 6 and PE-LD) and Their Improvement Using Various Flame Retardants. Materials. 2020; 13(24):5756. https://doi.org/10.3390/ma13245756
Chicago/Turabian StyleHohenwarter, Dieter, Hannelore Mattausch, Christopher Fischer, Matthias Berger, and Bernd Haar. 2020. "Analysis of the Fire Behavior of Polymers (PP, PA 6 and PE-LD) and Their Improvement Using Various Flame Retardants" Materials 13, no. 24: 5756. https://doi.org/10.3390/ma13245756
APA StyleHohenwarter, D., Mattausch, H., Fischer, C., Berger, M., & Haar, B. (2020). Analysis of the Fire Behavior of Polymers (PP, PA 6 and PE-LD) and Their Improvement Using Various Flame Retardants. Materials, 13(24), 5756. https://doi.org/10.3390/ma13245756




