Regional Waste Streams as Potential Raw Materials for Immediate Implementation in Cement Production
Abstract
1. Introduction
2. Materials and Methods
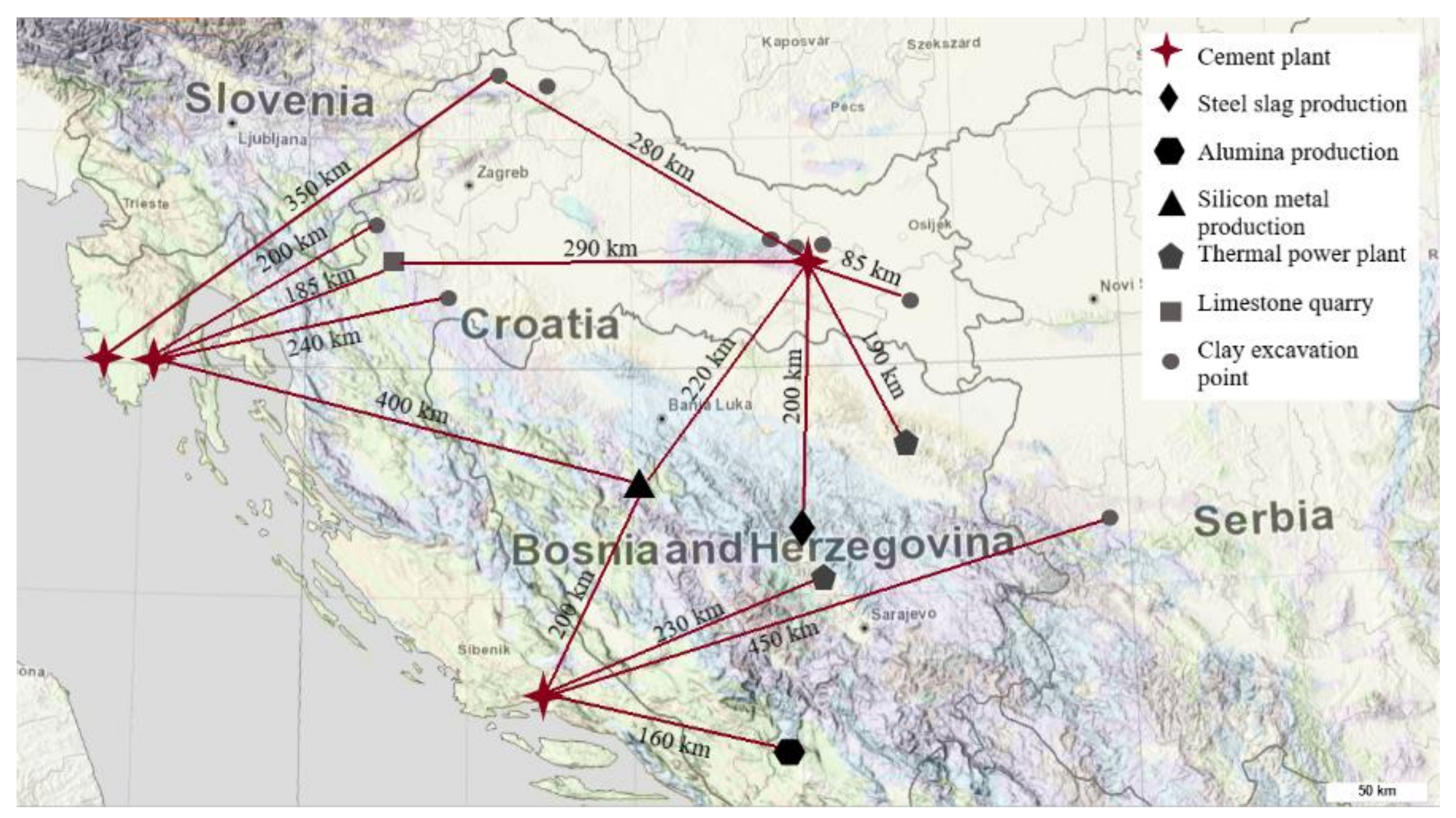
2.1. Materials
2.2. Characterisation Methods
3. Results
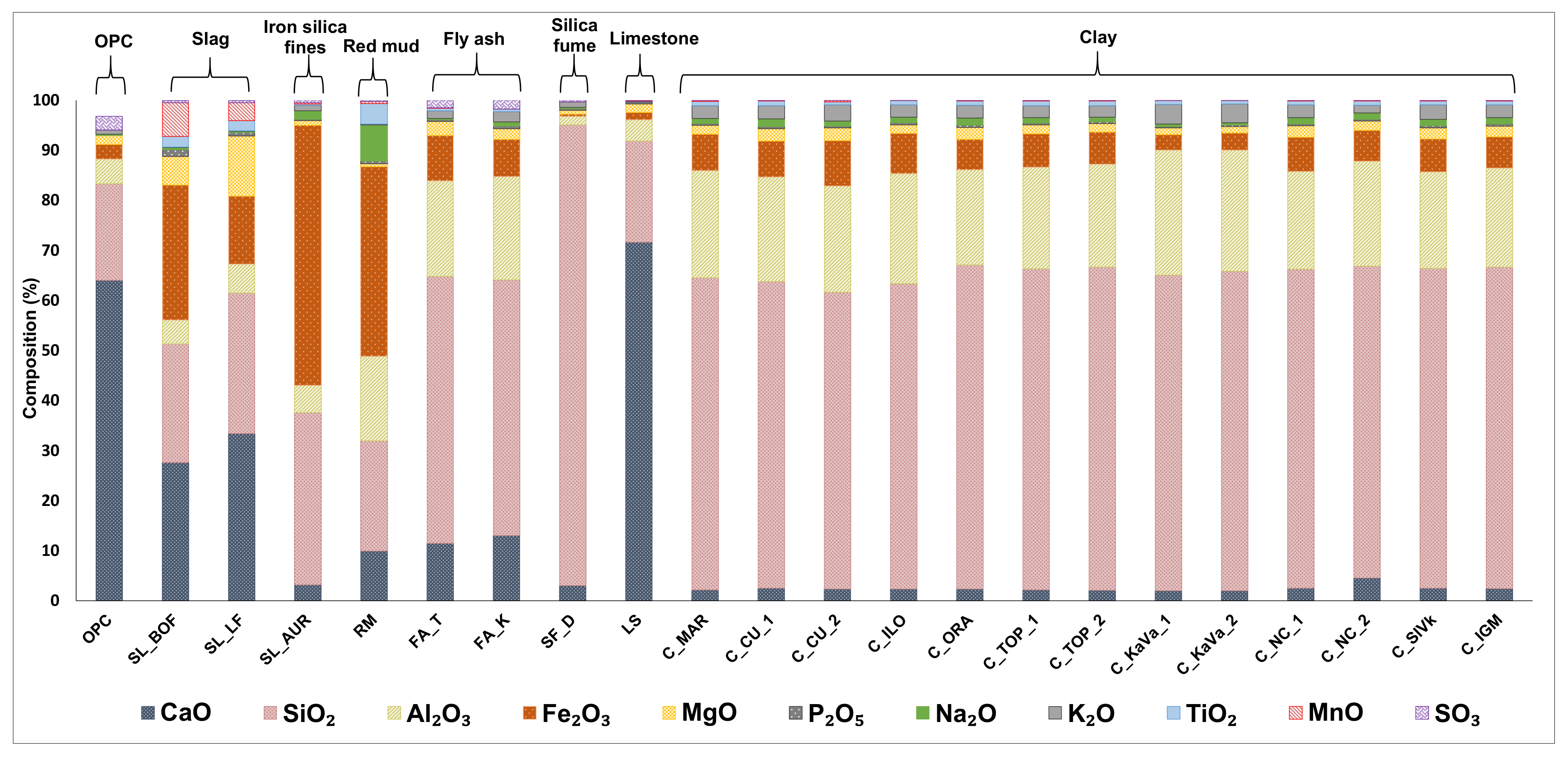
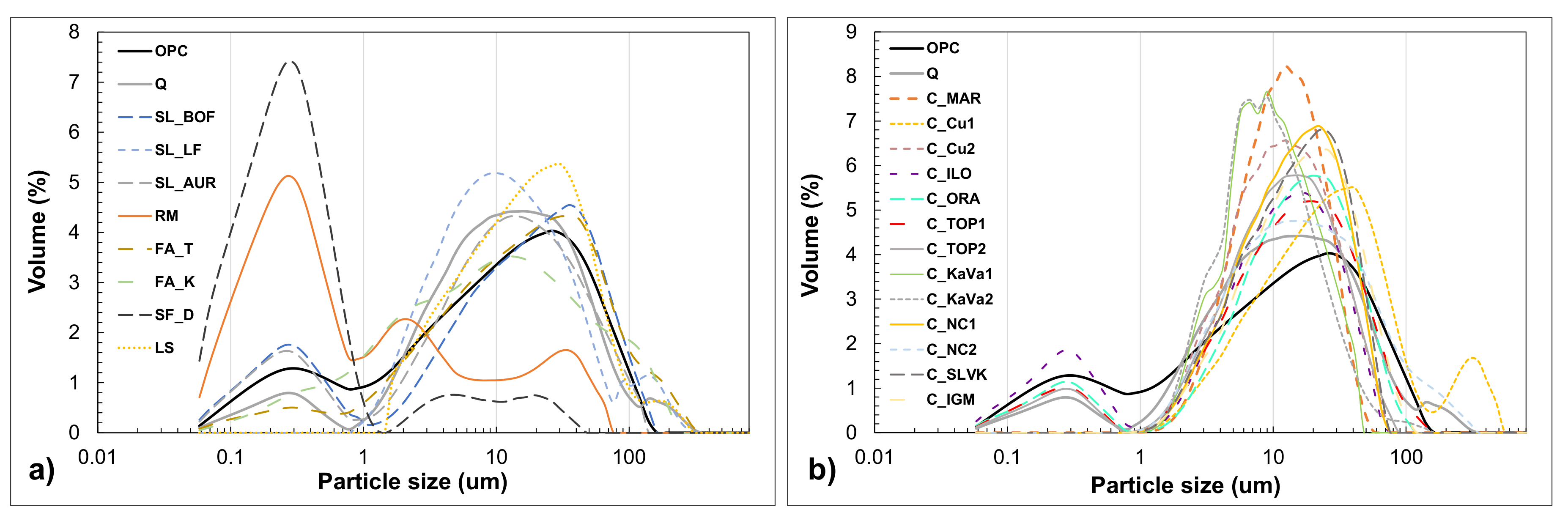
3.1. Physicochemical Properties
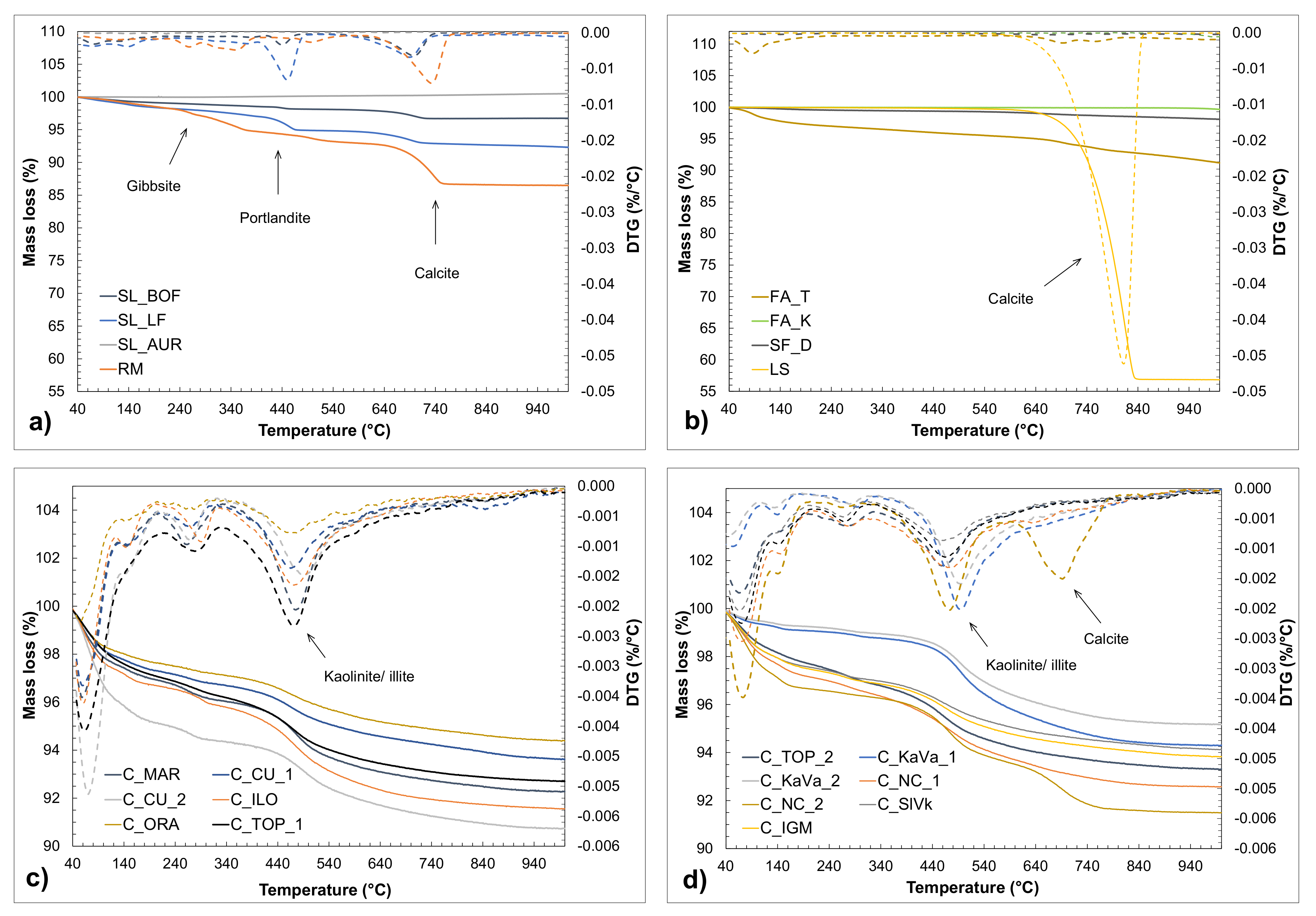
3.2. Phase Composition
3.3. Chemical Reactivity
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meyer, C. The greening of the concrete industry. Cem. Concr. Compos. 2009, 31, 601–605. [Google Scholar] [CrossRef]
- World Business Council for Sustainable Development. Cement Industry Energy and CO2 Performance: Getting the Numbers Right (GNR). Cem. Sustain. Initiat. Cem. 2016, p. 44. Available online: https://www.wbcsdcement.org/index.html (accessed on 5 June 2020).
- Scrivener, K.L.; John, V.M.; Gartner, E. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Celik, K.; Meral, C.; Mancio, M.; Mehta, P.K.; Monteiro, P.J.M. A comparative study of self-consolidating concretes incorporating high-volume natural pozzolan or high-volume fly ash. Constr. Build. Mater. 2014, 67, 14–19. [Google Scholar] [CrossRef]
- Manso, J.M.; Losáñez, M.; Polanco, J.A.; González, J.J. Ladle Furnace Slag in Construction. J. Mater. Civ. Eng. 2005, 17, 513–518. [Google Scholar] [CrossRef]
- Ćećez, M.; Šahinagić-Isović, M. Mortars with addition of local industrial by-products. Građevinar 2019, 71, 1–7. [Google Scholar] [CrossRef]
- Habert, G.; Billard, C.; Rossi, P.; Chen, C.; Roussel, N. Cement production technology improvement compared to factor 4 objectives. Cem. Concr. Res. 2010, 40, 820–826. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the calcined kaolinite content on the hydration of Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Blotevogel, S.; Ehrenberg, A.; Steger, L.; Doussang, L.; Kaknics, J.; Patapy, C.; Cyr, M. Ability of the R3 test to evaluate differences in early age reactivity of 16 industrial ground granulated blast furnace slags (GGBS). Cem. Concr. Res. 2020, 130, 105998. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Janković, K.; Bojović, D.; Lončar, L.; Stojanović, M.; Antić, L. Possibility of using bottom ash in precast concrete products. J. Croat. Assoc. Civ. Eng. 2018, 70, 413–419. [Google Scholar] [CrossRef]
- Snellings, R. Assessing, Understanding and Unlocking Supplementary Cementitious Materials. RILEM Tech. Lett. 2016, 1, 50–55. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary Cementitious Materials. Rev. Miner. Geochem. 2012, 74, 211–278. [Google Scholar] [CrossRef]
- The Geological Survey Organizations of Europe, E-Government Development Index (EGDI). Available online: http://www.europe-geology.eu/onshore-geology/geological-map/igme5000/ (accessed on 10 June 2020).
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- The International Organization for Standardization. ISO 13605:2018 Solid mineral fuels—Major and Minor Elements in Coal Ash and Coke Ash—Wavelength Dispersive X-Ray Fluorescence Spectrometric Method; The International Organization for Standardization: Vernier, Switzerland, 2018. [Google Scholar]
- ASTM International. ASTM C188-17, Standard Test Method for Density of Hydraulic Cement; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- European Standard. EN 450-1:2012 Fly Ash for concrete—Part 1: Definition, Specifications and Conformity Criteria; European Committee for Standardization: Brussels, Belgium, 2012. [Google Scholar]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Avet, F.; Scrivener, K. Simple and Reliable Quantification of Kaolinite in Clay Using an Oven and a Balance. In Calcined Clays for Sustainable Concrete, RILEM Bookseries; Springer: Dordrecht, The Netherlands, 2020; Volume 15, pp. 147–156. [Google Scholar]
- Li, X.; Snellings, R.; Antoni, M.; Alderete, N.M.; Ben Haha, M.; Bishnoi, S.; Cizer, Ö.; Cyr, M.; de Weerdt, K.; Dhandapani, Y.; et al. Reactivity tests for supplementary cementitious materials: RILEM TC 267-TRM phase 1. Mater. Struct. Constr. 2018, 51, 151. [Google Scholar] [CrossRef]
- European Committee for Standardization (CEN). EN 196-1:2016 Methods of Testing Cement—Part 1: Determination of Strength; CEN: Brussel, Belgium, 2016. [Google Scholar]
- ASTM International. C618—19, Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar] [CrossRef]
- Montes-Morán, M.A.; Concheso, A.; Canals-Batlle, C.; Aguirre, N.V.; Ania, M.C.O.; Martín, M.J.; Masaguer, V. Linz-Donawitz Steel Slag for the Removal of Hydrogen Sulfide at Room Temperature. Environ. Sci. Technol. 2012, 46, 8992–8997. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, A. An investigation on characterization and thermal analysis of the Auginish red mud. J. Therm. Anal. Calorim. 2005, 81, 357–361. [Google Scholar] [CrossRef]
- Taylor-Lange, S.C.; Lamon, E.L.; Riding, K.A.; Juenger, M.C. Calcined kaolinite–bentonite clay blends as supplementary cementitious materials. Appl. Clay Sci. 2015, 108, 84–93. [Google Scholar] [CrossRef]
- Koshy, N.; Dondrob, K.; Hu, L.; Wen, Q.; Meegoda, J.N. Mechanical Properties of Geopolymers Synthesized from Fly Ash and Red Mud under Ambient Conditions. Crystals 2019, 9, 572. [Google Scholar] [CrossRef]
- ASTM International. C1897−20, Standard Test Methods for Measuring the Reactivity of Supplementary Cementitious Materials by Isothermal Calorimetry and Bound Water; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Walker, R.; Pavía, S. Physical properties and reactivity of pozzolans, and their influence on the properties of lime–pozzolan pastes. Mater. Struct. 2010, 44, 1139–1150. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. Increasing the kaolinite content of raw clays using particle classification techniques for use as supplementary cementitious materials. Constr. Build. Mater. 2020, 244, 118335. [Google Scholar] [CrossRef]
- Netinger, I.; Rukavina, M.J.; Bjegović, D. Possibility of using domestic slag as concrete aggregate. Gradjevinar 2010, 62, 35–43. [Google Scholar]


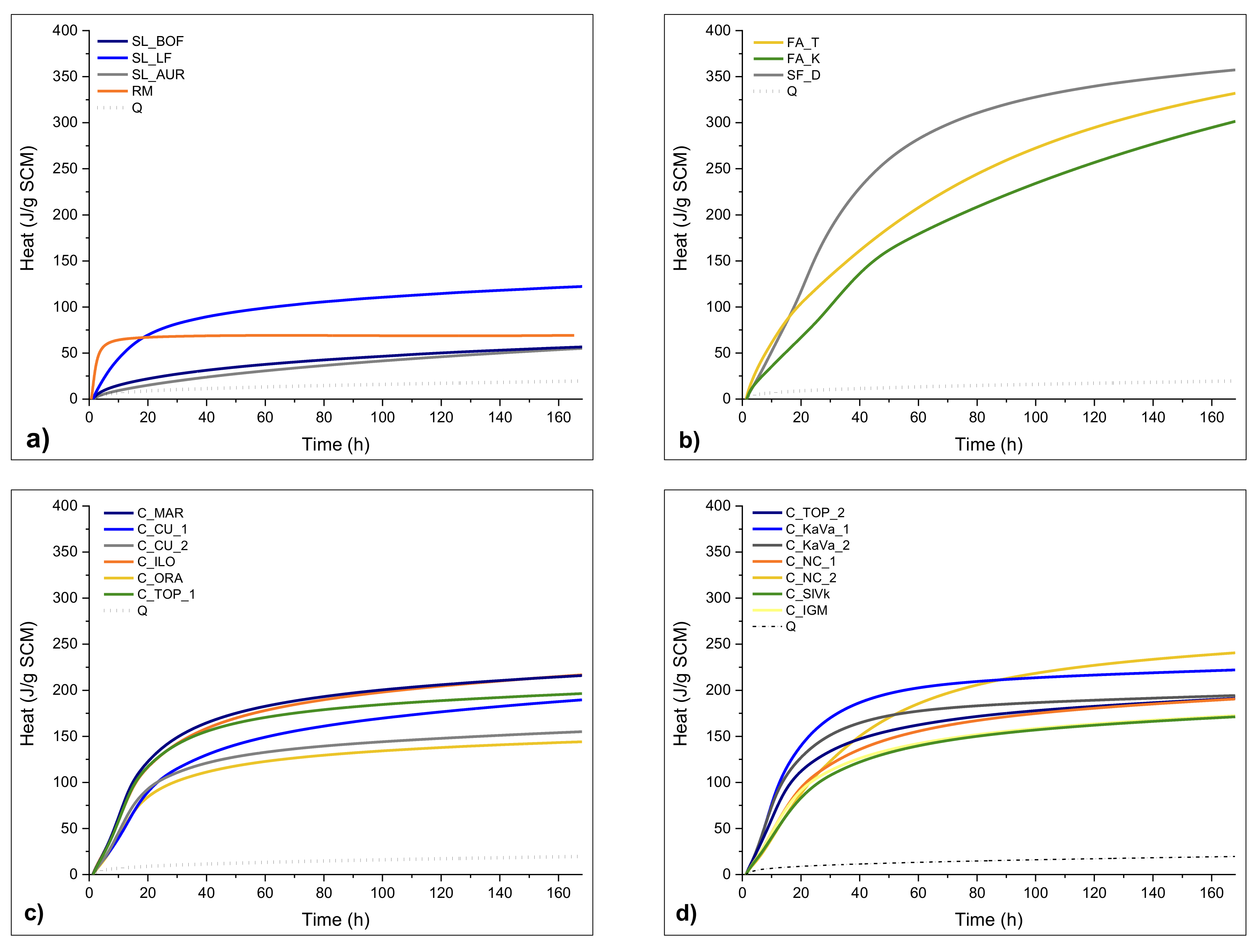
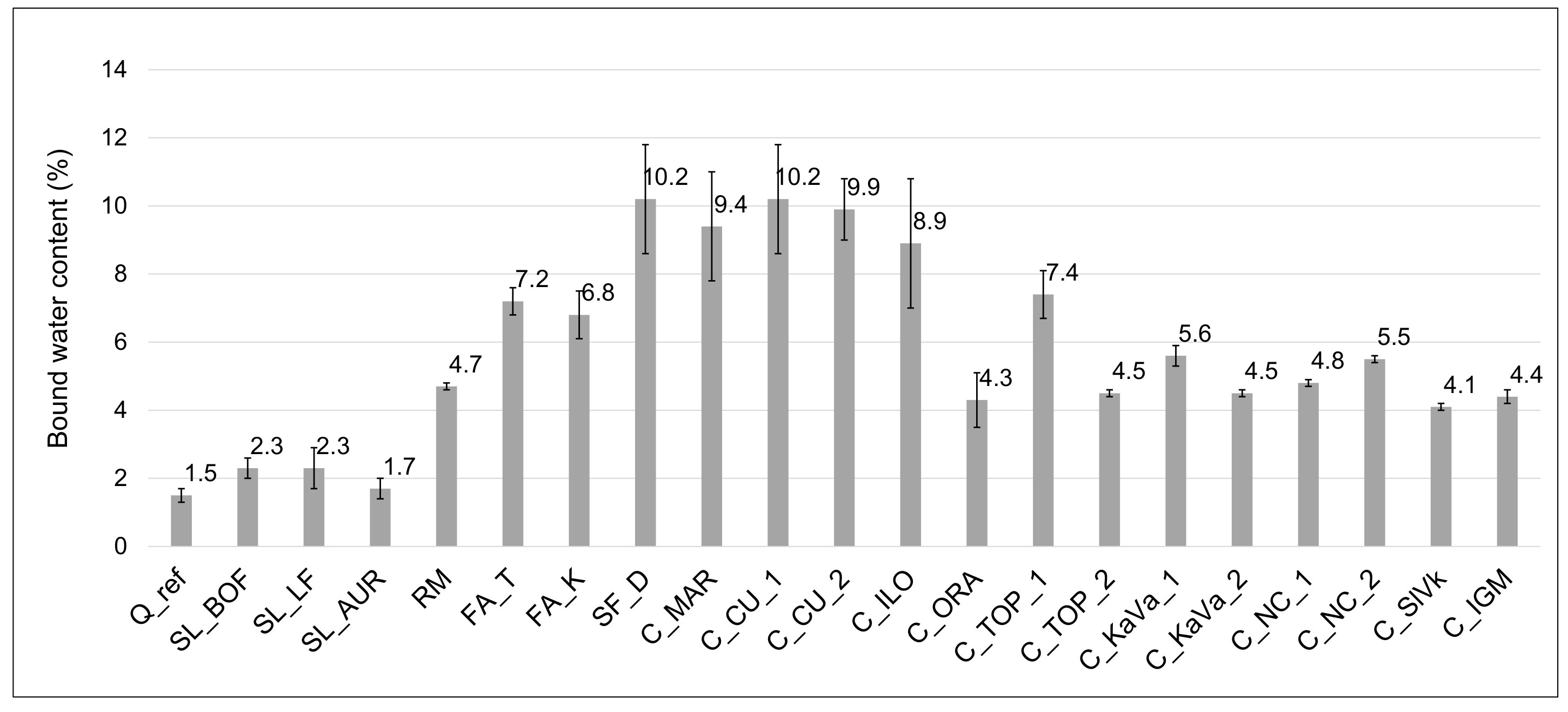
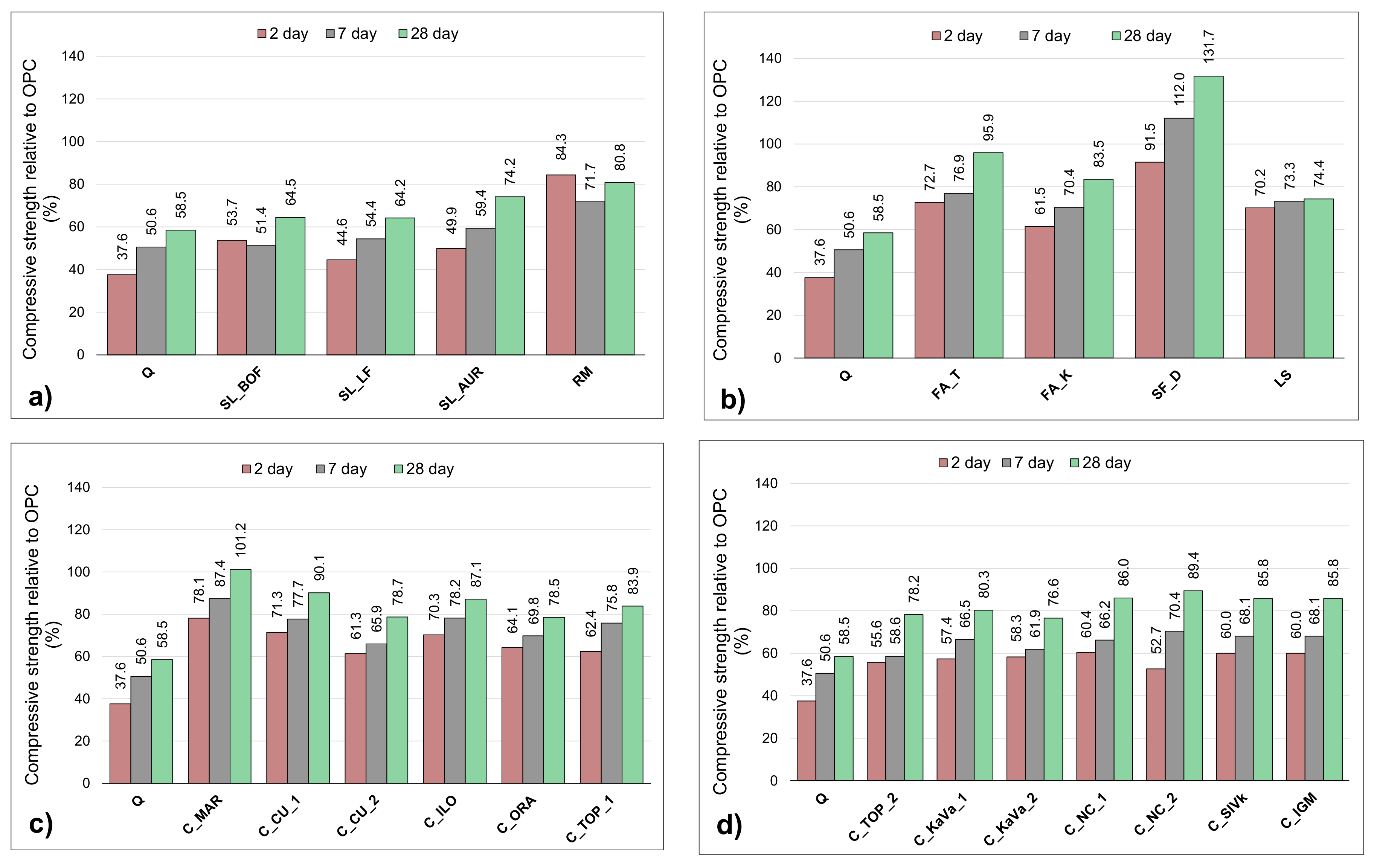
| Material | ∑ Primary Oxides ≥ 70% wt. | Na2Oeq ≤ 5% wt. | MgO ≤ 4% wt. | SO3 ≤ 3% wt. | P2O5 ≤ 5% wt. |
|---|---|---|---|---|---|
| SL_BOF | 55.51 | 0.51 | 5.68 | 0.46 | 1.37 |
| SL_LF | 47.45 | 0.31 | 12.00 | 0.44 | 0.72 |
| SL_AUR | 91.74 | 2.42 | 0.94 | 0.53 | 0.26 |
| RM | 76.77 | 7.35 | 0.61 | 0.24 | 0.47 |
| FA_T | 81.44 | 1.25 | 2.78 | 1.48 | 0.36 |
| FA_K | 79.10 | 2.20 | 2.15 | 1.72 | 0.54 |
| SF_D | 94.15 | 0.94 | 0.77 | 0.27 | 0.36 |
| LS | 25.96 | <0.010 | 1.69 | 0.08 | 0.42 |
| C_MAR | 91.02 | 2.70 | 1.78 | 0.07 | 0.36 |
| C_CU_1 | 89.38 | 3.26 | 2.42 | 0.07 | 0.44 |
| C_CU_2 | 89.53 | 3.09 | 2.57 | 0.07 | 0.40 |
| C_ILO | 90.99 | 2.76 | 1.75 | 0.08 | 0.36 |
| C_ORA | 89.78 | 3.18 | 2.41 | 0.07 | 0.40 |
| C_TOP_1 | 91.11 | 2.72 | 1.78 | 0.09 | 0.36 |
| C_TOP_2 | 91.53 | 2.47 | 1.74 | 0.10 | 0.34 |
| C_KaVa_1 | 91.12 | 3.08 | 1.38 | 0.07 | 0.28 |
| C_KaVa_2 | 91.47 | 2.97 | 1.28 | 0.08 | 0.28 |
| C_NC_1 | 90.03 | 2.92 | 2.34 | 0.12 | 0.35 |
| C_NC_2 | 89.37 | 2.29 | 1.88 | 0.08 | 0.35 |
| C_SlVk | 89.70 | 3.18 | 2.25 | 0.08 | 0.43 |
| C_IGM | 90.29 | 2.97 | 2.11 | 0.07 | 0.39 |
| Material | Density | Particle Size (µm) | ||
|---|---|---|---|---|
| (g/cm3) | D (v, 0.1) | D (v, 0.5) | D (v, 0.9) | |
| OPC | 3.09 | 0.32 | 9.95 | 50.8 |
| SL_BOF | 3.63 | 0.2 | 13.1 | 57.2 |
| SL_LF | 3.04 | 2.6 | 10.3 | 47.2 |
| SL_AUR | 2.4 | 0.2 | 9.5 | 46 |
| RM | 2.88 | 0.1 | 0.4 | 10.1 |
| FA_T | 2.08 | 1.5 | 15.2 | 73 |
| FA_K | 2.47 | 0.7 | 8.6 | 68 |
| SF_D | 2.13 | 0.1 | 0.3 | 3.5 |
| LS | 2.7 | 3.4 | 18 | 63.8 |
| C_MAR | 2.26 | 4 | 10.7 | 24.8 |
| C_CU_1 | 2.59 | 5 | 23.8 | 104.8 |
| C_CU_2 | 2.43 | 3.4 | 10.8 | 31.3 |
| C_ILO | 2.29 | 0.2 | 8.9 | 29.8 |
| C_ORA | 2.56 | 0.4 | 13 | 40.6 |
| C_TOP_1 | 2.52 | 0.5 | 12.2 | 43.5 |
| C_TOP_2 | 2.5 | 1.3 | 10 | 30.1 |
| C_KaVa_1 | 2.57 | 3.2 | 8.6 | 24.1 |
| C_KaVa_2 | 2.57 | 3 | 8.2 | 24.5 |
| C_NC_1 | 2.12 | 3.8 | 13.4 | 34.7 |
| C_NC_2 | 2.41 | 3.5 | 15.4 | 72.7 |
| C_SlVk | 2.5 | 3.8 | 14.1 | 36.7 |
| C_IGM | 2.57 | 4.1 | 15.4 | 44 |
| Material | Mass Loss 350–670 °C (%) | Kaolinite Content (%) |
|---|---|---|
| C_Cu1 | 1.63 | 11.6 |
| C_Cu2 | 1.52 | 10.8 |
| C_ORA | 1.31 | 9.7 |
| C_IlO | 2.68 | 19.1 |
| C_Mar | 2.47 | 17.6 |
| C_TOP 1 | 1.90 | 13.5 |
| C_TOP2 | 2.87 | 20.4 |
| C_NC_1 | 2.93 | 20.8 |
| C_NC_2 | 2.72 | 19.3 |
| C_KaVa_1 | 3.26 | 23.2 |
| C_KaVa_2 | 2.85 | 20.3 |
| C_SLVK | 2.11 | 15.0 |
| C_IGM | 2.26 | 16.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flegar, M.; Serdar, M.; Londono-Zuluaga, D.; Scrivener, K. Regional Waste Streams as Potential Raw Materials for Immediate Implementation in Cement Production. Materials 2020, 13, 5456. https://doi.org/10.3390/ma13235456
Flegar M, Serdar M, Londono-Zuluaga D, Scrivener K. Regional Waste Streams as Potential Raw Materials for Immediate Implementation in Cement Production. Materials. 2020; 13(23):5456. https://doi.org/10.3390/ma13235456
Chicago/Turabian StyleFlegar, Matea, Marijana Serdar, Diana Londono-Zuluaga, and Karen Scrivener. 2020. "Regional Waste Streams as Potential Raw Materials for Immediate Implementation in Cement Production" Materials 13, no. 23: 5456. https://doi.org/10.3390/ma13235456
APA StyleFlegar, M., Serdar, M., Londono-Zuluaga, D., & Scrivener, K. (2020). Regional Waste Streams as Potential Raw Materials for Immediate Implementation in Cement Production. Materials, 13(23), 5456. https://doi.org/10.3390/ma13235456






