Platinum(II) Complexes with Bulky Disubstitute Triazolopyrimidines as Promising Materials for Anticancer Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation and Analyses
2.2. Reactants and Methods
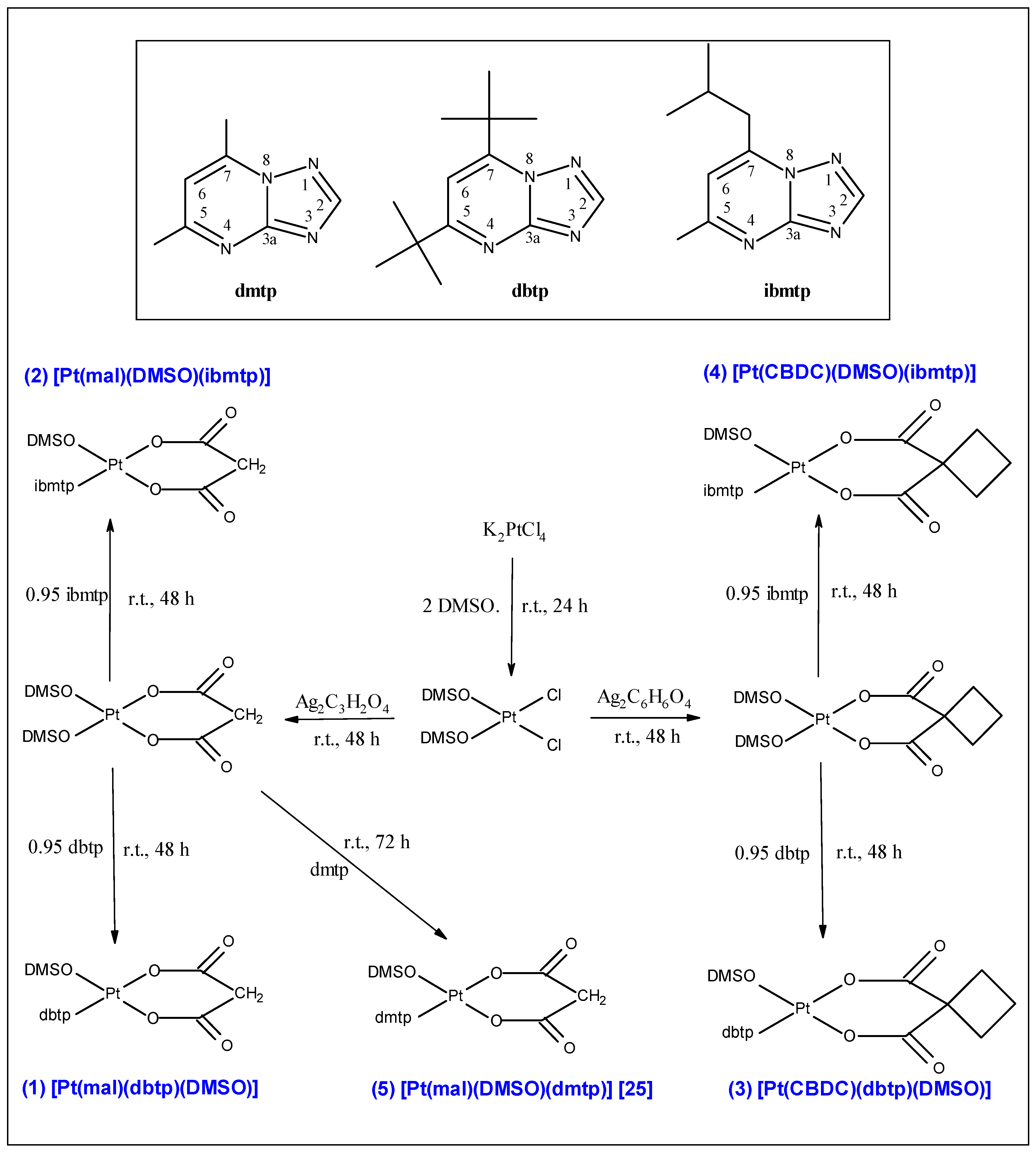
2.3. Preparation of Dicarboxylato Platinum(II) Complexes
2.4. Crystal Structure
2.5. Partition Coefficient
2.6. In Vitro Cytotoxicity
3. Results and Discussion
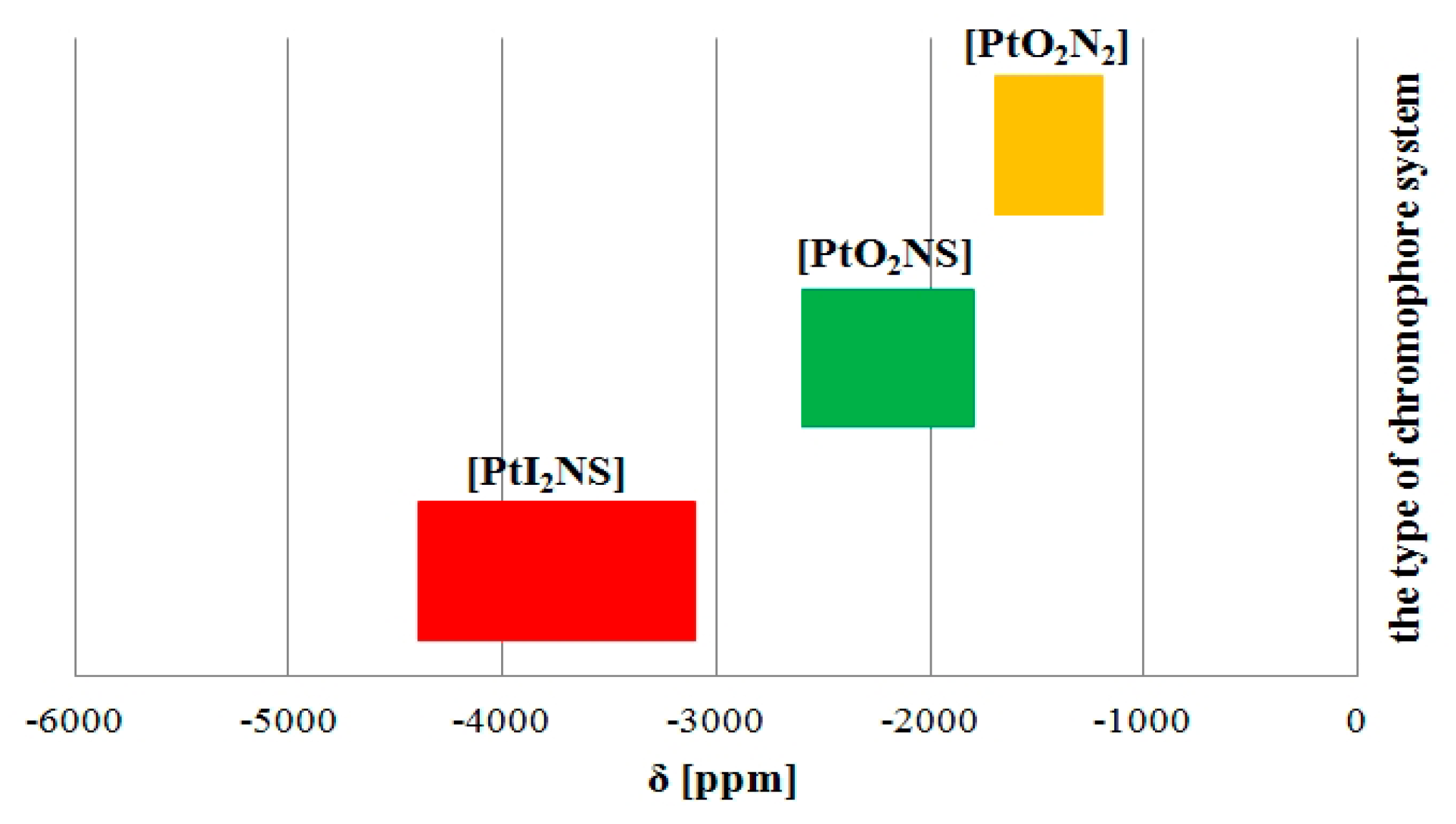
3.1. Multinuclear Magnetic Resonance Spectroscopy
3.2. Infrared Studies
3.3. X-ray Studies
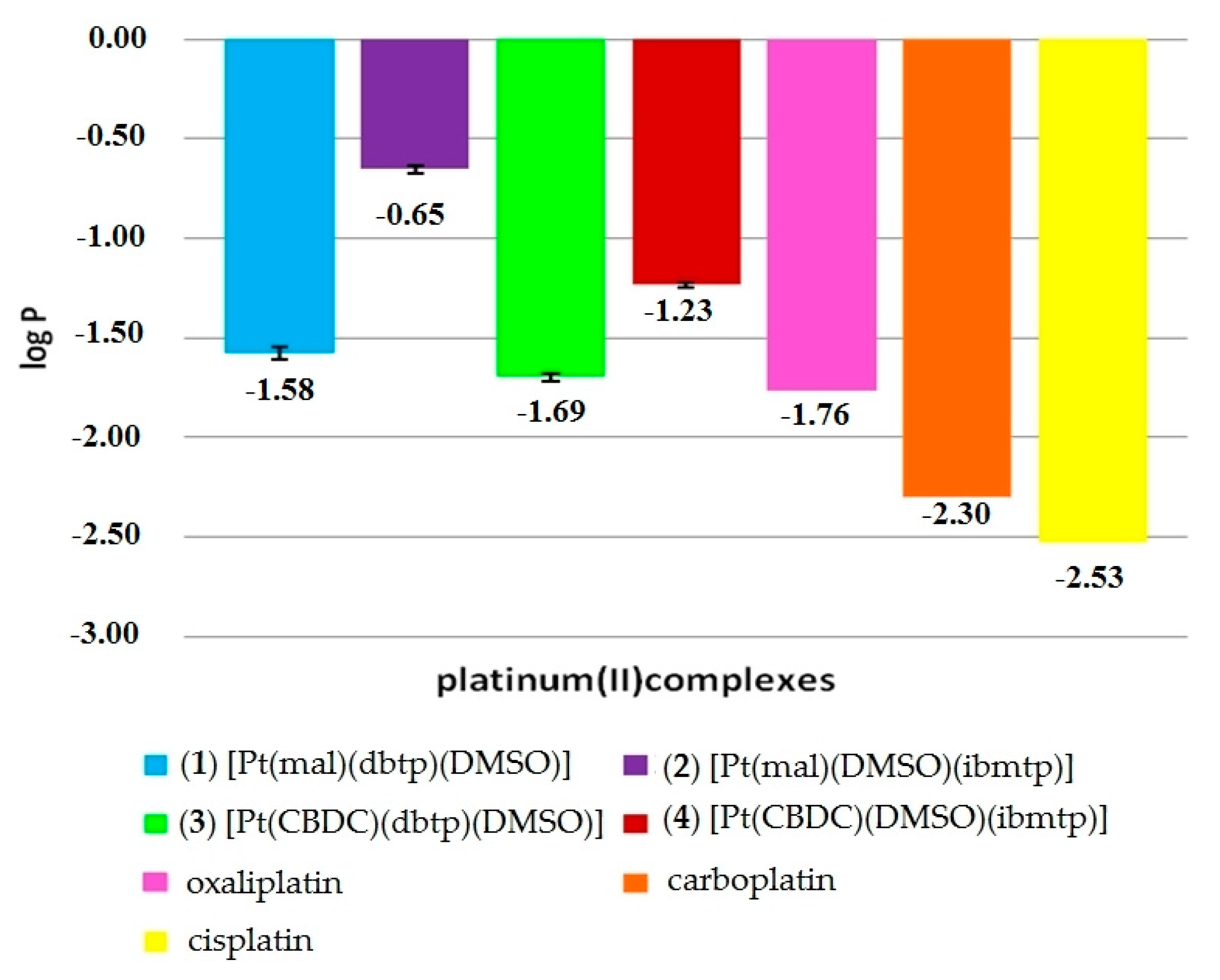
3.4. Lipophilicity
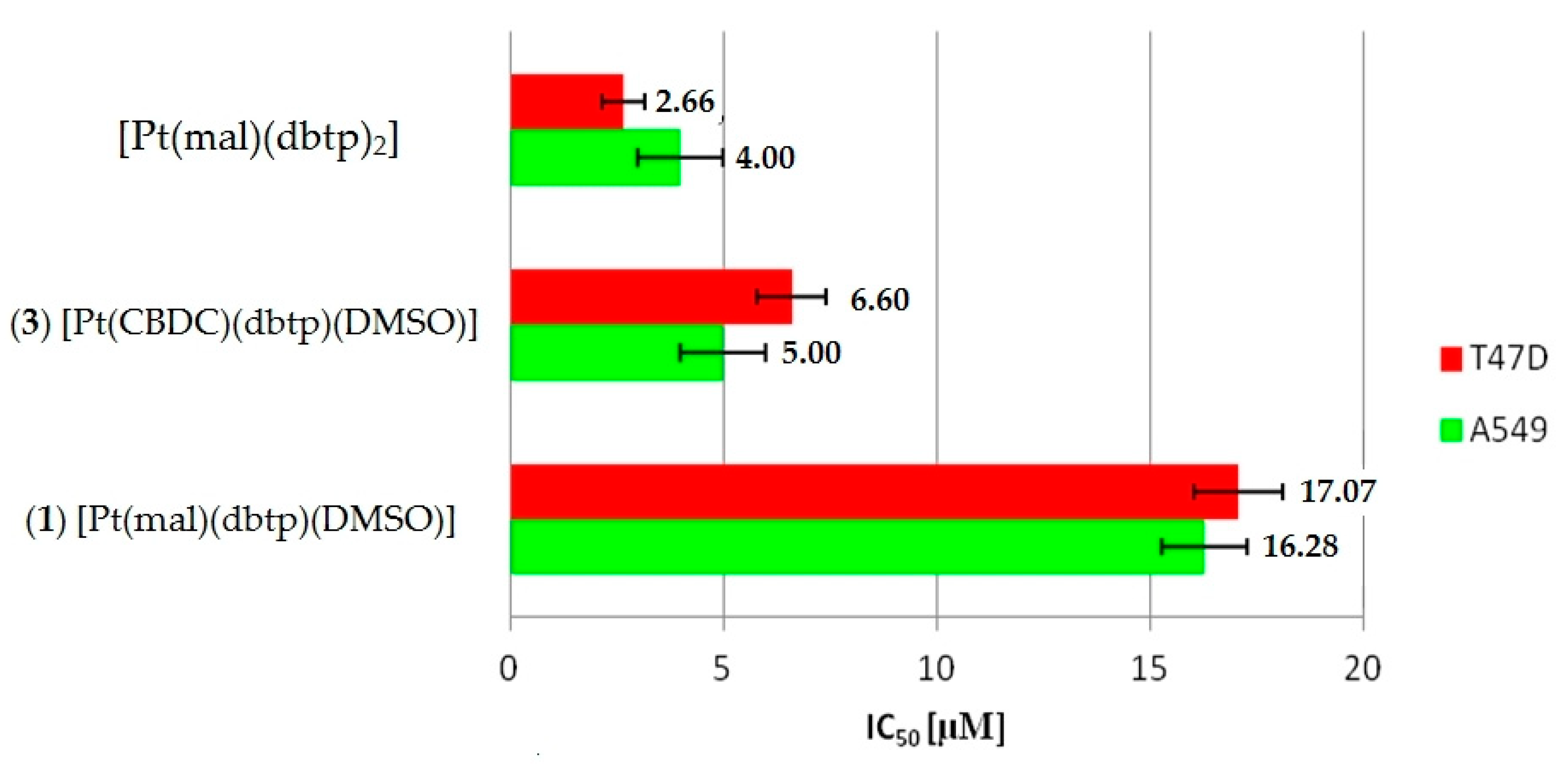
3.5. In Vitro Antiproliferative Activity
4. Conclusions
- (i)
- Relevant cytotoxic activity against A-549 (non-small cell lung carcinoma) and T47D (breast cancer) cells;
- (ii)
- Better selectivity against A549 and T47D than cisplatin;
- (iii)
- Lower toxicity against normal BALB/3T3 cells than that of cisplatin.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moucheron, C. From cisplatin to photoreactive Ru complexes: Targeting DNA for biomedical applications. New J. Chem. 2009, 33, 235–245. [Google Scholar] [CrossRef]
- Bharti, S.K.; Singh, S.K. Recent development in the field of anticancer metallopharmaceuticals. Int. J. PharmTech Res. 2009, 1, 1406–1420. [Google Scholar]
- Pucci, D.; Bellusci, A.; Bernardini, S.; Bloise, R.; Crispini, A.; Federici, G.; Liguori, P.; Lucas, M.F.; Russo, N.; Valentini, A. Bioactive fragments synergically involved in the design of new generation Pt(II) and Pd(II)-based anticancer compounds. Dalton Trans. 2008, 43, 5897–5904. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; Trotter, K.D.; Sutcliffe, O.B.; Plumb, J.A.; Waddell, B.; Briggsa, N.E.B.; Wheate, N.J. Combining aspects of the platinum anticancer drugs picoplatin and BBR3464 to synthesize a new family of sterically hindered dinuclear complexes; their synthesis, binding kinetics and cytotoxicity. Dalton Trans. 2012, 41, 11330–11339. [Google Scholar] [CrossRef][Green Version]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef]
- Van Rijt, S.H.; Sadler, P.J. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov. Today 2009, 14, 1089–1097. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Nukolova, N.V.; Kabanov, A.V.; Bronich, T.K. Nanocarriers for delivery of platinum anticancer drugs. Adv. Drug Deliv. Rev. 2013, 65, 1667–1685. [Google Scholar] [CrossRef]
- Hall, M.D.; Hambley, T.W. Platinum(IV) antitumor compounds: Their bioinorganic chemistry. Coord. Chem. Rev. 2002, 232, 49–67. [Google Scholar] [CrossRef]
- Graf, N.; Lippard, S.J. Redox activation of metal-based prodrugs as a strategy for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 993–1004. [Google Scholar] [CrossRef]
- TJohnstone, C.; Kulak, N.; Pridgen, E.M.; Farokhazd, O.C.; Langer, R.; Lippard, S.J. Nanoparticles encapsulation of mitaplatin and the effect thereof on in vivo properties. ACS Nano 2013, 7, 5675–5683. [Google Scholar]
- Kenny, R.G.; Marmion, C.J. Toward multi-targeted platinum and ruthenium drugs- a new paradigm in cancer drug treatment regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Z. Towards the rational design of platinum(II) and gold(III) complexes as antitumour agents. Dalton Trans. 2008, 12, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Schluga, P.; Hartinger, C.G.; Galanski, M.; Meelich, K.; Timerbaev, A.R.; Keppler, B.K. Tumour-inhibiting platinum(II) complexes with aminoalcohol ligands: Biologically important transformations studied by micellar electrokinetic chromatography, nuclear magnetic resonance spectroscopy and mass spectrometry. Analyst 2005, 130, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Goddard, R.; Pörschke, K.R.; Hamacher, A.; Kassack, M.U. Bispidine Analogues of Cisplatin, Carboplatin, and Oxaliplatin. Synthesis, Structures, and Cytotoxicity. Inorg. Chem. 2014, 53, 3371–3384. [Google Scholar] [CrossRef]
- Zhao, J.; Gou, S.; Liu, F.; Sun, Y.; Gao, C. Anticancer potency of platinum(II) complexes containing both chloride anion and chelated carboxylate as leaving groups. Inorg. Chem. 2013, 52, 8163–8170. [Google Scholar] [CrossRef]
- Pasetto, L.M.; D’Andrea, M.R.; Brandes, A.A.; Rossi, E.; Monfardini, S. The development of platinum compounds and their possible combination. Crit. Rev. Oncol. Hematol. 2006, 60, 59–75. [Google Scholar] [CrossRef]
- Warnecke, A.; Fichtner, I.; Garmann, D.; Jaehde, U.; Kratz, F. Synthesis and Biological Activity of Water-Soluble Maleimide Derivatives of the Anticancer Drug Carboplatin Designed as Albumin-Binding Prodrugs. Bioconjugate Chem. 2004, 15, 1349–1359. [Google Scholar] [CrossRef]
- Di Pasqua, A.J.; Centerwall, C.R.; Kerwood, D.J.; Dabrowiak, J.C. Formation of Carbonato and Hydroxo Complexes in the Reaction of Platinum Anticancer Drugs with Carbonate. Inorg. Chem. 2009, 48, 1192–1197. [Google Scholar] [CrossRef]
- Kuduk-Jaworska, J.; Waszkiewicz, K. Malatoplatinum(II) complexes—Carboplatin analogs. Transit. Met. Chem. 2000, 25, 443–449. [Google Scholar] [CrossRef]
- Štarha, P.; Trávníček, Z.; Popa, A.; Popa, I.; Muchová, T.; Brabec, V. How to modify 7-azaindole to form cytotoxic Pt(II) complexes: Highly in vitro anticancer effective cisplatin derivatives involving halogeno-substituted 7-azaindole. J. Inorg. Biochem. 2012, 115, 57–63. [Google Scholar] [CrossRef]
- Křikavová, R.; Hanousková, L.; Dvořák, Z.; Trávníček, Z. Dichlorido-platinum(II) complexes with kinetin derivatives as promising cytotoxic agents avoiding resistance of cancer cells: Contrasting results between cisplatin and oxaliplatin analogues. Polyhedron 2015, 90, 7–17. [Google Scholar] [CrossRef]
- Łakomska, I.; Hoffmann, K.; Wojtczak, A.; Sitkowski, J.; Maj, E.; Wietrzyk, J. Cytotoxic malonate platinum(II) complexes with 1,2,4-triazolo[1,5-a] pyrimidine derivatives: Structural characterization and mechanism of the suppression of tumor cell growth. J. Inorg. Biochem. 2014, 141, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Marqués-Gallego, P.; Contaldi, S.; den Dulk, H.; Monari, M.; Brouwer, J.; Jaehde, U.; Kalayda, G.V.; Reedijk, J. Relevance of the leaving group for antitumor activity of new platinum(II) compounds containing anthracene derivatives as a carrier ligand. J. Inorg. Biochem. 2009, 103, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, L.; Popa, I.; Starha, P.; Travnicek, Z. In Vitro Cytotoxic-Active Platinum(II) Complexes Derived from Carboplatin and Involving Purine Derivatives. Eur. J. Inorg. Chem. 2010, 22, 3441–3448. [Google Scholar] [CrossRef]
- Jakubowski, M.; Łakomska, I.; Sitkowski, J.; Wiśniewska, J. Dicarboxylato platinum(II) complexes containing dimethyl sulfoxide and triazolopyrimidine as potential anticancer agents: Synthesis, structural and biological studies in solution. New J. Chem. 2018, 42, 8113–8122. [Google Scholar] [CrossRef]
- Furrer, J.; Robust, A. A robust, sensitive, and versatile HMBC experiment for rapid structure elucidation by NMR: IMPACT-HMBC. Chem. Commun. 2010, 46, 3396–3398. [Google Scholar] [CrossRef]
- Kupce, E.; Freeman, R. Fast multidimensional NMR by polarization sharing. Magn. Reson. Chem. 2007, 45, 2–4. [Google Scholar] [CrossRef]
- Grodzicki, A.; Szłyk, E.; Pazderski, L.; Goliński, A. NMR Properties of 5,7-Disubstituted Derivatives of 1,2,4-Triazolo[ 1,5a] pyrimidine. Magn. Reson. Chem. 1996, 34, 725–727. [Google Scholar] [CrossRef]
- Rochon, F.D.; Massarweh, G. Synthesis, multinuclear magnetic resonance and crystal structures of Pt(II) complexes containing amines and bidentate carboxylate ligands. Inorg. Chim. Acta 2006, 359, 4095–4104. [Google Scholar] [CrossRef]
- Price, J.H.; Williamson, A.S.; Schramm, R.S.; Wayland, B.B. Palladium(II) and platinum(II) alkyl sulfoxide complexes. Examples of sulfur-bonded, mixed sulfur- and oxygen-bonded, and totally oxygen-bonded complexes. Inorg. Chem. 1972, 11, 1280–1284. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro, Version 1.171.38.43 Package of Programs, Rigaku OD; Agilent Technologies Ltd.: Yarnton, UK, 2015.
- Cambridge Crystallographic Data Centre Home Page. Available online: www.ccdc.cam.ac.uk/structures (accessed on 20 November 2020).
- Albert, A. Selective Toxicity: The Physico-Chemical Basis of Therapy; Chapman and Hall: London, UK, 1979; pp. 662–664. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Gou, S.; Zhao, J.; Sun, Y.; Cheng, L. Platinum(II) complexes with N-monoalkyl 1R,2R-diaminocyclohenxane derivatives as carrier ligands and 3-hydroxycyclobutane- 1,1-dicarboxylate as a leaving group: Potent cytotoxicity and DNA binding ability. Eur. J. Med. Chem. 2013, 69, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gou, S.; Hu, G.; Cheng, L. Antitumor platinum(II) complexes of N-monoalkyl 1R,2R-diamino-cyclohexanes with 3-(nitrooxy)cyclobutane-1,1-dicarboxylate as a leaving group. Eur. J. Med. Chem. 2014, 85, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Łakomska, I.; Hoffmann, K.; Muzioł, T.; Sitkowski, J. Multinuclear magnetic resonance and X-ray characterization of platinum(II) complexes with substituted-1,2,4-triazolo [1,5-a]pyrimidines. J. Mol. Struct. 2014, 1056, 146–151. [Google Scholar] [CrossRef]
- Łakomska, I.; Fandzloch, M.; Muzioł, T.; Sitkowski, J.; Wietrzyk, J. Structure-cytotoxicity relationship for different types of mononuclear platinum(II) complexes with 5,7-ditertbutyl-1,2,4- triazolo[1,5-a]pyrimidine. J. Inorg. Biochem. 2012, 115, 100–105. [Google Scholar] [CrossRef]
- Łakomska, I.; Fandzloch, M.; Popławska, B.; Sitkowski, J. Platinum(II) complexes with 5,7-disubstituted-1,2,4-triazolo[1,5-a]pyrimidines: Spectroscopical characterization and cytotoxic activity in vitro. Spectrochim. Acta Part A 2012, 91, 126–129. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Xie, M.; Lou, L.; Ye, Q.; Yu, Y.; Hou, S. Synthesis and anticancer activity of [2-hydroxy-1,3-diaminopropane-κ2N,N′] platinum(II) complexes. J. Inorg. Biochem. 2008, 102, 1942–1946. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, J.; Xie, C.; Hou, S.; Quan, H.; Ye, Q.; Lou, L. Synthesis, anticancer activity and toxicity of a water-soluble 4S,5S-derivative of heptaplatin, cis-{Pt(II)[(4S,5S)-4,5-bis(aminomethyl)-2-isopropyl- 1,3-dioxolane]·(3-hydroxyl-cyclobutane-1,1-dicarboxylate)}. J. Inorg. Biochem. 2014, 140, 126–130. [Google Scholar] [CrossRef]
- Yin, R.; Gou, S.; Sun, Y.; Liu, X. In vitro biological evaluation of platinum(II) complexes with 1-(methoxy substituted benzyl) azetidine-3,3-dicarboxylato ligands. Bioorg. Med. Chem. 2012, 20, 1461–1467. [Google Scholar] [CrossRef]
- Łakomska, I.; Szłyk, E.; Sitkowski, J.; Kozerski, L.; Nasulewicz, A.; Pełczyńska, M.; Wietrzyk, J.; Opolski, A. Multinuclear NMR spectroscopy and antitumor activity of novel platinum(II) complexes with 5,7-disubstituted-1,2,4-triazolo[1,5-a]pyrimidines. J. Inorg. Biochem. 2004, 98, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Szłyk, E.; Łakomska, I.; Surdykowski, A.; Głowiak, T.; Pazderski, L.; Sitkowski, J.; Kozerski, L. The X-ray structure and spectroscopy of platinum(II) complexes with 1,2,4-triazolo[1,5-a]pyrimidines and dimethylsulfoxide. Inorg. Chim. Acta 2002, 333, 93–99. [Google Scholar] [CrossRef]
- Łakomska, I.; Barwiołek, M.; Wojtczak, A.; Szłyk, E. X-ray structure and multinuclear NMR studies of platinum(II) and palladium(II) complexes with 5,7-ditertbutyl-1,2,4-triazolo[1,5-a]pyrimidine. Polyhedron 2007, 26, 5349–5355. [Google Scholar] [CrossRef]
- Clement, O.; Roszak, A.W.; Buncel, E. Synthesis, characterization and x-ray crystal structure determination of platinum(II)-diaminoalkane complexes. Inorg. Chim. Acta 1996, 253, 53–57. [Google Scholar] [CrossRef]
- Cornia, A. The bonding of thiazoles to platinum(II) complexes. X-ray crystal structure of cis- and trans-[Pt(dimethyl sulfoxide)(thiazole)Cl2]. Inorg. Chim. Acta 1997, 255, 405–410. [Google Scholar] [CrossRef]
- De Almeida, S.G.; Hubbard, J.L.; Farrell, N. Spectroscopic and structural properties of dimethyl-, diphenyl- and methylphenylsulfoxide platinum complexes. The crystal and molecular structures of cis-dichlorobis(methylphenylsulfoxide)-platinum(II) and potassiumtrichloro(phenylsulfoxide)- platinum(II)acetone solvate. Inorg. Chim. Acta 1992, 193, 149–157. [Google Scholar]
- Calligaris, M. Structure and bonding in metal sulfoxide complexes: An update. Coord. Chem. Rev. 2004, 248, 351–361. [Google Scholar] [CrossRef]
- Łakomska, I.; Babinska, M.; Wojtczak, A.; Sitkowski, J. Synthesis, characterization and in vitro cytotoxicity of three types of platinum(II) complexes containing 5,7-diethyl-1,2,4-triazolo[1,5-a]pyrimidine. Inorg. Chim. Acta 2016, 453, 516–521. [Google Scholar] [CrossRef]
- Buß, I.; Garmann, D.; Galanski, M.; Weber, G.; Kalayda, G.V.; Keppler, B.K.; Jaehde, U. Enhancing lipophilicity as a strategy to overcome resistance against platinum complexes? J. Inorg. Biochem. 2011, 105, 709–717. [Google Scholar] [CrossRef]
- Margiotta, N.; Marzano, C.; Gandin, V.; Osella, D.; Ravera, M.; Gabano, E.; Platts, J.A.; Petruzzella, E.; Hoeschele, J.D.; Natile, G. Revisiting [PtCl2(cis-1,4-DACH)]: An Underestimated Antitumor Drug with Potential Application to the Treatment of Oxaliplatin-Refractory Colorectal Cancer. J. Med. Chem. 2012, 55, 7182–7192. [Google Scholar] [CrossRef]
- Platts, J.A.; Oldfield, S.P.; Reif, M.M.; Palmucci, A.; Gabano, E. The RP-HPLC measurement and QSPR analysis of logPo/w values of several Pt(II) complexes. J. Inorg. Biochem. 2006, 100, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.S.; Lou, L.G.; Liu, W.P.; Yu, Y.; Chen, X.Z.; Hou, S.Q.; Gao, W.Q.; Liu, Y. Synthesis and in vitro cytotoxicity of novel lipophilic (diamine)platinum(II) complexes of salicylate derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 2146–2149. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, S.P.; Hall, M.D.; Platts, J.A. Calculation of Lipophilicity of a Large, Diverse Dataset of Anticancer Platinum Complexes and the Relation to Cellular Uptake. J. Med. Chem. 2007, 50, 5227–5237. [Google Scholar] [CrossRef] [PubMed]
- Sava, G.; Jaouen, G.; Hillard, E.A.; Bergamo, A. Targeted therapy vs. DNA-adduct formation-guided design: Thoughts about the future of metal-based anticancer drugs. Dalton Trans. 2012, 41, 8226–8234. [Google Scholar] [PubMed]
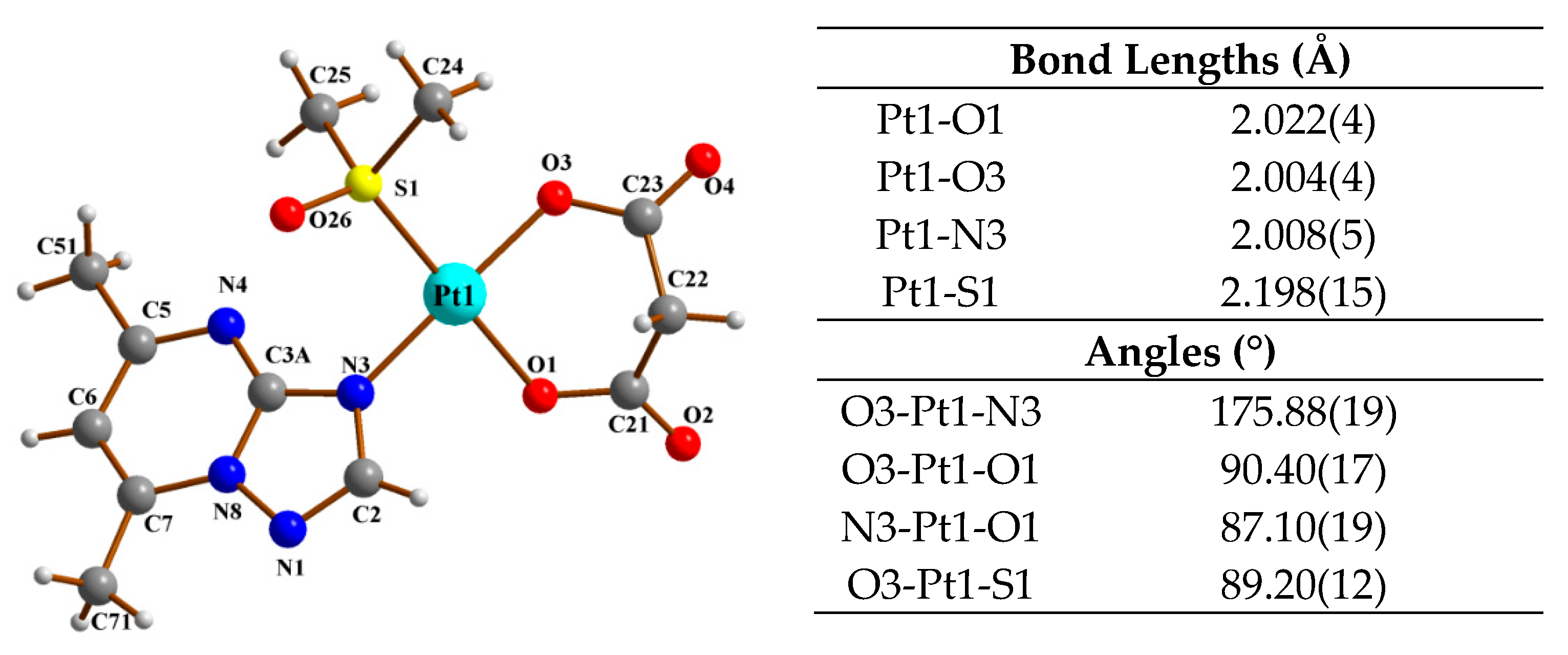
| Parameter | Result |
|---|---|
| Empirical formula | C12H16N4O5PtS |
| Formula weight | 523.44 |
| Temperature; K | 293(2) |
| Wavelength; Å | 0.71073 |
| Crystal system | Orthorhombic |
| Space group | Pbca |
| Unit cell dimensions; Å, | a = 11.7627(4) |
| b = 8.8447(3) | |
| c = 30.8525(11) | |
| α | |
| β | |
| γ | |
| Volume; Å3 | 3209.82(19) |
| Z | 8 |
| Density (calculated); Mg/m3 | 2.166 |
| Absorption coefficient; mm−1 | 8.903 |
| F(000) | 2000 |
| Crystal size; mm | 0.331 × 0.217 × 0.022 |
| Theta range for data collection | 2.177 to 28.678°. |
| Index ranges | −10 ≤ h ≤ 15, −11 ≤ k ≤ 11, −34 ≤ l ≤ 38 |
| Reflections collected | 20604 |
| Independent reflections | 3785 [R(int) = 0.0540] |
| Completeness to theta | 25.242°, 99.8% |
| Absorption correction | Analytical |
| Max. and min. transmission | 0.8291 and 0.1618 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3785/0/208 |
| Goodness-of-fit on F2 | 1.060 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0395, wR2 = 0.0760 |
| R indices (all data) | R1 = 0.0601, wR2 = 0.0802 |
| Largest diff. peak and hole; e.Å−3 | 2.06 d −3.949 |
| Compounds | 1H | 15N | ||||
|---|---|---|---|---|---|---|
| H(2) | H(6) | N(1) | N(3) | N(4) | N(8) | |
| (1) [Pt(mal)(dbtp)(DMSO)] | 9.19 (+0.39) | 7.55 (+0.24) | −105.8 (−0.6) | −252.5 (−86.2) | −121.6 (−5.2) | −160.2 (−3.7) |
| (2) [Pt(mal)(DMSO)(ibmtp)] | 9.15 (+0.73) | 7.59 (+0.22) | −110.3 (−4.3) | −252.1 (−62.7) | −120.1 (−6.0) | −158.3 (−1.2) |
| (3) [Pt(CBDC)(dbtp)(DMSO)] | 9.21 (+0.41) | 7.55 (+0.24) | −106.0 (−0.4) | −253.0 (−85.7) | −122.0 (−4.8) | −161.0 (−2.2) |
| (4) [Pt(CBDC)(DMSO)(ibmtp)] | 9.16 (+0.74) | 7.58 (+0.21) | −110.3 (−4.3) | −251.7 (−87.0) | −119.8 (−6.3) | −158.3 (−1.2) |
| (5) [Pt(mal)(DMSO)(dmtp)] [25] | 9.14 (+0.55) | 7.58 (+0.39) | −109.7 (+2.2) | −252.2 (−98.3) | −120.8 (−5.0) | −157.4 (−2.1) |
| Compounds | C(2) | C(3a) | C(5) | C(6) | C(7) | Pt |
|---|---|---|---|---|---|---|
| (1) [Pt(mal)(dbtp)(DMSO)] | 152.8 (−0.1) | 152.6 (−4.1) | 179.8 (+3.1) | 107.9 (+3.3) | 159.3 (+1.4) | −2609 (−985) |
| (2) [Pt(mal)(DMSO)(ibmtp)] | 153.2 (+2.0) | 152.3 (−4.6) | 169.5 (+0.2) | 114.3 (+0.9) | 151.9 (+0.7) | −2598 (−974) |
| (3) [Pt(CBDC)(dbtp)(DMSO)] | 153.0 (+0.1) | 152.7 (−4.2) | 179.7 (+3.2) | 107.0 (+4.2) | 159.0 (+1.7) | −2607 (−983) |
| (4) [Pt(CBDC)(DMSO)(ibmtp)] | 153.4 (+1.8) | 152.3 (−4.6) | 169.4 (+0.3) | 114.2 (+1.0) | 151.9 (+0.7) | −2605 (−981) |
| Compounds | νas(OCO—) | νpyrim. ring | νtriazole ring | νsym(OCO—) | Δ | Δ’ |
|---|---|---|---|---|---|---|
| (1) [Pt(mal)(dbtp)(DMSO)] | 1662 | 1617 | 1541 | 1344 | 318 | 197 |
| (2) [Pt(mal)(DMSO)(ibmtp)] | 1662 | 1636 | 1555 | 1351 | 311 | |
| (3) [Pt(CBDC)(dbtp)(DMSO)] | 1656 | 1618 | 1542 | 1339 | 317 | 268 |
| (4) [Pt(CBDC)(DMSO)(ibmtp)] | 1669 | 1636 | 1555 | 1341 | 328 |
| Compounds | IC50 a | |||
|---|---|---|---|---|
| A549 | LoVo | T47D | BALB/3T3 | |
| (1) [Pt(mal)(dbtp)(DMSO)] | 16.28 ± 9.9 | 27.92 ± 1.0 | 17.07 ± 10.5 | 30.25 ± 22.6 |
| (2) [Pt(mal)(DMSO)(ibmtp)] | 57.81 ± 13.5 | 38.35 ± 1.8 | 42.6 ± 19.0 | 45.95 ± 40.7 |
| (3) [Pt(CBDC)(dbtp)(DMSO)] | 5.00 ± 0.9 | 20.02 ± 2.8 | 6.60 ± 4.8 | 10.19 ± 6.2 |
| (4) [Pt(CBDC)(DMSO)(ibmtp)] | 57.29 ± 11.3 | 41.19 ± 1.6 | 21.87 ± 17.2 | 73.40 ± 29.7 |
| cisplatin | 9.00 ± 1.1 | 0.84 ± 0.3 | 3.11 ± 1.2 | 3.20 ± 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łakomska, I.; Śmiłowicz, D.; Jakubowski, M.; Sitkowski, J.; Wojtczak, A. Platinum(II) Complexes with Bulky Disubstitute Triazolopyrimidines as Promising Materials for Anticancer Agents. Materials 2020, 13, 5312. https://doi.org/10.3390/ma13235312
Łakomska I, Śmiłowicz D, Jakubowski M, Sitkowski J, Wojtczak A. Platinum(II) Complexes with Bulky Disubstitute Triazolopyrimidines as Promising Materials for Anticancer Agents. Materials. 2020; 13(23):5312. https://doi.org/10.3390/ma13235312
Chicago/Turabian StyleŁakomska, Iwona, Dariusz Śmiłowicz, Mateusz Jakubowski, Jerzy Sitkowski, and Andrzej Wojtczak. 2020. "Platinum(II) Complexes with Bulky Disubstitute Triazolopyrimidines as Promising Materials for Anticancer Agents" Materials 13, no. 23: 5312. https://doi.org/10.3390/ma13235312
APA StyleŁakomska, I., Śmiłowicz, D., Jakubowski, M., Sitkowski, J., & Wojtczak, A. (2020). Platinum(II) Complexes with Bulky Disubstitute Triazolopyrimidines as Promising Materials for Anticancer Agents. Materials, 13(23), 5312. https://doi.org/10.3390/ma13235312





