Histomorphometric Evaluation of Peri-Implant Bone Response to Intravenous Administration of Zoledronate (Zometa®) in an Osteoporotic Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Ovariectomy Rat Model
2.2. Sample Number Estimation
2.3. Implantation Procedures and Study Groups
- ZOL-Pre: weekly ZOL administration (0.04 mg/kg body weight) in the 4 weeks prior to implant placement [31].
- ZOL-Post: weekly ZOL administration in the 6 weeks from implant placement until the end of the study (=6 weeks) [14].
- ZOL-Pre+Post: weekly ZOL administration from 4 weeks prior to implant placement until the end of the study (=10 weeks).
- Saline: weekly intravenous injection with 1 mL saline from 4 weeks prior to implant placement until the end of the study; this group was considered the non-treated control.
2.4. Animal Euthanasia and Specimen Retrieval
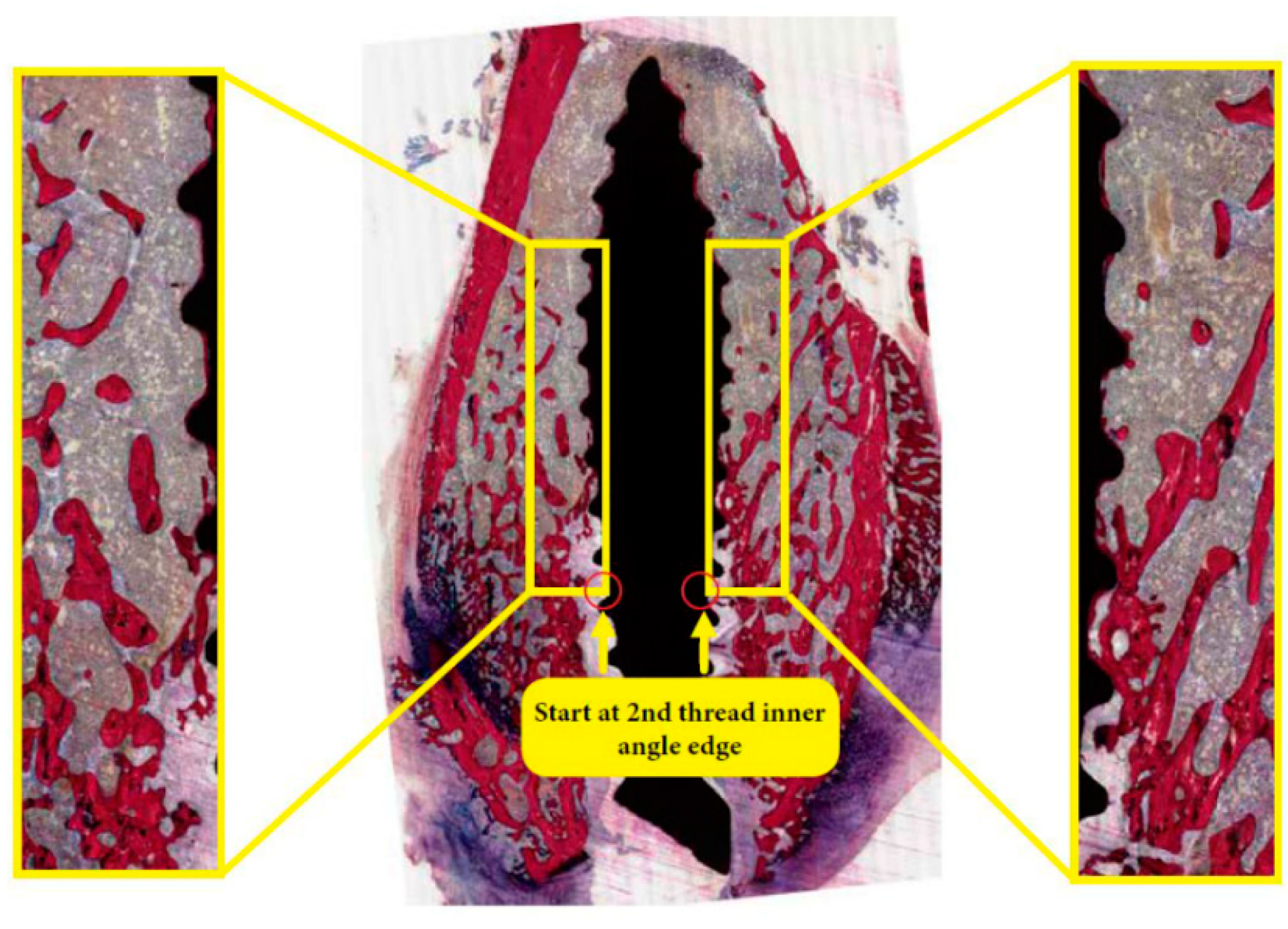
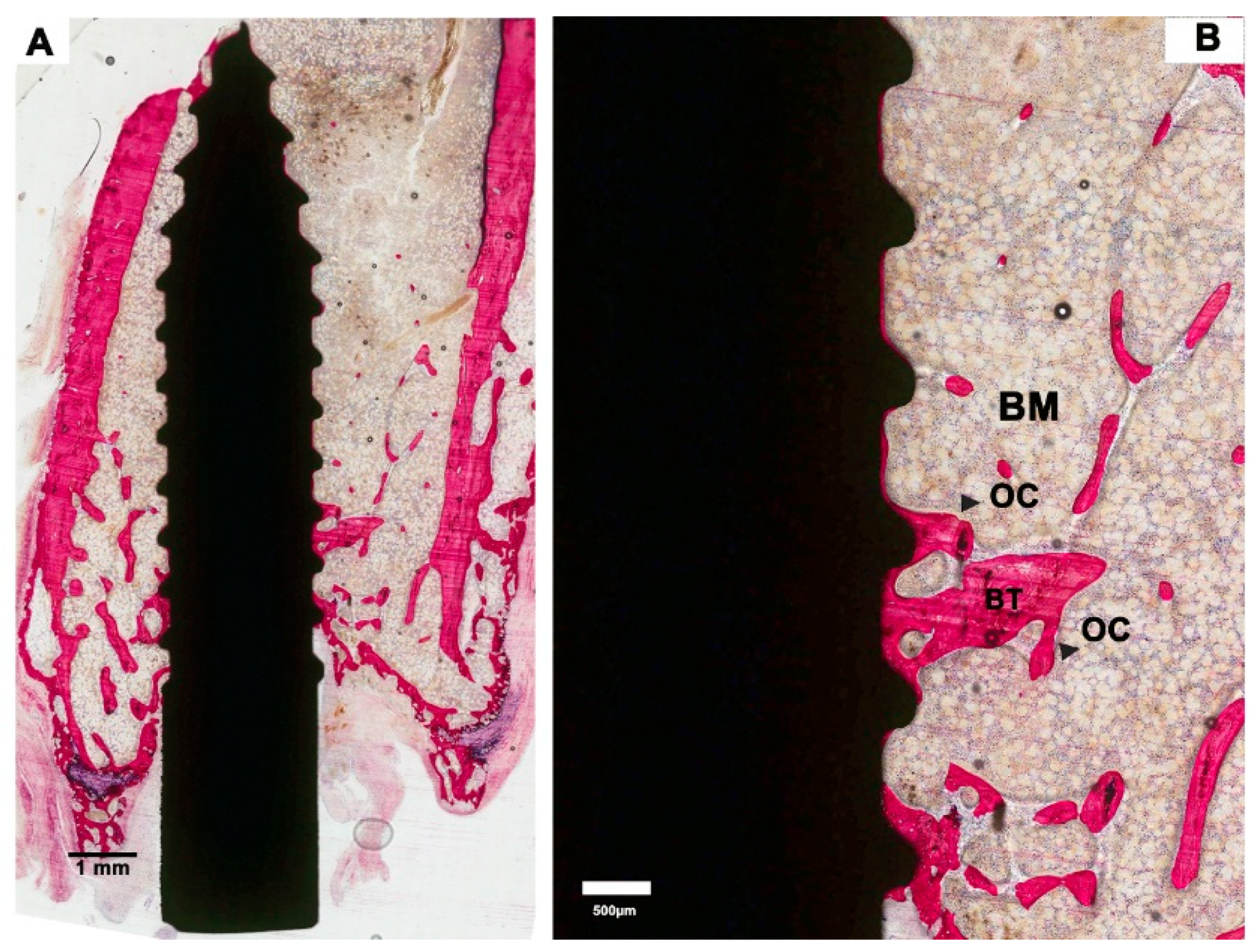
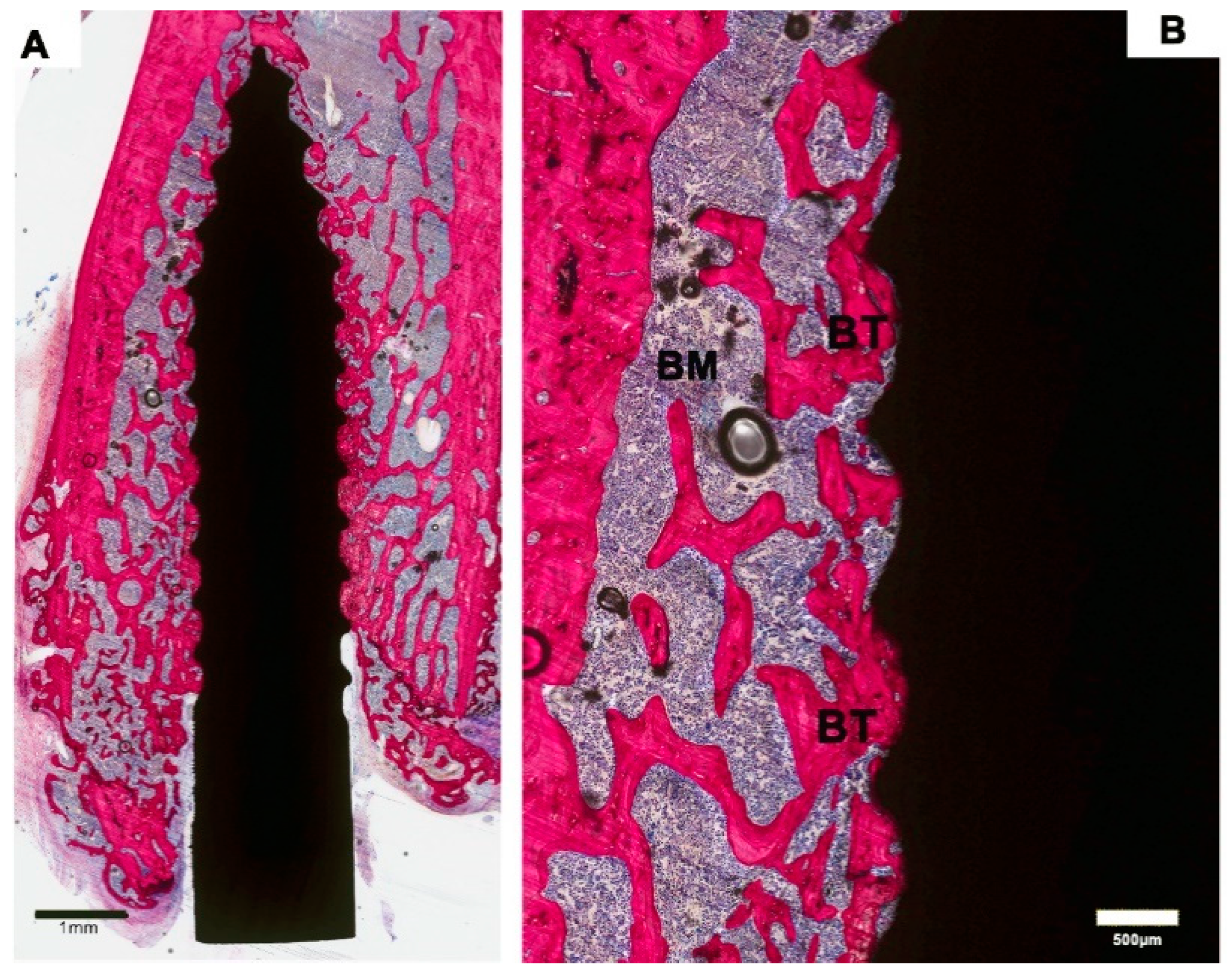
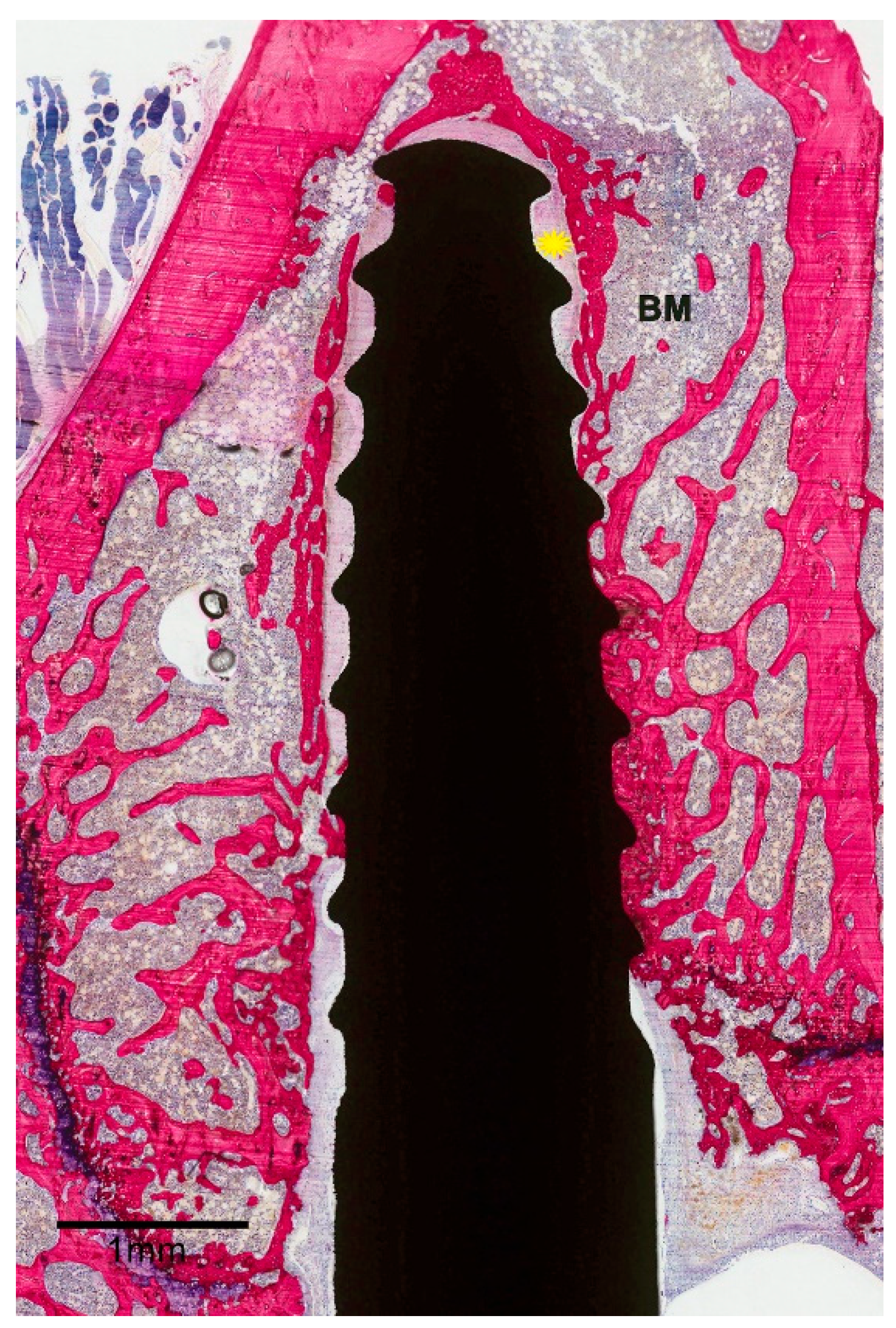
2.5. Histological and Histomorphometric Examination
2.6. Statistical Analysis
3. Results
3.1. Rat Model
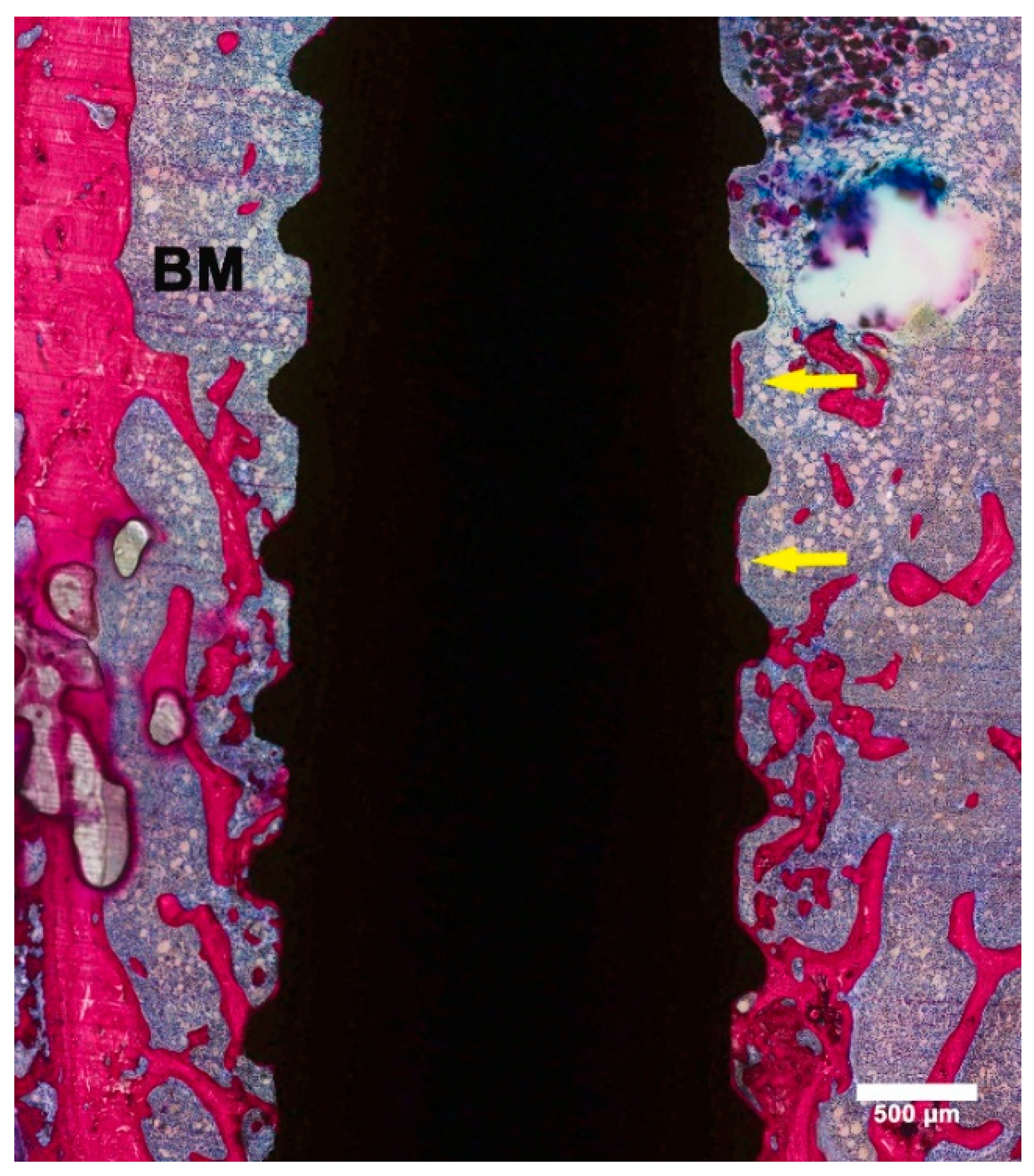
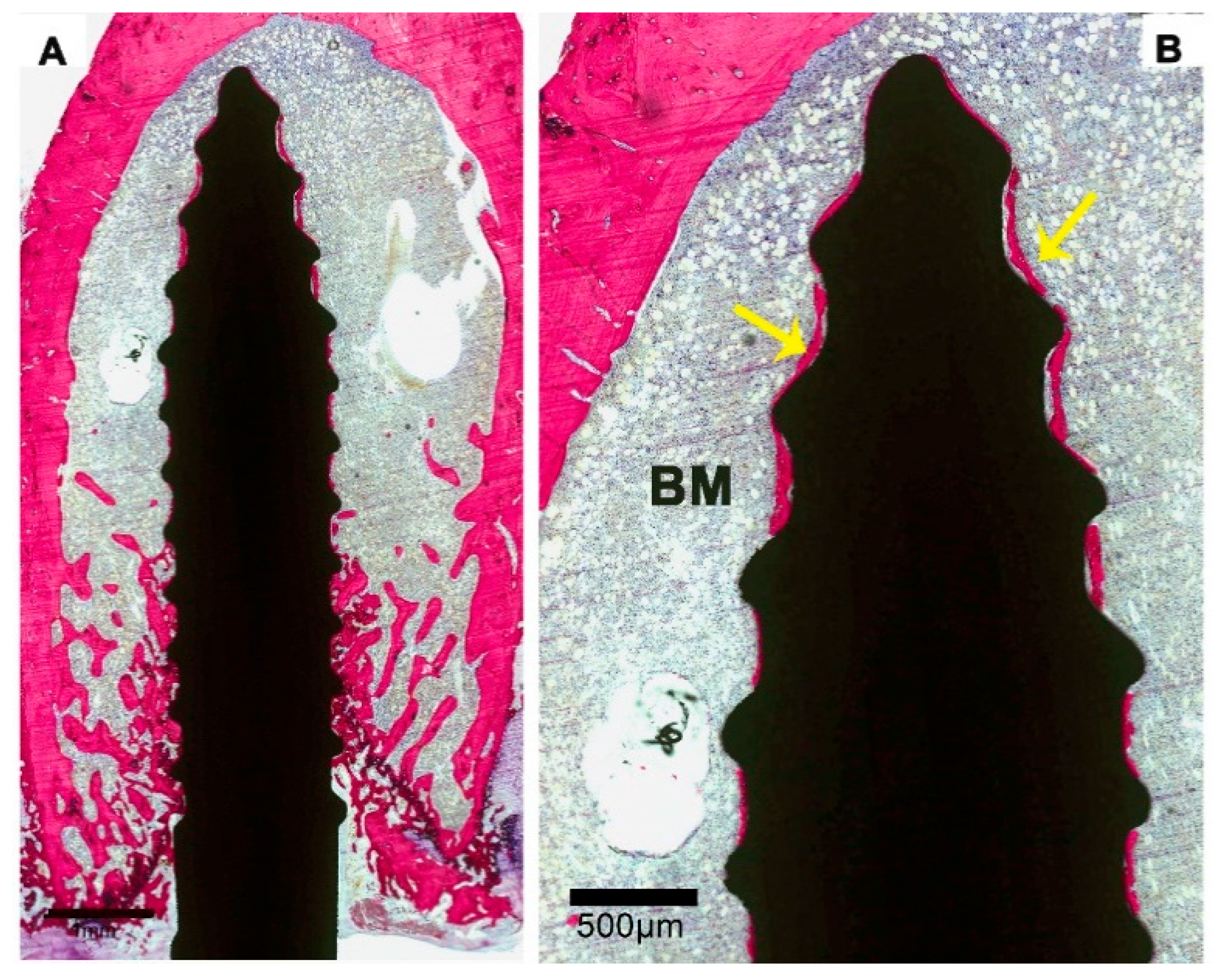
3.2. Histological Evaluation
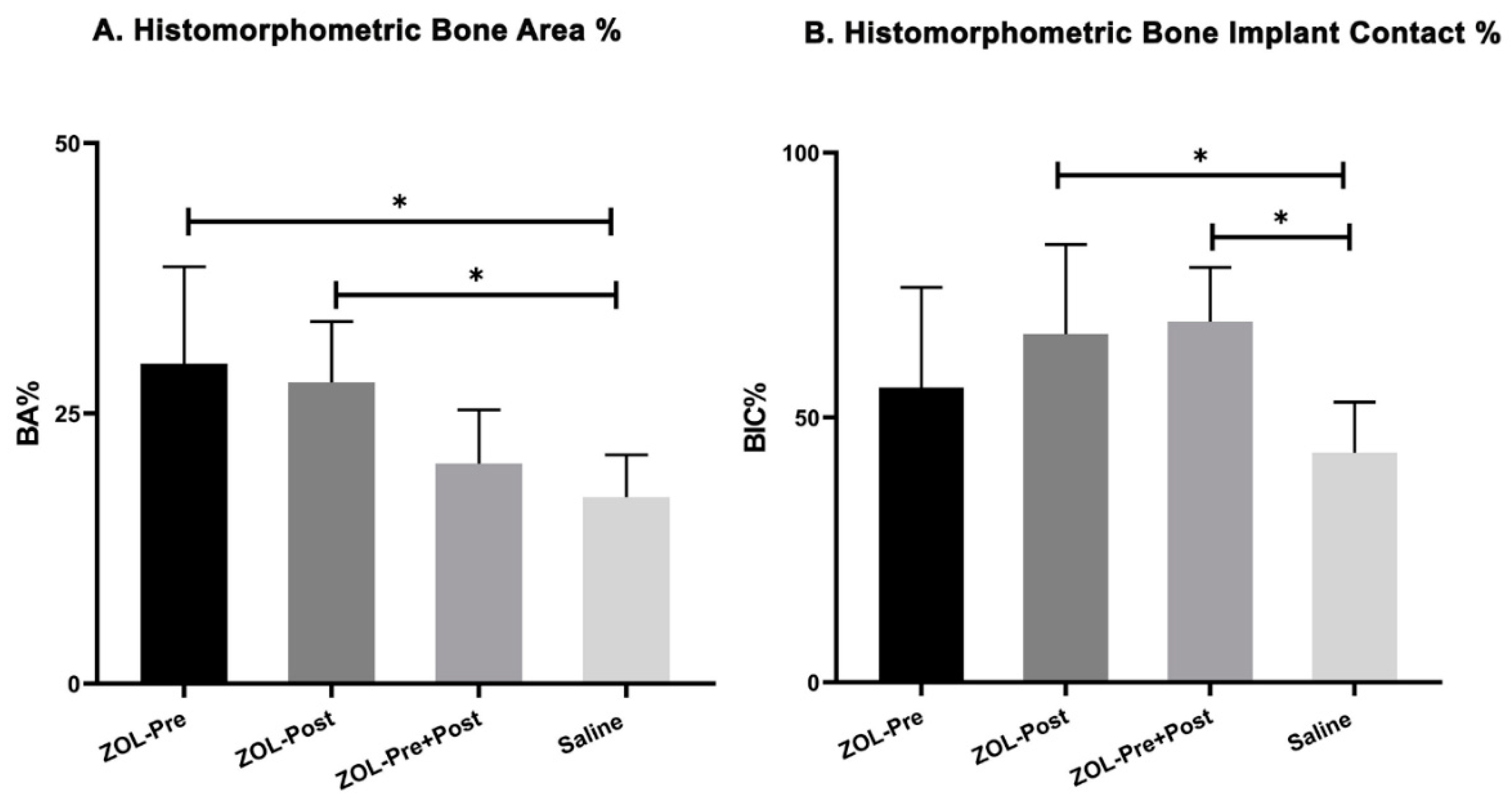
3.3. Histomorphometric Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alghamdi, H.S.; Jansen, J.A. The development and future of dental implants. Dent. Mater. J. 2020, 39, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ducommun, J.; El Kholy, K.; Rahman, L.; Schimmel, M.; Chappuis, V.; Buser, D. Analysis of trends in implant therapy at a surgical specialty clinic: Patient pool, indications, surgical procedures, and rate of early failures—A 15-year retrospective analysis. Clin. Oral Implant. Res. 2019, 30, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated Titanium Implants:Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef] [PubMed]
- Bone Mass Measurement: What the Numbers Mean. NIH Osteoporosis and Related Bone Diseases—National Resource Center. 2018. Available online: https://www.bones.nih.gov/health-info/bone/bone-health/bone-mass-measure (accessed on 10 November 2020).
- Begum, R.A.; Ali, L.; Akter, J.; Takahashi, O.; Fukui, T.; Rahman, M. Osteopenia and osteoporosis among 16–65 year old women attending outpatient clinics. J. Community Health 2014, 39, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Ye, M.; Gu, X.; Cao, Y.; Zheng, Y.; Guo, H.; Zhao, Y.; Zhan, H.; Shi, Y. Ovariectomy-Induced Osteopenia Influences the Middle and Late Periods of Bone Healing in a Mouse Femoral Osteotomy Model. Rejuvenation Res. 2015, 18, 356–365. [Google Scholar] [CrossRef]
- Chen, H.; Liu, N.; Xu, X.; Qu, X.; Lu, E. Smoking, Radiotherapy, Diabetes and Osteoporosis as Risk Factors for Dental Implant Failure: A Meta-Analysis. PLoS ONE 2013, 8, e71955. [Google Scholar] [CrossRef]
- De Medeiros, F.; Kudo, G.; Leme, B.; Saraiva, P.; Verri, F.; Honório, H.; Pellizzer, E.; Santiago, J.F. Dental implants in patients with osteoporosis: A systematic review with meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 480–491. [Google Scholar] [CrossRef]
- Trullenque-Eriksson, A.; Guisado-Moya, B. Retrospective Long-term Evaluation of Dental Implants in Totally and Partially Edentulous Patients. Part, I. Implant. Dent. 2014, 23, 732–737. [Google Scholar] [CrossRef]
- Alsaadi, G.; Quirynen, M.; Komárek, A.; Van Steenberghe, D. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J. Clin. Periodontol. 2007, 34, 610–617. [Google Scholar] [CrossRef]
- Temmerman, A.; Rasmusson, L.; Kubler, A.C.; Thor, A.; Quirynen, M. An open, prospective, non-randomized, controlled, multicentre study to evaluate the clinical outcome of implant treatment in women over 60 years of age with osteoporosis/osteopenia: 1-year results. Clin. Oral Implant. Res. 2017, 28, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.A.L.; Takayama, L.; Jorgetti, V.; Pereira, R.M.R. Comparative study of axial and femoral bone mineral density and parameters of mandibular bone quality in patients receiving dental implants. Osteoporos. Int. 2007, 18, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Basudan, A.M.; Shaheen, M.; De Vries, R.B.; Beucken, J.J.J.P.V.D.; Jansen, J.A.; Alghamdi, H.S. Antiosteoporotic Drugs to Promote Bone Regeneration Related to Titanium Implants: A Systematic Review and Meta-Analysis. Tissue Eng. Part B Rev. 2019, 25, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.S.; Jansen, J.A. Bone Regeneration Associated with Nontherapeutic and Therapeutic Surface Coatings for Dental Implants in Osteoporosis. Tissue Eng. Part B Rev. 2013, 19, 233–253. [Google Scholar] [CrossRef]
- Cattalini, J.P.; Boccaccini, A.R.; Lucangioli, S.; Mouriño, V. Bisphosphonate-Based Strategies for Bone Tissue Engineering and Orthopedic Implants. Tissue Eng. Part B Rev. 2012, 18, 323–340. [Google Scholar] [CrossRef]
- Fuchs, R.K.; Faillace, M.E.; Allen, M.R.; Phipps, R.J.; Miller, L.M.; Burr, D. Bisphosphonates do not alter the rate of secondary mineralization. Bone 2011, 49, 701–705. [Google Scholar] [CrossRef]
- Aspenberg, P. Bisphosphonates and implants. Acta Orthop. 2009, 80, 119–123. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Lezcano, V.; Thostenson, J.; Weinstein, R.S.; Manolagas, S.C.; Bellido, T. Connexin 43 Is Required for the Anti-Apoptotic Effect of Bisphosphonates on Osteocytes and Osteoblasts In Vivo. J. Bone Miner. Res. 2008, 23, 1712–1721. [Google Scholar] [CrossRef]
- Hu, L.; Wen, Y.; Xu, J.; Wu, T.; Zhang, C.; Wang, J.; Du, J.; Wang, S. Pretreatment with Bisphosphonate Enhances Osteogenesis of Bone Marrow Mesenchymal Stem Cells. Stem Cells Dev. 2017, 26, 123–132. [Google Scholar] [CrossRef]
- Gelažius, R.; Poskevicius, L.; Sakavicius, D.; Grimuta, V.; Juodzbalys, G. Dental Implant Placement in Patients on Bisphosphonate Therapy: A Systematic Review. J. Oral Maxillofac. Res. 2018, 9, e1. [Google Scholar] [CrossRef]
- Caraglia, M.; Marra, M.; Naviglio, S.; Botti, G.; Addeo, R.; Abbruzzese, A. Zoledronic acid: An unending tale for an antiresorptive agent. Expert Opin. Pharmacother. 2009, 11, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Papapoulos, S.E. Bisphosphonates: How do they work? Best Pr. Res. Clin. Endocrinol. Metab. 2008, 22, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Maricic, M. The role of zoledronic acid in the management of osteoporosis. Clin. Rheumatol. 2010, 29, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Sunyecz, J.A. Zoledronic acid infusion for prevention and treatment of osteoporosis. Int. J. Women’s Health 2010, 2, 353–360. [Google Scholar] [CrossRef][Green Version]
- Ying, G.; Bo, L.; Yanjun, J.; Lina, W.; Binquan, W. Effect of a local, one time, low-dose injection of zoledronic acid on titanium implant osseointegration in ovariectomized rats. Arch. Med Sci. 2016, 12, 941–949. [Google Scholar] [CrossRef]
- Dikicier, E.; Karacayli, U.; Dikicier, S.; Günaydın, Y. Effect of systemic administered zoledronic acid on osseointegration of a titanium implant in ovariectomized rats. J. Cranio Maxillofac. Surg. 2014, 42, 1106–1111. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Yang, X.; Xu, H.; Xie, D. Zoledronic acid enhances bone-implant osseointegration more than alendronate and strontium ranelate in ovariectomized rats. Osteoporos. Int. 2013, 24, 2115–2121. [Google Scholar] [CrossRef]
- He, Y.; Bao, W.; Wu, X.-D.; Huang, W.; Chen, H.; Li, Z. Effects of Systemic or Local Administration of Zoledronate on Implant Osseointegration: A Preclinical Meta-Analysis. BioMed Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; Beucken, J.J.J.P.V.D.; Jansen, J.A. Osteoporotic Rat Models for Evaluation of Osseointegration of Bone Implants. Tissue Eng. Part C Methods 2014, 20, 493–505. [Google Scholar] [CrossRef]
- Cardemil, C.; Omar, O.; Norlindh, B.; Wexell, C.L.; Thomsen, P. The effects of a systemic single dose of zoledronic acid on post-implantation bone remodelling and inflammation in an ovariectomised rat model. Biomaterials 2013, 34, 1546–1561. [Google Scholar] [CrossRef]
- Liu, M.; Guo, L.; Pei, Y.; Li, N.; Jin, M.; Ma, L.; Liu, Y.; Sun, B.; Li, C. Efficacy of zoledronic acid in treatment of osteoporosis in men and women-a meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 3855–3861. [Google Scholar] [PubMed]
- Hegde, V.V.; Jo, J.E.; Andreopoulou, P.; Lane, J.M. Effect of osteoporosis medications on fracture healing. Osteoporos. Int. 2016, 27, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; To, L.B.; Farrugia, A.N.; Findlay, D.M.; Green, J.; Gronthos, S.; Evdokiou, A.; Lynch, K.; Atkins, G.J.; Zannettino, A.C.W. The nitrogen-containing bisphosphonate, zoledronic acid, increases mineralisation of human bone-derived cells in vitro. Bone 2004, 34, 112–123. [Google Scholar] [CrossRef]
- Basso, F.G.; Pansani, T.N.; Soares, D.G.; Cardoso, L.M.; Hebling, J.; Costa, C.A.D.S. Influence of bisphosphonates on the adherence and metabolism of epithelial cells and gingival fibroblasts to titanium surfaces. Clin. Oral Investig. 2017, 22, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.M.; Devogelaer, J.-P.; Saag, K.; Roux, C.; Lau, C.-S.; Reginster, J.; Papanastasiou, P.; Ferreira, A.; Hartl, F.; Fashola, T.; et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): A multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2009, 373, 1253–1263. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals, Aclasta Product Information. 2020. Available online: http://guildlink.com.au/gc/ws/nv/pi.cfm?product=nvpaclin11115 (accessed on 18 November 2020).
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The Laboratory Rat as an Animal Model for Osteoporosis Research. Comp. Med. 2008, 58, 424–430. [Google Scholar] [PubMed]
- August, M.; Chung, K.; Chang, Y.; Glowacki, J. Influence of estrogen status on endosseous implant osseointegration. J. Oral Maxillofac. Surg. 2001, 59, 1285–1289. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Yamachika, E.; Nakanishi, M.; Ninomiya, T.; Nakatsuji, K.; Matsubara, M.; Moritani, N.; Kobayashi, Y.; Fujii, T.; Iida, S. Molecular alterations of newly formed mandibular bone caused by zoledronate. Int. J. Oral Maxillofac. Surg. 2018, 47, 1206–1213. [Google Scholar] [CrossRef]
- Qi, M.; Hu, J.; Li, J.; Li, J.; Dong, W.; Feng, X.; Yu, J. Effect of zoledronate acid treatment on osseointegration and fixation of implants in autologous iliac bone grafts in ovariectomized rabbits. Bone 2012, 50, 119–127. [Google Scholar] [CrossRef]
- Bobyn, J.D.; Hacking, S.A.; Krygier, J.J.; Harvey, E.J.; Little, D.G.; Tanzer, M. Zoledronic acid causes enhancement of bone growth into porous implants. J. Bone Jt. Surgery. Br. Vol. 2005, 87, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.E.; Rosenberg, E.; Santora, A.C.; Reszka, A.A. In Vitro and In Vivo Responses to High and Low Doses of Nitrogen-Containing Bisphosphonates Suggest Engagement of Different Mechanisms for Inhibition of Osteoclastic Bone Resorption. Calcif. Tissue Int. 2013, 92, 531–538. [Google Scholar] [CrossRef]
- Lambrinoudaki, I.; Vlachou, S.; Galapi, F.; Papadimitriou, D.; Papadias, K. Once-yearly zoledronic acid in the prevention of osteoporotic bone fractures in postmenopausal women. Clin. Interv. Aging 2008, 3, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, R.W.G.; Weening, E.C. Zolendroninezuur als intraveneuze infusie een nieuwe behandelingslijn bij de ziekte van Paget. Pharma Sel. 2006, 22, 33–35. [Google Scholar]
- Clokie, C.M.; Warshawsky, H. Morphologic and radioautographic studies of bone formation in relation to titanium implants using the rat tibia as a model. Int. J. Oral Maxillofac. Implant. 1995, 10, 155–165. [Google Scholar]
- Khojasteh, A.; Dehghan, M.M.; Nazeman, P. Immediate implant placement following 1-year treatment with oral versus intravenous bisphosphonates: A histomorphometric canine study on peri-implant bone. Clin. Oral Investig. 2019, 23, 1803–1809. [Google Scholar] [CrossRef]
- Minematsu, A.; Yoshimura, O.; Yotsuji, H.; Ichigo, H.; Takayanagi, K.; Kobayashi, R.; Hosoda, M.; Sasaki, H.; Maejima, H.; Matsuda, Y.; et al. The Effect of Low Calcium Diet on Bone in Ovariectomized Mice. J. Jpn. Phys. Ther. Assoc. 2000, 3, 13–16. [Google Scholar] [CrossRef][Green Version]
| Groups | Osteoporotic Condition | ||
|---|---|---|---|
| No. Placed Implants | No. Used Implants | No. Histological Sections | |
| ZOL-Pre | 6 | 5 * | 15 |
| ZOL-Post | 6 | 6 # | 18 |
| ZOL-Pre+Post | 6 | 6 | 18 |
| Saline | 6 | 5 * | 14 |
| Study Groups | ZOL-Pre | ZOL-Post | ZOL-Pre+Post | Saline |
|---|---|---|---|---|
| Bone area (BA%) (Mean ± SD) | 29.6 ± 9.0 | 27.9 ± 5.6 | 20.4 ± 5.0 | 17.3 ± 3.9 |
| Bone–implant contact (BIC%) (Mean ± SD) | 55.6 ± 19.0 | 65.8 ± 16.9 | 68.3 ± 10.0 | 43.3 ± 9.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basudan, A.M.; Shaheen, M.Y.; Niazy, A.A.; van den Beucken, J.J.J.P.; Jansen, J.A.; Alghamdi, H.S. Histomorphometric Evaluation of Peri-Implant Bone Response to Intravenous Administration of Zoledronate (Zometa®) in an Osteoporotic Rat Model. Materials 2020, 13, 5248. https://doi.org/10.3390/ma13225248
Basudan AM, Shaheen MY, Niazy AA, van den Beucken JJJP, Jansen JA, Alghamdi HS. Histomorphometric Evaluation of Peri-Implant Bone Response to Intravenous Administration of Zoledronate (Zometa®) in an Osteoporotic Rat Model. Materials. 2020; 13(22):5248. https://doi.org/10.3390/ma13225248
Chicago/Turabian StyleBasudan, Amani M., Marwa Y. Shaheen, Abdurahman A. Niazy, Jeroen J. J. P. van den Beucken, John A. Jansen, and Hamdan S. Alghamdi. 2020. "Histomorphometric Evaluation of Peri-Implant Bone Response to Intravenous Administration of Zoledronate (Zometa®) in an Osteoporotic Rat Model" Materials 13, no. 22: 5248. https://doi.org/10.3390/ma13225248
APA StyleBasudan, A. M., Shaheen, M. Y., Niazy, A. A., van den Beucken, J. J. J. P., Jansen, J. A., & Alghamdi, H. S. (2020). Histomorphometric Evaluation of Peri-Implant Bone Response to Intravenous Administration of Zoledronate (Zometa®) in an Osteoporotic Rat Model. Materials, 13(22), 5248. https://doi.org/10.3390/ma13225248








