Molecular Dynamic Investigations on the Adhesion Behaviors of Asphalt Mastic–Aggregate Interface
Abstract
1. Introduction
2. Molecular Model Generation
2.1. Asphalt Binder
2.2. Mineral Filler
2.3. Asphalt Mastic
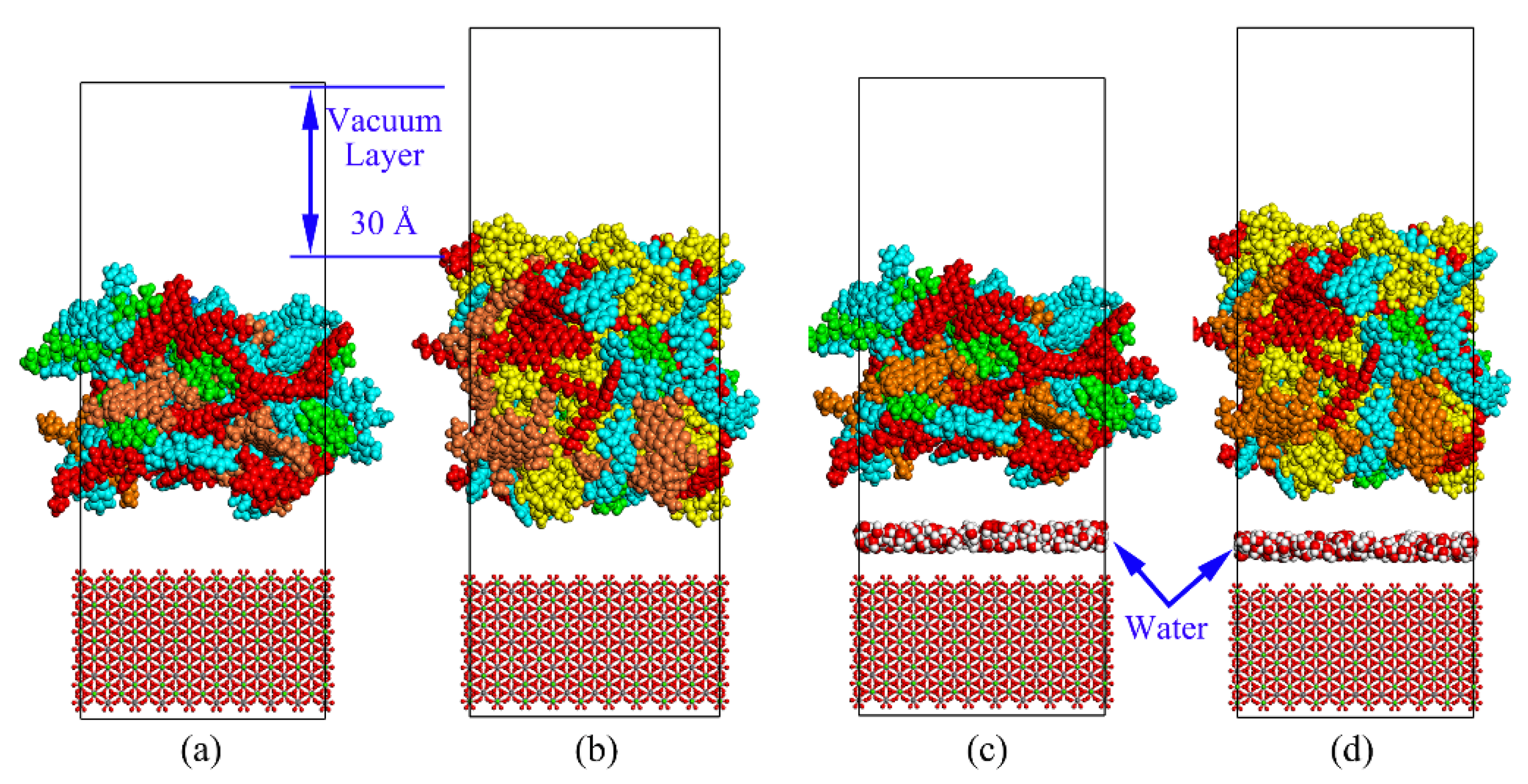
2.4. Asphalt Mastic–Aggregate System
2.5. Force Field and Simulation Details
3. Results and Discussion
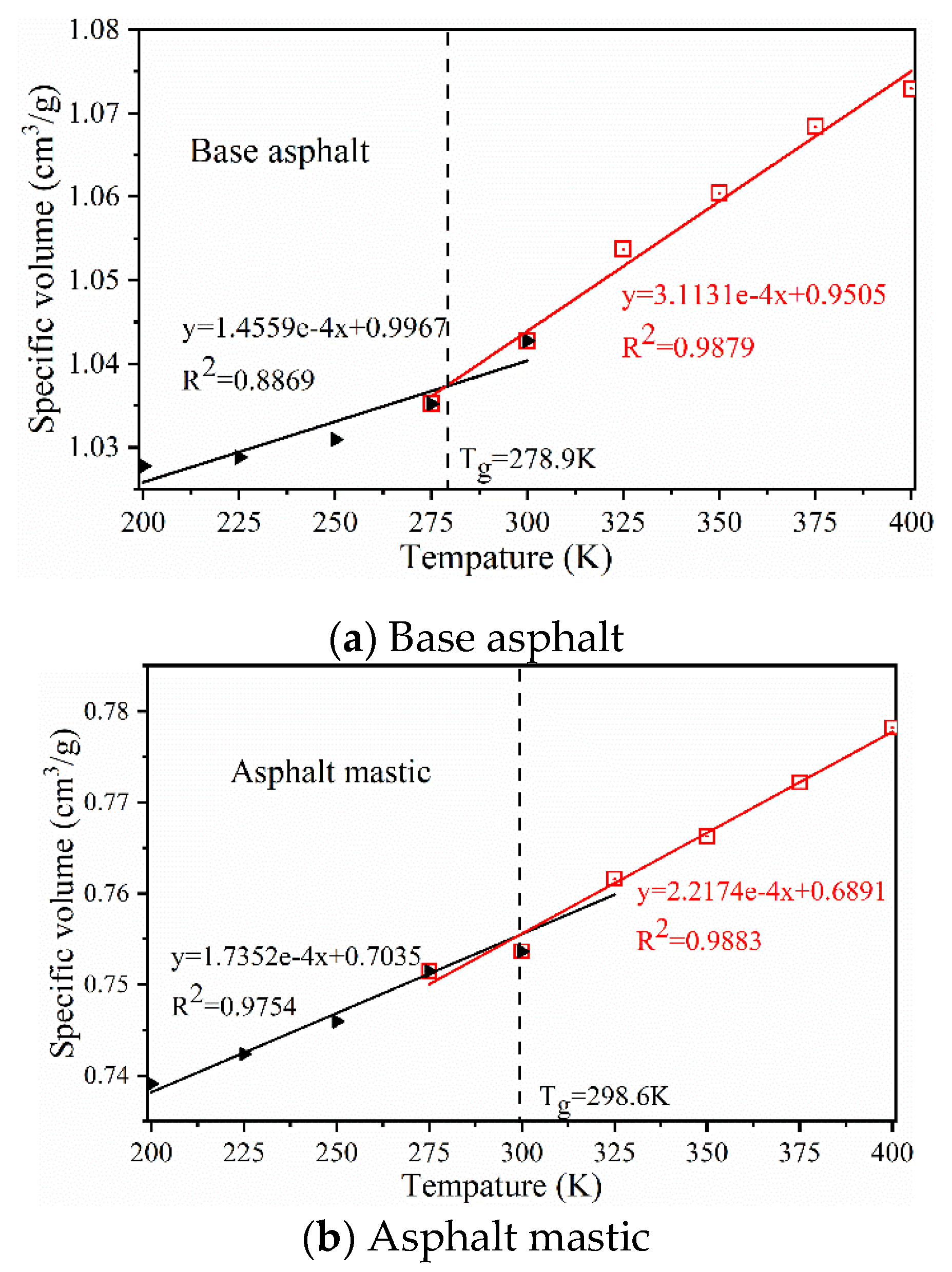
3.1. Thermodynamic Properties of Asphalt
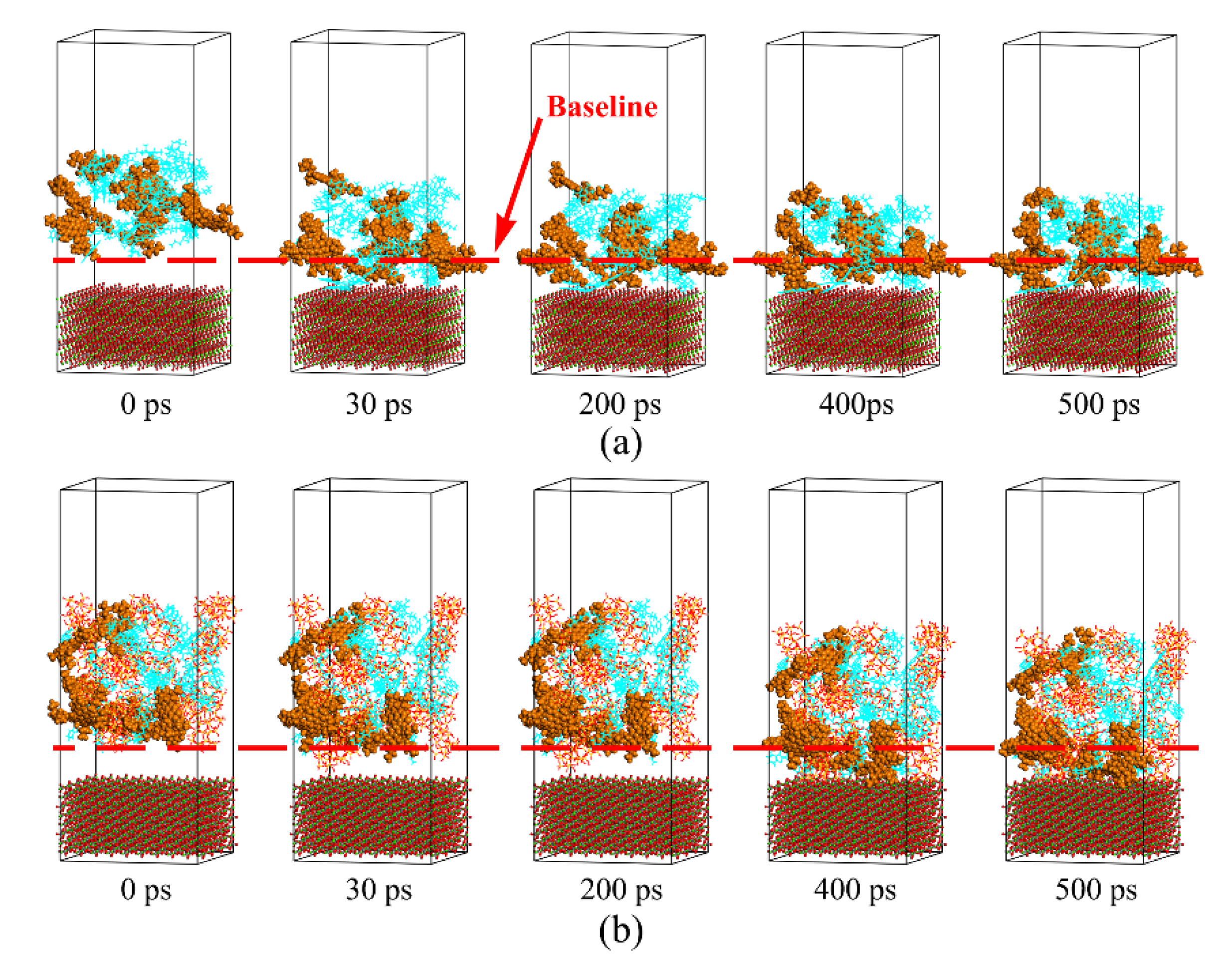
3.2. Aggregation Behaviors of Polar Components
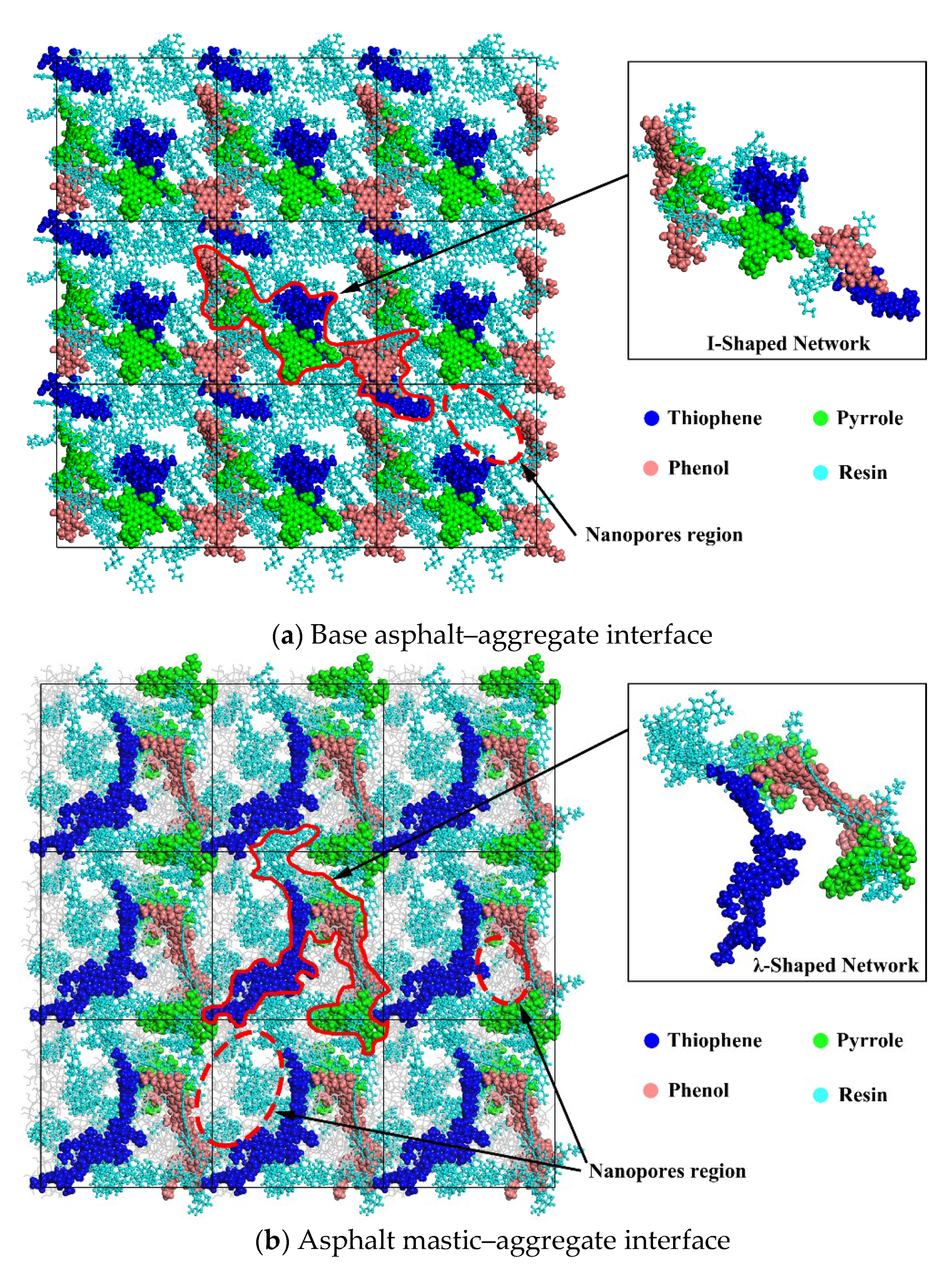
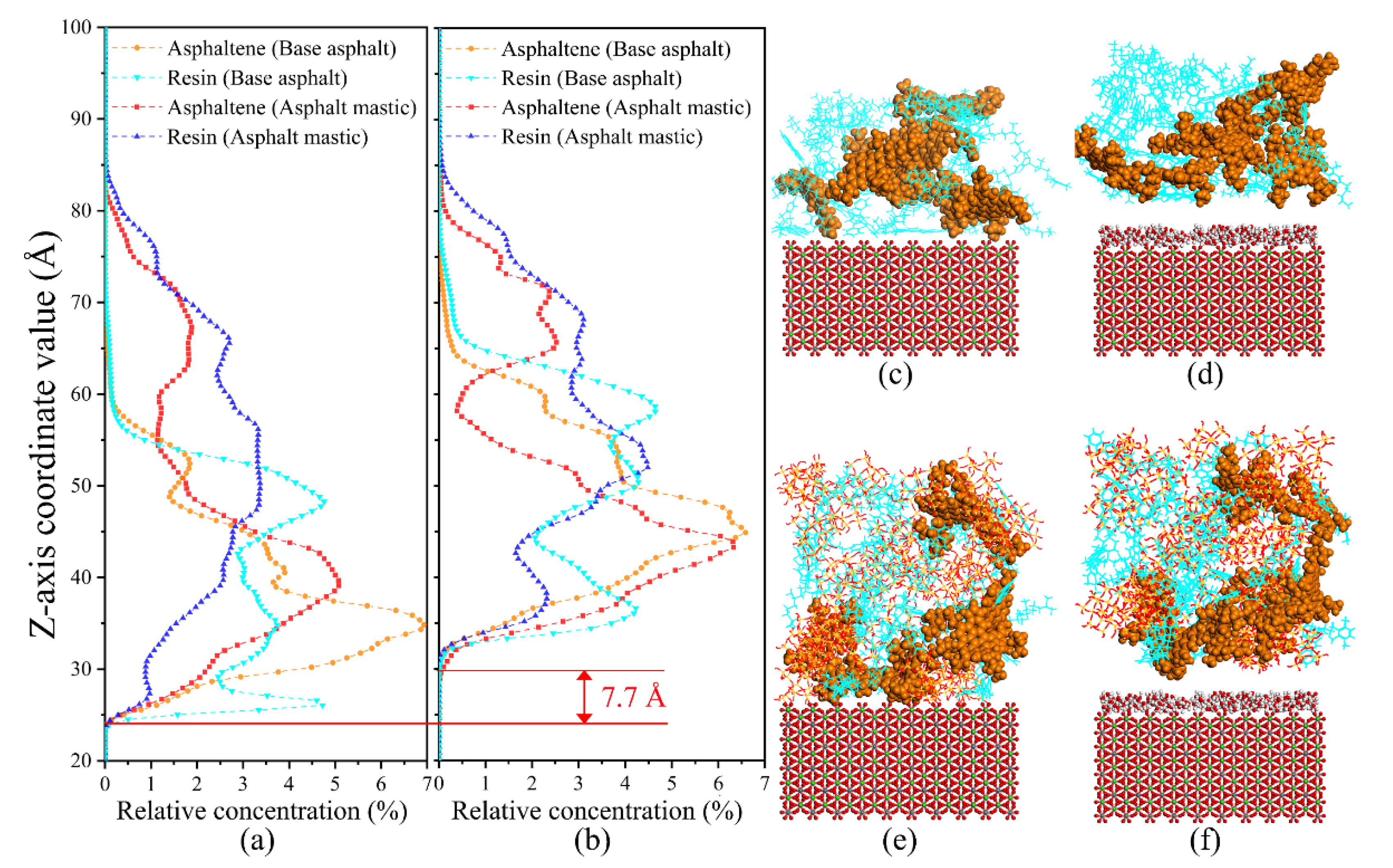
3.3. Distribution Characteristics of Polar Components
3.3.1. Relative Concentration
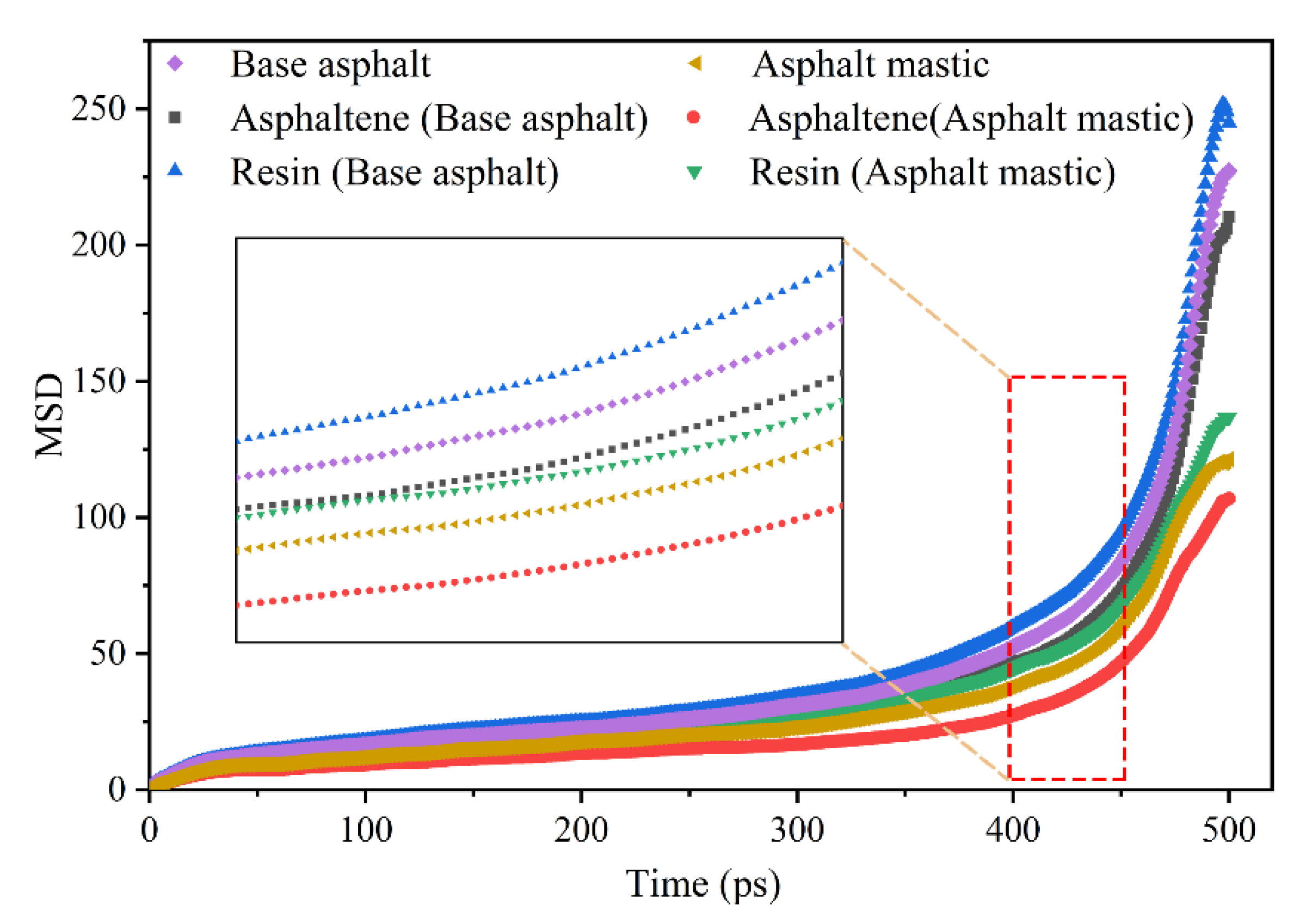
3.3.2. Mean Square Displacement
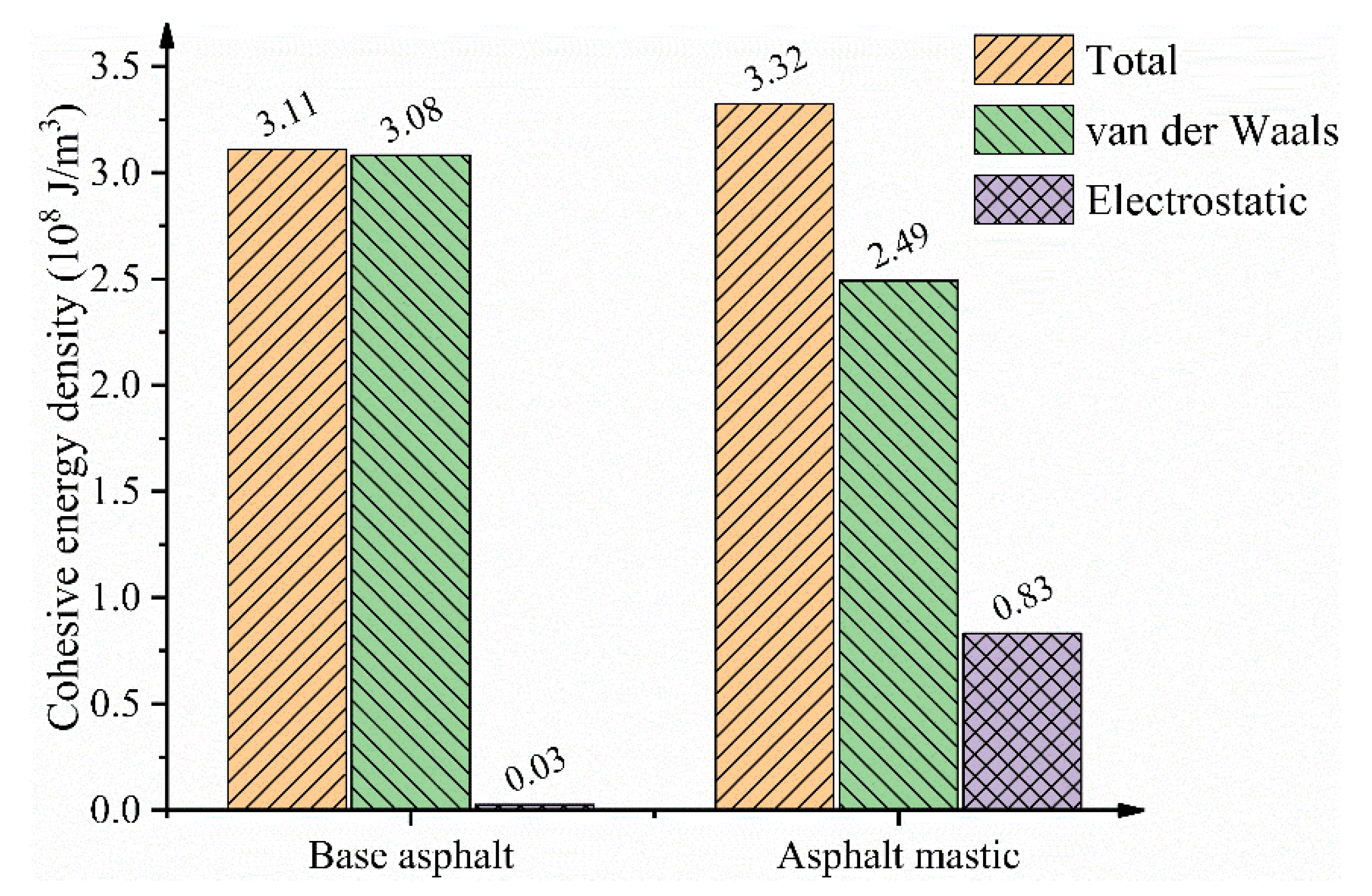
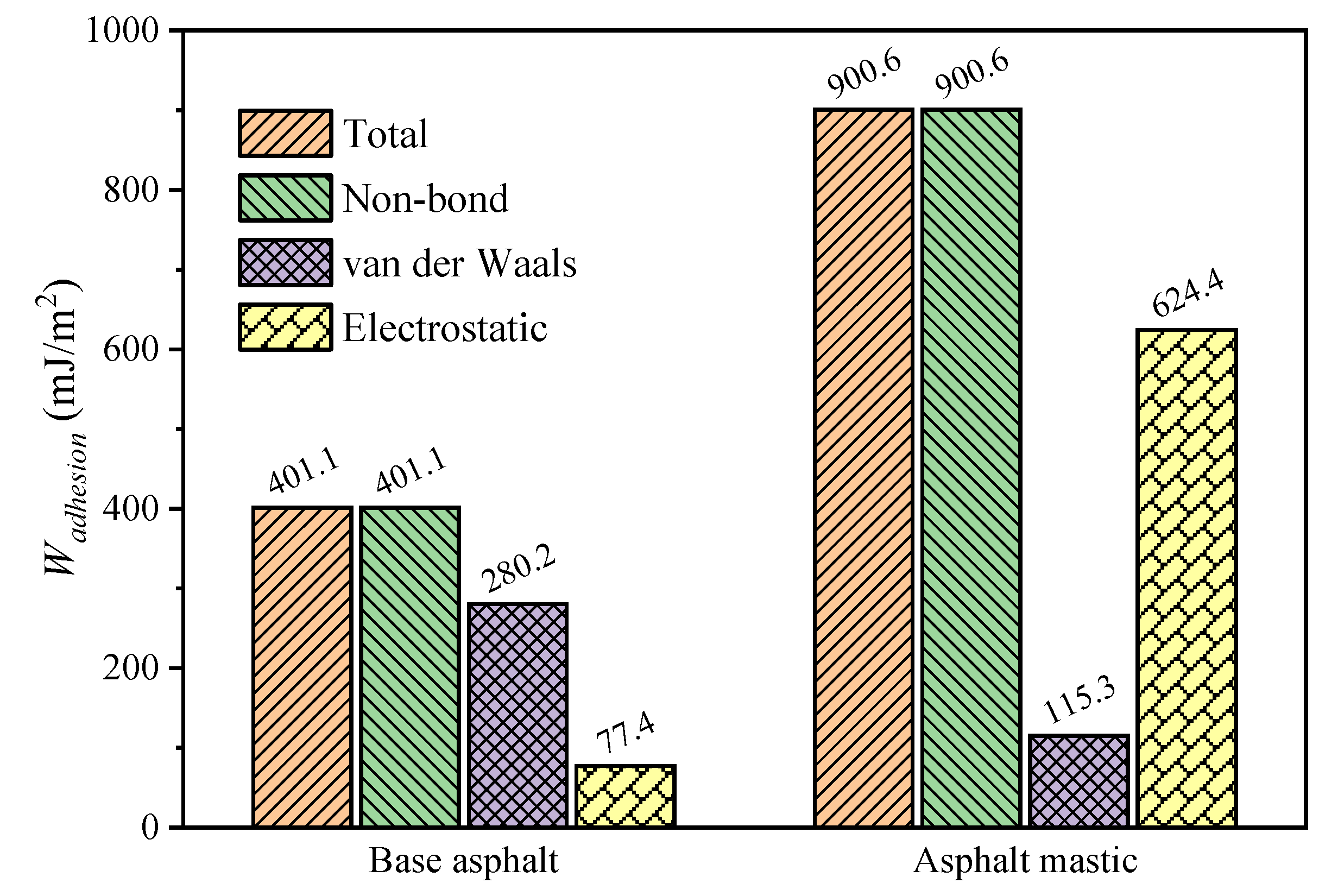
3.4. Interfacial Bonding Strength
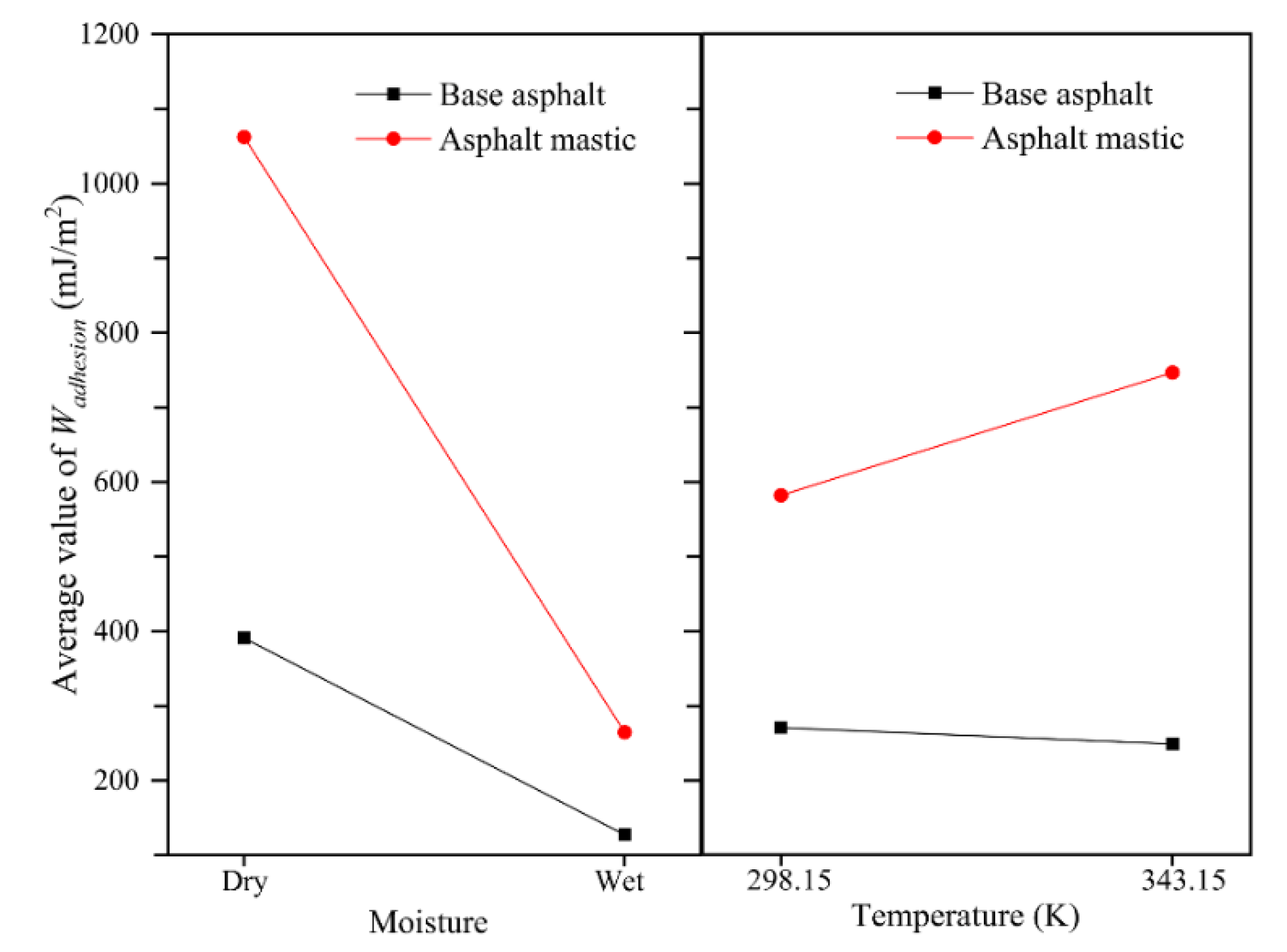
3.5. Impact of Temperature and Moisture on Adhesion Behaviors
4. Conclusions
- The asphalt mastic model under the actual F/A condition is established and validated by analyzing the difference of thermodynamic properties between asphalt mastic and base asphalt.
- The self-aggregation behaviors of polar components are significantly enhanced with the addition of silica particles. Because of the high polarity of silica particles, the molecular arrangement of polar components is changed from “parallel arrangement” into “stack distribution”, which results in the decrease of van der Waals energy.
- Silica particles could significantly change the distribution of polar components of base asphalt because of the adsorption effect of silica particles, and polar components adsorbed around the evenly dispersed silica particles in asphalt mastic exhibit a more uniform distribution state and lower mobility capability than base asphalt.
- The addition of silica particles with amounts of residual charges dramatically increases the electrostatic energy of the asphalt mastic–aggregate interface, thus strengthening the adhesion between asphalt mastic and aggregate.
- The coupling effect of moisture and temperature indicates that moisture could dramatically deteriorate the adhesion between asphalt and aggregate, especially for the asphalt mastic–aggregate interface. The increase of temperature enhances the work of adhesion of the asphalt mastic–aggregate interface, which is opposite to that of the base asphalt–aggregate interface.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Luo, X.; Luo, R.; Lytton, R.L. Crack initiation in asphalt mixtures under external compressive loads. Constr. Build. Mater. 2014, 72, 94–103. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Chen, J. Micromechanical analysis of asphalt mixture fracture with adhesive and cohesive failure. Eng. Fract. Mech. 2014, 132, 104–119. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, X.; Wang, H.; Wang, H.; Chen, J.; Li, C. Improvement of Asphalt-Aggregate Adhesion Using Plant Ash Byproduct. Materials 2019, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Xiao, S.; Yang, Q.; Chen, B.; Wang, F. Meso-scale analysis on shear failure characteristics of asphalt–aggregate interface. Mater. Struct. 2017, 50, 209. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, F.; Birgisson, B.; Lytton, R.L. Modelling cracking damage of asphalt mixtures under compressive monotonic and repeated loads using pseudo J-integral Paris’ law. Road Mater. Pavement Des. 2017, 19, 525–535. [Google Scholar] [CrossRef]
- Caro, S.; Masad, E.; Bhasin, A.; Little, D. Moisture susceptibility of asphalt mixtures, Part 1: Mechanisms. Int. J. Pavement Eng. 2008, 9, 81–98. [Google Scholar] [CrossRef]
- Chen, X.; Huang, B. Evaluation of moisture damage in hot mix asphalt using simple performance and superpave indirect tensile tests. Constr. Build. Mater. 2008, 22, 1950–1962. [Google Scholar] [CrossRef]
- Bhasin, A.; Little, D.N. Application of Microcalorimeter to Characterize Adhesion between Asphalt Binders and Aggregates. J. Mater. Civ. Eng. 2009, 21, 235–243. [Google Scholar] [CrossRef]
- Khotbehsara, M.M.; Manalo, A.C.; Aravinthan, T.; Reddy, K.R.; Ferdous, W.; Wong, H.; Nazari, A. Effect of elevated in-service temperature on the mechanical properties and microstructure of particulate-filled epoxy polymers. Polym. Degrad. Stab. 2019, 170, 108994. [Google Scholar] [CrossRef]
- Hefer, A.W. Adhesion in Bitumen-Aggregate Systems and Quantification of the Effect of Water on the Adhesive Bond; Texas A&M University: College Station, TX, USA, 2005. [Google Scholar]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
- Moraes, R.; Velasquez, R.; Bahia, H.U. Measuring the Effect of Moisture on Asphalt–Aggregate Bond with the Bitumen Bond Strength Test. Transp. Res. Rec. J. Transp. Res. Board 2011, 2209, 70–81. [Google Scholar] [CrossRef]
- Hefer, A.W.; Little, D.N.; Lytton, R.L. A synthesis of theories and mechanisms of bitumen-aggregate adhesion including recent advances in quantifying the effects of water. J. Assoc. Asph. Paving Technol. 2005, 74, 139–196. [Google Scholar]
- Miller, C.; Little, D.N.; Bhasin, A.; Gardner, N.; Herbert, B. Surface Energy Characteristics and Impact of Natural Minerals on Aggregate–Bitumen Bond Strengths and Asphalt Mixture Durability. Transp. Res. Rec. J. Transp. Res. Board 2012, 2267, 45–55. [Google Scholar] [CrossRef]
- ASTM. Standard Practice for Effect of Water on Bituminous-Coated Aggregate Using Boiling Water; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Tan, Y.; Xiaolin, L.; Jiantao, W. Internal Influence Factors of Asphalt-Aggregate Filler Interactions Based on Rheological Characteristics. J. Mater. Civ. Eng. 2012, 24, 1520–1528. [Google Scholar] [CrossRef]
- Alvarez, A.E.; Ovalles, E.; Caro, S. Assessment of the effect of mineral filler on asphalt–aggregate interfaces based on thermodynamic properties. Constr. Build. Mater. 2012, 28, 599–606. [Google Scholar] [CrossRef]
- Alvarez, A.E.; Gomez, K.L.; Gomez, D.C.; Reyes-Ortiz, O.J. Optimising the effect of natural filler on asphalt-aggregate interfaces based on surface free energy measurements. Road Mater. Pavement Des. 2018, 20, 1548–1570. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jeong, J.-H.; Kim, N. Use of surface free energy properties to predict moisture damage potential of Asphalt concrete mixture in cyclic loading condition. KSCE J. Civ. Eng. 2003, 7, 381–387. [Google Scholar] [CrossRef]
- Tan, Y.; Guo, M. Using surface free energy method to study the cohesion and adhesion of asphalt mastic. Constr. Build. Mater. 2013, 47, 254–260. [Google Scholar] [CrossRef]
- Fischer, H.R.; Dillingh, E.; Hermse, C. On the interfacial interaction between bituminous binders and mineral surfaces as present in asphalt mixtures. Appl. Surf. Sci. 2013, 265, 495–499. [Google Scholar] [CrossRef]
- Lyne, Å.L.; Wallqvist, V.; Birgisson, B. Adhesive surface characteristics of bitumen binders investigated by Atomic Force Microscopy. Fuel 2013, 113, 248–256. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Tan, T. Study on adhesion between asphalt binders and aggregate minerals under ambient conditions using particle-modified atomic force microscope probes. Constr. Build. Mater. 2015, 101, 159–165. [Google Scholar] [CrossRef]
- Xu, M.; Yi, J.; Feng, D.; Huang, Y.; Wang, D. Analysis of Adhesive Characteristics of Asphalt Based on Atomic Force Microscopy and Molecular Dynamics Simulation. ACS Appl. Mater. Interfaces 2016, 8, 12393–12403. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Fan, W.; Wang, J.; Liang, M.; Qian, C.; Luo, H.; Nan, G.; Yao, B.; Zhao, P. Study on adhesion of asphalt using AFM tip modified with mineral particles. Constr. Build. Mater. 2019, 207, 422–430. [Google Scholar] [CrossRef]
- Luo, D.; Guo, M.; Tan, Y. Molecular Simulation of Minerals-Asphalt Interfacial Interaction. Minerals 2018, 8, 176. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, H.; Gao, Y.; Wang, L. Study of diffusion characteristics of asphalt–aggregate interface with molecular dynamics simulation. Int. J. Pavement Eng. 2019, 21, 1–12. [Google Scholar] [CrossRef]
- Guo, M.; Tan, Y.; Wei, J. Using Molecular Dynamics Simulation to Study Concentration Distribution of Asphalt Binder on Aggregate Surface. J. Mater. Civ. Eng. 2018, 30, 04018075. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Z.; Wang, P.; Gong, X. Nanostructure characterization of asphalt-aggregate interface through molecular dynamics simulation and atomic force microscopy. Fuel 2017, 189, 155–163. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H. Molecular dynamics study of interfacial mechanical behavior between asphalt binder and mineral aggregate. Constr. Build. Mater. 2016, 121, 246–254. [Google Scholar] [CrossRef]
- Wang, H.; Lin, E.; Xu, G. Molecular dynamics simulation of asphalt-aggregate interface adhesion strength with moisture effect. Int. J. Pavement Eng. 2015, 18, 414–423. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H. Molecular dynamics study of oxidative aging effect on asphalt binder properties. Fuel 2017, 188, 1–10. [Google Scholar] [CrossRef]
- Sun, W.; Wang, H. Moisture effect on nanostructure and adhesion energy of asphalt on aggregate surface: A molecular dynamics study. Appl. Surf. Sci. 2020, 510, 145435. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Gu, F.; Xu, T.; Wang, H. Impact of minerals and water on bitumen-mineral adhesion and debonding behaviours using molecular dynamics simulations. Constr. Build. Mater. 2018, 171, 214–222. [Google Scholar] [CrossRef]
- Liu, J.; Yu, B.; Hong, Q. Molecular dynamics simulation of distribution and adhesion of asphalt components on steel slag. Constr. Build. Mater. 2020, 255, 119–332. [Google Scholar] [CrossRef]
- Abousnina, R.; Manalo, A.; Ferdous, W.; Lokuge, W.; Benabed, B.; Al-Jabri, K.S. Characteristics, strength development and microstructure of cement mortar containing oil-contaminated sand. Constr. Build. Mater. 2020, 252, 119–155. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Ma, H.L.; Huang, J.P.; Zhang, P.; Tao, J.L. Performances Comparison of Mine Filler Asphalt Mortar and Diatomite Asphalt Mortar. Appl. Mech. Mater. 2013, 1861–1864. [Google Scholar] [CrossRef]
- Antunes, V.; Freire, A.; Quaresma, L.; Micaelo, R. Influence of the geometrical and physical properties of filler in the filler–bitumen interaction. Constr. Build. Mater. 2015, 76, 322–329. [Google Scholar] [CrossRef]
- Xu, W.; Qiu, X.; Xiao, S.; Hong, H.; Wang, F.; Yuan, J. Characteristics and Mechanisms of Asphalt–Filler Interactions from a Multi-Scale Perspective. Materials 2020, 13, 2744. [Google Scholar] [CrossRef]
- Curtis, C.W.; Ensley, K.; Epps, J. Fundamental Properties of Asphalt-Aggregate Interactions Including Adhesion and Absorption; Strategic Highway Research Program: Washington, DC, USA, 1993; p. 603. [Google Scholar]
- Zhu, X.; Du, Z.; Ling, H.; Chen, L.; Wang, Y. Effect of filler on thermodynamic and mechanical behaviour of asphalt mastic: A MD simulation study. Int. J. Pavement Eng. 2018, 21, 1248–1262. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y. Understanding the temperature and loading frequency effects on physicochemical interaction ability between mineral filler and asphalt binder using molecular dynamic simulation and rheological experiments. Constr. Build. Mater. 2020, 244, 118311. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.; You, L. Chemo-physical analysis and molecular dynamics (MD) simulation of moisture susceptibility of nano hydrated lime modified asphalt mixtures. Constr. Build. Mater. 2015, 101, 536–547. [Google Scholar] [CrossRef]
- Su, M.; Si, C.; Zhang, Z.; Zhang, H. Molecular dynamics study on influence of Nano-ZnO/SBS on physical properties and molecular structure of asphalt binder. Fuel 2020, 263, 116777. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.; You, Z.; Bick, A.; Wang, M.; Guo, S. Property Analysis of Exfoliated Graphite Nanoplatelets Modified Asphalt Model Using Molecular Dynamics (MD) Method. Appl. Sci. 2017, 7, 43. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.; You, Z.; Bick, A.; Wang, M. Modulus simulation of asphalt binder models using Molecular Dynamics (MD) method. Constr. Build. Mater. 2018, 162, 430–441. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Xu, S.; Wu, S.; Liu, Q.; Fan, Z. Evaluation of thermo-mechanical properties of graphene/carbon-nanotubes modified asphalt with molecular simulation. Mol. Simul. 2017, 43, 312–319. [Google Scholar] [CrossRef]
- Long, Z.; You, L.; Tang, X.; Ma, W.; Ding, Y.; Xu, F. Analysis of interfacial adhesion properties of nano-silica modified asphalt mixtures using molecular dynamics simulation. Constr. Build. Mater. 2020, 255, 119354. [Google Scholar] [CrossRef]
- Murgich, J.; Rodriguez, J.; Aray, Y. Molecular Recognition and Molecular Mechanics of Micelles of Some Model Asphaltenes and Resins. Energy Fuels 1996, 10, 68–76. [Google Scholar] [CrossRef]
- Wiehe, I.; Liang, K. Asphaltenes, resins, and other petroleum macromolecules. Fluid Phase Equilibria 1996, 117, 201–210. [Google Scholar] [CrossRef]
- Corbett, L.W. Composition of asphalt based on generic fractionation, using solvent deasphaltening, elution-adsorption chromatography, and densimetric characterization. Anal. Chem. 1969, 41, 576–579. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Zhu, R.; Zhou, J.-M.; Chen, M.-B. Molecular dynamics simulation of diffusion of gases in pure and silica-filled poly(1-trimethylsilyl-1-propyne) [PTMSP]. Polymer 2006, 47, 5206–5212. [Google Scholar] [CrossRef]
- Rao, K.; Kundu, T.; Parker, S. Molecular Modeling of Mineral Surface Reactions in Flotation; CRC Press: New York, NY, USA, 2012. [Google Scholar]
- Xu, G.; Wang, H. Study of cohesion and adhesion properties of asphalt concrete with molecular dynamics simulation. Comput. Mater. Sci. 2016, 112, 161–169. [Google Scholar] [CrossRef]
- AASHTO. Standard Specification for Superpave Volumetric Mix Design; AASHTO: Washington, DC, USA, 2017. [Google Scholar]
- Faheem, A.F.; Bahia, H.U. Conceptual phenomenological model for interaction of asphalt binders with mineral fillers. Asph. Paving Technol. 2009, 78, 679–719. [Google Scholar]
- Zhang, J.P.; Pei, J.Z.; Li, Y.W. Research on Interaction between Asphalt and Filler Based on DSR Test. Adv. Mater. Res. 2013, 723, 480–487. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Zhu, C.; Pei, J. Evaluation indices of asphalt–filler interaction ability and the filler critical volume fraction based on the complex modulus. Road Mater. Pavement Des. 2016, 18, 1338–1352. [Google Scholar] [CrossRef]
- Kringos, N.; Scarpas, T.; Kasbergen, C.; Selvadurai, P. Modelling of combined physical–mechanical moisture-induced damage in asphaltic mixes, Part 1: Governing processes and formulations. Int. J. Pavement Eng. 2008, 9, 115–128. [Google Scholar] [CrossRef]
- Kringos, N.; Scarpas, A.; Copeland, A.; Youtcheff, J. Modelling of combined physical–mechanical moisture-induced damage in asphaltic mixes Part 2: Moisture susceptibility parameters. Int. J. Pavement Eng. 2008, 9, 129–151. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.C.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 2016, 22, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.; Greenfield, M.L. Analyzing Properties of Model Asphalts Using Molecular Simulation. Energy Fuels 2007, 21, 1712–1716. [Google Scholar] [CrossRef]
- Khabaz, F.; Khare, R. Glass Transition and Molecular Mobility in Styrene–Butadiene Rubber Modified Asphalt. J. Phys. Chem. B 2015, 119, 14261–14269. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Yang, Y.; Zhang, J.; Gu, F. Molecular dynamics investigation of interfacial adhesion between oxidised bitumen and mineral surfaces. Appl. Surf. Sci. 2019, 479, 449–462. [Google Scholar] [CrossRef]
- Xu, G. Characterization of Asphalt Properties and Asphalt-Aggregate Interaction Using Molecular Dynamics Simulation; Rutgers University Libraries: New Jersey, NJ, USA, 2017. [Google Scholar]
- Wang, P.; Dong, Z.-J.; Tan, Y.-Q.; Liu, Z.-Y. Investigating the Interactions of the Saturate, Aromatic, Resin, and Asphaltene Four Fractions in Asphalt Binders by Molecular Simulations. Energy Fuels 2015, 29, 112–121. [Google Scholar] [CrossRef]
- Lesueur, D. The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef] [PubMed]
- Little, D.N.; Bhasin, A. Using Surface Energy Measurements to Select Materials for Asphalt Pavement; Transport Research Board: Washington, DC, USA, 2006; p. 196. [Google Scholar]
- LeMarchand, C.A.; Schrøder, T.B.; Dyre, J.C.; Hansen, J.S. Cooee bitumen: Chemical aging. J. Chem. Phys. 2013, 139, 124506. [Google Scholar] [CrossRef] [PubMed]
- LeMarchand, C.A.; Schrøder, T.B.; Dyre, J.; Hansen, J.S. Cooee bitumen. II. Stability of linear asphaltene nanoaggregates. J. Chem. Phys. 2014, 141, 144308. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Han, M.; Tan, Y.; Meng, A.; Li, J.; Li, S. Research on bitumen molecule aggregation based on coarse-grained molecular dynamics. Constr. Build. Mater. 2020, 263, 263. [Google Scholar] [CrossRef]
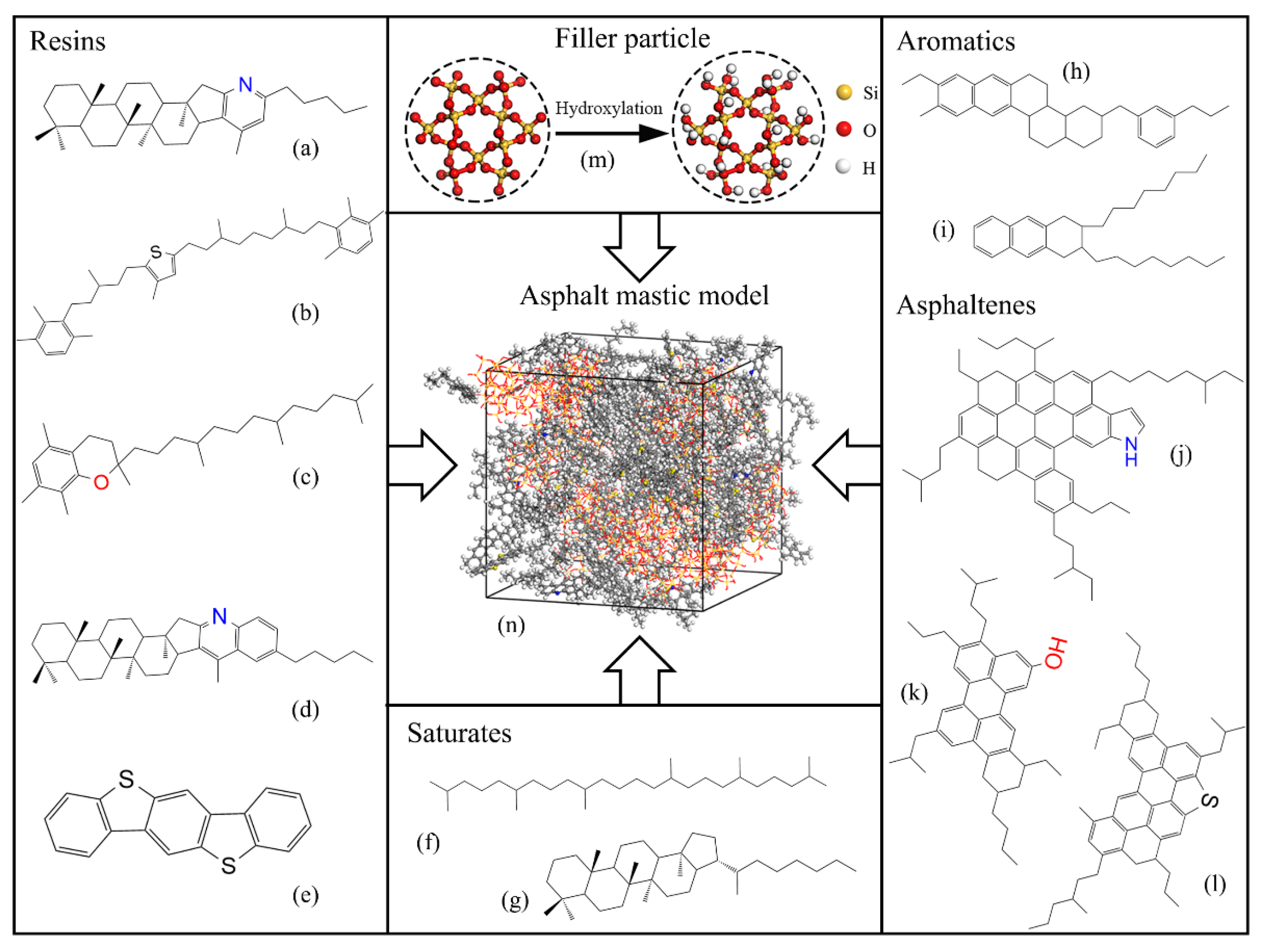




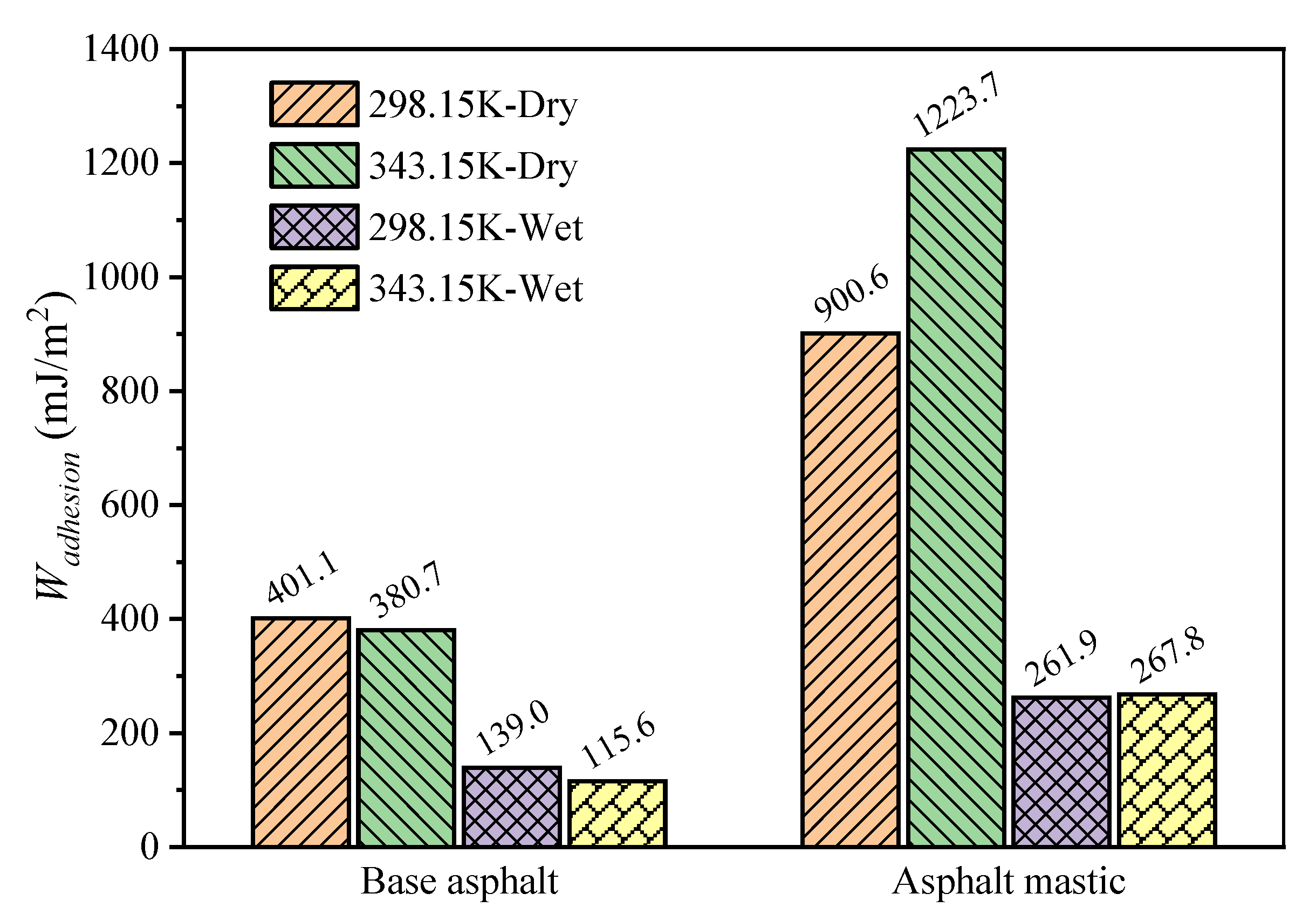

| SARA Fractions | Molecules | Molecules Number | Molecular Formula | Molar Mass (g/mol) | Mass Fraction (%) |
|---|---|---|---|---|---|
| Saturates (S) | Squalane | 4 | C30H62 | 422.9 | 5.2 |
| Hopane | 4 | C35H62 | 482.8 | 5.8 | |
| Aromatics (A) | PHPN | 11 | C35H44 | 464.8 | 15.7 |
| DOCHN | 13 | C30H46 | 406.8 | 16.2 | |
| Resins (R) | Pyridinohopane | 4 | C36H57N | 530.9 | 6.2 |
| Thio-isorenieratane | 4 | C40H60S | 572.9 | 7.0 | |
| Trimethylbenzene-oxane | 5 | C29H50O | 414.7 | 6.4 | |
| Quinolinohopane | 4 | C40H59N | 554.0 | 6.8 | |
| Benzobisbenzothiophene | 15 | C18H10S2 | 290.4 | 13.4 | |
| Asphaltenes (A) | Phenol | 3 | C42H54O | 575 | 5.3 |
| Pyrrole | 2 | C66H81N | 888.5 | 5.5 | |
| Thiophene | 3 | C51H62S | 707.2 | 6.5 |
| Properties | Calculated Results | Simulated Results in the Literatures |
|---|---|---|
| Density (298.15 K) | 0.998 | 0.92 (Long et al. [48]); 0.997 (Khabaz and Khare [65]);0.981 (Gao et al. [66]) |
| Glass-transition temperature (K) | 278.9 | 278.66 (Zhu et al. [41]); 275(Xu [67]) |
| Cohesive energy density (108 J/m3) | 3.11 | 3.32 (Xu and Wang [32]); 3.21(Wang et al. [68]) |
| Components | Number | Molecules | Molecular Formula | Dipole Moment (debye) |
|---|---|---|---|---|
| Saturates | A | Squalane | C30H62 | 0.077 |
| B | Hopane | C35H62 | 0.052 | |
| Aromatics | A | PHPN | C35H44 | 0.364 |
| B | DOCHN | C30H46 | 0.662 | |
| Resins | A | Pyridinohopane | C36H57N | 2.123 |
| B | Thio-isorenieratane | C40H60S | 1.567 | |
| C | Trimethylbenzene-oxane | C29H50O | 1.170 | |
| D | Quinolinohopane | C40H59N | 1.772 | |
| E | Benzobisbenzothiophene | C18H10S2 | 0 | |
| Asphaltenes | A | Phenol | C42H54O | 1.747 |
| B | Pyrrole | C66H81N | 0.846 | |
| C | Thiophene | C51H62S | 1.237 | |
| Silica particles | / | Silicon dioxide | SiO2 | 4.619 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Qiu, X.; Xiao, S.; Hu, G.; Wang, F.; Yuan, J. Molecular Dynamic Investigations on the Adhesion Behaviors of Asphalt Mastic–Aggregate Interface. Materials 2020, 13, 5061. https://doi.org/10.3390/ma13225061
Xu W, Qiu X, Xiao S, Hu G, Wang F, Yuan J. Molecular Dynamic Investigations on the Adhesion Behaviors of Asphalt Mastic–Aggregate Interface. Materials. 2020; 13(22):5061. https://doi.org/10.3390/ma13225061
Chicago/Turabian StyleXu, Wenyi, Xin Qiu, Shanglin Xiao, Ganghua Hu, Feng Wang, and Jie Yuan. 2020. "Molecular Dynamic Investigations on the Adhesion Behaviors of Asphalt Mastic–Aggregate Interface" Materials 13, no. 22: 5061. https://doi.org/10.3390/ma13225061
APA StyleXu, W., Qiu, X., Xiao, S., Hu, G., Wang, F., & Yuan, J. (2020). Molecular Dynamic Investigations on the Adhesion Behaviors of Asphalt Mastic–Aggregate Interface. Materials, 13(22), 5061. https://doi.org/10.3390/ma13225061





