Effect of Spraying Power on Oxidation Resistance of MoSi2-ZrB2 Coating for Nb-Si Based Alloy Prepared by Atmospheric Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of MoSi2-ZrB2 Coating
2.2. Isothermal Oxidation
2.3. Coating Characterization
3. Results
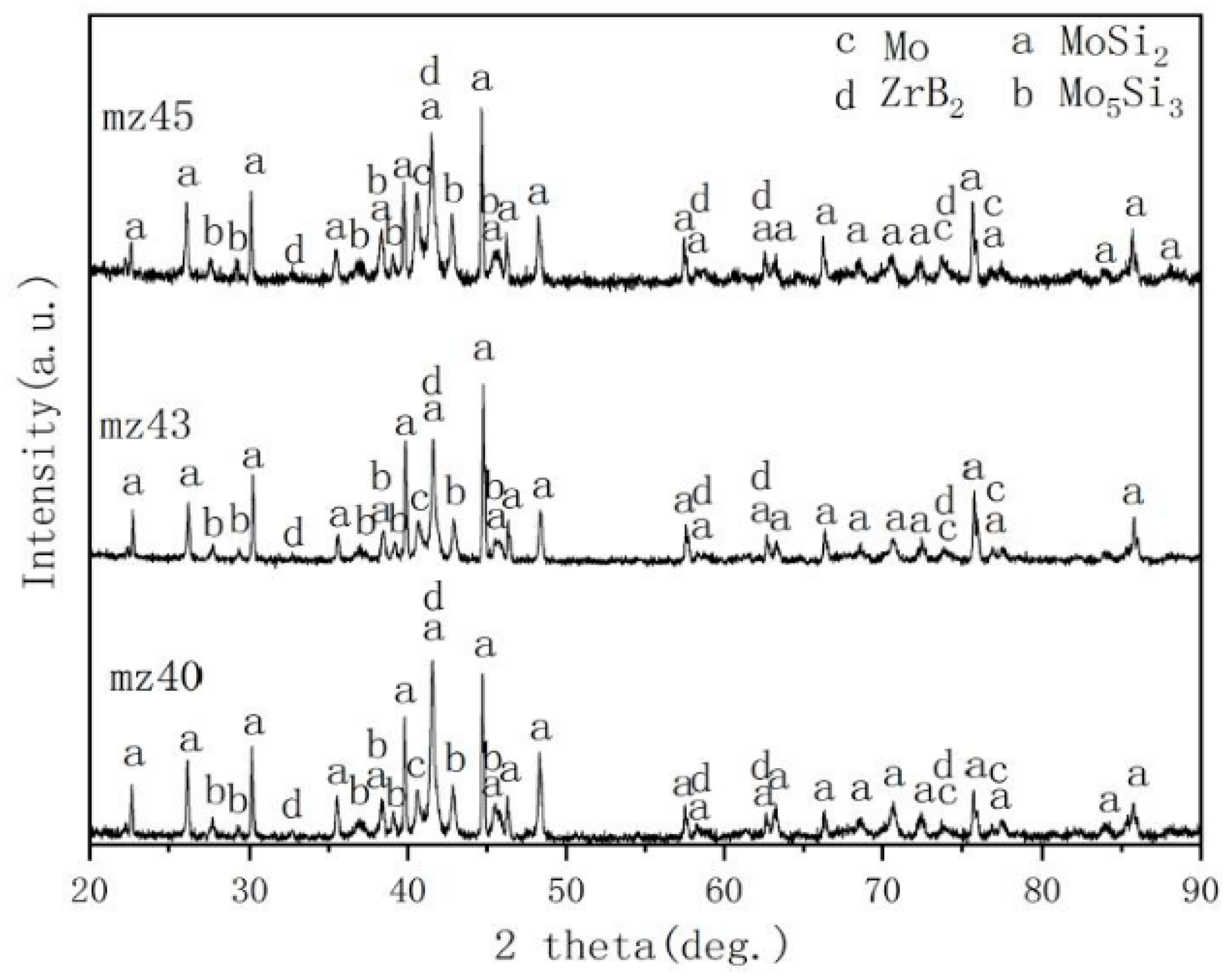
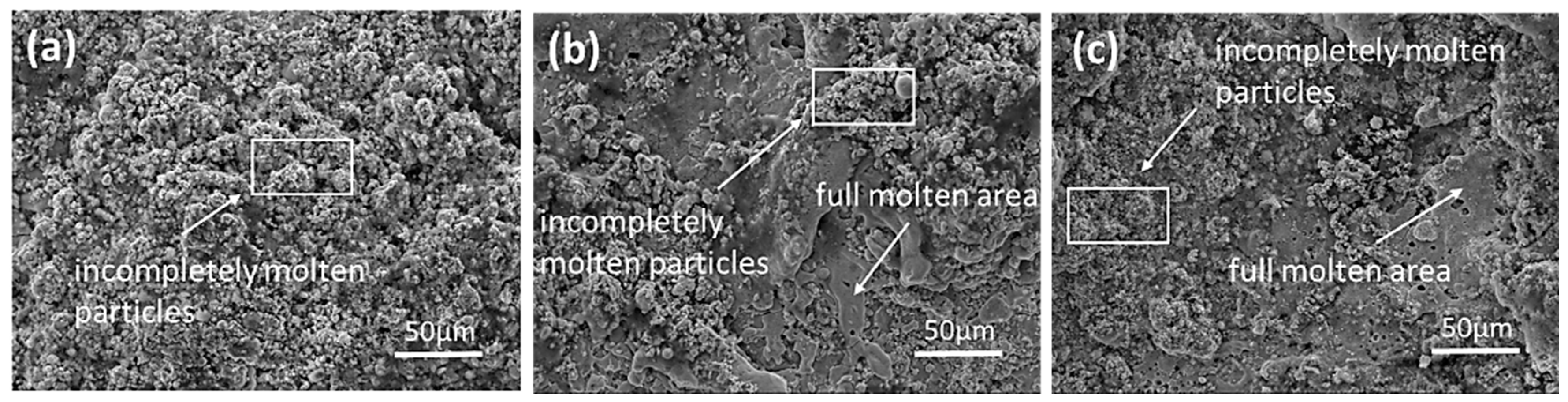
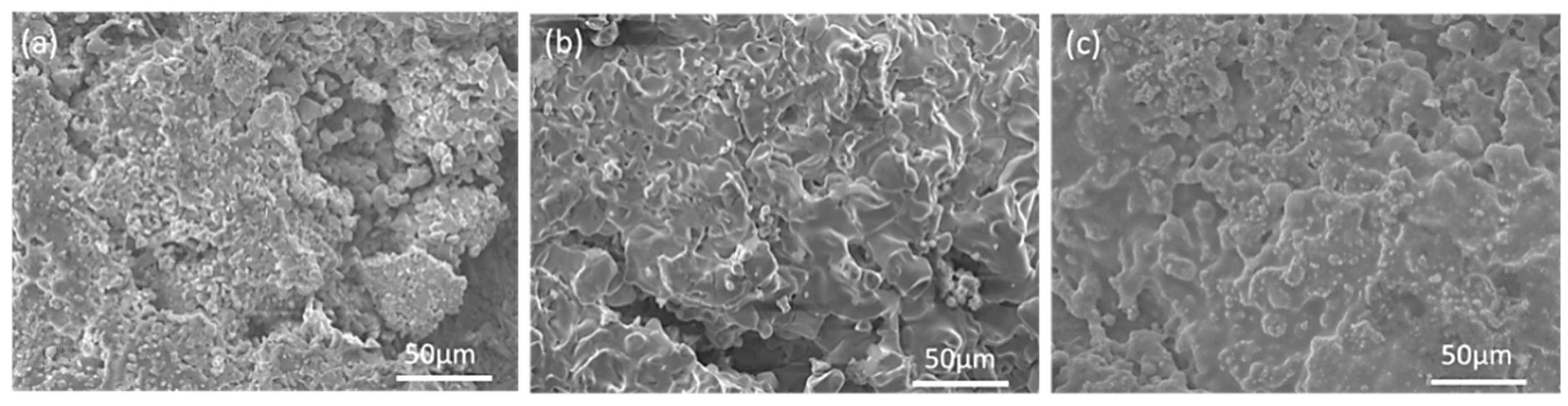
3.1. Microstructure of MoSi2-ZrB2 Coating
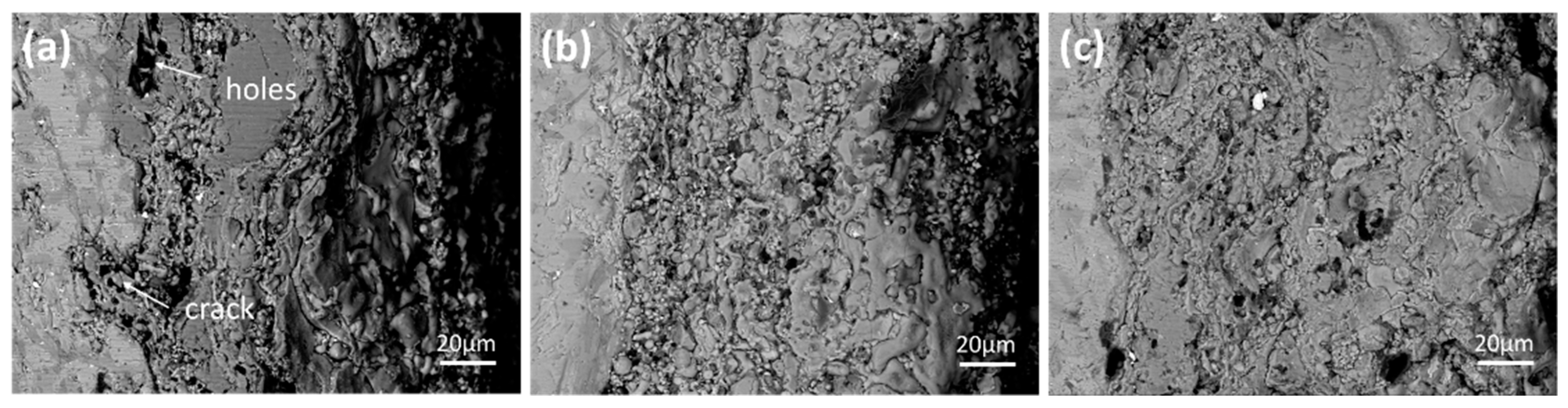
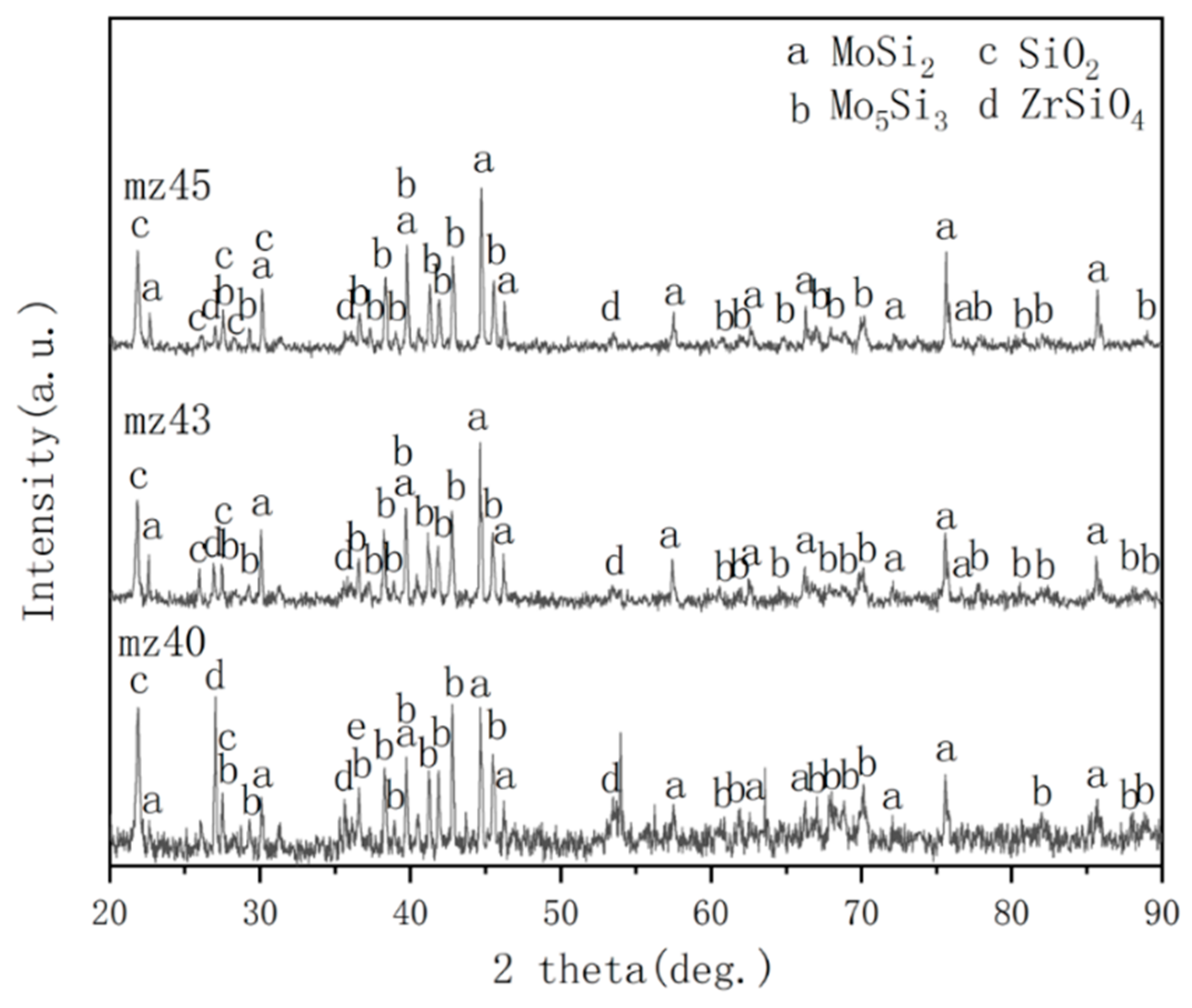
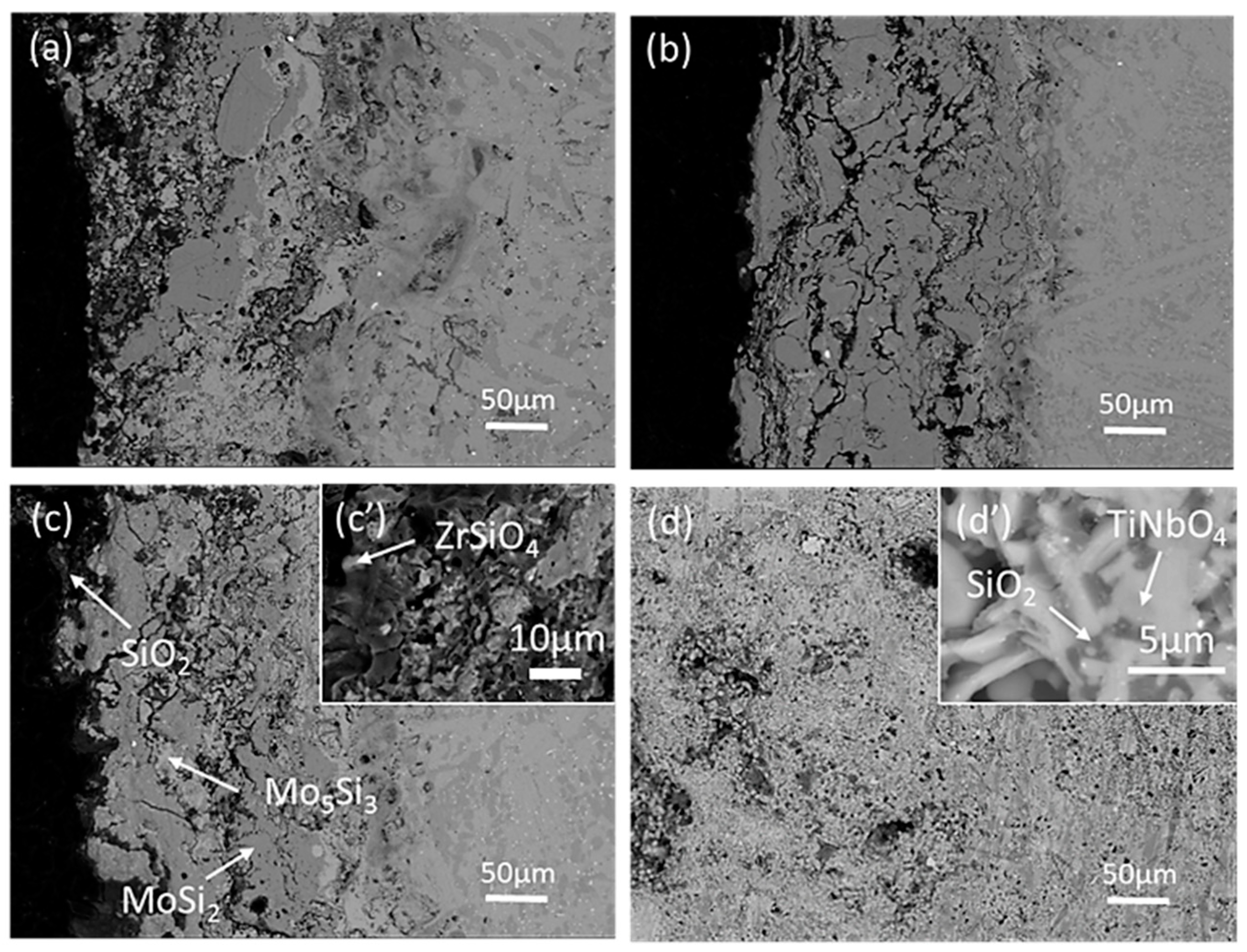
3.2. High Temperature Oxidation Resistance
4. Discussion
4.1. Effect of Spraying Powder on the Microstructure of Coatings
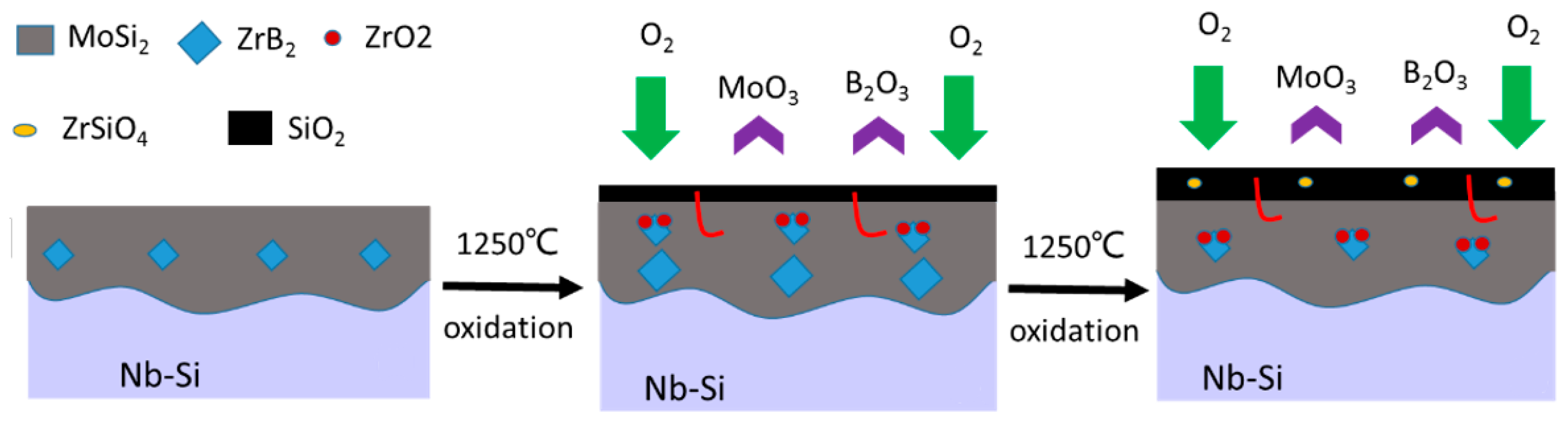
4.2. Oxidation Mechanism of MoSi2-ZrB2 Coatings
5. Conclusions
- The microstructure of MoSi2-ZrB2 coatings prepared by atmospheric plasma spraying mainly consisted of MoSi2. The higher spraying power could produce a much more compact coating.
- The MoSi2-ZrB2 coating prepared under 45 Kw showed the best oxidation resistance with the mass gain of 1.66 mg cm-2 after oxidation at 1250 °C for 60 h. However, the MoSi2-ZrB2 coatings prepared under 40 and 43 Kw showed worse oxidation resistance, due to the bad combination between coating and substrate at the lower spraying power.
- The excellent anti-oxidation protection of mz45 sample was mainly due to the formation of a silica glass layer, leading to a low diffusion rate of oxygen.
Author Contributions
Funding
Conflicts of Interest
References
- Bewlay, B.P.; Jackson, M.R.; Subramanian, P.R.; Zhao, J.-C. A review of very-high-temperature Nb-silicide-based composites. Met. Mater. Trans. A 2003, 34, 2043–2052. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, X. High temperature deformation behavior of an optimized Nb–Si based ultrahigh temperature alloy. Scr. Mater. 2016, 116, 16–20. [Google Scholar] [CrossRef]
- Guan, K.; Jia, L.; Kong, B.; Yuan, S.; Zhang, H. Study of the fracture mechanism of NbSS/Nb5Si3 in situ composite: Based on a mechanical characterization of interfacial strength. Mater. Sci. Eng. A 2016, 663, 98–107. [Google Scholar] [CrossRef]
- Geng, J.; Tsakiropoulos, P.; Shao, G. The effects of Ti and Mo additions on the microstructure of Nb-silicide based in situ composites. Intermetalic 2006, 14, 227–235. [Google Scholar] [CrossRef]
- Zhang, S.-N.; Guo, Y.; Kong, B.; Zhang, F.-X.; Zhang, H. High-temperature oxidation behavior of Nb-Si-based alloy with separate vanadium, tantalum, tungsten and zirconium addition. Rare Met. 2017, 36, 1–9. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Jackson, M.R.; Zhao, J.-C.; Subramanian, P.R.; Mendiratta, M.G.; Lewandowski, J.J. Ultrahigh-Temperature Nb-Silicide-Based Composites. MRS Bull. 2003, 28, 646–653. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Jackson, M.R.; Lipsitt, H.A. The balance of mechanical and environmental properties of a multielement niobium-niobium silicide-basedIn Situ composite. Met. Mater. Trans. A 1996, 27, 3801–3808. [Google Scholar] [CrossRef]
- Wang, L.; Jia, L.; Cui, R.; Zheng, L.; Zhang, H. Microstructure, Mechanical Properties and Oxidation Resistance of Nb-22Ti-14Si-2Hf-2Al-xCr Alloys. Chin. J. Aeronaut. 2012, 25, 292–296. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Sha, J.; Zhang, H. The effects of melting technologies on the microstructures and properties of Nb–16Si–22Ti–2Al–2Hf–17Cr alloy. Mater. Sci. Eng. A 2010, 527, 6140–6152. [Google Scholar] [CrossRef]
- Yao, D.; Cai, R.; Zhou, C.; Sha, J.; Jiang, H. Experimental study and modeling of high temperature oxidation of Nb-base in situ composites. Corros. Sci. 2009, 51, 364–370. [Google Scholar] [CrossRef]
- Kang, Y.; Qu, S.; Song, J.; Huang, Q.; Han, Y. Microstructure and mechanical properties of Nb–Ti–Si–Al–Hf–xCr–yV multi-element in situ composite. Mater. Sci. Eng. A 2012, 534, 323–328. [Google Scholar] [CrossRef]
- Geng, J.; Tsakiropoulos, P. A study of the microstructures and oxidation of Nb–Si–Cr–Al–Mo in situ composites alloyed with Ti, Hf and Sn. Intermetalic 2007, 15, 382–395. [Google Scholar] [CrossRef]
- Xiao, L.-R.; Cai, Z.-G.; Yi, D.-Q.; Ying, L.; Liu, H.-Q.; Huang, D.-Y. Morphology, structure and formation mechanism of silicide coating by pack cementation process. Trans. Nonferrous Met. Soc. China 2006, 16, s239–s244. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Liu, L.; Wang, Y. Oxidation and interdiffusion behavior of Niobium substrate coated MoSi2 coating prepared by spark plasma sintering. Appl. Surf. Sci. 2014, 320, 791–797. [Google Scholar] [CrossRef]
- Hidouci, A.; Pelletier, J. Microstructure and mechanical properties of MoSi2 coatings produced by laser processing. Mater. Sci. Eng. A 1998, 252, 17–26. [Google Scholar] [CrossRef]
- Sun, S.; Li, X.; Wang, H.; Jiang, Y.; Yi, D. Prediction on anisotropic elasticity, sound velocity, and thermodynamic properties of MoSi2 under pressure. J. Alloy. Compd. 2015, 652, 106–115. [Google Scholar] [CrossRef]
- Su, L.; Jia, L.; Weng, J.; Hong, Z.; Zhou, C.; Zhang, H. Improvement in the oxidation resistance of Nb–Ti–Si–Cr–Al–Hf alloys containing alloyed Ge and B. Corros. Sci. 2014, 88, 460–465. [Google Scholar] [CrossRef]
- Yokota, H.; Kudoh, T.; Suzuki, T. Oxidation resistance of boronized MoSi2. Surf. Coat. Technol. 2003, 169, 171–173. [Google Scholar] [CrossRef]
- Lange, A.; Braun, R. Magnetron-sputtered oxidation protection coatings for Mo–Si–B alloys. Corros. Sci. 2014, 84, 74–84. [Google Scholar] [CrossRef]
- Majumdar, S.; Dönges, B.; Gorr, B.; Christ, H.-J.; Schliephake, D.; Heilmaier, M. Mechanisms of oxide scale formation on yttrium-alloyed Mo–Si–B containing fine-grained microstructure. Corros. Sci. 2015, 90, 76–88. [Google Scholar] [CrossRef]
- Tian, X.; Guo, X.; Sun, Z.; Yin, Z.; Wang, L. Formation of B-modified MoSi2 coating on pure Mo prepared through HAPC process. Int. J. Refract. Met. Hard Mater. 2014, 45, 8–14. [Google Scholar] [CrossRef]
- Fu, Q.-G.; Li, H.; Wang, Y.-J.; Li, K.; Shi, X.-H. B2O3 modified SiC–MoSi2 oxidation resistant coating for carbon/carbon composites by a two-step pack cementation. Corros. Sci. 2009, 51, 2450–2454. [Google Scholar] [CrossRef]
- Pang, J.; Wang, W.; Zhou, C. Microstructure evolution and oxidation behavior of B modified MoSi2 coating on Nb–Si based alloys. Corros. Sci. 2016, 105, 1–7. [Google Scholar] [CrossRef]
- Wang, C.; Li, K.; Huo, C.; He, Q.; Shi, X. Oxidation behavior and microstructural evolution of plasma sprayed La2O3-MoSi2-SiC coating on carbon/carbon composites. Surf. Coat. Technol. 2018, 348, 81–90. [Google Scholar] [CrossRef]
- Sun, L.; Fu, Q.; Fang, X.-Q.; Sun, J. A MoSi2-based composite coating by supersonic atmospheric plasma spraying to protect Nb alloy against oxidation at 1500 °C. Surf. Coat. Technol. 2018, 352, 182–190. [Google Scholar] [CrossRef]
- Yanagihara, K.; Maruyama, T.; Nagata, K. Effect of third elements on the pesting suppression of Mo-Si-X intermetallics (X = Al, Ta, Ti, Zr and Y). Intermetalics 1996, 4, S133–S139. [Google Scholar] [CrossRef]
- Wang, C.; Li, K.; He, Q.; He, D.; Huo, C.; Su, Y.; Shi, X.; Li, H. Oxidation and ablation protection of plasma sprayed LaB6-MoSi2-ZrB2 coating for carbon/carbon composites. Corros. Sci. 2019, 151, 57–68. [Google Scholar] [CrossRef]
- Wang, L.; Fu, Q.; Liu, N.; Shan, Y. Supersonic plasma sprayed MoSi2-ZrB2 antioxidation coating for SiC–C/C composites. Surf. Eng. 2016, 32, 508–513. [Google Scholar] [CrossRef]
- Mao, J.; Ding, S.; Li, Y.; Li, S.; Liu, F.; Zeng, X.; Cheng, X.-D. Preparation and investigation of MoSi2/SiC coating with high infrared emissivity at high temperature. Surf. Coat. Technol. 2019, 358, 873–878. [Google Scholar] [CrossRef]
- Yan, J.; Liu, L.; Mao, Z.; Xu, H.; Wang, Y. Effect of Spraying Powders Size on the Microstructure, Bonding Strength, and Microhardness of MoSi2 Coating Prepared by Air Plasma Spraying. J. Therm. Spray Technol. 2014, 23, 934–939. [Google Scholar] [CrossRef]
- Sun, J.; Li, T.; Zhang, G.-P. Effect of thermodynamically metastable components on mechanical and oxidation properties of the thermal-sprayed MoSi2 based composite coating. Corros. Sci. 2019, 155, 146–154. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, J.; Fu, Q. Effect of mullite on the microstructure and oxidation behavior of thermal-sprayed MoSi2 coating at 1500 °C. Ceram. Int. 2020, 46, 10058–10066. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.; Lei, Q.; Fu, Q.-G.; Ma, C.; Yao, D.-J.; Wang, Y.-J.; Sun, C.; Wei, J.-F.; Han, Z.-H. Effect of spraying power on microstructure and bonding strength of MoSi2-based coatings prepared by supersonic plasma spraying. Appl. Surf. Sci. 2011, 257, 5566–5570. [Google Scholar] [CrossRef]
- Jia, Y.; Li, H.; Fu, Q.; Zhao, Z.; Sun, J. Ablation resistance of supersonic-atmosphere-plasma-spraying ZrC coating doped with ZrO2 for SiC-coated carbon/carbon composites. Corros. Sci. 2017, 123, 40–54. [Google Scholar] [CrossRef]
- Veytizou, C. Zircon formation from amorphous silica and tetragonal zirconia: Kinetic study and modelling. Solid State Ionics 2001, 139, 315–323. [Google Scholar] [CrossRef]
- Chu, Y.; Li, H.-J.; Luo, H.; Li, L.; Qi, L. Oxidation protection of carbon/carbon composites by a novel SiC nanoribbon-reinforced SiC–Si ceramic coating. Corros. Sci. 2015, 92, 272–279. [Google Scholar] [CrossRef]
- Fu, Q.-G.; Jing, J.-Y.; Tan, B.-Y.; Yuan, R.-M.; Zhuang, L.; Li, L. Nanowire-toughened transition layer to improve the oxidation resistance of SiC–MoSi2–ZrB2 coating for C/C composites. Corros. Sci. 2016, 111, 259–266. [Google Scholar] [CrossRef]
- Lee, W.Y.; More, K.L.; Lara-Curzio, E. Multilayered Oxide Interphase Concept for Ceramic-Matrix Composites. J. Am. Ceram. Soc. 2005, 81, 717–720. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Luo, H.; Xie, J.; Zhang, J.; Fu, Q. Effect of Y2O3 on the oxidation resistant of ZrSiO4/SiC coating prepared by supersonic plasma spraying technique for carbon/carbon composites. Surf. Coat. Technol. 2013, 235, 127–133. [Google Scholar] [CrossRef]
- Chu, Y.; Fu, Q.; Li, H.; Li, K.; Zou, X.; Gu, C. Influence of SiC nanowires on the properties of SiC coating for C/C composites between room temperature and 1500 °C. Corros. Sci. 2011, 53, 3048–3053. [Google Scholar] [CrossRef]


| Content | Parameters |
|---|---|
| Spraying power (kW) | 40–45 |
| Primary gas Ar (L/min) | 58 |
| Carrier gas Ar (L/min) | 10 |
| Second gas H2 (L/min) | 9 |
| Powder feed rate (g/min) | 17 |
| Spraying distance (mm) | 100 |
| Nozzle diameter (mm) | 5.5 |
| Elements | Mo | Si | O | B |
|---|---|---|---|---|
| MoSi2 | 34.29 | 65.71 | 0 | 0 |
| Element | Mo | Si | Zr | B | Nb | O |
|---|---|---|---|---|---|---|
| SiO2 | 0 | 38.26 | 0 | 0 | 0 | 61.74 |
| MoSi2 | 32.99 | 67.01 | 0 | 0 | 0 | 0 |
| Mo5Si3 | 52.02 | 35.4 | 0 | 2.93 | 3.08 | 6.57 |
| ZrSiO4 | 0 | 15.01 | 14.91 | 0 | 0 | 70.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuo, G.; Su, L.; Jiang, K.; Yang, J. Effect of Spraying Power on Oxidation Resistance of MoSi2-ZrB2 Coating for Nb-Si Based Alloy Prepared by Atmospheric Plasma. Materials 2020, 13, 5060. https://doi.org/10.3390/ma13225060
Zhuo G, Su L, Jiang K, Yang J. Effect of Spraying Power on Oxidation Resistance of MoSi2-ZrB2 Coating for Nb-Si Based Alloy Prepared by Atmospheric Plasma. Materials. 2020; 13(22):5060. https://doi.org/10.3390/ma13225060
Chicago/Turabian StyleZhuo, Guanqun, Linfen Su, Kaiyong Jiang, and Jianyong Yang. 2020. "Effect of Spraying Power on Oxidation Resistance of MoSi2-ZrB2 Coating for Nb-Si Based Alloy Prepared by Atmospheric Plasma" Materials 13, no. 22: 5060. https://doi.org/10.3390/ma13225060
APA StyleZhuo, G., Su, L., Jiang, K., & Yang, J. (2020). Effect of Spraying Power on Oxidation Resistance of MoSi2-ZrB2 Coating for Nb-Si Based Alloy Prepared by Atmospheric Plasma. Materials, 13(22), 5060. https://doi.org/10.3390/ma13225060




