Imidazolium-Based Ionic Liquid as Efficient Corrosion Inhibitor for AA 6061 Alloy in HCl Solution
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Gravimetric Tests
2.3. Electrochemical Analysis
2.4. Surface Analyses
3. Results and Discussion
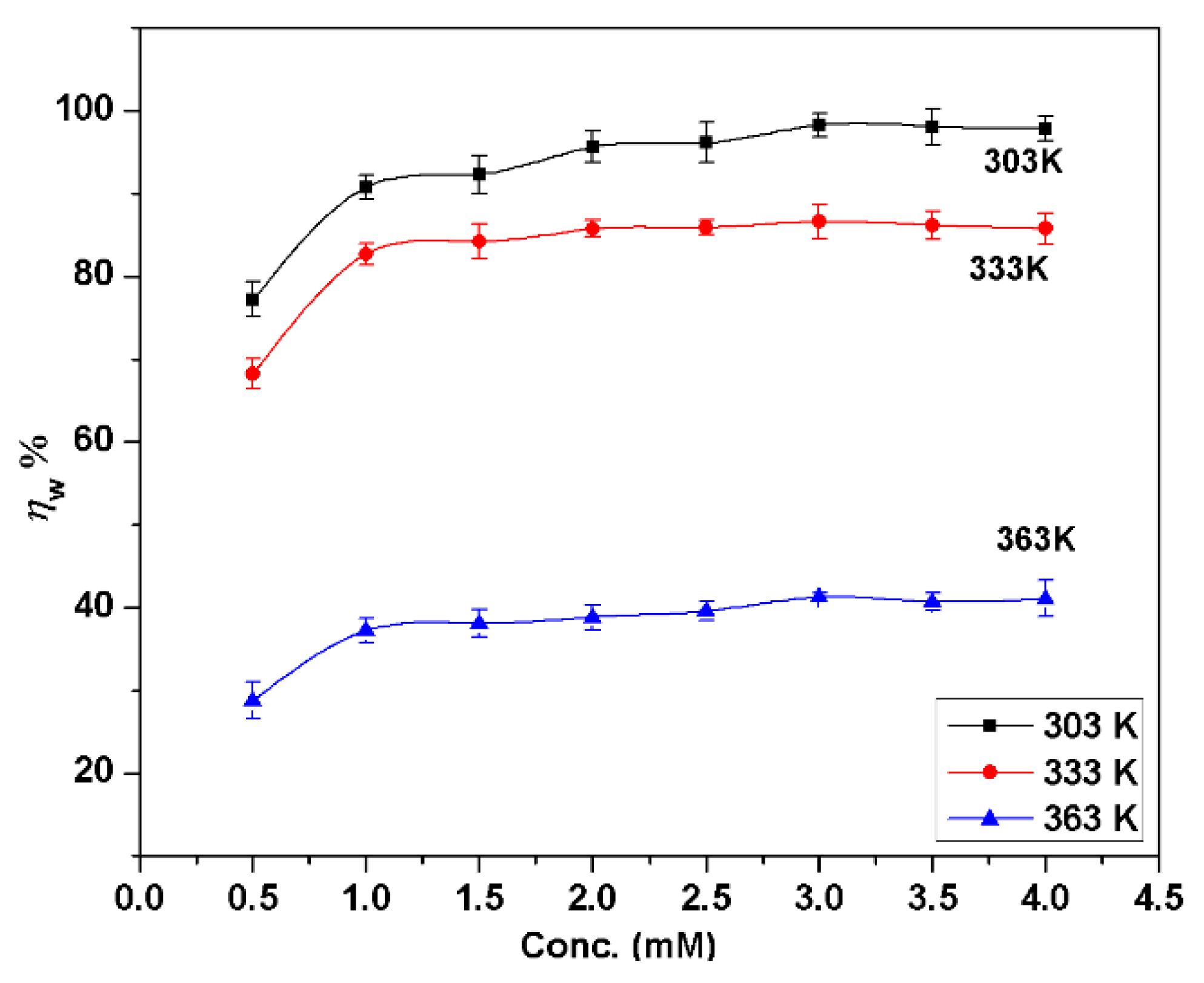
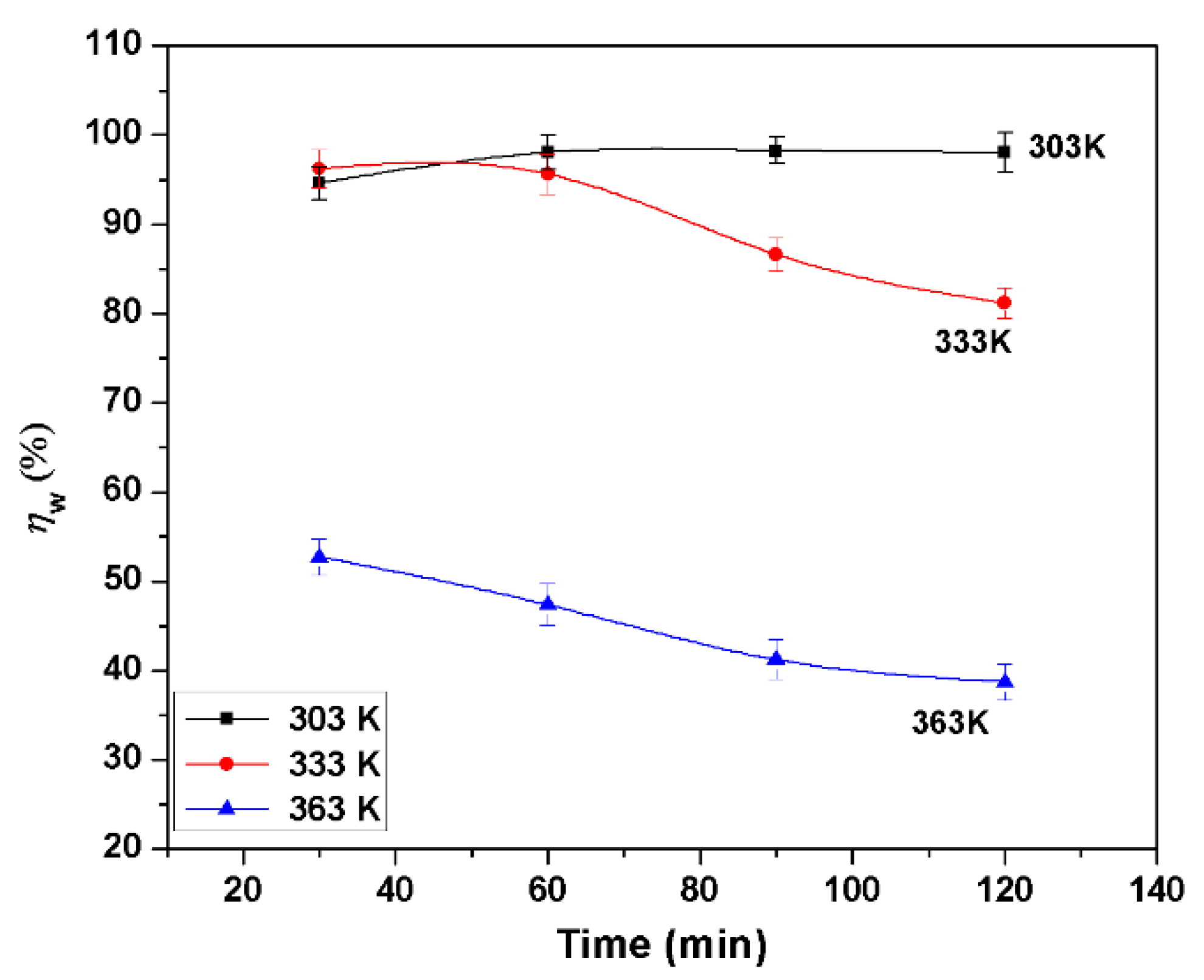
3.1. Gravimetric Investigations
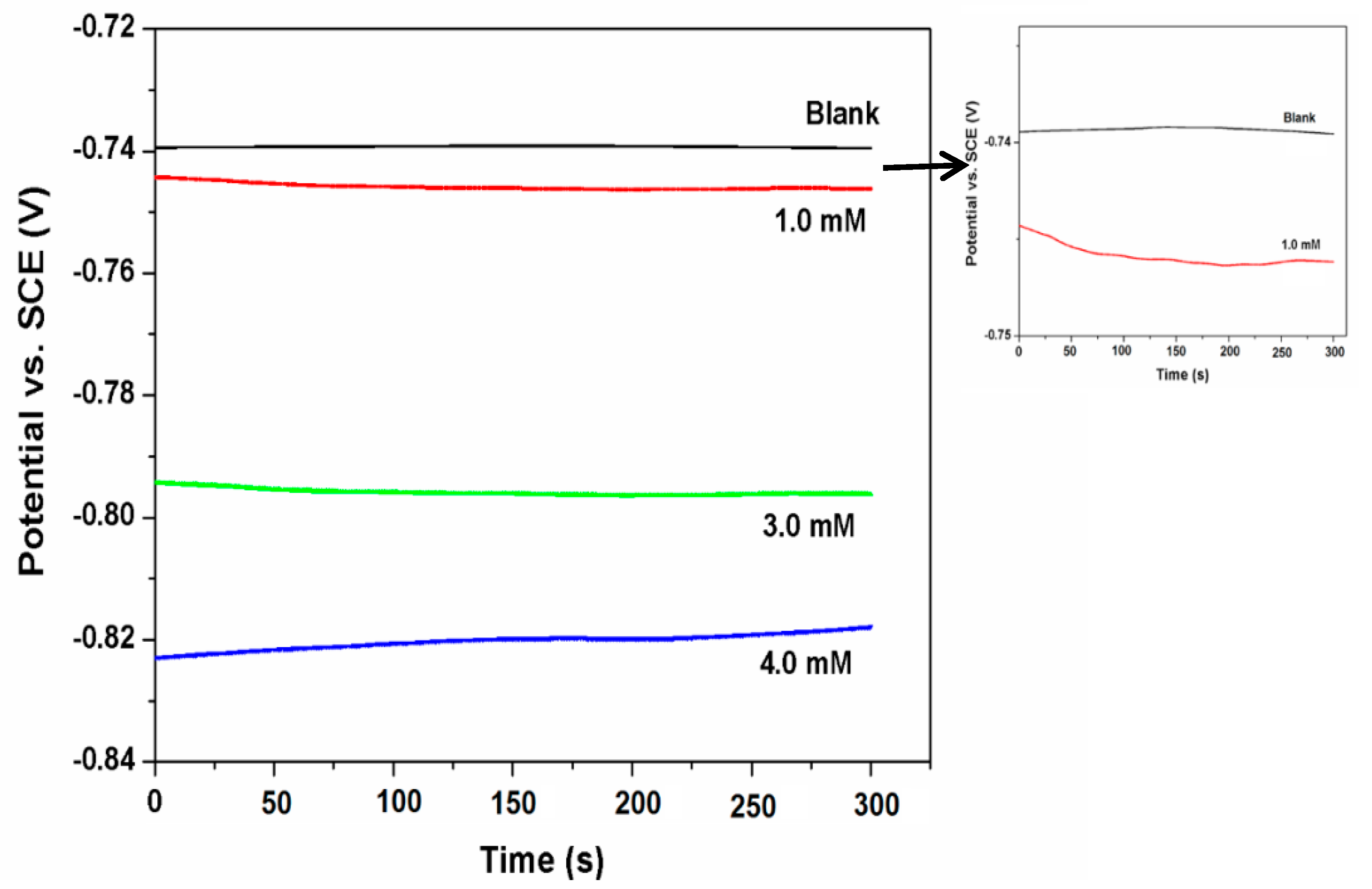
3.2. Open Circuit Potential (OCP)
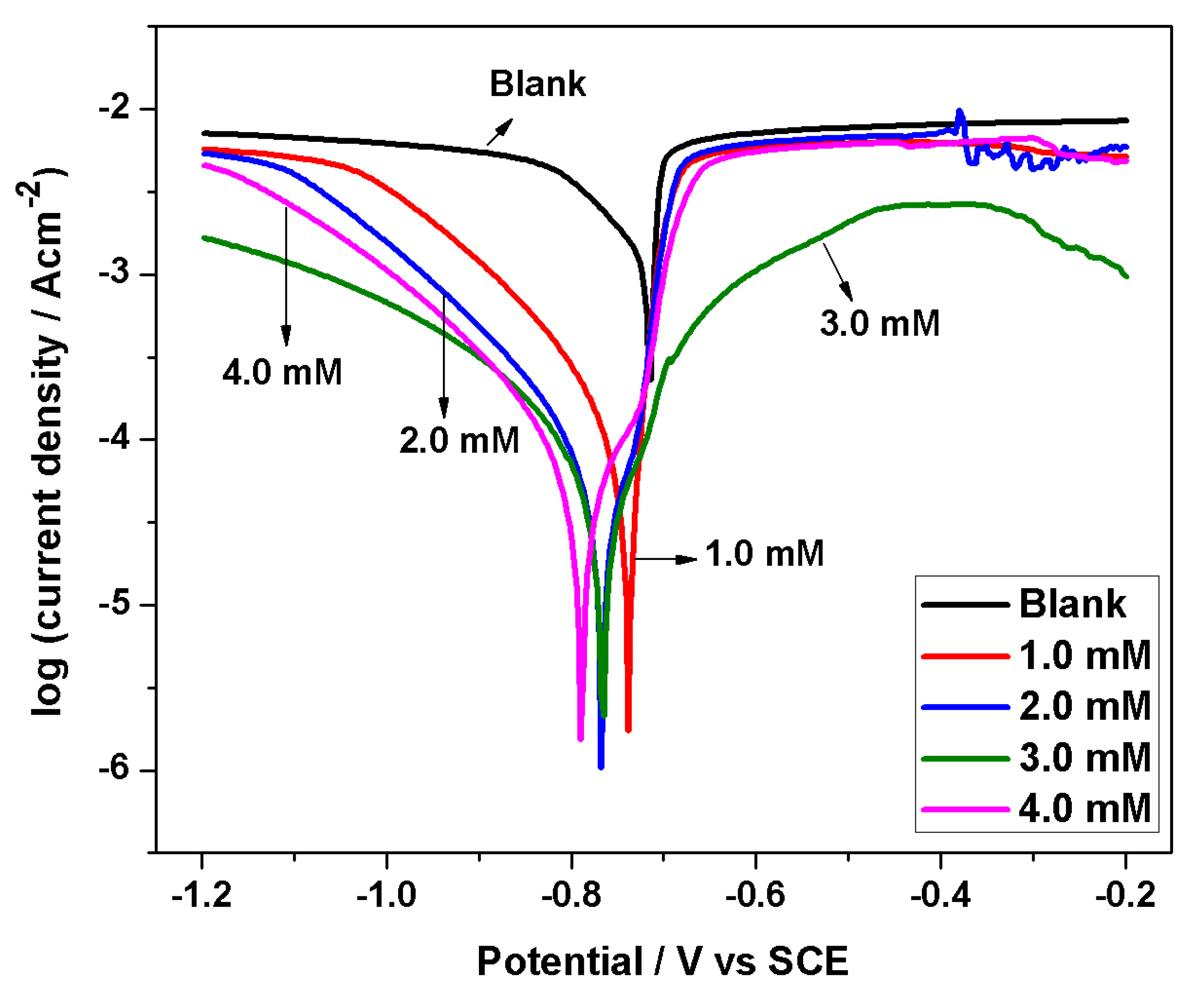
3.3. Potentiodynamic Polarization Study
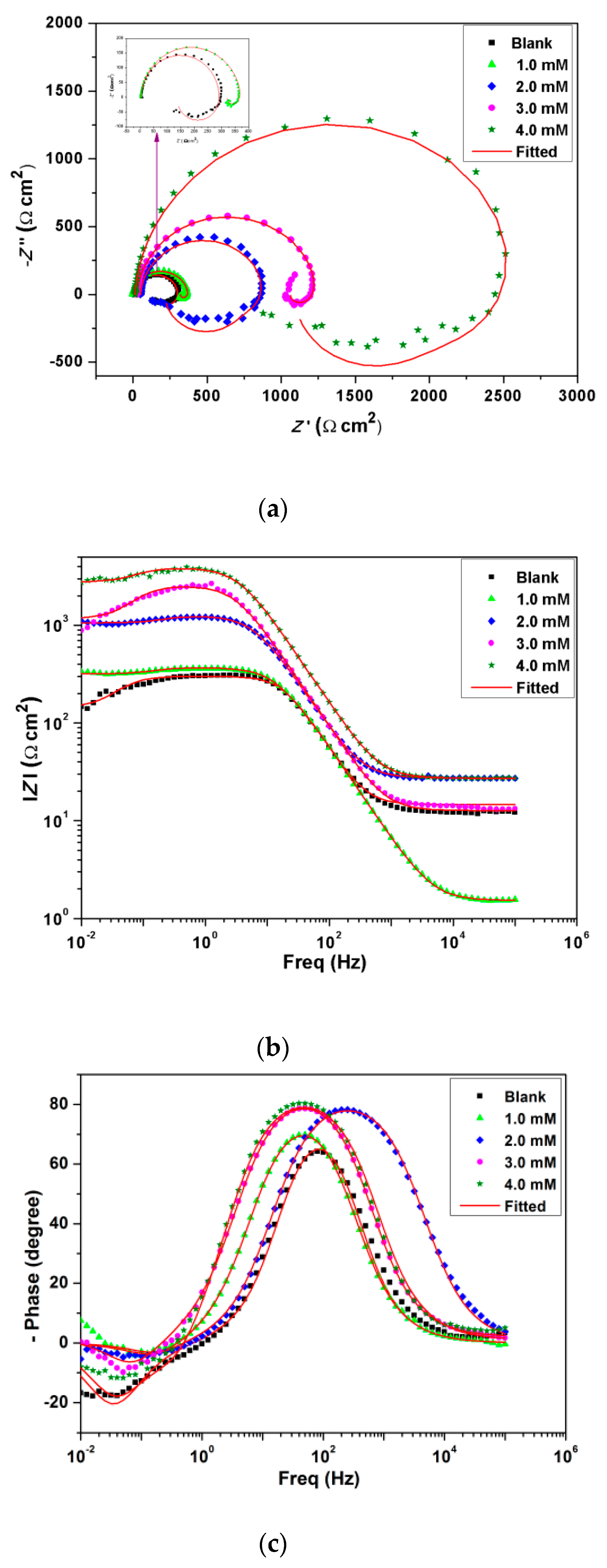
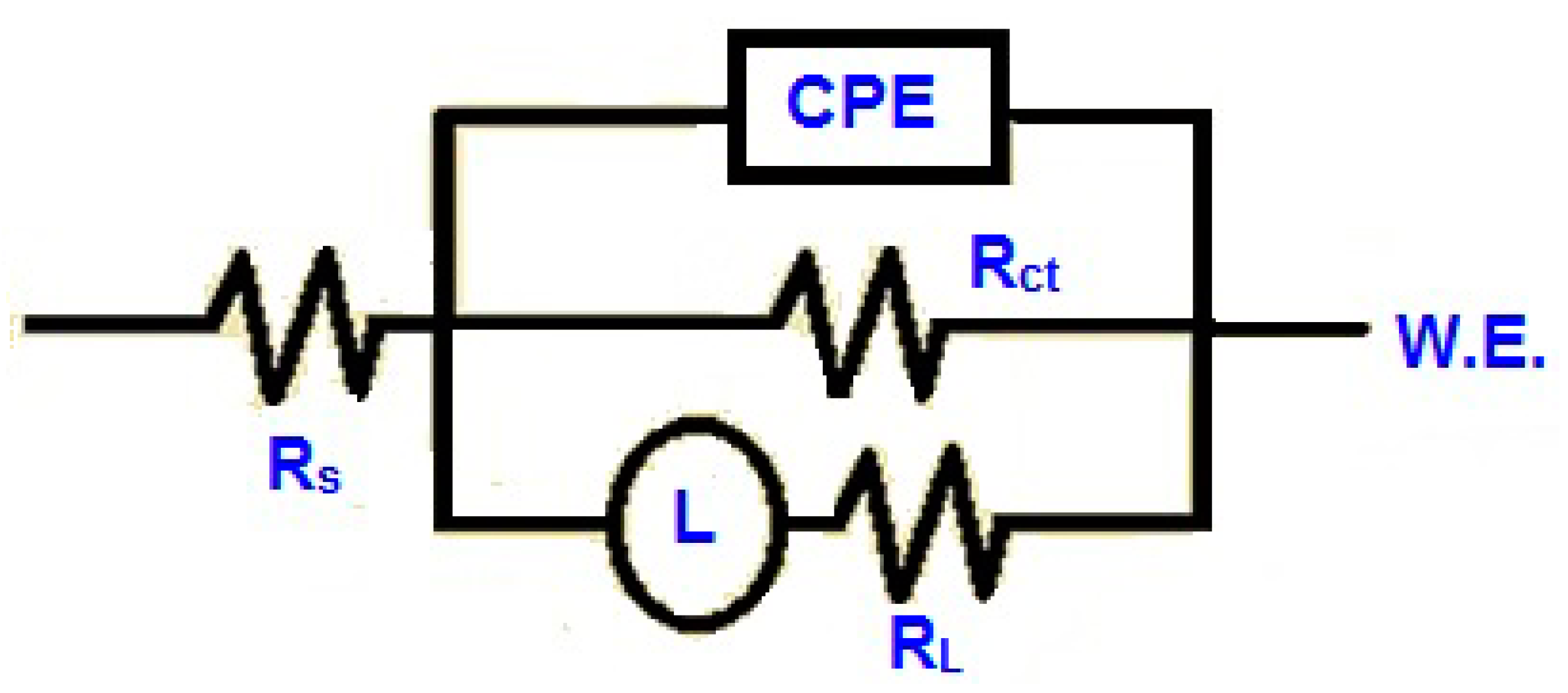
3.4. Electrochemical Impedance Spectroscopy (EIS) Analysis
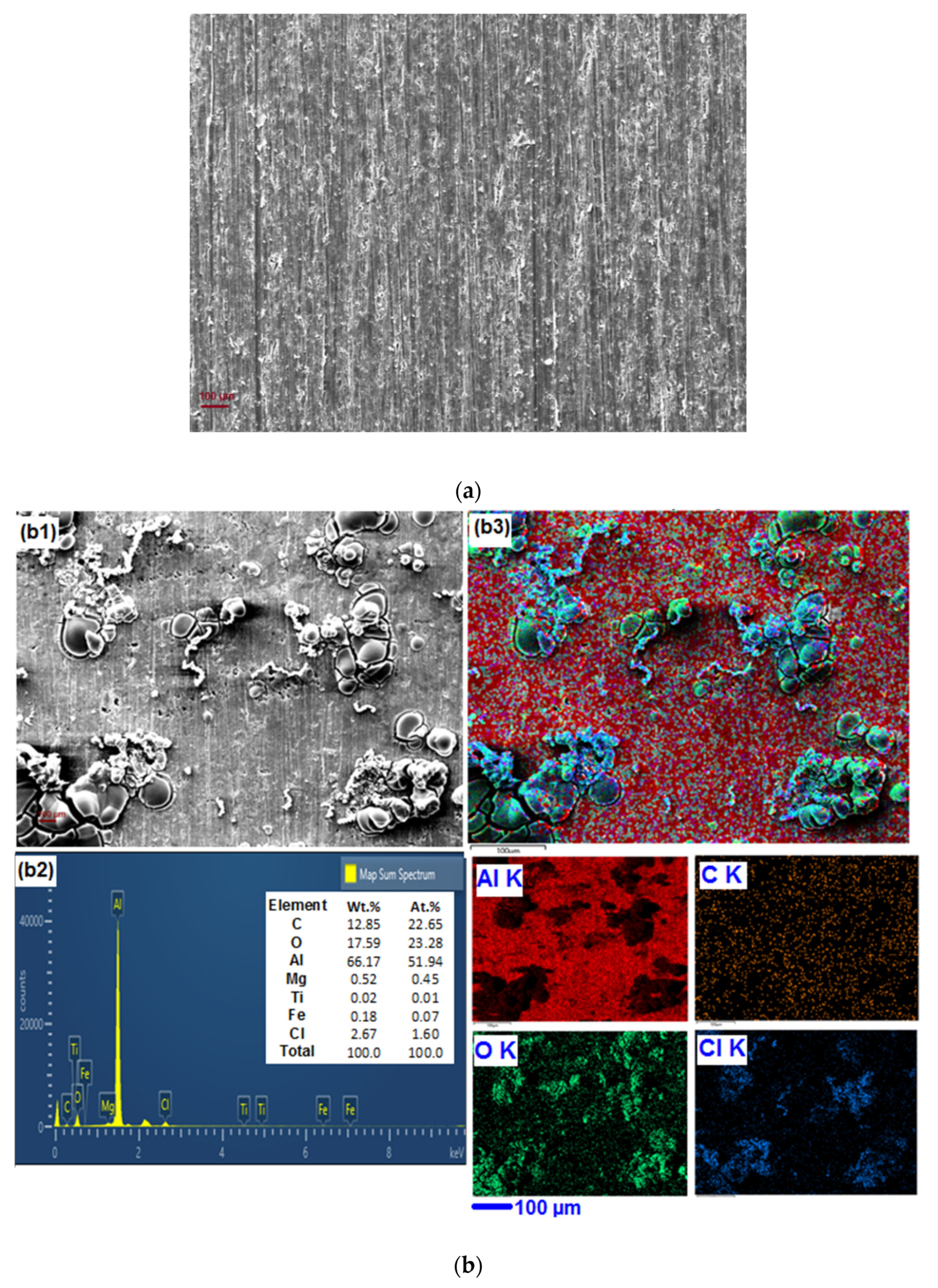
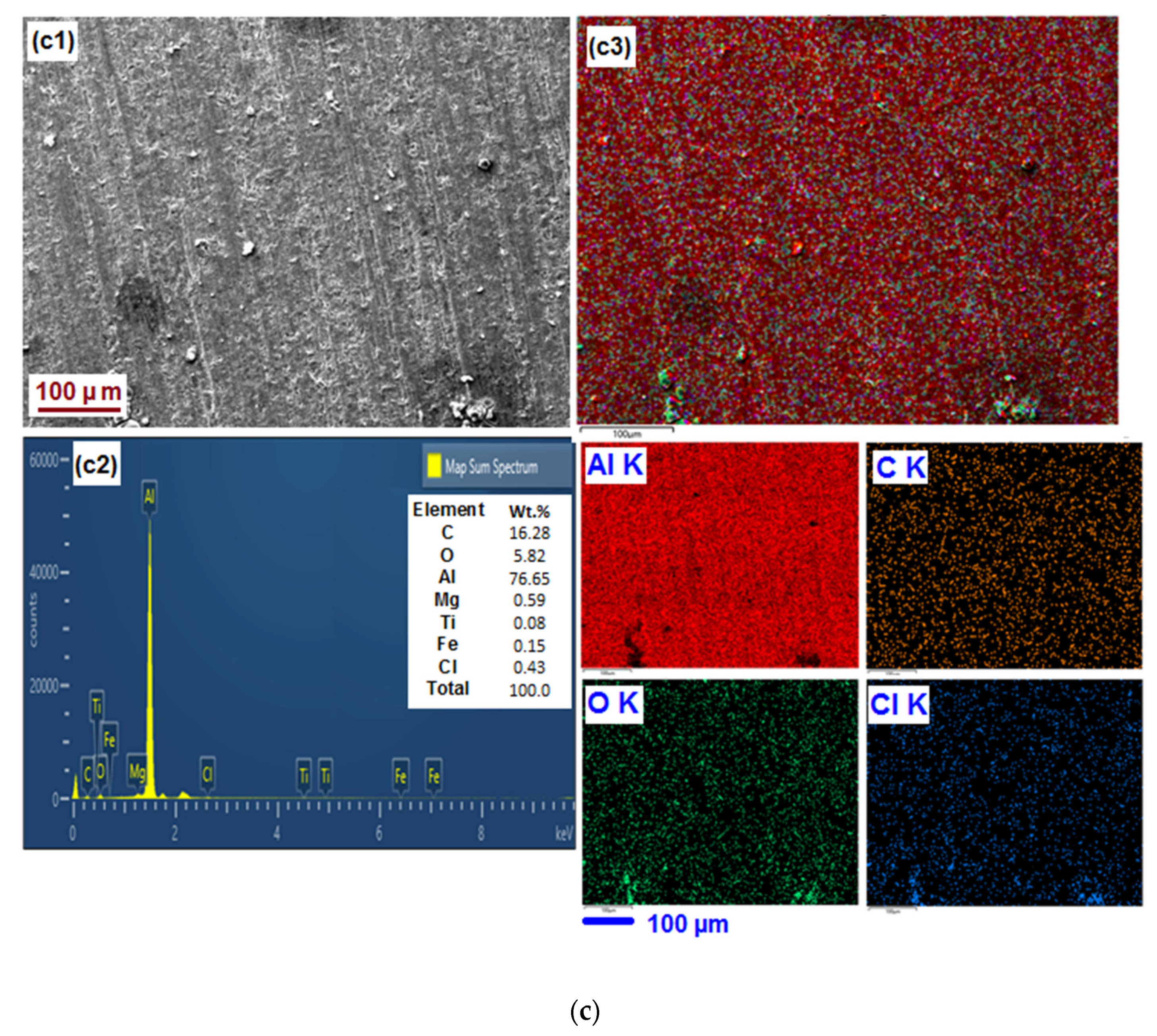
3.5. SEM-EDX Analyses and Elemental Mapping
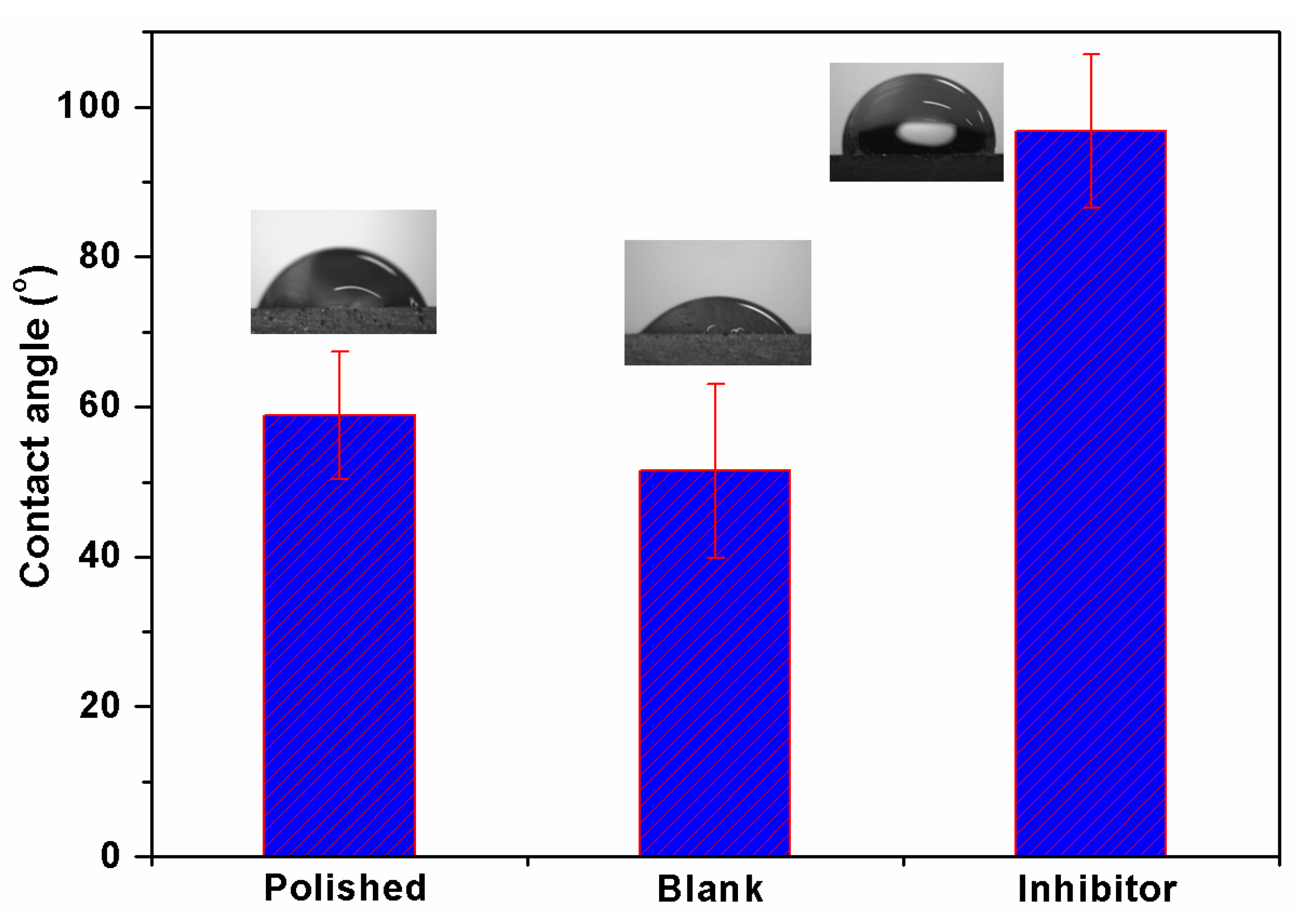
3.6. Contact Angle Analysis
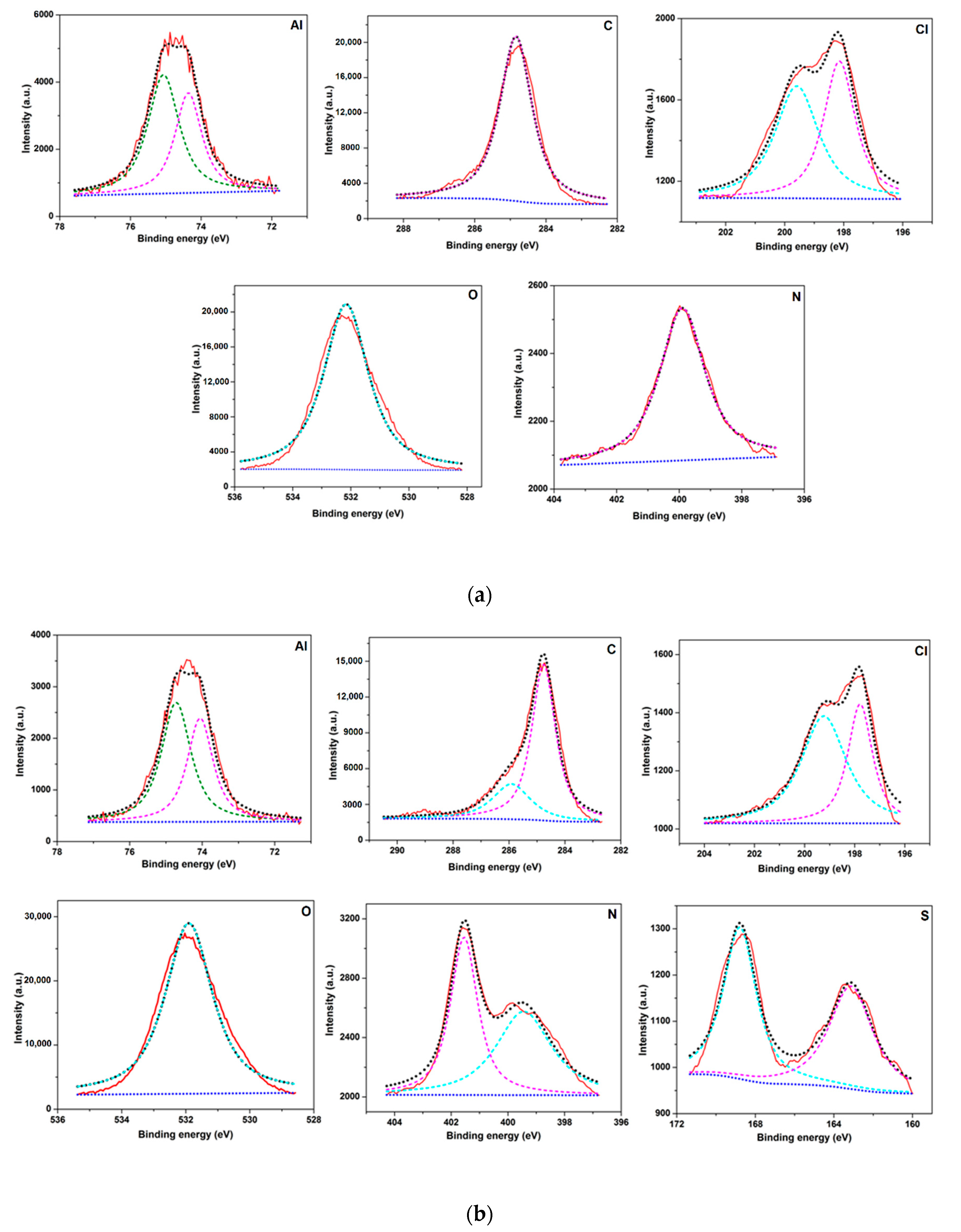
3.7. X-ray Photoelectron Spectroscopy (XPS) Analysis
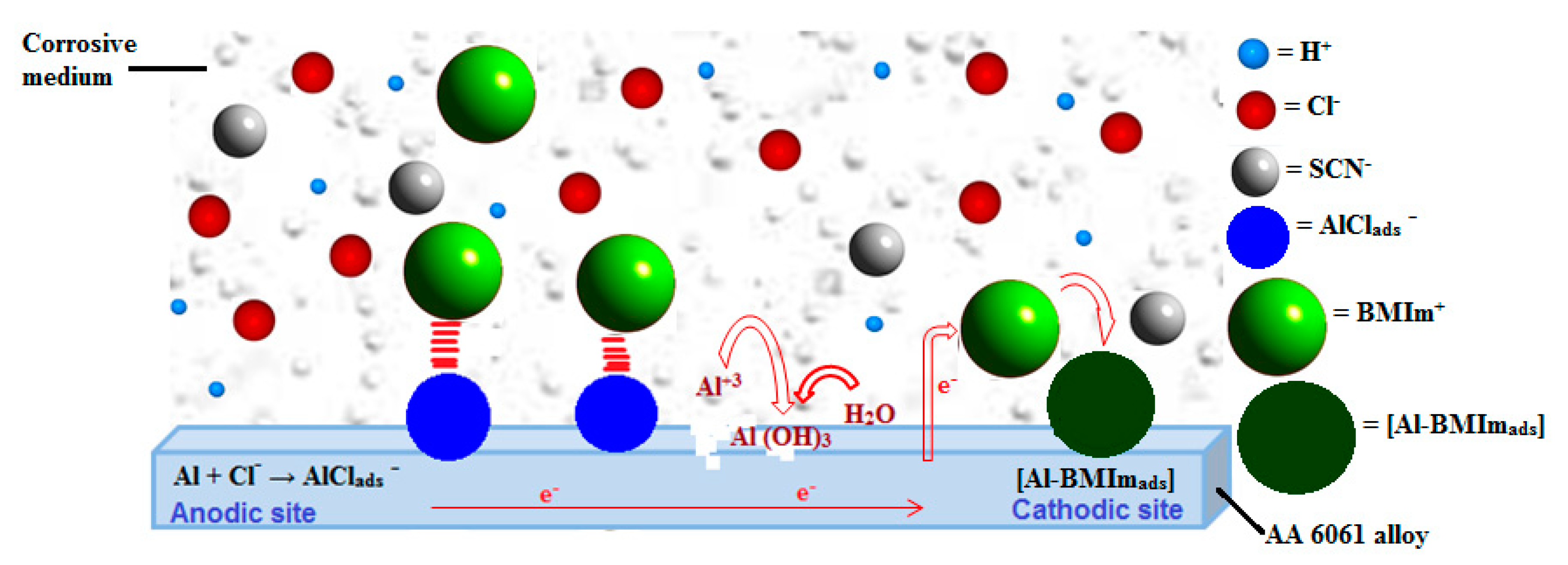
4. Discussion
5. Conclusions
- BMIm is an effective corrosion inhibitor for AA6061; the maximum inhibition efficiencies () were 98.2%, 86.6% and 41.2% at 303 K, 333 K, and 363 K, respectively, at 3.0 mM concentration. Inhibition efficiency increases with the inhibitor concentration, but decreases with the increase of temperature. increases with immersion time at 303 K from 30 to 90 min, and then decreases slightly but for other temperatures; it decreases regularly with an increase in immersion time. at 363 K was 71.4% if the samples had been preadsorbed in inhibitor and then tested.
- Polarization results show that BMIm can efficiently diminish corrosion of AA 6061 in HCl solution, and can be deemed as a mixed-type inhibitor with principal control on cathodic processes, thereby reducing the overall rate of corrosion. The EIS results indicate the development of a more protective passive film on the sample surface immersed in an acid solution containing inhibitor.
- Surface morphology analyses by SEM revealed less surface damage in the presence of inhibitor, verifying the effectiveness of the BMIm, while EDX and elemental mapping offers graphic and qualitative support to the results from experiments. A contact angle analysis suggested that the inhibited Al 6061 surface was hydrophobic in nature. XPS analysis established the formation of a protective film by the inhibitor on the AA6061 alloy surface.
- ILs have a promising future in the field of corrosion inhibition as green corrosion inhibitors for various alloys. Among the numerous existing ILs, imidazole-based ionic liquids have been most extensively used. More research needs to be carried out in other industrially relevant corrosive environments like CO2, H2S, and NaCl to determine the inhibitive behavior of IL-based compounds for various alloys in these environments.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.H.; Zhong, S.Y.; Song, Y.H.; Liu, H.; Huang, A.L.; Hu, Q.G.; Wang, G.R.; Chai, S.S. Effect of tantalum film on corrosion behaviour of AA6061 aluminium alloy in hydrochloric acid- and chloride-containing solutions. Trans. IMF 2020, 98, 243–249. [Google Scholar] [CrossRef]
- Goswami, R.; Lynch, S.; Holroyd, N.J.H.; Knight, S.P.; Holtz, R.L. Evolution of Grain Boundary Precipitates in Al 7075 Upon Aging and Correlation with Stress Corrosion Cracking Behavior. Metall. Mater. Trans. A 2013, 44A, 1268–1278. [Google Scholar] [CrossRef]
- Khaled, K.F.; Al-Qahtani, M.M. The inhibitive effect of some tetrazole derivatives towards Al corrosion in acid solution: Chemical electrochemical and theoretical studies. Mater. Chem. Phys. 2009, 113, 150–158. [Google Scholar] [CrossRef]
- Xhanariab, K.; Finšgar, M. Organic corrosion inhibitors for aluminium and its alloys in acid solutions: A review. RSC Adv. 2016, 6, 62833–62857. [Google Scholar] [CrossRef]
- Padash, R.; Rahimi-Nasrabadi, M.; Rad, A.S.; Sobhani-Nasab, A.; Jesionowski, T.; Ehrlich, H. A theoretical study of two novel Schiff bases as inhibitors of carbon steel corrosion in acidic medium. Appl. Phys. A 2019, 125, 78. [Google Scholar] [CrossRef]
- He, X.K.; Jiang, Y.M.; Li, C.; Wang, W.C.; Hou, B.L.; Wu, L.Y. Inhibition properties and adsorption behavior of imidazoleand 2-phenyl-2-imidazoline on AA5052 in 1.0 M HCl solution. Corros. Sci. 2014, 83, 124–136. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I.; Lekka, M.; Andreatta, F.; Fedrizzi, L. Corrosion behaviour and chemical stability of transparent hybrid sol-gel coatings deposited on aluminium in acidic and alkaline solutions. Prog. Org. Coat. 2018, 124, 286–295. [Google Scholar] [CrossRef]
- Deng, S.D.; Li, X.H. Inhibition by JasminumnudiflorumLindl leaves extract of the corrosion of aluminum in HCl solution. Corros. Sci. 2012, 64, 253–262. [Google Scholar] [CrossRef]
- Fares, M.M.; Maayta, A.K.; Al-Qudah, M.M. Pectin as promising green corrosion inhibitor of aluminum in hydrochloric acid solution. Corros. Sci. 2012, 60, 112–117. [Google Scholar] [CrossRef]
- Oguzie, E.E. Corrosion inhibition of aluminum in acidic and alkaline media by Sansevieriatrifasciata extract. Corros. Sci. 2007, 49, 1527–1539. [Google Scholar] [CrossRef]
- Palou, R.M.; Olivares-Xomelt, O.; Likhanova, N.V. Environmentally Friendly Corrosion Inhibitors. In Development in Corrosion Protection; IntechOpen: London, UK, 2014; pp. 431–465. [Google Scholar]
- Kowsari, E.; Arman, S.Y.; Shahini, M.H.; Zandi, H.; Ehsani, A.; Naderi, R.; Hanza, A.P.; Mehdipour, M. In situ synthesis, electrochemical and quantum chemical analysis of an amino acid-derived ionic liquid inhibitor for corrosion protection of mild steel in 1M HCl solution. Corros. Sci. 2016, 112, 73–85. [Google Scholar] [CrossRef]
- Shetty, S.K.; Shetty, A.N. Eco-friendly benzimidazolium based ionic liquid as a corrosion inhibitor for aluminum alloy composite in acidic media. J. Mol. Liq. 2017, 225, 426–438. [Google Scholar] [CrossRef]
- Uerdingen, M.; Treber, C.; Balser, M.; Schmitt, G.; Werner, C. Corrosion behavior of ionic liquids. Green Chem. 2005, 7, 321–325. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are ionic liquids chemically stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.M.; Zhang, Y.W.; Zhou, X.Q.; Cao, Y.Y.; Mu, T.C. Thermal stabilities and decomposition mechanism of amino-and hydroxyl-functionalized ionic liquids. Thermochim. Acta 2014, 578, 59–67. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Efimova, A.; Varga, J.; Matuschek, G.; Saraji-Bozorgzad, M.R.; Denner, T.; Zimmermann, R.; Schmidt, P. Thermal Resilience of Imidazolium-Based Ionic Liquids—Studies on Short- and Long-Term Thermal Stability and Decomposition Mechanism of 1-Alkyl-3-methylimidazolium Halides by Thermal Analysis and Single-Photon Ionization Time-of-Flight Mass Spectrometry. J. Phys. Chem. B 2018, 122, 8738–8749. [Google Scholar] [CrossRef]
- Qiang, Y.J.; Zhang, S.T.; Guo, L.; Zheng, X.W.; Xiang, B.; Chen, S.J. Experimental and theoretical studies of four allylimidazolium-based ionic liquids as green inhibitors for copper corrosion in sulfuric acid. Corros. Sci. 2017, 119, 68–78. [Google Scholar] [CrossRef]
- Trombetta, F.; de Souza, R.F.; de Souza, M.O.; Borges, C.B.; Panno, N.F.; Agostini Martini, E.M. Stability of aluminium in 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid and ethylene glycol mixtures. Corros. Sci. 2011, 53, 51–58. [Google Scholar] [CrossRef]
- Wu, Q.L.; Zhang, Z.H.; Dong, X.M.; Yang, J.Q. Corrosion behavior of low-alloy steel containing 1% chromium in CO2 environments. Corros. Sci. 2013, 75, 400–408. [Google Scholar] [CrossRef]
- Bousskri, A.; Anejjar, A.; Messali, M.; Salghi, R.; Benali, O.; Karzazi, Y.; Jodeh, S.; Zougagh, M.; Ebenso, E.E.; Hammouti, B. Corrosion inhibition of carbon steel in aggressive acidic media with 1-(2-(4-chlorophenyl)-2-oxoethyl)pyridaziniumbromide. J. Mol. Liq. 2015, 211, 1000–1008. [Google Scholar] [CrossRef]
- Ibrahim, M.A.M.; Messali, M.; Moussa, Z.; Alzahrani, A.Y.; Alamry, S.N.; Hammouti, B. Corrosion inhibition of carbon steel by imidazolium and pyridiniumcations ionic liquids in acidic environment. Port. Electrochim. Acta 2011, 29, 375–389. [Google Scholar] [CrossRef]
- Sherif, E.M.; Abdo, H.S.; Abedin, S.Z.E. Corrosion inhibition of cast iron in Arabian gulf seawater by two different ionic liquids. Materials 2015, 8, 3883–3895. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.C.; Manh, T.L.; Sierra, R.C.; Flores, J.V.M.; Rojas, L.L.; Estrada, E.M.A. Study of corrosion behavior of API 5L X52 steel in sulfuric acid in the presence of ionic liquid 1-ethyl 3-methylimidazolium thiocyanate as corrosion inhibitor. J. Mol. Liq. 2019, 289, 111106. [Google Scholar] [CrossRef]
- Li, X.H.; Deng, S.D.; Fu, H. Inhibition by tetradecylpyridinium bromide of the corrosion of aluminium in hydrochloric acid solution. Corros. Sci. 2011, 53, 1529–1536. [Google Scholar] [CrossRef]
- Zhao, T.P.; Mu, G.N. The adsorption and corrosion inhibition of anion surfactants on aluminium surface in hydrochloric acid. Corros. Sci. 1999, 41, 1937–1944. [Google Scholar] [CrossRef]
- Li, X.H.; Deng, S.D.; Xie, X.G. Experimental and theoretical study on corrosion inhibition of o-phenanthroline for aluminum in HCl solution. J. Taiwan Inst. Chem. E 2014, 45, 1865–1875. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Shabani, B.; Aligholipour, B.; Seifzadeh, D. The effect of some Schiff bases on the corrosion of aluminum in hydrochloric acid solution. Appl. Surf. Sci. 2006, 252, 4039–4047. [Google Scholar] [CrossRef]
- Cao, C.N. Corrosion Electrochemistry Mechanism, 2nd ed.; Chemical Industrial Engineering Press: Beijing, China, 2004. [Google Scholar]
- Mousavi, M.; Mohammadalizadeh, M.; Khosravan, A. Theoretical investigation of corrosion inhibition effect of imidazole and its derivatives on mild steel using cluster model. Corros. Sci. 2011, 53, 3086–3091. [Google Scholar] [CrossRef]
- Abdallah, M.; Sobhi, M.; Altass, H.M. Corrosion inhibition of aluminum in hydrochloric acid by pyrazinamide derivatives. J. Mol. Liq. 2016, 223, 1143–1150. [Google Scholar] [CrossRef]
- Arukalam, I.O.; Madu, I.O.; Ijomah, N.T.; Ewulonu, C.M.; Onyeagoro, G.N. Acid corrosion inhibition and adsorption behavior of ethyl hydroxyethyl cellulose on mild steel corrosion. J. Mater. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Pinto, G.M.; Nayak, J.; Shetty, A.N. Corrosion inhibition of 6061 Al–15 vol. pct. SiC(p) composite and its base alloy in a mixture of sulfuric acid and hydrochloric acid by4-(N,N-dimethylamino) benzaldehyde thiosemicarbazone. Mater. Chem. Phys. 2011, 125, 628–640. [Google Scholar] [CrossRef]
- Yurt, A.; Ulutas, S.; Dal, H. Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some Schiff bases. Appl. Surf. Sci. 2006, 253, 919–925. [Google Scholar] [CrossRef]
- Bessone, J.; Mayer, C.; Jutter, K.; Lorenz, W. AC-impedance measurements on aluminium barrier type oxide films. Electrochim. Acta 1983, 28, 171–175. [Google Scholar] [CrossRef]
- Brett, C.M.A. On the electrochemical behaviour of aluminium in acidic chloride solution. Corros. Sci. 1992, 33, 203–210. [Google Scholar] [CrossRef]
- MetikoŠ-Hukovi, C.M.; Babi, C.R.; Gruba, C.Z. Corrosion protection of aluminum in acidic chloride solutions with nontoxic inhibitors. J. Appl. Electrochem. 1998, 28, 433–439. [Google Scholar] [CrossRef]
- Abd El Rehim, S.S.; Hassan, H.H.; Amin, M.A. Corrosion inhibition of aluminum by 1,1(lauryl amido) propyl ammonium chloride in HCl solution. Mat. Chem. Phys. 2001, 70, 64–72. [Google Scholar] [CrossRef]
- Noor, E.A. Evaluation of inhibitive action of some quaternary N-heterocyclic compounds on the corrosion of Al–Cu alloy in hydrochloric acid. Mat. Chem. Phys. 2009, 114, 533–541. [Google Scholar] [CrossRef]
- Bessone, J.B.; Salinas, D.R.; Mayer, C.E.; Ebert, M.; Lorenz, W.J. An EIS study of aluminium barrier-type oxide films formed in different media. Electrochim. Acta 1992, 37, 2283–2290. [Google Scholar] [CrossRef]
- Flores, E.N.; Chong, Z.; Omanovic, S. Characterization of Ni, NiMo, NiW and NiFe electroactive coatings as electrocatalysts for hydrogen evolution in an acidic medium. J. Mol. Catal. A Chem. 2005, 226, 179–197. [Google Scholar] [CrossRef]
- McCafferty, E.; Hackerman, N. Double Layer Capacitance of Iron and Corrosion Inhibition with Polymethylene Diamines. J. Electrochem. Soc. 1972, 119, 146. [Google Scholar] [CrossRef]
- Lagrenee, M.; Mernari, B.; Bouanis, M.; Traisnel, M.; Bentiss, F. Study of the mechanism and inhibiting efficiency of 3,5-bis(4-metylthiophenyl)-4H-1,2,4-triazole on mild steel corrosion in acidic media. Corros. Sci. 2002, 44, 573–588. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Akbarian, M.; Karati, M.R.; Mahdavian, M.; Alibakhshi, E.; Kardar, P. Corrosion protection of steel with zinc phosphate conversion coating andpost-treatment by hybrid organic-inorganic sol-gel based silane film. J. Electrochem. Soc. 2017, 164, C224–C230. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Milić, S.M.; Petrović, M.B. Films formed on copper surface in chloride media in the presence of azoles. Corros. Sci. 2009, 51, 1228–1237. [Google Scholar] [CrossRef]
- Tamilarasan, R.; Sreekanth, A. Spectroscopic and DFT investigations on the corrosion inhibition behavior of tris (5-methyl-2-thioxo-1,3,4-thiadiazole) borate on high carbon steel and aluminium in HCl media. RSC Adv. 2013, 3, 23681–23691. [Google Scholar] [CrossRef]
- Piao, H.; McIntyre, N.S. Adventitious carbon growth on aluminium and gold-aluminium alloy surfaces. Surf. Interface Anal. 2002, 33, 591–594. [Google Scholar] [CrossRef]
- Barr, T.L.; Seal, S. Nature of the use of adventitious carbon as a binding energy standard. J. Vac. Sci. Technol. Vac. Surf. Film. 1995, 13, 1239–1246. [Google Scholar] [CrossRef]
- Ryl, J.; Wysocka, J.; Cieslik, M.; Gerengi, H.; Ossowski, T.; Krakowiak, S.; Niedzialkowski, P. Understanding the origin of high corrosion inhibition efficiency of beeproducts towards aluminium alloys in alkaline environments. Electrochim. Acta 2019, 304, 263–274. [Google Scholar] [CrossRef]
- Zarrok, H.; Zarrouk, A.; Hammouti, B.; Salghi, R.C.; Bentiss, J.M. Corrosion control of carbon steel in phosphoric acid by purpald—Weight loss, electrochemical and XPS studies. Corros. Sci. 2012, 64, 243–252. [Google Scholar] [CrossRef]
- Tourabi, M.; Nohair, K.; Traisnel, M.; Jama, C.; Bentiss, F. Electrochemical and XPS studies of the corrosion inhibition of carbon steel in hydrochloric acid pickling solutions by 3,5-bis(2-thienylmethyl)-4-amino-1,2,4-triazole. Corros. Sci. 2013, 75, 123–133. [Google Scholar] [CrossRef]
- Arellanes-Lozada, P.; Olivares-Xometl, O.; Likhanova, N.V.; Lijanova, I.V.; Vargas-García, J.R.; Hernández-Ramírez, R.E. Adsorption and performance of ammonium-based ionic liquids as corrosion inhibitors of steel. J. Mol. Liq. 2018, 265, 151–163. [Google Scholar] [CrossRef]
- Likhanova, N.V.; Domínguez-Aguilar, M.A.; Olivares-Xometl, O.; Nava-Entzana, N.; Arce, E.; Dorantes, H. The effect of ionic liquids with imidazolium and pyridiniumcations on the corrosion inhibition of mild steel in acidic environment. Corros. Sci. 2010, 52, 2088–2097. [Google Scholar] [CrossRef]
- Zhang, D.S.; Li, L.D.; Cao, L.X.; Yang, N.F.; Huang, C.B. Studies of corrosion inhibitors for zinc–manganese batteries: Quinoline quaternary ammonium phenolates. Corros. Sci. 2001, 43, 1627–1636. [Google Scholar] [CrossRef]
- Bereket, G.; Pinarbsi, A. Electrochemical thermodynamic and kinetic studies of the behaviour of aluminium in hydrochloric acid containing various benzotriazole derivatives. Corros. Eng. Sci. Technol. 2004, 39, 308–312. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Petrovic, M.B. Copper corrosion inhibitors. Int. J. Electrochem. Sci. 2008, 3, 1–28. [Google Scholar]
- Fuchs-Godec, R. The adsorption, CMC determination and corrosion inhibition of some N-alkyl quaternary ammonium salts on carbon steel surface in 2M H2SO4. Colloid Surf. A 2006, 280, 130–139. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Vishal, Y.; Quraishi, M.A. Dendrimers: A new class of corrosion inhibitors for mild steel in 1M HCl: Experimental and quantum chemical studies. J. Mol. Liq. 2016, 224, 1282–1293. [Google Scholar] [CrossRef]
- Haque, J.; Srivastava, V.; Verma, C.; Quraishi, M.A. Propionic Acid as New and Green Corrosion Inhibitor for Mild Steel in 1 M Hydrochloric Acid Solution. J. Mol. Liq. 2017, 225, 848–855. [Google Scholar] [CrossRef]
- Thirumalairaj, B.; Jaganathan, M. Corrosion protection of mild steel by a new binary inhibitor system in hydrochloric acid solution. Egypt. J. Pet. 2016, 25, 423–432. [Google Scholar] [CrossRef]
| Fe | Si | Cu | Mn | Mg | Ti | Zn | Al |
|---|---|---|---|---|---|---|---|
| 0.15 | 0.53 | 0.26 | 0.06 | 0.92 | 0.04 | 0.02 | Bal. |
| Inhibitor Conc. (mM) | Ecorr (V vs. SCE) | Icorr (µAcm−2) | βc (mV dec−1) | βa (mV dec−1) | Corrosion Rate (mmy−1) | Polarization Resistance (Ω) | IE (%) |
|---|---|---|---|---|---|---|---|
| 0 | −0.71 | 1030 | 80 | 139 | 12.06 | 4.9 | -- |
| 1 | −0.73 | 59.3 | 98 | 21 | 0.689 | 124.6 | 94.2 |
| 2 | −0.77 | 29.5 | 77 | 40 | 0.271 | 504.3 | 97.1 |
| 3 | −0.76 | 22.3 | 68 | 90 | 0.254 | 582.0 | 97.8 |
| 4 | −0.79 | 20.7 | 60 | 52 | 0.223 | 665.6 | 98.0 |
| BMIm Conc. (mM) | Rs (Ω cm2) | CPE (μF cm−2) | Rct (Ω cm2) | Rp (Ω cm2) | Cdl (μF cm−2) | χ2 | η (%) |
|---|---|---|---|---|---|---|---|
| Blank | 17.8 | 9.3 × 10−5 | 39.1 | 18.74 | 4.7 × 10−5 | 0.0035 | - |
| 1.0 | 15.2 | 3.9 × 10−5 | 3.7 × 102 | 317.7 | 2.8 × 10−5 | 0.0022 | 94.0 |
| 2.0 | 23.6 | 2.0 × 10−5 | 7.8 × 102 | 672.1 | 1.7 × 10−5 | 0.0029 | 97.2 |
| 3.0 | 27.3 | 2.2 × 10−5 | 1.2 × 103 | 1032.3 | 1.6 × 10−5 | 0.0011 | 98.2 |
| 4.0 | 27.2 | 1.3 × 10−5 | 3.8 × 103 | 2756.9 | 9.1 × 10−6 | 0.0032 | 98.4 |
| Element | 1 M HCl | 1 M HCl + 3 mM IL |
|---|---|---|
| Al | 12.3 | 13.6 |
| C | 52.6 | 41.6 |
| Cl | 1.6 | 0.98 |
| O | 31.8 | 39.3 |
| N | 1.2 | 03.3 |
| S | 0.4 | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Huang, A.; Lin, D.; Talha, M.; Liu, H.; Lin, Y. Imidazolium-Based Ionic Liquid as Efficient Corrosion Inhibitor for AA 6061 Alloy in HCl Solution. Materials 2020, 13, 4672. https://doi.org/10.3390/ma13204672
Wang X, Huang A, Lin D, Talha M, Liu H, Lin Y. Imidazolium-Based Ionic Liquid as Efficient Corrosion Inhibitor for AA 6061 Alloy in HCl Solution. Materials. 2020; 13(20):4672. https://doi.org/10.3390/ma13204672
Chicago/Turabian StyleWang, Xiaohong, Ailing Huang, Dongquan Lin, Mohd Talha, Hao Liu, and Yuanhua Lin. 2020. "Imidazolium-Based Ionic Liquid as Efficient Corrosion Inhibitor for AA 6061 Alloy in HCl Solution" Materials 13, no. 20: 4672. https://doi.org/10.3390/ma13204672
APA StyleWang, X., Huang, A., Lin, D., Talha, M., Liu, H., & Lin, Y. (2020). Imidazolium-Based Ionic Liquid as Efficient Corrosion Inhibitor for AA 6061 Alloy in HCl Solution. Materials, 13(20), 4672. https://doi.org/10.3390/ma13204672






