Consequences of Different Mechanical Surface Preparation of Ni-Base Alloys during High Temperature Oxidation
Abstract
1. Introduction
2. Materials and Methods
3. Results
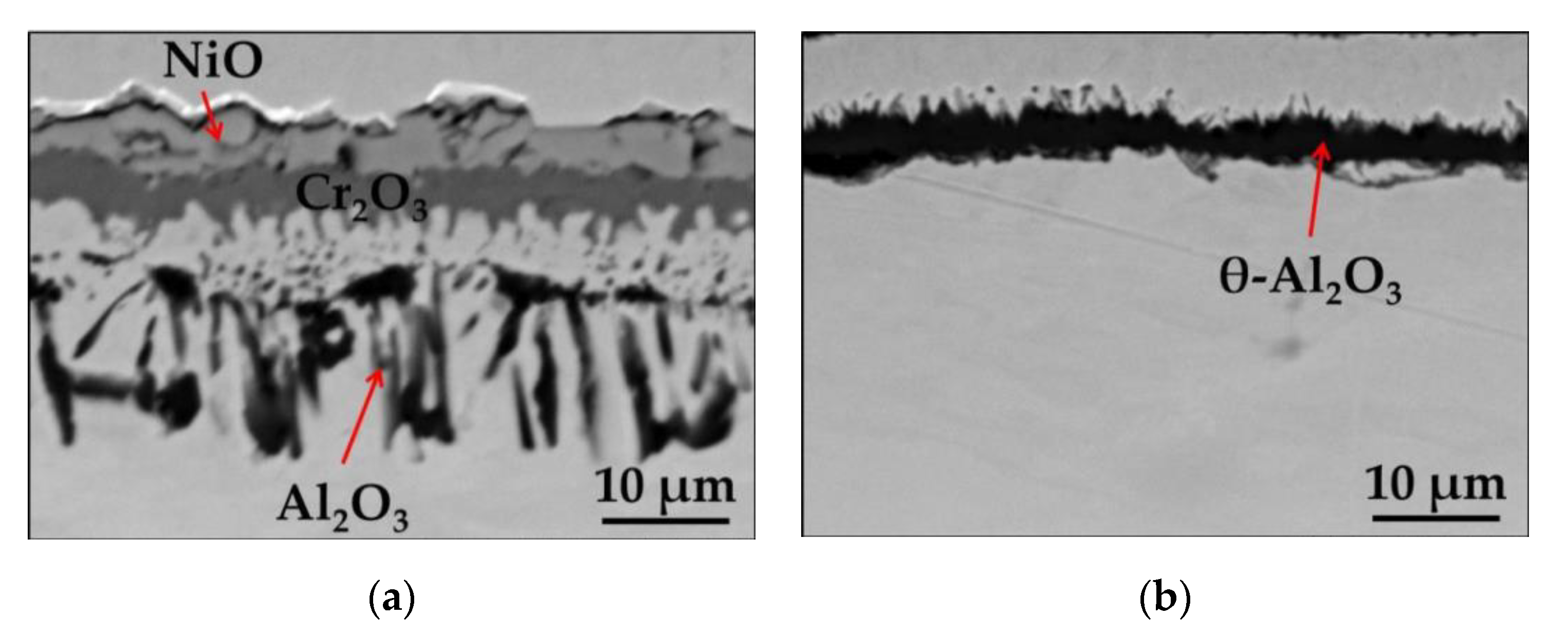
3.1. Oxidation of Commercial Ni-Base Superalloy MAR 247
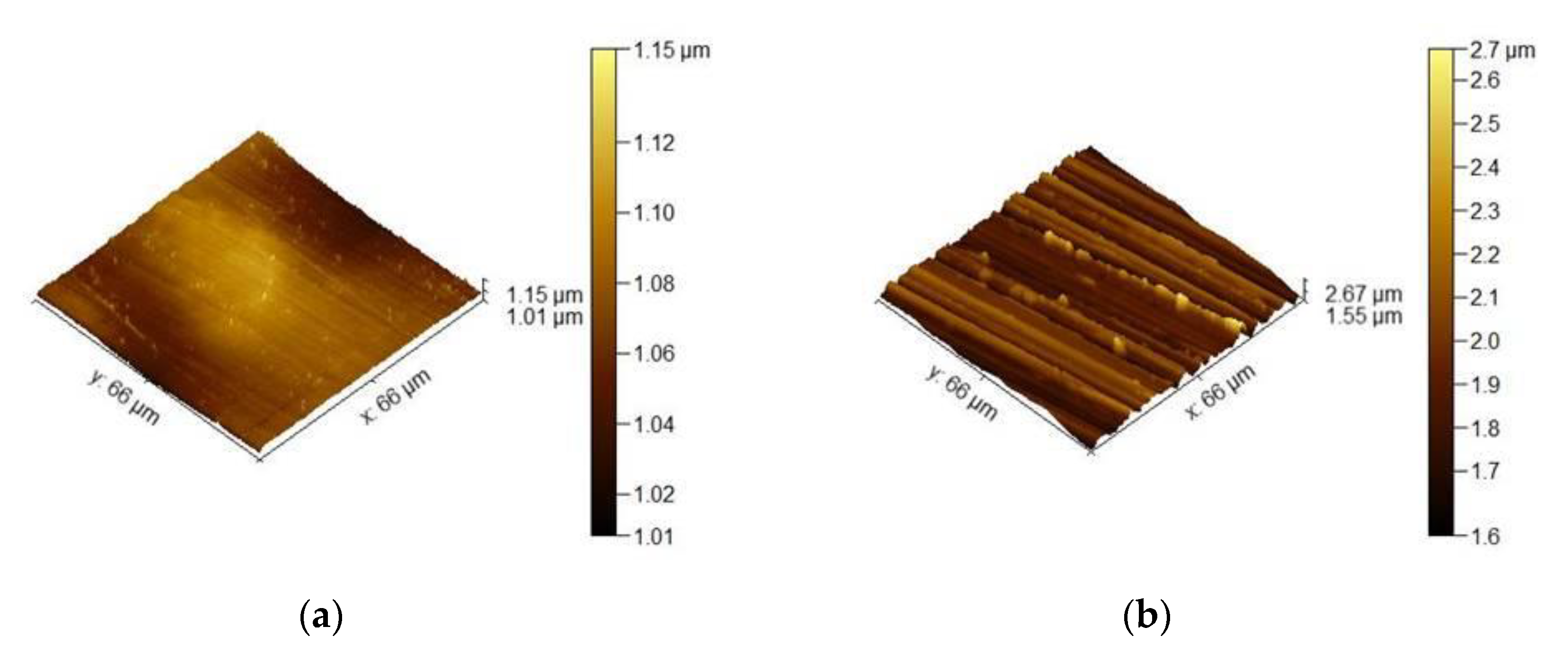
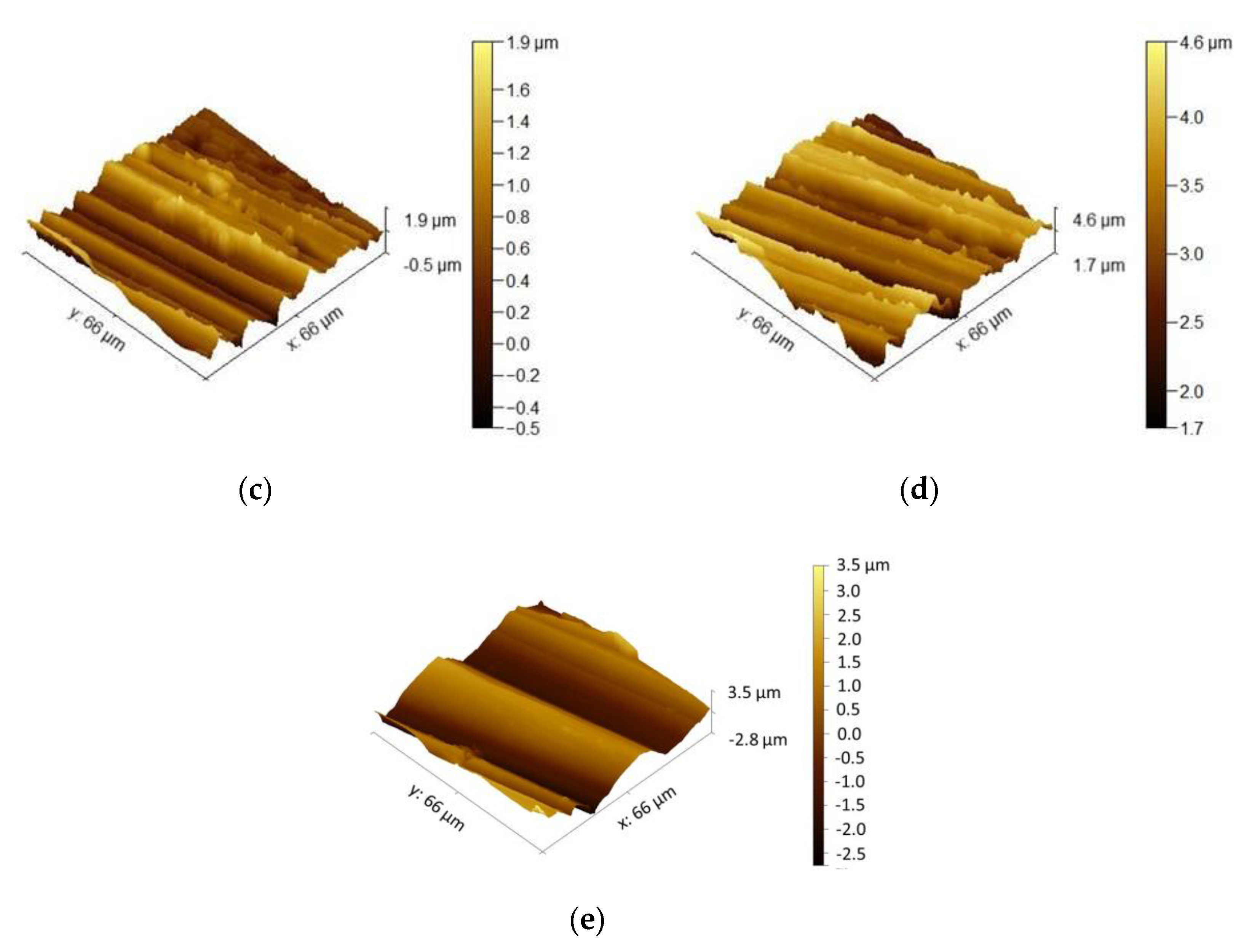
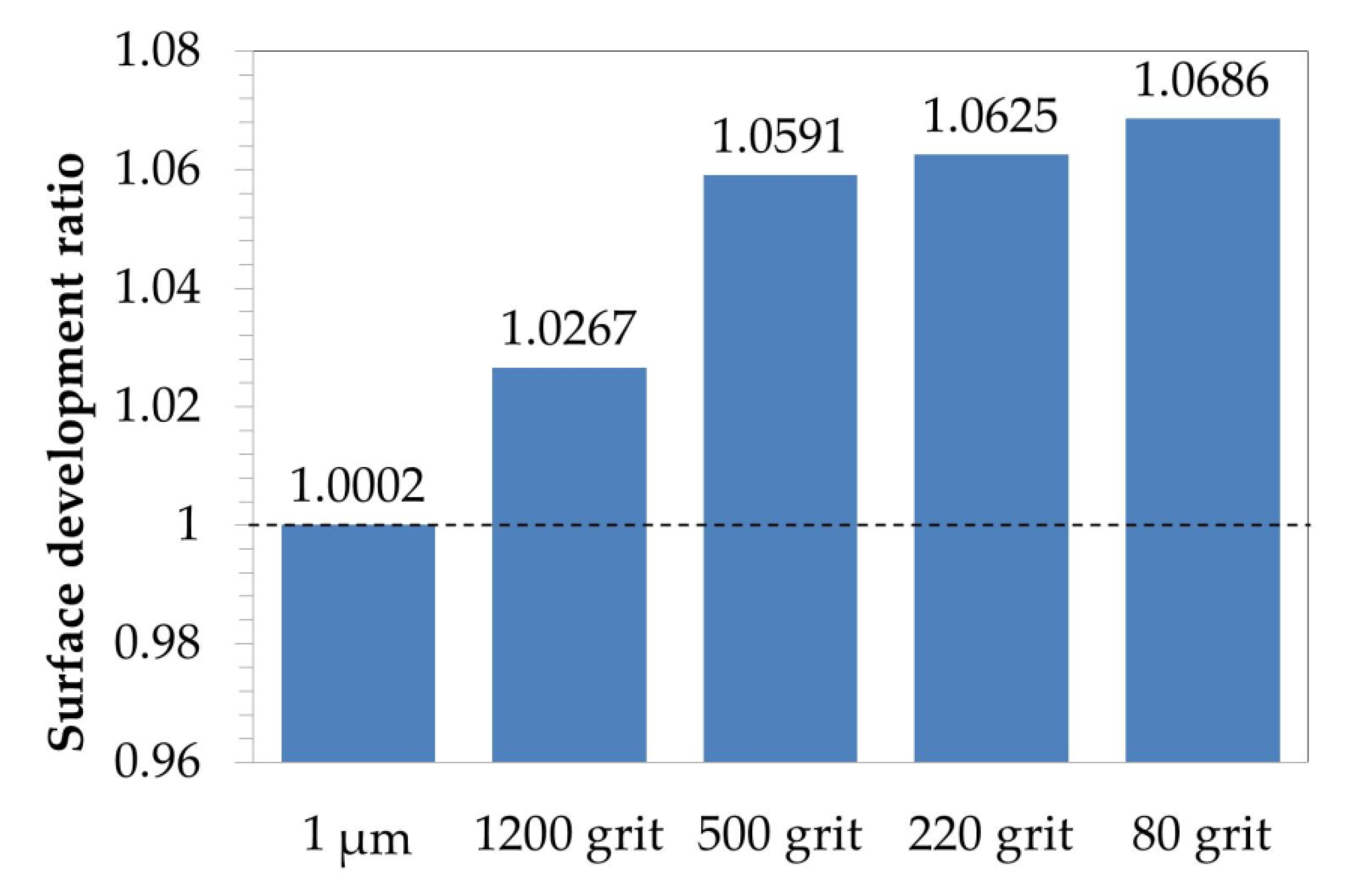
3.1.1. Surface Roughness Description
3.1.2. Short-Term Isothermal Air Oxidation
3.1.3. Rapid Cyclic Oxidation Test
3.1.4. Slow Cyclic Oxidation Test
3.2. Oxidation of Model Alloy Ni-14Cr-4Al
3.3. Consideration about Factors Altering Oxidation Behaviour of Ni-Base Alloys
3.3.1. Surface Roughness
3.3.2. Residual Stresses
3.3.3. Defects in the Near-Surface Region
4. Discussion
5. Conclusions
- Surface preparation method resulting in different surface roughness significantly influences the oxidation behavior of studied alloys. Namely, on a polished surface, a multilayered oxide scale was found consisting of NiO, Cr2O3, and internal zone of Al2O3, while on the ground surface, mainly protective Al2O3 formation was observed.
- The observed effect was found not only during short-term exposure, but also during long-term cyclic exposure at rapid and slow cyclic conditions.
- Surface roughness evaluation revealed an increase in surface development ratio with the rougher surface preparation method. However, this factor was claimed not to be responsible for the change in oxidation behavior of the studied alloys.
- It was found that the surface preparation method resulting in higher surface roughness also introduces higher compressive stresses into the near-surface region of the studied material. The material with higher compressive stress formed more protective oxide scale. Then, a higher compressive stress value was claimed to be one of the factors responsible for a change in the oxidation behavior of studied Ni-base alloys.
- It was confirmed that the sample with a rougher surface contains defects in the near-surface region. The presence of defects was claimed as a second factor influencing the oxidation behavior of the studied alloys, causing the formation of protective oxide scale.
- It was shown that, by the simple surface mechanical preparation method, the alloy can be moved from the region of alumina formation into chromia forming alloys. Then, in the case of studied alloys, polishing showed a negative effect in terms of high temperature oxidation behavior.
Author Contributions
Funding
Conflicts of Interest
References
- Young, D.J. High Temperature Oxidation and Corrosion of Metals, 2nd ed.; Elsevier: Cambridge, UK, 2008. [Google Scholar]
- Giggins, C.S.; Pettit, F.S. Oxidation of Ni-Cr-Al alloys between 1100 °C and 1200 °C. Solid. State Sci. 1971, 118, 1782–1790. [Google Scholar]
- Birks, N.; Meier, G.H.; Pettit, F.S. High-Temperature Oxidation of Metals, 2nd ed.; Cambridge Univeristy Press: Cambridge, UK, 2006. [Google Scholar]
- Davis, J.R. ASM Specialty Handbook, Heat Resistant Materials; ASM International: Materials Park, OH, USA, 1997. [Google Scholar]
- Leng, W.; Pillai, R.; Naumenko, D.; Galiullin, T.; Quadakkers, W.J. Effect of substrate alloy composition on the oxidation behaviour and degradation of aluminide coatings on two Ni base superalloys. Corros. Sci. 2020, 167, 108494. [Google Scholar] [CrossRef]
- Xiang, X.; Yang, F.; Zhang, G.; Wang, X. Effect of steel substrates on the formation and deuterium permeation resistance of aluminide coatings. Coatings 2019, 9, 95. [Google Scholar] [CrossRef]
- Nowak, W.J.; Tomków, M.; Wierzba, P.; Gancarczyk, K.; Wierzba, B. The role of substrate surface roughness on in-pack aluminization kinetics of Ni-base superalloy. J. Manuf. Mater. Process 2020, 4, 15. [Google Scholar] [CrossRef]
- Pęska, M.; Karczewski, K.; Rzeszotarska, M.; Polański, M. Direct synthesis of Fe-Al alloys from elemental powders using laser engineered net shaping. Materials 2020, 13, 531. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Y.; Deng, C.; Yang, K.; Li, F. Interdiffusion behaviour of NiCoCrAlY coating and N5 single crystal superalloy. Coatings 2020, 10, 393. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Xiao, P. Effect of surface curvature on oxidation of a MCrAlY coating. Corros. Sci. 2020, 163, 108256. [Google Scholar] [CrossRef]
- Chen, H.; Fan, M.; Zhu, W.; Norton, A.D. High temperature oxidation behaviour of combustion flame sprayed CoNiCrAlY coatings. Surf. Coat. Technol. 2020, 385, 125431. [Google Scholar] [CrossRef]
- Hejrani, E.; Sebold, D.; Nowak, W.J.; Mauer, G.; Naumenko, D.; Vaßen, R.; Quadakkers, W.J. Isothermal and cyclic oxidation behavior of free standing MCrAlY coatings manufactured by high-velocity atmospheric plasma spraying. Surf. Coat. Technol. 2017, 313, 191–201. [Google Scholar] [CrossRef]
- Rapp, R.A. The transition from internal to external oxidation and the formation of interruption bands in silver-indium alloys. Acta Metall. 1961, 9, 730–741. [Google Scholar] [CrossRef]
- Akhiani, H.; Szpunar, J.A. Effect of surface roughness on the texture and oxidation behavior of Zircaloy-4 cladding tube. Appl. Surf. Sci. 2013, 285, 832–839. [Google Scholar] [CrossRef]
- Serafin, D.; Bartkowiak, T.; Nowak, W.J.; Wierzba, B. Influence of microgeometry of iron surface on the oxidation process—A comparison of multiscale geometric methods and their applicability. Appl. Surf. Sci. 2020, 527, 146838. [Google Scholar] [CrossRef]
- Gumpel, P.; Hortnagl, A. Influence of the surface condition on corrosion behavior of stainless steel. Mater. Corros. 2016, 67, 607–620. [Google Scholar] [CrossRef]
- Uran, S.; Veal, B.; Grimsditch, M.; Pearson, J.; Berger, A. Effect of surface roughness on oxidation: Changes in scale thickness, somposition, and residual stress. Oxid. Met. 2020, 54, 73–85. [Google Scholar] [CrossRef]
- Nowak, W.J.; Serafin, D.; Wierzba, B. Effect of surface mechanical treatment on the oxidation behavior of FeAl-model alloy. J. Mater. Sci. 2019, 54, 9185–9196. [Google Scholar] [CrossRef]
- Serafin, D.; Nowak, W.J.; Wierzba, B. Mechanically prepared copper surface in oxidizing and non-oxidizing conditions. Appl. Surf. Sci. 2019, 492, 607–616. [Google Scholar] [CrossRef]
- Serafin, D.; Nowak, W.J.; Wierzba, B. The effect of surface preparation on high temperature oxidation of Ni, Cu and Ni-Cu alloy. Appl. Surf. Sci. 2019, 476, 442–451. [Google Scholar] [CrossRef]
- Serafin, D.; Nowak, W.J.; Wierzba, B. Differences in oxides morphology as a result of surface preparation of Ni-Fe alloy. Surf. Coat. Technol. 2020, 382, 125421. [Google Scholar] [CrossRef]
- Pradhan, D.; Mahobia, G.S.; Chattopadhyay, K.; Singh, V. Effect of surface roughness on corrosion behavior of the superalloy IN718 in simulated marine environment. J. Alloys Compd. 2018, 740, 250–263. [Google Scholar] [CrossRef]
- Nowak, W.J.; Wierzba, B. Effect of surface treatment on high-temperature oxidation behavior of IN 713C. J. Mater. Eng. Perform. 2018, 27, 5280–5290. [Google Scholar] [CrossRef]
- Nowak, W.J. Effect of surface roughness on early stage oxidation behavior of Ni-base superalloy IN 625. Appl. Syst. Innov. 2018, 1, 32. [Google Scholar] [CrossRef]
- Pei, H.; Wen, Z.; Li, Z.; Zhang, Y.; Yue, Z. Influence of surface roughness on the oxidation behavior of a Ni-4.0Cr-5.7Al single crystal superalloy. Appl. Surf. Sci. 2018, 440, 790–803. [Google Scholar] [CrossRef]
- Pfeifer, J.P.; Holzbrecher, H.; Quadakkers, W.J.; Speier, J. Quantitative analysis of oxide films on ODS-alloys using MCs+-SIMS and e-beam SNMS. J. Anal. Chem. 1993, 346, 186–191. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Elschner, A.; Speier, W.; Nickel, H. Composition and growth mechanisms of alumina scales on FeCrAl-based alloys determined by SNMS. Appl. Surf. Sci. 1991, 52, 271–287. [Google Scholar] [CrossRef]
- Nowak, W.J. Characterization of oxidized Ni-based superalloys by GD-OES. J. Anal. At. Spectrom. 2017, 32, 1730–1738. [Google Scholar] [CrossRef]
- Nowak, W.J.; Wierzba, P.; Naumenko, D.; Quadakkers, W.J.; Sieniawski, J. Water vapour effect on high temperature oxidation behaviour of superalloy Rene 80. Adv. Manuf. Sci. Technol. 2016, 40, 41–52. [Google Scholar] [CrossRef]
- Kansy, J. Microcomputer program for analysis of positron annihilation lifetime spectra. Nucl. Instrum. Methods Phys. Res. Sect. A 1996, 374, 235. [Google Scholar] [CrossRef]
- Horodek, P.; Siemek, K.; Dryzek, J.; Wróbel, M. Positron annihilation and complementary studies of copper sandblasted with alumina particles at different pressures. Materials 2017, 10, 1343. [Google Scholar] [CrossRef]
- Siemek, K.; Kulik, M.; Eseev, M.K.; Kobets, A.G.; Wróbel, M.; Orlov, O.S.; Sidorin, A.A. Surface and subsurface defects studies of dental alloys exposed to sandblasting. Acta Metall. Sin. Engl. Lett. 2019, 32, 1181–1194. [Google Scholar] [CrossRef]
- Horodek, P.; Siemek, K.; Dryzek, J.; Wróbel, M. Impact of abradant size on damaged zone of 304 AISI steel characterized by positron annihilation spectroscopy. Metall. Mater. Trans. A 2019, 50, 1502–1508. [Google Scholar] [CrossRef]
- Horodek, P.; Siemek, K.; Dryzek, J.; Kobets, A.G.; Wróbel, M. Positron annihilation and complementary studies of stainless steel exposed to sandblasting at different angles. Tribol. Lett. 2017, 65, 1–7. [Google Scholar] [CrossRef]
- Jalowicka, A.; Nowak, W.; Young, D.J.; Nischwitz, V.; Naumenko, D.; Quadakkers, W.J. Boron depletion in a nickel base superalloy induced by high temperature oxidation. Oxid. Met. 2015, 83, 393–413. [Google Scholar] [CrossRef]
- Campillo Robles, J.M.; Ogando, E.; Plazaola, F. Positron lifetime calculation for the elements of the periodic table. J. Phys. Condens. Matter. 2007, 19, 176222. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, E.; Tsutsumi, T.; Ueno, K.; Ohmura, M.; Kamimura, Y. Positron lifetime calculations on vacancy clusters and dislocations in Ni and Fe. Comput. Mater. Sci. 1999, 14, 28–35. [Google Scholar] [CrossRef]
- De Heer, J. The principle of Le Chatelier and Braun. J. Chem. Educ. 1957, 34, 375. [Google Scholar] [CrossRef]
- El-Khabeery, M.; Fattouh, M. Residual stress distribution caused by milling. Int. J. Mach. Tools Manuf. 1989, 29, 391–401. [Google Scholar] [CrossRef]
- Wagner, C. Theoretical analysis of the diffusion processes determining the oxidation rate of alloys. J. Electrochem. Soc. 1956, 99, 369. [Google Scholar] [CrossRef]
- Wagner, C. Reaktionstypen bei der oxydation von legierungen. Z. Elektrochem. 1959, 63, 772–782. [Google Scholar] [CrossRef]
- Hou, P.Y.; Cannon, R.M. Spallation behavior of thermally grown nickel oxide on nickel. Oxid. Met. 2009, 71, 237–256. [Google Scholar] [CrossRef]
- Naumenko, D.; Shemet, V.; Singheiser, L.; Quadakkers, W.J. Failure mechanisms of thermal barrier coatings on MCrAlY-type bondcoats associated with the formation of the thermally grown oxide. J. Mater. Sci. 2009, 44, 1687–1703. [Google Scholar] [CrossRef]
- Berthod, P. Kinetics of high temperature oxidation and chromia volatilization for a binary Ni-Cr alloy. Oxid. Met. 2005, 64, 235–252. [Google Scholar] [CrossRef]
- Monceau, D.; Bouhanek, K.; Peraldi, R.; Malie, A.; Pieraggi, B. Transition in high-temperature oxidation kinetics of Pd-modified aluminide coatings: Role of oxygen partial pressure, heating rate, and surface treatment. J. Mater. Res. 2000, 15, 665–675. [Google Scholar] [CrossRef]

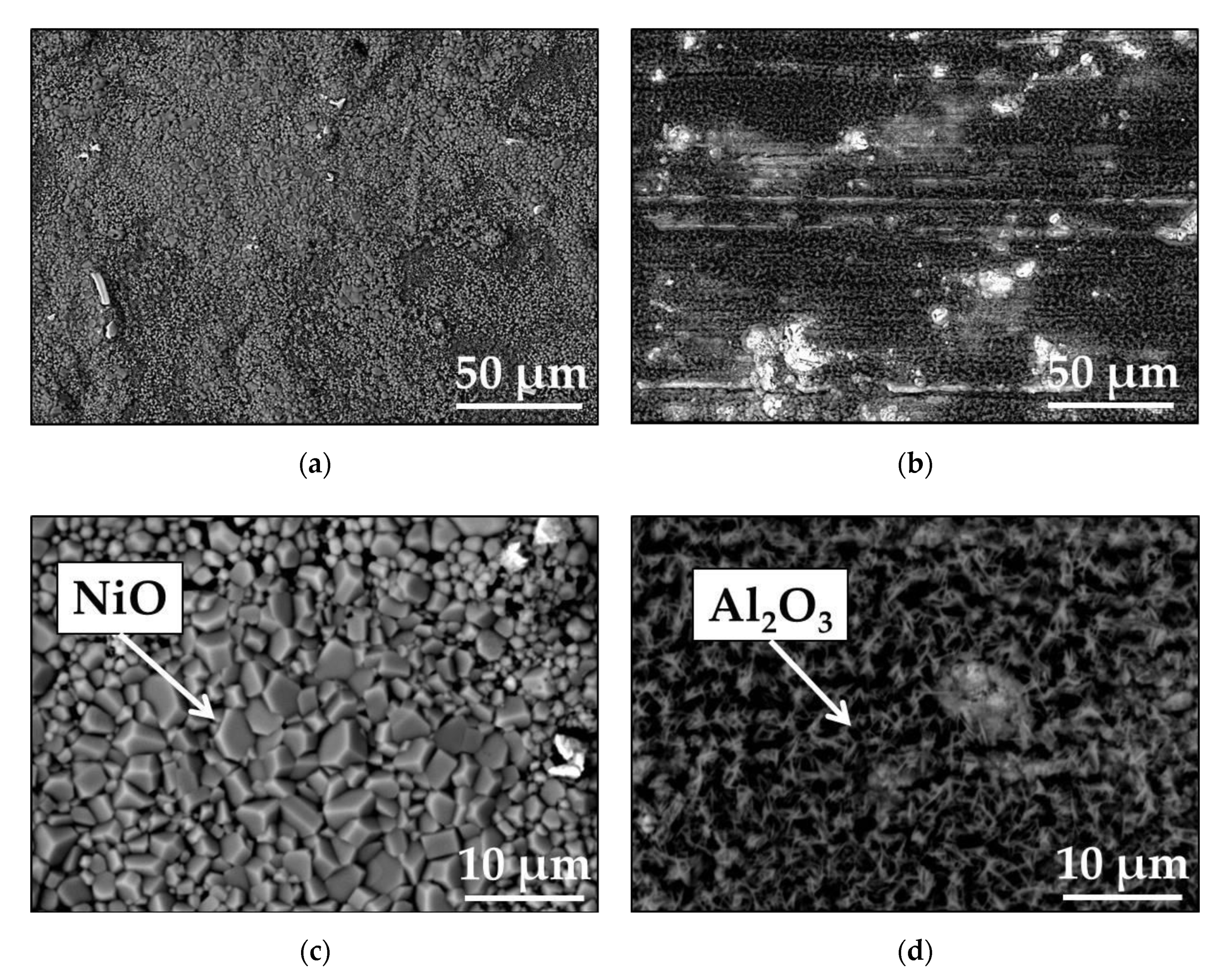
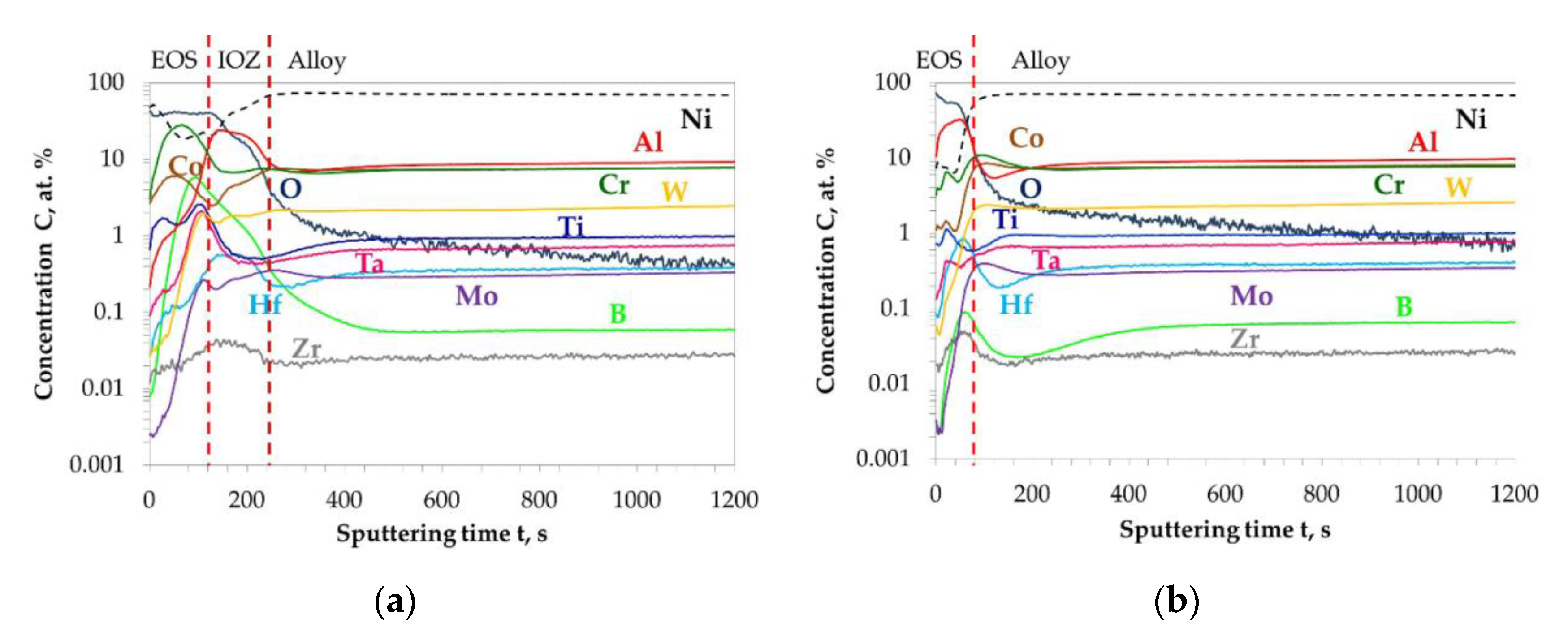
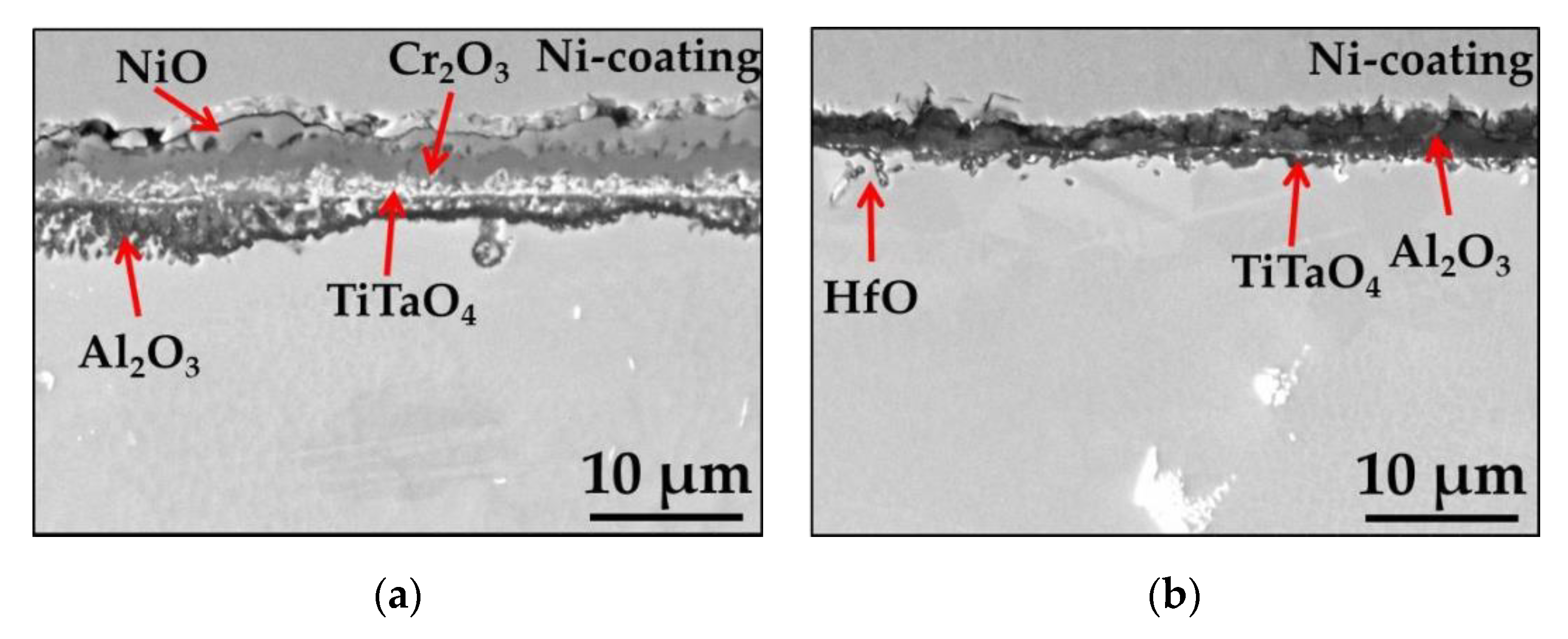
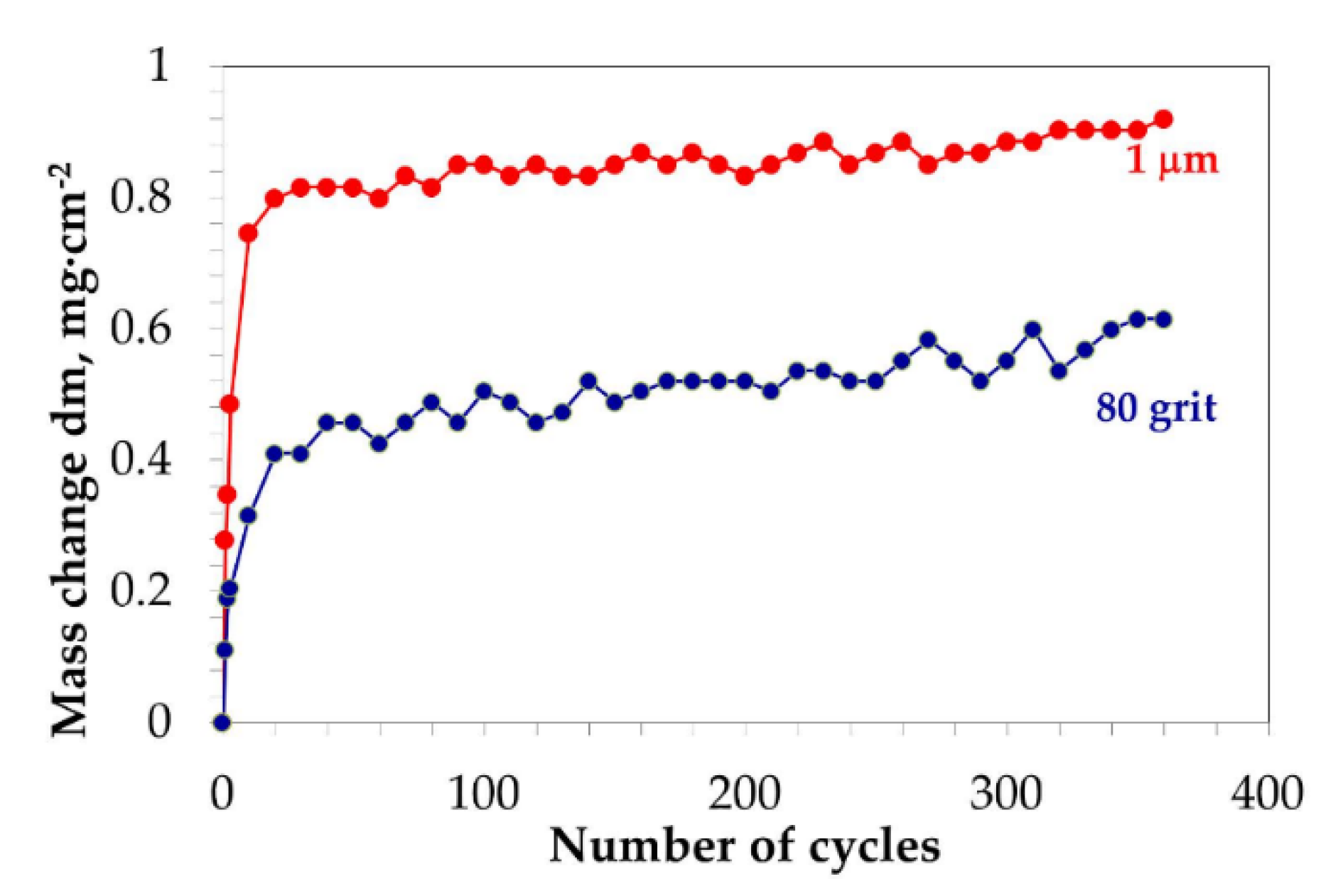
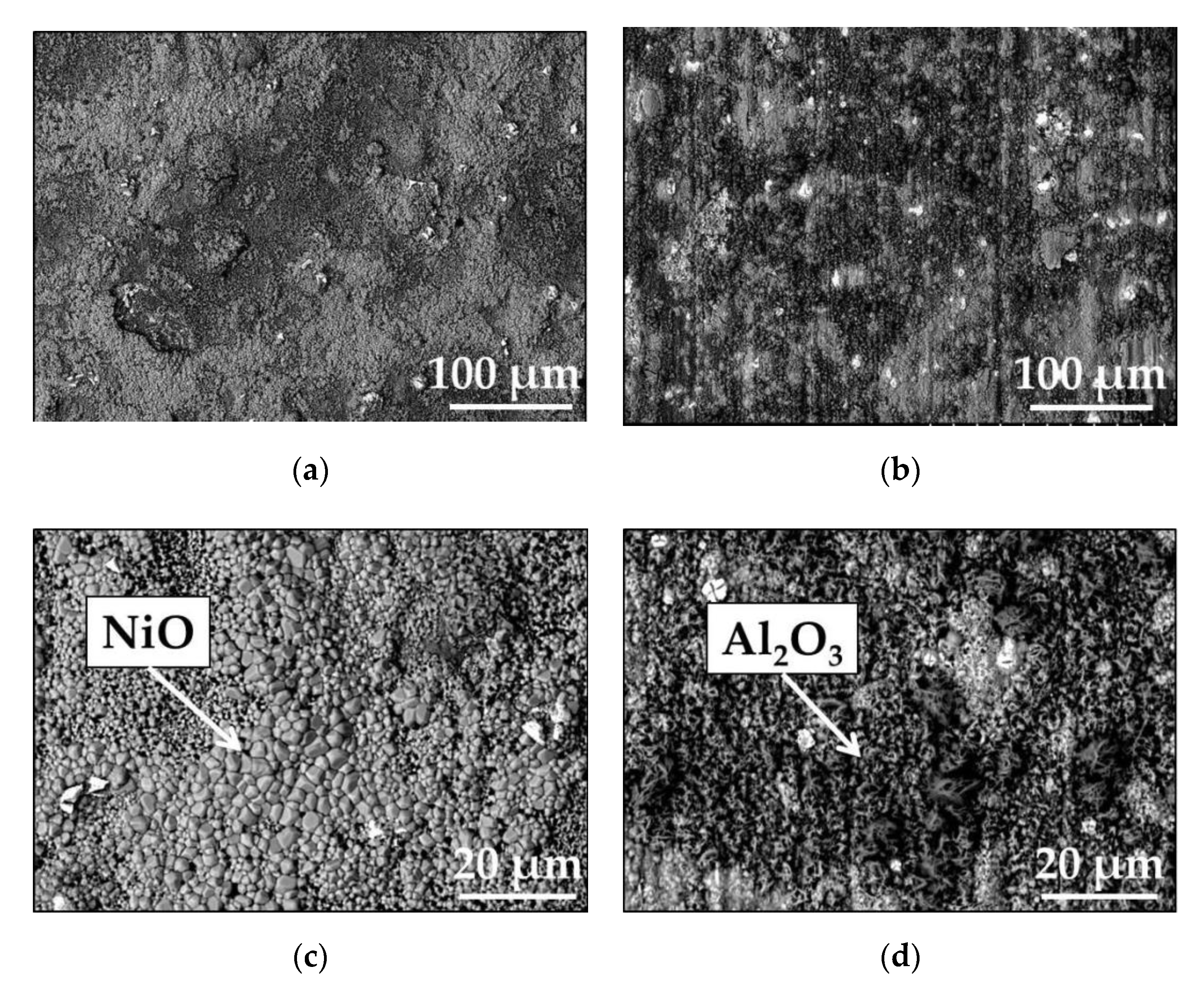
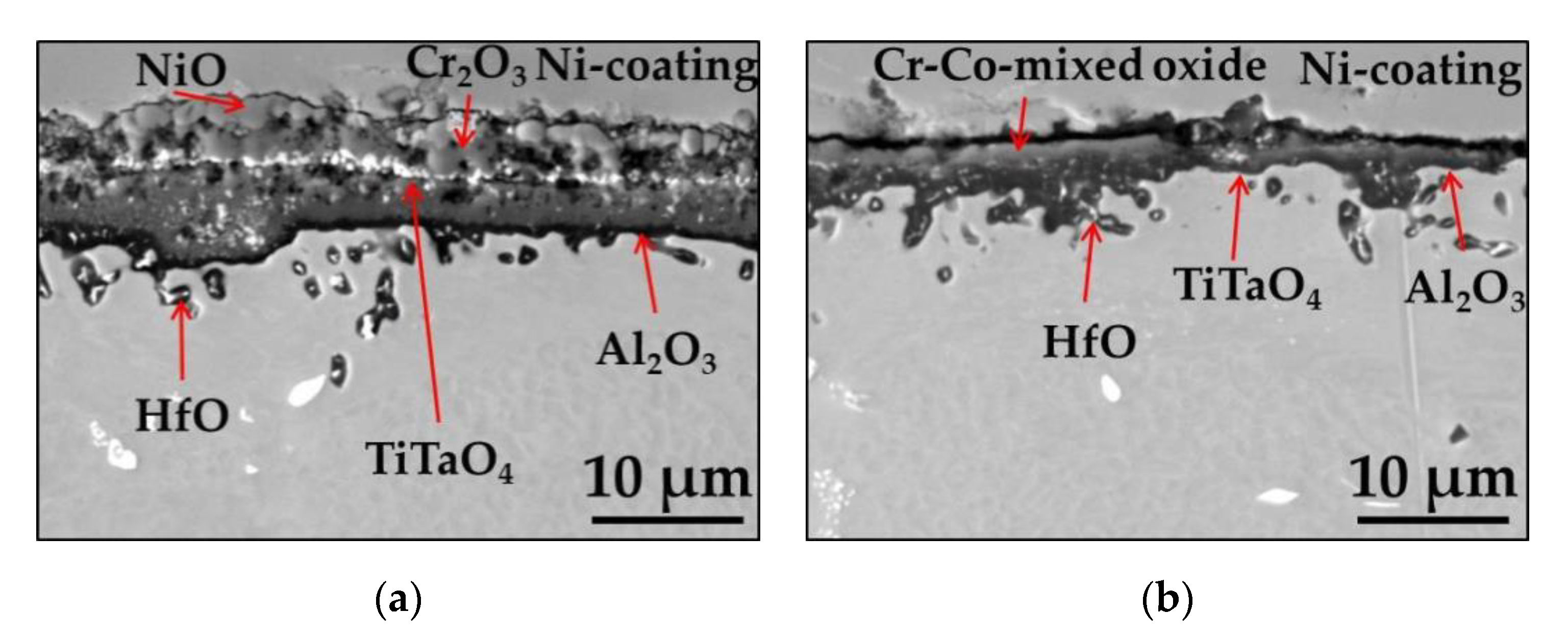
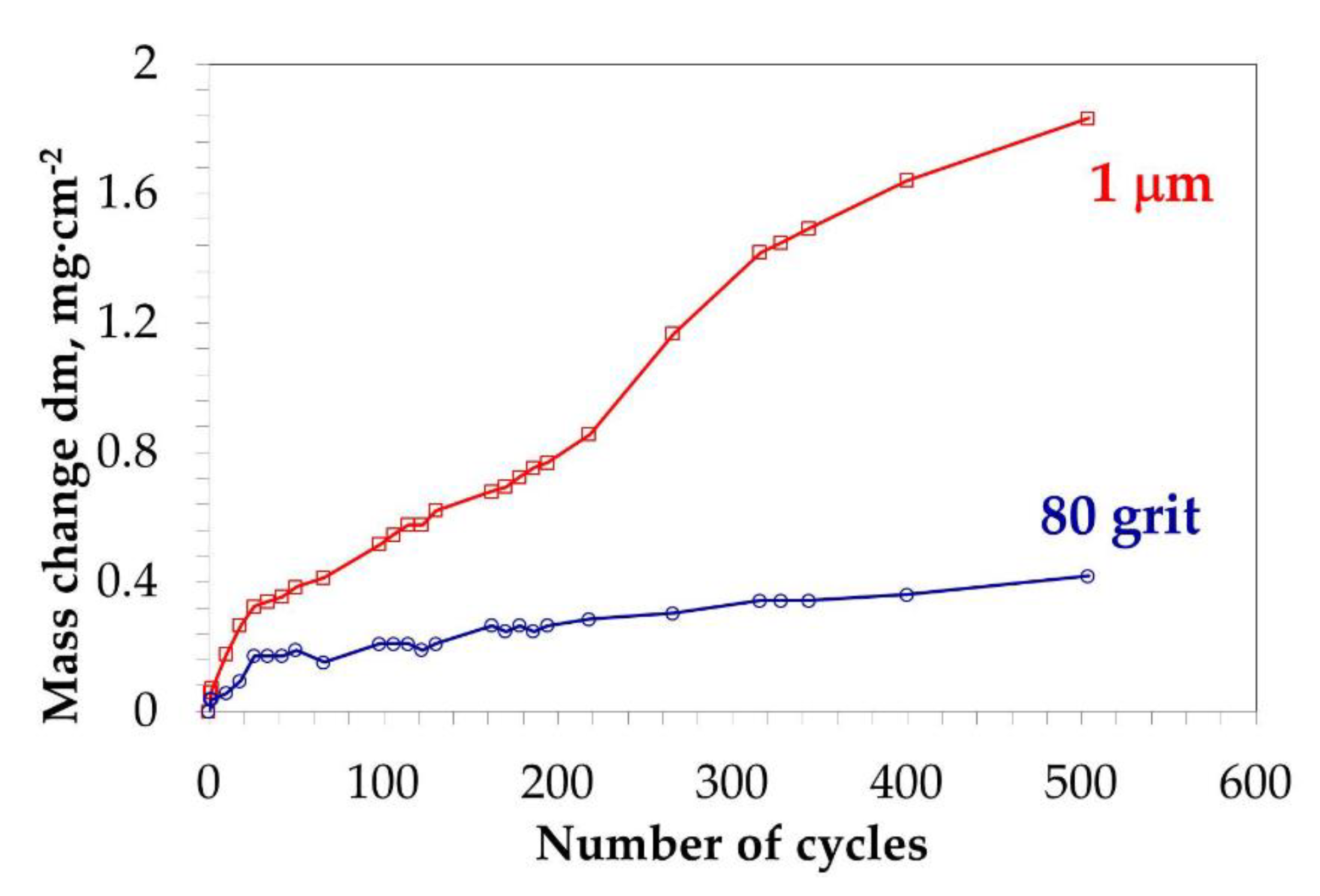
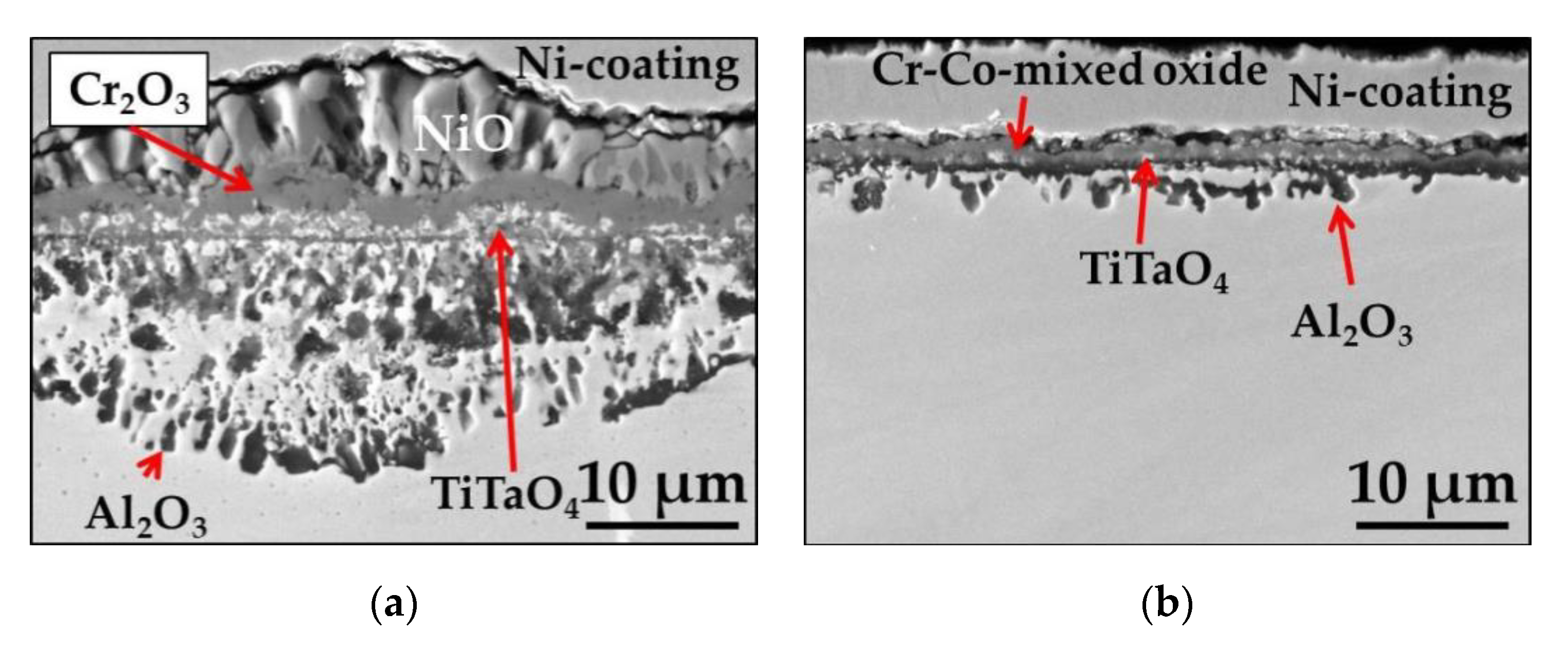

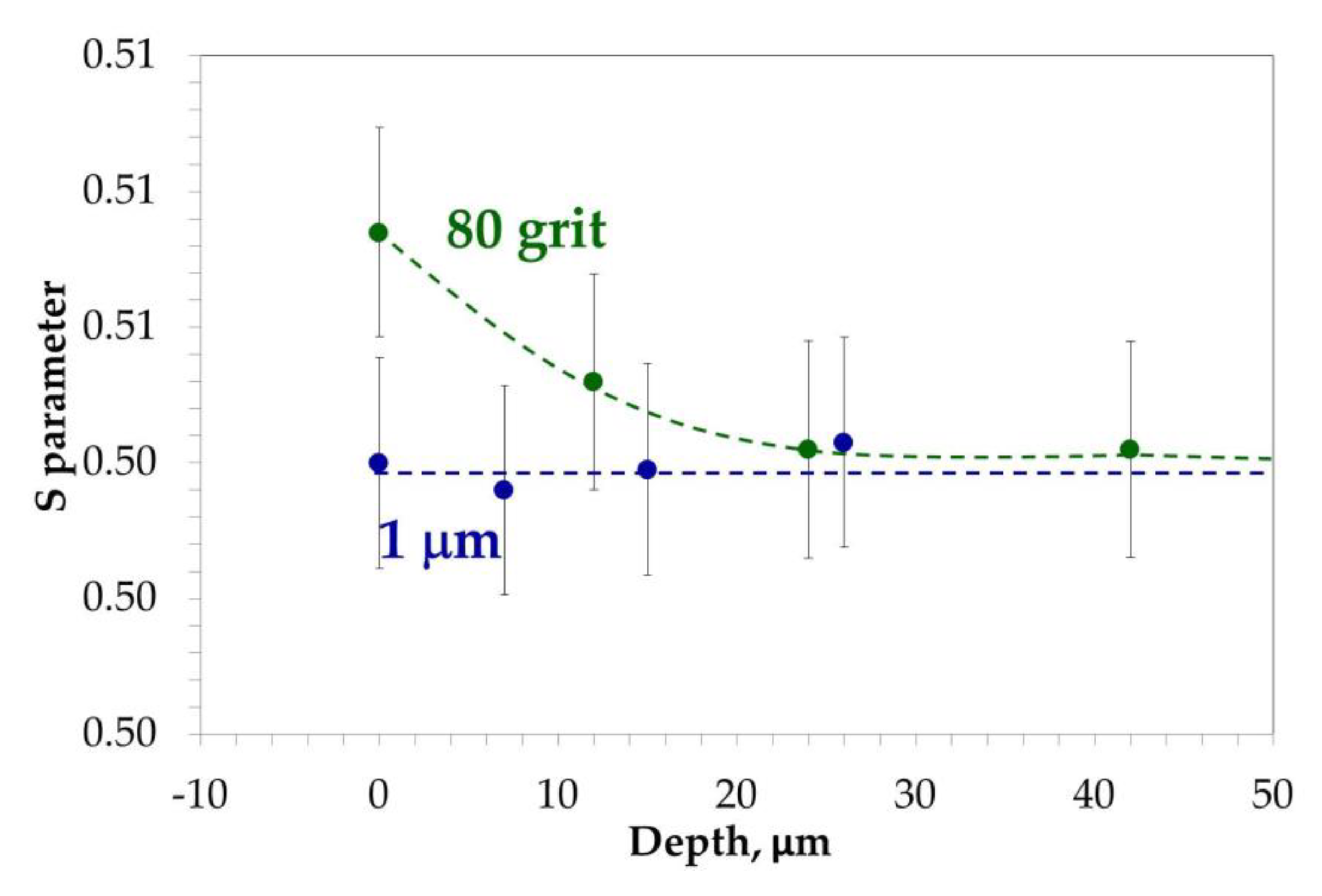
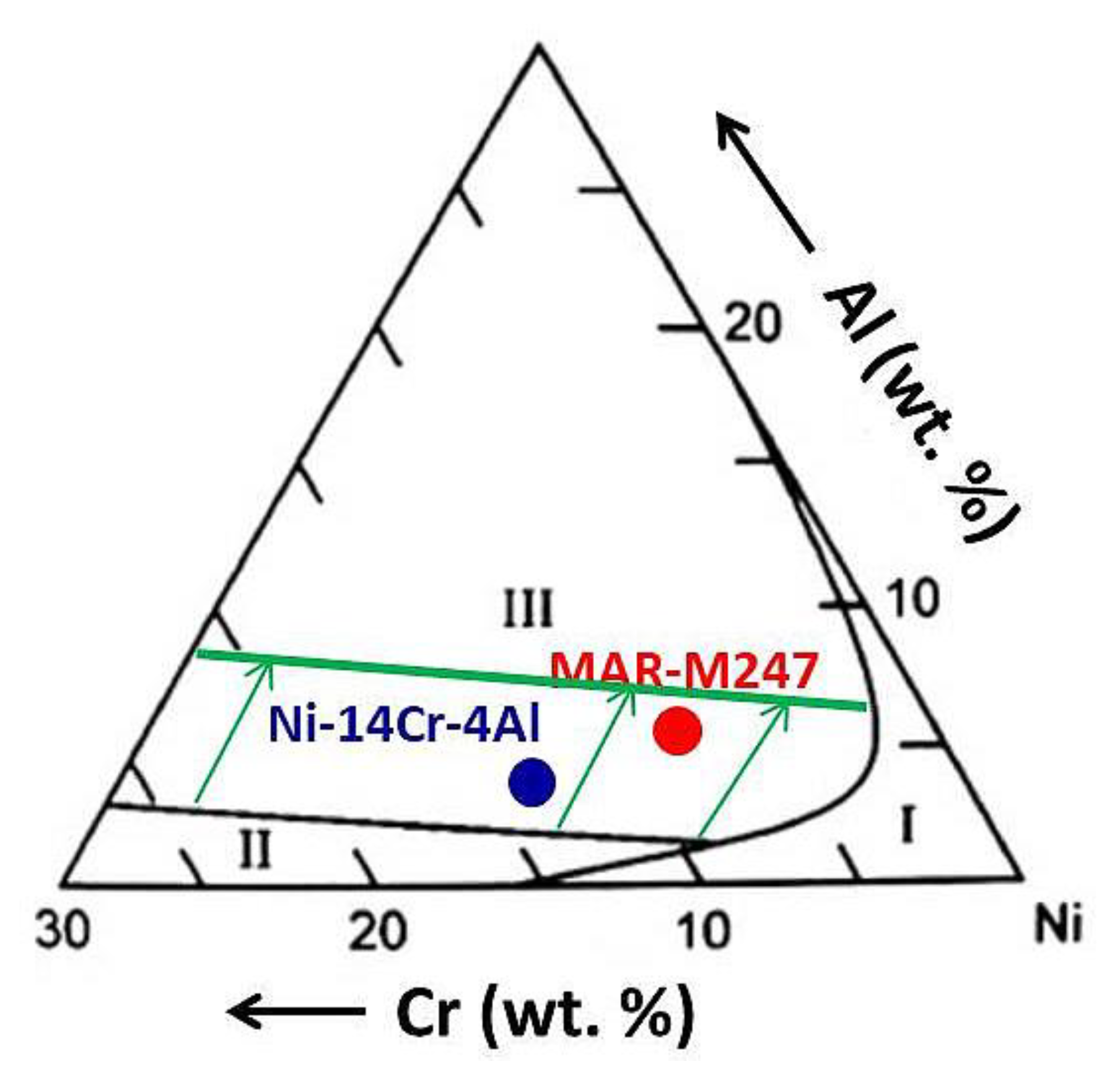
| Elements (wt.%) | Ni | Cr | Ta | Co | Mo | W | Al | Ti | Zr | B | Fe | Hf | C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mar M 247 | Base | 8.5 | 3 | 20 | 0.7 | 10 | 5.5 | 1 | 0.05 | 0.02 | 0.16 | 1.5 | 0.14 |
| Model alloy | Base | 14 | - | - | - | - | 4 | - | - | - | - | - | - |
| Sample Preparation | τ1 (ps) | I1 (%) | τ2 (ps) | I2 (%) | τmean (ps) |
|---|---|---|---|---|---|
| 1 µm | 122 | 100 | - | - | 122 |
| 80 grit | 127 | 78.6 | 230 | 21.4 | 149 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
J. Nowak, W.; Siemek, K.; Ochał, K.; Kościelniak, B.; Wierzba, B. Consequences of Different Mechanical Surface Preparation of Ni-Base Alloys during High Temperature Oxidation. Materials 2020, 13, 3529. https://doi.org/10.3390/ma13163529
J. Nowak W, Siemek K, Ochał K, Kościelniak B, Wierzba B. Consequences of Different Mechanical Surface Preparation of Ni-Base Alloys during High Temperature Oxidation. Materials. 2020; 13(16):3529. https://doi.org/10.3390/ma13163529
Chicago/Turabian StyleJ. Nowak, Wojciech, Krzysztof Siemek, Kamil Ochał, Barbara Kościelniak, and Bartek Wierzba. 2020. "Consequences of Different Mechanical Surface Preparation of Ni-Base Alloys during High Temperature Oxidation" Materials 13, no. 16: 3529. https://doi.org/10.3390/ma13163529
APA StyleJ. Nowak, W., Siemek, K., Ochał, K., Kościelniak, B., & Wierzba, B. (2020). Consequences of Different Mechanical Surface Preparation of Ni-Base Alloys during High Temperature Oxidation. Materials, 13(16), 3529. https://doi.org/10.3390/ma13163529






