Chestnut-Derived Activated Carbon as a Prospective Material for Energy Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pyrolysis and Activation Process of Biochar
2.3. Characterization Techniques
2.4. Electrode Preparation
3. Results and Discussion
3.1. CHNS and Proximate Analyses
3.2. Surface Area Analysis
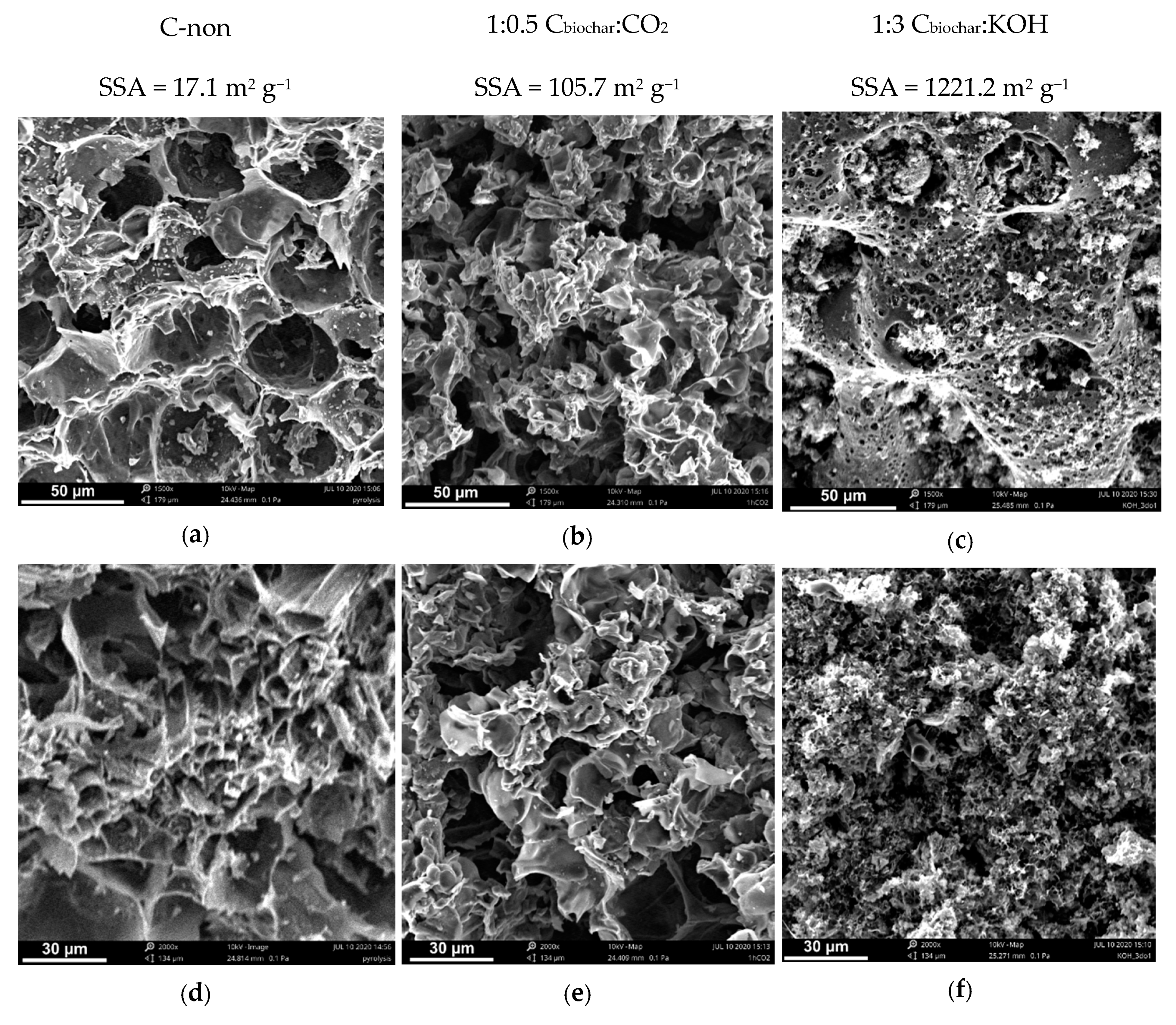
3.3. SEM Analysis
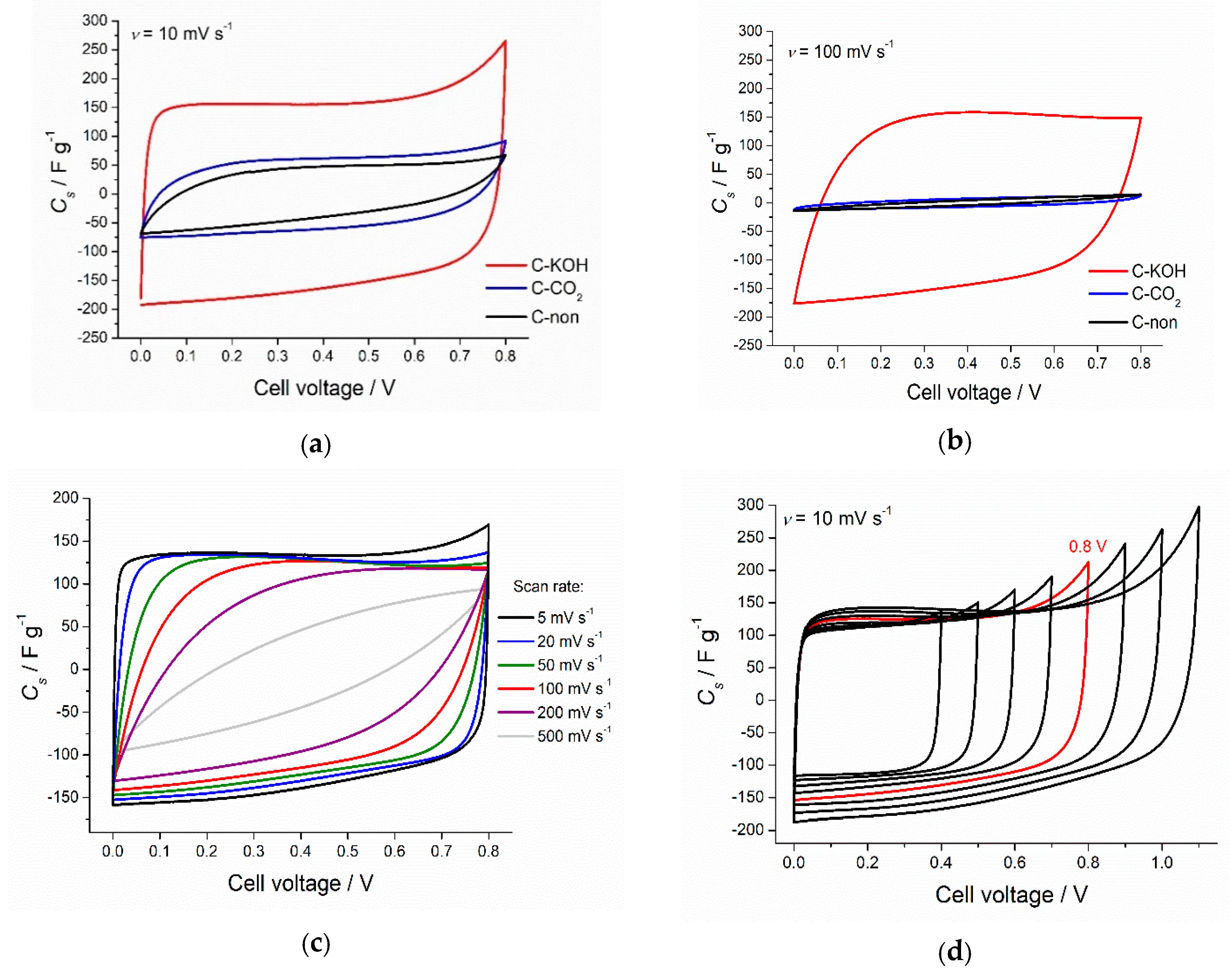
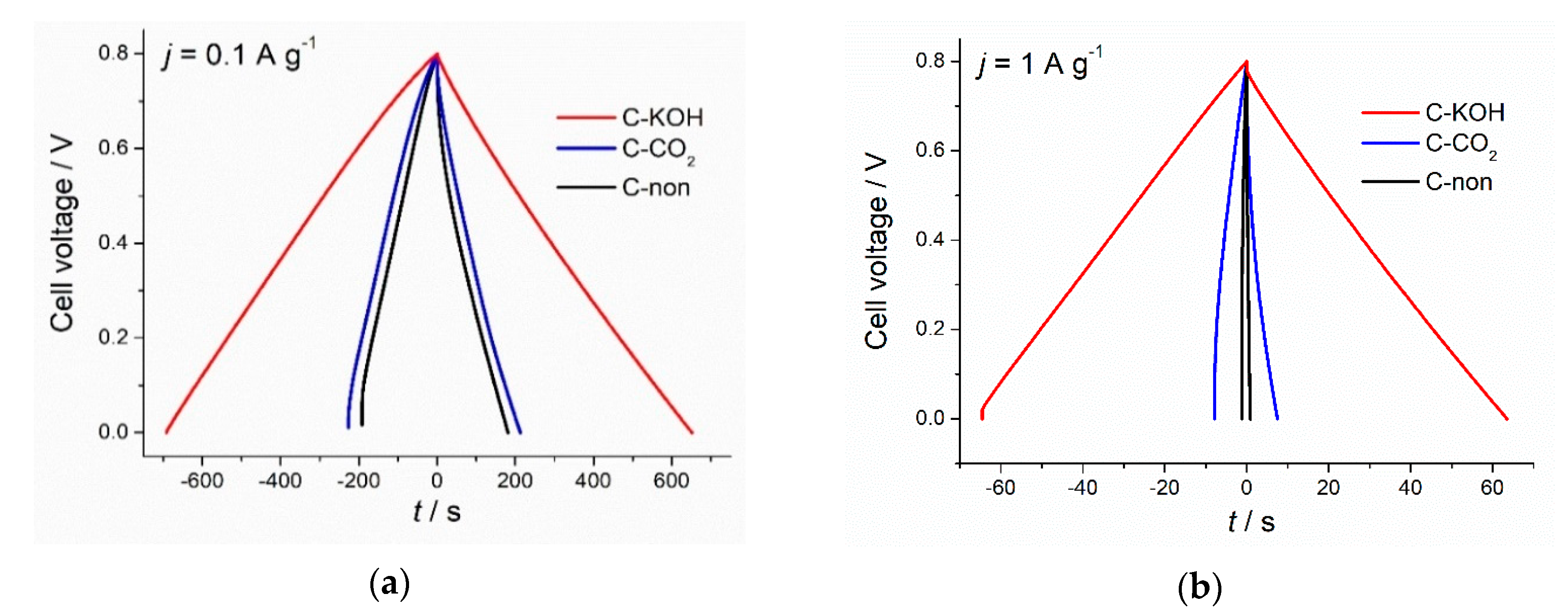
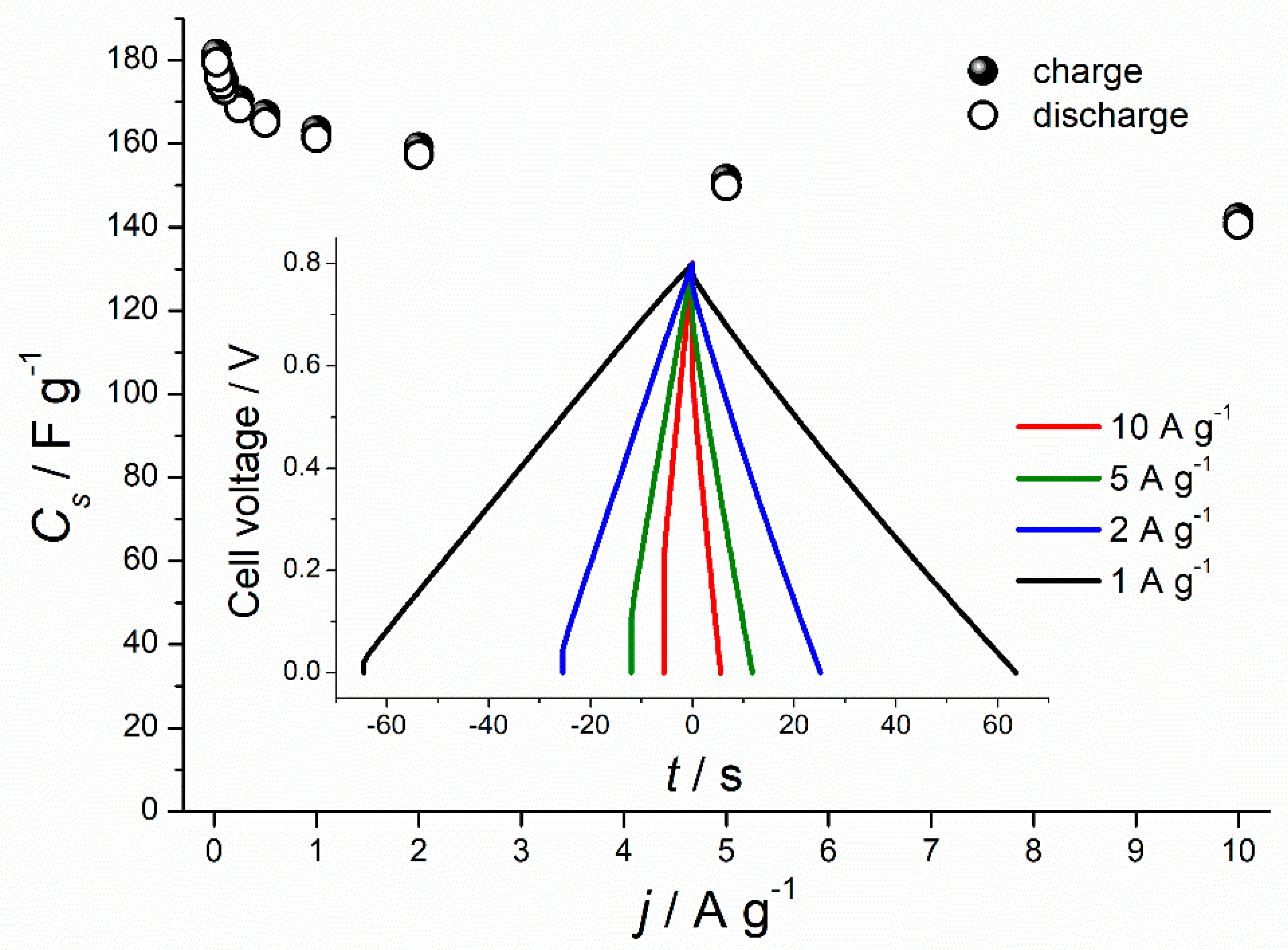
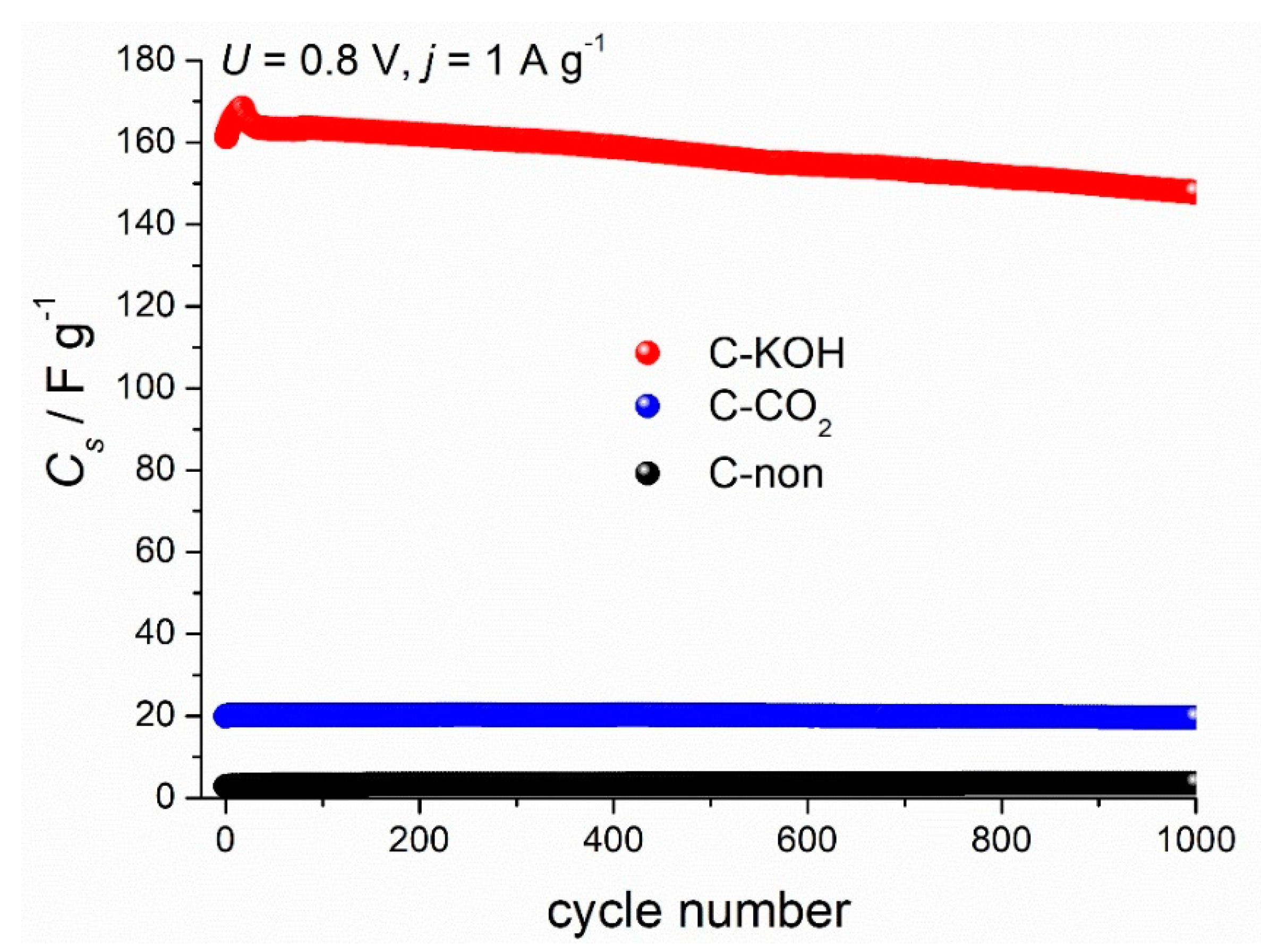
3.4. Electrochemical Analysis Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lin, G.; Ma, R.; Zhou, Y.; Liu, Q.; Dong, X.; Wang, J. KOH activation of biomass-derived nitrogen-doped carbons for supercapacitor and electrocatalytic oxygen reduction. Electrochim. Acta 2018, 261, 49–57. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, Q.-W.; Wang, Q.; Ma, J. Nitrogen doped porous carbon as excellent dual anodes for Li- and Na-ion batteries. Chin. Chem. Lett. 2020, 31, 583–588. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, Y.; Liu, W.; Yang, L.; Zhang, B.; Wang, L.P.; Jia, G.; Xu, Z.J. Biomass-Derived Porous Carbon-Based Nanostructures for Microwave Absorption. Nano-Micro Lett. 2019, 11, 24. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Tan, X.-F.; Liu, S.; Liu, Y.; Gu, Y.-L.; Zeng, G.-M.; Hu, X.; Wang, X.; Jiang, L. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Januszewicz, K.; Klugmann-Radziemska, E. Synthesis of reduced graphene oxide nanosheets using nanofibers from methane and biogas thermal decomposition with various catalysts. Chem. Pap. 2018, 72, 1991–1999. [Google Scholar] [CrossRef]
- Vishnuganth, M.; Remya, N.; Kumar, M.; Selvaraju, N. Photocatalytic degradation of carbofuran by TiO2-coated activated carbon: Model for kinetic, electrical energy per order and economic analysis. J. Environ. Manag. 2016, 181, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Roonasi, P.; Mazinani, M. Synthesis and application of barium ferrite/activated carbon composite as an effective solar photocatalyst for discoloration of organic dye contaminants in wastewater. J. Environ. Chem. Eng. 2017, 5, 3822–3827. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, F.; Li, J.; Chen, J.; Tang, M. The effects of different factors on the removal mechanism of Pb(ii) by biochar-supported carbon nanotube composites. RSC Adv. 2020, 10, 5988–5995. [Google Scholar] [CrossRef]
- Tzvetkov, G.; Mihaylova, S.; Stoitchkova, K.; Tzvetkov, P.; Spassov, T. Mechanochemical and chemical activation of lignocellulosic material to prepare powdered activated carbons for adsorption applications. Powder Technol. 2016, 299, 41–50. [Google Scholar] [CrossRef]
- Ryms, M.; Januszewicz, K.; Kazimierski, P.; Łuczak, J.; Klugmann-Radziemska, E.; Lewandowski, W.M. Post-Pyrolytic Carbon as a Phase Change Materials (PCMs) Carrier for Application in Building Materials. Materials 2020, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pu, W.; Hou, H.; Hu, J.; Liu, B.; Li, J.; Cheng, K.; Huang, L.; Yuan, X.; Yang, C.; et al. Activated microporous-mesoporous carbon derived from chestnut shell as a sustainable anode material for high performance microbial fuel cells. Bioresour. Technol. 2018, 249, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, D.; Liu, Y.; Wang, H.; Wang, L. Recent advances and challenges in biomass-derived porous carbon nanomaterials for supercapacitors. Chem. Eng. J. 2020, 397, 125418. [Google Scholar] [CrossRef]
- Fic, K.; Platek, A.; Piwek, J.; Frackowiak, E. Sustainable materials for electrochemical capacitors. Mater. Today 2018, 21, 437–454. [Google Scholar] [CrossRef]
- Ghosh, S.; Santhosh, R.; Jeniffer, S.; Raghavan, V.; Jacob, G.; Nanaji, K.; Kollu, P.; Jeong, S.K.; Grace, A.N. Natural biomass derived hard carbon and activated carbons as electrochemical supercapacitor electrodes. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kaal, J.; Schneider, M.P.W.; Schmidt, M.W.I. Rapid molecular screening of black carbon (biochar) thermosequences obtained from chestnut wood and rice straw: A pyrolysis-GC/MS study. Biomass Bioenergy 2012, 45, 115–129. [Google Scholar] [CrossRef]
- Vinciguerra, V.; Spina, S.; Luna, M.; Petrucci, G.; Romagnoli, M. Structural analysis of lignin in chestnut wood by pyrolysis-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2011, 92, 273–279. [Google Scholar] [CrossRef]
- Özçimen, D.; Ersoy-Meriçboyu, A. A study on the carbonization of grapeseed and chestnut shell. Fuel Process. Technol. 2008, 89, 1041–1046. [Google Scholar] [CrossRef]
- Yang, H.; Li, K.; Xiao, H.; Cai, N.; Dong, Z.; Xu, C.; Chen, Y.; Yang, H.; Tu, X.; Chen, H. Pyrolysis of Chinese chestnut shells: Effects of temperature and Fe presence on product composition. Bioresour. Technol. 2019, 287, 121444. [Google Scholar] [CrossRef]
- Piwek, J.; Platek, A.; Fic, K.; Frackowiak, E. Carbon-based electrochemical capacitors with acetate aqueous electrolytes. Electrochim. Acta 2016, 215, 179–186. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Pei, L.; Ying, D.; Xu, X.; Zhao, L.; Jia, J.; Cao, X. Converting Ni-loaded biochars into supercapacitors: Implication on the reuse of exhausted carbonaceous sorbents. Sci. Rep. 2017, 7, 41523. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mou, B.; Liang, Y.; Dong, H.; Zheng, M.; Xiao, Y.; Liu, Y. Component Degradation-Enabled Preparation of Biomass-Based Highly Porous Carbon Materials for Energy Storage. ACS Sustain. Chem. Eng. 2019, 7, 15259–15266. [Google Scholar] [CrossRef]
- Qian, L.; Guo, F.; Jia, X.; Zhan, Y.; Zhou, H.; Jiang, X.; Tao, C. Recent development in the synthesis of agricultural and forestry biomass-derived porous carbons for supercapacitor applications: A review. Ionics 2020, 26, 3705–3723. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Li, A.; Li, H.; Cui, D.; Dong, M.; Lin, J.; Fan, J.; Zhang, J.; Hou, H.; et al. Advanced porous hierarchical activated carbon derived from agricultural wastes toward high performance supercapacitors. J. Alloys Compd. 2020, 820, 153111. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Z.; Dai, J.; Yan, Y. Waste Biomass Based-Activated Carbons Derived from Soybean Pods as Electrode Materials for High-Performance Supercapacitors. ChemistrySelect 2018, 3, 5726–5732. [Google Scholar] [CrossRef]
- Okonkwo, C.A.; Lv, T.; Hong, W.; Li, G.; Huang, J.; Deng, J.; Jia, L.; Wu, M.; Liu, H.; Guo, M. The synthesis of micromesoporous carbon derived from nitrogen-rich spirulina extract impregnated castor shell based on biomass self-doping for highly efficient supercapacitor electrodes. J. Alloys Compd. 2020, 825, 154009. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Ukkakimapan, P.; Sattayarut, V.; Wanchaem, T.; Yordsri, V.; Phonyiem, M.; Ichikawa, S.; Obata, M.; Fujishige, M.; Takeuchi, K.; Wongwiriyapan, W.; et al. Preparation of activated carbon via acidic dehydration of durian husk for supercapacitor applications. Diam. Relat. Mater. 2020, 107, 107906. [Google Scholar] [CrossRef]
- Bin Mujib, S.; Vessalli, B.; Bizzo, W.A.; Mazon, T.; Singh, G. Cassava- and bamboo-derived carbons with higher degree of graphitization for energy storage. Nanomater. Energy 2020, 9, 54–65. [Google Scholar] [CrossRef]
- Sudhan, N.; Subramani, K.; Karnan, M.; Ilayaraja, N.; Sathish, M. Biomass-Derived Activated Porous Carbon from Rice Straw for a High-Energy Symmetric Supercapacitor in Aqueous and Non-aqueous Electrolytes. Energy Fuels 2016, 31, 977–985. [Google Scholar] [CrossRef]
- Hong, P.; Liu, X.; Zhang, X.; Peng, S.; Wang, Z.; Yang, Y.; Zhao, R.; Wang, Y. Hierarchically porous carbon derived from the activation of waste chestnut shells by potassium bicarbonate (KHCO3) for high-performance supercapacitor electrode. Int. J. Energy Res. 2019, 44, 988–999. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, J.; Hao, L.; Xue, R.; Sun, G.; Yi, B. High rate performance activated carbons prepared from ginkgo shells for electrochemical supercapacitors. Carbon 2013, 56, 146–154. [Google Scholar] [CrossRef]
- Cheng, L.; Guo, P.; Wang, R.; Ming, L.; Leng, F.; Li, H.; Zhao, G.X. Electrocapacitive properties of supercapacitors based on hierarchical porous carbons from chestnut shell. Colloids Surf. A 2014, 446, 127–133. [Google Scholar] [CrossRef]
- Hong, P.; Liu, X.; Zhang, X.; Peng, S.; Zou, T.; Wang, Z.; Yang, Y.; Zhao, R.; Chen, Y.; Wang, Y. Potassium sulphate (K2SO4) activation of chestnut shell to oxygen-enriched porous carbons with enhanced capacitive properties. Int. J. Energy Res. 2020, 44, 5385–5396. [Google Scholar] [CrossRef]
- Wan, L.; Li, X.; Li, N.; Xie, M.; Du, C.; Zhang, Y.; Chen, J. Multi-heteroatom-doped hierarchical porous carbon derived fromchestnut shell with superior performance in supercapacitors. J. Alloys Compd. 2019, 790, 760–771. [Google Scholar] [CrossRef]
- Cui, Y.; Cheng, L.; Wen, C.; Sang, Y.; Guo, P.; Zhao, X. Capacitive behavior of chestnut shell-based porous carbon electrode in ionic liquid electrolytes. Colloids Surf. A 2016, 508, 173–177. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Fu, N.; Chen, H.; Lin, H.; Han, S. Biomass-derived nitrogen-doped porous carbon with superior capacitive performance and high CO2 capture capacity. Electrochim. Acta 2018, 266, 161–169. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, H.; Zhang, H.; Liu, Z.; Zhang, L.; Chen, J.; Zhou, Q.; Pan, G.-B. In-situ preparation of water chestnut-based carbon aerogel and its application in binder-less electric double layer electrode and stress sensing. Vacuum 2020, 181, 109731. [Google Scholar] [CrossRef]
- Jiang, K.-M.; Cheng, C.-G.; Ran, M.; Lu, Y.-G.; Wu, Q.-L. Preparation of a biochar with a high calorific value from chestnut shells. New Carbon Mater. 2018, 33, 183–187. [Google Scholar] [CrossRef]
- Kar, T.; Keleş, S. Fast Pyrolysis of Chestnut Cupulae: Yields and Characterization of the Bio-Oil. Energy Explor. Exploit. 2013, 31, 847–858. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T. Alternative Fuels from Forestry Biomass Residue: Torrefaction Process of Horse Chestnuts, Oak Acorns, and Spruce Cones. Energies 2020, 13, 2468. [Google Scholar] [CrossRef]
- Gómez-Serrano, V.; Cuerda-Correa, E.; Fernández-González, C.; Alexandre-Franco, M.; Macias-Garcia, A. Preparation of activated carbons from chestnut wood by phosphoric acid-chemical activation. Study of microporosity and fractal dimension. Mater. Lett. 2005, 59, 846–853. [Google Scholar] [CrossRef]
- Haryanti, N.H.; Husain, S.; Safitri, M. Preliminary study of activated carbon from water chestnut (Eleocharis dulcis). J. Phys. 2020, 1572. [Google Scholar] [CrossRef]
- Cha, J.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Contescu, C.; Adhikari, S.; Gallego, N.C.; Evans, N.D.; Biss, B.E. Activated Carbons Derived from High-Temperature Pyrolysis of Lignocellulosic Biomass. J. Carbon Res. 2018, 4, 51. [Google Scholar] [CrossRef]
- Januszewicz, K.; Kazimierski, P.; Klein, M.; Kardaś, D.; Łuczak, J. Activated Carbon Produced by Pyrolysis of Waste Wood and Straw for Potential Wastewater Adsorption. Materials 2020, 13, 2047. [Google Scholar] [CrossRef] [PubMed]
- Raymundo-Piñero, E.; Azaïs, P.; Cacciaguerra, T.; Cazorla-Amorós, D.; Linares-Solano, A.; Béguin, F. KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organisation. Carbon 2005, 43, 786–795. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Otowa, T.; Tanibata, R.; Itoh, M. Production and adsorption characteristics of MAXSORB: High-surface-area active carbon. Gas Separ. Purific. 1993, 7, 241–245. [Google Scholar] [CrossRef]
- Lu, W.; Cao, X.; Hao, L.; Zhou, Y.; Wang, Y. Activated carbon derived from pitaya peel for supercapacitor applications with high capacitance performance. Mater. Lett. 2020, 264, 127339. [Google Scholar] [CrossRef]
- Sun, G.; Song, W.; Liu, X.; Long, D.; Qiao, W.; Ling, L. Capacitive matching of pore size and ion size in the negative and positive electrodes for supercapacitors. Electrochim. Acta 2011, 56, 9248–9256. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Relation between the Ion Size and Pore Size for an Electric Double-Layer Capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, Y.; Shen, K.; Huo, J.; Liu, Y.; Guo, S. Ion-matching porous carbons with ultra-high surface area and superior energy storage performance for supercapacitors. J. Mater. Chem. A 2019, 7, 9163–9172. [Google Scholar] [CrossRef]
- Fic, K.; Meller, M.; Lota, G.; Frąckowiak, E. Quinone/hydroquinone rodex couple as a siurce of enormous capacitance of activated carbon electrodes. Mater. Res. Soc. 2013, 1505, 17–45. [Google Scholar] [CrossRef]
- Dai, G.; Zhang, L.; Liao, Y.; Shi, Y.; Xie, J.; Lei, F.; Fan, L. Multi-Scale Model for Describing the Effect of Pore Structure on Carbon-Based Electric Double Layer. J. Phys. Chem. C 2020, 124, 3952–3961. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J.; Xing, L.-B.; Zhou, J.; Cui, H.; Si, W.; Zhuo, S. KOH-Activated Porous Carbons Derived from Chestnut Shell with Superior Capacitive Performance. Chin. J. Chem. 2016, 34, 1093–1102. [Google Scholar] [CrossRef]
- Hoppe, H.-W.; Strehblow, H.-H. XPS and UPS examinations of the formation of passive layers on Ni in 1M sodium hydroxide and 0.5M sulphuric acid. Surf. Interface Anal. 1989, 14, 121–131. [Google Scholar] [CrossRef]
- Yılmaz, I.; Gelir, A.; Yargı, Ö.; Sahinturk, U.; Ozdemir, O.K. Electrodeposition of zinc and reduced graphene oxide on porous nickel electrodes for high performance supercapacitors. J. Phys. Chem. Solids 2020, 138, 109307. [Google Scholar] [CrossRef]
- Salleh, N.A.; Kheawhom, S.; Mohamad, A.A. Characterizations of nickel mesh and nickel foam current collectors for supercapacitor application. Arab. J. Chem. 2020, 13, 6838–6846. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Lu, M.; Tao, P.-Y.; Zhang, Y.-J.; Gong, X.-T.; Yang, Z.; Zhang, G.-Q.; Li, H.-L. Hierarchically porous and heteroatom doped carbon derived from tobacco rods for supercapacitors. J. Power Sources 2016, 307, 391–400. [Google Scholar] [CrossRef]
- Liu, C.; Han, G.; Chang, Y.; Xiao, Y.; Li, M.; Zhou, W.; Fu, D.; Hou, W. Properties of Porous Carbon Derived from Cornstalk Core in High-Performance Electrochemical Capacitors. ChemElectroChem 2015, 3, 323–331. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Ma, X.; Idrees, F.; Xu, B.; Hao, X.; Lin, W. From Rice Bran to High Energy Density Supercapacitors: A New Route to Control Porous Structure of 3D Carbon. Sci. Rep. 2014, 4, 7260. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, P.; Yan, X.; Lang, J.; Peng, C.; Xue, Q. Promising Porous Carbon Derived from Celtuce Leaves with Outstanding Supercapacitance and CO2 Capture Performance. ACS Appl. Mater. Interfaces 2012, 4, 5800–5806. [Google Scholar] [CrossRef]
- Xu, G.; Han, J.; Ding, B.; Nie, P.; Pan, J.; Dou, H.; Li, H.; Zhang, X. Biomass-derived porous carbon materials with sulfur and nitrogen dual-doping for energy storage. Green Chem. 2015, 17, 1668–1674. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Q.; Zhang, Y.; Tan, Y.; Tian, W.; Zhu, L.; Jiang, L. Preparing two-dimensional microporous carbon from Pistachio nutshell with high areal capacitance as supercapacitor materials. Sci. Rep. 2014, 4, 5545. [Google Scholar] [CrossRef]
- Gao, Y.; Li, L.; Jin, Y.; Wang, Y.; Yuan, C.; Wei, Y.; Chen, G.; Ge, J.; Lu, H. Porous carbon made from rice husk as electrode material for electrochemical double layer capacitor. Appl. Energy 2015, 153, 41–47. [Google Scholar] [CrossRef]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.-P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High surface area activated carbon from rice husk as a high performance supercapacitor electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef]
- Saha, D.; Li, Y.; Bi, Z.; Chen, J.; Keum, J.K.; Hensley, D.K.; Grappe, H.A.; Meyer, I.H.M.; Dai, S.; Paranthaman, M.P.; et al. Studies on Supercapacitor Electrode Material from Activated Lignin-Derived Mesoporous Carbon. Langmuir 2014, 30, 900–910. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, A.; Rafat, M. Supercapacitor performance of activated carbon derived from rotten carrot in aqueous, organic and ionic liquid based electrolytes. J. Saudi Chem. Soc. 2018, 22, 993–1002. [Google Scholar] [CrossRef]
- Du, S.; Wang, L.; Fu, X.; Chen, M.; Wang, C. Bioresource Technology Hierarchical porous carbon microspheres derived from porous starch for use in high-rate electrochemical double-layer capacitors. Bioresour. Technol. 2013, 139, 406–409. [Google Scholar] [CrossRef]




| Sample | Elemental Analysis (wt.%) | ||||
|---|---|---|---|---|---|
| C | H | N | S | O * | |
| Chestnut, raw material | 45.97 | 6.65 | 2.53 | 0.2 | 44.65 |
| Chestnut, biochar (C-non) | 75.14 | 1.32 | 2.41 | 0.0 | 21.13 |
| CO2 activated carbon (C–CO2) | 75.04 | 1.11 | 2.61 | 0.0 | 21.24 |
| KOH activated carbon (C–KOH) | 60.54 | 0.85 | 2.63 | 0.0 | 35.98 |
| Sample | C:Activating Agent Ratio | SBET (m2 g−1) | Vp (cm3 g−1) |
|---|---|---|---|
| C-non | - | 17.1 | 0.0094 |
| C–CO2 | 1:0.5 * | 105.7 | 0.056 |
| C–KOH | 1:3 ** | 1221.2 | 0.625 |
| Biomass Type | Thermal Treatment | Activation Process (C: Activated Agent Ratio, Temp., Time) | SSA/m2 g−1 | C/F g−1 (in 6 M KOH, Symmetric Device) | Reference |
|---|---|---|---|---|---|
| Tobacco rods | HTC * (200 °C, 12 h, autoclave) | 1:3 C:KOH, 800 °C, 1 h | 2115 | 263 @ 0.5 A g−1 | [60] |
| Cornstalk core | Pre-carbonization 300 °C 2 h; pyrolysis 800 °C 3 h | 1:6 C:KOH, 800 °C, 3 h | 2139 | 186.8 @ 2 A g−1 | [61] |
| Rice bran | Pyrolysis 700 °C | 1:4 C:KOH, 850 °C, 1 h | 2475 | 323 @ 0.1 A g−1 | [62] |
| Ginkgo shells | Pyrolysis 600 °C | 1:2 C:KOH, 700 °C, 1 h; 1% Co(NO3)2 for 12 h; 900 °C for 2 h | 1775 | 237 @ 2 mV s−1 | [32] |
| Celtuce | Pyrolysis 600 °C | 1:4 C:KOH, 800 °C, 1 h | 3404 | 273 @ 0.5 A g−1 | [63] |
| Broad beans | Carbonization 800 °C | 1:3 C:KOH, 650 °C, 1 h | 655 | 202 @ 0.5 A g−1 measured in 3-electrode cell | [64] |
| Pistachio shell | Pyrolysis 750 °C | 1:3 C:KOH, 750 °C, - | 1069 | 261 @ 0.2 A g−1 | [65] |
| Rice husk | Carbonization 450 °C, 1 h | 1:4 C:KOH, 800 °C, several hours | 3145 | 367 @ 2.27 A g−1 | [66] |
| Rice husk | 1:1.6 (C: NaOH) 400 °C, 4 h | 1:5 C:KOH, 850 °C, 1 h | 2696 | 147 @ 0.1 A g−1 | [67] |
| Lignin | 1000 °C, 15 min (1 °C min−1 to 400 °C and 2 °C to 1000 °C) | 1:2 C:KOH, 1000 °C, ramp rate 10 °C min−1 | 1148 | 91.7 @ 2 mV s−1, measured in 3-electrode cell | [68] |
| Rotten carrot | 100 °C, 24 h | 1:2 C:ZnCl2, 900 °C, 2 h | 1155 | 137 @ 10 mV s−1 | [69] |
| Starch | Pretreatment with 10 wt.% (NH4)2HPO4 aqueous solution, 210 °C, 3 h; carbonization 600 °C, 2 h | 1:4 C:KOH, 800 °C, 2 h | 3251 | 304 @ 0.05 A g−1 | [70] |
| Chestnut shell | Drying 80 °C | 1:2 C:ZnCl2, 700 °C, 1.5 h | 1987 | 105.4 @ 0.1 A g−1 | [33] |
| Chestnut shell | Freeze-dried, 12 h | 1:0.25 C:melamine, 800 °C, 2 h | 691.8 | 402.8 @ 0.5 A g−1 measured in 3-electrode cell | [35] |
| Chestnut shell | 90 °C, 24 h | 1.8:1 C:K2SO4, 800 ° C, 2 h | 1412 | 265 @ 0.1 A g−1 measured in 3-electrode cell | [34] |
| Chestnut shell | 60 °C, 2 h | 1:3 (C:KHCO3), 850 °C;2.5, 2 h | 2298 | 387 @ 2A g−1 measured in 3-electrode cell | [31] |
| Chestnut shell | 1 M HNO3, 24 h | 1:2.5, 750 °C, 4 h | 1347.9 | 174 @ 0.5 A g−1 | [55] |
| Horse chestnut seed | Pyrolysis 800 °C, 30 min | 1:3, 800 °C, 1 h | 1252.5 | 173 @ 0.1 A g−1 | This work |
| 161 @ 1 A g−1 | |||||
| 140 @ 10 A g−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Januszewicz, K.; Cymann-Sachajdak, A.; Kazimierski, P.; Klein, M.; Łuczak, J.; Wilamowska-Zawłocka, M. Chestnut-Derived Activated Carbon as a Prospective Material for Energy Storage. Materials 2020, 13, 4658. https://doi.org/10.3390/ma13204658
Januszewicz K, Cymann-Sachajdak A, Kazimierski P, Klein M, Łuczak J, Wilamowska-Zawłocka M. Chestnut-Derived Activated Carbon as a Prospective Material for Energy Storage. Materials. 2020; 13(20):4658. https://doi.org/10.3390/ma13204658
Chicago/Turabian StyleJanuszewicz, Katarzyna, Anita Cymann-Sachajdak, Paweł Kazimierski, Marek Klein, Justyna Łuczak, and Monika Wilamowska-Zawłocka. 2020. "Chestnut-Derived Activated Carbon as a Prospective Material for Energy Storage" Materials 13, no. 20: 4658. https://doi.org/10.3390/ma13204658
APA StyleJanuszewicz, K., Cymann-Sachajdak, A., Kazimierski, P., Klein, M., Łuczak, J., & Wilamowska-Zawłocka, M. (2020). Chestnut-Derived Activated Carbon as a Prospective Material for Energy Storage. Materials, 13(20), 4658. https://doi.org/10.3390/ma13204658








