Effects of Methyl Terminal and Carbon Bridging Groups Ratio on Critical Properties of Porous Organosilicate Glass Films
Abstract
1. Introduction
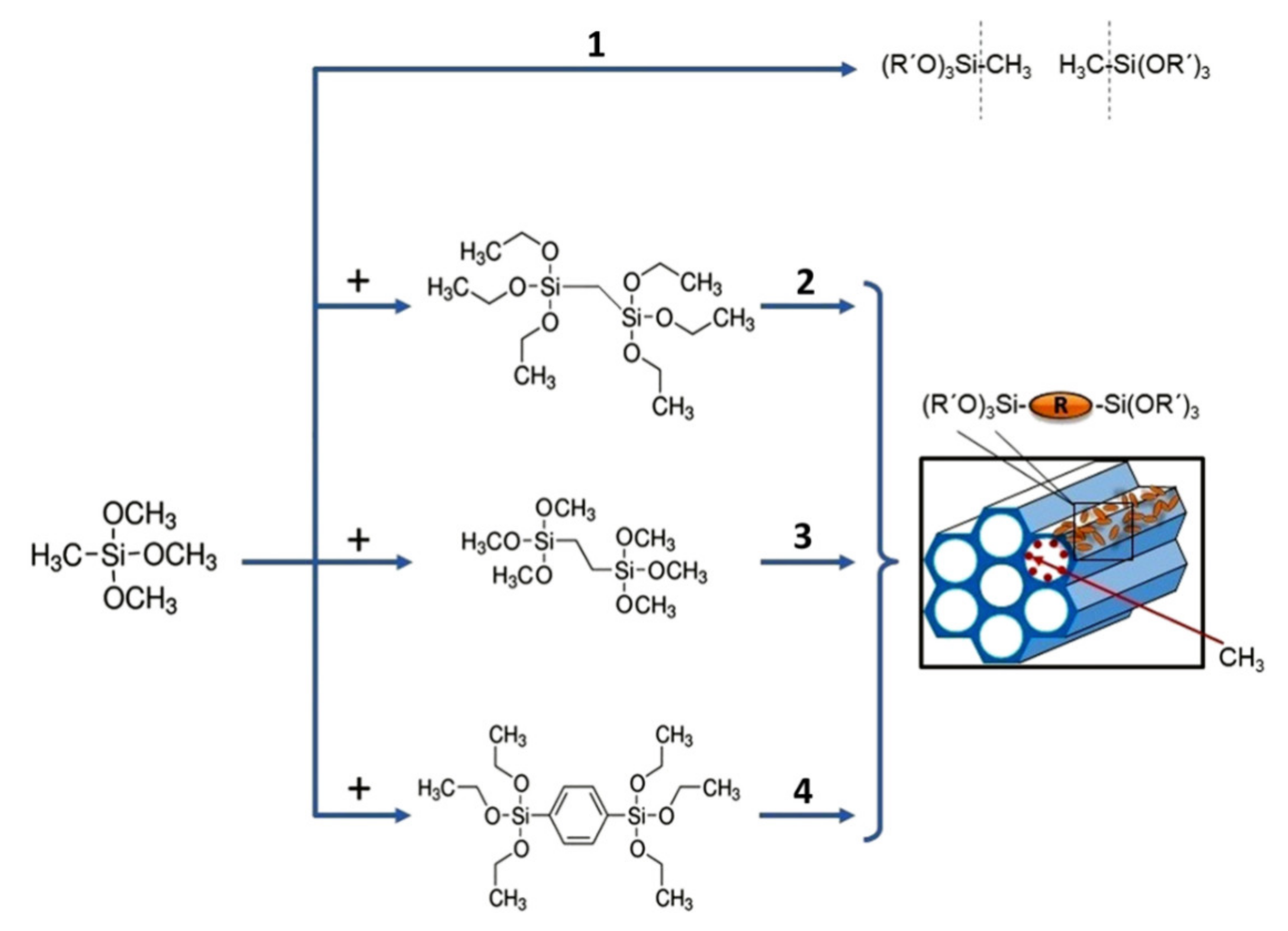
2. Experimental Design, Materials and Methods
3. Results and Discussion
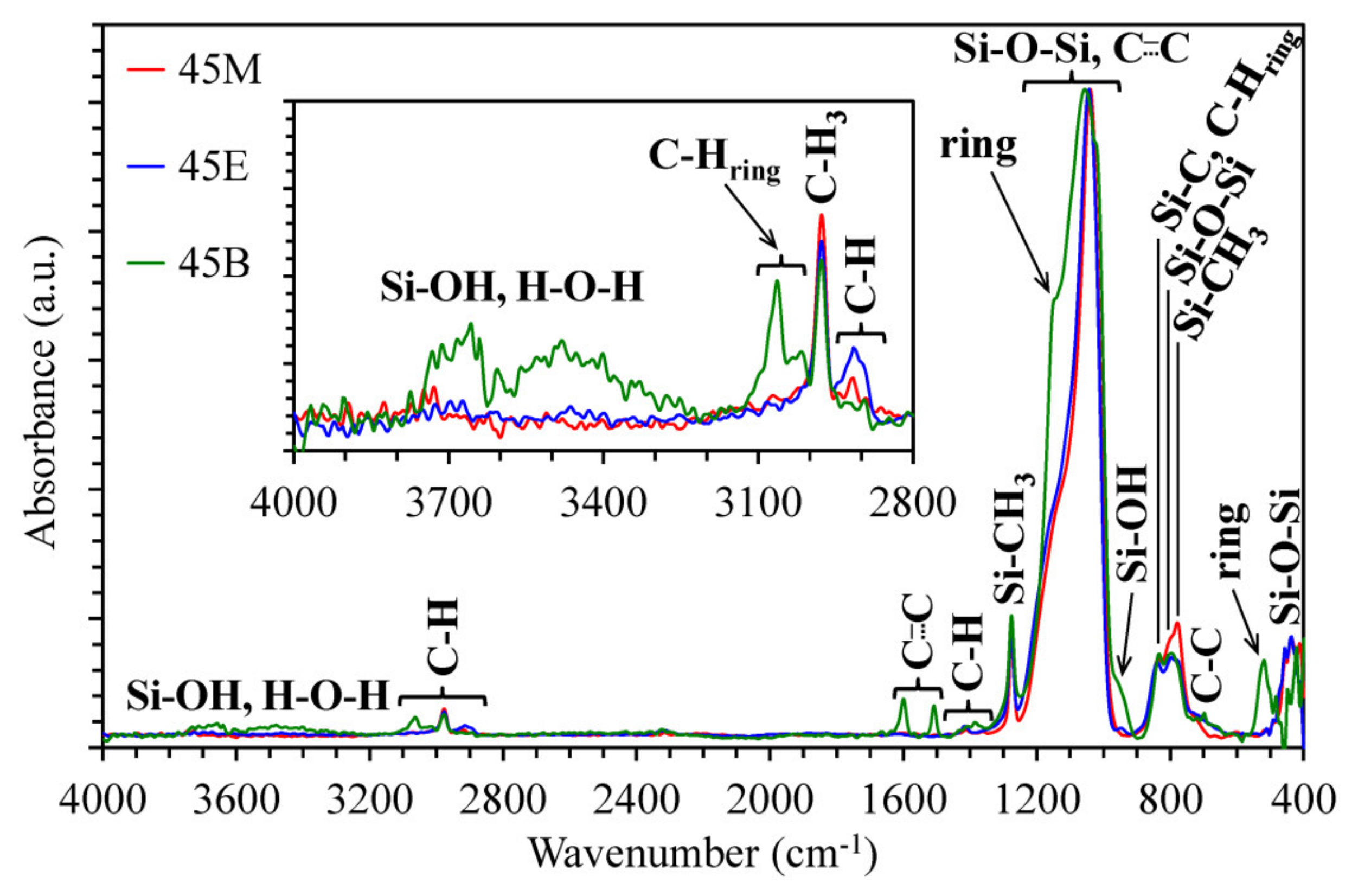
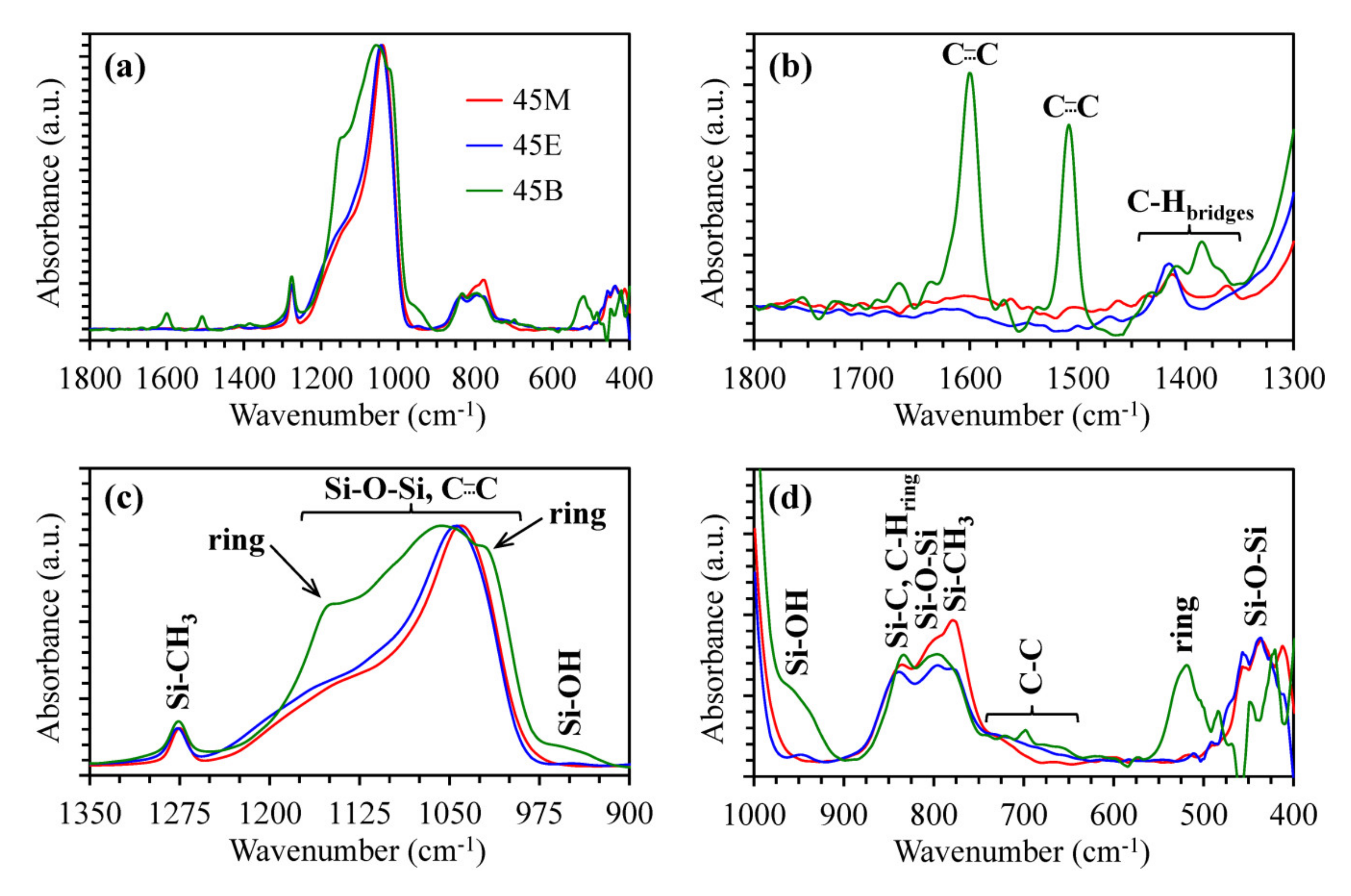
3.1. Chemical Composition of Methylene, Ethylene and 1,4-phenylene-bridged OSG Films
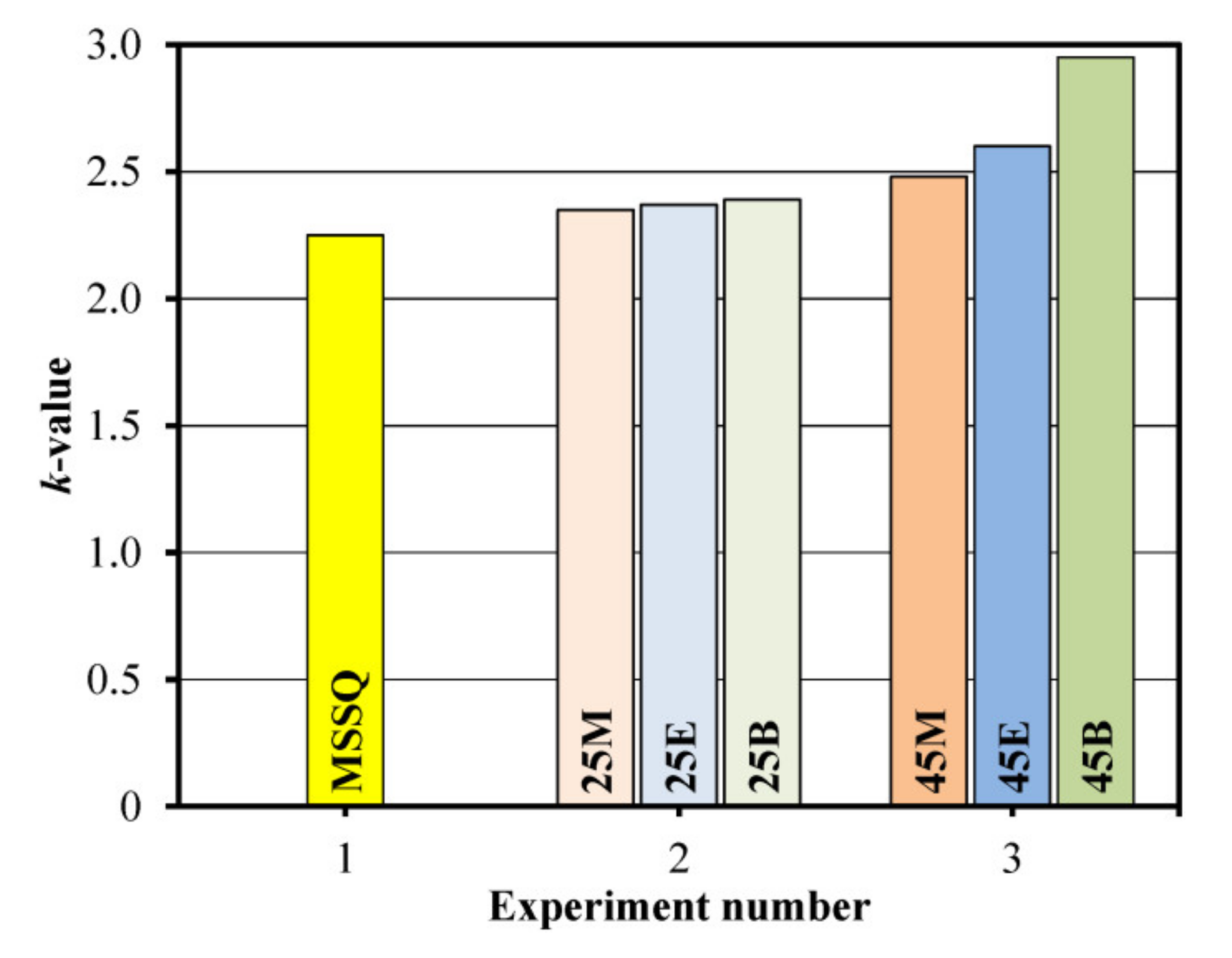
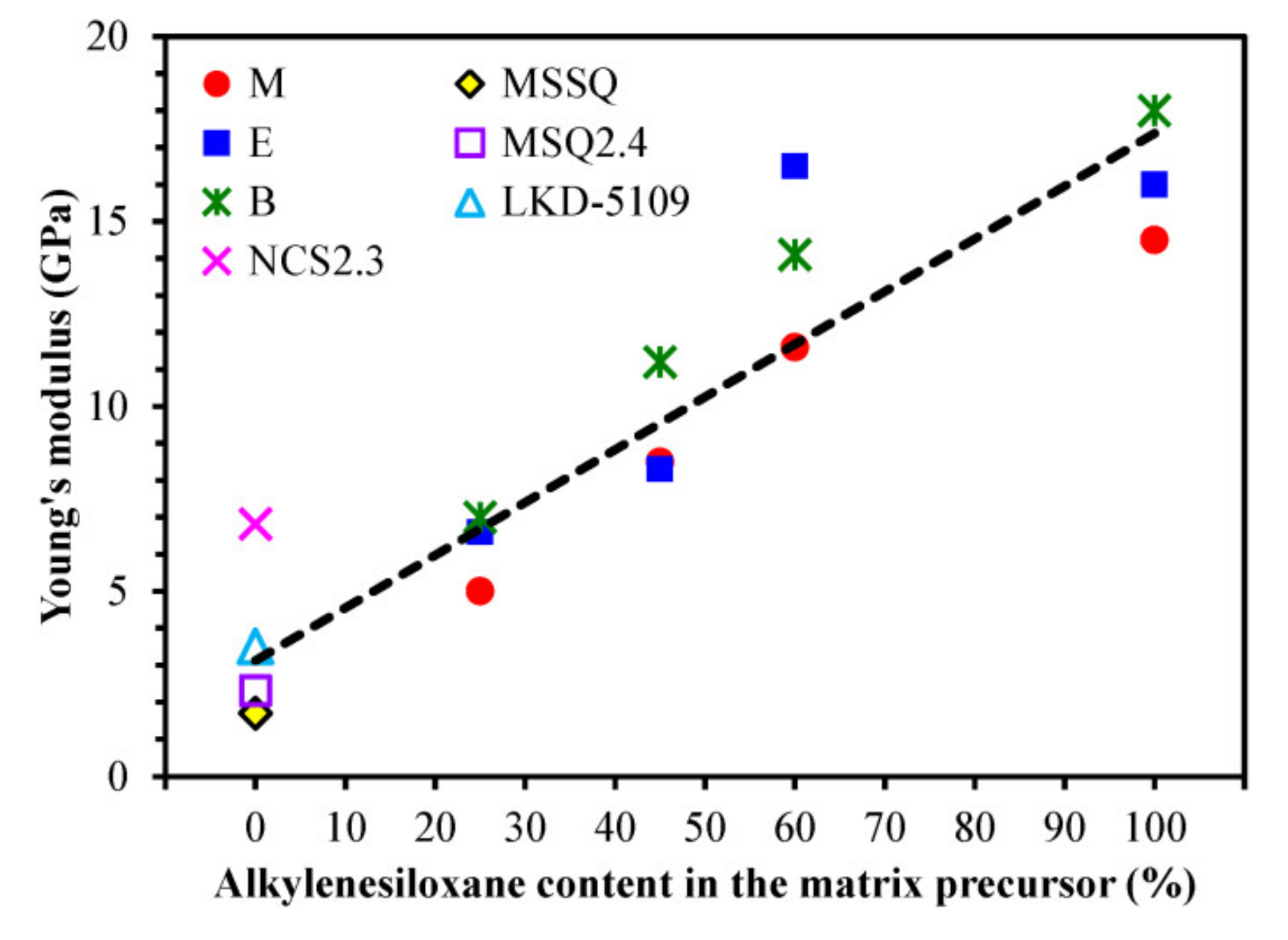
3.2. Impact of Bridge Type and Composition on the Properties of Carbon-bridged Low-k Films
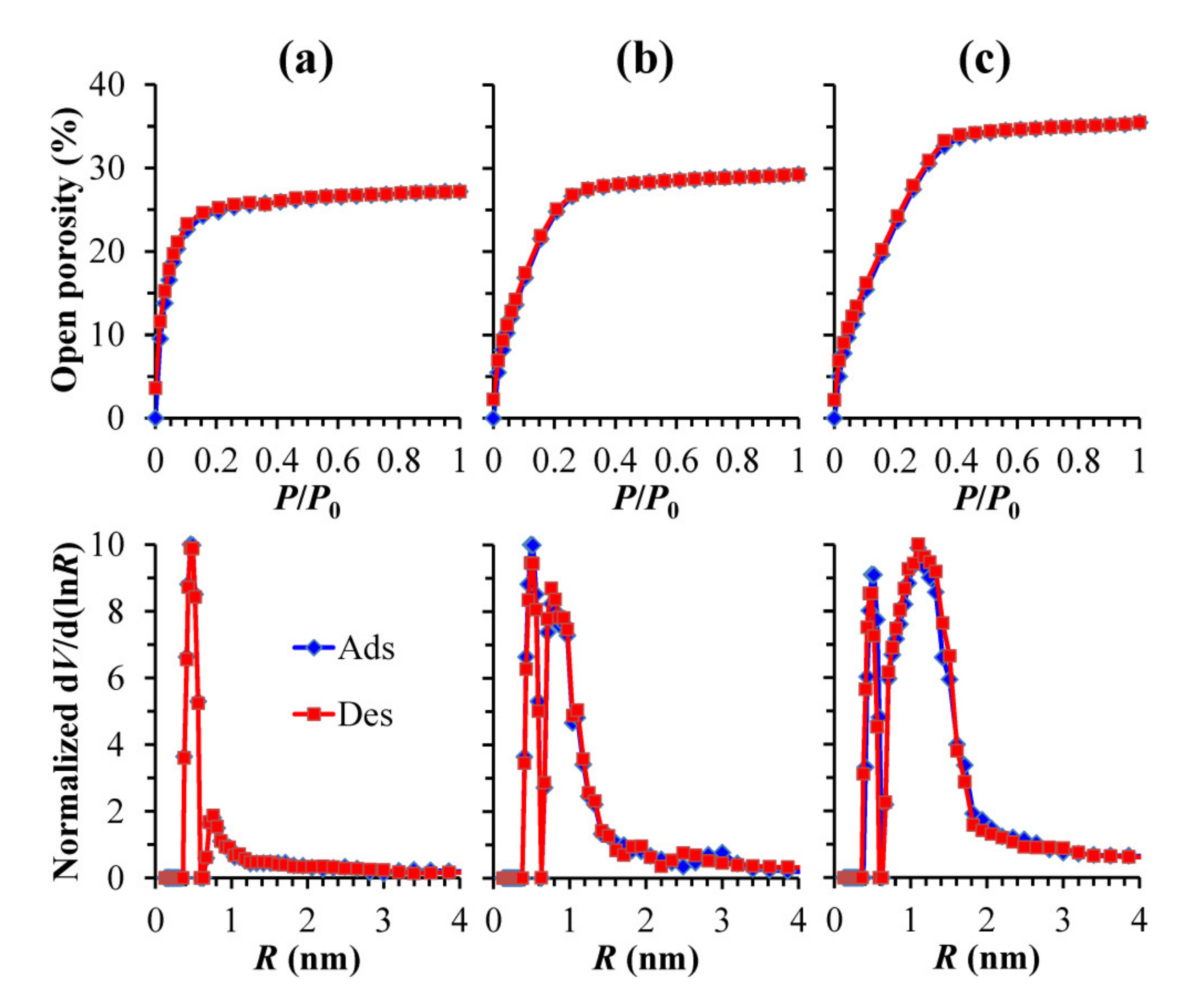
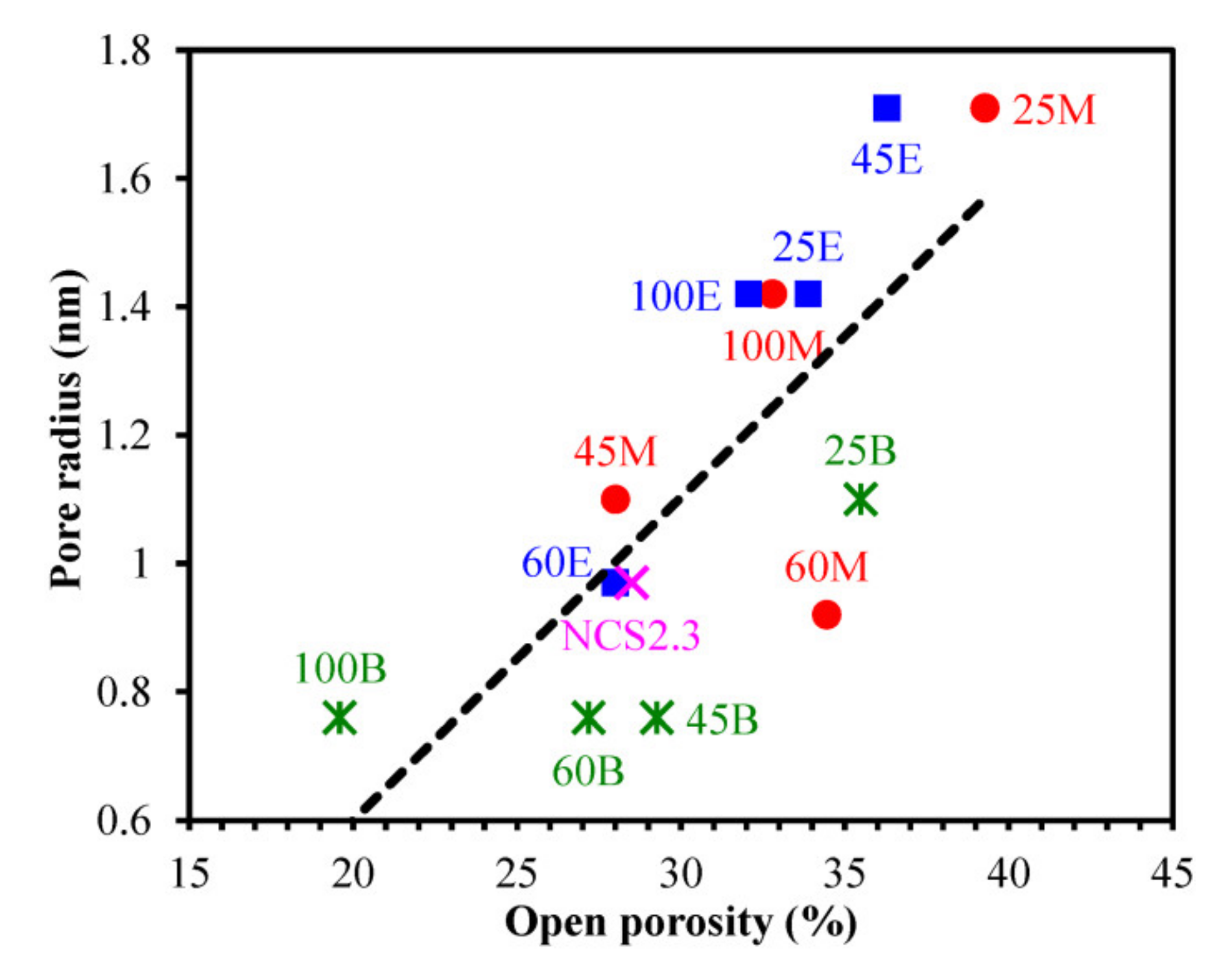
3.3. Porosity and Pore Size Distribution (EP Data)
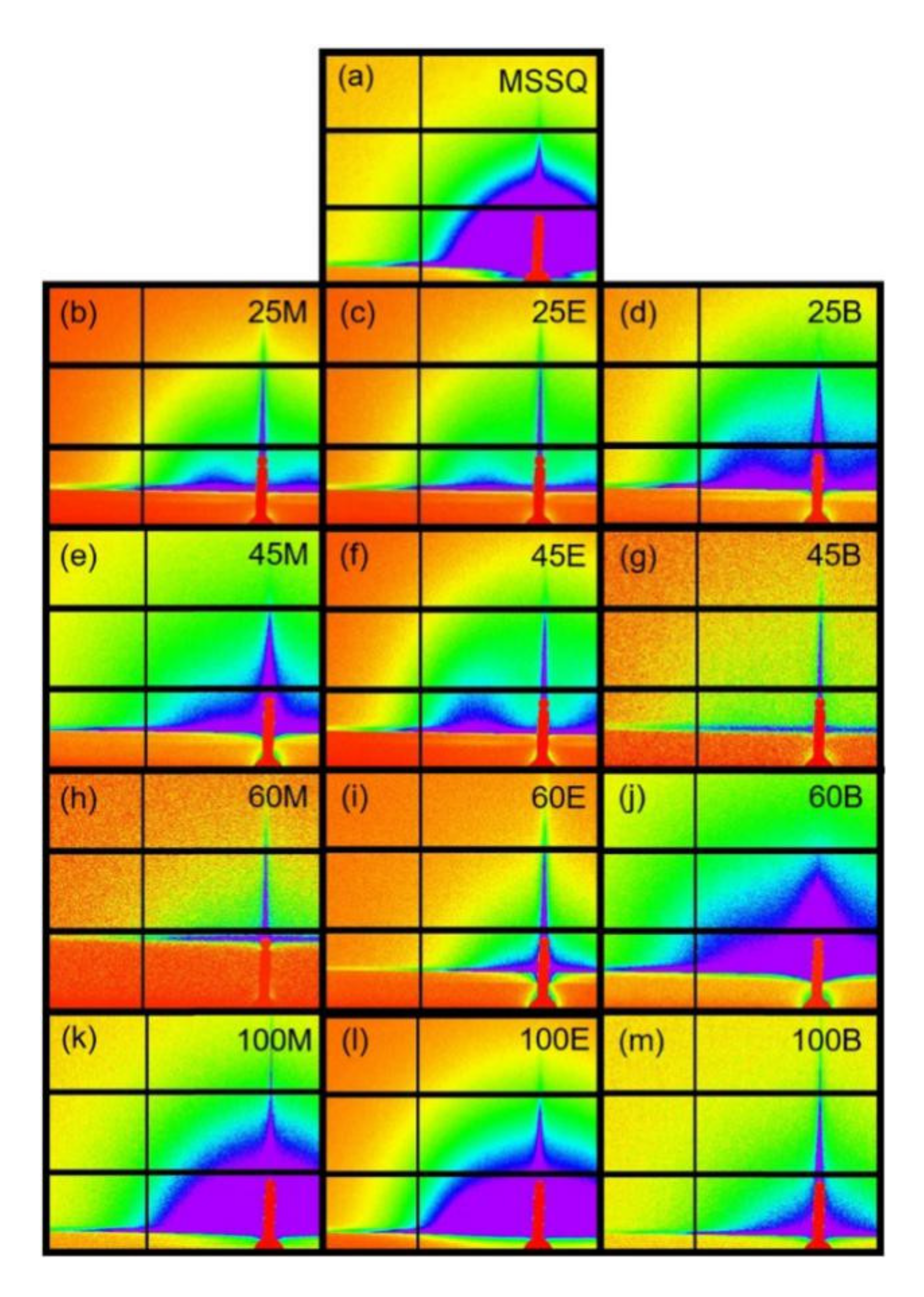
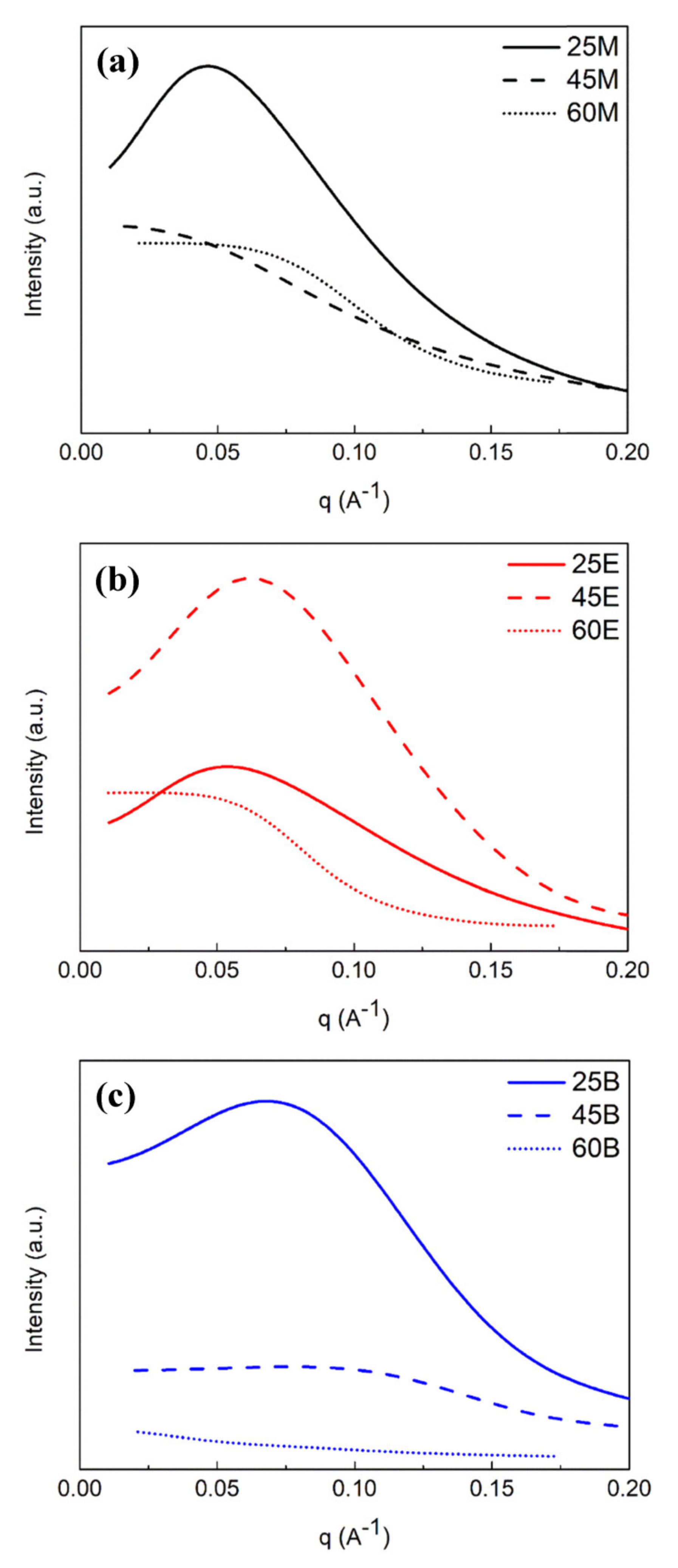
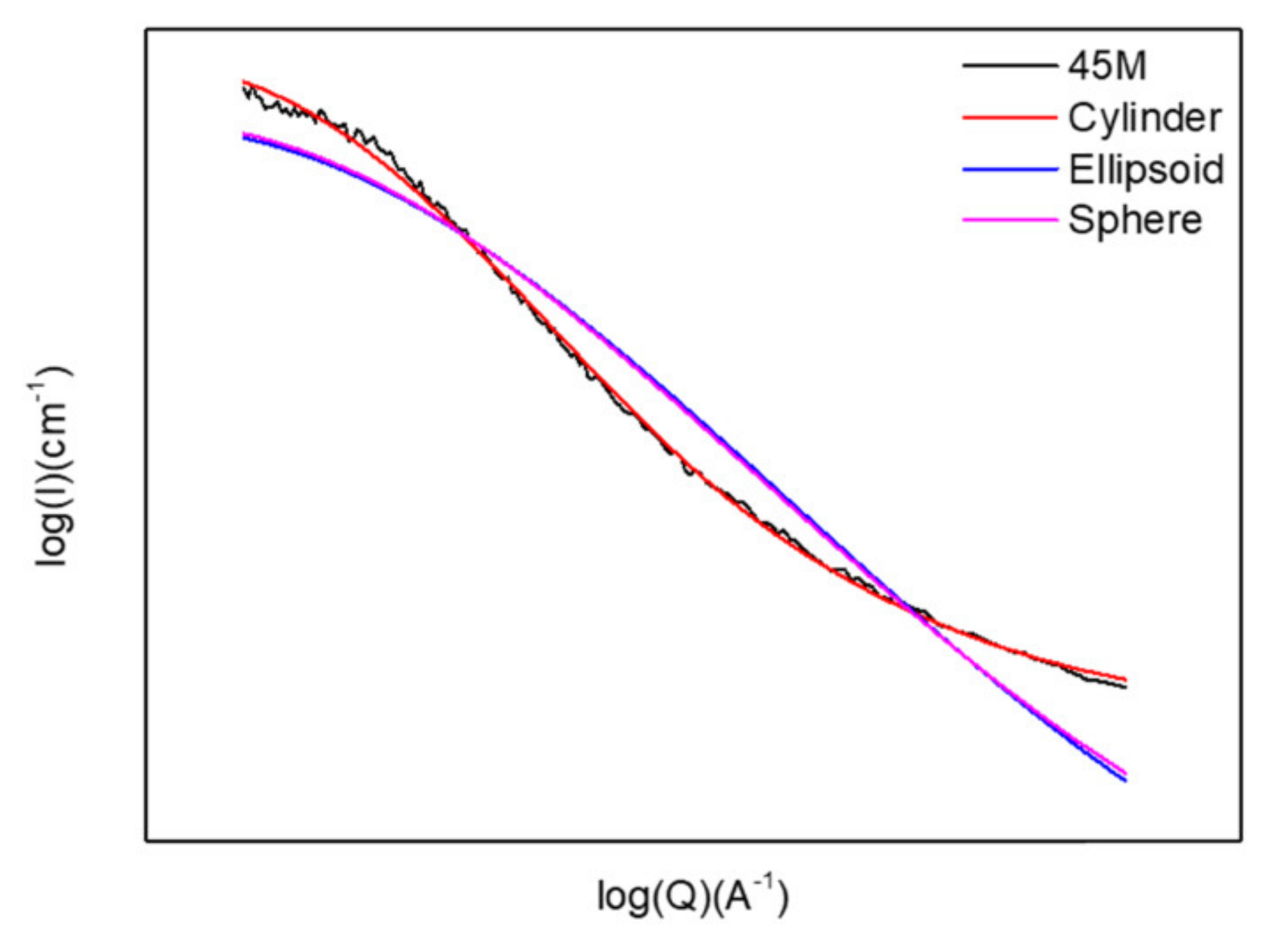
3.4. Pore Geometries Characterized by GISAXS
- –CH2–segment with two rotational axes for BTESM;
- –CH2–CH2– segment with three rotational axes for BTMSE;
- 1,4-phenylene unit with Si in a para-configuration having only one rotational axis for BTESB.
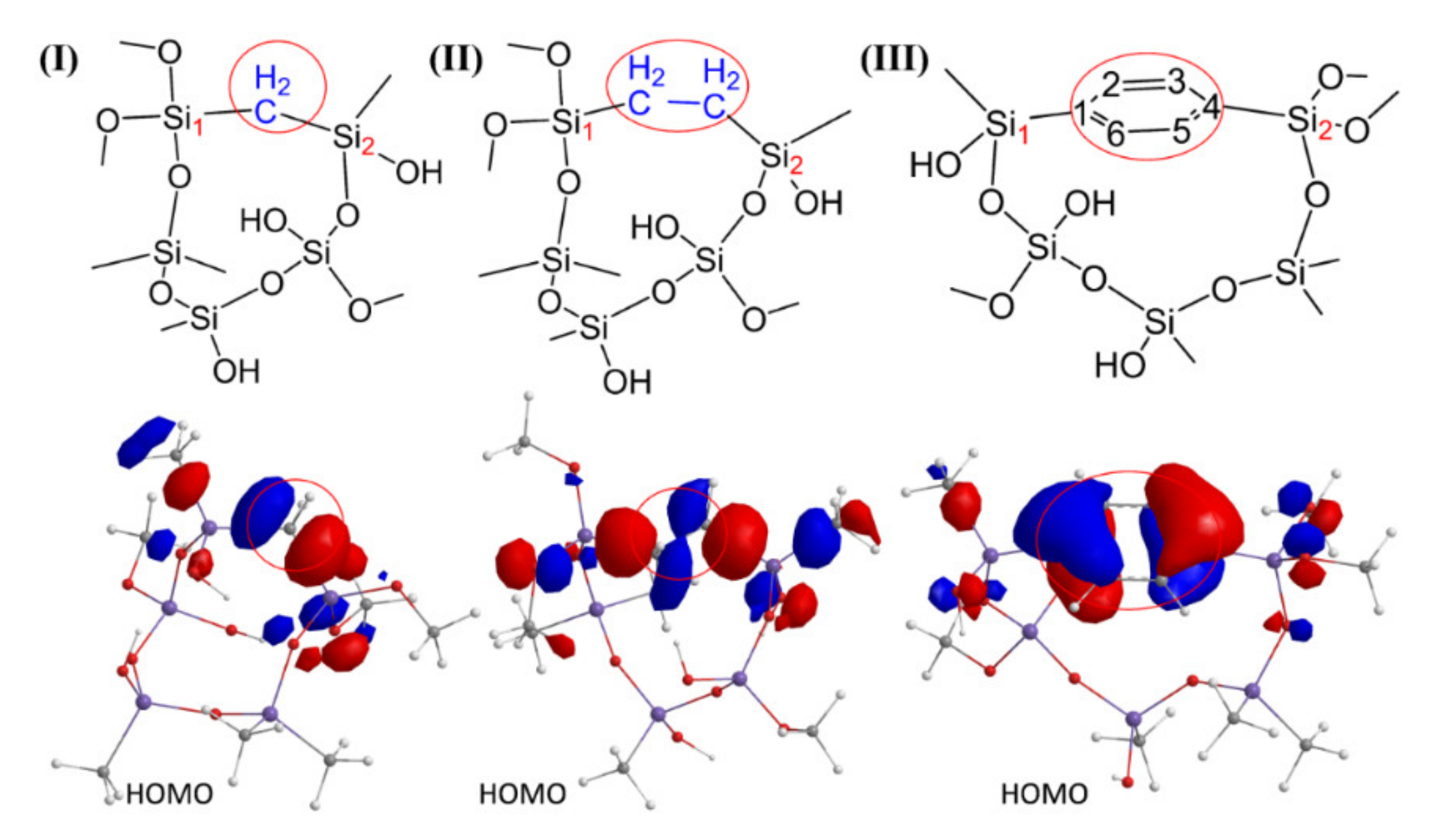
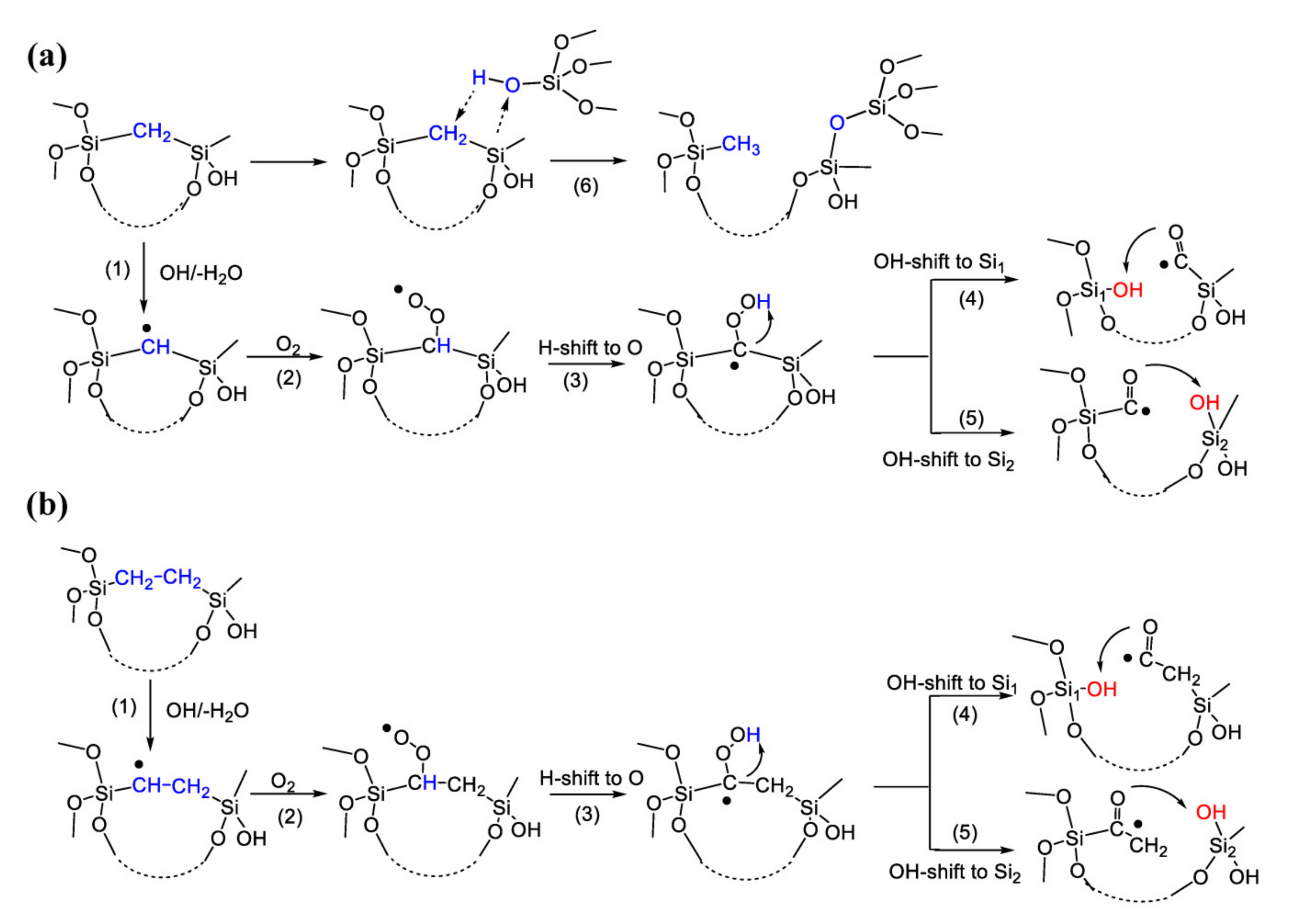
3.5. Bridge Destruction by Residual Oxygen (DFT Data)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maex, K.; Baklanov, M.R.; Shamiryan, D.; Lacopi, F.; Brongersma, S.H.; Yanovitskaya, Z.S. Low dielectric constant materials for microelectronics. J. Appl. Phys. 2003, 93, 8793–8841. [Google Scholar] [CrossRef]
- Grill, A. PECVD low and ultralow dielectric constant materials: From invention and research to products. J. Vac. Sci. Technol. B 2016, 34, 020801. [Google Scholar] [CrossRef]
- Baklanov, M.R.; Adelmann, C.; Zhao, L.; De Gendt, S. Advanced Interconnects: Materials, Processing, and Reliability. Ecs J. Solid State Sci. Technol. 2014, 4, Y1–Y4. [Google Scholar] [CrossRef]
- Palov, A.P.; Voronina, E.N.; Rakhimova, T.V.; Lopaev, D.V.; Zyryanov, S.M.; Mankelevich, Y.A.; Krishtab, M.B.; Baklanov, M.R. Effect of porosity and pore size on dielectric constant of organosilicate based low-k films: An analytical approach. J. Vac. Sci. Technol. BNanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2016, 34, 041205. [Google Scholar] [CrossRef]
- Van der Voort, P.; Esquivel, D.; De Canck, E.; Goethals, F.; Van Driessche, I.; Romero-Salguero, F.J. Periodic Mesoporous Organosilicas: From simple to complex bridges; a comprehensive overview of functions, morphologies and applications. Chem Soc Rev. 2013, 42, 3913–3955. [Google Scholar] [CrossRef]
- Li, H.; Knaup, J.M.; Kaxiras, E.; Vlassak, J.J. Stiffening of organosilicate glasses by organic cross-linking. Acta Mater. 2011, 59, 44–52. [Google Scholar] [CrossRef]
- Volksen, W.; Miller, R.D.; Dubois, G. Low dielectric constant materials. Chem Rev. 2010, 110, 56–110. [Google Scholar] [CrossRef]
- Vanstreels, K.; Li, H.; Vlassak, J.J. Mechanical Reliability of Low-k Dielectrics. In Advanced Interconnects for ULSI Technology; Baklanov, M., Ho, P.S., Zschech, E., Eds.; John Wiley & Sons Inc.: Chichester, West Sussex, UK, 2012; pp. 339–367. [Google Scholar]
- Lu, Y.; Ganguli, R.; Drewien, C.A.; Anderson, M.T.; Brinker, C.J.; Gong, W.; Guo, Y.; Soyez, H.; Dunn, B.; Huang, M.H.; et al. Continuous formation of supported cubic and hexagonal mesoporous films by sol–gel dip-coating. Nature 1997, 389, 364–368. [Google Scholar] [CrossRef]
- Baklanov, M.R.; Mogilnikov, K.P.; Polovinkin, V.G.; Dultsev, F.N. Determination of pore size distribution in thin films by ellipsometric porosimetry. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2000, 18, 1385. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Che, M.-L.; Chuang, S.; Leu, J. The Mechanical Property, Microstructure, and Pore Geometry of a Methyltrimethoxysilane Modified Silica Zeolite (MSZ) Film. J. Electrochem. Soc. 2012, 159, G23–G28. [Google Scholar] [CrossRef]
- Pedersen, J.S. Analysis of small-angle scattering data from colloids and polymer solutions: Modeling and least-squares fitting. Adv. Colloid Interface Sci. 1997, 70, 171–210. [Google Scholar] [CrossRef]
- SasView—Small Angle Scattering Analysis. Available online: http://www.sasview.org (accessed on 11 August 2020).
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 2011, 7, 1564–1583. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Jaguar, Version 9.6 Software for Technical Computation; Schrodinger, Inc.: New York, NY, USA, 2017.
- Blin, J.L.; Carteret, C. Investigation of the Silanols Groups of Mesostructured Silica Prepared Using a Fluorinated Surfactant: Influence of the Hydrothermal Temperature. J. Phys. Chem. C 2007, 111, 14380–14388. [Google Scholar] [CrossRef]
- Grill, A.; Neumayer, D.A. Structure of low dielectric constant to extreme low dielectric constant SiCOH films: Fourier transform infrared spectroscopy characterization. J. Appl. Phys. 2003, 94, 6697–6707. [Google Scholar] [CrossRef]
- Baklanov, M.R.; de Marneffe, J.-F.; Shamiryan, D.; Urbanowicz, A.M.; Shi, H.; Rakhimova, T.V.; Huang, H.; Ho, P.S. Plasma processing of low-k dielectrics. J. Appl. Phys. 2013, 113, 041101. [Google Scholar] [CrossRef]
- Seregin, D.S.; Naumov, S.; Chang, W.Y.; Wu, Y.H.; Wang, Y.; Kotova, N.M.; Vishnevskiy, A.S.; Wei, S.; Zhang, J.; Vorotilov, K.A.; et al. Effect of the C-bridge on UV properties of organosilicate films. Thin Solid Film 2019, 685, 329–334. [Google Scholar] [CrossRef]
- Database of KnowItAll® Informatics System 2018 Academic Edition; Bio-Rad Laboratories, Inc.: Hercules, CA, USA, 2018.
- Kim, S.; Toivola, Y.; Cook, R.F.; Char, K.; Chu, S.-H.; Lee, J.-K.; Yoon, D.Y.; Rhee, H.-W. Organosilicate Spin-on Glasses. J. Electrochem. Soc. 2004, 151, F37. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Kim, B.R.; Kang, J.W.; Lee, K.Y.; Son, J.M.; Ko, M.J. Physical properties of low-k films based on the co-condensation of methyltrimethoxysilane with a bridged silsesquioxane. J. Mater. Sci. 2007, 42, 4591–4602. [Google Scholar] [CrossRef]
- Redzheb, M.; Prager, L.; Krishtab, M.; Armini, S.; Vanstreels, K.; Franquet, A.; Van Der Voort, P.; Baklanov, M.R. UV cure of oxycarbosilane low-k films. Microelectron. Eng. 2016, 156, 103–107. [Google Scholar] [CrossRef]
- Redzheb, M.; Prager, L.; Naumov, S.; Krishtab, M.; Armini, S.; Van Der Voort, P.; Baklanov, M.R. Effect of the C-bridge length on the ultraviolet-resistance of oxycarbosilane low-k films. Appl. Phys. Lett. 2016, 108, 012902. [Google Scholar] [CrossRef]
- Vishnevskiy, A.S.; Seregin, D.S.; Vorotilov, K.A.; Sigov, A.S.; Mogilnikov, K.P.; Baklanov, M.R. Effect of water content on the structural properties of porous methyl-modified silicate films. J. Sol.-Gel Sci. Technol. 2019, 92, 273–281. [Google Scholar] [CrossRef]
- Nenashev, R.N.; Kotova, N.M.; Vishnevskii, A.S.; Vorotilov, K.A. Effect of the Brij 30 porogen on the properties of sol–gel derived thin polymethylsilsesquioxane films. Inorg. Mater. 2016, 52, 968–972. [Google Scholar] [CrossRef]
- Nenashev, R.N.; Kotova, N.M.; Vishnevskii, A.S.; Vorotilov, K.A. Effect of Methyltrimethoxysilane Hydrolysis and Condensation Conditions on the Properties of Thin Polymethylsilsesquioxane Films. Inorg. Mater. 2016, 52, 625–629. [Google Scholar] [CrossRef]
- Ovchinnikov, I.; Vishnevskiy, A.; Seregin, D.; Rezvanov, A.; Schneider, D.; Sigov, A.; Vorotilov, K.A.; Baklanov, M. Evaluation of mechanical properties of porous OSG films by PFQNM AFM and benchmarking with traditional instrumentation. Langmuir ACS J. Surf. Colloids 2020. [Google Scholar] [CrossRef]
- Nenashev, R.N.; Vishnevskiy, A.S.; Kotova, N.M.; Vorotilov, K.A. Properties of Sol–Gel Derived Thin Organoalkylenesiloxane Films. Inorg. Mater. 2018, 54, 405–411. [Google Scholar] [CrossRef]
- Ikeda, M.; Nakahira, J.; Iba, Y.; Kitada, H.; Nishikawa, N.; Miyajima, M.; Fukuyama, S.; Shimizu, N.; Ikeda, K.; Ohba, T.; et al. A highly reliable nano-clustering silica with low dielectric constant (k>2.3) and high elastic modulus (E=10 GPa) for copper damascene process. In In Proceedings of the IEEE 2003 International Interconnect Technology Conference (Cat. No.03TH8695), Burlingame, CA, USA, 4–4 June 2003; pp. 71–73. [Google Scholar] [CrossRef]
- Gaire, C.; Ou, Y.; Arao, H.; Egami, M.; Nakashima, A.; Picu, R.C.; Wang, G.C.; Lu, T.M. Mechanical properties of porous methyl silsesquioxane and nanoclustering silica films using atomic force microscope. J. Porous Mater. 2008, 17, 11–18. [Google Scholar] [CrossRef][Green Version]
- Das, A.; Kokubo, T.; Furukawa, Y.; Struyf, H.; Vos, I.; Sijmus, B.; Iacopi, F.; Van. Aelst, J.; Le, Q.T.; Carbonell, L.; et al. Characterisation and integration feasibility of JSR’s low-k dielectric LKD-5109. Microelectron. Eng. 2002, 64, 25–33. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Baklanov, M.R.; Mogilnikov, K.P. Non-destructive characterisation of porous low-k dielectric films. Microelectron. Eng. 2002, 64, 335–349. [Google Scholar] [CrossRef]
- Jousseaume, V.; Rolland, G.; Babonneau, D.; Simon, J.P. Influence of polymer porogens on the porosity and mechanical properties of spin coated Ultra Low k dielectrics. Thin Solid Film. 2009, 517, 4413–4418. [Google Scholar] [CrossRef]
- Van Der Voort, P.; Leus, K.; De Canck, E. Introduction to Porous Materials; John Wiley & Sons: Cambridge, UK, 2019. [Google Scholar]
- Rasadujjaman, M.; Wang, Y.; Zhang, L.; Naumov, S.; Attallah, A.G.; Liedke, M.O.; Koehler, N.; Redzheb, M.; Vishnevskiy, A.S.; Seregin, D.S.; et al. A detailed ellipsometric porosimetry and positron annihilation spectroscopy study of porous organosilicate-glass films with various ratios of methyl terminal and ethylene bridging groups. Microporous Mesoporous Mater. 2020, 306, 110434. [Google Scholar] [CrossRef]
- Asefa, T.; MacLachlan, M.J.; Grondey, H.; Coombs, N.; Ozin, G.A. Metamorphic Channels in Periodic Mesoporous Methylenesilica. Angew. Chem. Int. Ed. 2000, 39, 1808–1811. [Google Scholar] [CrossRef]
- Hatton, B.D.; Landskron, K.; Hunks, W.J.; Bennett, M.R.; Shukaris, D.; Perovic, D.D.; Ozin, G.A. Materials chemistry for low-k materials. Mater. Today 2006, 9, 22–31. [Google Scholar] [CrossRef]
- Mankelevich, Y.; Voronina, E.; Rakhimova, T.V.; Palov, A.; Lopaev, D.V.; Zyryanov, S.; Baklanov, M.R. Multi-step reaction mechanism for F atom interactions with organosilicate glass and SiO x films. J. Phys. D. Appl. Phys. 2016, 49, 345203. [Google Scholar] [CrossRef]
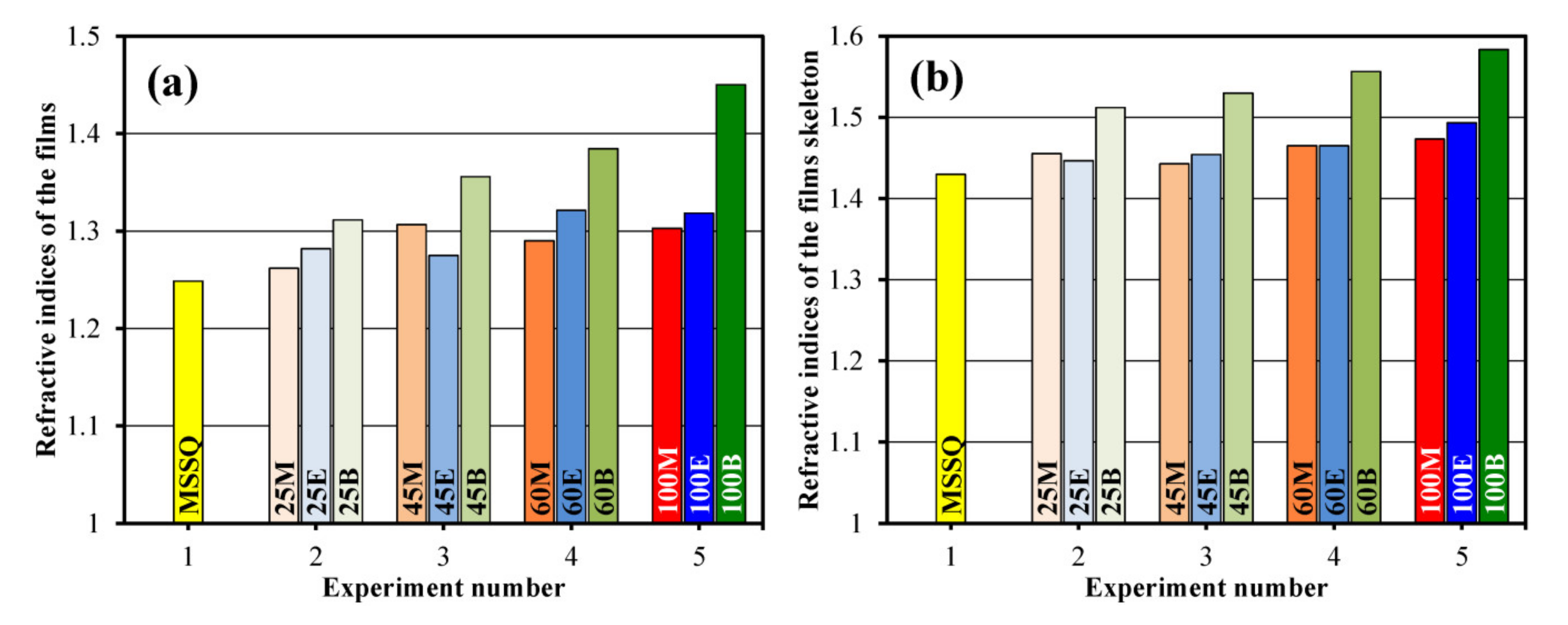


| Exp. No | Sample No | Sol Composition (mol%) | Thickness d (nm) | Shrinkage Δd (%) | Refractive Index | Pore Radius (nm) | Porosity (%) | Young’s Modulus (GPa) | k-Value | Skeleton Refractive Index |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MSSQ | MTMS = 100 | 298 | 15 | 1.25 | 3.00 | 39.2 | 1.7 * | 2.25 | 1.43 |
| 2 | 25M | MTMS = 75 BTESM = 25 | 243 | 14 | 1.26 | 1.71 | 39.3 | 5.0 | 2.35 | 1.46 |
| 25E | MTMS = 75 BTMSE = 25 | 238 | 20 | 1.28 | 1.42 | 33.9 | 6.6 | 2.37 | 1.45 | |
| 25B | MTMS = 75 BTESB = 25 | 312 | 19 | 1.31 | 1.10 | 35.5 | 7.0 | 2.39 | 1.51 | |
| 3 | 45M | MTMS = 55 BTESM = 45 | 260 | 16 | 1.31 | 1.10 | 28.0 | 8.5 | 2.48 | 1.44 |
| 45E | MTMS = 55 BTMSE = 45 | 419 | 19 | 1.28 | 1.71 | 36.3 | 8.3 | 2.60 | 1.45 | |
| 45B | MTMS = 55 BTESB = 45 | 160 | 17 | 1.36 | 0.76 | 29.3 | 11.2 | 2.95 | 1.53 | |
| 4 | 60M | MTMS = 40 BTESM = 60 | 327 | 27 | 1.29 | 0.92 | 34.4 | 11.6 | - | 1.47 |
| 60E | MTMS = 40 BTMSE = 60 | 227 | 30 | 1.32 | 0.97 | 28.0 | 16.5 | - | 1.47 | |
| 60B | MTMS = 40 BTESB = 60 | 110 | 38 | 1.39 | 0.76 | 27.2 | 14.1 | - | 1.56 | |
| 5 | 100M | MTMS = 0 BTESM = 100 | 183 | 26 | 1.30 | 1.42 | 32.8 | 14.5 | - | 1.47 |
| 100E | MTMS = 0 BTMSE = 100 | 203 | 27 | 1.32 | 1.42 | 32.1 | 16.0 | - | 1.49 | |
| 100B | MTMS = 0 BTESB = 100 | 162 | 32 | 1.45 | 0.76 | 19.6 | 18.0 | - | 1.58 |
| Exp. No | Sample No | Sol Composition (mol%) | Grazing-incidence Small-angle X-ray Scattering Data | EP Pore Diam. (nm) | |||
|---|---|---|---|---|---|---|---|
| Diam. (nm) | Length L (nm) | Aspect Ratio (diam./length) | Pore-to-pore Distance (nm) | ||||
| 1 | MSSQ | MTMS = 100 | 4.6 | 3.4 | 1.35 | - | 6.00 |
| 2 | 25M | MTMS = 75 BTESM = 25 | 3.8 | 2.2 | 1.73 | 19.0 | 3.42 |
| 25E | MTMS = 75 BTMSE = 25 | 2.6 | 1.2 | 2.17 | 10.8 | 2.84 | |
| 25B | MTMS = 75 BTESB = 25 | 2.2 | 1.0 | 2.20 | 8.1 | 2.20 | |
| 3 | 45M | MTMS = 55 BTESM = 45 | 2.0 | 0.6 | 3.33 | - | 2.20 |
| 45E | MTMS = 55 BTMSE = 45 | 3.4 | 2.0 | 1.70 | 8.6 | 3.42 | |
| 45B | MTMS = 55 BTESB = 45 | 3.2 | 1.8 | 1.78 | - | 1.52 | |
| 4 | 60M | MTMS = 40 BTESM = 60 | 2.0 | 1.0 | 2.00 | - | 1.84 |
| 60E | MTMS = 40 BTMSE = 60 | 1.8 | 0.6 | 3.00 | - | 1.94 | |
| 60B | MTMS = 40 BTESB = 60 | 3.0 | 1.5 | 2.00 | - | 1.52 | |
| 5 | 100M | MTMS = 0 BTESM = 100 | 2.0 | 0.8 | 2.50 | - | 2.84 |
| 100E | MTMS = 0 BTMSE = 100 | 1.8 | 0.8 | 2.25 | - | 2.84 | |
| 100B | MTMS = 0 BTESB = 100 | 1.6 | 0.5 | 3.20 | - | 1.52 | |
| Reaction | –Si–CH2–Si– | –Si–CH2–CH2–Si– | –Si–1,4-Ar–Si– |
|---|---|---|---|
| 1 | ΔH = −13 ΔG = −15 | ΔH = −26 ΔG = −27 | ΔH = −3 ΔG = −4 |
| 2 | ΔH = −19 ΔG = −11 | ΔH = −26 ΔG = −14 | ΔH = −47 ΔG = −34 |
| 3 | ΔH = −13 ΔG = −15 | ΔH = −32 ΔG = −32 | ΔH = +15 ΔG = +15 |
| 4 | ΔH = −63 ΔG = −63 | ΔH = −51 ΔG = −50 | ΔH = +18 ΔG = +17 |
| 5 | ΔH = −57 ΔG = −57 | ΔH = −46 ΔG = −46 | ΔH = −26 ΔG = −13 |
| 6 | ΔH = −18 ΔG = −20 | - | ΔH = −5 ΔG = −8 |
| 7 | - | - | ΔH = +25 ΔG = +22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnevskiy, A.S.; Naumov, S.; Seregin, D.S.; Wu, Y.-H.; Chuang, W.-T.; Rasadujjaman, M.; Zhang, J.; Leu, J.; Vorotilov, K.A.; Baklanov, M.R. Effects of Methyl Terminal and Carbon Bridging Groups Ratio on Critical Properties of Porous Organosilicate Glass Films. Materials 2020, 13, 4484. https://doi.org/10.3390/ma13204484
Vishnevskiy AS, Naumov S, Seregin DS, Wu Y-H, Chuang W-T, Rasadujjaman M, Zhang J, Leu J, Vorotilov KA, Baklanov MR. Effects of Methyl Terminal and Carbon Bridging Groups Ratio on Critical Properties of Porous Organosilicate Glass Films. Materials. 2020; 13(20):4484. https://doi.org/10.3390/ma13204484
Chicago/Turabian StyleVishnevskiy, Alexey S., Sergej Naumov, Dmitry S. Seregin, Yu-Hsuan Wu, Wei-Tsung Chuang, Md Rasadujjaman, Jing Zhang, Jihperng Leu, Konstantin A. Vorotilov, and Mikhail R. Baklanov. 2020. "Effects of Methyl Terminal and Carbon Bridging Groups Ratio on Critical Properties of Porous Organosilicate Glass Films" Materials 13, no. 20: 4484. https://doi.org/10.3390/ma13204484
APA StyleVishnevskiy, A. S., Naumov, S., Seregin, D. S., Wu, Y.-H., Chuang, W.-T., Rasadujjaman, M., Zhang, J., Leu, J., Vorotilov, K. A., & Baklanov, M. R. (2020). Effects of Methyl Terminal and Carbon Bridging Groups Ratio on Critical Properties of Porous Organosilicate Glass Films. Materials, 13(20), 4484. https://doi.org/10.3390/ma13204484







