Micro- and Nanoscale Spectroscopic Investigations of Threonine Influence on the Corrosion Process of the Modified Fe Surface by Cu Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. AFM Measurements
2.3. FT-IR and SEIRA Measurements
2.4. RS and SERS Measurements
2.5. Nano-SEIRA Measurements
2.6. Data Analysis
2.7. Fitting Procedure
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stupnišek-Lisac, E.; Lončarić Božić, A.; Cafuk, I. Low-toxicity copper corrosion inhibitors. Corrosion 1998, 54, 713–720. [Google Scholar] [CrossRef]
- Tang, Z. A review of corrosion inhibitors for rust preventative fluids. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100759–100775. [Google Scholar] [CrossRef]
- Stoyanova, A.E.; Sokolova, E.I.; Raicheva, S.N. The inhibition of mild steel corrosion in 1 M HCL in the presence of linear and cyclic thiocarbamides—Effect of concentration and temperature of the corrosion medium on their protective action. Corros. Sci. 1997, 39, 1595–1604. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Li, Y.; Wang, F.H. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid Interface Sci. 2007, 310, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Sarkar, T.K.; Purkait, T. Amino acid compounds as eco-friendly corrosion inhibitor for N80 steel in HCl solution: Electrochemical and theoretical approaches. J. Mol. Liq. 2015, 212, 731–738. [Google Scholar] [CrossRef]
- Kesavan, D.; Gopiraman, M.; Sulochana, N. Green inhibitors for corrosion of metals: A review. Chem. Sci. Rev. Lett. 2012, 1, 1–8. Available online: https://chesci.com/wp-content/uploads/2016/12/V1i1_1_CS10204205.pdf (accessed on 20 August 2020).
- Amin, M.A.; Khaled, K.F.; Mohsen, Q.; Arida, H.A. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green corrosion inhibitors from natural sources and biomass wastes. Molecules 2019, 24, 48. [Google Scholar] [CrossRef]
- Palaniappan, N.; Cole, I.S.; Kuznetsov, A.E.; Balasubramanian, K.; Thomas, K.R.J. Experimental and computational studies of a graphene oxide barrier layer covalently functionalized with amino acids on Mg AZ13 alloy in salt medium. RSC Adv. 2019, 9, 32441–32447. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Cai, Q.R.; Gao, L.X.; Lee, K.Y. Effect of serine, threonine and glutamic acid on the corrosion of copper in aerated hydrochloric acid solution. Corros. Sci. 2008, 50, 3615–3621. [Google Scholar] [CrossRef]
- Makarenko, N.V.; Kharchenko, U.V.; Zemnukhova, L.A. Effect of amino acids on corrosion of copper and steel in acid medium. Russ. J. Appl. Chem. 2011, 84, 1362–1365. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, M.; Hu, J.; Wang, C.; Xu, S.; Han, C.C. Preparation and optimization of silver nanoparticles embedded electrospun membrane for implant associated infections prevention. ACS Appl. Mater. Interfaces 2013, 5, 11014–11021. [Google Scholar] [CrossRef] [PubMed]
- Kusior, A.; Kollbek, K.; Kowalski, K.; Borysiewicz, M.; Wojciechowski, T.; Adamczyk, A.; Trenczek-Zajac, A.; Radecka, M.; Zakrzewska, K. Sn and Cu oxide nanoparticles deposited on TiO2 nanoflower 3D substrates by inert gas condensation technique. Appl. Surf. Sci. 2016, 380, 193–202. [Google Scholar] [CrossRef]
- Abbasi Kesbi, F.; Rashid, A.M.; Astinchap, B. Preparation of ultrafine grained copper nanoparticles via immersion deposit method. Appl. Nanosci. 2018, 8, 221–230. [Google Scholar] [CrossRef]
- Ustunol, I.B.; Gonzalez-Pech, N.I.; Grassian, V.H. pH-dependent adsorption of α-amino acids, lysine, glutamic acid, serine and glycine, on TiO2 nanoparticle surfaces. J. Colloid Interface Sci. 2019, 554, 362–375. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Tierolf, M.W.A.M.; Valerio, V.P.M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachiteia, I.; Zadpoor, A.A. Self-defending additively manufactured bone implants bearing silver and copper nanoparticles. J. Mater. Chem. B 2020, 8, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M. Electrochemical synthesis of copper oxide particles with controlled oxidation state, shape and size. Mater. Res. Express. 2019, 6, 0850a3. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kim, K.; Shin, I.-S.; Shin, K.S. Antioxidative metallic copper nanoparticles prepared by modified polyol method and their catalytic activities. J. Nanopart. Res. 2020, 22, 8. [Google Scholar] [CrossRef]
- Magdassi, S.; Grouchko, M.; Kamyshny, A. Copper nanoparticles for printed electronics: Routes towards achieving oxidation stability. Materials 2010, 3, 4626–4638. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Bogdanović, U.; Lazić, V.; Vodnik, V.; Budimir, M.; Marković, Z.; Dimitrijević, S. Copper nanoparticles with high antimicrobial activity. Mater. Lett. 2014, 128, 75–78. [Google Scholar] [CrossRef]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, C.; Ren, G.; Ren, L. Study on behaviour and mechanism of Cu2+ ion release from Cu bearing antibacterial stainless steel. Mater. Technol. 2015, 30, B126–B132. [Google Scholar] [CrossRef]
- Hameed, H.A.; Ariffin, A.; Luddin, N.; Husein, A. Evaluation of antibacterial properties of copper nanoparticles surface coating on titanium dental implant. J. Pharm. Sci. Res. 2018, 10, 1157–1160. Available online: https://www.jpsr.pharmainfo.in/Documents/Volumes/vol10Issue05/jpsr10051840.pdf (accessed on 15 August 2020).
- Li, D.; Chen, S.; Zhao, S.; Ma, H. The corrosion inhibition of the self- assembled Au, and Ag nanoparticles films on the surface of copper. Colloids Surf. A 2006, 273, 16–23. [Google Scholar] [CrossRef]
- El-Ansary, A.; Faddah, L.M. Nanoparticles as biochemical sensors. Nanotechnol. Sci. Appl. 2010, 3, 65–76. [Google Scholar] [CrossRef]
- Bozzini, B.; De Gaudenzi, G.P.; Mele, C. An in-situ FT-IR investigation of the anodic behaviour of WC-Co hardmetal. Mater. Corros. 2003, 54, 694–696. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, K.; Dong, C.; Wu, J.; Li, X.; Huang, Y. In situ Raman spectroscopy study of corrosion products on the surface of carbon steel in solution containing Cl− and SO42−. Eng. Faul. Anal. 2011, 18, 1981–1989. [Google Scholar] [CrossRef]
- Piergies, N.; Pięta, E.; Paluszkiewicz, C.; Domin, H.; Kwiatek, W.M. Polarization effect in tip-enhanced infrared nanospectroscopy studies of the selective Y5 receptor antagonist Lu AA33810. Nano Res. 2018, 11, 4401–4411. [Google Scholar] [CrossRef]
- Pięta, E.; Paluszkiewicz, C.; Kwiatek, W.M. Multianalytical approach to surface- and tip-enhanced infrared spectroscopy study of molecule-metal conjugate: Deducing the adsorption geometry. Phys. Chem. Chem. Phys. 2018, 20, 27992–28000. [Google Scholar] [CrossRef]
- Pięta, E.; Petibois, C.; Paluszkiewicz, C.; Kwiatek, W.M. Physico-chemical analysis of molecular binding to the colloidal metal nanostructure: Multiple micro- and nanospectroscopy study. Appl. Surf. Sci. 2020, 499, 143975. [Google Scholar] [CrossRef]
- Doering, W.E.; Nie, S. Single-molecule and single-nanoparticle SERS: Examining the roles of surface active sites and chemical enhancement. J. Phys. Chem. B 2002, 106, 311–317. [Google Scholar] [CrossRef]
- Aroca, R.F. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Blaber, M.G.; Arnold, M.D.; Harris, N.; Ford, M.J.; Cortie, M.B. Plasmon absorption in nanosphers: A comparision of sodium, potassium, aluminium, silver and gold. Physica B Cond. Mat. 2007, 394, 184–187. [Google Scholar] [CrossRef]
- Hartstein, A.; Kirtley, J.R.; Tsang, J.C. Enhancement of the infrared absorption from molecular monolayers with thin metal overlayers. Phys. Rev. Lett. 1980, 15, 201–204. [Google Scholar] [CrossRef]
- Osawa, M.M.; Ikeda, M. Surface-enhanced infrared absorption of p-nitrobenzoic acid deposited on silver island films: Contribution of electromagnetic and chemical mechanisms. J. Phys. Chem. 1991, 95, 9914–9919. [Google Scholar] [CrossRef]
- Dazzi, A.; Prazeres, R.; Glotin, F.; Ortega, J.M. Subwavelength infrared spectromicroscopy using an AFM as a local absorption sensor. Infrared Phys. Technol. 2006, 49, 113–121. [Google Scholar] [CrossRef]
- Dazzi, A.; Prater, C.B. AFM-IR: Technology and applications in nanoscale infrared spectroscopy and chemical imaging. Chem. Rev. 2017, 117, 5146–5173. [Google Scholar] [CrossRef]
- Ruggeri, F.S.; Habchi, J.; Cerreta, A.; Dietler, G. AFM-based single molecule techniques: Unraveling the amyloid Pathogenic Species. Curr. Pharm. Des. 2016, 22, 3950–3970. [Google Scholar] [CrossRef]
- Dazzi, A.; Prater, C.B.; Hu, Q.C.; Chase, D.B.; Rabolt, J.F.; Marcott, C. AFM-IR: Combining atomic force microscopy and infrared spectroscopy for nanoscale chemical characterization. Appl. Spectrosc. 2012, 66, 1365–1384. [Google Scholar] [CrossRef]
- Święch, D.; Ozaki, Y.; Kim, Y.; Proniewicz, E. Surface- and tip-enhanced Raman scattering of bradykinin onto the colloidal suspended Ag surface. Phys. Chem. Chem. Phys. 2015, 17, 17140–17149. [Google Scholar] [CrossRef]
- Piergies, N.; Dazzi, A.; Deniset-Beseau, A.; Mathurin, J.; Oćwieja, M.; Paluszkiewicz, C.; Kwiatek, W.M. Nanoscale image of the drug/metal mono-layer interaction: Tapping AFM-IR investigations. Nano Res. 2020, 13, 1020–1028. [Google Scholar] [CrossRef]
- Chisanga, M.; Muhamadali, H.; Ellis, D.I.; Goodacre, R. Enhancing disease diagnosis: Biomedical applications of surface-enhanced Raman scattering. Appl. Sci. 2019, 9, 1163. [Google Scholar] [CrossRef]
- Xie, W.; Schlücker, S. Surface-enhanced Raman spectroscopic detection of molecular chemo- and plasmo-catalysis on noble metal nanoparticles. Chem. Commun. 2018, 54, 2326–2336. [Google Scholar] [CrossRef]
- Vendrell, M.; Maiti, K.K.; Dhaliwal, K.; Chang, Y.T. Surface-enhanced Raman scattering in cancer detection and imaging. Trends Biotechnol. 2013, 31, 249–257. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Sommertune, J.; Örnek, C.; Ryl, J.; Kurilo, I.I.; Claessona, P.M.; Pan, J. Corrosion inhibition of aluminium alloy AA6063-T5 by vanadates: Local surface chemical events elucidated by confocal Raman micro-spectroscopy. Corros. Sci. 2019, 148, 237–250. [Google Scholar] [CrossRef]
- Johnson, C.M.; Böhmler, M. Nano-FTIR microscopy and spectroscopy studies of atmospheric corrosion with a spatial resolution of 20 nm. Corros. Sci. 2016, 108, 60–65. [Google Scholar] [CrossRef]
- Ruggeri, F.S.; Longo, G.; Faggiano, S.; Lipiec, E.; Pastore, A.; Dietler, G. Infrared nanospectroscopy characterization of oligomeric and fibrillar aggregates during amyloid formation. Nat. Commun. 2015, 6, 7831. [Google Scholar] [CrossRef]
- Singh, G.P.; Moon, A.P.; Sengupta, S.; Deo, G.; Sangal, S.; Mondal, K. Corrosion behavior of IF steel in various media and its comparison with mild steel. J. Mater. Eng. Perform. 2015, 24, 1961–1974. [Google Scholar] [CrossRef]
- Veneranda, M.; Aramendia, J.; Bellot-Gurlet, L.; Colomban, P.; Castro, K.; Madariaga, J.M. FTIR spectroscopic semi-quantification of iron phases: A new method to evaluate the protection ability index (PAI) of archaeological artefacts corrosion systems. Corros. Sci. 2018, 133, 68–77. [Google Scholar] [CrossRef]
- Inoue, K.; Kwon, S.-K.; Suzuki, S.; Saito, M.; Waseda, Y. Atomic-scale structure and morphology of ferric oxyhydroxides formed by corrosion of an iron–silicon alloy. Mater. Trans. 2006, 47, 243–246. [Google Scholar] [CrossRef][Green Version]
- Johnson, G.E.; Colby, R.; Laskin, J. Soft landing of bare nanoparticles with controlled size, composition, and morphology. Nanoscale 2015, 7, 3491–3503. [Google Scholar] [CrossRef]
- Musić, S.; Gotić, M.; Popović, S. X-ray diffraction and Fourier transform-infrared analysis of the rust formed by corrosion of steel in aqueous solutions. J. Mater. Sci. 1993, 28, 5744–5752. [Google Scholar] [CrossRef]
- Hedberga, J.; Karlssonb, H.L.; Hedberga, Y.; Blomberga, E.; Wallinder, I.O. The importance of extracellular speciation and corrosion of copper nanoparticles on lung cell membrane integrity. Colloid Surf. B 2016, 141, 291–300. [Google Scholar] [CrossRef]
- Debbichi, L.; Marco de Lucas, M.C.; Pierson, J.F.; Krüger, P. Vibrational properties of CuO and Cu4O3 from first-principles calculations, and Raman and infrared Spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Refait, P. On the formation of b-FeOOH (akaganéite) in chloride-containing environments. Corros. Sci. 2007, 49, 844–857. [Google Scholar] [CrossRef]
- Pawlukojc´, A.; Leciejewicz, J.; Tomkinson, J.; Parker, S.F. Neutron scattering, infrared, Raman spectroscopy and ab initio study of L-threonine. Spectrochim. Acta A 2001, 57, 2513–2523. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta A 2011, 78, 1187–1195. [Google Scholar] [CrossRef]
- Hernández, B.; Pflüger, F.; Adenier, A.; Nsangou, M.; Kruglik, S.G.; Ghomi, M. Energy maps, side chain conformational flexibility, and vibrational features of polar amino acids L-serine and L-threonine in aqueous environment. J. Phys. Chem. 2011, 135, 08B601. [Google Scholar] [CrossRef]
- Wolpert, M.; Hellwig, P. Infrared spectra and molar absorption coefficients of the 20 alpha amino acids in aqueous solutions in the spectral range from 1800 to 500 cm−1. Spectrochim. Acta A 2006, 64, 987–1001. [Google Scholar] [CrossRef]
- Stewart, S.; Fredericks, P.M. Surface-enhanced Raman spectroscopy of amino acids adsorbed on an electrochemically prepared silver surface. Spectrochim. Acta A 1999, 55, 1641–1660. [Google Scholar] [CrossRef]
- Negri, P.; Schultz, Z.D. Online SERS detection of the 20 proteinogenic L-amino acids separated by capillary zone electrophoresis. Analyst 2014, 139, 5989–5998. [Google Scholar] [CrossRef]
- Guzzetti, K.A.; Brizuela, A.B.; Romano, E.; Brandán, S.A. Structural and vibrational study on zwitterions of L-threonine in aqueous phase using the FT-Raman and SCRF calculations. J. Mol. Struct. 2013, 1045, 171–179. [Google Scholar] [CrossRef]
- Quesada-Moreno, M.M.; Márquez-García, A.A.; Avilés-Moreno, J.R.; López-González, J.J. Conformational landscape of L-threonine in neutral, acid and basic solutions from vibrational circular dichroism spectroscopy and quantum chemical calculations. Tetrahedron 2013, 24, 1537–1547. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyer, R.A., Ed.; John Wiley & Sons Ltd: Chichester, UK, 2000. [Google Scholar]
- Costa, D.; Savio, L.; Pradier, C.-M. Adsorption of amino acids and peptides on metal and oxide surfaces in water environment: A synthetic and prospective review. J. Phys. Chem. B 2016, 120, 7039–7052. [Google Scholar] [CrossRef]
- Talley, C.E.; Jusinski, L.; Hollars, C.W.; Lane, S.M.; Huser, T. Intracellular pH sensors based on surface-enhanced Raman scattering. Anal. Chem. 2004, 76, 7064–7068. [Google Scholar] [CrossRef]
- Moskovits, M. Surface selection rules. J. Chem. Phys. 1982, 77, 4408–4416. [Google Scholar] [CrossRef]
- Creighton, J.A. Surface Raman electromagnetic enhancement factors for molecules at the surface of small isolated metal spheres: The determination of adsorbate orientation from SERS relative intensities. Surf. Sci. 1983, 124, 209–219. [Google Scholar] [CrossRef]
- Suh, J.S.; Moskovits, M. Surface-enhanced Raman spectroscopy of amino acids and nucleotide bases adsorbed on silver. J. Am. Chem. Soc. 1986, 108, 4711–4718. [Google Scholar] [CrossRef]
- Suh, J.S.; Kim, J. Three distinct geometries of surface-adsorbed carboxylate groups. J. Raman Spectrosc. 1998, 29, 143–148. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A unified approach to surface-enhanced Raman spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617. [Google Scholar] [CrossRef]
- Dou, X.; Jung, Y.M.; Yamamoto, H.; Doi, S.; Ozaki, Y. Near-Infrared excited surface-enhanced Raman scattering of biological molecules on gold colloid I: Effects of pH of the solutions of amino acids and of their polymerization. Appl. Spectrosc. 1999, 53, 133–138. [Google Scholar] [CrossRef]
- Morad, M.S. Effect of amino acids containing sulfur on the corrosion of mild steel in phosphoric acid solutions containing Cl−, F− and Fe3+ ions: Behavior under polarization conditions. J. Appl. Electrochem. 2005, 35, 889–895. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; García, P.F.; Merck, G.K.; Bodensteiner, F.A.; Heissler, S.; Günther, S.; Berensmeier, S. On the nature of interactions of amino acids with bare magnetite nanoparticles. J. Phys. Chem. C 2015, 119, 23032–23041. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Grassian, V.H. Histidine adsorption on TiO2 nanoparticles: An integrated spectroscopic, thermodynamic, and molecular-based approach toward understanding nano-bio interactions. Langmuir 2014, 30, 8751–8760. [Google Scholar] [CrossRef]
- Begonja, S.; Rodenas, L.G.; Borghi, E.B.; Morando, P.J. Adsorption of cysteine on TiO2 at different pH values: Surface complexes characterization by FTIR-ATR and Langmuir isotherms analysis. Colloids Surf. A 2012, 403, 114–120. [Google Scholar] [CrossRef]
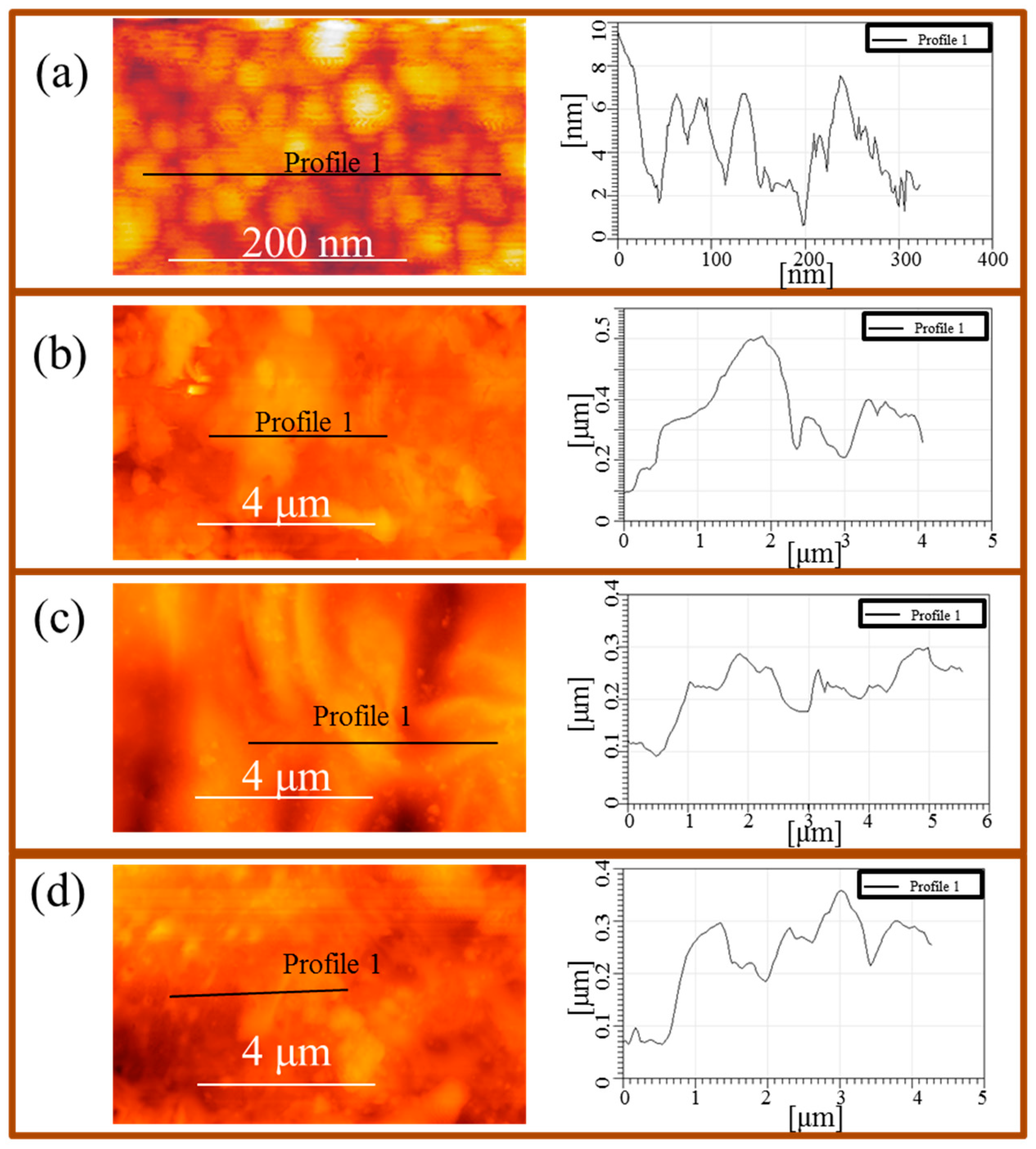
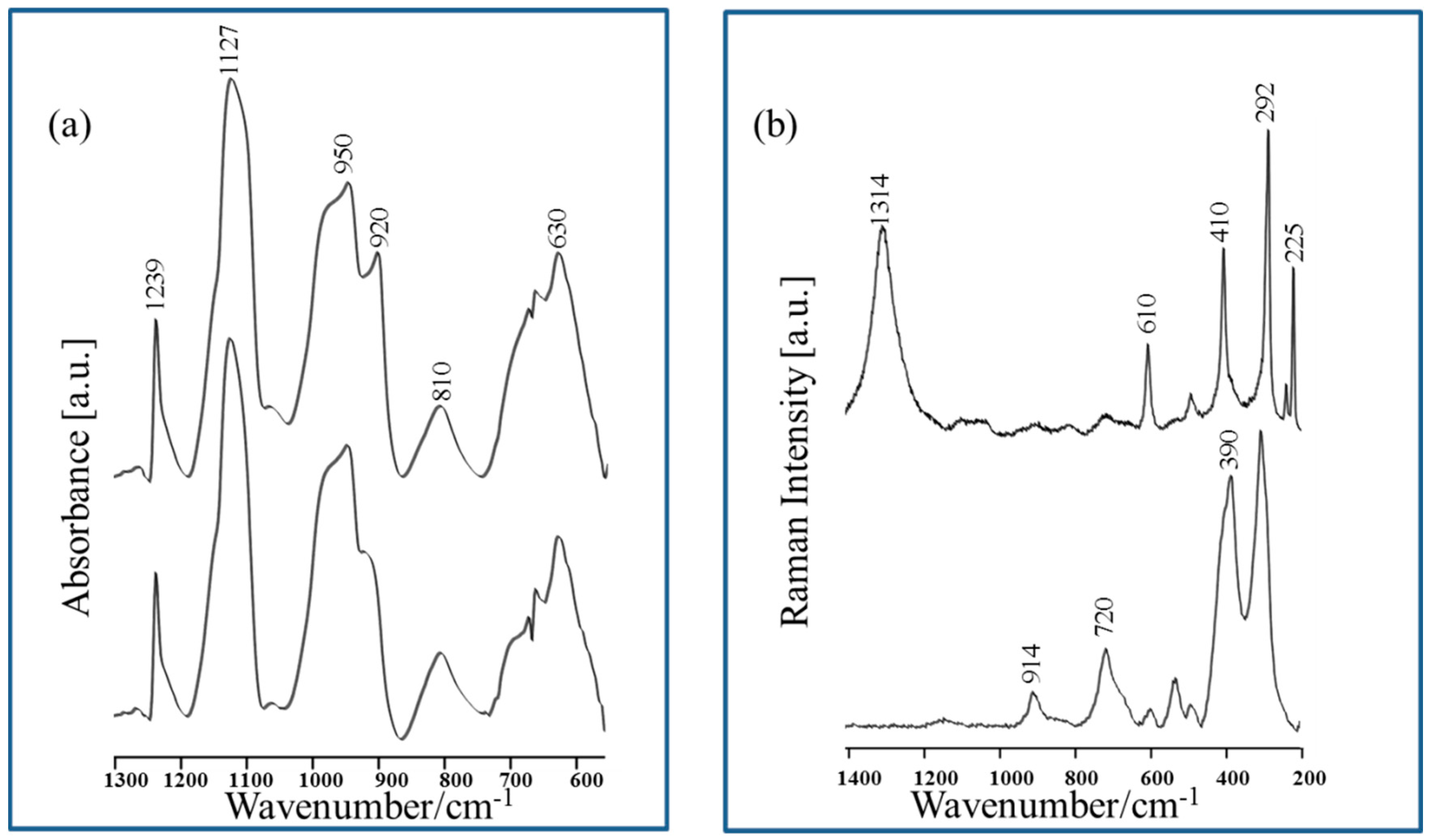
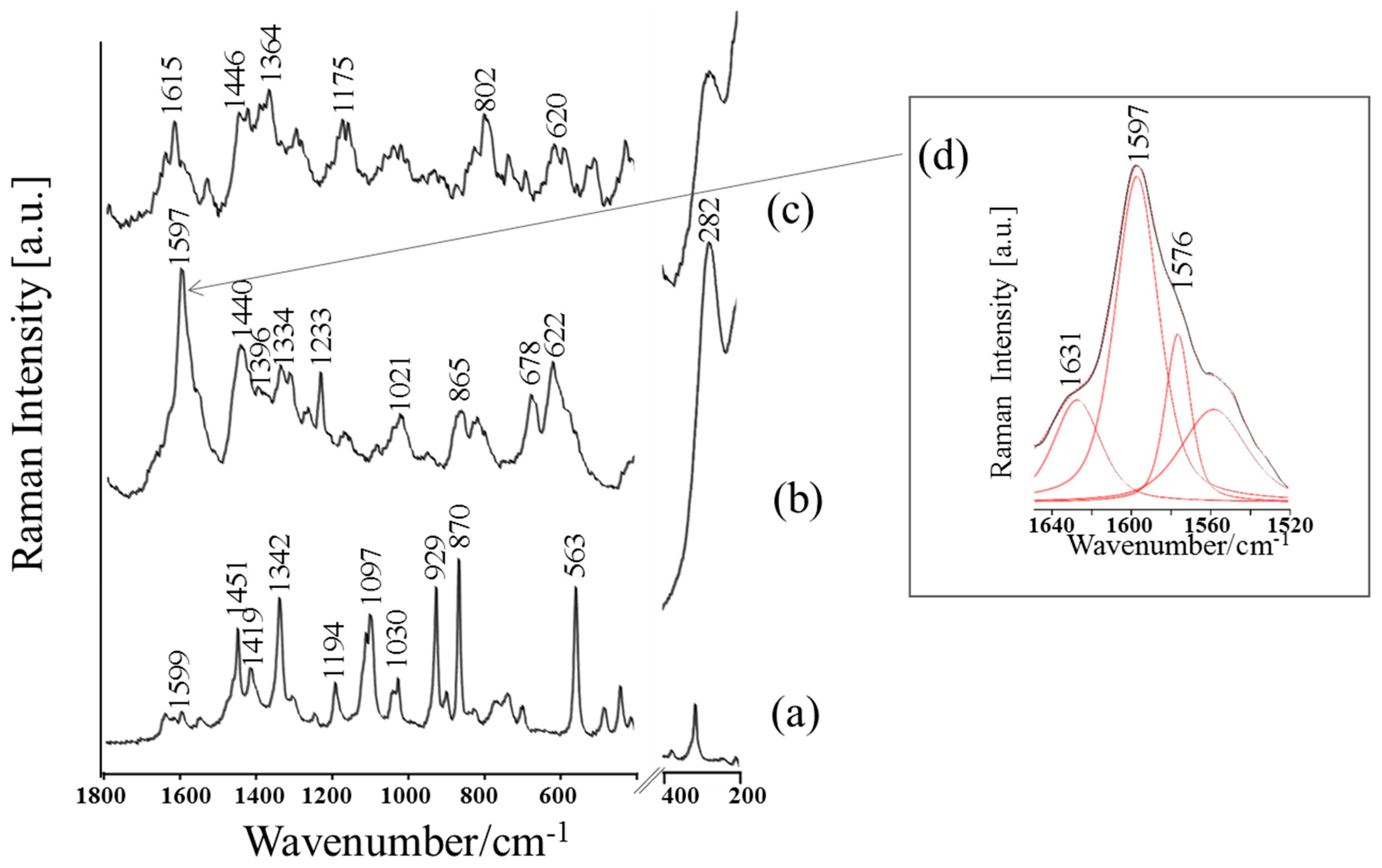
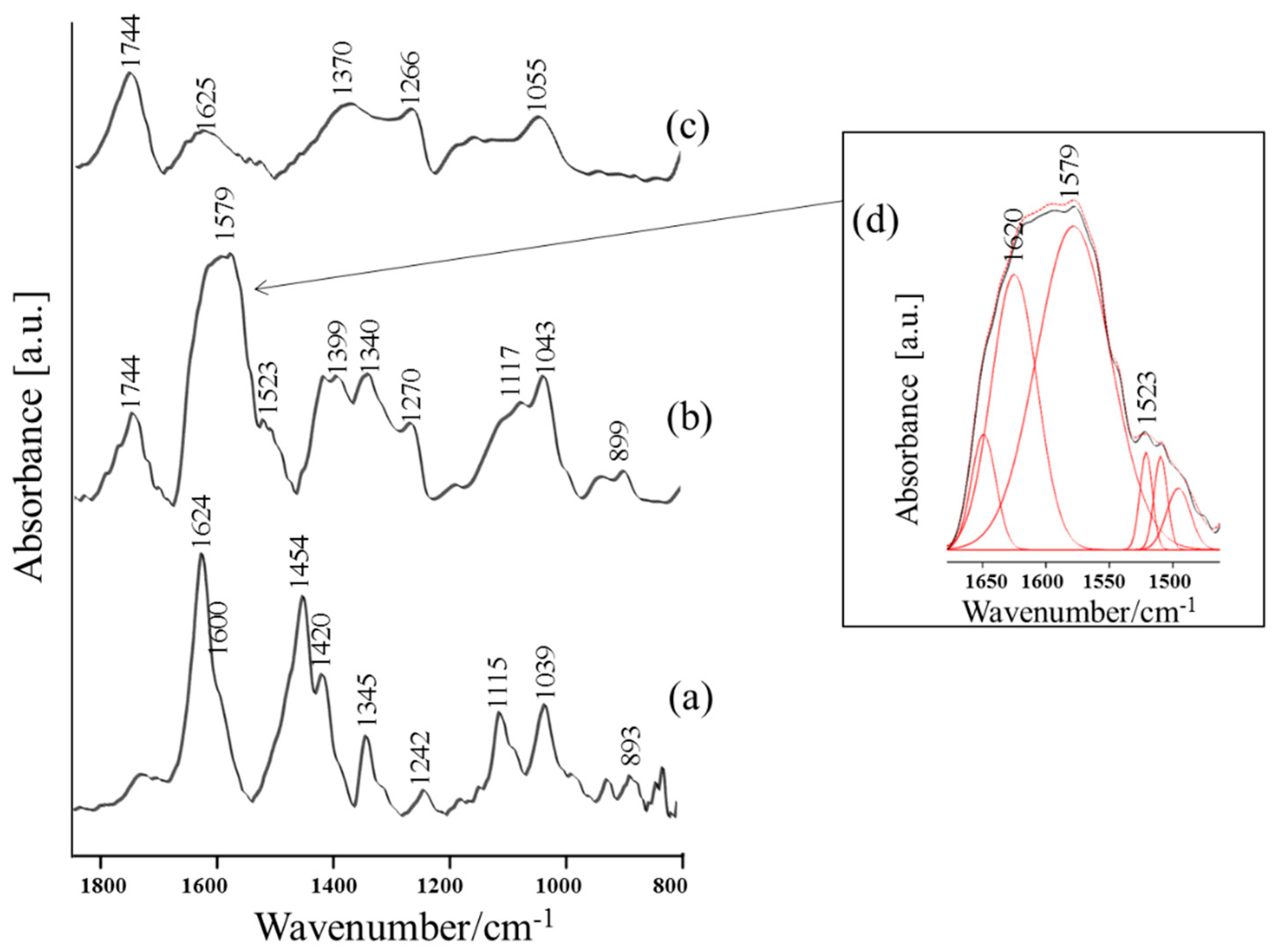

| Band Assignments | RS | SERS | FT-IR | SEIRA | Nano-SEIRA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ν (cm−1) | FWHM (cm−1) | ν (cm−1) | FWHM (cm−1) | ν (cm−1) | FWHM (cm−1) | ν (cm−1) | FWHM (cm−1) | s-pol | p-pol | |
| ν(C=O) | − | − | − | − | 1723 vw | 30 | 1744 m | 45 | 1725 vs | 1724 m |
| δas(NH3+) | 1641 vw | 15 | 1631 sh | 23 | 1624 vs | 39 | 1620 sh | 54 | 1666 vs | 1672 m |
| δas(NH3+)/ νas(COO) | 1599 vw | 16 | 1597 vs/ 1576 sh | 28 20 | 1598 sh | 27 | 1579 vs | 68 | 1572 m | 1572 m |
| δs(NH3+) | 1547 vw | 13 | 1545 sh | 26 | − | − | 1523 sh | 23 | − | − |
| δ(CH3)/ δ(NH3+) | 1451 m | 9 | 1440 s | 25 | 1454 vs/ 1420 sh | 40 44 | 1450 sh | 60 | 1481 m | 1468 m |
| νs (COO)/ δ(CH) | 1419 m | 16 | 1396 sh | 27 | 1385 sh | 22 | 1399 s | 28 | 1436 sh | 1438 sh |
| δ(CH3)/ δ(CH) | 1342 s | 15 | 1334 m | 7 | 1345 m | 46 | 1340 m | 63 | − | − |
| δ(CH) | 1302 sh | 27 | 1315 m | 20 | 1320 sh | 29 | − | − | − | − |
| δ(NH3+)/ ν(C-OH) | 1246 vw | 11 | 1233 m | 13 | 1242 w | 49 | 1270 sh | 34 | 1272 m | 1260 w |
| ρr(CH),ν(C-C), δ(OH) | 1194 m | 11 | 1168 w | 8 | 1180 vw | 47 | 1200 w | 36 | 1156 m | 1140 w |
| δ(C-O) ν(CC), δ(NH) | 1114 sh | 22 | − | − | 1115 m | 43 | 1117 sh | 45 | − | − |
| ν(C-O) | 1097 s | 16 | − | − | 1086 sh | 19 | − | − | 1090 w | 1089 w |
| ν(C-OH), ν(C-N) | 1030 m | 9 | 1021 m | 27 | 1039 m | 26 | 1043 m | 35 | − | − |
| ν (CC) | 929 s | 9 | − | − | 929 w | 53 | 945 w | 34 | − | − |
| ν(C-N), ν(C-C) | 902 w | 11 | − | − | 893 w | 35 | 899 w | 28 | − | − |
| ν(CCN)/ δip(COO) | 870 s | 8 | 865 m | 20 | − | − | − | − | − | − |
| δ(COO) | − | − | 823 m | 18 | − | − | − | − | − | − |
| δoop(COO) | 678 m | 35 | ||||||||
| ν(Cu-O), ρw(COO) | − | − | 622 m | 23 | − | − | − | − | − | − |
| ρr(COO) | 563 s | 12 | − | − | − | − | − | − | − | − |
| ν(Cu-O)/ Cu-Thr | − | − | 282 s | 45 | − | − | − | − | − | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Święch, D.; Paluszkiewicz, C.; Piergies, N.; Pięta, E.; Kollbek, K.; Kwiatek, W.M. Micro- and Nanoscale Spectroscopic Investigations of Threonine Influence on the Corrosion Process of the Modified Fe Surface by Cu Nanoparticles. Materials 2020, 13, 4482. https://doi.org/10.3390/ma13204482
Święch D, Paluszkiewicz C, Piergies N, Pięta E, Kollbek K, Kwiatek WM. Micro- and Nanoscale Spectroscopic Investigations of Threonine Influence on the Corrosion Process of the Modified Fe Surface by Cu Nanoparticles. Materials. 2020; 13(20):4482. https://doi.org/10.3390/ma13204482
Chicago/Turabian StyleŚwięch, Dominika, Czesława Paluszkiewicz, Natalia Piergies, Ewa Pięta, Kamila Kollbek, and Wojciech M. Kwiatek. 2020. "Micro- and Nanoscale Spectroscopic Investigations of Threonine Influence on the Corrosion Process of the Modified Fe Surface by Cu Nanoparticles" Materials 13, no. 20: 4482. https://doi.org/10.3390/ma13204482
APA StyleŚwięch, D., Paluszkiewicz, C., Piergies, N., Pięta, E., Kollbek, K., & Kwiatek, W. M. (2020). Micro- and Nanoscale Spectroscopic Investigations of Threonine Influence on the Corrosion Process of the Modified Fe Surface by Cu Nanoparticles. Materials, 13(20), 4482. https://doi.org/10.3390/ma13204482








