The Improvement of Moisture Resistance and Organic Compatibility of SrAl2O4: Eu2+, Dy3+ Persistent Phosphors Coated with Silica–Polymer Hybrid Shell
Abstract
1. Introduction
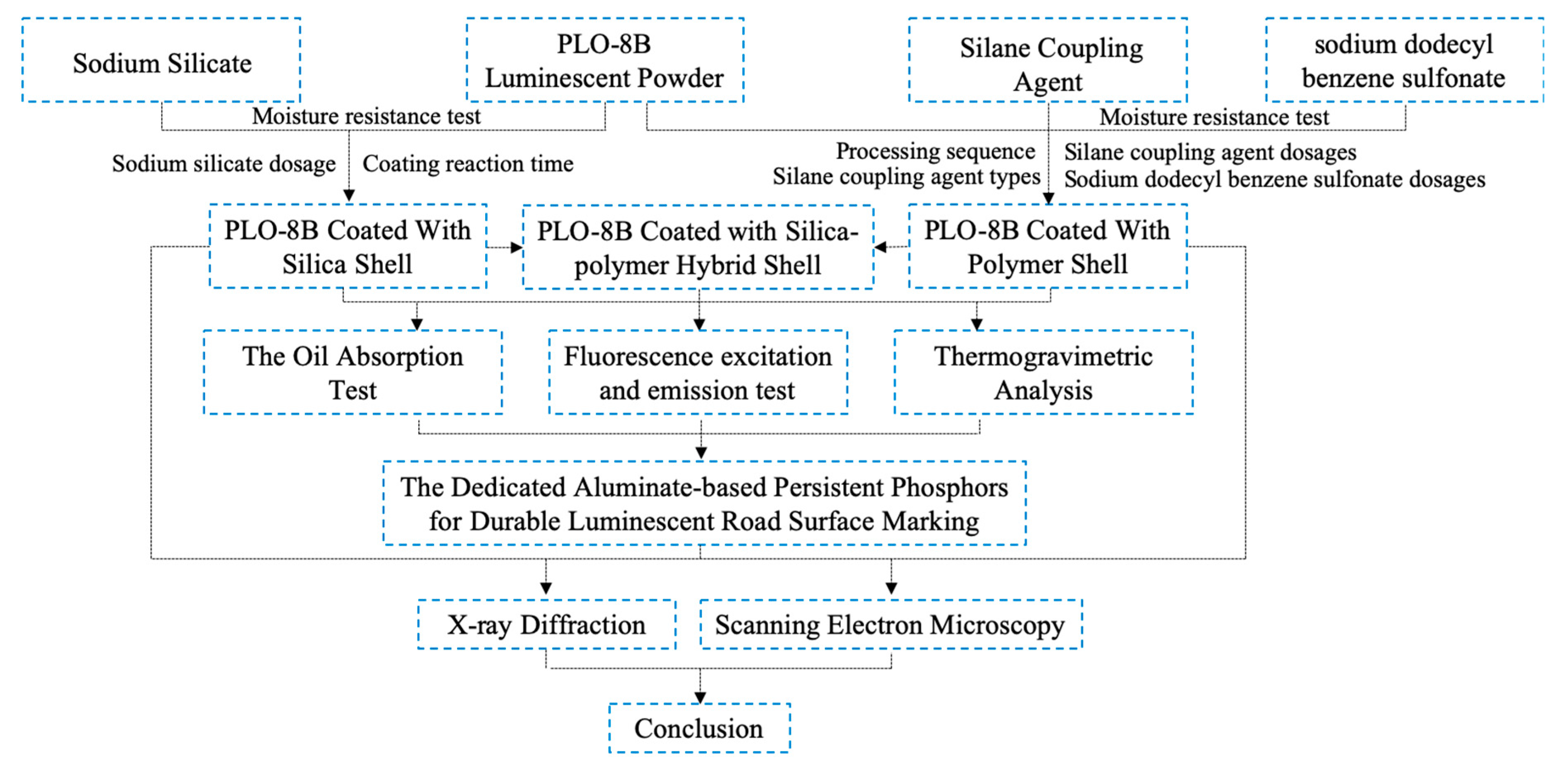
2. Experiments
2.1. Raw Materials
2.2. Preparation
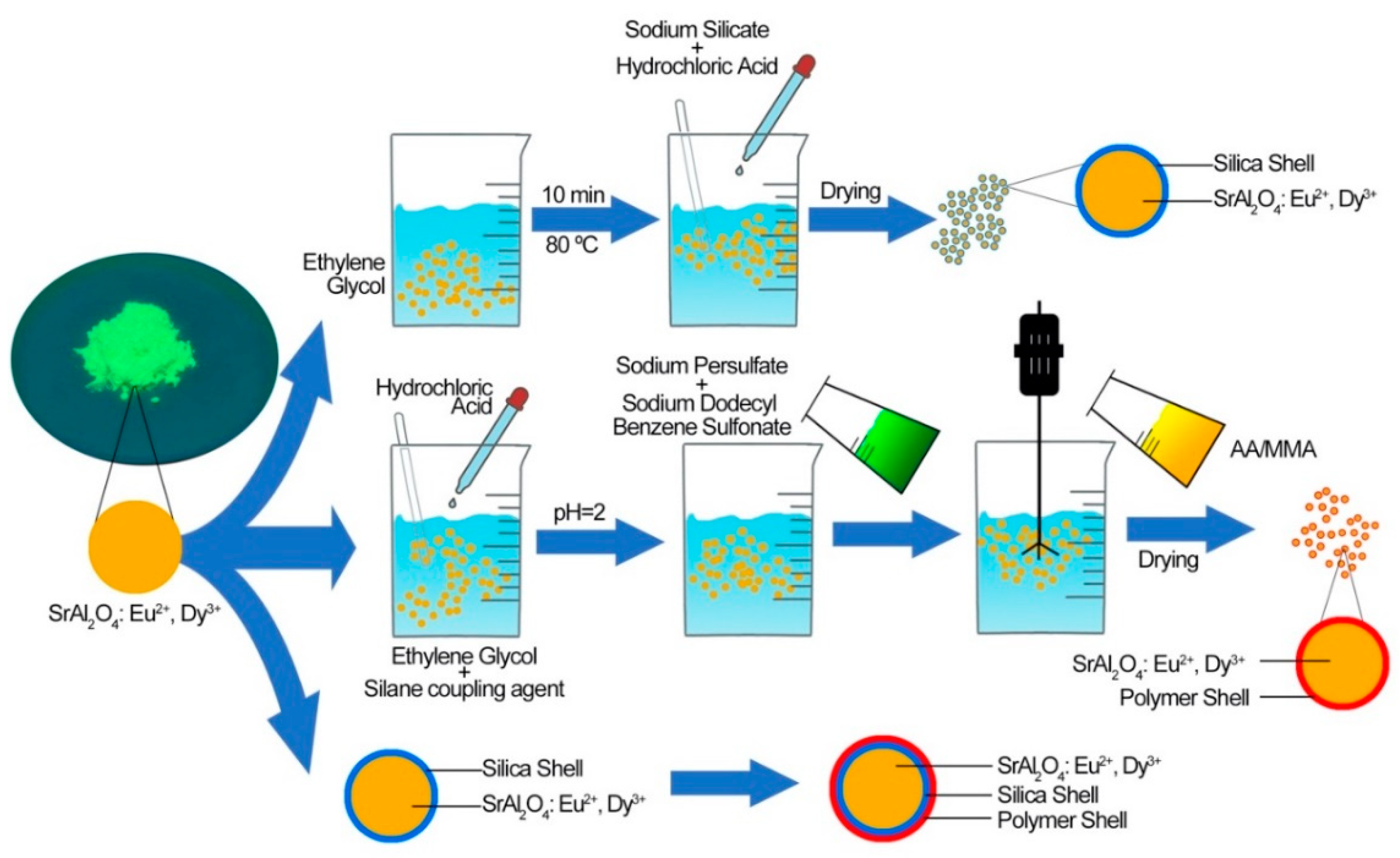
2.2.1. Preparation of Persistent Phosphors Coated with Silica Shell
2.2.2. Preparation of Persistent Phosphors Coated with Polymer Shell
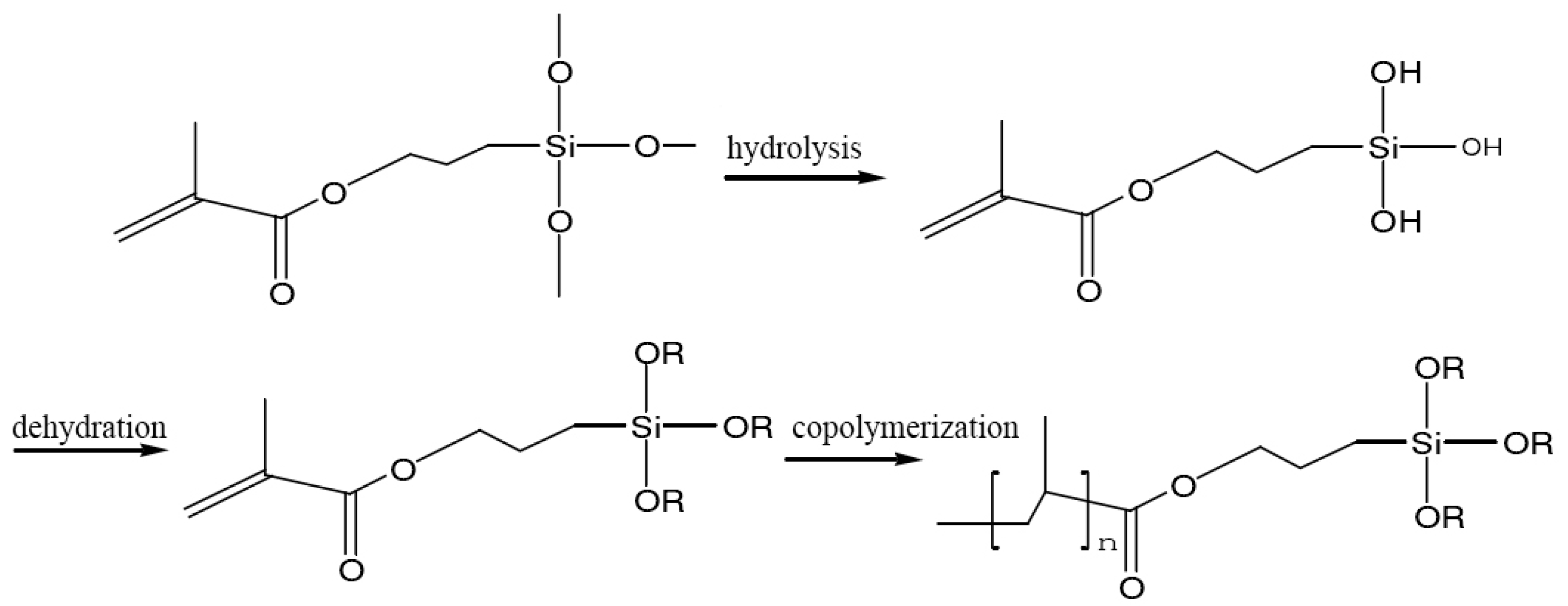
2.2.3. Preparation of Persistent Phosphors Coated with Silica-Polymer Hybrid Shell
2.3. Characterization Method
2.3.1. The Moisture Resistance Test
- SPf: the solution pH after the samples soaked for 6 h;
- SPi: the initial solution pH soaked with samples.
2.3.2. The Oil Absorption Test
2.3.3. Thermogravimetric Analysis
2.3.4. Fluorescence Excitation and Emission Test
2.3.5. X-Ray Diffraction
2.3.6. Scanning Electron Microscopy
3. Results and Discussions
3.1. Moisture Resistance of Silica Shell Coating
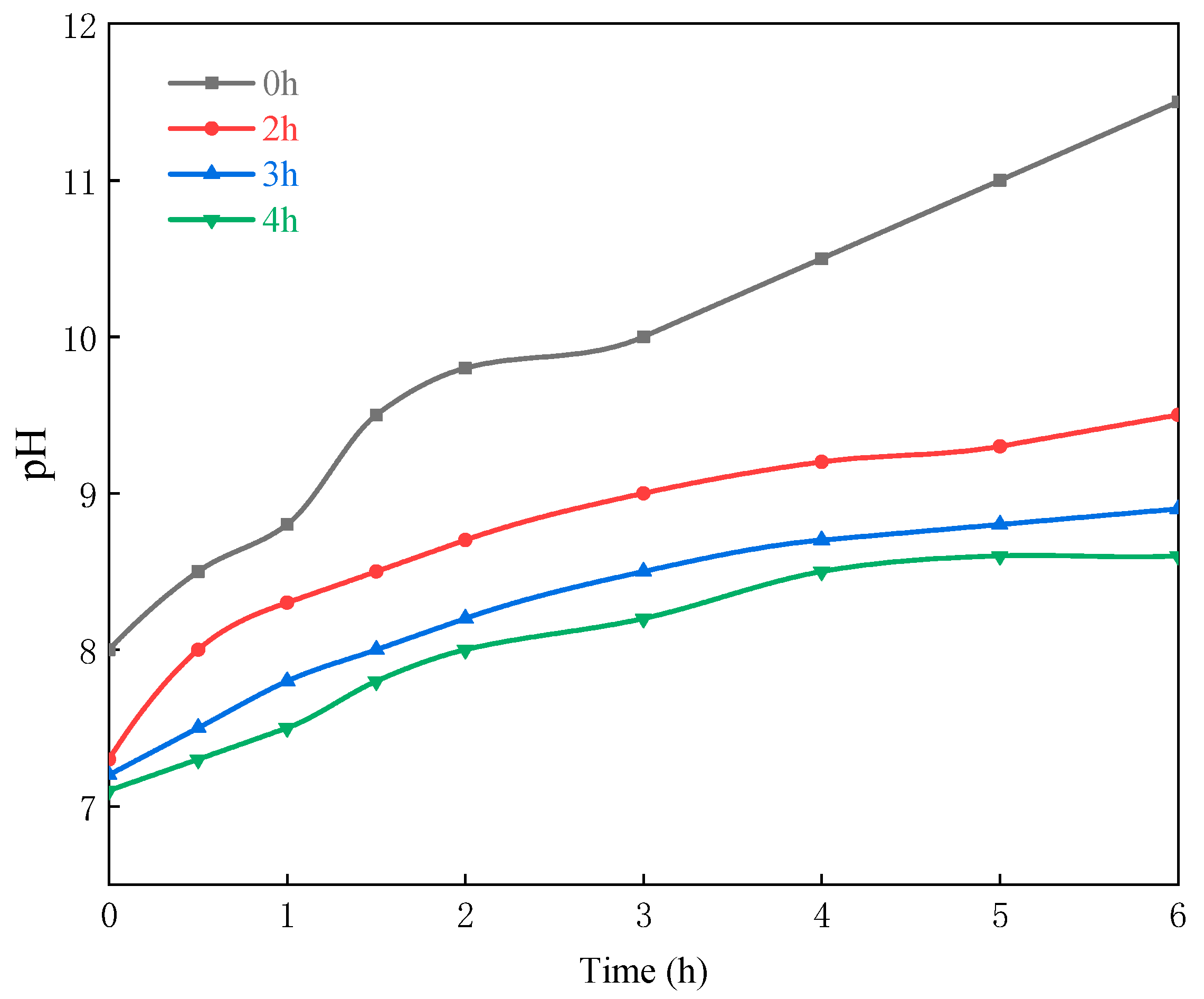
3.1.1. Effect of the Coating Reaction Time
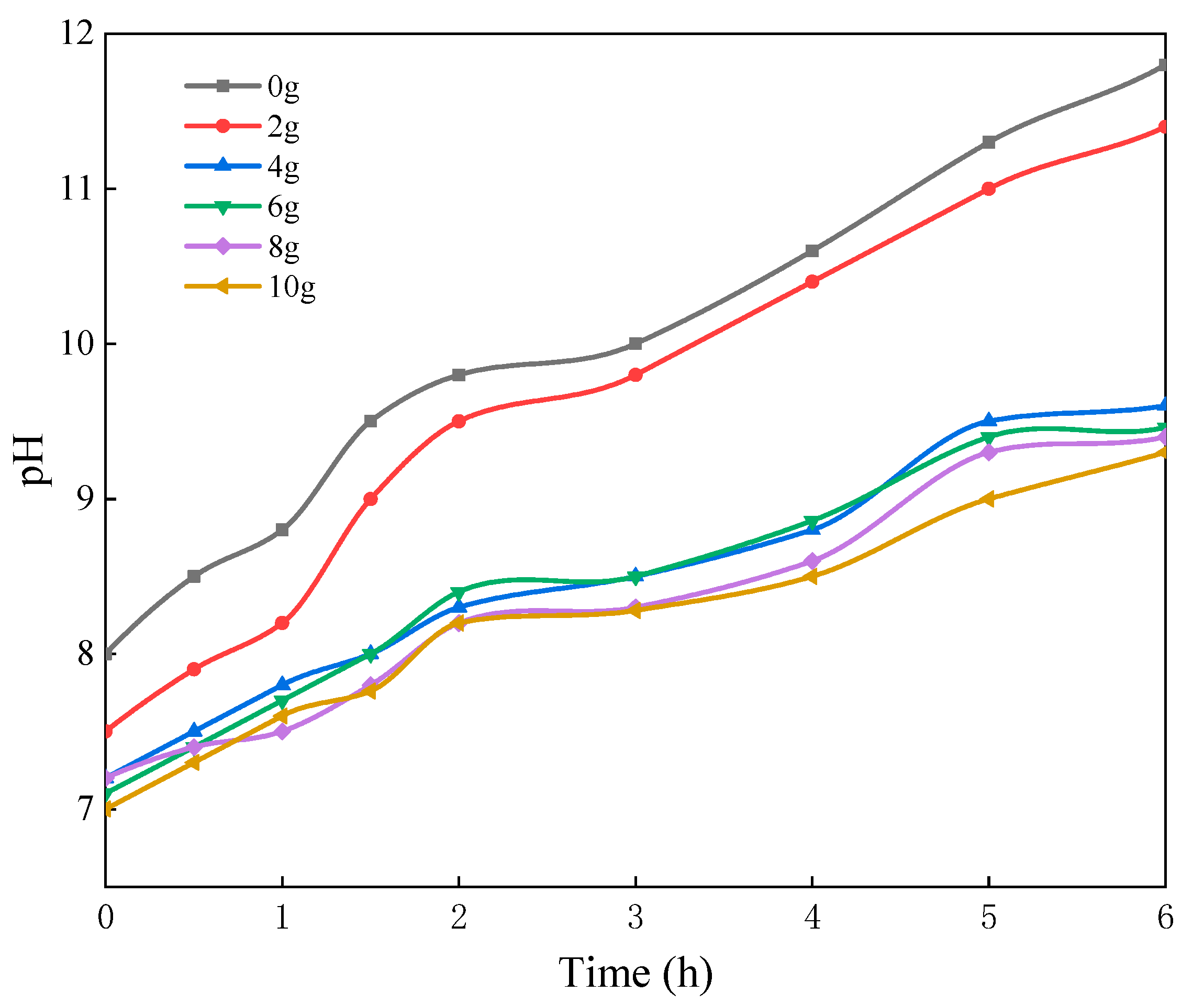
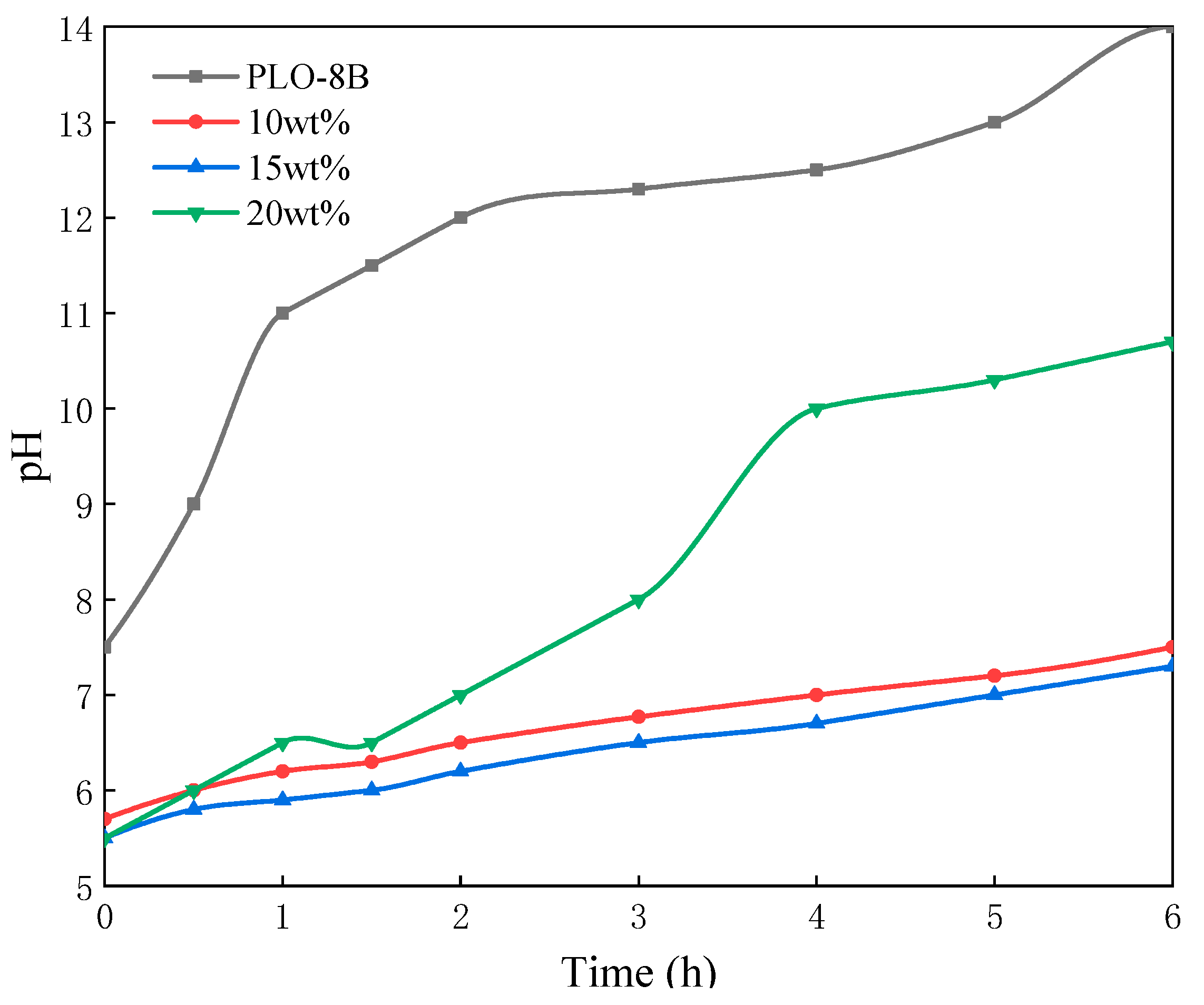
3.1.2. Effect of the Sodium Silicate Dosage
3.2. Moisture Resistance of Polymer Shell Coating
3.2.1. Effect of the Processing Sequence
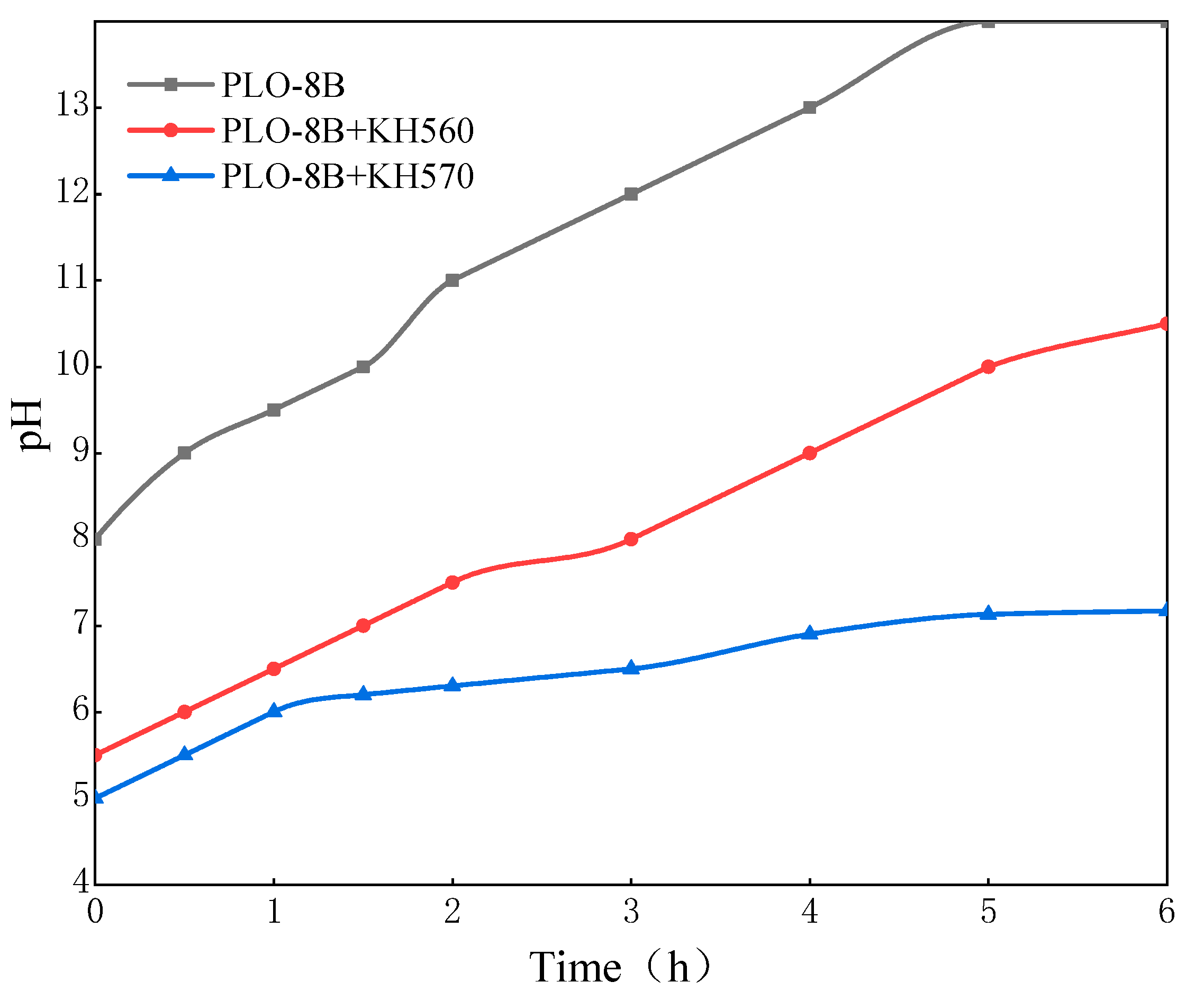
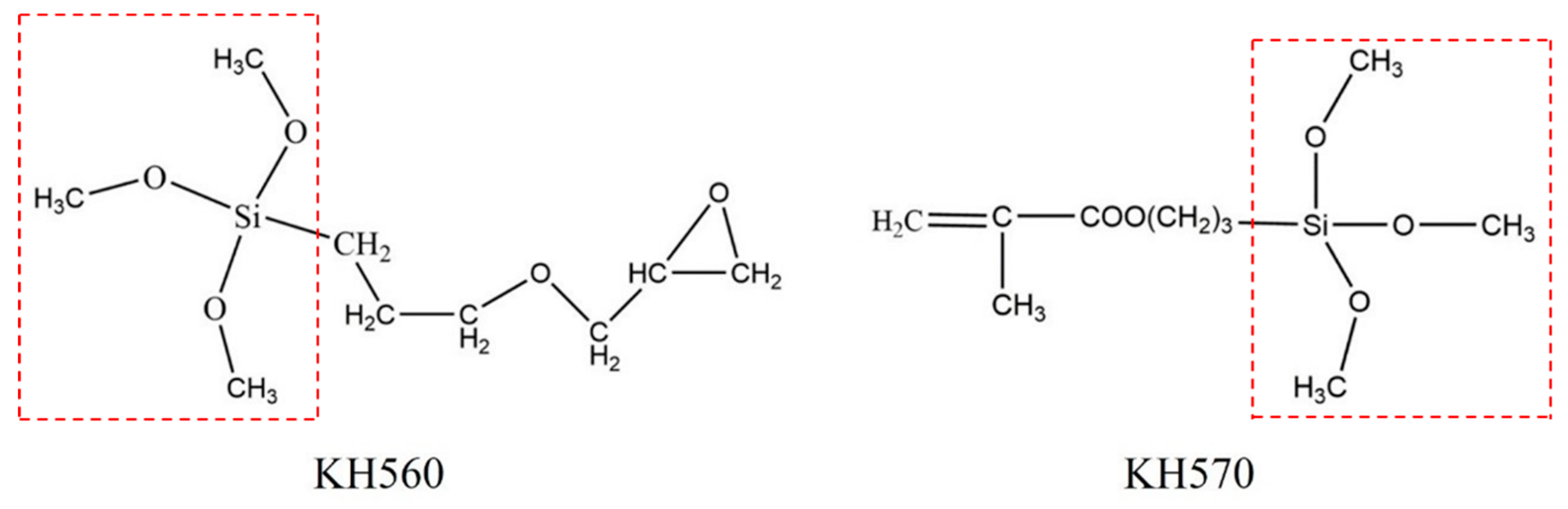
3.2.2. Effect of the Silane Coupling Agent Types
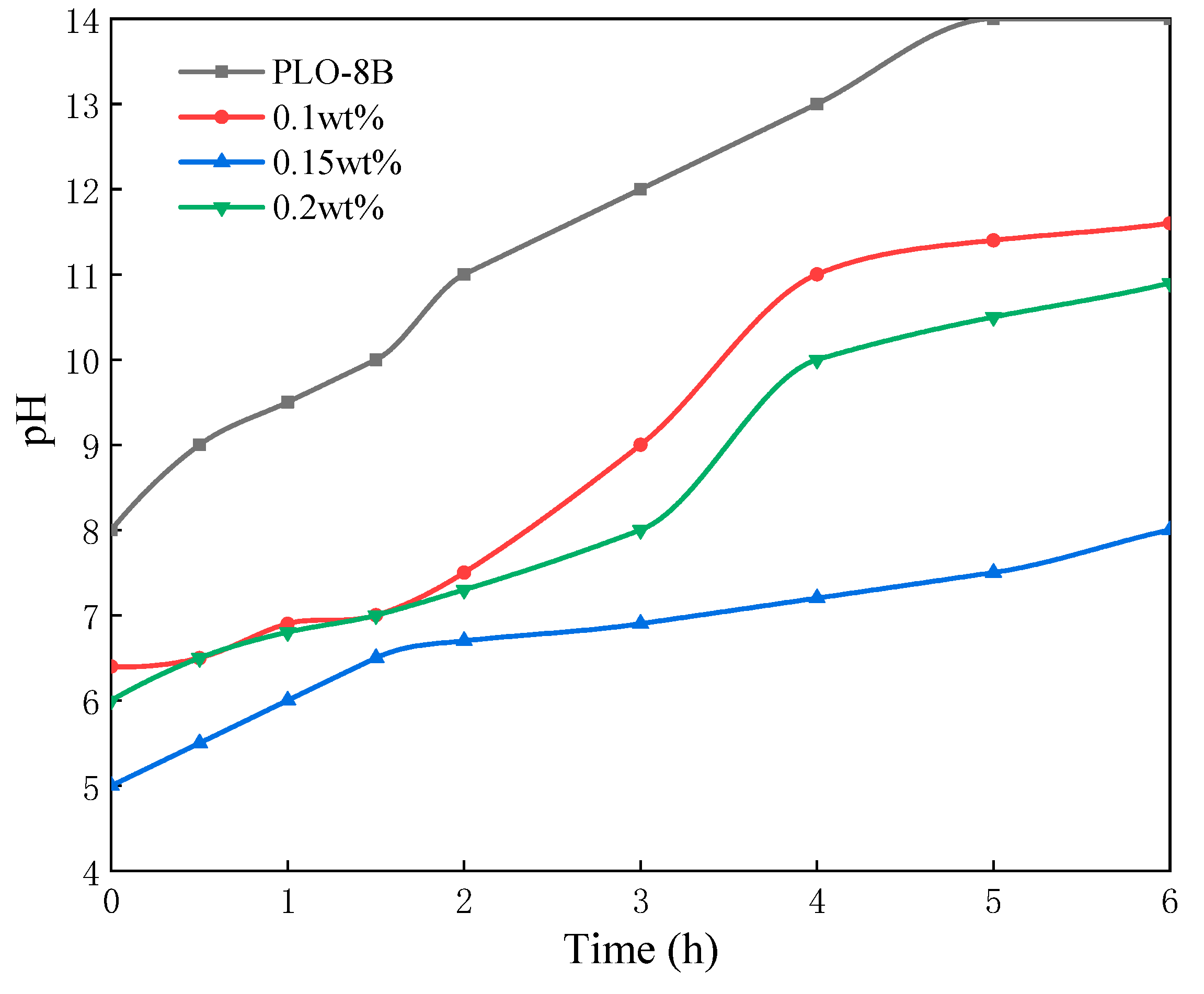
3.2.3. Effect of Silane Coupling Agent Dosages
3.2.4. Effect of Sodium Dodecyl Benzene Sulfonate Dosages
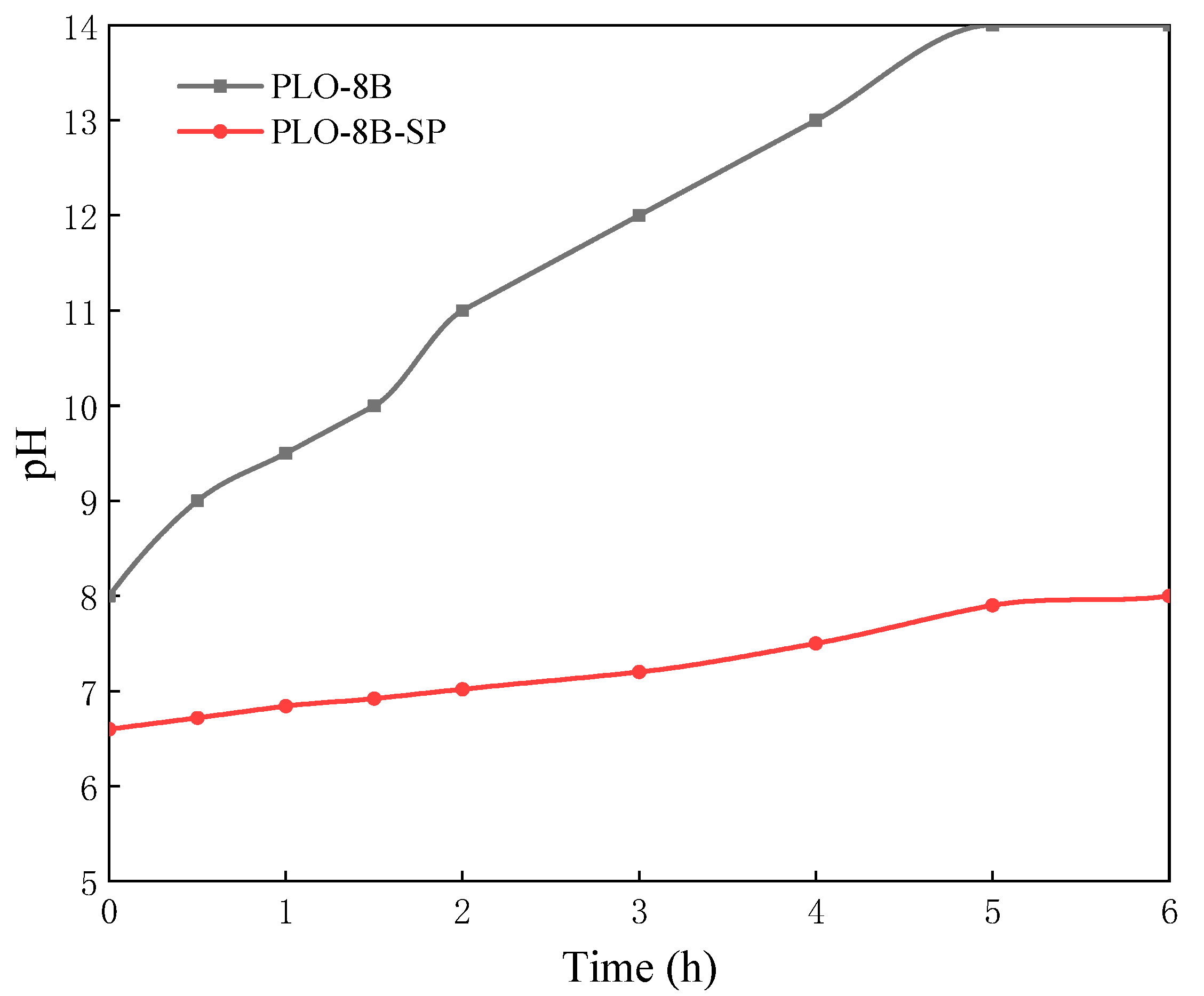
3.3. Moisture Resistance of Silica-Polymer Hybrid Shell Coating
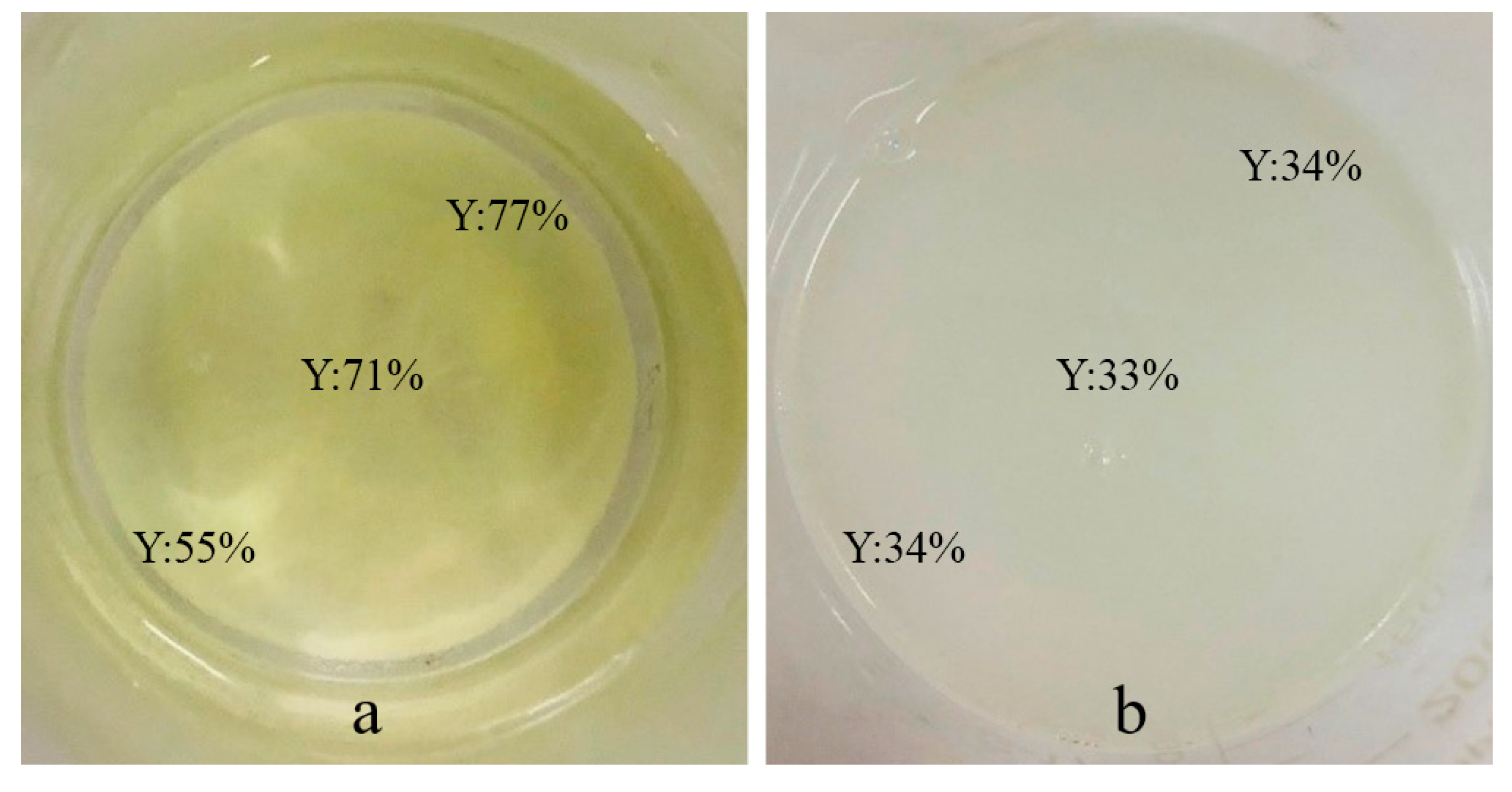
3.4. Organic Compatibility of Persistent Phosphors
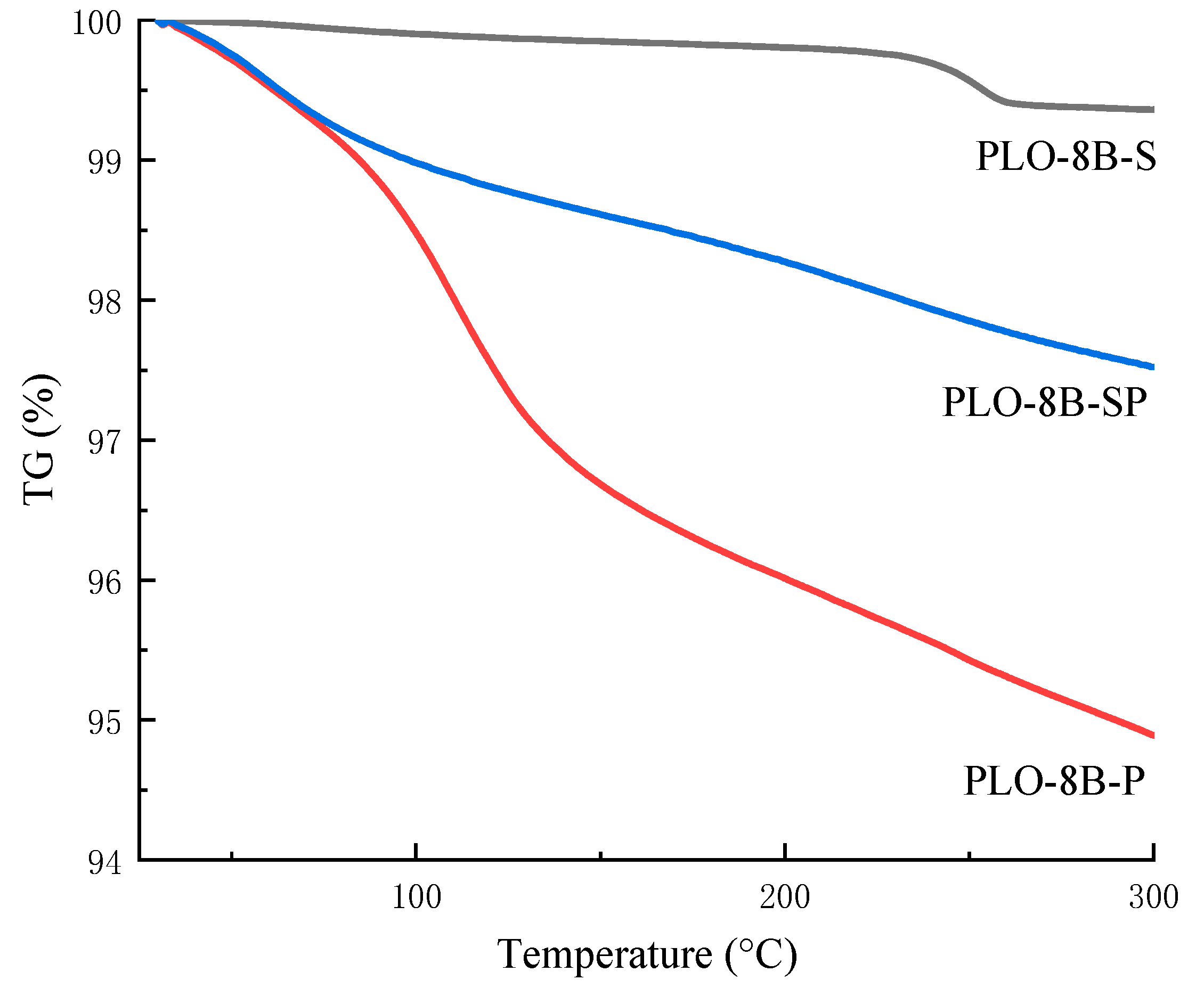
3.5. Thermal Stability of Persistent Phosphors
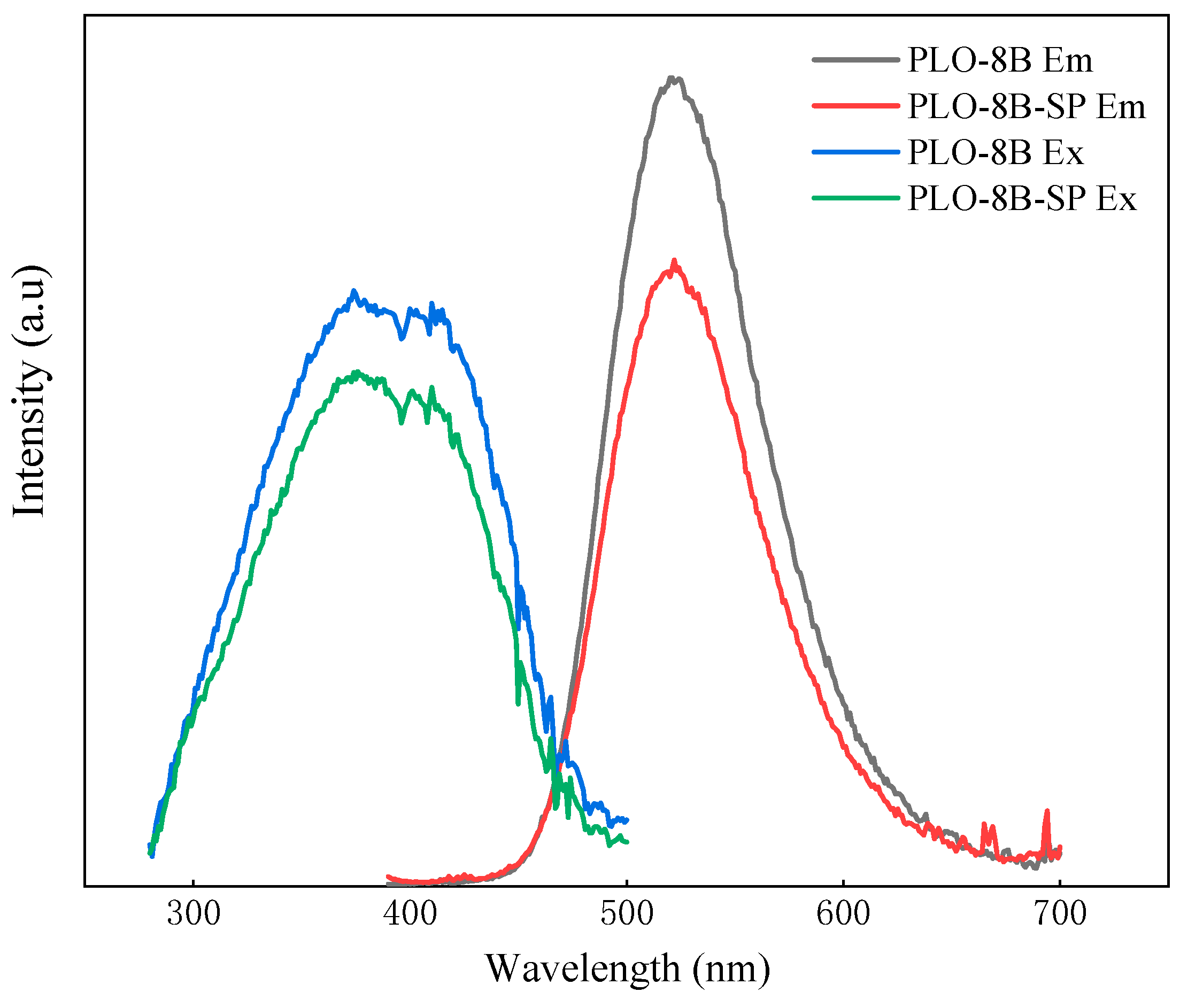
3.6. Luminous Performance of Persistent Phosphors
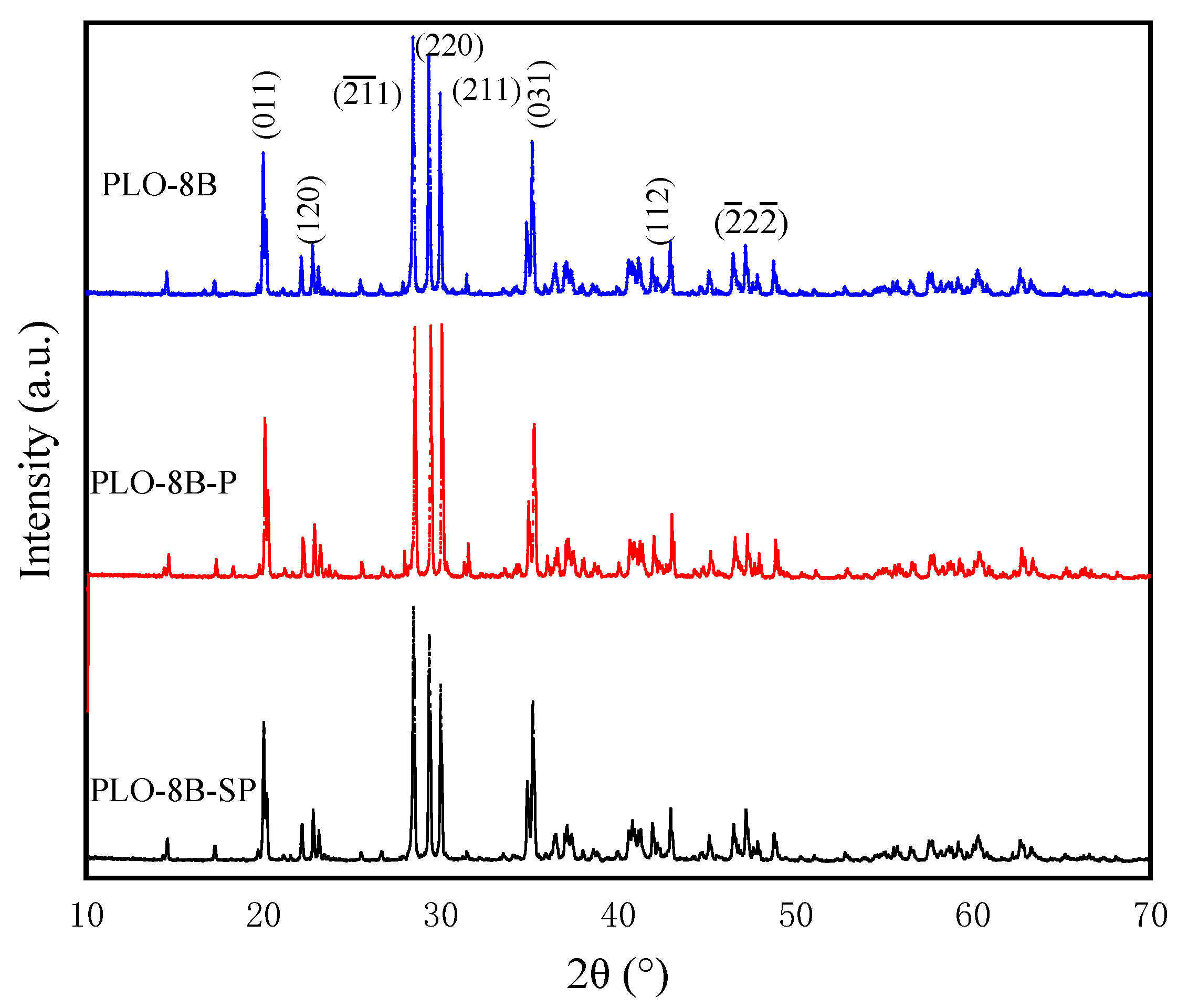
3.7. X-Ray Diffraction (XRD) Analysis
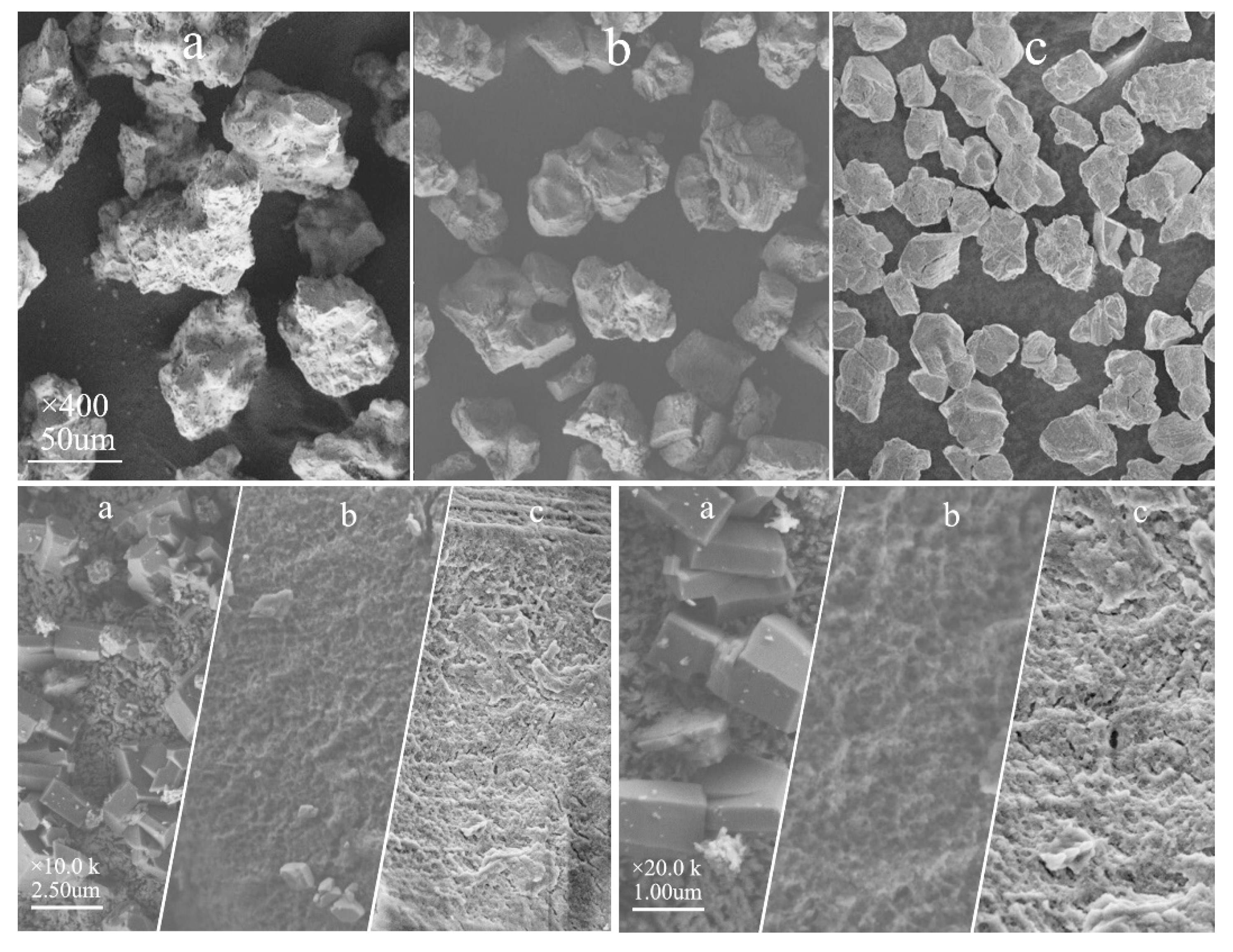
3.8. Scanning Electron Microscopy (SEM) Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naidoo, S.; Steyn, W.J. Performance of thermoplastic road-marking material. J. S. Afr. Inst. Civ. Eng. 2018, 60, 9–22. [Google Scholar] [CrossRef]
- Pan, P.; Wu, S.; Xiao, Y.; Liu, G. A review on hydronic asphalt pavement for energy harvesting and snow melting. Renew. Sustain. Energy Rev. 2015, 48, 624–634. [Google Scholar] [CrossRef]
- Liu, T.; Li, R.; Pei, J.; Xing, X.; Guo, Q. Effect of different fibers reinforcement on the properties of PZT/PVA composites. Mater. Chem. Phys. 2020, 239, 122063. [Google Scholar] [CrossRef]
- Ge, L.; Sun, L.; Zhang, X. New Generation of Spray Polyurea Road Marking Paint. Paint Coat. Ind. 2006, 3, 36–38. [Google Scholar]
- Liu, J.; Wang, B. The Double-Component Epoxy Resin Road Marking Coating; TaiYuan University of Technology: TaiYuan, China, 2007. [Google Scholar]
- Rojas-Hernandez, R.E.; Rubio-Marcos, F.; Rodriguez, M.Á.; Fernandez, J.F. Long lasting phosphors: SrAl2O4:Eu, Dy as the most studied material. Renew. Sustain. Energy Rev. 2018, 81, 2759–2770. [Google Scholar] [CrossRef]
- Delgado, T.; Afshani, J.; Hagemann, H. Spectroscopic study of a single crystal of SrAl2O4:Eu2+:Dy3+. J. Phys. Chem. C 2019, 123, 8607–8613. [Google Scholar] [CrossRef]
- Yeilay Kaya, S.; Karacaoglu, E.; Karasu, B. Effect of Al/Sr ratio on the luminescence properties of SrAl2O4:Eu2+, Dy3+ phosphors. Ceram. Int. 2012, 38, 3701–3706. [Google Scholar] [CrossRef]
- Praticò, F.G.; Vaiana, R.; Noto, S. Photoluminescent road coatings for open-graded and dense-graded asphalts: Theoretical and experimental investigation. J. Mater. Civ. Eng. 2018, 30, 1–9. [Google Scholar] [CrossRef]
- Praticò, F.G.; Noto, S.; Moro, A. Optimisation of photoluminescent painting treatments on different surface layers. In Proceedings of the 4th Chinese–European Workshop on Functional Pavement Design CEW 2016, Delft, The Netherlands, 29 June–1 July 2016; pp. 1533–1542. [Google Scholar]
- Giuliani, F.; Autelitano, F. Photoluminescent road surface dressing: A first laboratory experimental investigation. Mater. Tech. 2014, 102, 6–7. [Google Scholar]
- Glow in the Dark Roads not Glowing. Available online: https://www.bbc.com/news/technology-27187827 (accessed on 16 January 2020).
- Bacero, R.; To, D.; Arista, J.P.; Dela Cruz, M.K.; Villaneva, J.P.; Uy, F.A. Evaluation of Strontium Aluminate in Traffic Paint Pavement Markings for Rural and Unilluminated Roads. J. East. Asia Soc. Transp. Stud. 2015, 11, 1726–1744. [Google Scholar]
- Botterman, J.; Smet, P.F. Persistent phosphor SrAl2O4: Eu, Dy in outdoor conditions: Saved by the trap distribution. Opt. Express 2015, 23, A868–A881. [Google Scholar] [CrossRef] [PubMed]
- Botterman, J.; Smet, P. Glow-in-the-dark traffic markings: Feasible or not? In Proceedings of the 2nd International Workshop on Persistent and Photostimulable Phosphors (IWPPP 2013), Guangzhou, China, 17–21 November 2013. [Google Scholar]
- Mohajerani, A.; Bakaric, J.; Jeffrey-Bailey, T. The urban heat island effect, its causes, and mitigation, with reference to the thermal properties of asphalt concrete. J. Environ. Manag. 2017, 197, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shuen, H.; Yong, P. Research of Silica Solcoated Afterglow Luminescence Materials. Paint Coat. Ind. China 2007, 7, 18–21. [Google Scholar]
- Zhuang, J.; Xia, Z.; Liu, H.; Zhang, Z.; Liao, L. The improvement of moisture resistance and thermal stability of Ca3SiO4Cl2:Eu2+ phosphor coated with SiO2. Appl. Surf. Sci. 2011, 257, 4350–4353. [Google Scholar] [CrossRef]
- Xia, Z.; Li, G.; Chen, D.; Xiao, H. Synthesis and calcination temperature dependent photoluminescence properties of novel bromosilicate phosphors. Mater. Lett. 2009, 63, 2600–2602. [Google Scholar] [CrossRef]
- Guo, B. Study on the Surface Modification of Long Afterglow Phosphors by Amino Silane Coupling Agent. Rare Earth 2011, 32, 6–10. [Google Scholar]
- Xing, W. Preparation of Silicone-Acrylic Emulsion Strontium Aluminates Luminous Coating; Shenyang Ligong University: Shenyang, China, 2015. [Google Scholar]
- Lü, X.; Zhong, M.; Shu, W.; Yu, Q.; Xiong, X.; Wang, R. Alumina encapsulated SrAl2O4: Eu2+, Dy3+ phosphors. Powder Technol. 2007, 177, 83–86. [Google Scholar]
- Zhang, J.Y.; Zhang, Z.T.; Tang, Z.L.; Wang, T.M. Hydrolysis mechanism and method to improve water resistance of long after-glow phosphor. In Proceedings of the Materials Science Forum; Trans Tech Publications Ltd.: Zurich-Uetikon, Switzerland, 2003; Volume 423, pp. 147–150. [Google Scholar]
- China Climate Bulletin. 2018. Available online: http://www.cma.gov.cn/root7/auto13139/201903/t20190319_517664.html (accessed on 16 January 2020).
- Jin, W.X.; Sun, C.H.; Zuo, J.Q.; Li, W.J. Spatiotemporal Characteristics of Summer Precipitation with Different Durations in Central East China. Clim. Environ. Res. 2015, 20, 465–476. [Google Scholar]
- Iso, E. General Methods of Test for Pigments and Extenders Part 9: Determination of pH Value of an Aqueous Suspension; German Institute for Standardisation: Berlin, Germany, 1995; ISO787-5-1. [Google Scholar]
- Shirokoff, J.; Lye, L. A Review of Asphalt Binders Characterized by X-ray Diffraction. Innov. Corros. Mater. Sci. Former. Recent Pat. Corros. Sci. 2019, 9, 28–40. [Google Scholar] [CrossRef]
- Mazumder, M.; Ahmed, R.; Ali, A.W.; Lee, S.-J. SEM and ESEM techniques used for analysis of asphalt binder and mixture: A state of the art review. Constr. Build. Mater. 2018, 186, 313–329. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, R.; Xiao, Y.; Chen, X.; Zhang, X.; Liu, G. Mapping of publications on asphalt pavement and bitumen materials: A bibliometric review. Constr. Build. Mater. 2020, 234, 117370. [Google Scholar] [CrossRef]
- Xiao, Y.; Wan, M.; Jenkins, K.J.; Wu, S.P.; Cui, P.Q. Using activated carbon to reduce the volatile organic compounds from bituminous materials. J. Mater. Civ. Eng. 2017, 29, 4017166. [Google Scholar] [CrossRef]
- Cui, P.; Wu, S.; Xiao, Y.; Wan, M.; Cui, P. Inhibiting effect of Layered Double Hydroxides on the emissions of volatile organic compounds from bituminous materials. J. Clean. Prod. 2015, 108, 987–991. [Google Scholar] [CrossRef]
- Swati, G.; Chawla, S.; Mishra, S.; Rajesh, B.; Vijayan, N.; Sivaiah, B.; Dhar, A.; Haranath, D. e enhancement and decay characteristics of long afterglow nanophosphors for dark-vision display applications. Appl. Surf. Sci. 2015, 333, 178–185. [Google Scholar] [CrossRef]
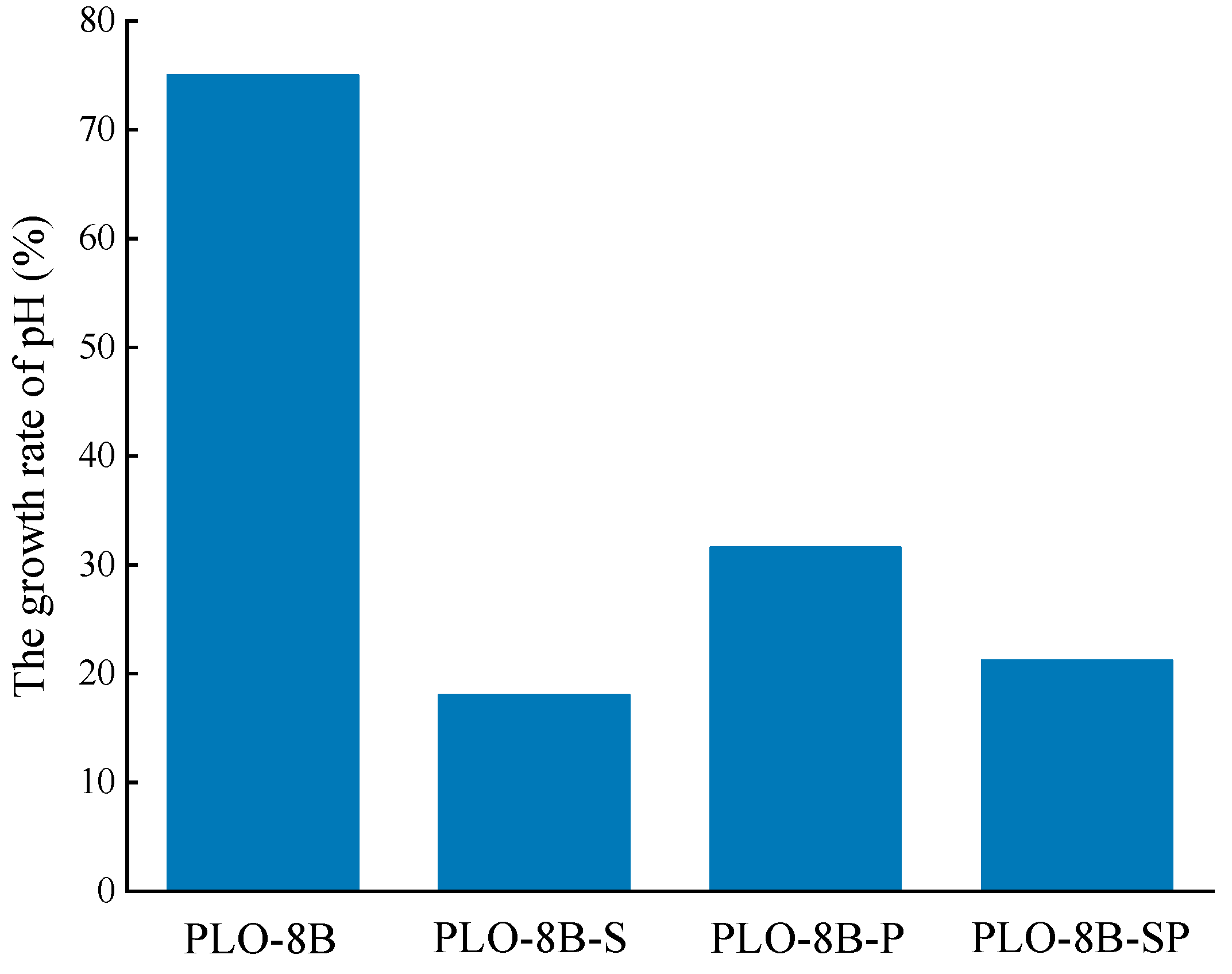

| Reagent | Weight |
|---|---|
| PLO-8B luminescent powder (PLP) | 5 g |
| Ethylene glycol | 50 g |
| Sodium silicate | 4 g |
| Reagent | Weight |
|---|---|
| PLO-8B luminescent powder (PLP) | 10 g |
| Silane coupling agent | 1 g |
| Anhydrous ethanol | 70 g |
| Acrylic acid monomer (AA) | 0.5 g |
| Methyl methacrylate (MMA) | 0.5 g |
| Sodium persulfate | 1 g |
| Sodium dodecyl benzene sulfonate | 0.15 g |
| Water | 30 g |
| Step | Reagent | Weight |
|---|---|---|
| Inorganic coating | PLO-8B luminescent powder (PLP) | 5 g |
| Ethylene glycol | 50 g | |
| Sodium silicate | 4 g | |
| Organic coating | Silane coupling agent | 0.2 g |
| Anhydrous ethanol | 70 g | |
| Acrylic acid monomer (AA) | 0.1 g | |
| Methyl methacrylate (MMA) | 0.1 g | |
| Sodium persulfate | 1 g | |
| Sodium dodecyl benzene sulfonate | 0.15 g | |
| Water | 30 g |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, L.; Chen, Y.; Yu, L.; Li, R.; Zhang, L.; Pei, J. The Improvement of Moisture Resistance and Organic Compatibility of SrAl2O4: Eu2+, Dy3+ Persistent Phosphors Coated with Silica–Polymer Hybrid Shell. Materials 2020, 13, 426. https://doi.org/10.3390/ma13020426
Lyu L, Chen Y, Yu L, Li R, Zhang L, Pei J. The Improvement of Moisture Resistance and Organic Compatibility of SrAl2O4: Eu2+, Dy3+ Persistent Phosphors Coated with Silica–Polymer Hybrid Shell. Materials. 2020; 13(2):426. https://doi.org/10.3390/ma13020426
Chicago/Turabian StyleLyu, Lei, Yuxian Chen, Liting Yu, Rui Li, Liu Zhang, and Jianzhong Pei. 2020. "The Improvement of Moisture Resistance and Organic Compatibility of SrAl2O4: Eu2+, Dy3+ Persistent Phosphors Coated with Silica–Polymer Hybrid Shell" Materials 13, no. 2: 426. https://doi.org/10.3390/ma13020426
APA StyleLyu, L., Chen, Y., Yu, L., Li, R., Zhang, L., & Pei, J. (2020). The Improvement of Moisture Resistance and Organic Compatibility of SrAl2O4: Eu2+, Dy3+ Persistent Phosphors Coated with Silica–Polymer Hybrid Shell. Materials, 13(2), 426. https://doi.org/10.3390/ma13020426





