Repeated Thermal Shock Behavior of ZrB2–SiC–Graphite Composite under Pre-Stress in Air and Ar Atmospheres
Abstract
1. Introduction
2. Experimental Procedure
2.1. Specimen Preparation
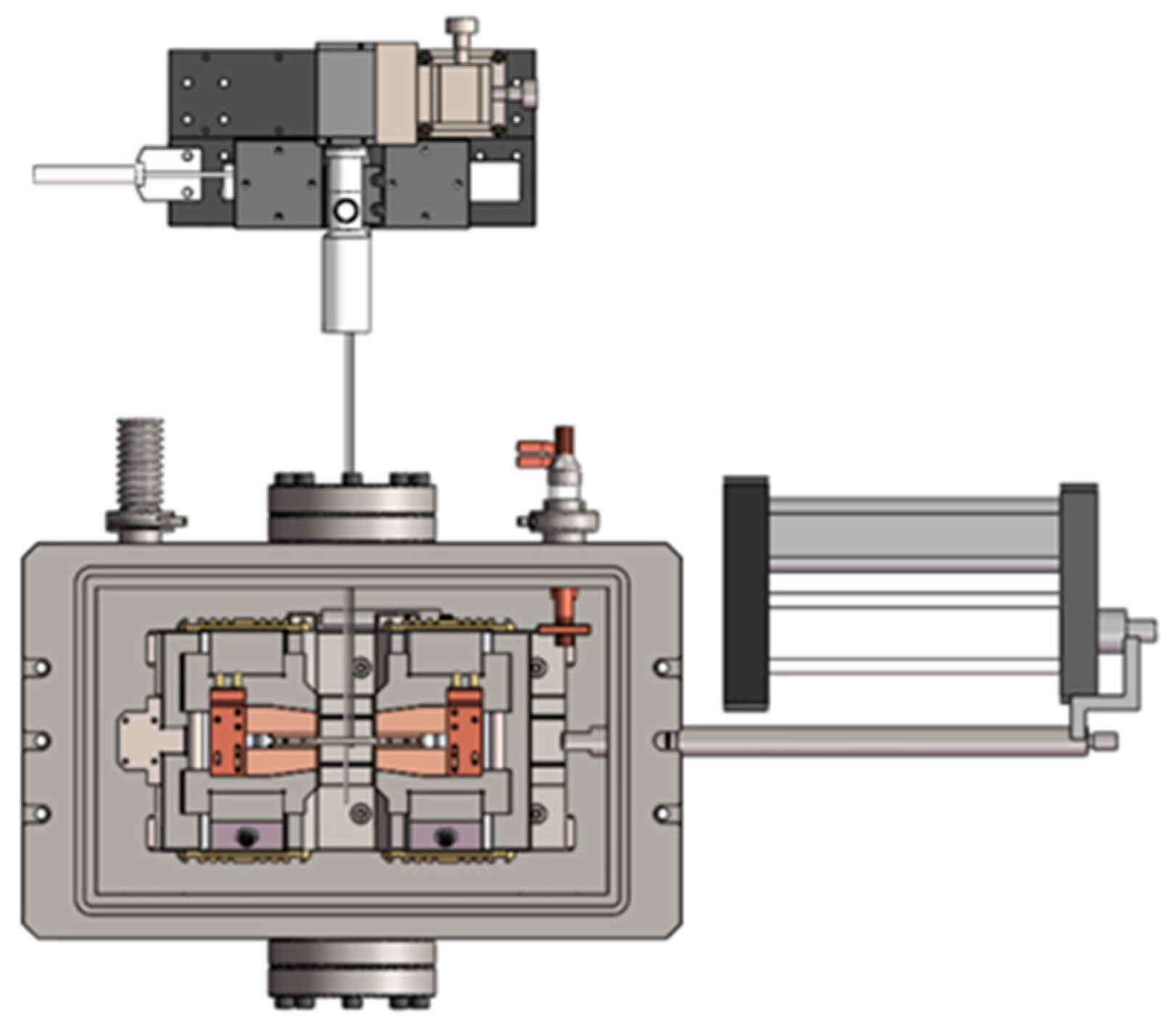
2.2. Repeated Thermal Shock Tests
2.3. Flexural Tests
3. Results and Discussion
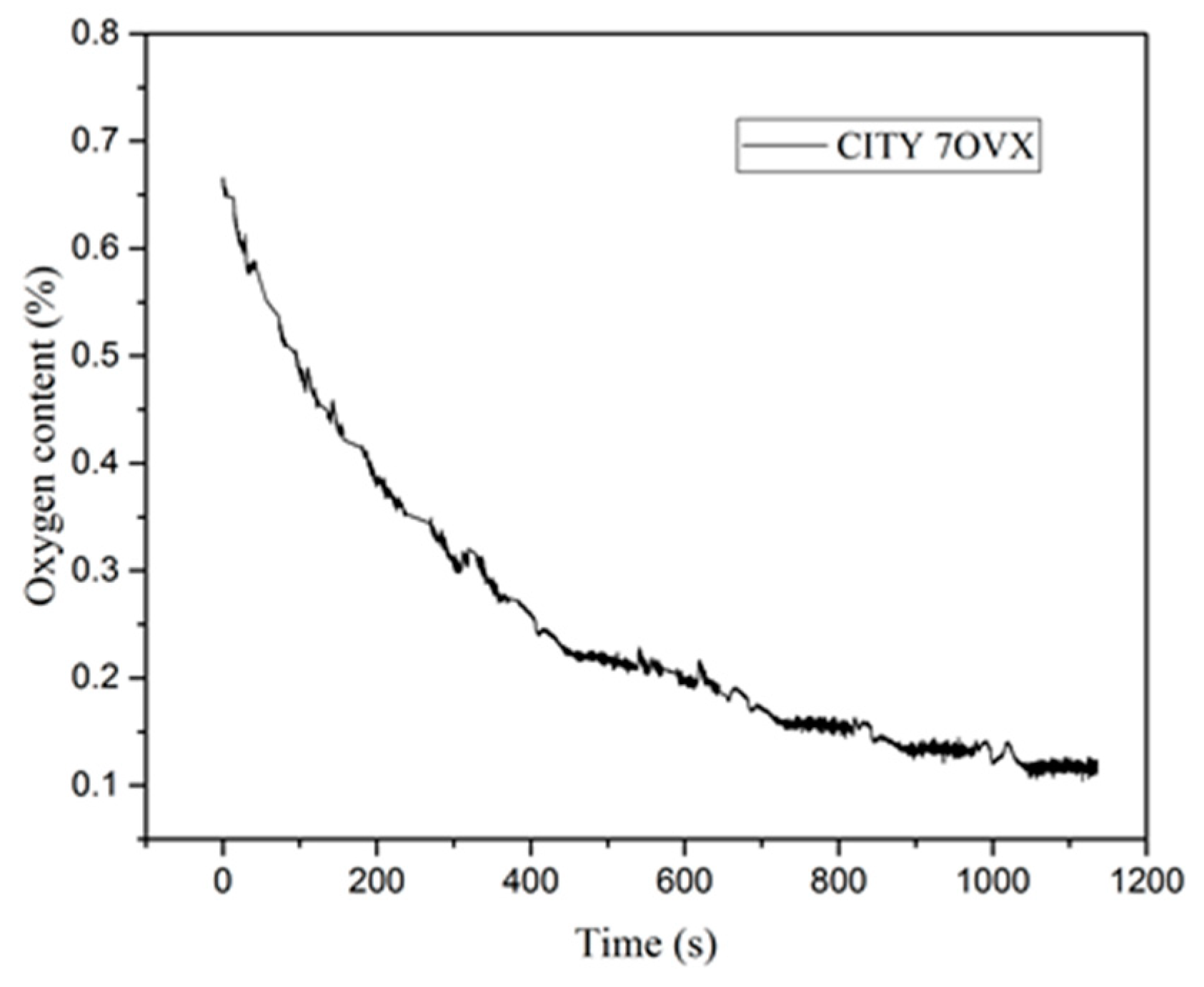
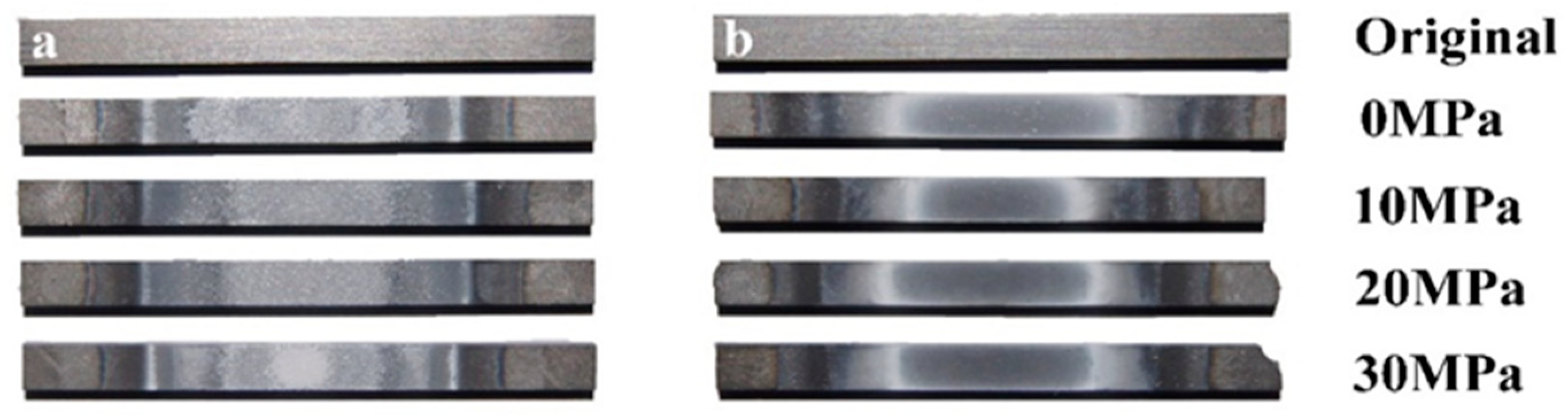
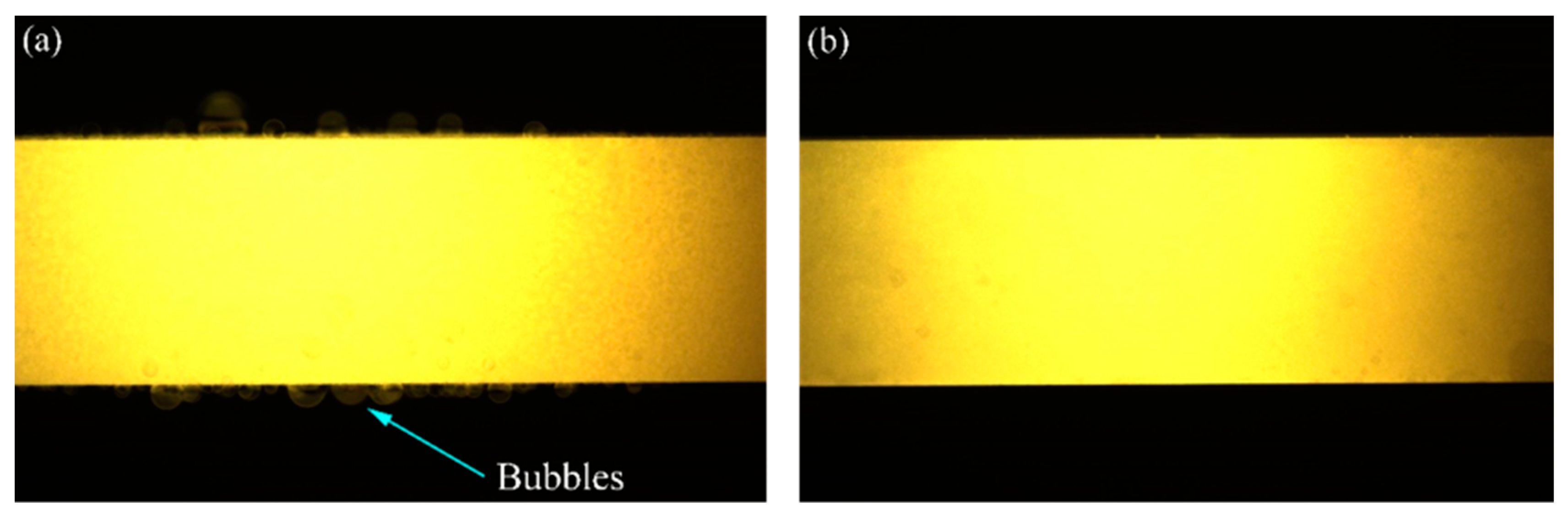
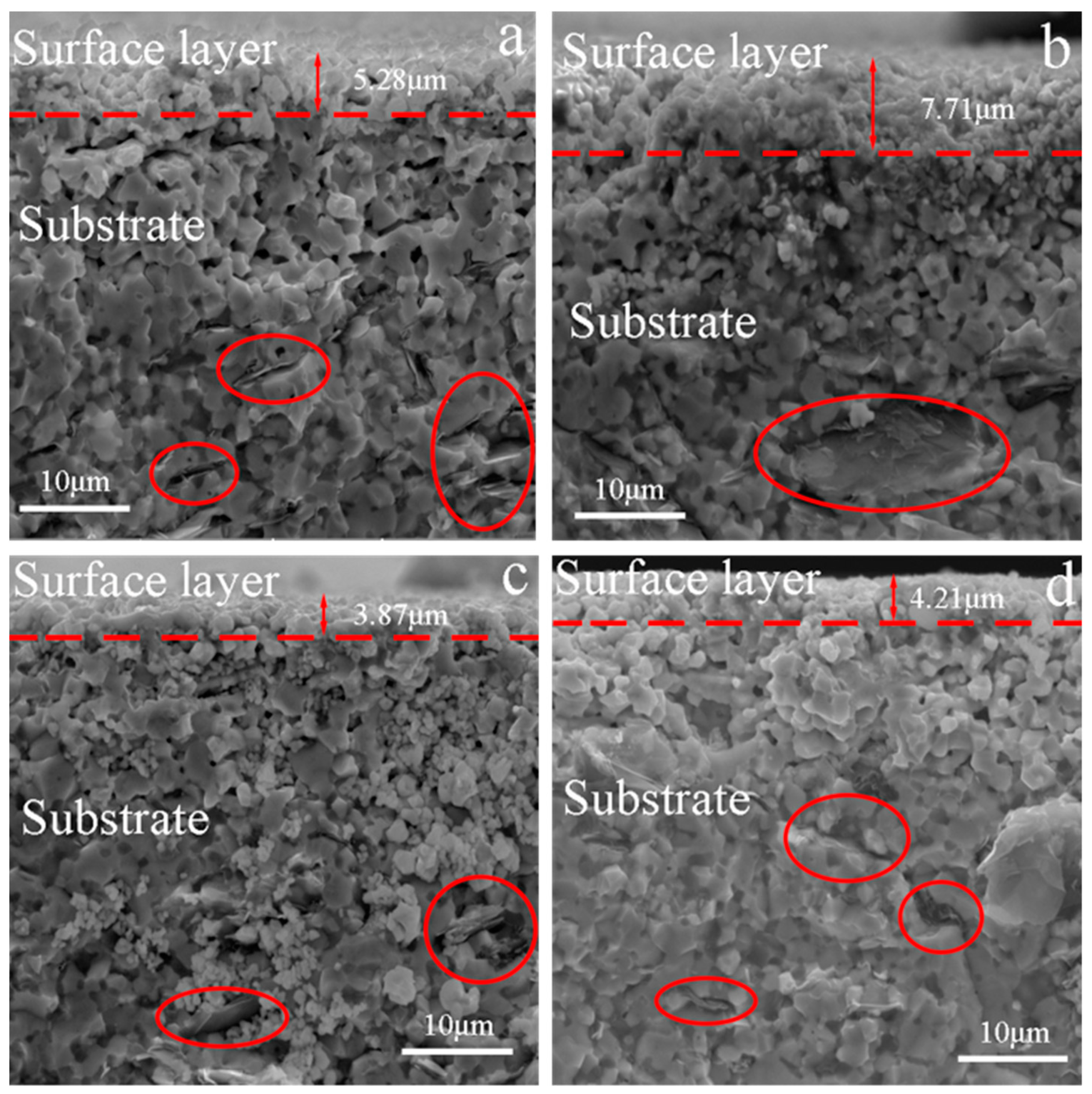
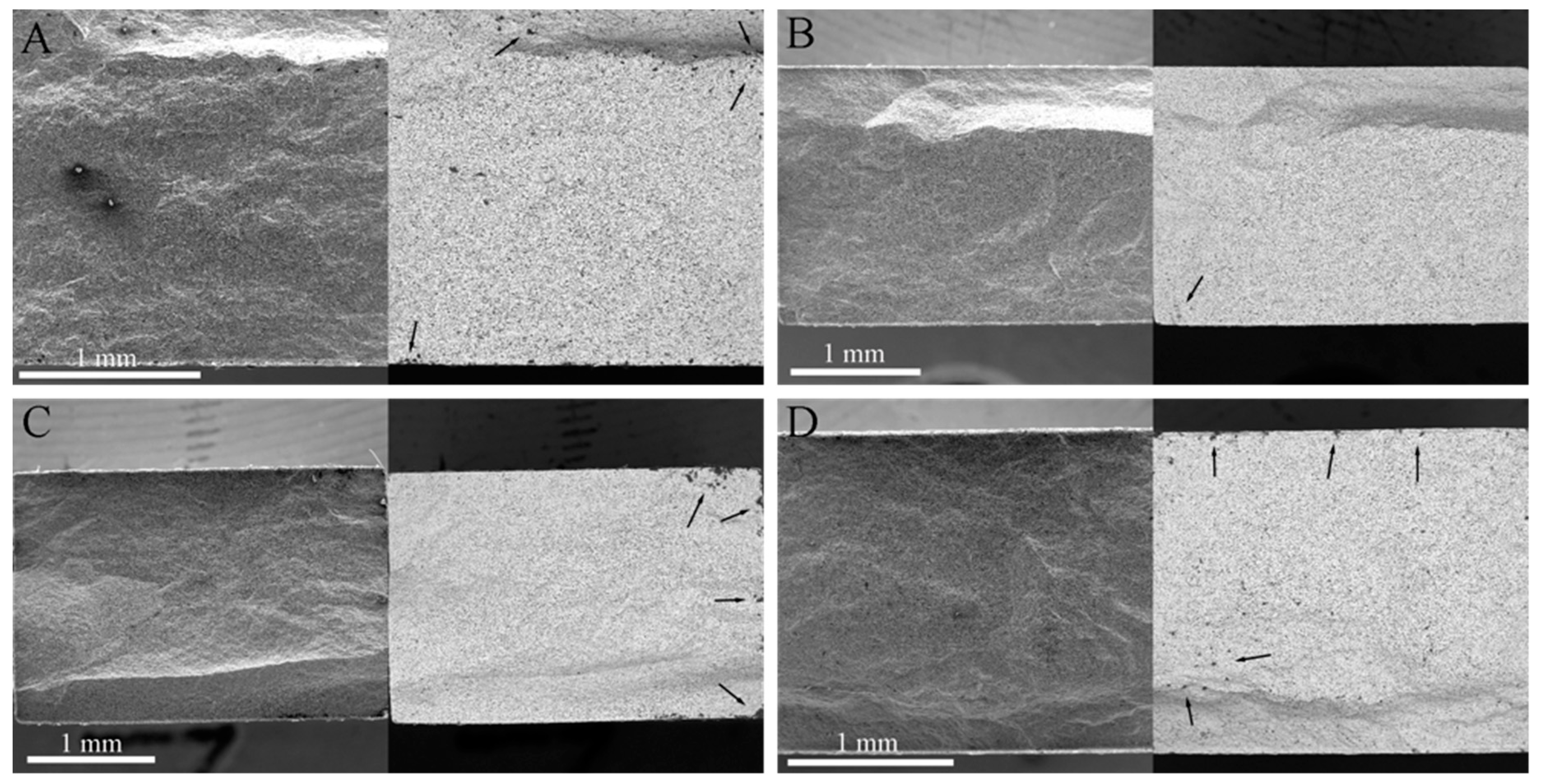
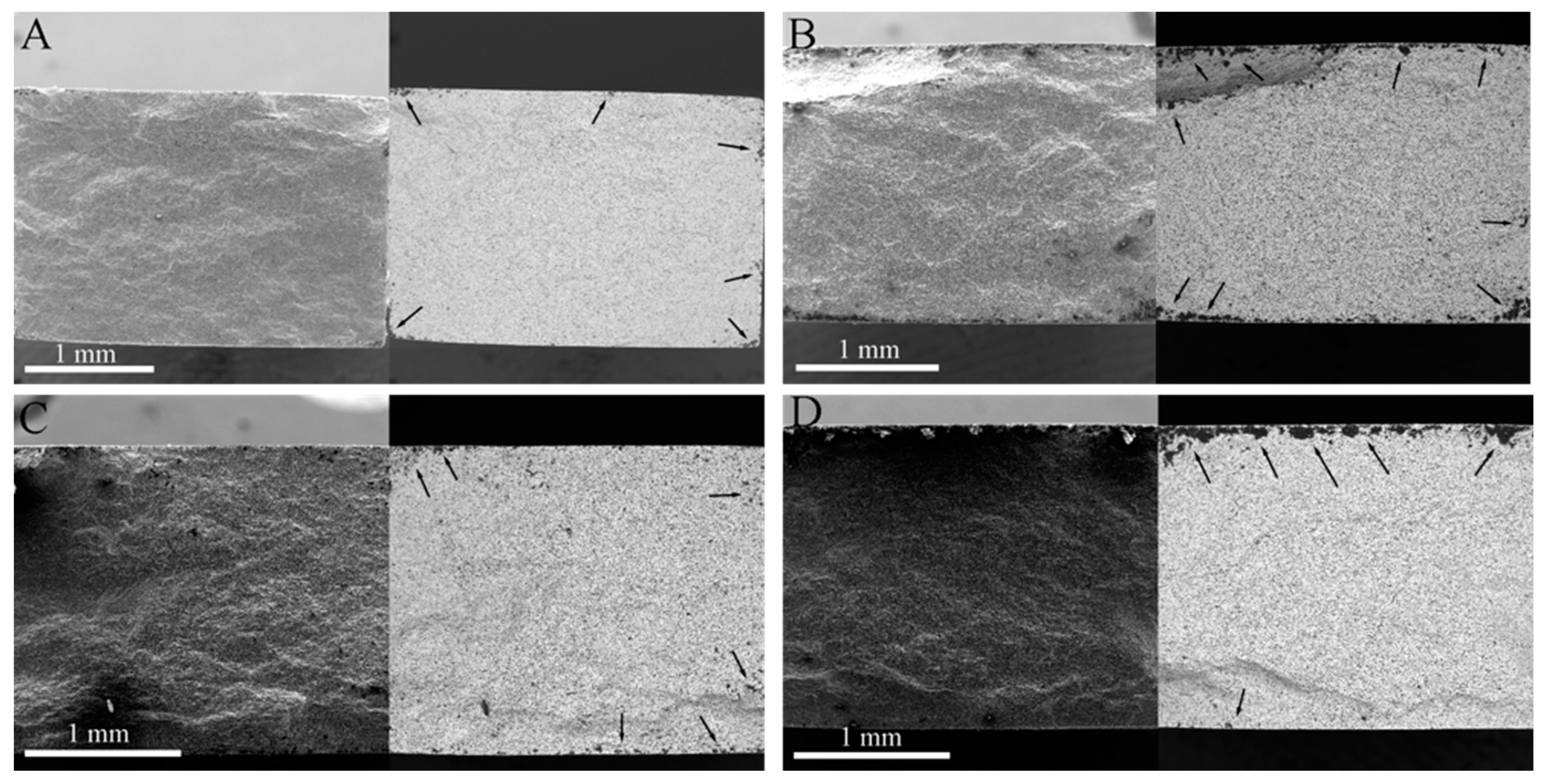
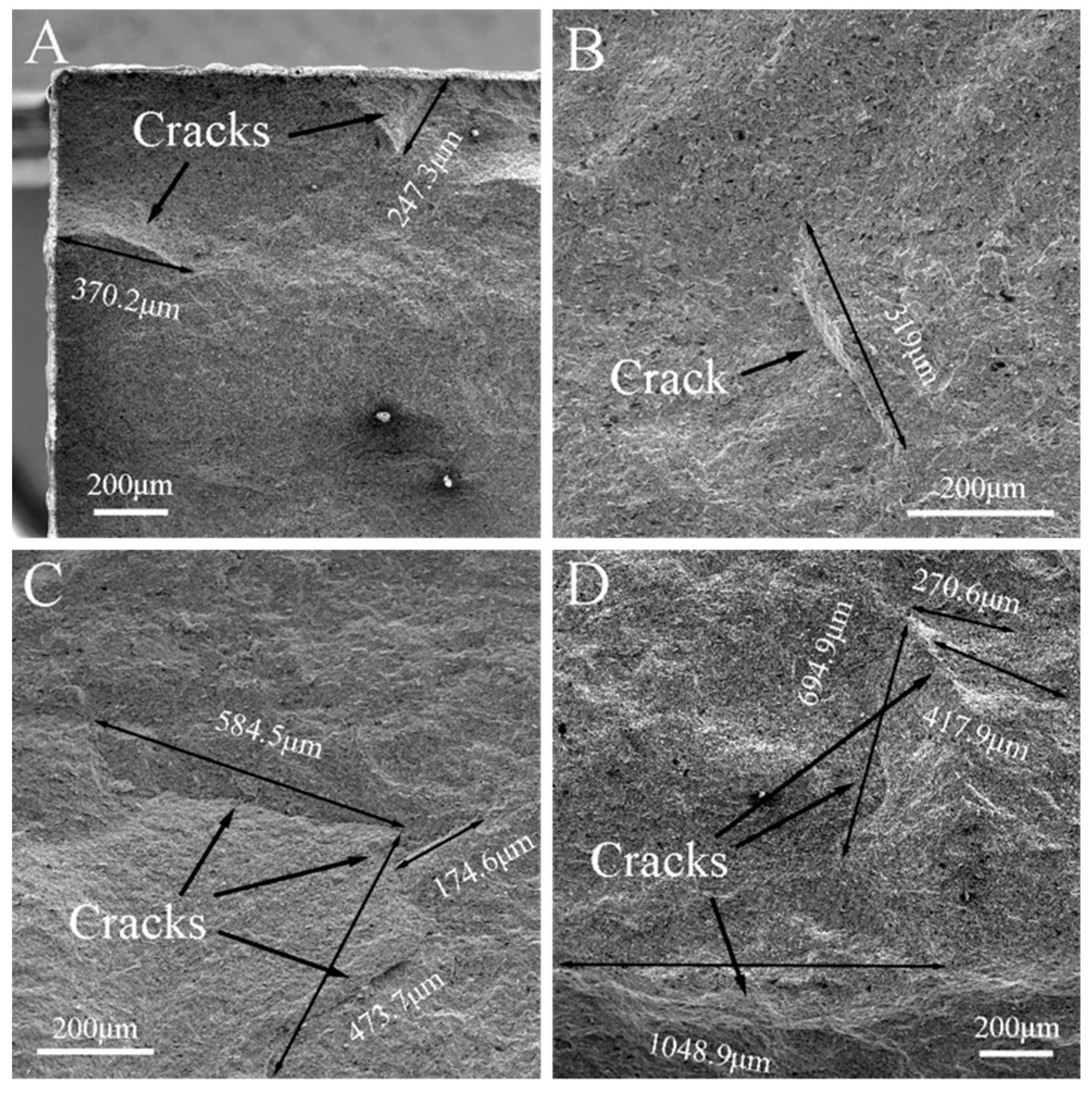
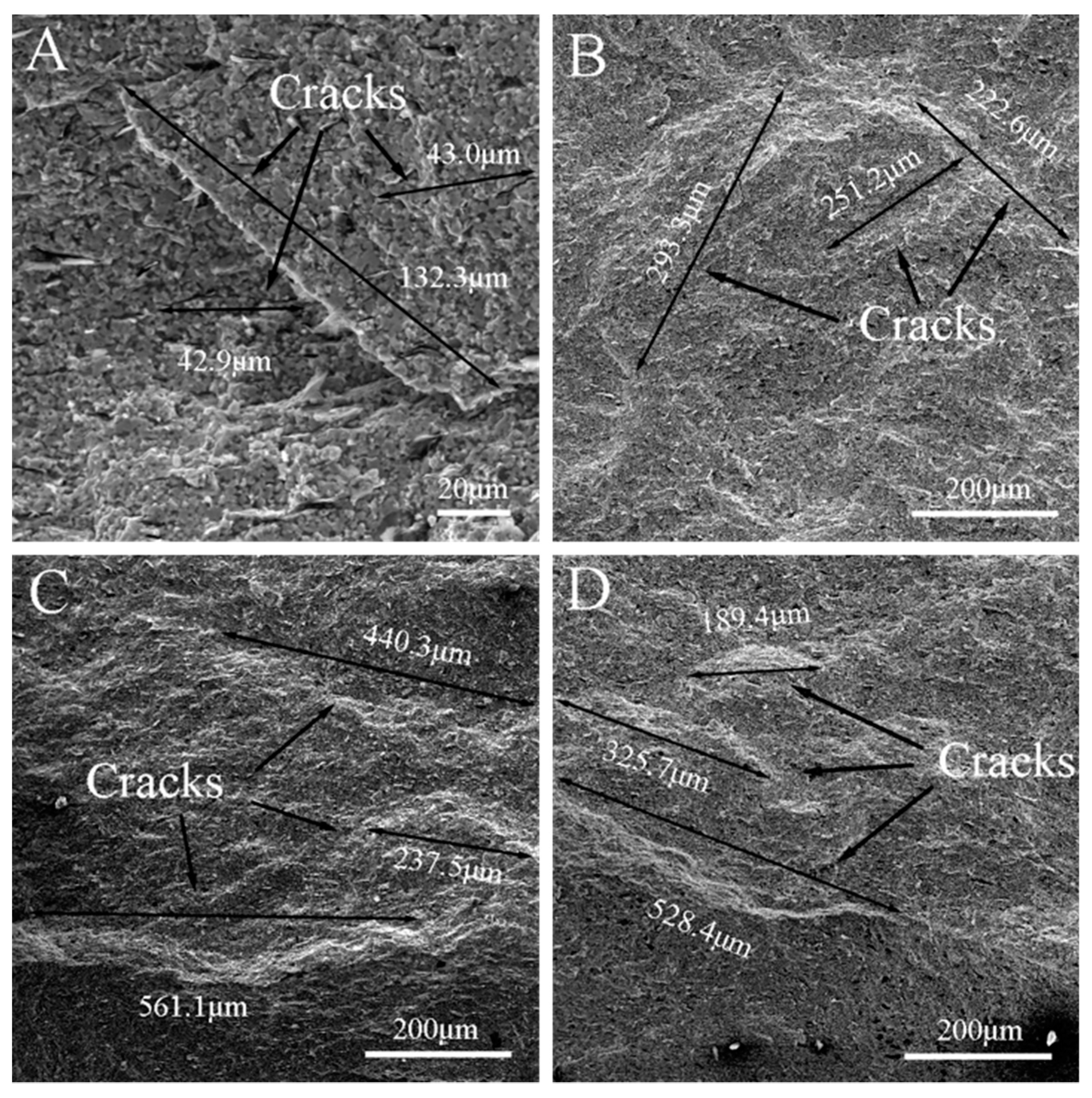
3.1. Surface Analysis
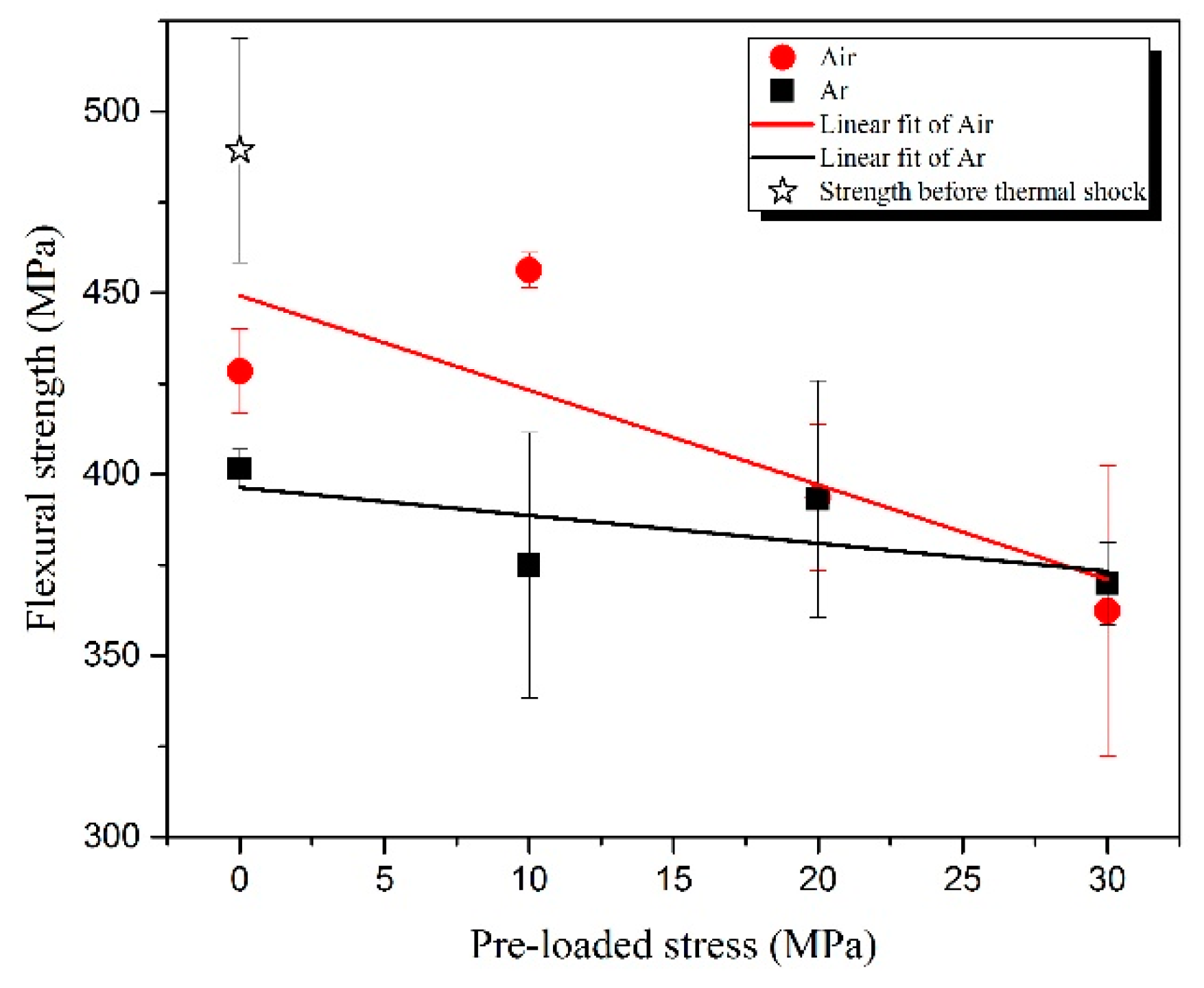
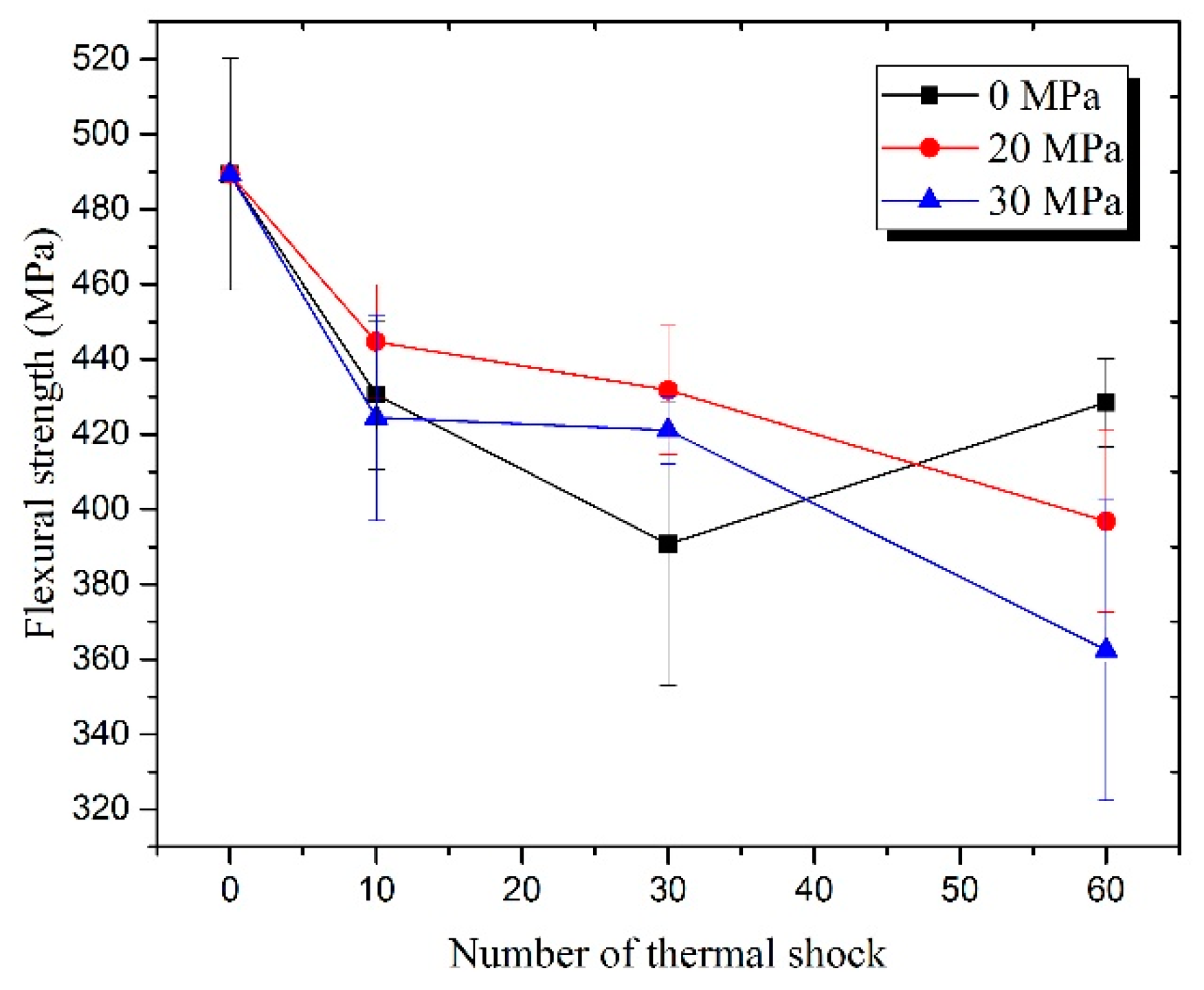
3.2. Residual Flexural Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Opeka, M.M.; Talmy, I.G.; Wuchina, E.J.; Zaykoski, J.A.; Causey, S.J. Mechanical, Thermal, and Oxidation Properties of Refractory Hafnium and Zirconium Compounds. J. Eur. Ceram. Soc. 1999, 19, 2405–2414. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E.; Talmy, I.G.; Zaykoski, J.A. Refractory Diborides of Zirconium and Hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Monteverde, F.; Cecere, A.; Savino, R. Thermo-Chemical Surface Instabilities of SiC-ZrB2 Ceramics in High Enthalpy Dissociated Supersonic Airflows. J. Eur. Ceram. Soc. 2017, 37, 2325–2341. [Google Scholar] [CrossRef]
- Guérineau, V.; Julian-Jankowiak, A. Oxidation Mechanisms under Water Vapour Conditions of ZrB2-SiC and HfB2-SiC Based Materials up to 2400 °C. J. Eur. Ceram. Soc. 2018, 38, 421–432. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Yu, P.; Shen, S. Temperature-Dependent Fracture Strength and the Effect of Oxidation for ZrB2-SiC Ceramics. J. Eur. Ceram. Soc. 2018, 38, 1112–1117. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Luo, L.; An, L. Oxidation Behaviour of ZrB2–SiC (Al/Y) Ceramics at 1700 °C. J. Eur. Ceram. Soc. 2016, 36, 3769–3774. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, Y.; Han, W.; Zhang, X.; Zhang, Y.; Zhang, H. Microstructure, Mechanical Behavior and Oxidation Resistance of Disorderly Assembled ZrB2-Based Short Fibrous Monolithic Ceramics. J. Eur. Ceram. Soc. 2019, 39, 2794–2804. [Google Scholar] [CrossRef]
- Zhang, Z.; Nan, C.; Xu, J.; Gao, Z.; Li, M.; Wang, J. Oxidation Behaviors of C–ZrB2–SiC Composite at 2100 °C in Air and O2. J. Mater. Sci. Technol. 2014, 30, 1223–1229. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, G.-J.; Vleugels, J.; Van der Biest, O. High Temperature Strength of Hot Pressed ZrB2–20 vol% SiC Ceramics Based on ZrB2 Starting Powders Prepared by Different Carbo/Boro-Thermal Reduction Routes. J. Eur. Ceram. Soc. 2013, 33, 1609–1614. [Google Scholar] [CrossRef]
- Zou, J.; Rubio, V.; Binner, J. Thermoablative Resistance of ZrB2-SiC-WC Ceramics at 2400 °C. Acta Mater. 2017, 133, 293–302. [Google Scholar] [CrossRef]
- Wuchina, E.; Opila, E.; Opeka, M.; Fahrenholtz, W.G.; Talmy, I.G. UHTCs Ultra-High Temperature Ceramic Materials for Extreme Environment Applications. Electrochem. Soc. Interface 2007, 16, 30–36. [Google Scholar]
- Fahrenholtz, W.G.; Hilmas, G.E. Ultra-High Temperature Ceramics: Materials for Extreme Environments. Scr. Mater. 2017, 129, 94–99. [Google Scholar] [CrossRef]
- Savino, R.; Criscuolo, L.; Di Martino, G.D.; Mungiguerra, S. Aero-Thermo-Chemical Characterization of Ultra-High-Temperature Ceramics for Aerospace Applications. J. Eur. Ceram. Soc. 2018, 38, 2937–2953. [Google Scholar] [CrossRef]
- Meng, S.; Liu, G.; Sun, S. Prediction of Crack Depth during Quenching Test for an Ultra High Temperature Ceramic. Mater. Des. 2010, 31, 556–559. [Google Scholar] [CrossRef]
- Shao, Y.; Song, F.; Liu, B.; Li, W.; Li, L.; Jiang, C. Observation of Ceramic Cracking during Quenching. J. Am. Ceram. Soc. 2017, 100, 520–523. [Google Scholar] [CrossRef]
- Jin, X.; Dong, L.; Xu, H.; Liu, L.; Li, N.; Zhang, X.; Han, J. Effects of Porosity and Pore Size on Mechanical and Thermal Properties as Well as Thermal Shock Fracture Resistance of Porous ZrB2–SiC Ceramics. Ceram. Int. 2016, 42, 9051–9057. [Google Scholar] [CrossRef]
- Chen, L.; Wang, A.; Suo, X.; Hu, P.; Zhang, X.; Zhang, Z. Effect of Surface Heat Transfer Coefficient Gradient on Thermal Shock Failure of Ceramic Materials under Rapid Cooling Condition. Ceram. Int. 2018, 44, 8992–8999. [Google Scholar] [CrossRef]
- Jin, X.; Li, P.; Hou, C.; Wang, X.; Fan, X.; Lu, C.; Xiao, G.; Shu, X. Oxidation Behaviors of ZrB2 Based Ultra-High Temperature Ceramics under Compressive Stress. Ceram. Int. 2019, 45, 7278–7285. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, C.; Song, F.; Li, J.; Shao, Y.; Xu, X.; Yan, P. Size Effect of Thermal Shock Crack Patterns in Ceramics and Numerical Predictions. J. Eur. Ceram. Soc. 2015, 35, 1263–1271. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, X.; Han, J.; Hu, P.; He, R. Thermal Shock Behavior of Porous ZrB2–SiC Ceramics. Mater. Sci. Eng. A 2013, 588, 175–180. [Google Scholar] [CrossRef]
- Hu, P.; Wang, Z.; Sun, X. Effect of Surface Oxidation on Thermal Shock Resistance of ZrB2–SiC–G Composite. Int. J. Refract. Met. Hard Mater. 2010, 28, 280–285. [Google Scholar] [CrossRef]
- Wang, A.; Hu, P.; Zhang, X.; Zhang, D.; Fang, C. Thermal Shock Behavior of ZrB2-based Sharp Leading Edges Evaluated by a Novel Water Spraying Method. Ceram. Int. 2018, 44, 2376–2382. [Google Scholar] [CrossRef]
- Wang, A.; Hu, P.; Fang, C.; Zhang, D.; Zhang, X. Evaluations of Cooling Rate and Initial Temperature on Thermal Shock Behavior of ZrB2-SiC Ceramic. J. Alloy. Compd. 2018, 741, 509–513. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, F.; Zhang, Z.; Hu, P.; Wang, A.; Ling, Y.; Liang, W.; Suo, X.; Zhang, X. An Aqueous Polymer Quenching Medium for Instantaneous Thermal Shock Cooling Rate Study of Ceramic Materials. J. Alloys Compd. 2017, 724, 234–239. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Z.; Sun, X.; Han, J. Microstructure, Mechanical Properties and Thermal Shock Resistance of Zirconium Diboride Containing Silicon Carbide Ceramic Toughened by Carbon Black. Mater. Chem. Phys. 2010, 122, 470–473. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, Z.; Hu, P.; Han, W.B.; Hong, C.Q. Mechanical Properties and Thermal Shock Resistance of ZrB2–SiC Ceramic Toughened with Graphite Flake and SiC Whiskers. Scr. Mater. 2009, 61, 809–812. [Google Scholar] [CrossRef]
- Wang, S.; Xu, C.; Ding, Y.; Zhang, X. Thermal Shock Behavior of ZrB2–SiC Composite Ceramics with Added TaSi2. Int. J. Refract. Met. Hard Mater. 2013, 41, 507–516. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Sun, X.; Han, W.; Hong, C. Effect of Graphite Flake on the Mechanical Properties of Hot Pressed ZrB2–SiC Ceramics. Mater. Lett. 2008, 62, 4360–4362. [Google Scholar] [CrossRef]
- Guo, S.; Naito, K.; Kagawa, Y. Mechanical and Physical Behaviors of Short Pitch-Based Carbon Fiber-Reinforced HfB2–SiC Matrix Composites. Ceram. Int. 2013, 39, 1567–1574. [Google Scholar] [CrossRef]
- Wenbo, H.; Shanbao, Z.; Jihong, Z. Single-Cycle Thermal Shock Resistance of ZrB2–SiCnp Ceramic Composites. Ceram. Int. 2014, 40, 16665–16669. [Google Scholar] [CrossRef]
- Gui, K.; Hu, P.; Hong, W.; Zhang, X.; Dong, S. Microstructure, Mechanical Properties and Thermal Shock Resistance of ZrB2-SiC-Cf Composite with Inhibited Degradation of Carbon Fibers. J. Alloys Compd. 2017, 706, 16–23. [Google Scholar] [CrossRef]
- Silvestroni, L.; Sciti, D.; Hilmas, G.E.; Fahrenholtz, W.G.; Watts, J. Effect of a Weak Fiber Interface Coating in ZrB2 Reinforced with Long SiC Fibers. Mater. Des. 2015, 88, 610–618. [Google Scholar] [CrossRef]
- Asl, M.S.; Nayebi, B.; Ahmadi, Z.; Zamharir, M.J.; Shokouhimehr, M. Effects of carbon additives on the properties of ZrB2–based composites: A review. Ceram. Int. 2018, 44, 7334–7348. [Google Scholar] [CrossRef]
- Weng, S.; Fu, T.; Peng, X.; Chen, X. Anisotropic Phase Transformation in B2 Crystalline CuZr Alloy. Nanoscale Res. Lett. 2019, 14, 283. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Chen, X.; Yue, X.; Fu, T.; Peng, X. Inapparent Strengthening Effect of Twin Interface in Cu/Pd Multilayered Films with a Large Lattice Mismatch. Nanomaterials 2019, 9, 1778. [Google Scholar] [CrossRef] [PubMed]
- Squire, T.H.; Marschall, J. Material property requirements for analysis and design of UHTC components in hypersonic applications. J. Eur. Ceram. Soc. 2010, 30, 2239–2251. [Google Scholar] [CrossRef]
- Gangireddy, S.; Halloran, J.W.; Wing, Z.N. Non-contact mechanical property measurements at ultrahigh temperatures. J. Eur. Ceram. Soc. 2010, 30, 2183–2189. [Google Scholar] [CrossRef]
- Meng, S.; Qi, F.; Chen, H.; Wang, Z.; Bai, G. The repeated thermal shock behaviors of a ZrB2–SiC composite heated by electric resistance method. Int. J. Refract. Met. Hard Mater. 2011, 29, 44–48. [Google Scholar] [CrossRef]
- Qi, F.; Meng, S.; Guo, H. Repeated thermal shock behavior of the ZrB2–SiC–ZrC ultrahigh-temperature ceramic. Mater. Des. 2012, 35, 133–137. [Google Scholar] [CrossRef]
- Niu, J.; Meng, S.; Jin, H.; Li, J.; Yi, F.; Zhou, Y. Thermal stability and nanostructure evolution of amorphous SiCN ceramics during laser ablation in an argon atmosphere. J. Eur. Ceram. Soc. 2019, 39, 4535–4544. [Google Scholar] [CrossRef]
- Gangireddy, S.; Halloran, J.W.; Wing, Z.N. Flexural creep of zirconium diboride–Silicon carbide up to 2200 °C in minutes with non-Contact electromagnetic testing. J. Eur. Ceram. Soc. 2013, 33, 2901–2908. [Google Scholar] [CrossRef]
- Jin, H.; Meng, S.; Yang, Q.; Zhu, Y. Thermal shock resistance of a ZrB2–SiC–graphite composite in low oxygen partial pressure environment. Ceram. Int. 2013, 39, 5591–5596. [Google Scholar] [CrossRef]
- Jin, H.; Meng, S.; Zhu, Y.; Zhou, Y. Effect of environment atmosphere on thermal shock resistance of the ZrB2–SiC–graphite composite. Mater. Des. 2013, 50, 509–514. [Google Scholar] [CrossRef]
- Chen, X.; Peng, X.; Wei, Z.; Yue, X.; Fu, T. Repeated thermal shock behavior of ZrB2-SiC-graphite composite under prestress. Ceram. Int. 2016, 42, 18012–18018. [Google Scholar] [CrossRef]
- Chen, X.; Peng, X.; Wei, Z.; Yue, X.; Fu, T. Effect of tensile stress on thermal fatigue life of ZrB2-SiC-graphite composite. Mater. Des. 2017, 126, 91–97. [Google Scholar] [CrossRef]
- Yue, X.; Peng, X.; Wei, Z.; Chen, X.; Fu, T. Effect of heating rate on the strength of ZrB2–SiC composite subjected to cyclic thermal shock. Ceram. Int. 2019, 45, 15400–15405. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G. Thermodynamic Analysis of ZrB2-SiC Oxidation: Formation of a SiC-Depleted Region. J. Am. Ceram. Soc. 2007, 90, 143–148. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, X.; Wei, Z.; Zangvil, A. A Thermo-Chemo-Mechanical Model for the Oxidation of Zirconium Diboride. J. Am. Ceram. Soc. 2015, 98, 629–636. [Google Scholar] [CrossRef]
- Haibo, O.; Cuiyan, L.; Jianfeng, H.; Liyun, C.; Jie, F.; Jing, L.; Zhanwei, X. Self-healing ZrB2 –SiO2 oxidation resistance coating for SiC coated carbon/carbon composites. Corros. Sci. 2016, 110, 265–272. [Google Scholar] [CrossRef]
- Rezaie, A.; Fahrenholtz, W.G.; Hilmas, G.E. Evolution of structure during the oxidation of zirconium diboride–silicon carbide in air up to 1500 °C. J. Eur. Ceram. Soc. 2007, 27, 2495–2501. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, G. Oxidation resistance and strength retention of ZrB2–SiC ceramics. J. Eur. Ceram. Soc. 2010, 30, 2387–2395. [Google Scholar] [CrossRef]



| Specimen and Test Name | Pre-Loaded Stress σ0 (MPa) | Gaseous Environment | Cycles |
|---|---|---|---|
| ZSG0 | 0 | Air | 60 |
| ZSG10 | 10 | ||
| ZSG20 | 20 | ||
| ZSG30 | 30 | ||
| ZSG0-Ar | 0 | Ar | |
| ZSG10-Ar | 10 | ||
| ZSG20-Ar | 20 | ||
| ZSG30-Ar | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, X.; Peng, X.; Wei, Z.; Chen, X.; Chen, X.; Fu, T. Repeated Thermal Shock Behavior of ZrB2–SiC–Graphite Composite under Pre-Stress in Air and Ar Atmospheres. Materials 2020, 13, 370. https://doi.org/10.3390/ma13020370
Yue X, Peng X, Wei Z, Chen X, Chen X, Fu T. Repeated Thermal Shock Behavior of ZrB2–SiC–Graphite Composite under Pre-Stress in Air and Ar Atmospheres. Materials. 2020; 13(2):370. https://doi.org/10.3390/ma13020370
Chicago/Turabian StyleYue, Xing, Xianghe Peng, Zhen Wei, Xiaosheng Chen, Xiang Chen, and Tao Fu. 2020. "Repeated Thermal Shock Behavior of ZrB2–SiC–Graphite Composite under Pre-Stress in Air and Ar Atmospheres" Materials 13, no. 2: 370. https://doi.org/10.3390/ma13020370
APA StyleYue, X., Peng, X., Wei, Z., Chen, X., Chen, X., & Fu, T. (2020). Repeated Thermal Shock Behavior of ZrB2–SiC–Graphite Composite under Pre-Stress in Air and Ar Atmospheres. Materials, 13(2), 370. https://doi.org/10.3390/ma13020370





