Abstract
Sodium iron hexafluoride (Na3FeF6), as a colorless iron fluoride, is expected to be an ideal host for rare earth ions to realize magneto-optical bi-functionality. Herein, monodispersed terbium ions (Tb3+) doped Na3FeF6 particles are successfully synthesized by a facile one-pot hydrothermal process. X-ray diffraction (XRD) and Field emission scanning electron microscopy (FESEM) reveal that the Tb3+ doped Na3FeF6 micro-particles with regular octahedral shape can be assigned to a monoclinic crystal structure (space group P21/c). Under ultraviolet light excitation, the Na3FeF6:Tb3+ octahedral particles given orange-red light emission originated from the 5D4→7FJ transitions of the Tb3+ ions. In addition, the magnetism measurement indicates that Na3FeF6:Tb3+ octahedral particles are paramagnetic with high magnetization at room temperature. Therefore, the Na3FeF6:Tb3+ powders may find potential applications in the biomedical field as magnetic-optical bi-functional materials.
1. Introduction
Bi-functional materials with distinct magnetic and fluorescent (luminescent) properties have received considerable attention [1,2] due to their potential applications in magnetic resonance imaging [3], targeted drug delivery [4], sensors [5,6], optical isolators [7,8,9], high accuracy communication [10], and aircraft guidance [11]. To date, there have been a few reports about the synthesis of magnetically-functionalized luminescent materials based on quantum dots (QDs) and organic dyes [12]. However, QDs features notorious disadvantages including chemical instability, potential toxicity, luminescent intermittence and weakly magnetic, while organic dyes typically exhibit rapid photobleaching and a low fluorescence quantum yield [13]. As a result, biological applications of these materials have been seriously restricted.
Rare-earth (RE) ions doped inorganic materials can be considered as alternative luminescent materials in which the above limitations are partly circumvented [14,15]. Nowadays, great efforts have been devoted to the design and fabrication of magneto-optical bi-functional systems based on RE-doped up-conversion or down-conversion materials, such as Gd2O3: Er3+/Yb3+ [16], GdPO4: Eu3+ [17], YVO4: Er3+ [18], Tb0.94Pr0.06VO4 [19] and NaYF4: Yb, Ho [20]. However, studies of magneto-optical effects usually have to rely on materials with a high magnetic movement, which are usually non-transparent. On the other hand, the introduction of strong magnetic (ferromagnetic) materials can be achieved by fabricating magnetic-core/luminescent-shell structures, such as the Fe3O4@LaF3:Yb3+, Er3+ [21], Fe3O4@α-NaYF4/Yb [13] and Fe3O4@ZnO:Er3+,Yb3+ [22]. However, the preparation processes for core-shell structures is complicated, and more importantly, magnetic oxide, Fe3O4, strongly absorbs visible light and quenches fluorescence of the RE ions [23]. Therefore, use of a colorless, strongly magnetic host is of great importance for the development of magneto-optical bifunctional materials.
In this work, colorless Tb3+ ions doped sodium iron hexafluoride (Na3FeF6:Tb3+) containing a high centration of paramagnetic ion (Fe3+) is synthesized through a simple hydrothermal process. The Na3FeF6:Tb3+ particles give distinct visible emission under excitation by UV light and its luminescence intensity is optimized by adjusting Tb3+ doping concentration. The investigation of the magnetic property reveals that the Na3FeF6:Tb3+ particles are paramagnetic at room temperature. These results indicate that Na3FeF6:Tb3+ particles might be promising as a new platform for exploiting magnetic-optical functionalities.
2. Materials and Methods
2.1. Synthesis of Na3FeF6:Tb3+ Particles
The reagents used in this work were analytical-grade Fe(NO3)3·9H2O (99.99%), NH4HF2 (99%), NaF (99%), HF (40%), and Tb(NO3)3·6H2O (99.99%) (Xiya Reagent, Shandong, China). Samples with a different molar ratio of Tb3+ to Fe3+ (5%, 10%, 15%, 18%, and 20%) were synthesized by a hydrothermal method under the same conditions. Here, we take Na3FeF6:18%Tb3+ as an example to present the detailed preparation procedure. The process mainly involves four steps: (1) 14 mL of Fe(NO3)3·9H2O solution (0.1 M), 14 mL of NaF solution (0.5 M), 42 mL of NH4HF2 solution (0.5 M), and 3 mL of HF were mixed under vigorous magnetic stirring for 30 min; (2) 2.7 mL Tb(NO3)3·6H2O was added to the above solution under vigorous magnetic stirring for 3 h; (3) after stirring for 3 h, the above solution was transferred into a Teflon-lined stainless steel autoclave (capacity 100 mL), which was heated at 190 °C for 12 h and cooled naturally to room temperature; (4) the obtained sample was washed by deionized water for several times and dried at 60 °C overnight.
2.2. Characterization
Phase identification of the as-prepared samples were carried out by X-ray diffraction (XRD) (X’Pert Pro, PANalytical BV, Netherland) with Cu Kα radiation (λ = 1.5418 Å). The microstructure and element mapping of particles were observed with a Field emission scanning electron microscopy (FESEM) (Hitachi Ltd., Tokyo, Japan) equipped with an energy dispersive spectroscopy (EDS). UV-Vis (ultraviolet-visible) absorption, transmission, and reflectance spectra of particles were acquired in an UV-Vis spectrophotometer (Model: U3600P) with an integrating sphere using BaSO4 as a standard reference. Photoluminescence excitation and emission spectra were obtained using two spectrometers (Omni-λ3007 and Omni-λ180D; Zolix, Beijing, China) and a 150 W Xenon lamp as the excitation source. The Commission International de I’Eclairage (CIE) chromaticity coordinates of sample were calculated by CIE 1931 software (V.1.6.0.2). Magnetic properties were collected on a Quantum Design superconducting quantum interference device (SQUID) magnetometer (MPMS XL-7).
3. Results and Discussion
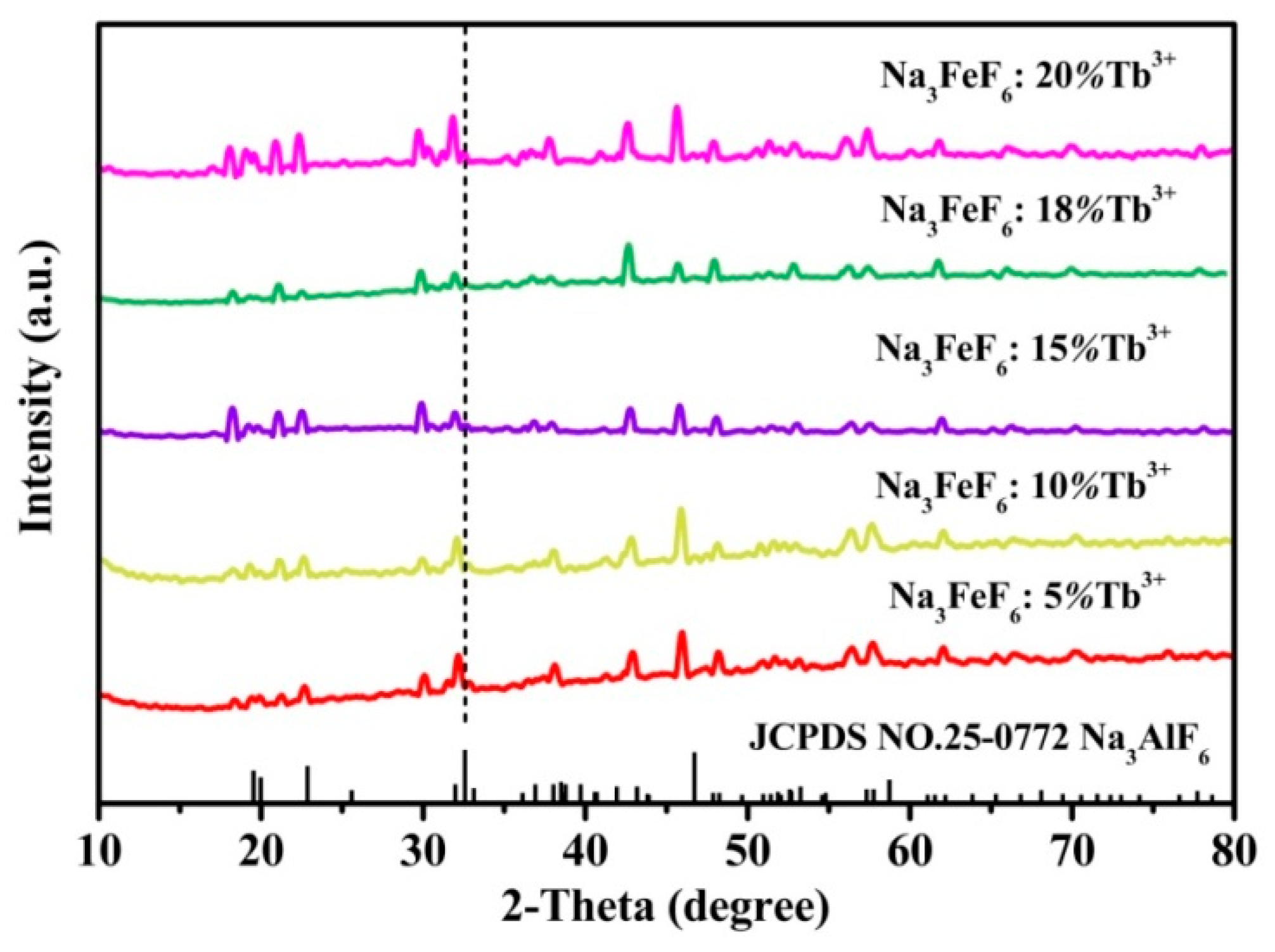
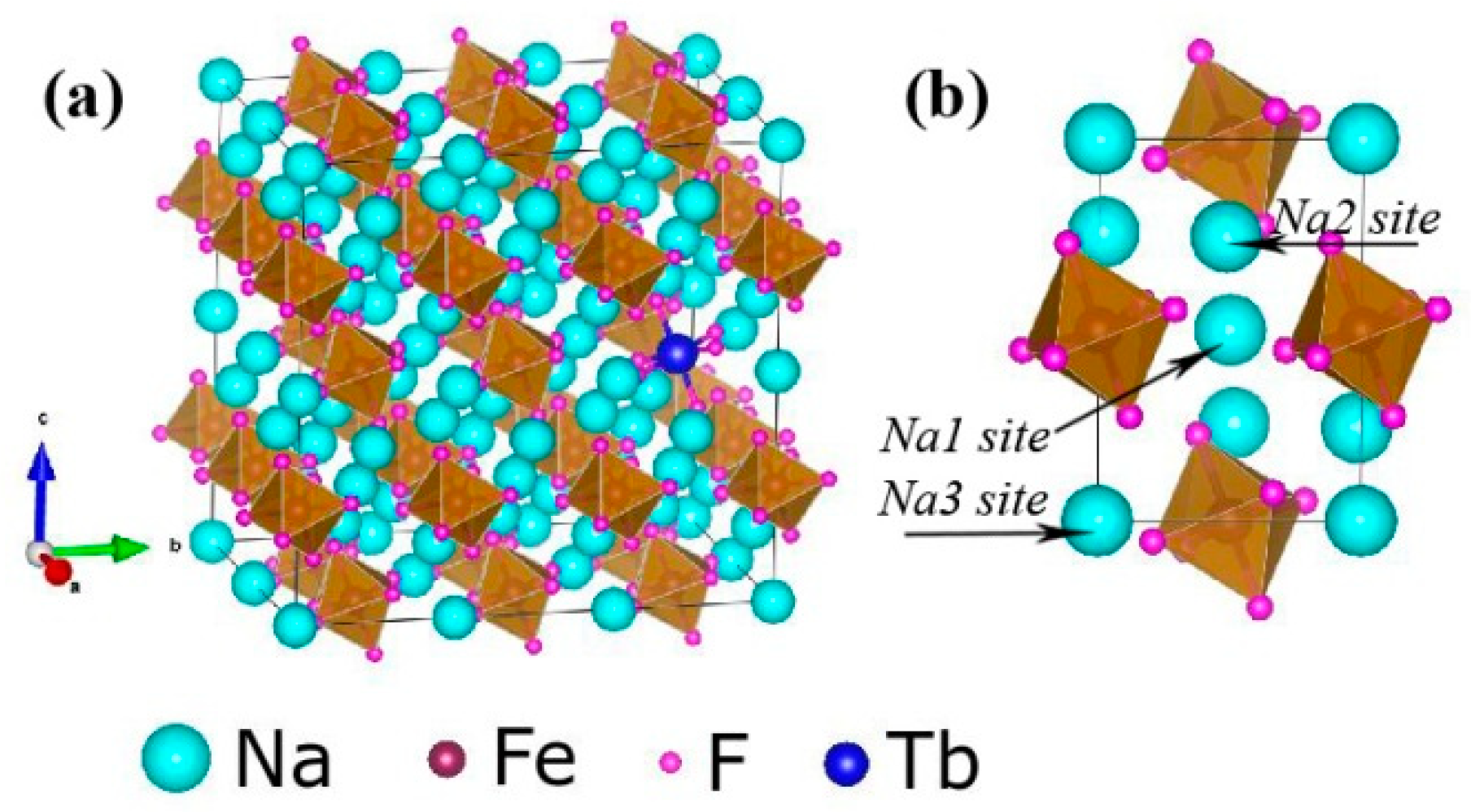
Figure 1 shows the typical XRD patterns of the Tb3+ doped Na3FeF6 samples synthesized with different doping concentrations of Tb3+ (5%, 10%, 15%, 18%, and 20%). The diffraction peaks of all samples clearly match that of the standard pattern of Na3AlF6 (JCPDS no. 12-0907), indicating the structure of obtained samples is isomorphic with cryolite-like structures (Na3AlF6 and Na3CrF6) that belongs to the space group P21/c [24,25,26]. This result agrees with previous report about the structure of Na3FeF6 [27]. The three-dimensional crystal structure of Na3FeF6:Tb3+ is shown in Figure 2. There are three different sodium sites, namely Na1, Na2, and Na3, as highlighted in Figure 2b. Na1 site is located at the distorted octahedral site of (NaF6), Na2 site is located at the bi-pyramid site of (NaF5), and the Na3 site is located at the distorted tetrahedral site of (NaF4). As can be observed from the crystal structure (Figure 2a), Na1 octahedral and Na3 tetrahedral share corners. Na1 octahedral share edges with Na2 bipyramid. Furthermore, all Fe atoms are located at the distorted FeF6 octahedral sites. FeF6 octahedra share corners with Na1 octahedral and Na3 tetrahedral share edges with Na2 bi-pyramid. In this structure, Fe3+ sites can be taken by Tb3+ ions in Tb doped Na3FeF6. According to the Bragg equation (2dSinθ = nλ), d increases with the decreasing of θ. Figure 1 shows that diffraction peak of Na3FeF6:Tb3+ is all shifted to the left compared with that of Na3AlF6 due to the larger ionic size of Fe3+ as compared with that of Al3+. As concentration of the Tb ions increases from 5% to 18%, the diffraction peak gradually shifts to the left, diffraction angle θ decreases. This result can be explained by the substitution of Fe3+ (ionic radius = 0.65 Å) [28] by Tb3+ with a larger ionic radius (0.92 Å) [29]. Therefore, the lattice constant would increase with the increase in the concentration of Tb ions in the lattice. The diffraction peaks of the Na3FeF6 with 18% Tb3+ doping are the highest, indicating the best crystallinity. The increase of Tb3+ concentration above 18% leads to growth of lattice strain that prevents the further enhancement of crystallization. To further confirm the ions of Tb3+ is present in the form of Tb-F and Na3FeF6:18%Tb3+ powder was analyzed by XPS (Figure S1). The XPS spectrum shows the presence of Na, F, Fe and Tb elements. Figure S1b shown the XPS spectra of Tb(Ds-4s), Na(2p), F(2s), Fe(3p), Tb(4d) from Na3FeF6:18%Tb3+ and the relatively strong peaks at around 7.5, 152 eV can be assigned to the binding energy of Tb (Ds-4s) and Tb (4d), respectively. The peak around 24.7 eV is attributed to the binding energy of Na(2p). The binding energy of F(2s) around 30.3 eV and F(1s) around 684.9 eV are found in spectra of XPS (Figure 1b,d). The Fe(3p) peaks show a doublet around 56.3 and 59.1 eV, corresponding to structure of FeF3 and FeF2, respectively. The result in accordance with the discussion of the XRD patterns of the Tb3+ doped Na3FeF6.
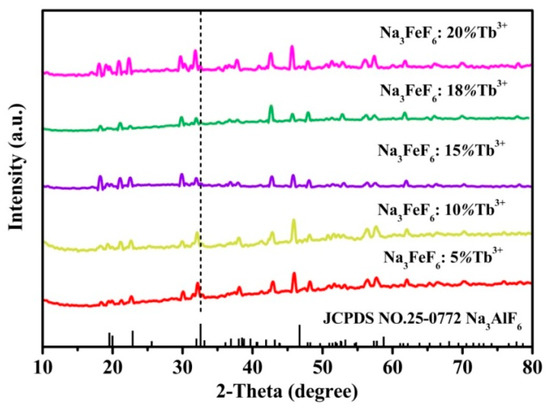
Figure 1.
X-ray diffraction (XRD) patterns for samples of the Na3FeF6:Tb3+ with different Tb3+-doping concentrations.
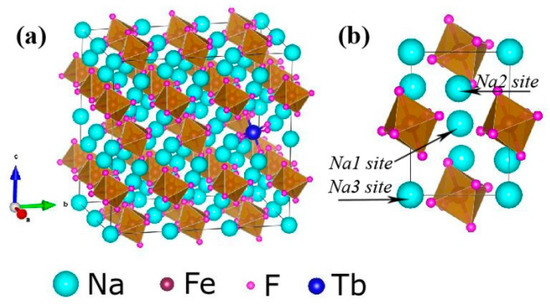
Figure 2.
(a) Three-dimensional crystal structure of Na3FeF6:Tb3+. (b) Three different sodium sites in the Na3FeF6 crystal structure.
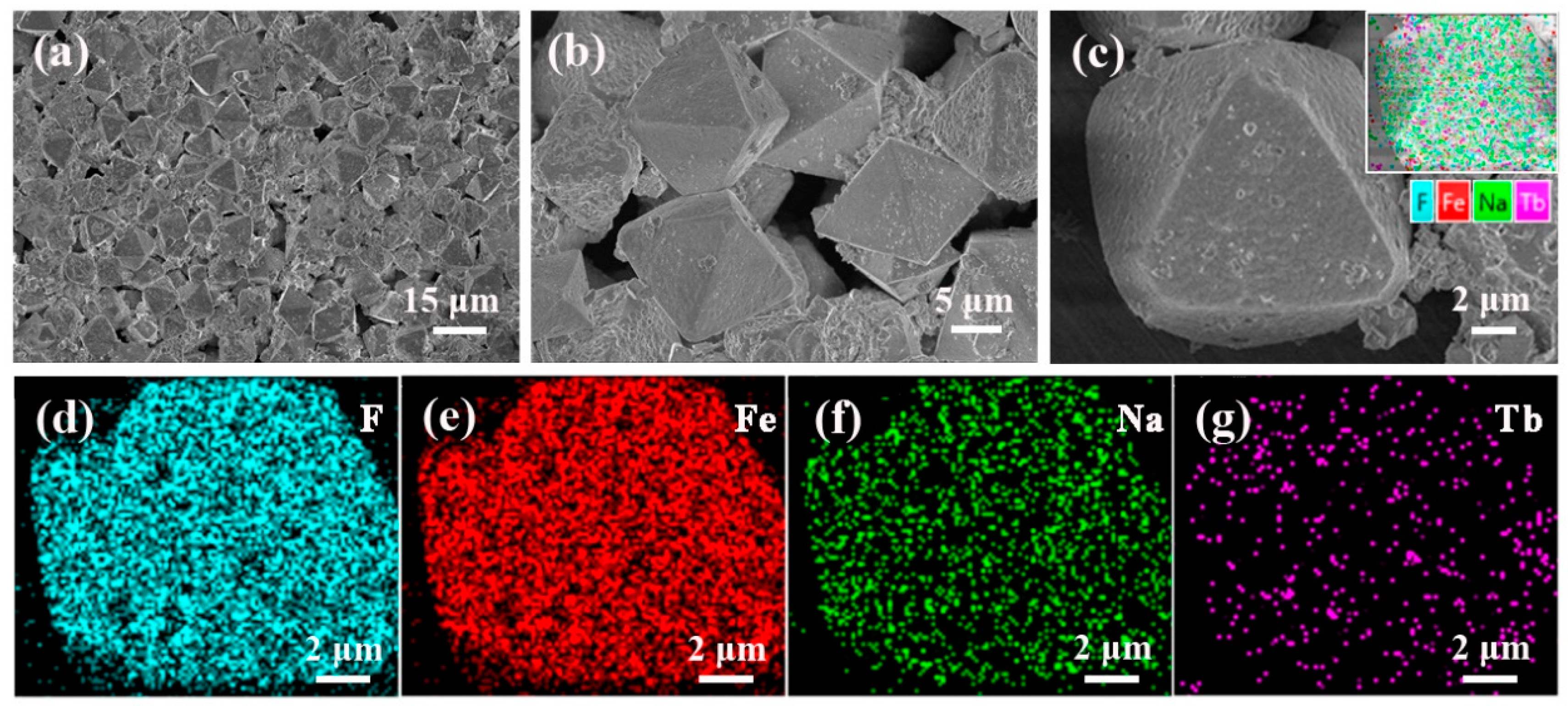
The Na3FeF6:18%Tb3+ particles are then observed by FESEM equipped with an energy dispersive spectroscopy (EDS) device. Figure 3a–c show the FESEM images with low magnification (a) and high magnification (b,c). It can be observed from Figure 3a that the as-prepared samples consist of randomly distributed octahedral particles with a relatively uniform size and shape (edge lengths are approximately 10 µm). As the magnification increases (Figure 3c), it can be seen clearly that the surfaces of the octahedron are almost smooth, but covered by a few small sized particles. EDS analysis was then used to determine the distribution of elements, as illustrated in Figure 3c. The results confirm the dominance of four elements:F, Fe, Na and Tb. In addition, the corresponding EDS mapping images given in Figure 3d–g reveal that all the elements are distribute homogeneously in the particles and Tb ions are successfully doped into the lattice of Na3FeF6.
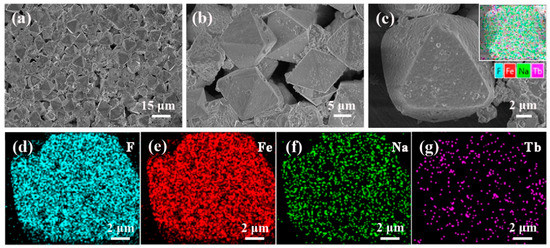
Figure 3.
(a–c) Field emission scanning electron microscopy (FESEM) images of the Na3FeF6:18%Tb3+ powders and (d–g) the corresponding energy dispersive spectroscopy (EDS) mapping for elements image F, Fe, Na, and Tb.
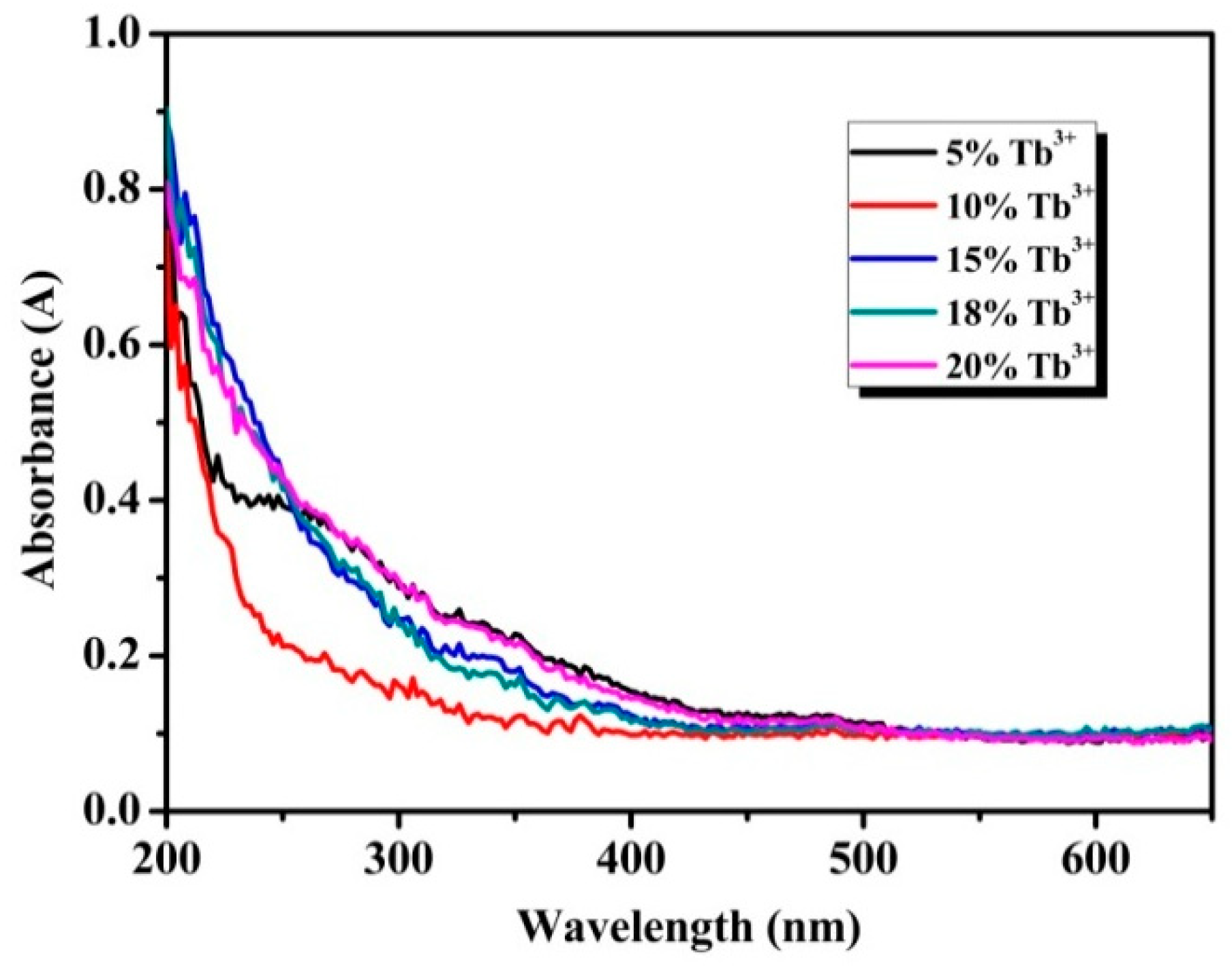
To confirm the optical response of the particles in the UV-Vis range, absorption spectra was detected by an UV-Vis spectrophotometer. As shown in Figure 4, all samples exhibit obvious ultraviolet absorption at wavelength short than 300 nm, which can be attributed to transition of the 4f electronic ground state to the 5d energy levels, namely 4f8→4f75d1 energy levels transitions of Tb3+ [30]. The f-f transitions of the Tb3+ in the wavelength region of 300-400 nm are relatively weak and these peaks at 355 and 380 nm by f-f transitions of Tb3+ are almost invisible in the absorption spectra [31]. The transmission spectra and the reflectance spectra of the Na3FeF6:18%Tb3+ particles correspond to the absorption spectra (as shown in Figure S2).
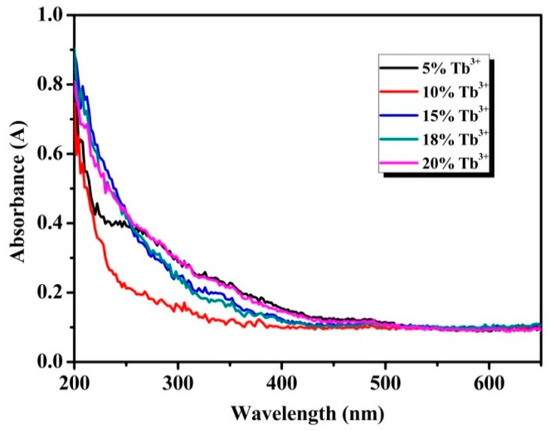
Figure 4.
Ultraviolet-visible (UV-Vis) absorption spectra of Na3FeF6:Tb3+ particles with different Tb3+-doping concentrations.
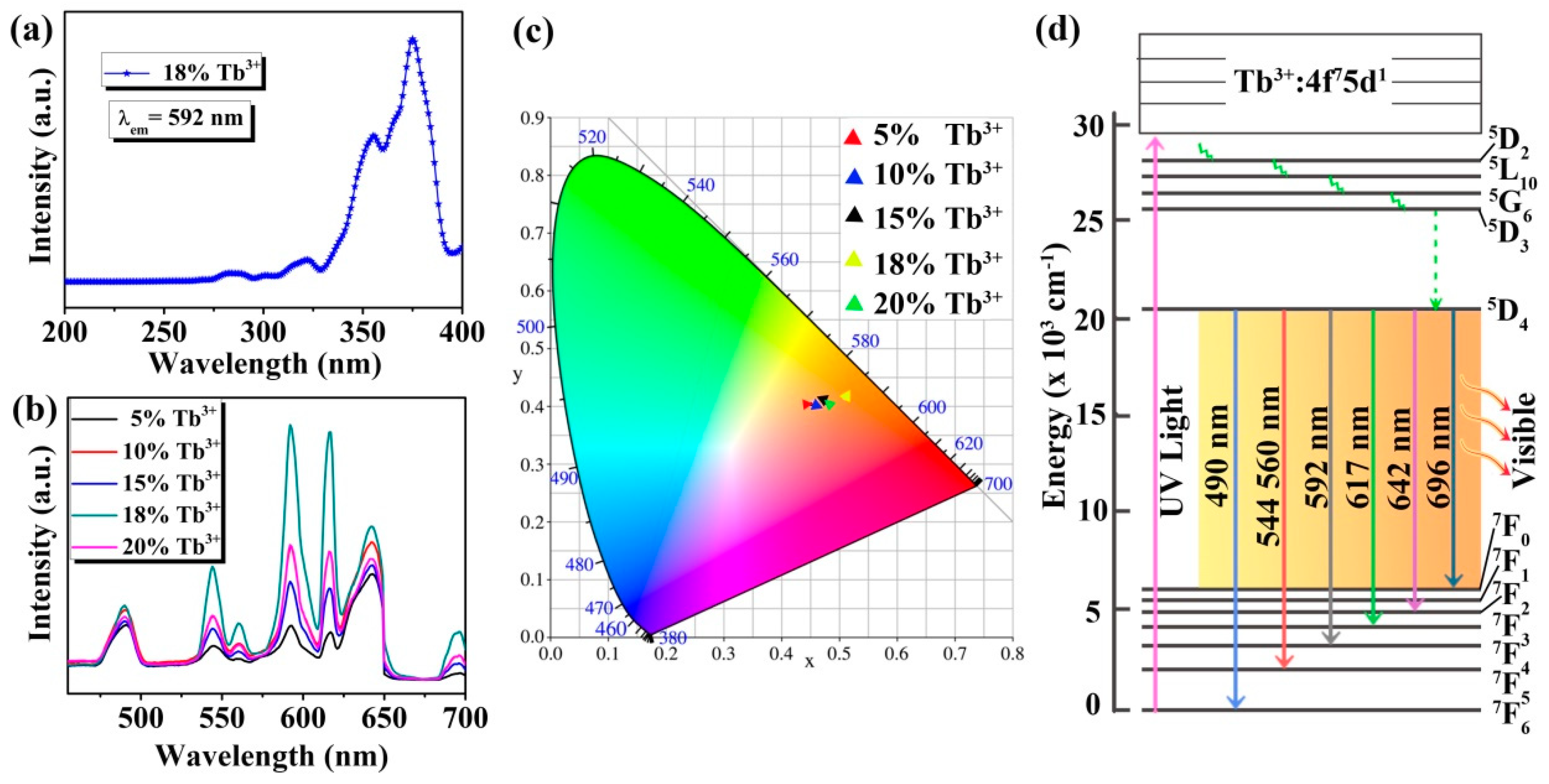
In order to further study the optical properties of the Na3FeF6:Tb3+ particles, excitation and emission spectra are measured by fluorescence spectrometers. Figure 5 presents the excitation and emission spectra, and CIE 1931 chromaticity coordinates of the samples together with the energy level diagram of Tb ions. As shown in Figure 5a, the excitation spectra of Na3FeF6:18%Tb3+ are measured for the emission wavelength of 592 nm. It can be observed that the excitation spectra consist of sharp and intense bands with peak positions at 355 and 375 nm along with weak bands at 280 and 320 nm, which can be assigned to the 7F6→5L10, 7F6→5G6, 7F6→3H6 and 7F6→5D1 transitions of the Tb3+, respectively [32,33,34].
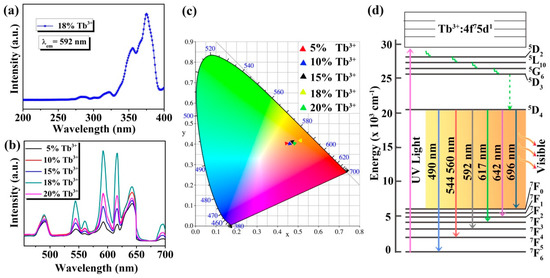
Figure 5.
(a) Excitation spectrum of Na3FeF6:18%Tb3+; (b) emission spectra of Na3FeF6:Tb3+ particles with different Tb3+-doping concentrations; (c) CIE 1931 chromaticity diagram of Na3FeF6 doped with different concentration of Tb3+; (d) simplified energy levels diagram of the Tb ions.
Since the peak of 375 nm is the strongest in the excitation spectrum, the emission spectra are recorded at this excitation wavelength for Na3FeF6:Tb3+ particles with different Tb3+-doping concentrations. As can be seen from Figure 5b, the emission spectra in the region of 455–700 nm exhibit seven peaks at 490, 544, 560, 592, 617, 642, and 696 nm due to 5D4→7F6, 7F5, 7F5, 7F4, 7F3, 7F2 and 7F0 transitions, respectively [35,36,37,38]. Among the seven peaks, five peaks at 490, 544, 592, 617, and 642 nm are much stronger, while the other two peaks (560 and 696 nm) are relatively weak. In addition, among the five samples, the luminescence intensity is strongest when the doping concentration of Tb3+ is 18%, and the highest luminescence peak is at 592 nm. The emission spectrum is converted to the CIE 1931 chromaticity coordinates using the photoluminescence data to better characterize the emission color of the samples. From the CIE 1931 chromaticity diagram (Figure 5c), it is found that all samples emit orange-red light, which is different from the traditional green light emission of Tb3+ ions. This may be due to the use of a new host (Na3FeF6) which favors the emission in the longer wavelengths. Furthermore, Figure 5c shows that as the doping concentration of Tb ions increases, the luminescence intensity first increases and then decreases, and the luminescence is strongest at the doping concentration of 18%, which is consistent with the emission spectrum (Figure 5b). The CIE coordinates of Na3FeF6:18%Tb3+ are X = 0.5103 and Y = 0.4155, which show a typical orange-red color.
In order to better understand the luminescence mechanism of the samples, we combined the energy level diagram of Tb ions (Figure 5d) and take the luminescence at 592 nm as an example to explain the involved electronic transitions. Upon excitation by ultraviolet light (UV-light), Tb3+ ions are promoted from the ground state (7F6) to the excited state (for example 5L10, 5G6). Subsequently, the level 5D4 of Tb3+ ions is populated by radiation-free transition. Finally, the Tb3+ ions relax to the ground state (7F4) by giving visible emission at around 592 nm. The visible luminescence at other wavelengths is similar to the emission at 592 nm.
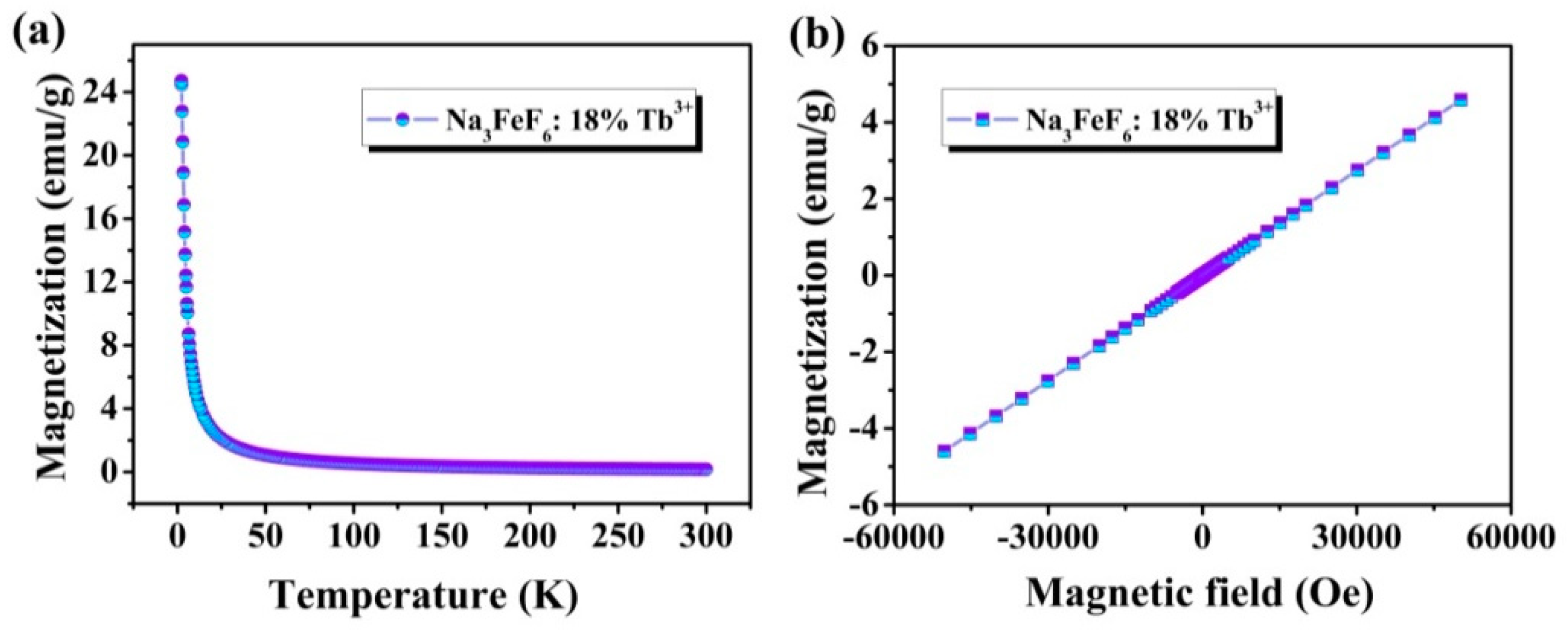
Figure 6a shows the temperature-dependence magnetization plots (M-T) in a temperature range between 5 K and 300 K in a 2000 Oe field of Na3FeF6:Tb3+ particles. It is found that the magnetization decreases rapidly from about 24.74 emu/g at 5 K to 1.06 emu/g at 50 K, and then slowly decreases with a temperature increase from 50 K to 300 K, typical for paramagnetic materials. The magnetization versus magnetic field (M-H) curves at 300 K of Na3FeF6:18%Tb3+ particles obtained by SQUID magnetometry are presented in Figure 6b. As the strength of the applied magnetic field increasing, the ideal linear correlation between the magnetization and the applied magnetic field was obtained, indicating that Na3FeF6:Tb3+ possesses paramagnetism. The results show that the synthesized samples might be used as magneto-optical bifunctional materials.
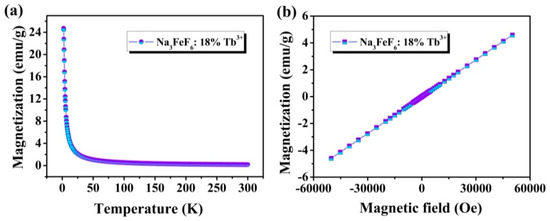
Figure 6.
(a) Temperature-dependent magnetization (M-T) curves measured at 2000 Oe for Na3FeF6:18%Tb3+ particles; (b) magnetization versus magnetic field (M-H) curve at 300 K of Na3FeF6:18%Tb3+ particles.
4. Conclusions
In summary, monodispersed Na3FeF6:Tb3+ octahedral particles have been successfully synthesized by a facile one-pot hydrothermal process and the results of XRD and SEM indicated that Na3FeF6:Tb3+ octahedral belong to a monoclinic crystal structure (space group P21/c). The Na3FeF6:Tb3+ octahedral particles emit orange-red colored light attributed to the 5D4 → 7FJ transitions of the Tb3+ ions. The luminescence intensity of the Na3FeF6:Tb3+ reaches maximum at Tb3+ doping concentration of 18%. The M-T and M-H curves confirm that Na3FeF6:Tb3+ particles are paramagnetic with a high magnetic moment. These results indicate that the obtained Na3FeF6:Tb3+ octahedral particles might be used as a magnetic-optical bi-functional material for various potential applications in biomedical fields and magneto-optical modulation.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/13/2/320/s1.
Author Contributions
Z.Z. conceptualization, methodology, data curation and writing-original draft preparation; X.L. preparation of sample, supervision of the work, editing, and review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 61575091, 61675094, and 51471082), excellent Team of Spectrum Technology and Application of Henan province (Grant No. 18024123007).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gai, S.; Yang, P.; Li, C.; Wang, W.; Dai, Y.; Niu, N.; Lin, J. Synthesis of Magnetic, Up-Conversion Luminescent, and Mesoporous Core-Shell-Structured Nanocomposites as Drug Carriers. Adv. Funct. Mater. 2010, 20, 1166–1172. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, Y.; Zhang, H.; Dong, G.; Han, J.; Qiu, J. Dynamically tuning the up-conversion luminescence of Er3+/Yb3+ co-doped sodium niobate nano-crystals through magnetic field. Sci. Rep. 2016, 6, 31327. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Su, C.H.; Liao, M.Y.; Yeh, C.S. Magneto-optical FeGa2O4 nanoparticles as dual-modality high contrast efficacy T-2 imaging and cathodoluminescent agents. PCCP 2009, 11, 6331–6334. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Liu, T.Y.; Su, C.H.; Lo, Y.W.; Chen, J.H.; Yeh, C.S. Superparamagnetic hollow and paramagnetic porous Gd2O3 particles. Chem. Mater. 2008, 20, 3840–3848. [Google Scholar] [CrossRef]
- Jung, H.K.; Kim, C.H.; Hong, A.R.; Lee, S.H.; Kim, T.C.; Jang, H.S.; Kim, D.H. Luminescent and magnetic properties of cerium-doped yttrium aluminum garnet and yttrium iron garnet composites. Ceram. Int. 2019, 45, 9846–9851. [Google Scholar] [CrossRef]
- Chen, H.; Qi, B.; Moore, T.; Colvin, D.C.; Crawford, T.; Gore, J.C.; Alexis, F.; Mefford, O.T.; Anker, J.N. Synthesis of brightly PEGylated luminescent magnetic upconversion nanophosphors for deep tissue and dual MRI imaging. Small 2014, 10, 160–168. [Google Scholar] [CrossRef]
- Hu, Q.; Jia, Z.; Yin, Y.; Mu, W.; Zhang, J.; Tao, X. Crystal growth, thermal and optical properties of TSLAG magneto-optical crystals. J. Alloys Compd. 2019, 805, 496–501. [Google Scholar] [CrossRef]
- Li, J.; Tang, T.; Luo, L.; Li, N.; Zhang, P. Spin Hall effect of reflected light in dielectric magneto-optical thin film with a double-negative metamaterial substrate. Opt. Express 2017, 25, 19117–19128. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, D.; Xu, J.; Tian, T.; Jia, R.; Wang, Z. Fabrication and magneto-optical property of yttria stabilized Tb2O3 transparent ceramics. J. Eur. Ceram. Soc. 2019, 39, 5005–5009. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, Y.; He, H.; Qiu, J.; Dong, G. Simultaneous broadband near-infrared emission and magnetic properties of single phase Ni2+-doped β-Ga2O3 nanocrystals via mediated phase-controlled synthesis. J. Mater. Chem. C 2015, 3, 2886–2896. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Q.; He, H.; Zhang, J.; Dong, G.; Han, J.; Qiu, J. Simultaneous luminescence modulation and magnetic field detection via magneto-optical response of Eu3+-doped NaGdF4 nanocrystals. J. Mater. Chem. C 2015, 3, 10140–10145. [Google Scholar] [CrossRef]
- Gu, H.; Zheng, R.; Zhang, X.; Xu, B. Facile one-pot synthesis of bifunctional heterodimers of nanoparticles: A conjugate of quantum dot and magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 5664–5665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Braun, G.B.; Pallaoro, A.; Zhang, Y.; Shi, Y.; Cui, D.; Moskovits, M.; Zhao, D.; Stucky, G.D. Mesoporous multifunctional upconversion luminescent and magnetic “nanorattle” materials for targeted chemotherapy. Nano Lett. 2011, 12, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhou, Y.; Li, X.; Li, Y.; Zhang, W.; Fu, H.; Zhao, J.; Pan, L.; Liu, X.; Qiu, J. Synthesis and phase transformation of NaGdF4: Yb–Er thin films using electro-deposition method at moderate temperatures. CrystEngComm 2018, 20, 6919–6924. [Google Scholar] [CrossRef]
- Jia, H.; Liu, Z.; Liao, L.; Gu, Y.; Ding, C.; Zhao, J.; Zhang, W.; Hu, X.; Feng, X.; Chen, Z. Upconversion Luminescence from Ln3+ (Ho3+, Pr3+) Ion-Doped BaCl2 Particles via NIR Light of Sun Excitation. J. Phys. Chem. C 2018, 122, 9606–9610. [Google Scholar] [CrossRef]
- Tan, C.; Ma, B.; Zhang, J.; Zuo, Y.; Zhu, W.; Liu, Y.; Li, W.; Zhang, Y. Pure red upconversion photoluminescence and paramagnetic properties of Gd2O3: Yb3⁺, Er3⁺ nanotubes prepared via a facile hydrothermal process. Mater. Lett. 2012, 73, 147–149. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, M.; You, H.; Yang, M.; Song, Y.; Huang, Y. Mutifuntional GdPO4: Eu3+ hollow spheres: Synthesis and magnetic and luminescent properties. Inorg. Chem. 2011, 50, 10608–10613. [Google Scholar] [CrossRef]
- Ma, Z.W.; Zhang, J.P.; Wang, X.; Yu, Y.; Han, J.B.; Du, G.H.; Li, L. Magnetic field induced great photoluminescence enhancement in an Er3+: YVO4 single crystal used for high magnetic field calibration. Opt. Lett. 2013, 38, 3754–3757. [Google Scholar] [CrossRef]
- Zhu, X.; Tu, H.; Hu, Z.; Zhuang, N. Enhancement of magneto-optical performance of Tb0.94Pr0.06VO4 single crystals by Pr doping. Mater. Lett. 2019, 242, 195–198. [Google Scholar] [CrossRef]
- Chen, P.; Zhong, Z.; Jia, H.; Zhou, J.; Han, J.; Liu, X.; Qiu, J. Magnetic field enhanced upconversion luminescence and magnetic-optical hysteresis behaviors in NaYF4: Yb, Ho nanoparticles. RSC Adv. 2016, 6, 7391–7395. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.S.; Yang, Y.; Zhang, F.; Dong, W.F.; Zhou, S.Y.; Pei, W.H.; Chen, H.D.; Sun, H.B. Magnetic/upconversion luminescent mesoparticles of Fe3O4@ LaF3: Yb3+, Er3+ for dual-modal bioimaging. Chem. Commun. 2012, 48, 11238–11240. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Cui, B.; Li, G.; Wang, Y.; Li, N.; Chang, Z.; Wang, Y. A multifunctional β-CD-modified Fe3O4@ ZnO: Er3+, Yb3+ nanocarrier for antitumor drug delivery and microwave-triggered drug release. Mater. Sci. Eng. C 2015, 46, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, S.; Teng, X.; Luo, Y.; Li, G. Bifunctional magnetic- luminescent nanocomposites: Y2O3/Tb nanorods on the surface of iron oxide/silica core-shell nanostructures. J. Phys. Chem. C 2008, 112, 9623–9626. [Google Scholar] [CrossRef]
- Brunton, G. The crystal structure of Na3CrF6. Mater. Res. Bull. 1969, 4, 621–626. [Google Scholar] [CrossRef]
- Shakoor, R.A.; Lim, S.Y.; Kim, H.; Nam, K.W.; Kang, J.K.; Kang, K.; Choi, J.W. Mechanochemical synthesis and electrochemical behavior of Na3FeF6 in sodium and lithium batteries. Solid State Ion. 2012, 218, 35–40. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lin, C.W.; Lin, P.H.; Li, C.W.; Liang, Y.M.; Liu, J.C.; Chen, S.S. Fluoride recovery from spent fluoride etching solution through crystallization of Na3AlF6 (synthetic cryolite). Sep. Purif. Technol. 2014, 137, 53–58. [Google Scholar] [CrossRef]
- Jia, H.; Zhou, Y.; Wang, X.; Zhang, W.; Feng, X.; Li, Z.; Fu, H.; Zhao, J.; Liu, Z.; Liu, X. Luminescent properties of Eu-doped magnetic Na3FeF6. RSC Adv. 2018, 8, 38410–38415. [Google Scholar] [CrossRef]
- Pang, Y.L.; Abdullah, A.Z. Effect of low Fe3+ doping on characteristics, sonocatalytic activity and reusability of TiO2 nanotubes catalysts for removal of Rhodamine B from water. J. Hazard. Mater. 2012, 235, 326–335. [Google Scholar] [CrossRef]
- Li, X.; Dong, M.; Hu, F.; Qin, Y.; Zhao, L.; Wei, X.; Chen, Y.; Duan, C.; Yin, M. Efficient sensitization of Tb3+ emission by Dy3+ in CaMoO4 phosphors: Energy transfer, tunable emission and optical thermometry. Ceram. Int. 2016, 42, 6094–6099. [Google Scholar] [CrossRef]
- Bi, F.; Dong, X.; Wang, J.; Liu, G. Electrospinning preparation and photoluminescence properties of Y3Al5O12: Tb3+ nanostructures. Luminescence 2015, 30, 751–759. [Google Scholar] [CrossRef]
- Singh, V.; Singh, N.; Pathak, M.S.; Singh, P.K.; Natarajan, V. Tb3+ doped Ca2La8 (SiO4) 6O2 oxyapatite phosphors. Optik 2018, 171, 356–362. [Google Scholar] [CrossRef]
- Kumar, J.S.; Pavani, K.; Sasikala, T.; Jayasimhadri, M.; Jang, K.; Moorthy, L.R. Concentration dependent luminescence characteristics of 5D4 and 5D3 excited states of Tb3+ ions in CFB glasses. Proc. SPIE 2011, 79401H. [Google Scholar] [CrossRef]
- Cho, I.; Kang, J.G.; Sohn, Y. Photoluminescence imaging of SiO2@ Y2O3: Eu (III) and SiO2@ Y2O3: Tb (III) core-shell nanostructures. Bull. Korean Chem. Soc. 2014, 35, 575–580. [Google Scholar] [CrossRef]
- Wang, D.Y.; Chen, Y.C.; Huang, C.H.; Cheng, B.M.; Chen, T.M. Photoluminescence investigations on a novel green-emitting phosphor Ba3Sc (BO3)3: Tb3+ using synchrotron vacuum ultraviolet radiation. J. Mater. Chem. 2012, 22, 9957–9962. [Google Scholar] [CrossRef]
- Van Do, P.; Quang, V.X.; Thanh, L.D.; Tuyen, V.P.; Ca, N.X.; Hoa, V.X.; Van Tuyen, H. Energy transfer and white light emission of KGdF4 polycrystalline co-doped with Tb3+/Sm3+ ions. Opt. Mater. 2019, 92, 174–180. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Huang, L.; Lu, P.; Sun, Q.; Wang, Y.; Tang, J.; Belfiore, L.A.; Kipper, M.J. Polyvinylpyrrolidone nanofibers encapsulating an anhydrous preparation of fluorescent SiO2-Tb3+ nanoparticles. Nanomaterials 2019, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Blasse, G.; Bril, A. Investigations of Tb3+-activated phosphors. Philips Res. Rep. 1967, 22, 481–504. [Google Scholar]
- Scholl, M.S.; Trimmier, J.R. Luminescence of YAG: Tm, Tb. J. Electrochem. Soc. 1986, 133, 643–648. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).