Magneto-Fluorescent Hybrid Sensor CaCO3-Fe3O4-AgInS2/ZnS for the Detection of Heavy Metal Ions in Aqueous Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Fe3O4 Nanoparticles
2.2.2. Synthesis of AgInS2/ZnS QDs
2.2.3. Preparation of CaCO3-Fe3O4-AgInS2/ZnS (CFA) Fluorescent Sensor
2.3. Equipments
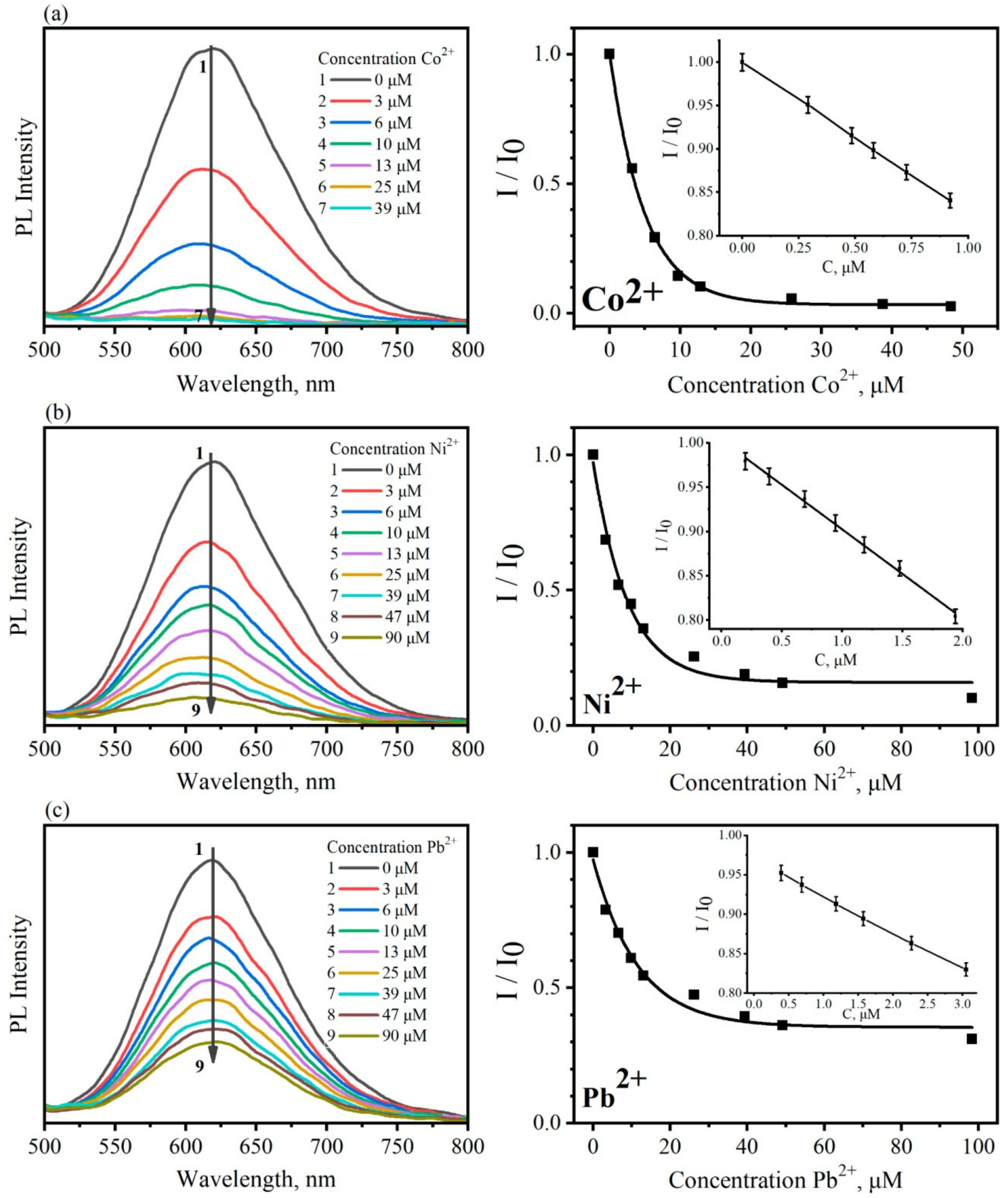
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roy-Chowdhury, A.; Datta, R.; Sarkar, D. Heavy metal pollution and remediation. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 94, pp. 359–373. [Google Scholar]
- Hembrom, S.; Singh, B.; Gupta, S.K.; Nema, A.K. A comprehensive evaluation of heavy metal contamination in foodstuff and associated human health risk: A global perspective. In Contemporary Environmental Issues and Challenges in Era of Climate Change; Singh, P., Singh, R.P., Srivastava, V., Eds.; Springer: Singapore, 2020; pp. 33–63. ISBN 978-981-32-9594-0. [Google Scholar]
- Dubey, S.; Shri, M.; Gupta, A.; Rani, V.; Chakrabarty, D. Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ. Chem. Lett. 2018, 16, 1169–1192. [Google Scholar] [CrossRef]
- Mallampati, S.R.; Mitoma, Y.; Okuda, T.; Sakita, S.; Kakeda, M. Total immobilization of soil heavy metals with nano-Fe/Ca/CaO dispersion mixtures. Environ. Chem. Lett. 2013, 11, 119–125. [Google Scholar] [CrossRef]
- Taseidifar, M.; Makavipour, F.; Pashley, R.M.; Rahman, A.F.M.M. Removal of heavy metal ions from water using ion flotation. Environ. Technol. Innov. 2017, 8, 182–190. [Google Scholar] [CrossRef]
- Luo, X.; Lei, X.; Cai, N.; Xie, X.; Xue, Y.; Yu, F. Removal of heavy metal ions from water by magnetic cellulose-based beads with embedded chemically modified magnetite nanoparticles and activated carbon. ACS Sustain. Chem. Eng. 2016, 4, 3960–3969. [Google Scholar] [CrossRef]
- Buledi, J.A.; Amin, S.; Haider, S.I.; Bhanger, M.I.; Solangi, A.R. A review on detection of heavy metals from aqueous media using nanomaterial-based sensors. Environ. Sci. Pollut. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. TrAC Trends Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.; Dong, H.; Liu, K.; He, N. Progress on sensors based on nanomaterials for rapid detection of heavy metal ions. Sci. China Chem. 2017, 60, 329–337. [Google Scholar] [CrossRef]
- Kunkel, R.; Manahan, S.E. Atomic absorption analysis of strong heavy metal chelating agents in water and waste water. Anal. Chem. 1973, 45, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- POHL, P. Determination of metal content in honey by atomic absorption and emission spectrometries. TrAC Trends Anal. Chem. 2009, 28, 117–128. [Google Scholar] [CrossRef]
- Bua, D.G.; Annuario, G.; Albergamo, A.; Cicero, N.; Dugo, G. Heavy metals in aromatic spices by inductively coupled plasma-mass spectrometry. Food Addit. Contam. Part B 2016, 9, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Habila, M.A.; ALOthman, Z.A.; El-Toni, A.M.; Soylak, M. Combination of syringe-solid phase extraction with inductively coupled plasma mass spectrometry for efficient heavy metals detection. CLEAN-Soil Air Water 2016, 44, 720–727. [Google Scholar] [CrossRef]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical sensors. Anal. Chem. 2010, 82, 4723–4741. [Google Scholar] [CrossRef] [PubMed]
- Root, H.D.; Thiabaud, G.; Sessler, J.L. Reduced texaphyrin: A ratiometric optical sensor for heavy metals in aqueous solution. Front. Chem. Sci. Eng. 2020, 14, 19–27. [Google Scholar] [CrossRef]
- Rudd, N.D.; Wang, H.; Fuentes-Fernandez, E.M.A.; Teat, S.J.; Chen, F.; Hall, G.; Chabal, Y.J.; Li, J. Highly efficient luminescent metal–organic framework for the simultaneous detection and removal of heavy metals from water. ACS Appl. Mater. Interfaces 2016, 8, 30294–30303. [Google Scholar] [CrossRef]
- Lou, Y.; Zhao, Y.; Chen, J.; Zhu, J.J. Metal ions optical sensing by semiconductor quantum dots. J. Mater. Chem. C 2014, 2, 595–613. [Google Scholar] [CrossRef]
- Devi, P.; Rajput, P.; Thakur, A.; Kim, K.H.; Kumar, P. Recent advances in carbon quantum dot-based sensing of heavy metals in water. TrAC-Trends Anal. Chem. 2019, 114, 171–195. [Google Scholar] [CrossRef]
- Chaniotakis, N.; Buiculescu, R. Semiconductor quantum dots in chemical sensors and biosensors. In Nanosensors for Chemical and Biological Applications: Sensing with Nanotubes, Nanowires and Nanoparticles; Woodhead Publishing: Sawston, UK, 2014; ISBN 9780857096609. [Google Scholar]
- Bach, L.G.; Nguyen, T.D.; Thuong, N.T.; Van, H.T.T.; Lim, K.T. Glutathione capped cdse quantum dots: synthesis, characterization, morphology, and application as a sensor for toxic metal ions. J. Nanosci. Nanotechnol. 2019, 19, 1192–1195. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, J.; Nie, J.; Du, B.; Xu, D. Ultrasensitive and selective detection of Cu2+ in aqueous solution with fluorescence enhanced CdSe quantum dots. Sens. Actuators B Chem. 2014, 190, 279–287. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Carrillo-Carrion, C. Analytical strategies based on quantum dots for heavy metal ions detection. J. Biomed. Opt. 2014, 19, 101503. [Google Scholar] [CrossRef]
- Raevskaya, A.; Lesnyak, V.; Haubold, D.; Dzhagan, V.; Stroyuk, O.; Gaponik, N.; Zahn, D.R.T.; Eychmüller, A. A fine size selection of brightly luminescent water-soluble Ag–In–S and Ag–In–S/ZnS quantum dots. J. Phys. Chem. C 2017, 121, 9032–9042. [Google Scholar] [CrossRef]
- Jain, S.; Bharti, S.; Bhullar, G.K.; Tripathi, S.K. I-III-VI core/shell QDs: Synthesis, characterizations and applications. J. Lumin. 2020, 219, 116912. [Google Scholar] [CrossRef]
- Stroyuk, O.; Weigert, F.; Raevskaya, A.; Spranger, F.; Würth, C.; Resch-Genger, U.; Gaponik, N.; Zahn, D.R.T. Inherently broadband photoluminescence in Ag–In–S/ZnS quantum dots observed in ensemble and single-particle studies. J. Phys. Chem. C 2019, 123, 2632–2641. [Google Scholar] [CrossRef]
- Cambrea, L.R.; Yelton, C.A.; Meylemans, H.A. ZnS-AgInS2 fluorescent nanoparticles for low level metal detection in water. In ACS Symposium Series; American Chemical Society: Washington, USA, 2015; Volume 1210, ISBN 9780841231092. [Google Scholar]
- Baimuratov, A.S.; Rukhlenko, I.D.; Noskov, R.E.; Ginzburg, P.; Gun’ko, Y.K.; Baranov, A.V.; Fedorov, A.V. Giant optical activity of quantum dots, rods and disks with screw dislocations. Sci. Rep. 2015, 5, 14712. [Google Scholar] [CrossRef] [PubMed]
- Stroyuk, O.; Raevskaya, A.; Spranger, F.; Selyshchev, O.; Dzhagan, V.; Schulze, S.; Zahn, D.R.T.; Eychmüller, A. Origin and dynamics of highly efficient broadband photoluminescence of aqueous glutathione-capped size-selected Ag–In–S quantum dots. J. Phys. Chem. C 2018, 122, 13648–13658. [Google Scholar] [CrossRef]
- Podgurska, I.; Rachkov, A. Influence of ions of heavy metals on the photoluminescence of nanocrystals AgInS2 /ZnS. Sens. Electron. Microsyst. Technol. 2017, 14, 41–47. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, T.; Deng, M.; Tang, X.; Han, S.; Liu, A.; Bai, Y.; Qu, D.; Huang, X.; Qiu, F. Selective and sensitive detection of copper(II) based on fluorescent zinc-doped AgInS2 quantum dots. J. Lumin. 2018, 201, 182–188. [Google Scholar] [CrossRef]
- Podgurska, I.; Rachkov, A.; Borkovska, L. Effect of Pb 2+ ions on photoluminescence of ZnS-coated AgInS 2 nanocrystals. Phys. Status Solidi 2018, 215, 1700450. [Google Scholar] [CrossRef]
- Mokadem, Z.; Mekki, S.; Saïdi-Besbes, S.; Agusti, G.; Elaissari, A.; Derdour, A. Triazole containing magnetic core-silica shell nanoparticles for Pb 2+, Cu 2+ and Zn 2+ removal. Arab. J. Chem. 2017, 10, 1039–1051. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Y.; Hu, Y.; Li, J.-P.; Dong, L.; Lin, L.-N.; Yu, S.-H. Synthesis of superparamagnetic CaCO3 mesocrystals for multistage delivery in cancer therapy. Small 2010, 6, 2436–2442. [Google Scholar] [CrossRef]
- Du, C.; Shi, J.; Shi, J.; Zhang, L.; Cao, S. PUA/PSS multilayer coated CaCO3 microparticles as smart drug delivery vehicles. Mater. Sci. Eng. C 2013, 33, 3745–3752. [Google Scholar] [CrossRef]
- Gusliakova, O.; Atochina-Vasserman, E.N.; Sindeeva, O.; Sindeev, S.; Pinyaev, S.; Pyataev, N.; Revin, V.; Sukhorukov, G.B.; Gorin, D.; Gow, A.J. Use of submicron vaterite particles serves as an effective delivery vehicle to the respiratory portion of the lung. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Sha, F.; Zhu, N.; Bai, Y.; Li, Q.; Guo, B.; Zhao, T.; Zhang, F.; Zhang, J. Controllable synthesis of various CaCO 3 morphologies based on a CCUS idea. ACS Sustain. Chem. Eng. 2016, 4, 3032–3044. [Google Scholar] [CrossRef]
- Parakhonskiy, B.; Zyuzin, M.V.; Yashchenok, A.; Carregal-Romero, S.; Rejman, J.; Möhwald, H.; Parak, W.J.; Skirtach, A.G. The influence of the size and aspect ratio of anisotropic, porous CaCO3 particles on their uptake by cells. J. Nanobiotechnology 2015, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ma, G.-H.; Hu, G.; Yu, D.; Mcleish, T.; Su, Z.-G.; Shen, Z.-Y. Preparation of hierarchical hollow CaCO 3 particles and the application as anticancer drug carrier. J. Am. Chem. Soc. 2008, 130, 15808–15810. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Dunnick, J.K.; Elwell, M.R.; Radovsky, A.E.; Benson, J.M.; Hahn, F.F.; Nikula, K.J.; Barr, E.B.; Hobbs, C.H. Comparative carcinogenic effects of nickel subsulfide, nickel oxide, or nickel sulfate hexahydrate chronic exposures in the lung. Cancer Res. 1995, 55, 5251–5256. [Google Scholar]
- Thomson, R.M.; Parry, G.J. Neuropathies associated with excessive exposure to lead. Muscle Nerve 2006, 33, 732–741. [Google Scholar] [CrossRef]
- Fathy, M.; Zayed, M.A.; Moustafa, Y.M. Synthesis and applications of CaCO3/HPC core–shell composite subject to heavy metals adsorption processes. Heliyon 2019, 5, e02215. [Google Scholar] [CrossRef]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef]
- Marchenko, I.; Yashchenok, A.; Borodina, T.; Bukreeva, T.; Konrad, M.; Möhwald, H.; Skirtach, A. Controlled enzyme-catalyzed degradation of polymeric capsules templated on CaCO3: Influence of the number of LbL layers, conditions of degradation, and disassembly of multicompartments. J. Control. Release 2012, 162, 599–605. [Google Scholar] [CrossRef]
- Martynenko, I.V.; Kusić, D.; Weigert, F.; Stafford, S.; Donnelly, F.C.; Evstigneev, R.; Gromova, Y.; Baranov, A.V.; Rühle, B.; Kunte, H.-J.; et al. Magneto-fluorescent microbeads for bacteria detection constructed from superparamagnetic Fe3O4 nanoparticles and AIS/ZnS quantum dots. Anal. Chem. 2019, 91, 12661–12669. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, Y.; Ogawa, T.; Tsuzuki, M.; Kuzuya, T. Photoluminescence properties and its origin of AgInS 2 quantum dots with chalcopyrite structure. J. Phys. Chem. C 2011, 115, 1786–1792. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, A.; Gao, Y.; He, C.; Wu, G.; Chen, Y.; Kai, X.; Zhu, C. Functionalized CdS quantum dots-based luminescence probe for detection of heavy and transition metal ions in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Baranov, A.V.; Orlova, A.O.; Maslov, V.G.; Toporova, Y.A.; Ushakova, E.V.; Fedorov, A.V.; Cherevkov, S.A.; Artemyev, M.V.; Perova, T.S.; Berwick, K. Dissociative CdSe/ZnS quantum dot-molecule complex for luminescent sensing of metal ions in aqueous solutions. J. Appl. Phys. 2010, 108, 074306. [Google Scholar] [CrossRef]
- Shar, G.A.; Soomro, G.A. Spectrophotometric determination of cobalt (ii), nickel (ii) and copper (ii) with 1-(2 pyridylazo)-2-naphthol in micellar medium. Nucleus 2004, 41, 77–82. [Google Scholar]
- Mahapatra, N.; Panja, S.; Mandal, A.; Halder, M. A single source-precursor route for the one-pot synthesis of highly luminescent CdS quantum dots as ultra-sensitive and selective photoluminescence sensor for Co 2+ and Ni 2+ ions. J. Mater. Chem. C 2014, 2, 7373. [Google Scholar] [CrossRef]
- Parani, S.; Oluwafemi, O.S. Selective and sensitive fluorescent nanoprobe based on AgInS 2 -ZnS quantum dots for the rapid detection of Cr (III) ions in the midst of interfering ions. Nanotechnology 2020, 31, 395501. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurshanov, D.A.; Khavlyuk, P.D.; Baranov, M.A.; Dubavik, A.; Rybin, A.V.; Fedorov, A.V.; Baranov, A.V. Magneto-Fluorescent Hybrid Sensor CaCO3-Fe3O4-AgInS2/ZnS for the Detection of Heavy Metal Ions in Aqueous Media. Materials 2020, 13, 4373. https://doi.org/10.3390/ma13194373
Kurshanov DA, Khavlyuk PD, Baranov MA, Dubavik A, Rybin AV, Fedorov AV, Baranov AV. Magneto-Fluorescent Hybrid Sensor CaCO3-Fe3O4-AgInS2/ZnS for the Detection of Heavy Metal Ions in Aqueous Media. Materials. 2020; 13(19):4373. https://doi.org/10.3390/ma13194373
Chicago/Turabian StyleKurshanov, Danil A., Pavel D. Khavlyuk, Mihail A. Baranov, Aliaksei Dubavik, Andrei V. Rybin, Anatoly V. Fedorov, and Alexander V. Baranov. 2020. "Magneto-Fluorescent Hybrid Sensor CaCO3-Fe3O4-AgInS2/ZnS for the Detection of Heavy Metal Ions in Aqueous Media" Materials 13, no. 19: 4373. https://doi.org/10.3390/ma13194373
APA StyleKurshanov, D. A., Khavlyuk, P. D., Baranov, M. A., Dubavik, A., Rybin, A. V., Fedorov, A. V., & Baranov, A. V. (2020). Magneto-Fluorescent Hybrid Sensor CaCO3-Fe3O4-AgInS2/ZnS for the Detection of Heavy Metal Ions in Aqueous Media. Materials, 13(19), 4373. https://doi.org/10.3390/ma13194373






