Thermo-Fluid-Dynamic Modeling of the Melt Pool during Selective Laser Melting for AZ91D Magnesium Alloy
Abstract
1. Introduction
2. Modeling
- (1)
- The powder material is considered to be a homogeneous whole, and the porosity of the powder material is indicated by mathematical methods.
- (2)
- The flow in the melt pool is considered to be incompressible laminar flow.
- (3)
- The evaporation of AZ91D magnesium alloy is ignored.
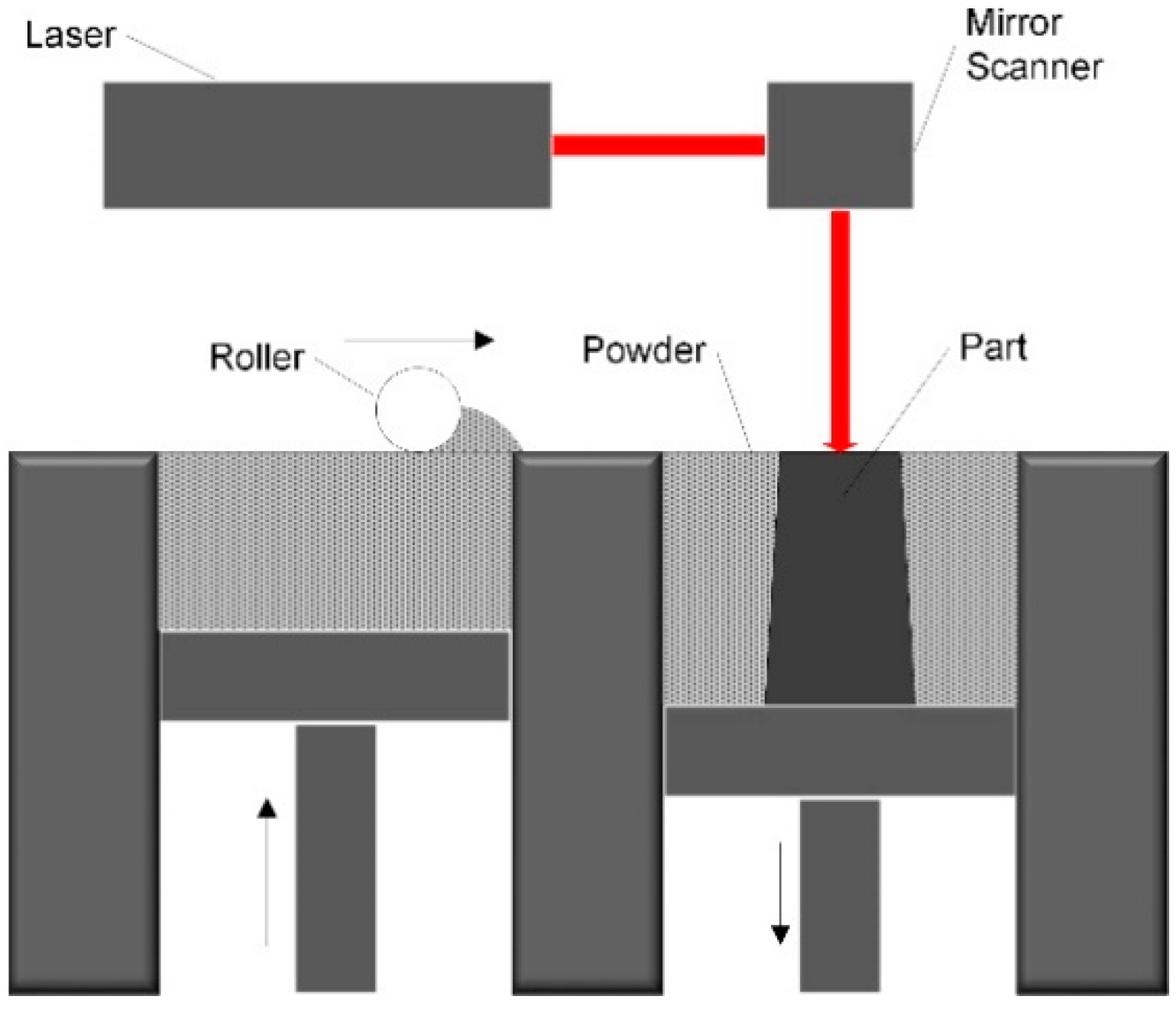
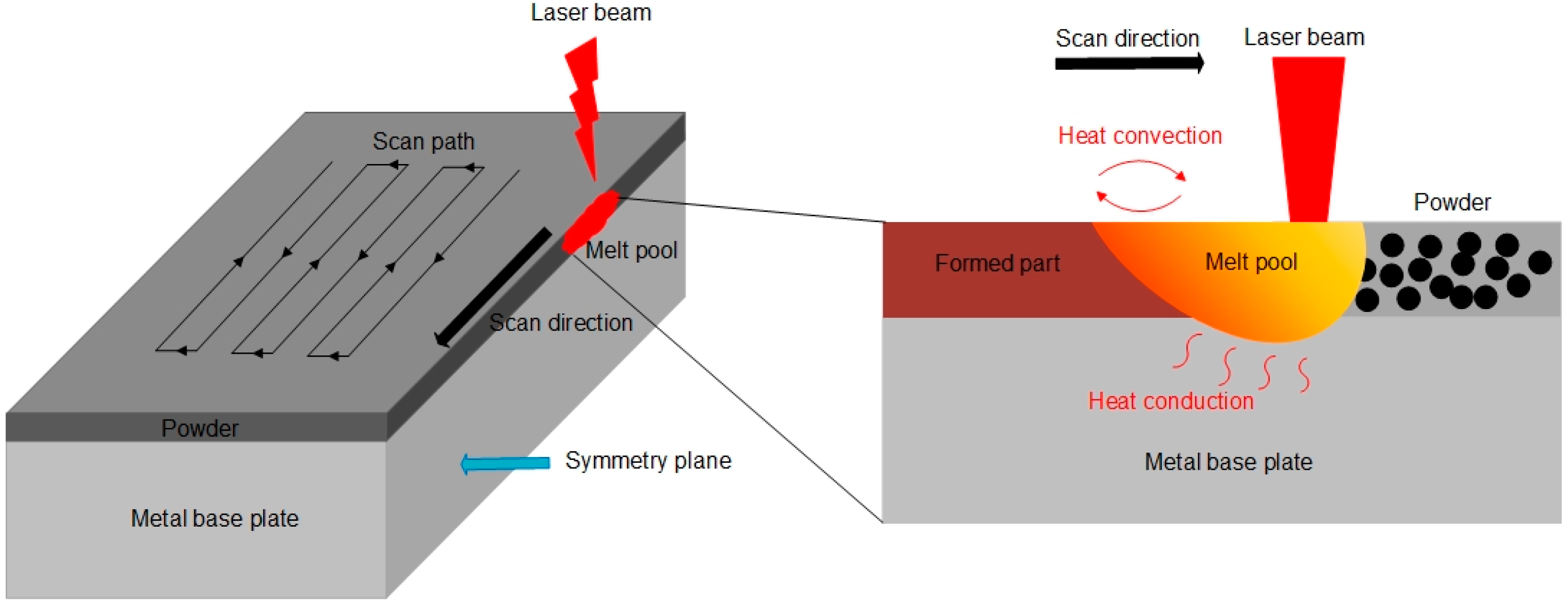
2.1. Physical Model
2.2. Powder Bed Properties
2.3. Governing Transporting Equations
- (1)
- Momentum conservation equation:
- (2)
- Energy conservation equation:
- (3)
- Heat source modeling
- (4)
- Boundary conditions
3. Results and Discussion
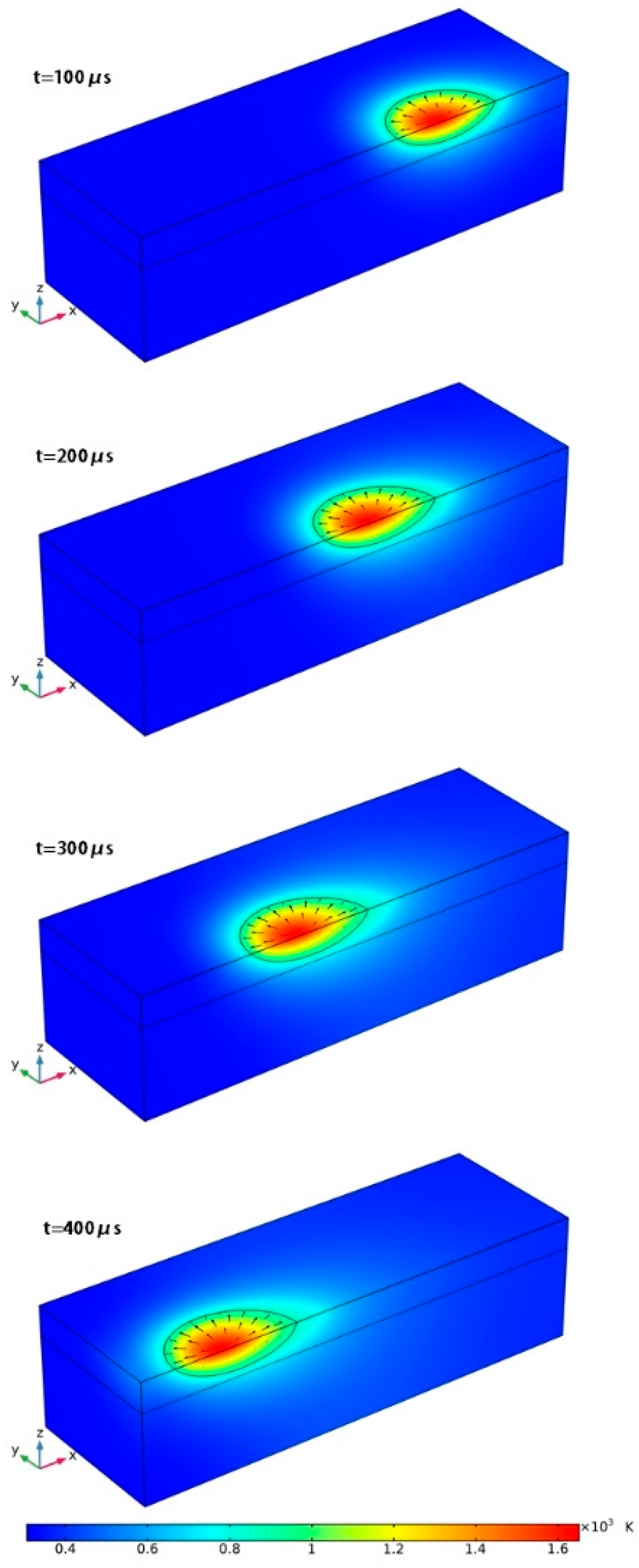
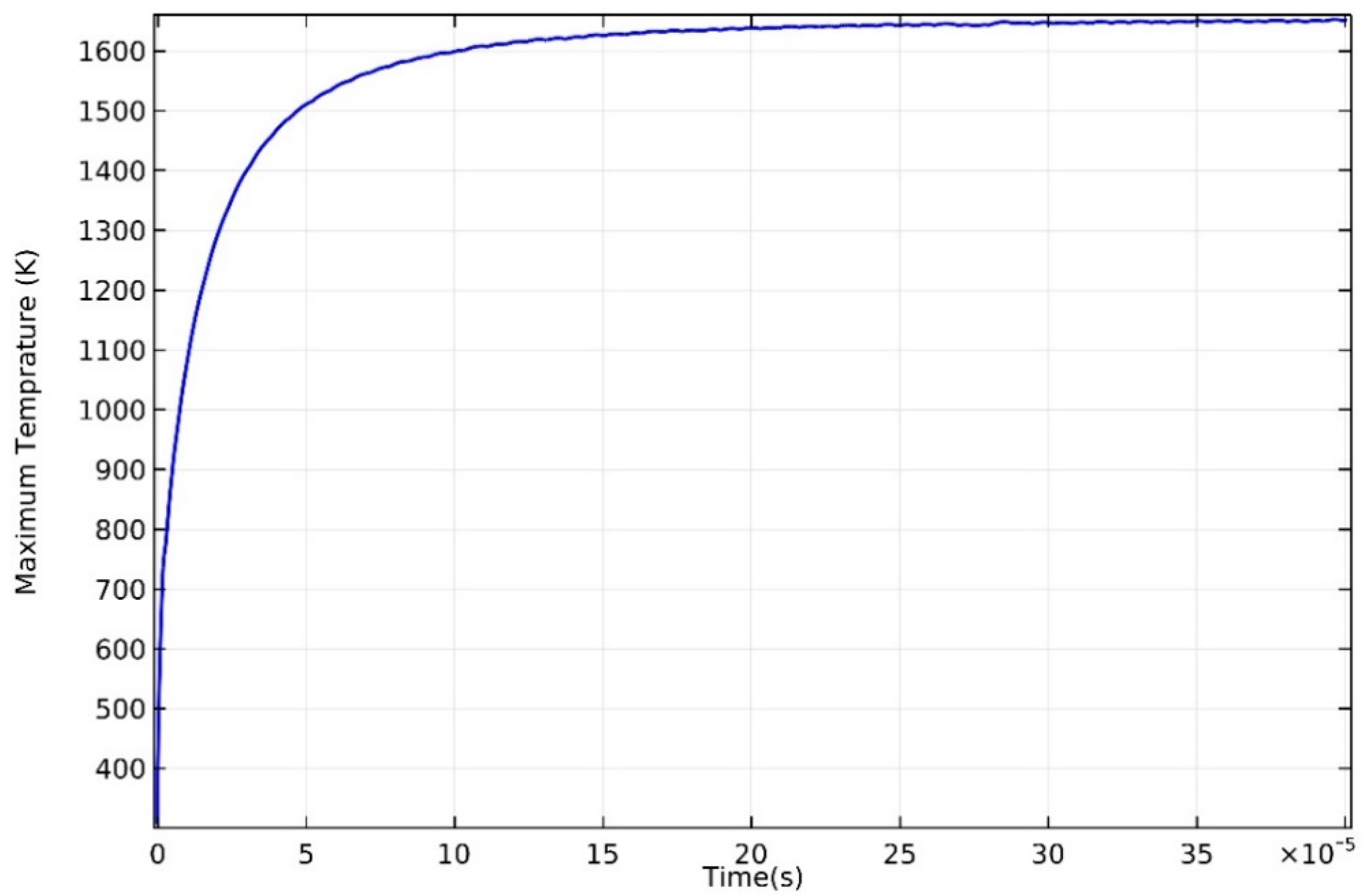
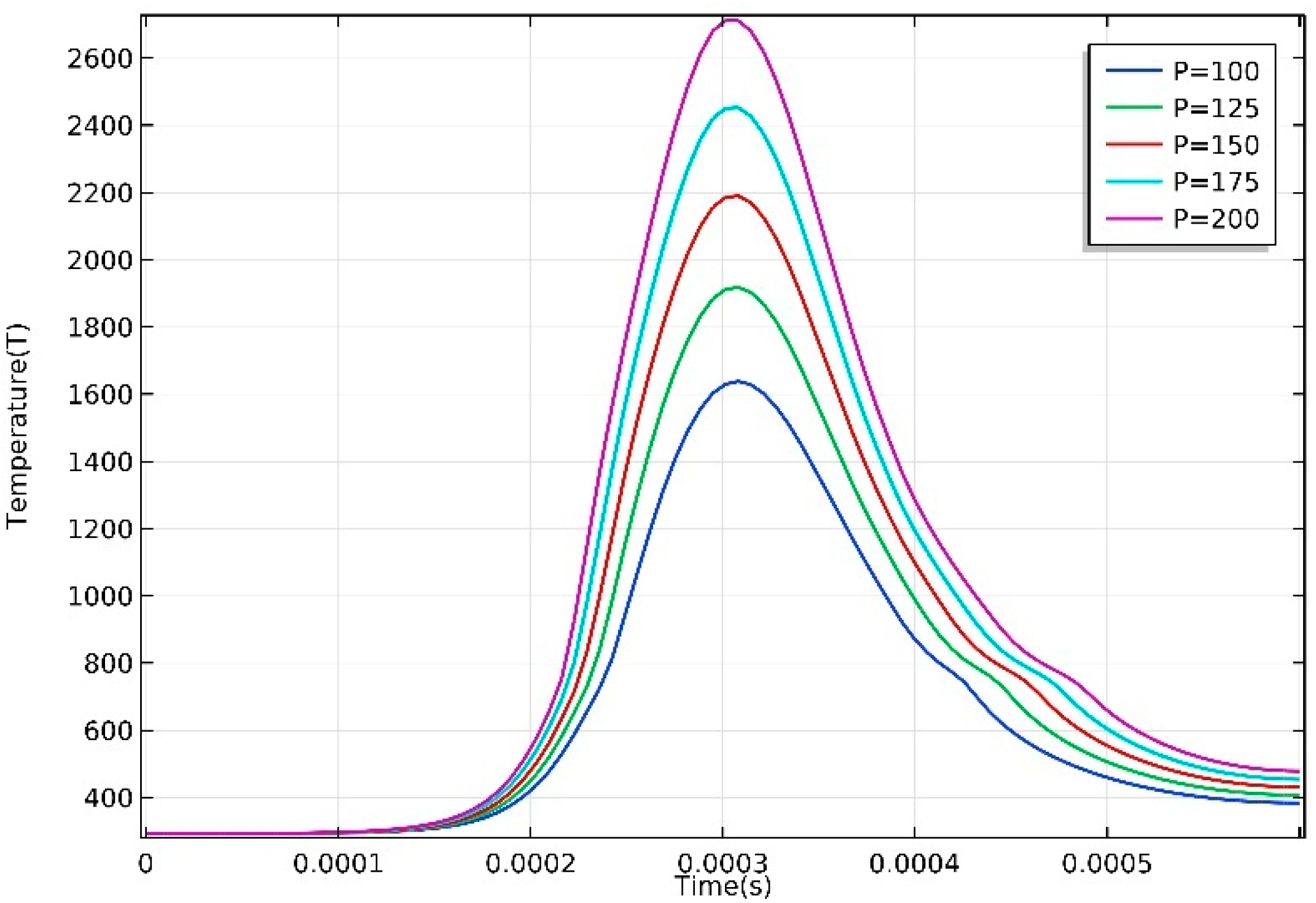
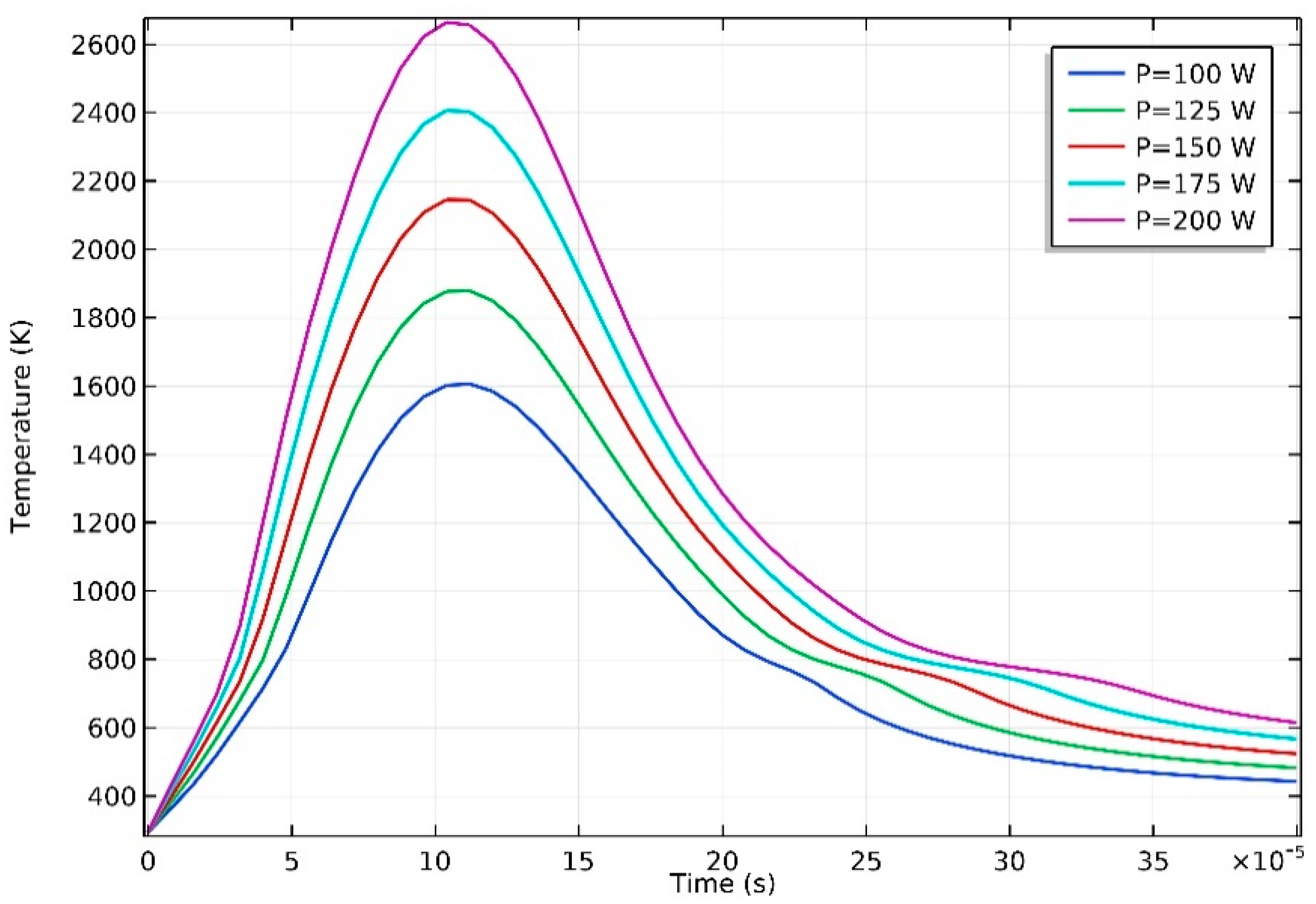
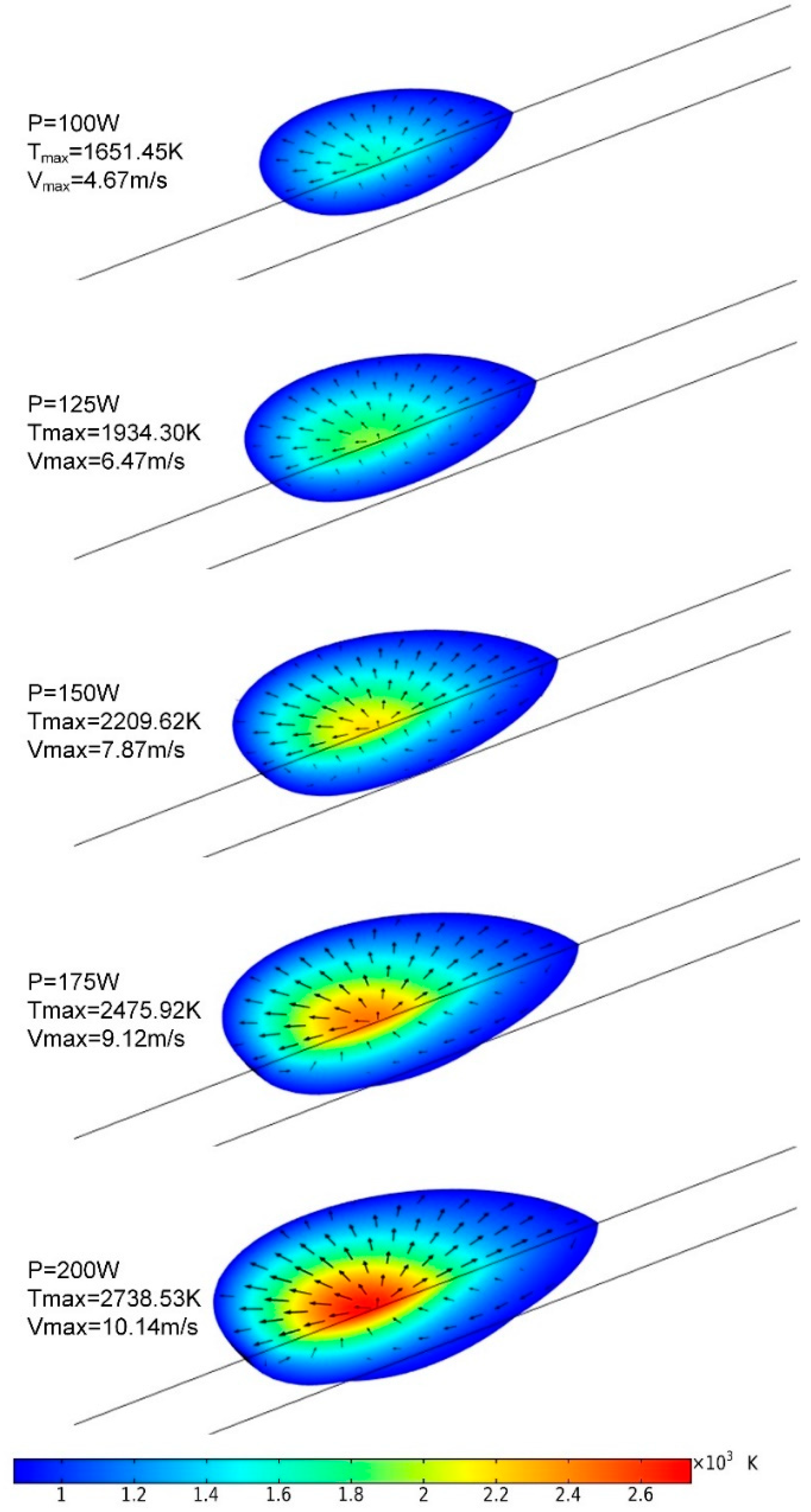
3.1. Temperature Distribution
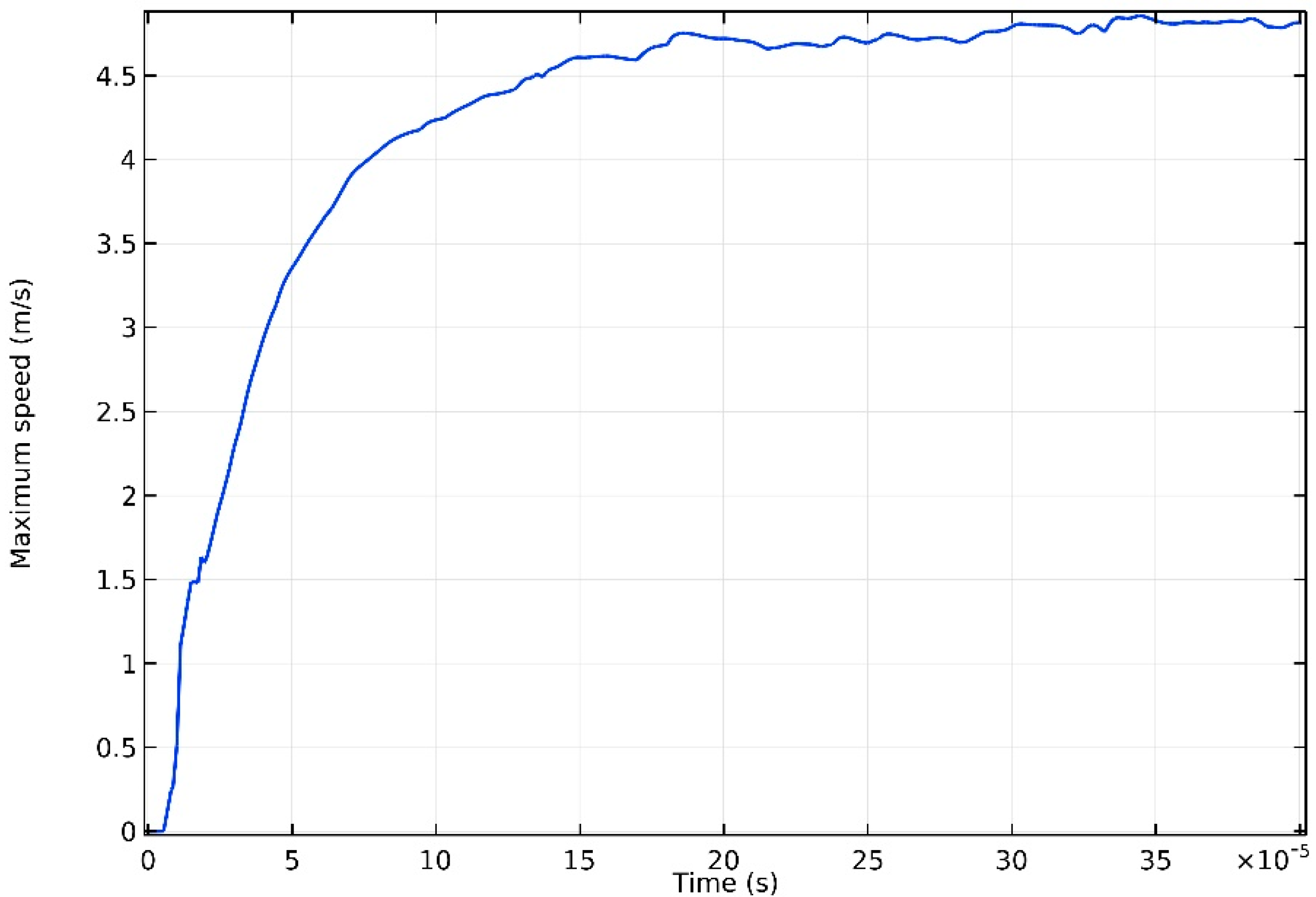
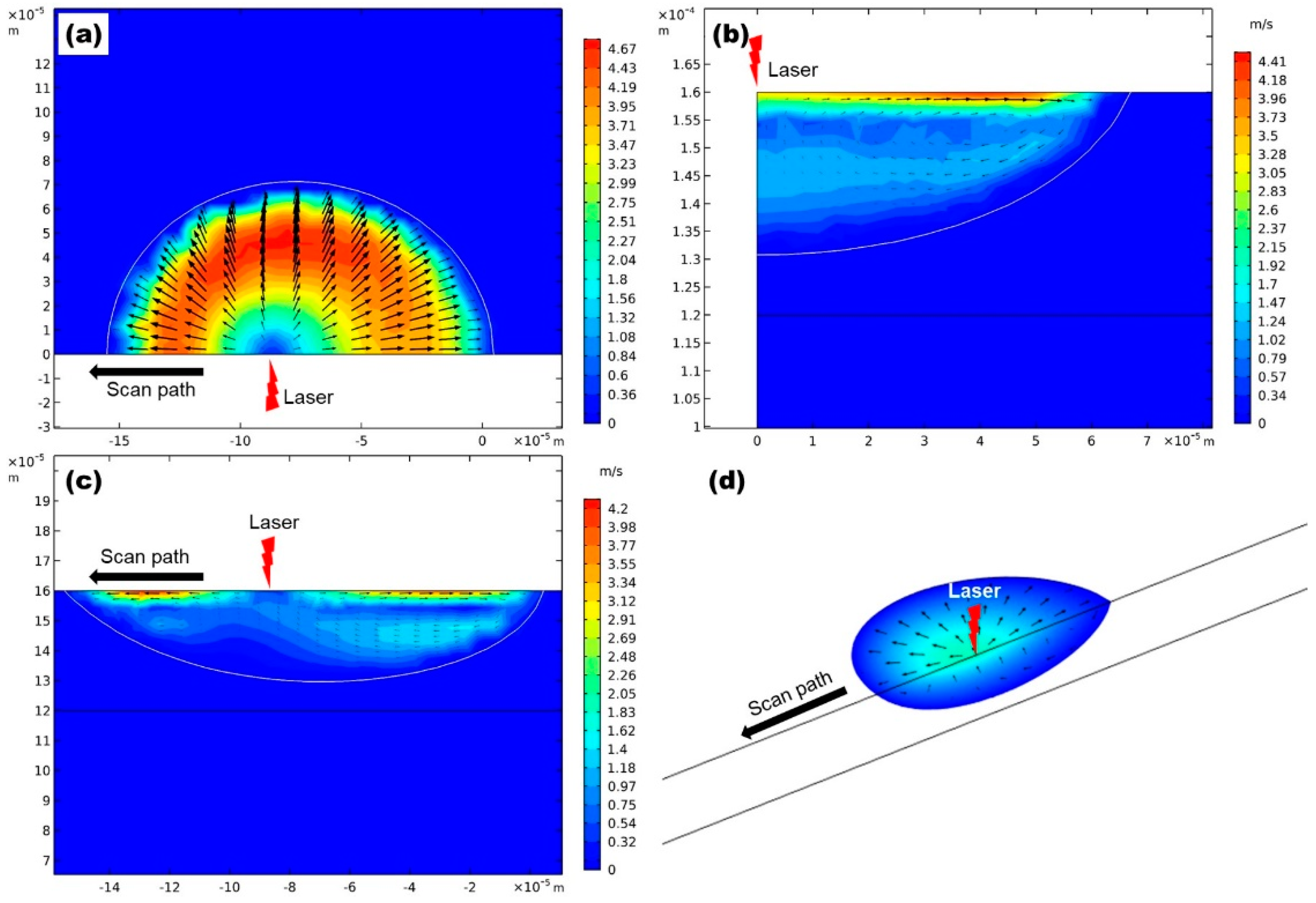
3.2. Flow in the Melt Pool
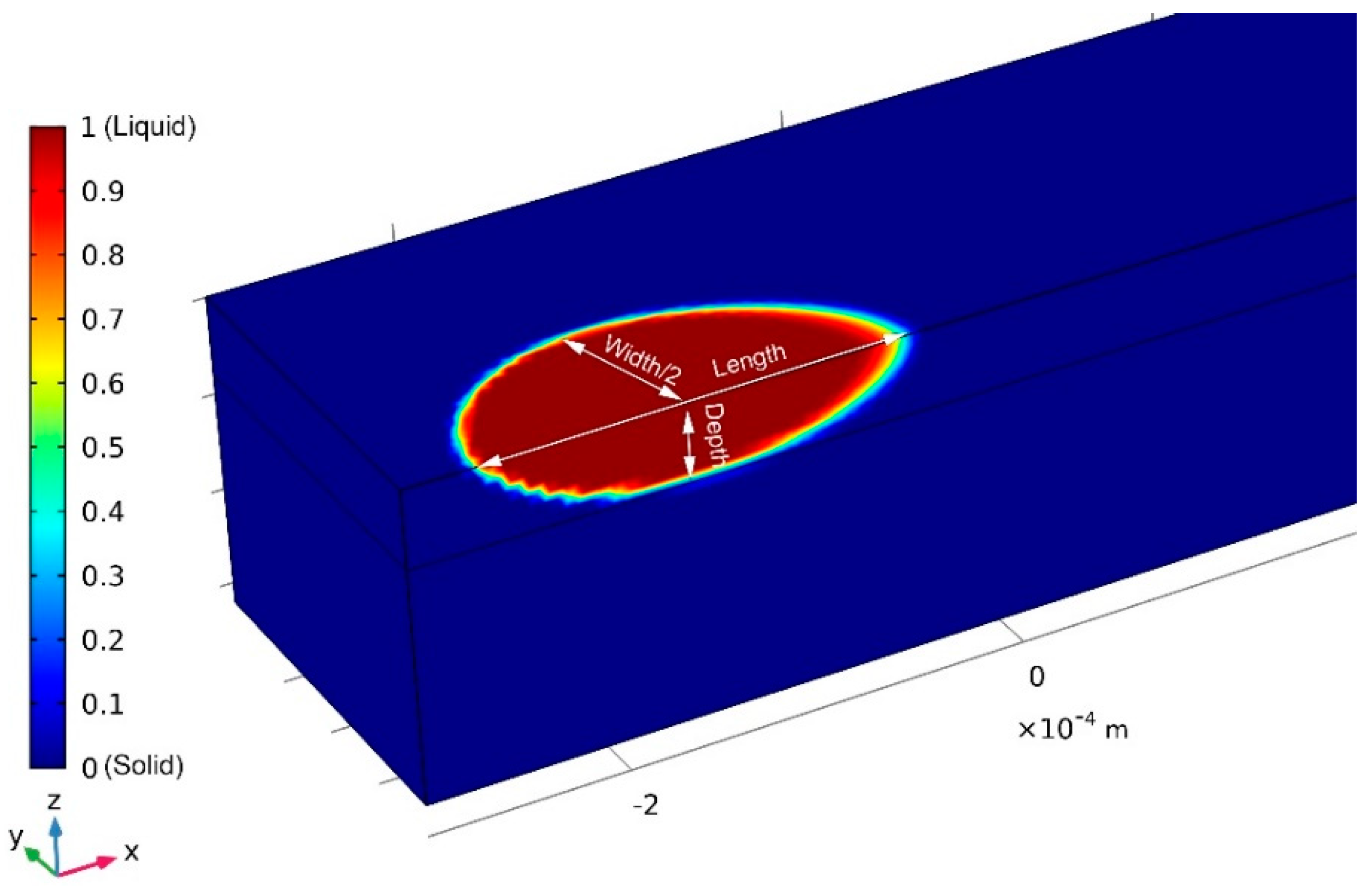
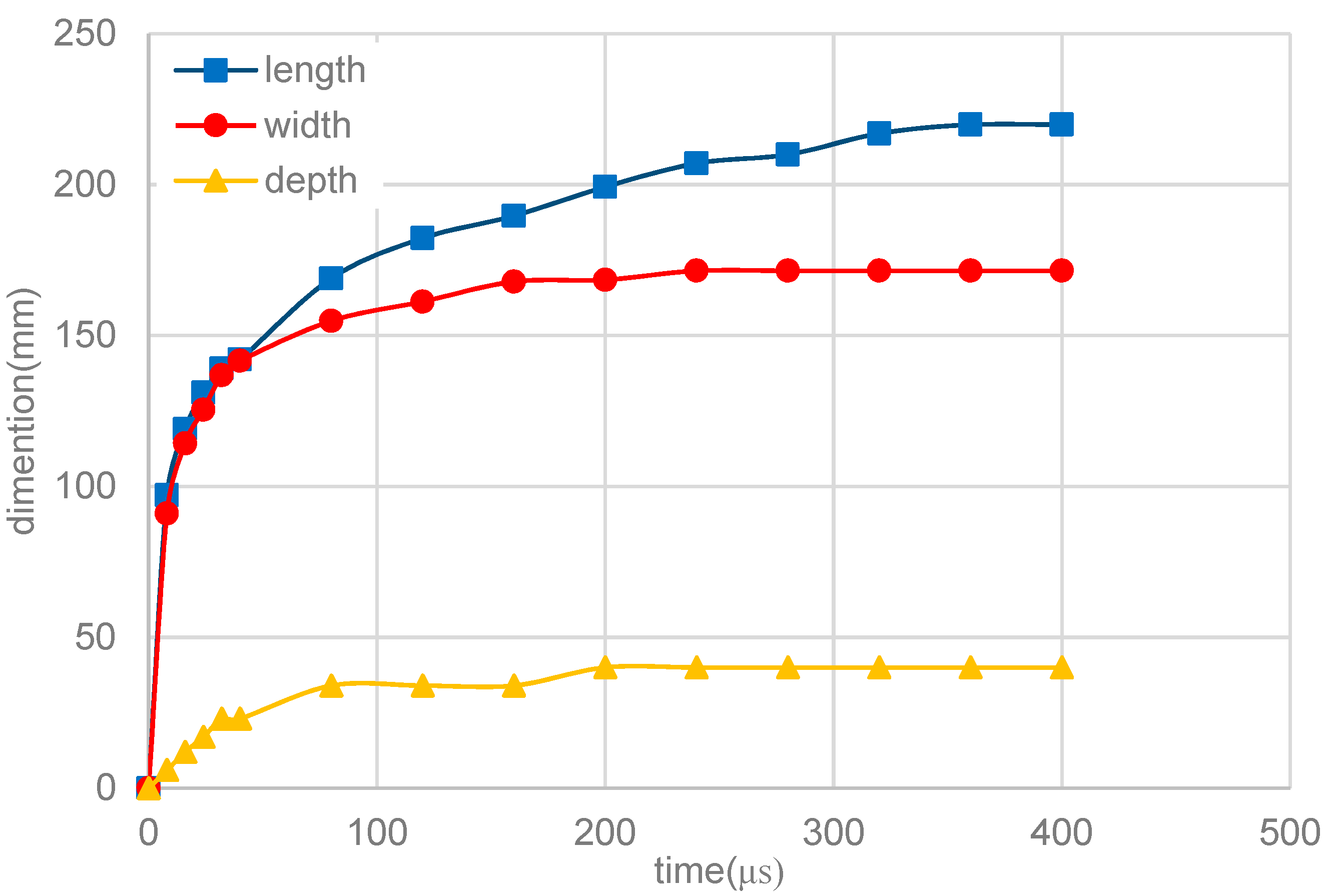
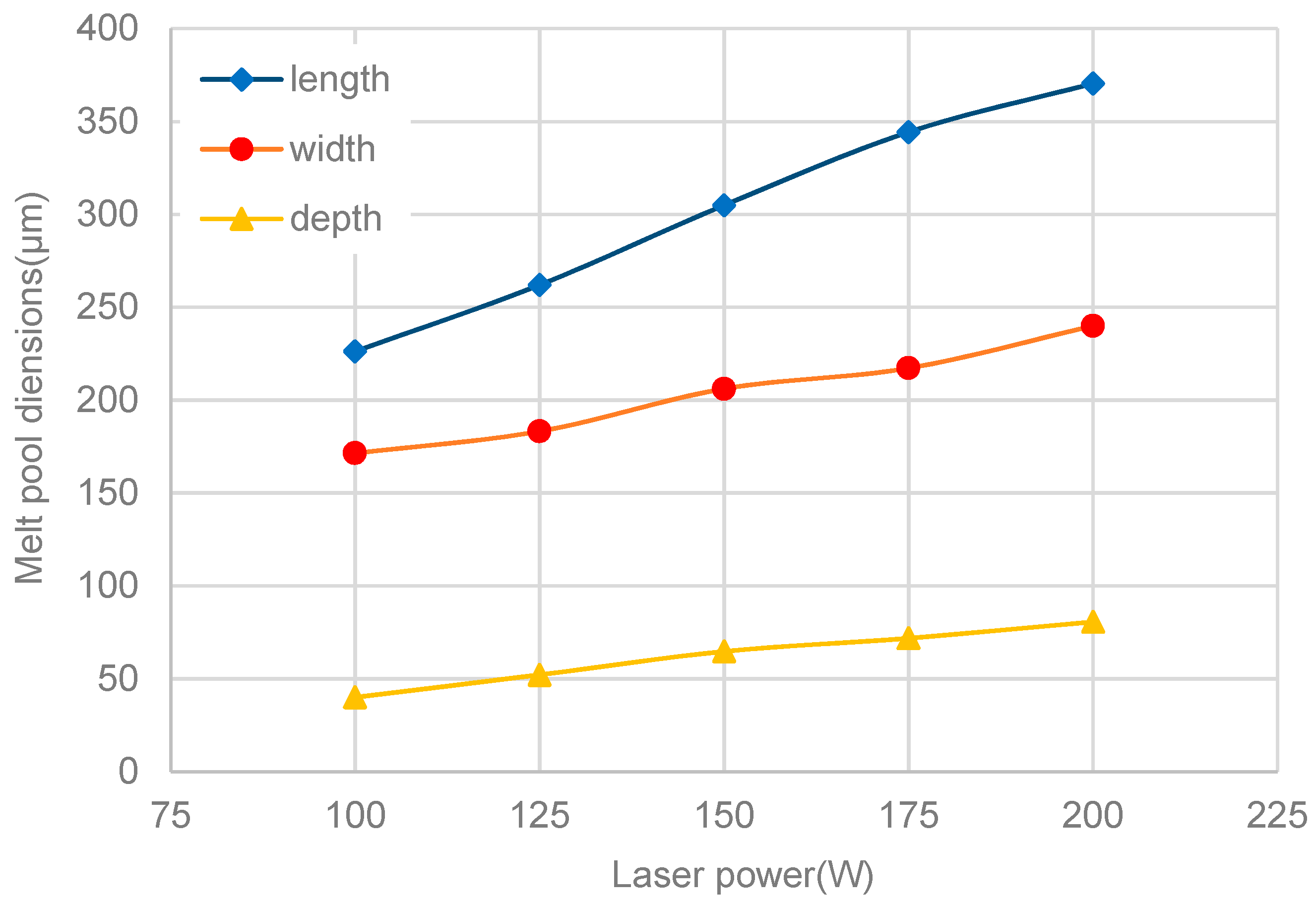
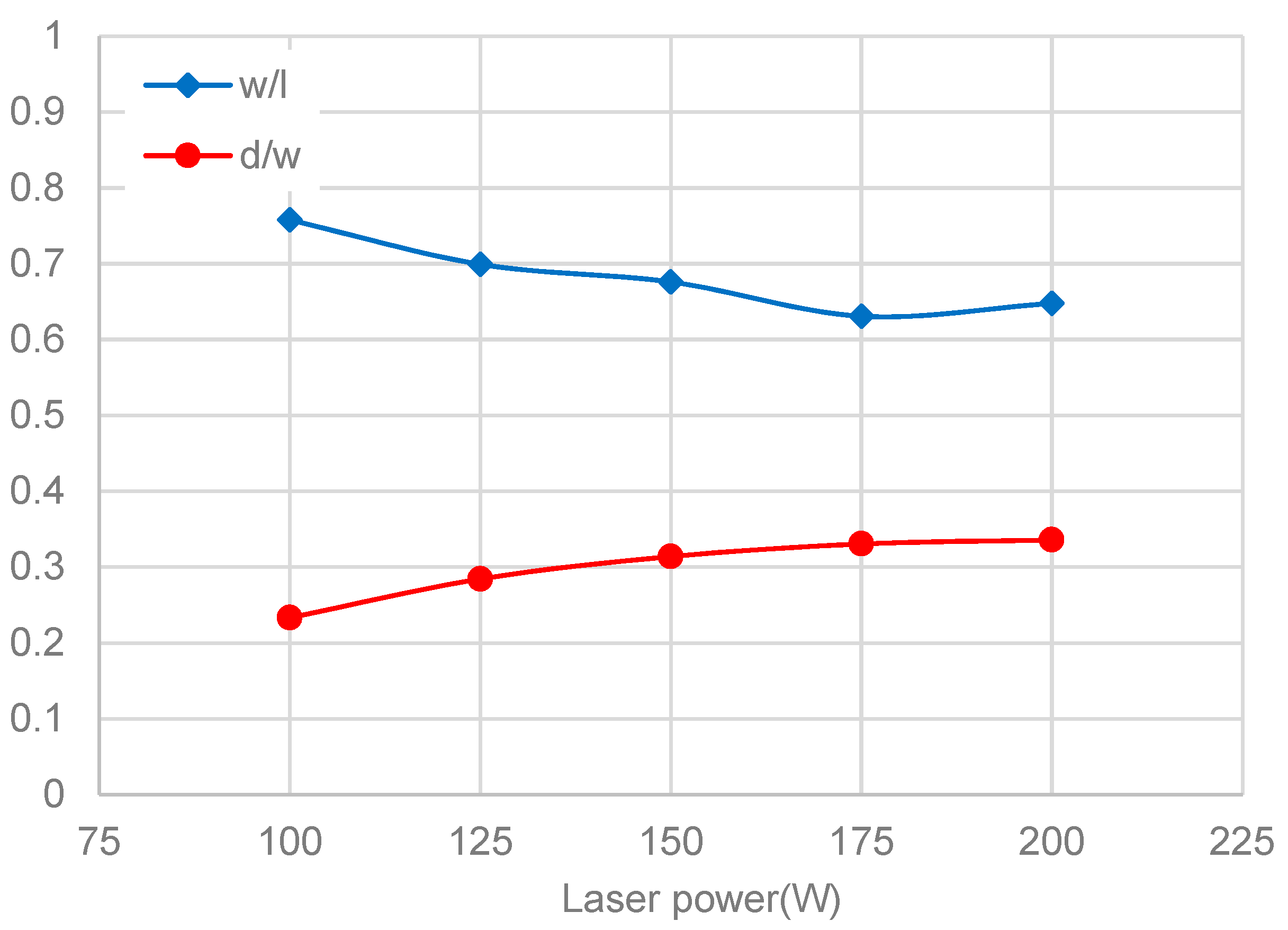
3.3. Melt-Pool Size and Shape
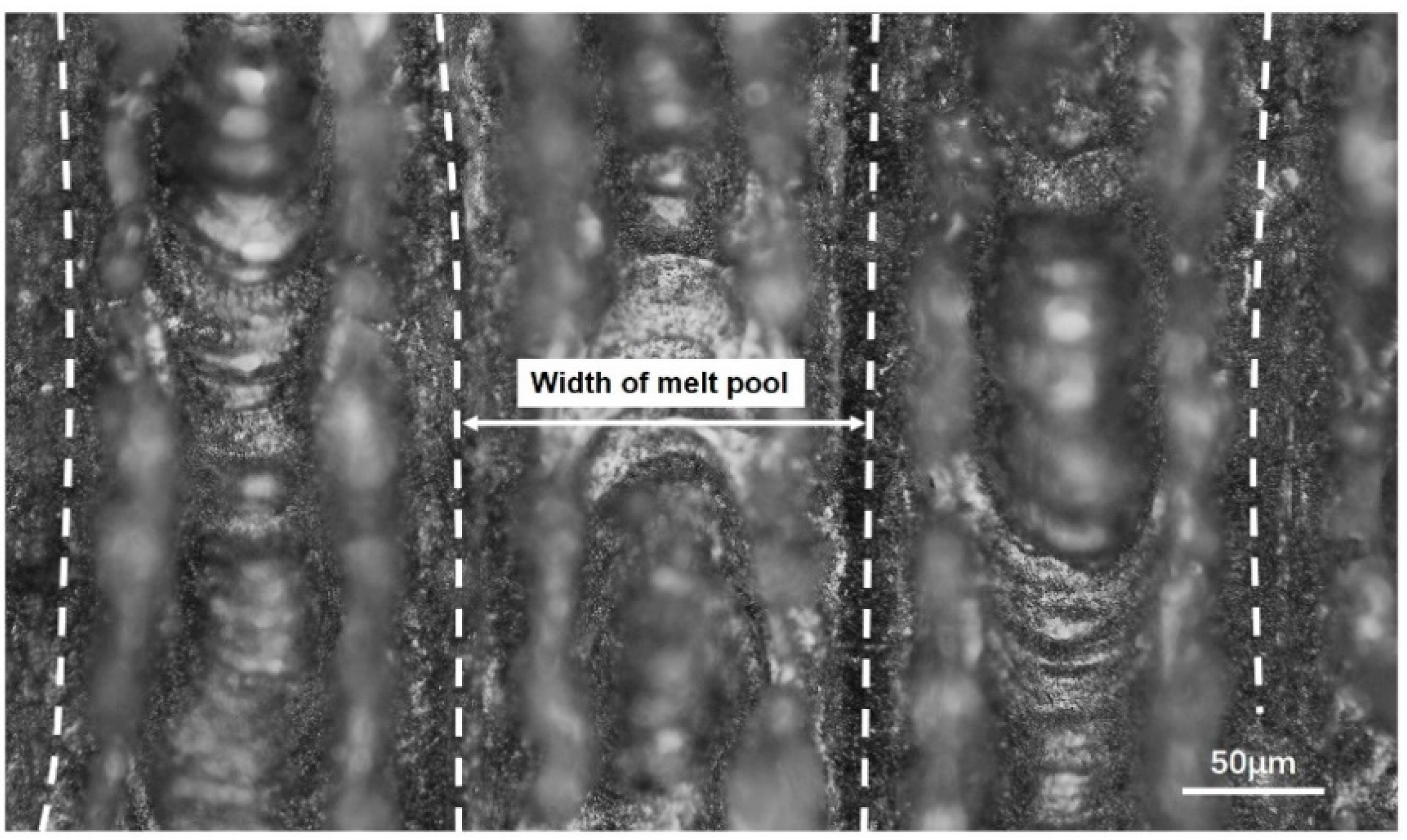
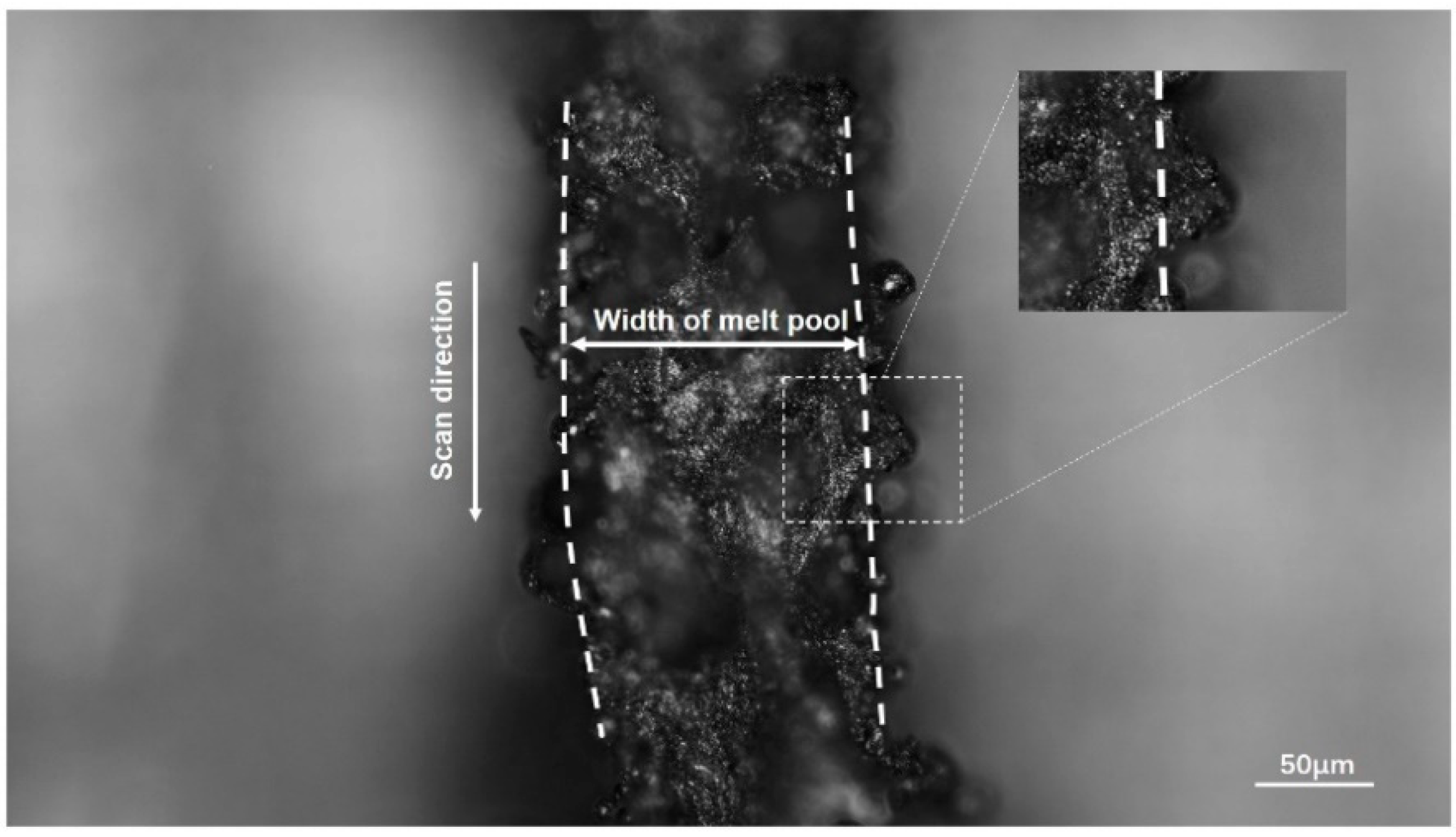
3.4. Experiment Verification
4. Conclusions
- (1)
- The temperature of the domain increased rapidly when the laser started to irradiate, and the maximum temperature showed a strong correlation with input laser energy. Severe temperature gradient and high cooling rate were observed, which is attributed to the high thermal conductivity of AZ91D magnesium alloy.
- (2)
- Violent flow caused by the Marangoni effect was observed in the melt pool, and the maximum velocity was found to increase when the laser power would rise. The high-speed flow enhanced convection heat transfer and made the melt pool wider and longer but shallower.
- (3)
- The dimensions of the melt pool stabilized rapidly., accordingly with the temperature and flow velocity. The size of the melt pool increased as the laser power rose, but the length of the melt pool increased faster than its width and depth. The shape of the melt pool became narrower and deeper as the laser power increased, which may lead to poor stability of the melt pool’s shape.
- (4)
- The width of the melt pool was acquired by single scan melt experiment, which was in good agreement with the results predicted by the FE model (with an average error of 1.49%).
Author Contributions
Funding
Conflicts of Interest
References
- Mishra, A.K.; Kumar, A. Modelling of SLM Additive Manufacturing for Magnesium Alloy; Springer: Singapore, 2018; pp. 123–140. [Google Scholar]
- Easton, M.; Beer, A.; Barnett, M.; Davies, C.; Dunlop, G.; Durandet, Y.; Blacket, S.; Hilditch, T.; Beggs, P. Magnesium alloy applications in automotive structures. JOM 2008, 60, 57–62. [Google Scholar] [CrossRef]
- Külekci, M.K. Magnesium and its alloys applications in automotive industry. Int. J. Adv. Manuf. Technol. 2007, 39, 851–865. [Google Scholar] [CrossRef]
- Nabae, H.; Hemmi, M.; Hirota, Y.; Ide, T.; Suzumori, K.; Endo, G. Super-low friction and lightweight hydraulic cylinder using multi-directional forging magnesium alloy and its application to robotic leg. Adv. Robot. 2018, 32, 524–534. [Google Scholar] [CrossRef]
- Mao, L.; Shen, L.; Chen, J.; Zhang, X.; Kwak, M.; Wu, Y.; Fan, R.; Zhang, L.; Pei, J.; Yuan, G.; et al. A promising biodegradable magnesium alloy suitable for clinical vascular stent application. Sci. Rep. 2017, 7, 46343. [Google Scholar] [CrossRef]
- Singh, R.K.R.; Jafari, S.; Harandi, S.E. Corrosion fatigue fracture of magnesium alloys in bioimplant applications: A review. Eng. Fract. Mech. 2015, 137, 97–108. [Google Scholar] [CrossRef]
- Zhang, W.-N.; Wang, L.-Z.; Feng, Z.-X.; Chen, Y.-M. Research progress on selective laser melting (SLM) of magnesium alloys: A review. Optik 2020, 207, 163842. [Google Scholar] [CrossRef]
- ISO/ASTM 52900:2015. Additive Manufacturing—General Principles—Terminology; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- Paolini, A.; Kollmannsberger, S.; Rank, E. Additive manufacturing in construction: A review on processes, applications, and digital planning methods. Addit. Manuf. 2019, 30, 100894. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, T.; Qian, F.; Chen, W.; Chandrasekaran, S.; Yao, B.; Song, Y.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M.; et al. 3D printed functional nanomaterials for electrochemical energy storage. Nano Today 2017, 15, 107–120. [Google Scholar] [CrossRef]
- King, D.; Tansey, T. Alternative materials for rapid tooling. J. Mater. Process. Technol. 2002, 121, 313–317. [Google Scholar] [CrossRef]
- Zhang, B.; Fenineche, N.-E.; Zhu, L.; Liao, H.; Coddet, C. Studies of magnetic properties of permalloy (Fe–30%Ni) prepared by SLM technology. J. Magn. Magn. Mater. 2012, 324, 495–500. [Google Scholar] [CrossRef]
- Savalani, M.M.; Pizarro, J.M. Effect of preheat and layer thickness on selective laser melting (SLM) of magnesium. Rapid Prototyp. J. 2016, 22, 115–122. [Google Scholar] [CrossRef]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Hagedorn, Y.-C.; Meiners, W.; Meng, G.; Batista, R.J.S.; Wissenbach, K.; Poprawe, R. Densification behavior, microstructure evolution, and wear performance of selective laser melting processed commercially pure titanium. Acta Mater. 2012, 60, 3849–3860. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.; Zhang, B.; Liao, H.; Coddet, C. Effects of processing parameters on microstructure and mechanical property of selective laser melted Ti6Al4V. Mater. Des. 2012, 35, 120–125. [Google Scholar] [CrossRef]
- Spierings, A.B.; Voegtlin, M.; Bauer, T.; Wegener, K. Powder flowability characterisation methodology for powder-bed-based metal additive manufacturing. Prog. Addit. Manuf. 2016, 1, 9–20. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Cochrane, R.; Dalgarno, K. Densification mechanism and microstructural evolution in selective laser sintering of Al–12Si powders. J. Mater. Process. Technol. 2011, 211, 113–121. [Google Scholar] [CrossRef]
- Ng, C.; Savalani, M.; Lau, M.; Man, H. Microstructure and mechanical properties of selective laser melted magnesium. Appl. Surf. Sci. 2011, 257, 7447–7454. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, J. Microstructure and mechanical properties of ZK60-Er magnesium alloys. Mater. Sci. Eng. A 2015, 633, 154–160. [Google Scholar] [CrossRef]
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of energy input on formability, microstructure and mechanical properties of selective laser melted AZ91D magnesium alloy. Mater. Sci. Eng. A 2014, 611, 212–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Kampffmeyer, M.C.; Zhao, X.; Tan, M. Deep Reinforcement Learning for Query-Conditioned Video Summarization. Appl. Sci. 2019, 9, 750. [Google Scholar] [CrossRef]
- Cao, L. Mesoscopic-scale simulation of pore evolution during laser powder bed fusion process. Comput. Mater. Sci. 2020, 179, 109686. [Google Scholar] [CrossRef]
- Tran, H.-C.; Lo, Y.-L. Heat transfer simulations of selective laser melting process based on volumetric heat source with powder size consideration. J. Mater. Process. Technol. 2018, 255, 411–425. [Google Scholar] [CrossRef]
- Tepylo, N.; Huang, X.; Patnaik, P.C. Laser-Based Additive Manufacturing Technologies for Aerospace Applications. Adv. Eng. Mater. 2019, 21, 1900617. [Google Scholar] [CrossRef]
- Chen, Q.; Guillemot, G.; Gandin, C.-A.; Bellet, M. Numerical modelling of the impact of energy distribution and Marangoni surface tension on track shape in selective laser melting of ceramic material. Addit. Manuf. 2018, 21, 713–723. [Google Scholar] [CrossRef]
- Gusarov, A.; Smurov, I. Radiation transfer in metallic powder beds used in laser processing. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 2517–2527. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Mai, S.; Wang, D.; Song, C. Investigation into spatter behavior during selective laser melting of AISI 316L stainless steel powder. Mater. Des. 2015, 87, 797–806. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, P.; Liu, Z.; Feng, Z.; Wang, C.; Guo, Y. Thermofluid field of molten pool and its effects during selective laser melting (SLM) of Inconel 718 alloy. Addit. Manuf. 2018, 21, 567–578. [Google Scholar] [CrossRef]
- Le, T.-N.; Lo, Y.-L. Effects of sulfur concentration and Marangoni convection on melt-pool formation in transition mode of selective laser melting process. Mater. Des. 2019, 179, 107866. [Google Scholar] [CrossRef]
- Chan, C.; Mazumder, J.; Chen, M.M. A two-dimensional transient model for convection in laser melted pool. Met. Mater. Trans. A 1984, 15, 2175–2184. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M. Dimensionless analysis on selective laser melting to predict porosity and track morphology. J. Mater. Process. Technol. 2019, 273, 116238. [Google Scholar] [CrossRef]
- Gu, D.; Xia, M.; Dai, D. On the role of powder flow behavior in fluid thermodynamics and laser processability of Ni-based composites by selective laser melting. Int. J. Mach. Tools Manuf. 2019, 137, 67–78. [Google Scholar] [CrossRef]
- Mukherjee, T.; Wei, H.; De, A.; Debroy, T. Heat and fluid flow in additive manufacturing—Part I: Modeling of powder bed fusion. Comput. Mater. Sci. 2018, 150, 304–313. [Google Scholar] [CrossRef]
- Cao, L. Workpiece-scale numerical simulations of SLM molten pool dynamic behavior of 316L stainless steel. Comput. Math. Appl. 2020. [Google Scholar] [CrossRef]
- Li, Y.; Gu, D. Thermal behavior during selective laser melting of commercially pure titanium powder: Numerical simulation and experimental study. Addit. Manuf. 2014, 1, 99–109. [Google Scholar] [CrossRef]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Mumtaz, K.; Erasenthiran, P.; Hopkinson, N. High density selective laser melting of Waspaloy®. J. Mater. Process. Technol. 2008, 195, 77–87. [Google Scholar] [CrossRef]
- Hussein, A.; Hao, L.; Yan, C.; Everson, R.M. Finite element simulation of the temperature and stress fields in single layers built without-support in selective laser melting. Mater. Des. 2013, 52, 638–647. [Google Scholar] [CrossRef]
- Jixue, Z.; Yuansheng, Y.; Wenhui, T.; Jie, W.; Junwei, F.; Bin, W. Effect of Cooling Rate on the Solidified Microstructure of Mg-Gd-Y-Zr Alloy. Rare Met. Mater. Eng. 2010, 39, 1899–1902. [Google Scholar] [CrossRef]
- Garibaldi, M.; Ashcroft, I.; Simonelli, M.; Hague, R. Metallurgy of high-silicon steel parts produced using Selective Laser Melting. Acta Mater. 2016, 110, 207–216. [Google Scholar] [CrossRef]
- Montero-Sistiaga, M.L.; Mertens, R.; Vrancken, B.; Wang, X.; Van Hooreweder, B.; Kruth, J.-P.; Van Humbeeck, J. Changing the alloy composition of Al7075 for better processability by selective laser melting. J. Mater. Process. Technol. 2016, 238, 437–445. [Google Scholar] [CrossRef]
- Simonelli, M.; Tse, Y.; Tuck, C. Effect of the build orientation on the mechanical properties and fracture modes of SLM Ti–6Al–4V. Mater. Sci. Eng. A 2014, 616, 1–11. [Google Scholar] [CrossRef]
- Guan, Y.; Zhou, W.; Li, Z.L.; Zheng, H. Influence of overlapping tracks on microstructure evolution and corrosion behavior in laser-melt magnesium alloy. Mater. Des. (1980–2015) 2013, 52, 452–458. [Google Scholar] [CrossRef]
- Zhang, B.; Liao, H.; Coddet, C. Microstructure evolution and density behavior of CP Ti parts elaborated by Self-developed vacuum selective laser melting system. Appl. Surf. Sci. 2013, 279, 310–316. [Google Scholar] [CrossRef]
- Bonifaz, E.A. Finite Element Analysis of heat flow in single pass arc welds. Am. Weld. J. 2000, 79, 121-s–125-s. [Google Scholar]
- Foroozmehr, A.; Badrossamay, M.; Foroozmehr, E.; Golabi, S. Finite Element Simulation of Selective Laser Melting process considering Optical Penetration Depth of laser in powder bed. Mater. Des. 2016, 89, 255–263. [Google Scholar] [CrossRef]
- Leitz, K.-H.; Singer, P.; Plankensteiner, A.; Tabernig, B.; Kestler, H.; Sigl, L. Multi-physical simulation of selective laser melting. Met. Powder Rep. 2017, 72, 331–338. [Google Scholar] [CrossRef]
| Chemical Composition | Al | Zn | Mn | Fe, Cu, etc. | Mg |
|---|---|---|---|---|---|
| Content (wt%) | 9.08 | 0.65 | 0.23 | ≤0.0051 | others |
| Computational domain dimensions (mm) | |
| Layer thickness (mm) | 0.04 |
| Laser spot size (mm) | 0.08 |
| Laser power (W) | 100, 125, 150, 175, 200 |
| Scan velocity (mm/s) | 1000 |
| Porosity (φ) | 0.475 |
| Solidus temperature | 743 K |
| Liquidus temperature | 868 K |
| Specific heat capacity | 1014 J/kg-K (solid, 293 K) |
| 1230 J/kg-K (liquid) | |
| Thermal conductivity (Solid) | 72 × 103 W/K |
| (Liquid) | 82.9 × 103 W/K |
| Latent heat of fusion | 373 kJ/kg |
| Dynamic viscosity | 3 × 10−3 Pa∙s |
| Thermal expansion coefficient | 2.6 × 10−5 K−1 |
| Volumetric thermal expansion coefficient () | 9.541 × 10−5 K−1 |
| Temperature coefficient for surface tension () | −2.13 × 10−4 N/m-K |
| Emissivity () | 0.18 |
| State of Powder | |
|---|---|
| Liquid (completely melted) | |
| Semi-melted | |
| . | Solid (unmelted) |
| Average width of melt channel in experiment I (μm) | 153.14 |
| Average width of melt pool in simulation model (μm) | 171.40 |
| Error | 10.65% |
| Average width of melt channel in experiment II (μm) | 173.95 |
| Average width of melt pool in simulation model (μm) | 171.40 |
| Error | 1.49% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Yan, J.; Niu, X. Thermo-Fluid-Dynamic Modeling of the Melt Pool during Selective Laser Melting for AZ91D Magnesium Alloy. Materials 2020, 13, 4157. https://doi.org/10.3390/ma13184157
Shen H, Yan J, Niu X. Thermo-Fluid-Dynamic Modeling of the Melt Pool during Selective Laser Melting for AZ91D Magnesium Alloy. Materials. 2020; 13(18):4157. https://doi.org/10.3390/ma13184157
Chicago/Turabian StyleShen, Hongyao, Jinwen Yan, and Xiaomiao Niu. 2020. "Thermo-Fluid-Dynamic Modeling of the Melt Pool during Selective Laser Melting for AZ91D Magnesium Alloy" Materials 13, no. 18: 4157. https://doi.org/10.3390/ma13184157
APA StyleShen, H., Yan, J., & Niu, X. (2020). Thermo-Fluid-Dynamic Modeling of the Melt Pool during Selective Laser Melting for AZ91D Magnesium Alloy. Materials, 13(18), 4157. https://doi.org/10.3390/ma13184157






