Implementation of Recycling Cigarette Butts in Lightweight Bricks and a Proposal for Ending the Littering of Cigarette Butts in Our Cities
Abstract
1. Introduction
2. Calorific Value of Cigarette Butts and Energy Reductions
- mass of control brick (no CB content)
- mass of clay in brick containing CBs
- mass of CBs in brick
- 2 MJ/kg energy required for firing clay
- CV = Measured calorific value of used CBs, 16.53 MJ/kg.
3. Laboratory Study on the Manufacturing and Properties of Bricks Containing CBs
3.1. Manufacture of Samples
3.2. Properties of the Bricks
- TC = thermal conductivity (W m−1 K−1)
- Dd = dry density of clay bricks (kg m−3).
4. Brick Manufacturing Processes—Industrial Scale
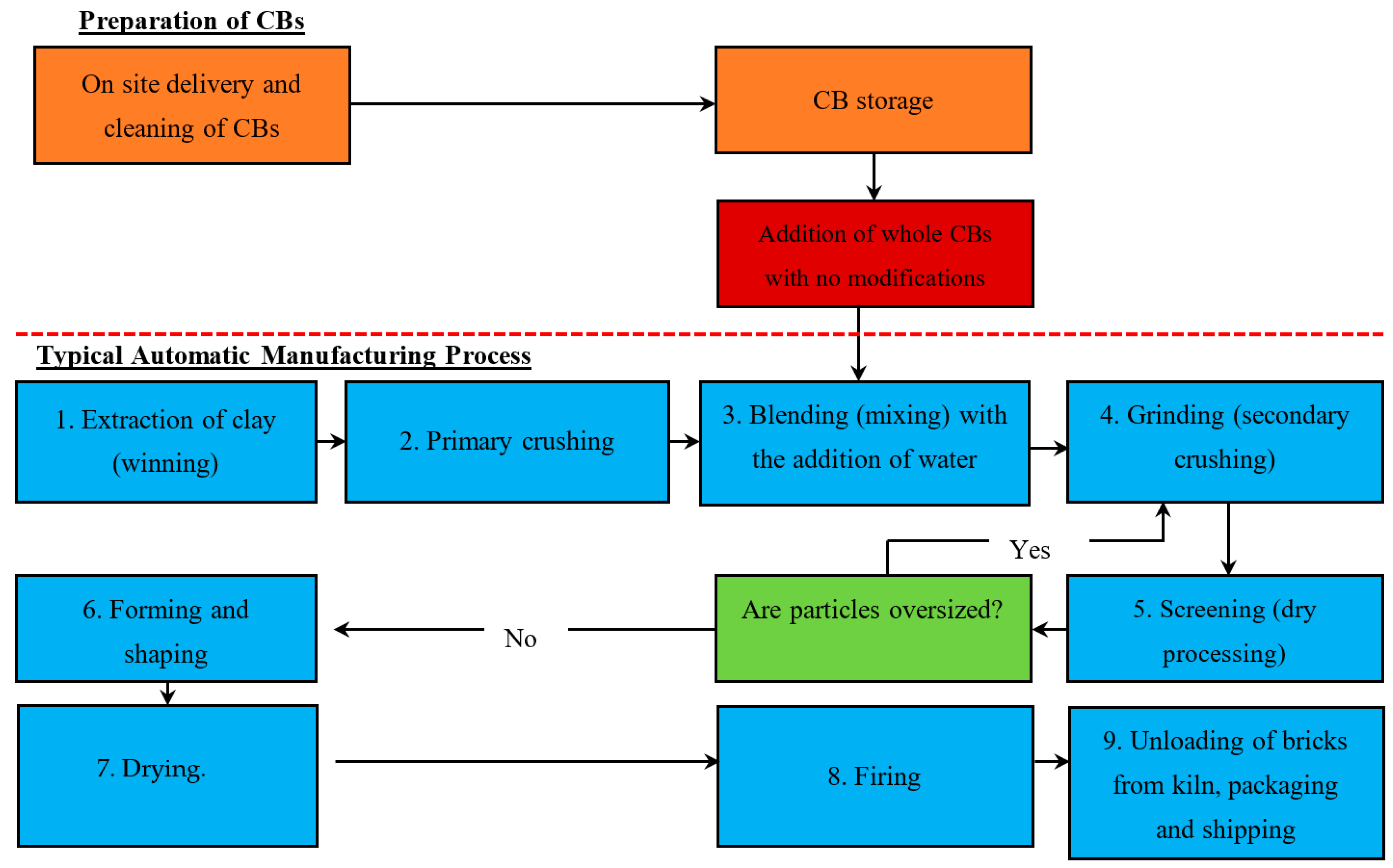
4.1. Automatic Processes
4.1.1. Stage 1. Winning
4.1.2. Stage 2. Primary Crushing
4.1.3. Stage 3. Blending
4.1.4. Stage 4. Grinding
4.1.5. Stage 5. Screening
4.1.6. Stage 6. Forming and Shaping
4.1.7. Stage 7. Drying
4.1.8. Stage 8. Firing
4.1.9. Stage 9. Unloading, Packaging and Shipping
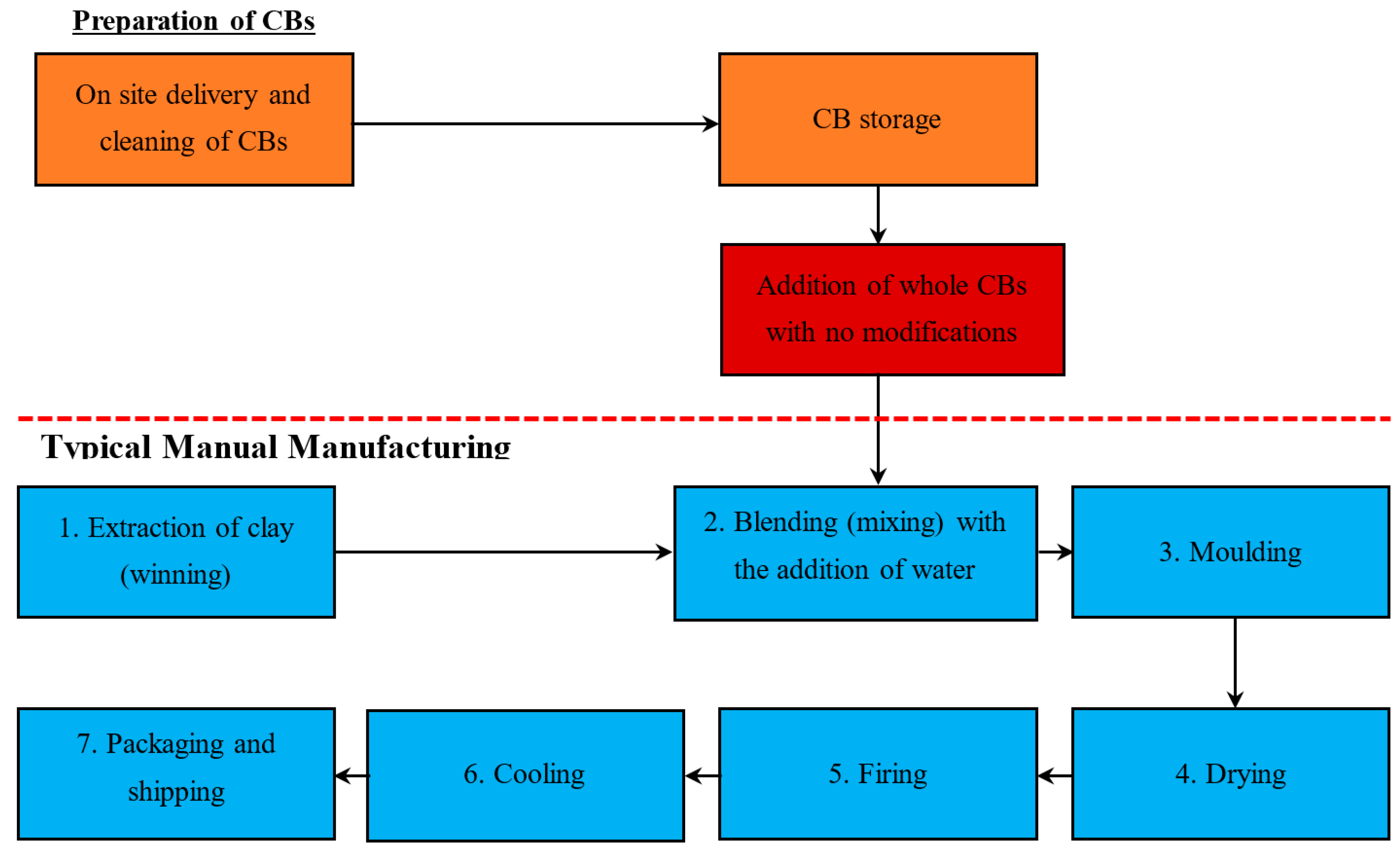
4.2. Manual Processes
4.2.1. Stage 1. Winning
4.2.2. Stage 2. Mixing
4.2.3. Stage 3. Moulding
4.2.4. Stage 4. Drying
4.2.5. Stage 5. Firing
4.2.6. Stage 6. Cooling
4.2.7. Stage 7. Packaging and Transportation
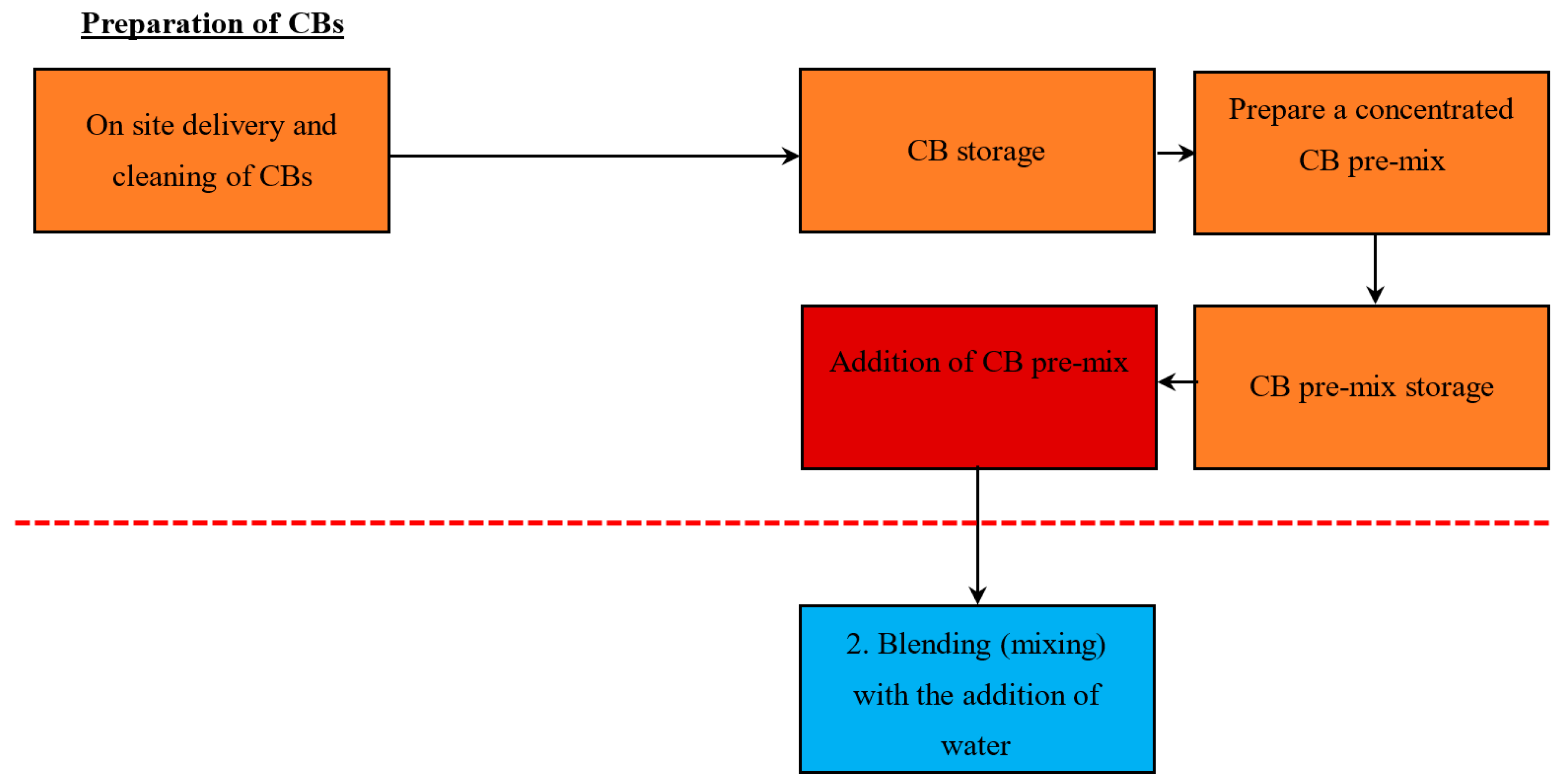
5. CB Incorporation into the Automatic and Manual Process
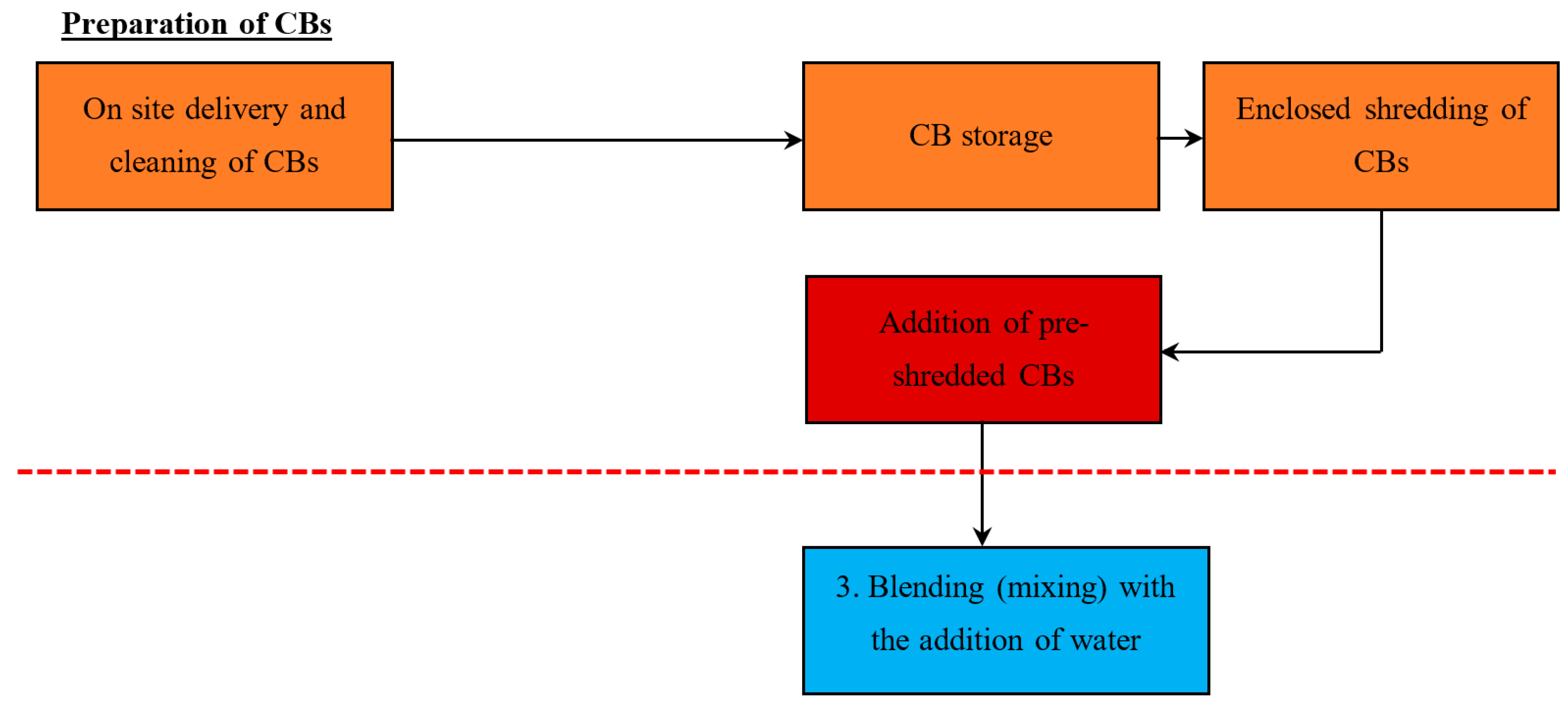
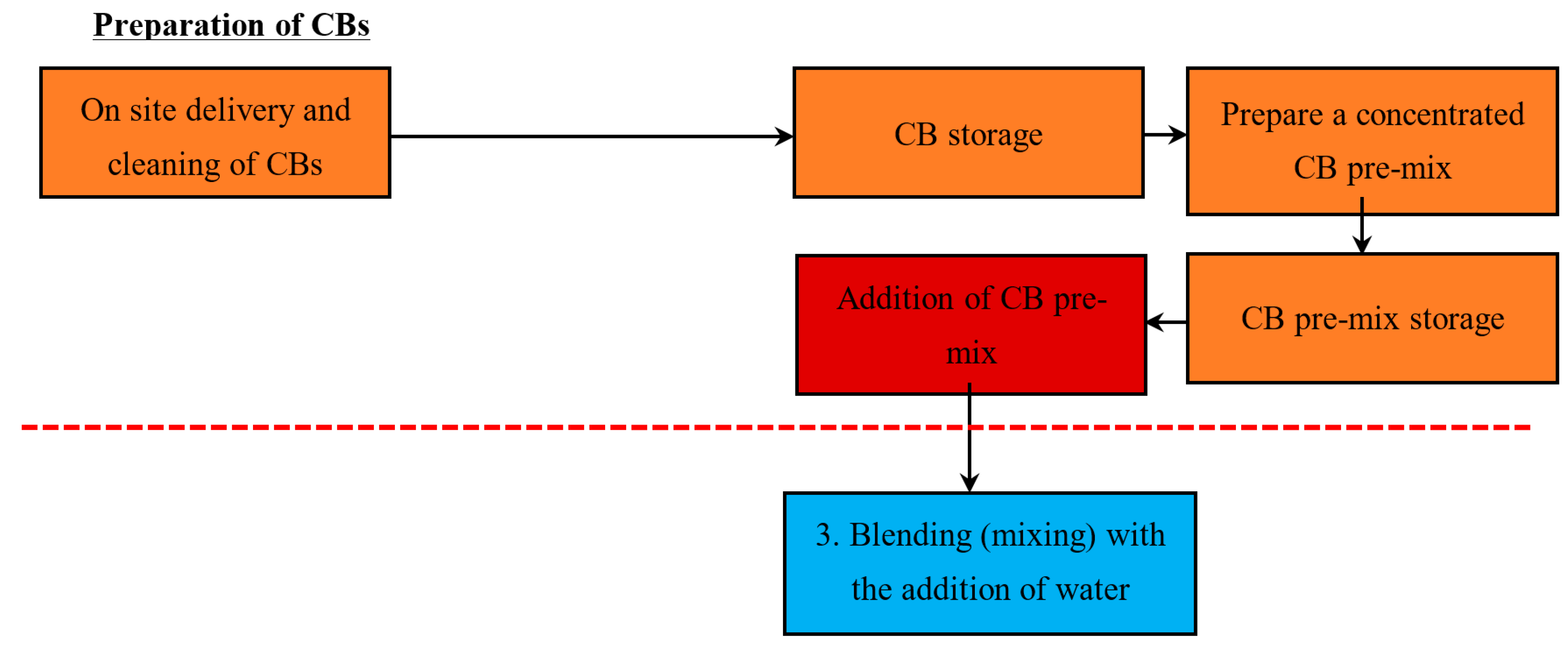
5.1. Incorporation into Automatic Processes
5.1.1. Method 1—Addition of Whole CBs with No Modification
5.1.2. Method 2—Addition of Pre-Shredded CBs
5.1.3. Method 3—Addition of a Concentrated CB Pre-Mix (with a High % Content of CB)
5.1.4. Appropriate Personal Protective Equipment for the Automatic Manufacturing Process
5.1.5. Odour Issues and Reduction Methods for the Automatic Manufacturing Process
- Intensity of CB Odour
- Constant based on how sensitive the human nose is at detecting odour from CB
- Cigarette butt content (%) to which 50% of the population can detect an odour (AMOL)
- Cigarette butt content.
5.1.6. Controlling Odour Level
5.1.7. Odour Elimination Using UV Light
5.2. Incorporation into Manual Processes
5.2.1. Method 1—Addition of Whole CBs with No Modification
5.2.2. Method 2—Addition of a Concentrated CB Pre-Mix (with a High % Content of CB)
5.2.3. Appropriate PPE for the Manual Process
5.2.4. Odour Issues and Reduction Methods for the Manual Process
6. Recycling CBs on an Industrial Scale: Collection and Processing
6.1. CB Collection Systems
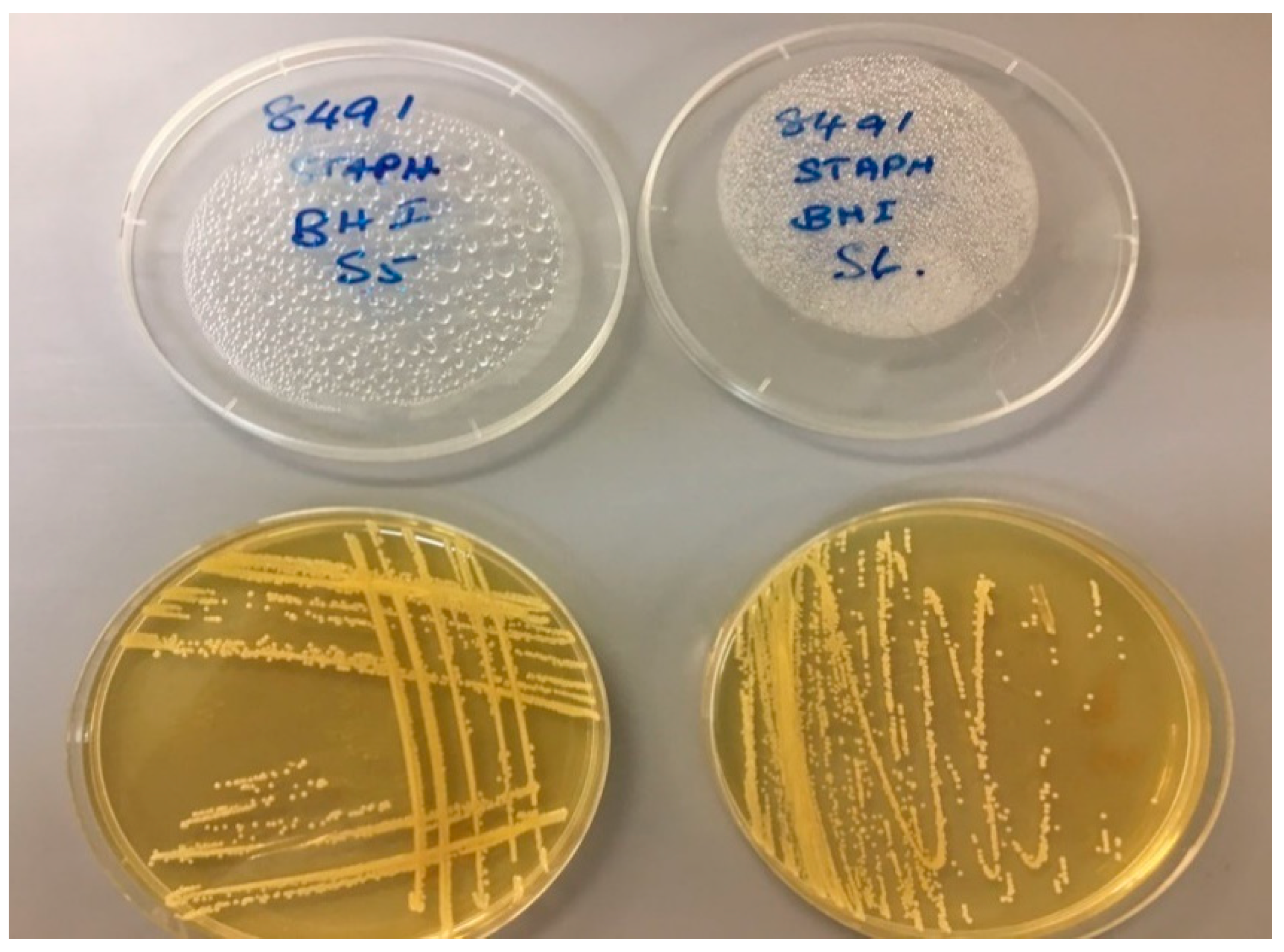
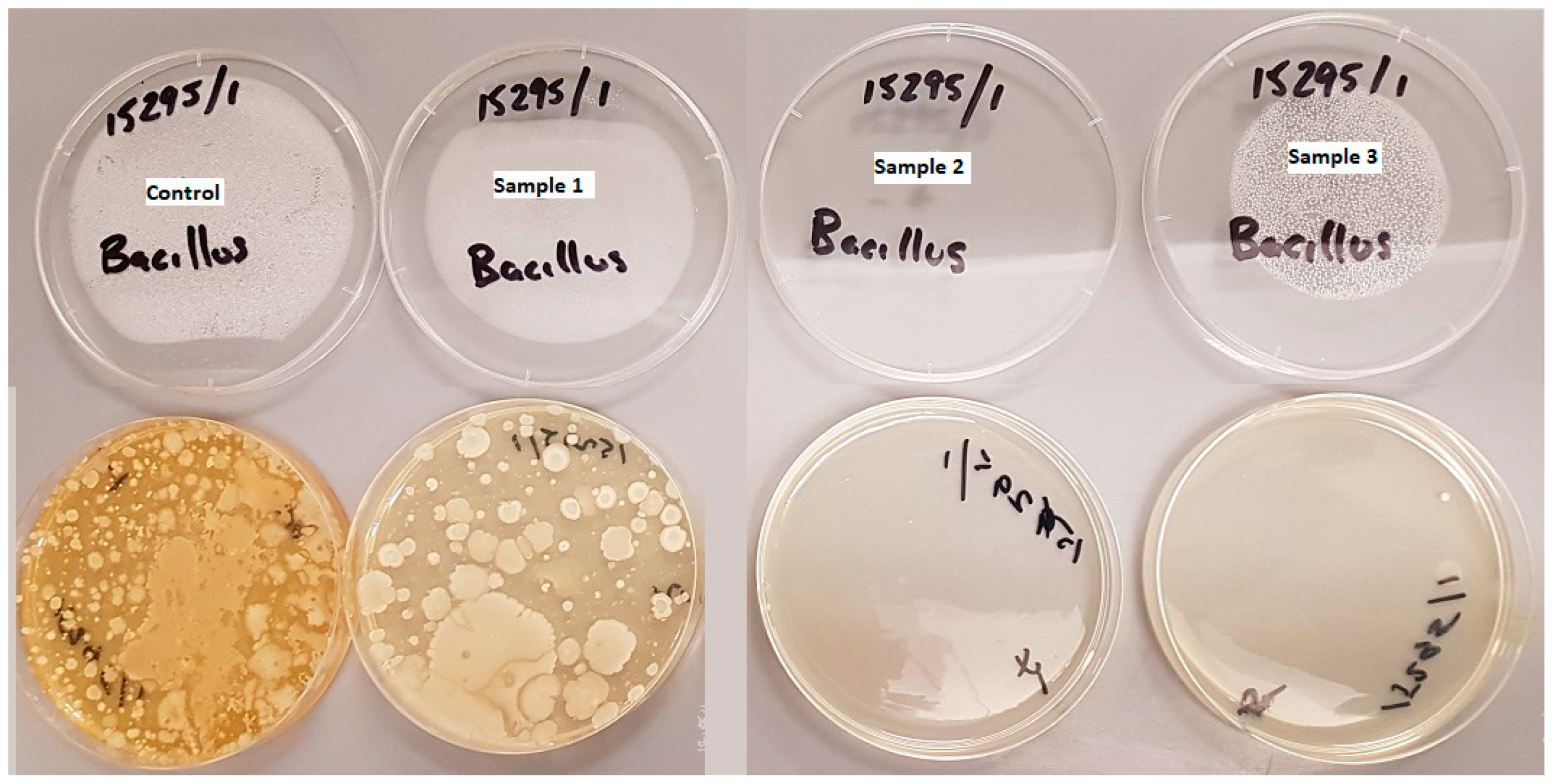
6.2. Preliminary Bacteriological Investigations
6.2.1. Pilot Investigation 1
6.2.2. Pilot Investigation 2
6.2.3. Presence of Viruses
6.3. Potential Cleaning Methods for CBs
6.3.1. Naphthalene
6.3.2. Ozone
6.3.3. Hydrogen Peroxide
6.3.4. Non-Ionising Ultraviolet Light Radiation
6.3.5. Dry and Moist Heat Treatment
6.3.6. Notes on Nicotine
6.4. Safe Handling of CBs
7. Implementation Guide
- For CB collection, aim to develop a close relationship with CB collection companies to facilitate the delivery of CBs to manufacturing sites. These CBs are normally collected from modern bins or receptacles.
- Once the CBs are collected, a sterilisation method (Section 6) should be used to clean the CBs from bacteria. The odour may be purged from CBs in this stage (Section 5). If mothballs (containing naphthalene) are used, they should be put into the bags containing CBs to inactivate any bacteria that may be present. This can be done by the collector or by workers on-site. Care should be taken to not breathe in fumes when the bags are opened. The CBs will then be stored on-site.
- When ready, CBs can be incorporated into the brick clay mix through a method suggested in Section 5 for incorporation into Automatic Processes and 5.2 for incorporation into Manual Processes. Once the CBs have been incorporated, the remaining steps that are common within the brick manufacturing process can be followed.
- Always ensure that relevant OH&S standards are followed, the correct PPE is worn, and the fumes are not breathed. Refer to Section 5.1.4_5.1.4_Appropriate_PPE, 5.2.3_5.2.3_Appropriate_PPE, and 6.4_6.4._Safe_Handling for more detail on the safe handling of CBs.
8. Conclusions
Proposal
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- The Tobacco Atlas. ‘Consumption’, American Cancer Society. 2018. Available online: https://tobaccoatlas.org/topic/consumption/ (accessed on 18 December 2019).
- Carlozo, L.R. ‘Kicking Butts’, Chicago Tribune. 2018. Available online: http://www.chicagotribune.com/news/ct-xpm-2008-06-18-0806170174-story.html (accessed on 27 March 2019).
- Keep Australia Beautiful. Cigarette Butts. 2017. Available online: http://www.kabc.wa.gov.au/report-littering/cigarette-butts (accessed on 17 November 2019).
- World Health Organization. Tobacco and Its Environmental Impact: An Overview; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Mackay, J.; Eriksen, M.; Eriksen, M.P. The Tobacco Atlas; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Srbinoska, M.; Radojičić, V.; Đulančić, N.; Kirkova, S. Possibilities for managing the cigarette butts waste. In Proceedings of the 26th International Conference Ecological Truth & Environmental Research, Bor Lake, Serbia, 12–15 June 2018. [Google Scholar]
- Hoffmann, D.H.I. The Changing Cigarette, 1950–1995. J. Toxicol. Environ. Health 1997, 50, 307–364. [Google Scholar] [CrossRef] [PubMed]
- Australian Government. Cigarettes and Poison. 2013. Available online: http://www.quitnow.gov.au/internet/quitnow/publishing.nsf/Content/cigarettes-and-poison (accessed on 6 October 2019).
- Hecht, S.S. Research Opportunities Related to Establishing Standards for Tobacco Products Under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob. Res. 2012, 14, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Hoffmann, I.; El-Bayoumy, K. The less harmful cigarette: A controversial issue. A tribute to Ernst L. Wynder. Chem. Res. Toxicol. 2001, 14, 767–790. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Djordjevic, M.V.; Brunnemann, K.D. Changes in cigarette design and composition over time and how they influence the yields of smoke constituents. J. Smok. Relat. Disord 1995, 6, 9–23. [Google Scholar]
- Hon, N.-S. Photodegradation of cellulose acetate fibers. J. Polym. Sci. A Polym. Chem. 1977, 15, 725–744. [Google Scholar] [CrossRef]
- Dieng, H.; Rajasaygar, S.; Ahmad, A.H.; Ahmad, H.; Rawi, C.S.M.; Zuharah, W.F.; Satho, T.; Miake, F.; Fukumitsu, Y.; Saad, A.R.; et al. Turning cigarette butt waste into an alternative control tool against an insecticide-resistant mosquito vector. Acta Trop. 2013, 128, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Marinello, S.; Lolli, F.; Gamberini, R.; Rimini, B. A second life for cigarette butts? A review of recycling solutions. J. Hazard. Mater. 2019, 384, 121–245. [Google Scholar] [CrossRef]
- Micevska, T.; Warne, M.; Pablo, F.; Patra, R. ariation in, and causes of, toxicity of cigarette butts to a cladoceran and microtox. Arch. Environ. Contam. Toxicol. 2006, 50, 205–212. [Google Scholar] [CrossRef]
- Slaughter, E.; Gersberg, R.M.; Watanabe, K.; Rudolph, J.; Stransky, C.; Novotny, T.E. Toxicity of cigarette butts, and their chemical components, to marine and freshwater fish. Tob. Control 2011, 20 (Suppl. 1), i25–i29. [Google Scholar] [CrossRef]
- Rebischung, F.; Chabot, L.; Biaudet, H.; Pandard, P. Cigarette butts: A small but hazardous waste, according to European regulation. Waste Manag. 2018, 82, 9–14. [Google Scholar] [CrossRef]
- Clean Up Australia Report. 2017. Available online: https://www.cleanup.org.au/rubbish-report (accessed on 1 September 2019).
- Torkashvand, J.; Farzadkia, M. A systematic review on cigarette butt management as a hazardous waste and prevalent litter: Control and recycling. Environ. Sci. Pollut. Res. 2019, 26, 11618–11630. [Google Scholar] [CrossRef] [PubMed]
- Kurmus, H.; Mohajerani, A. The toxicity and valorization options of cigarette butts. Waste Manag. 2020, 104, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Kurmus, H.; Mohajerani, A. Leachate Analysis of Heavy Metals in Cigarette Butts and Bricks Incorporated with Cigarette Butts. Materials 2020, 13, 2843. [Google Scholar] [CrossRef] [PubMed]
- Mohajerani, A.; Tanriverdi, Y.; Nguyen, B.T.; Wong, K.K.; Dissanayake, H.N.; Johnson, L.; Whitfield, D.; Thomson, G.; Alqattan, E.; Rezaei, A. Physico-mechanical properties of asphalt concrete incorporated with encapsulated cigarette butts. Constr. Build. Mater. 2017, 153, 69–80. [Google Scholar] [CrossRef]
- Mohajerani, A.; Kadir, A.A.; Larobina, L. A practical proposal for solving the world’s cigarette butt problem: Recycling in fired clay bricks. Waste Manag. 2016, 52, 228–244. [Google Scholar] [CrossRef]
- Neil, J.; Ravinda, K.D. Civil Engineering Materials, 5th ed; Macmillan Education Press: London, UK, 1997. [Google Scholar]
- Muñoz Velasco, P.; Morales Ortíz, M.P.; Mendívil Giró, M.A.; Muñoz Velasco, L. Fired clay bricks manufactured by adding wastes as sustainable construction material—A review. Constr. Build. Mater. 2014, 63, 97–107. [Google Scholar] [CrossRef]
- Prasertsan, S. Preliminary Study on Brick Making Industry in ASEAN Countries. In Final Report (Unpublished); NRCT: Bangkok, Thailand, 1995. [Google Scholar]
- Australian Standard. AS/NZS 1289.5.1.1. Method 5.1.1. In Methods for Testing Soils for Engineering Purposes—Soil Compaction and Density Tests—Determination of the Dry Density/Moisture Content Relations of a Soil Using Standard Compactive Effort; SAI Global: Standards Australia: Sydney, Australia, 2003. [Google Scholar]
- Sutcu, M.; Akkurt, S. AS/NZS 4456.1:2008 (Masonry Units and Segmental Pavers and Flags, 2008); SAI Global: Standards Australia: Sydney, Australia, 2008. [Google Scholar]
- Eliche-Quesada, D.; Martínez-Martínez, S.; Pérez-Villarejo, L.; Iglesias-Godino, F.J.; Martínez-García CCorpas-Iglesias, F.A. Valorization of biodiesel production residues in making porous clay brick. Fuel Process. Technol. 2012, 103, 166–173. [Google Scholar] [CrossRef]
- Sutcu, M.; Akkurt, S. The use of recycled paper processing residues in making porous brick with reduced thermal conductivity. Ceram. Int. 2009, 35, 2625–2631. [Google Scholar] [CrossRef]
- Kadir, A.A.; Mohajerani, A. Possible utilization of cigarette butts in light-weight fired clay bricks. Int. J. Civ. Environ. Eng. 2008, 2, 137–141. [Google Scholar]
- Kadir, A.A.; Mohajerani, A. Recycling cigarette butts in lightweight fired clay bricks. Constr. Mater. 2011, 164, 219–229. [Google Scholar] [CrossRef]
- USEPA. Hazardous Waste Characteristics Scoping Study; Office of Solid Waste; US Environmental Protection Agency: Washington, DC, USA, 1996.
- EPAV. Guidelines for Hazard Classification of Solid Prescribed Industrial Waste; Environmental Protection Agency of Victoria: Melbourne, Australia, 2005.
- Midwest Research Institute (MRI). Brick and Structural Clay Product Manufacturing; United States Environmental Protection Agency: Washington, DC, USA, 1997.
- Mortar Industry Association (MIA). Brick and Block Production; Mortar Industry Association: London, UK, 2013. [Google Scholar]
- Ibstock Brick Ltd. How Clay Bricks Are Made; Ibstock: Leicestershire, UK, 2005; Available online: https://www.ibstockbrick.co.uk/wp-content/uploads/2015/08/TIS-A1-HOW-BRICKS-ARE-MADE-3.pdf (accessed on 4 October 2019).
- Africa Clay Brick Association of South. Clay Brick Technical Guide; ABC Press: Cape Town, South Africa, 2015. [Google Scholar]
- Brick Industry Association. Technical Notes on Brick Construction; The Brick Industry Association: Reston, VA, USA, 2006. [Google Scholar]
- Alternatives Development. Enabling Policies in the Indian Brick Sector-Current Status and Future Trends; Alternatives Development: New Delhi, India, 2012. [Google Scholar]
- Jrup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Charles, S.M.; Batterman, S.A.; Jia, C. Composition and emissions of VOCs in main- and side-stream smoke of research cigarettes. Atmos. Environ. 2007, 41, 5371–5384. [Google Scholar] [CrossRef]
- Martuzevicius, D.; Prasauskas, T.; Setyan, A.; O’connell, G.; Cahours, X.; Julien, R.; Colard, S. Characterization of the Spatial and Temporal Dispersion Differences Between Exhaled E-Cigarette Mist and Cigarette Smoke. Nicotine Tob. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Poppendieck, D.; Khurshid, S.; Emmerich, S. Measuring Airborne Emissions from Cigarette Butts: Literature Review and Experimental Plan; National Institute of Standards and technology U.S Department of commerce: Gaithersburg, MA, USA, 2016.
- Sherman, C.; Weaver, E. The Senses: Smell and Taste’, The Dana Alliance for Brain Initiatives. 2016. Available online: https://www.dana.org/uploadedFiles/Pdfs/Brain-Brief-Senses-Smell-and-Taste-FINAL.pdf (accessed on 14 November 2019).
- Encyclopædia Britannica. 2018. Available online: https://www.britannica.com/science/methane (accessed on 1 October 2019).
- Miljøstyrelsen, V.F. Industrial Odour Control; Agency, D.E.P., Ed.; Danish Minestry of the Enviroment: Copenhagen, Denmark, 2002; p. 29. [Google Scholar]
- Laliberte, G. What is the Difference between Odour Concentration and Odour Intensity for Regulators? Available online: http://www.odotech.com/en/odour_concentration_vs_intensity/ (accessed on 16 October 2019).
- Normand Brais. ‘Garbage Room Odors Remediation’, Sanuvox. 2017. Available online: https://sanuvox.com/wp-content/uploads/2019/04/OdorControl_EN.pdf (accessed on 15 December 2019).
- City of Melbourne. Cigarette Butt Disposal. 2018. Available online: https://www.melbourne.vic.gov.au/business/waste-recycling/Pages/cigarette-butt-disposal.aspx (accessed on 5 October 2019).
- Centers for Disease Control and Prevention. E. coli (Escherichia coli). 2018. Available online: https://www.cdc.gov/ecoli/general/index.html (accessed on 4 October 2019).
- SA Health. Salmonella Infection—Including Symptoms, Treatment and Prevention. 2017. Available online: https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/health+topics/health+conditions+prevention+and+treatment/infectious+diseases/salmonella+infection/salmonella+infection+-+including+symptoms+treatment+and+prevention (accessed on 4 October 2019).
- Centers for Disease Control and Prevention. Pseudomonas aeruginosa in Healthcare Settings. 2018. Available online: https://www.cdc.gov/hai/organisms/pseudomonas.html (accessed on 4 October 2019).
- Fraser, S.L. Enterococcal Infections. 2018. Available online: https://emedicine.medscape.com/article/216993-overview#a4 (accessed on 4 October 2019).
- Centers for Disease Control and Prevention. Staphylococcus aureus in Healthcare Settings. 2011. Available online: https://www.cdc.gov/hai/organisms/staph.html (accessed on 4 October 2019).
- Healthdirect. Staph Infections. 2018. Available online: https://www.healthdirect.gov.au/staph-infections (accessed on 4 October 2019).
- MedlinePlus. Streptococcal Infections. 2018. Available online: https://medlineplus.gov/streptococcalinfections.html (accessed on 4 October 2019).
- Schmid-Hempel, P.; Frank, S.A. Pathogenesis, virulence, and infective dose. PLoS Pathog. 2007, 3, e147. [Google Scholar] [CrossRef] [PubMed]
- Food Standards Australia New Zealand 2013. Listeria Monocytogenes; Food Standards Australia New Zealand: Sydney, Australia, 2013.
- Centers for Disease Control and Prevention. Legionella (Legionnaires’ Disease and Pontiac Fever). 2018. Available online: https://www.cdc.gov/legionella/about/causes-transmission.html (accessed on 4 October 2019).
- Bacillus Species. 2017. Available online: http://www.antimicrobe.org/b82.asp (accessed on 4 January 2020).
- Government of UK. Bacillus Species (Food Poisoning). 2008. Available online: https://www.gov.uk/government/collections/bacillus-species-food-poisoning (accessed on 4 October 2019).
- Wells, C.L.; Wilkins, T.D. Clostridia: Sporeforming anaerobic bacilli. In Medical Microbiology, 4th ed; University of Texas Medical Branch at Galveston: Gaalveston, TX, USA, 1996. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8219/ (accessed on 18 September 2019).
- Todar, K. Pathogenic Clostridia, including Botulism and Tetanus (Page 1). Available online: http://textbookofbacteriology.net/clostridia.html (accessed on 4 October 2019).
- Garrec, N.; Picard-Bonnaud, F.; Pourcher, A. Occurrence of Listeria sp. and L. monocytogenes in sewage sludge used for land application: Effect of dewatering, liming and storage in tank on survival of Listeria species. FEMS Immunol. Med. Microbiol. 2003, 35, 275–283. [Google Scholar] [CrossRef]
- Larsson, L.; Szponar, B.; Ridha, B.; Pehrson, C.; Dutkiewicz, J.; Krysińska-Traczyk, E.; Sitkowska, J. Identification of bacterial and fungal components in tobacco and tobacco smoke. Tob. Induc. Dis. 2008, 4, 4. [Google Scholar] [CrossRef]
- World Health Organisation. Hepatitis A. 2016. Available online: http://www.who.int/mediacentre/factsheets/fs328/en (accessed on 15 December 2019).
- Hepatitis Australia. Transmission of Hepatitis B. 2015. Available online: http://www.hepatitisaustralia.com/hepatitis-b-facts/transmission (accessed on 15 October 2019).
- Centers for Disease Control and Prevention. Hepatitis C Questions and Answers for the Public. 2016. Available online: https://www.cdc.gov/hepatitis/hcv/cfaq.htm (accessed on 15 November 2019).
- Aidsinfo. The Basics of HIV Prevention. 2016. Available online: https://aidsinfo.nih.gov/education-materials/fact-sheets/20/48/the-basics-of-hiv-prevention (accessed on 15 September 2019).
- Buckpitt, A.; Kephalopoulos, S.; Koistinen, K.; Kotzias, D.; Morawska, L.; Sagunski, H. WHO Guidelines for Indoor Air Quality: Selected Pollutants. Available online: https://www.ncbi.nlm.nih.gov/books/NBK138704/ (accessed on 18 September 2019).
- Bond, E. Manual of Fumigation for Insect Control; FAO Plant Production And Protection Paper; FAO: Rome, Italy, 2007; Volume 54, pp. 131–138. [Google Scholar]
- Government of New South Wales. Naphthalene in Moth Balls and Toilet Deoderant Cakes. Available online: http://www.health.nsw.gov.au/environment/factsheets/Pages/naphthalene.aspx (accessed on 18 September 2019).
- Rokade, Y.; Sayyed, R. Naphthalene derivatives: A new range of antimicrobials with high therapeutic value. Rasayan J. Chem. 2009, 2, 972–980. [Google Scholar]
- Gisvolds, W.A. Textbook of Organic Medicinal and Pharmaceutical Chemistry; Wolters Kluwer, Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Mkpenie, V.; Ebong, G.; Obot, I.B.; Abasiekong, B.; Mkpenie, V.; Ebong, G.; Obot, I.; Abasiekong, B. Evaluation of the effect of azo group on the biological activity of 1-(4-methylphenylazo)-2-naphthol. J. Chem. 2008, 5, 431–434. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. ‘Ozone’, PubChem Compound Database. CID=24823. 2005. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/ozone#section=Top (accessed on 11 January 2020).
- Guzel-Seydim, Z.B.; Greene, A.K.; Seydim, A.C. Use of ozone in the food industry. LWT Food Sci. Technol. 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Khadre, M.; Yousef, A.; Kim, J.G. Microbiological aspects of ozone applications in food: A review. J. Food Sci. 2001, 66, 1242–1252. [Google Scholar] [CrossRef]
- Naitou, S.; Takahara, H. Recent Developments in Food and Agricultural uses of Ozone as an Antimicrobial Agent-Food Packaging Film Sterilizing Machine using Ozone. Ozone Sci. Eng. 2008, 30, 81–87. [Google Scholar] [CrossRef]
- Sharma, M.; Hudson, J.B. Ozone gas is an effective and practical antibacterial agent. Am. J. Infect. Control 2008, 36, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.K.; Few, B.K.; Serafini, J.C. A Comparison of Ozonation and Chlorination for the Disinfection of Stainless Steel Surfaces. J. Dairy Sci. 1993, 76, 3617–3620. [Google Scholar] [CrossRef]
- Ozone Oxidation is Nature’s Sanitation Powerhouse. 2017. Available online: http://www.delozone.com/ozone-technology/about-ozone.php (accessed on 11 October 2019).
- Burleson, G.R.; Murray, T.; Pollard, M. Inactivation of viruses and bacteria by ozone, with and without sonication. Appl. Microbiol. 1975, 29, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.; Charig, A.; Demoranville, I. Effect of ozone on storage of cranberries. In Proceedings of the American Society for Horticultural Science; American Society for Horticultural Science: Alexandria, VA, USA, 1968; Volume 93, p. 792. [Google Scholar]
- Rice, R.G.; Farquhar, J.W.; Bollyky, L.J. Review of the applications of ozone for increasing storage times of perishable foods. Ozone Sci. Eng. 1982, 4, 147–163. [Google Scholar]
- Hoof, F. Professional risks associated with ozone. Ozonation Man. Water Waste Water Treat. 1982, 200–201. [Google Scholar]
- Batakliev, T.; Georgiev, V.; Anachkov, M.; Rakovsky, S.; Zaikov, G.E. Ozone decomposition. Interdiscip. Toxicol. 2014, 7, 47–59. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. ‘Hydrogen Peroxide’, PubChem Compound Database; CID=784. 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/hydrogen_peroxide (accessed on 11 September 2019).
- Chung, S.; Kern, R.; Koukol, R.; Barengoltz, J.; Cash, H. Vapor hydrogen peroxide as alternative to dry heat microbial reduction. Adv. Space Res. 2008, 42, 1150–1160. [Google Scholar] [CrossRef]
- Fu, T.Y.; Gent, P.; Kumar, V. Efficacy, efficiency and safety aspects of hydrogen peroxide vapour and aerosolized hydrogen peroxide room disinfection systems. J. Hosp. Infect. 2012, 80, 199–205. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef]
- Kahnert, A.; Seiler, P.; Stein, M.; Aze, B.; McDonnell, G.; Kaufmann, S.H. Decontamination with vaporized hydrogen peroxide is effective against Mycobacterium tuberculosis. Lett. Appl. Microbiol. 2005, 40, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Fichet, G.; Antloga, K.; Comoy, E.; Deslys, J.; McDonnell, G. Prion inactivation using a new gaseous hydrogen peroxide sterilisation process. J. Hosp. Infect. 2007, 67, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Pottage, T.; Richardson, C.; Parks, S.; Walker, J.T.; Bennett, A.M. Evaluation of hydrogen peroxide gaseous disinfection systems to decontaminate viruses. J. Hosp. Infect. 2010, 74, 55–61. [Google Scholar] [CrossRef]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and potential applications of ultraviolet light in the food industry–a critical review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Rock, C.; Curless, M.S.; Nowakowski, E.; Ross, T.; Carson, K.A.; Trexler, P.; Carroll, K.; Maragakis, L.L. UV-C Light Disinfection of Carbapenem-Resistant Enterobacteriaceae from High-Touch Surfaces in a Patient Room and Bathroom. Infect. Control Hosp. Epidemiol. 2016, 37, 996–997. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.M.; Lee, S.Y. Inhibitory Effects of UV Treatment and a Combination of UV and Dry Heat against Pathogens on Stainless Steel and Polypropylene Surfaces. J. Food Sci. 2012, 77, M61–M64. [Google Scholar] [CrossRef]
- Cutler, T.D.; Zimmerman, J.J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim. Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef]
- Jagger, J. Introduction to Research in Ultra-Violet Photobiology; Prentice-Hall: Englewood Cliffs, NJ, USA, 1967. [Google Scholar]
- Petersson, L.P.; Albrecht, U.-V.; Sedlacek, L.; Gemein, S.; Gebel, J.; Vonberg, R.-P. Portable UV light as an alternative for decontamination. Am. J. Infect. Control 2014, 42, 1334–1336. [Google Scholar] [CrossRef]
- Angela, T.; Robert, P.S.; Antony, R.Y. UVA1 Induces Cyclobutane Pyrimidine Dimers but Not 6-4 Photoproducts in Human Skin In Vivo. J. Investig. Dermatol. 2011. [Google Scholar] [CrossRef]
- Mallet, J.D.; Rochette, P.J. Wavelength-dependent ultraviolet induction of cyclobutane pyrimidine dimers in the human cornea. Photochem. Photobiol. Sci. 2013, 12, 1310–1318. [Google Scholar] [CrossRef]
- Smelt, J.P.; Brul, S. Thermal inactivation of microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Darmady, E.M.; Hughes, K.E.; Jones, J.D.; Prince, D.; Tuke, W. Sterilization by dry heat. J. Clin. Pathol. 1961, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Cafe, T. Physical Constants for Investigators. Available online: http://www.tcforensic.com.au/docs/article10.html#2.1 (accessed on 13 April 2019).
- Pavia, C.S.; Pierre, A.; Nowakowski, J. Antimicrobial activity of nicotine against a spectrum of bacterial and fungal pathogens. J. Med. Microbiol. 2000, 49, 675–676. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adeleye, I.; Onubogu, C.; Ayolabi, C.; Isawumi, A.; Nshiogu, M. Screening of crude extracts of twelve medicinal plants and “wondercure” concoction used in Nigeria unorthodox medicine for activity against mycobacterium tuberculosis isolated from tuberculosis patients sputum. Afr. J. Infect. Dis. 2008, 2, 2. [Google Scholar] [CrossRef]
- Bakht, J.; Azra; Shafi, M. Antimicrobial activity of Nicotiana tabacum using different solvents extracts. Pak. J. Bot. 2012, 44, 459–463. [Google Scholar]
- Stewart, G.G. A history of the medicinal use of tobacco 1492–1860. Med. Hist. 1967, 11, 228–268. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A. Medicinal uses of tobacco in history. J. R. Soc. Med. 2004, 97, 292–296. [Google Scholar] [CrossRef]
- Mayer, B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch. Toxicol. 2014, 88, 5–7. [Google Scholar] [CrossRef]
- Zuskin, E.; Mustajbegovic, J.; Schachter, E.N.; Kern, J.; Doko-Jelinic, J.; Godnic-Cvar, J. Respiratory findings in workers employed in the brick-manufacturing industry. J. Occup. Environ. Med. 1998, 40, 814–820. [Google Scholar] [CrossRef]
- Myers, J.E.; Cornell, J.E. Respiratory health of brickworkers in Cape Town, South Africa: Symptoms, signs and pulmonary function abnormalities. Scand. J. Work Environ. Health 1989, 15, 188–194. [Google Scholar] [CrossRef]
- Raza, A.; Qamer, M.; Afsheen, S.; Adnan, M.; Naeem, S.; Atiq, M. Particulate Matter Associated Lung Function Decline in Brick Kiln Workers of Jalalpur Jattan, Pakistan. Pak. J. Zool. 2014, 46, 237–243. [Google Scholar]
- Sanjel, S.; Khanal, S.N.; Thygerson, S.M.; Carter, W.S.; Johnston, J.D.; Joshi, S.K. Respiratory symptoms and illnesses related to the concentration of airborne particulate matter among brick kiln workers in Kathmandu valley, Nepal. Ann. Occup. Environ. Med. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Fishwick, D.; Sumner, J.; Barber, C.M.; Robinson, E.; Codling, A.; Lewis, L.; Young, C.; Warren, N. P61 Respiratory ill health in the silica exposed brick manufacturing sector. Thorax 2015, 70, A106. [Google Scholar] [CrossRef]




| Test/Properties | Standard | Brick Soil |
|---|---|---|
| Specific gravity | AS 1289.3.5.1 | 2.69 |
| Liquid limit (%) | AS 1289.3.1.1 | 32 |
| Plastic limit (%) | AS 1289.3.2.1 | 19 |
| Plasticity index (%) | AS 1289.3.1.1 | 13 |
| Australian soil classification | AS 1726-1993 | CL |
| Optimum moisture content (%) | AS 1289.5.1.1 | 16 |
| Maximum dry density (Mg/m3) | AS 1289.5.1.1 | 1.78 |
| Organic content (%) | BS 1377-3 | 1.23 |
| Sample Identification | Moisture Content (%) | Compressive Strength (MPa) | Water Absorption: Cold (%) | Initial Rate of Absorption (kg m−2 min−1) | Diametric Shrinkage (%) | Height Shrinkage (%) | Average Density (kg m−3) | Thermal Conductivity (W m−1 K−1) |
|---|---|---|---|---|---|---|---|---|
| CB (0%) (0 kg m−3) | 15.0 | 43.17 | 8.15 | 0.31 | 5.38 | 6.16 | 2134.0 | 1.107 |
| CB (1%) (20 kg m−3) | 15.5 | 27.49 | 10.53 | 0.47 | 4.00 | 4.34 | 1991.0 | 0.906 |
| CB (1%) (20 kg m−3) | 17.5 | 25.77 | 11.51 | 0.39 | 5.39 | 5.98 | 1964.0 | 0.873 |
| Sample | Salmonella spp. /15g | Escherichia coli MPN/g | Pseudomonas aeruginosa MPN/g | Enterococcus spp. MPN/g | Coagulase + ve Staphylococcus spp. cfu/g | Streptococcus spp. cfu/g |
|---|---|---|---|---|---|---|
| Control (unused CBs) | Not Detected | <2 | <2 | <2 | <100 | <100 |
| Sample 1 | Not Detected | <2 | <2 | 2 | <100 | <100 |
| Sample 2 | Not Detected | <2 | <2 | <2 | 800 | <100 |
| Sample | Salmonella spp. /15g | Escherichia coli MPN/g | Pseudomonas aeruginosa MPN/g | Enterococcus spp. MPN/g | Coagulase + ve Staphylococcus spp. cfu/g | Streptococcus spp. cfu/g | Listeria spp. /25g | Bacillus spp. cfu/g | Clostridia spp. /g | Total Leginella spp. cfu/g |
|---|---|---|---|---|---|---|---|---|---|---|
| Control (unused CBs) | Not Detected | <2 | <2 | 4 | <100 | <100 | Not Detected | 70,000 | Not Detected | <1000 |
| Sample 1 | Not Detected | <2 | 2 | <2 | <100 | <100 | Not Detected | 70,000 | Not Detected | <1000 |
| Sample 2 Stored CBs | Not Detected | <2 | <2 | <2 | <100 | <100 | Detected | 39,000 | Not Detected | <1000 |
| Sample 3 Dried CBs | Not Detected | <2 | <2 | <2 | <100 | <100 | Not Detected | 440 | Not Detected | <1000 |
| Sample 4 with Mothballs | Not Detected | <2 | <2 | <2 | <100 | <100 | Not Detected | 4100 | Not Detected | <1000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohajerani, A.; Qun Hui, S.; Shen, C.; Suntovski, J.; Rodwell, G.; Kurmus, H.; Hana, M.; Rahman, M.T. Implementation of Recycling Cigarette Butts in Lightweight Bricks and a Proposal for Ending the Littering of Cigarette Butts in Our Cities. Materials 2020, 13, 4023. https://doi.org/10.3390/ma13184023
Mohajerani A, Qun Hui S, Shen C, Suntovski J, Rodwell G, Kurmus H, Hana M, Rahman MT. Implementation of Recycling Cigarette Butts in Lightweight Bricks and a Proposal for Ending the Littering of Cigarette Butts in Our Cities. Materials. 2020; 13(18):4023. https://doi.org/10.3390/ma13184023
Chicago/Turabian StyleMohajerani, Abbas, Siu Qun Hui, Cary Shen, James Suntovski, Glen Rodwell, Halenur Kurmus, Marven Hana, and Md Tareq Rahman. 2020. "Implementation of Recycling Cigarette Butts in Lightweight Bricks and a Proposal for Ending the Littering of Cigarette Butts in Our Cities" Materials 13, no. 18: 4023. https://doi.org/10.3390/ma13184023
APA StyleMohajerani, A., Qun Hui, S., Shen, C., Suntovski, J., Rodwell, G., Kurmus, H., Hana, M., & Rahman, M. T. (2020). Implementation of Recycling Cigarette Butts in Lightweight Bricks and a Proposal for Ending the Littering of Cigarette Butts in Our Cities. Materials, 13(18), 4023. https://doi.org/10.3390/ma13184023







