Excited-State Dynamics of Room-Temperature Phosphorescent Organic Materials Based on Monobenzil and Bisbenzil Frameworks
Abstract
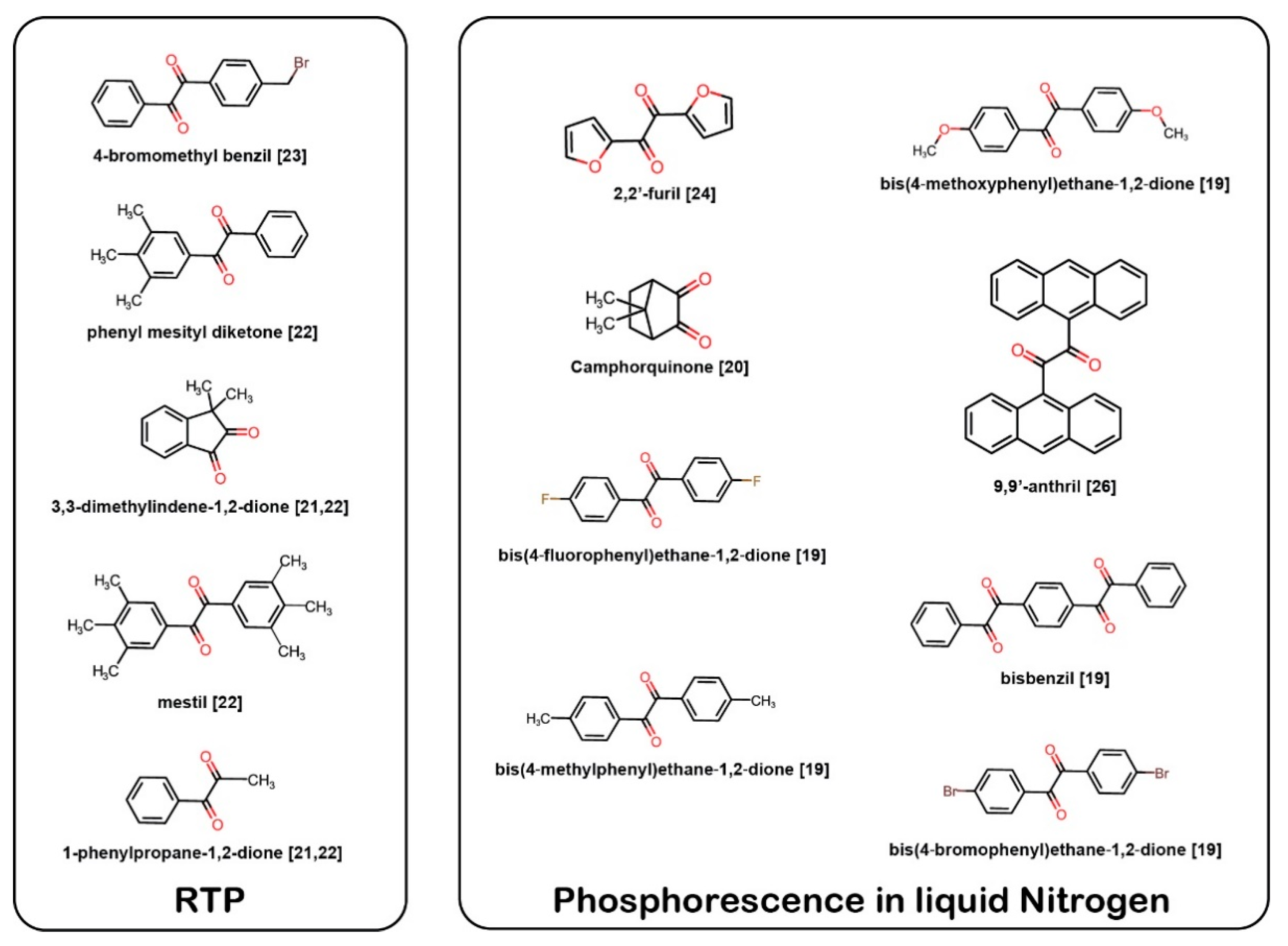
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Photostability
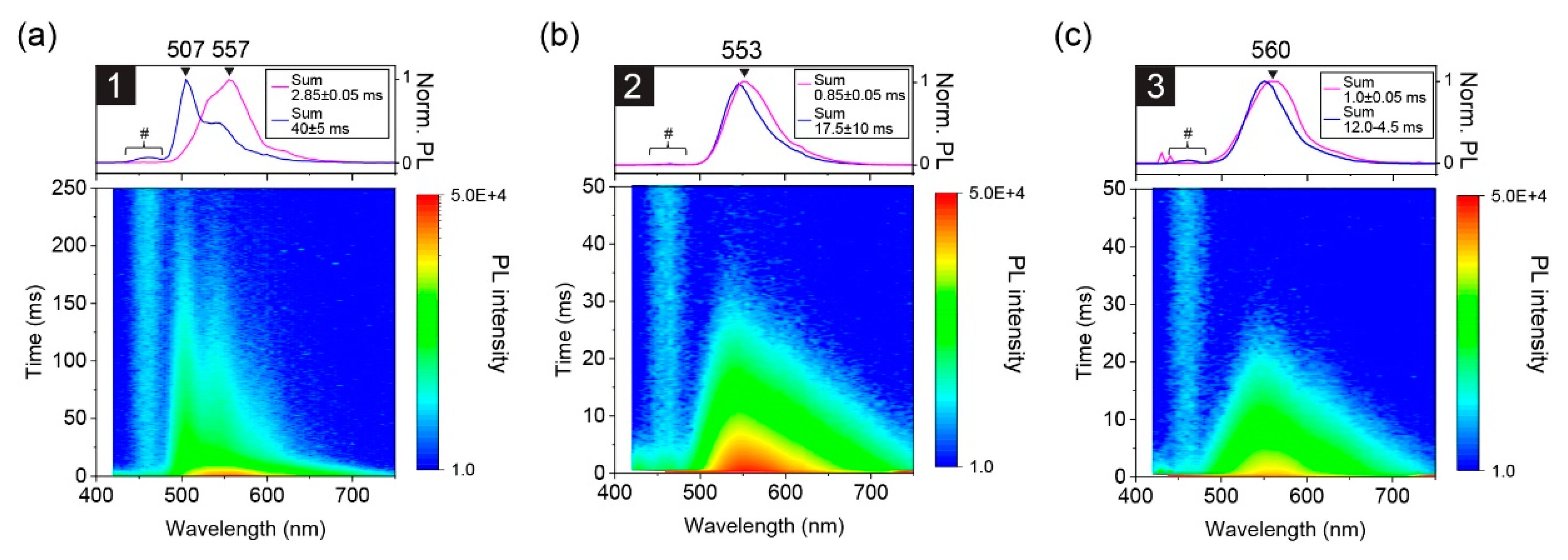
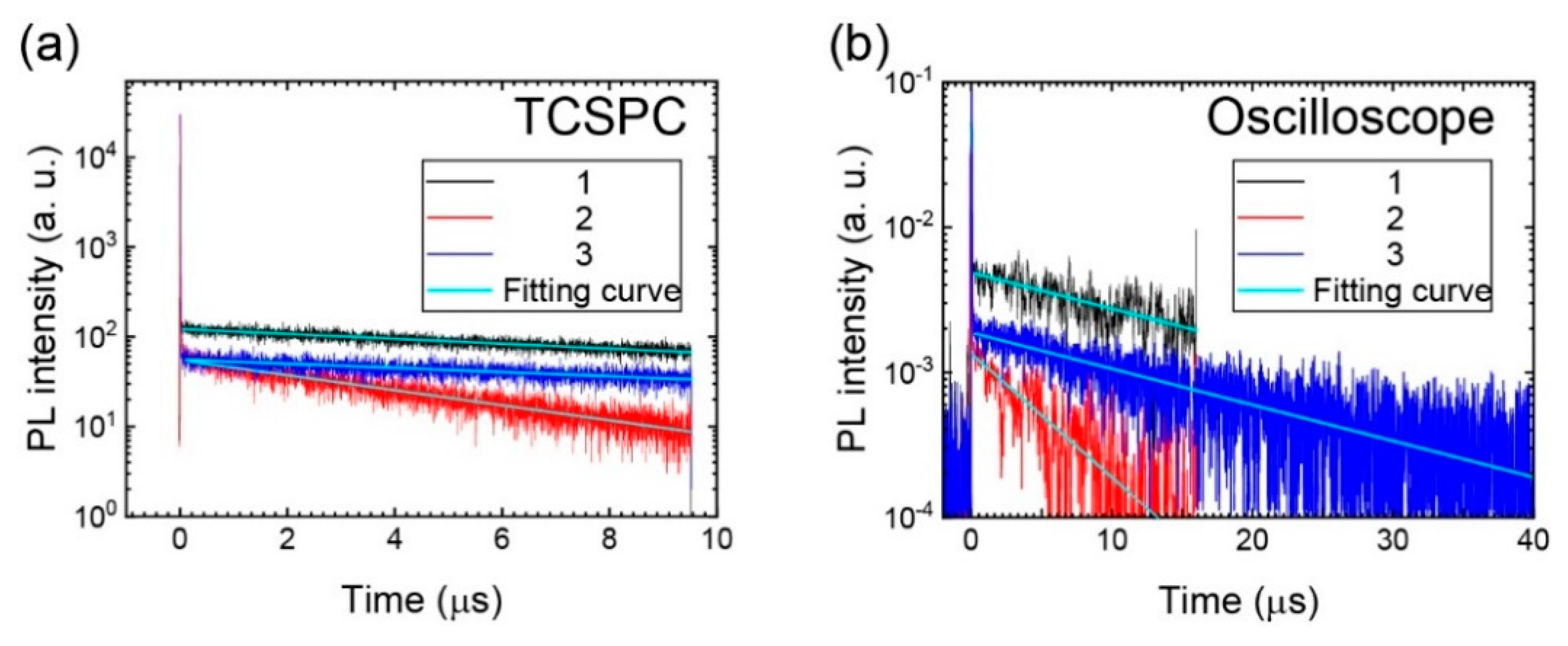
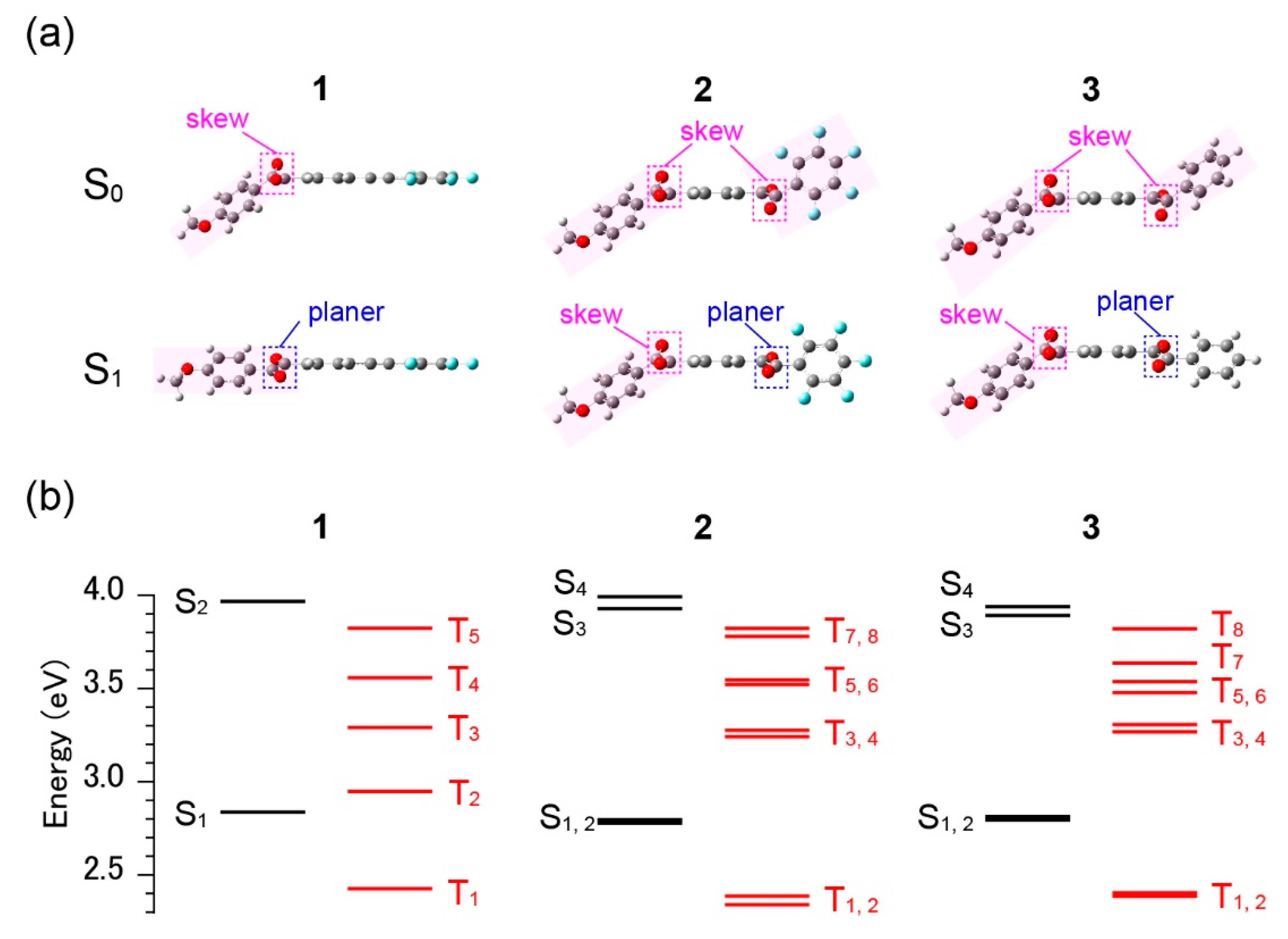
3.2. Excited-State Dynamics
4. Conclusions
- The lifetime of RTP for the present benzil derivatives is the microsecond regime.
- The monobenzil derivative possesses a longer lifetime and more intense RTP emission than the bisbenzil derivatives.
- Fluorination at one side of the phenyl groups of benzil does not significantly affect the behavior of the excited triplet state but shortened the lifetime of the excited singlet state.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Diaz-Garcia, M.E.; Fernandez-Gonzalez, A.; Badia-Laino, R. The triplet state: Emerging applications of room temperature phosphorescence spectroscopy. Appl. Spectrosco. Rev. 2007, 42, 605–624. [Google Scholar] [CrossRef]
- Haneder, S.; Da Como, E.; Feldmann, J.; Lupton, J.M.; Lennartz, C.; Erk, P.; Fuchs, E.; Molt, O.; Munster, I.; Schildknecht, C.; et al. Controlling the radiative rate of deep-blue electrophosphorescent organometallic complexes by singlet-triplet gap engineering. Adv. Mater. 2008, 20, 3325–3330. [Google Scholar] [CrossRef]
- Wu, W.; Sun, J.; Cui, X.; Zhao, J. Observation of the room temperature phosphorescence of Bodipy in visible light-harvesting Ru(ii) polyimine complexes and application as triplet photosensitizers for triplet–triplet-annihilation upconversion and photocatalytic oxidation. J. Mater. Chem. C 2013, 1, 4577–4589. [Google Scholar] [CrossRef]
- Fateminia, S.M.A.; Mao, Z.; Xu, S.; Yang, Z.; Chi, Z.; Liu, B. Organic Nanocrystals with Bright Red Persistent Room-Temperature Phosphorescence for Biological Applications. Angew. Chem. Int. Ed. 2017, 56, 12160–12164. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Cai, C.; Lin, H. Conversion of Carbon Dots from Fluorescence to Ultralong Room-Temperature Phosphorescence by Heating for Security Applications. Adv. Mater. 2018, 30, e1800783. [Google Scholar] [CrossRef]
- Green, D.C.; Holden, M.A.; Levenstein, M.A.; Zhang, S.; Johnson, B.R.G.; Gala de Pablo, J.; Ward, A.; Botchway, S.W.; Meldrum, F.C. Controlling the fluorescence and room-temperature phosphorescence behaviour of carbon nanodots with inorganic crystalline nanocomposites. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Bilen, C.S.; Harrison, N.; Morantz, D.J. Unusual Room-Temperature Afterglow in Some Crystalline Organic-Compounds. Nature 1978, 271, 235–237. [Google Scholar] [CrossRef]
- Hirata, Y.; Lim, E.C. Nonradiative Electronic Relaxation of Gas-Phase Aromatic Carbonyl-Compounds—Benzaldehyde. J. Chem. Phys. 1980, 72, 5505–5510. [Google Scholar] [CrossRef]
- Lei, Y.; Dai, W.; Tian, Y.; Yang, J.; Li, P.; Shi, J.; Tong, B.; Cai, Z.; Dong, Y. Revealing Insight into Long-Lived Room-Temperature Phosphorescence of Host−Guest Systems. J. Phys. Chem. Lett. 2019, 10, 6019–6025. [Google Scholar] [CrossRef]
- Donkerbroek, J.J.; Elzas, J.J.; Gooijer, C.; Frei, R.W.; Velthorst, N.H. Some Aspects of Room-Temperature Phosphorescence in Liquid Solutions. Talanta 1981, 28, 717–723. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Shen, X.Y.; Zhao, H.; Lam, J.W.Y.; Tang, L.; Lu, P.; Wang, C.L.; Liu, Y.; Wang, Z.M.; Zheng, Q.; et al. Crystallization-Induced Phosphorescence of Pure Organic Luminogens at Room Temperature. J. Phys. Chem. C 2010, 114, 6090–6099. [Google Scholar] [CrossRef]
- Kwon, M.S.; Yu, Y.; Coburn, C.; Phillips, A.W.; Chung, K.; Shanker, A.; Jung, J.; Kim, G.; Pipe, K.; Forrest, S.R.; et al. Suppressing molecular motions for enhanced room-temperature phosphorescence of metal-free organic materials. Nat. Commun. 2015, 6, 8947. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S. Recent Advances in Materials with Room-Temperature Phosphorescence: Photophysics for Triplet Exciton Stabilization. Adv. Opt. Mater. 2017, 5, 1700116. [Google Scholar] [CrossRef]
- Li, D.; Lu, F.; Wang, J.; Hu, W.; Cao, X.M.; Ma, X.; Tian, H. Amorphous Metal-Free Room-Temperature Phosphorescent Small Molecules with Multicolor Photoluminescence via a Host-Guest and Dual-Emission Strategy. J. Am. Chem. Soc. 2018, 140, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Liu, Z.; Bian, Z.; Huang, C. Recent Advances in Organic Light-Emitting Diodes Based on Pure Organic Room Temperature Phosphorescence Materials. Front. Chem 2019, 7, 305. [Google Scholar] [CrossRef]
- Zhao, W.J.; He, Z.K.; Lam, J.W.Y.; Peng, Q.; Ma, H.L.; Shuai, Z.G.; Bai, G.X.; Hao, J.H.; Tang, B.Z. Rational Molecular Design for Achieving Persistent and Efficient Pure Organic Room-Temperature Phosphorescence. Chem 2016, 1, 592–602. [Google Scholar] [CrossRef]
- Morantz, D.J.; Wright, A.J.C. Structures of the Excited States of Benzil and Related Dicarbonyl Molecules. J. Chem. Phys. 1971, 54, 692–697. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Tan, Y.Q.; Li, H.; Zhang, Y.R.; Yuan, W.Z.; Zhang, Y.M.; Sun, J.Z.; Tang, B.Z. Crystallization-induced phosphorescence of benzils at room temperature. Sci. China-Chem. 2013, 56, 1183–1186. [Google Scholar] [CrossRef]
- Tsai, L.; Charney, E. The triplet states of alpha-dicarbonyls. Camphorquinone. J. Phys. Chem. 1969, 73, 2462–2463. [Google Scholar] [CrossRef]
- Arnett, J.F.; Newkome, G.; Mattice, W.L.; McGlynn, S.P. Excited electronic states of the α-dicarbonyls. J. Am. Chem. Soc. 1974, 96, 4385–4392. [Google Scholar] [CrossRef]
- Arnett, J.F.; Mcglynn, S.P. Photorotamerism of Aromatic Alpha-Dicarbonyls. J. Phys. Chem. 1975, 79, 626–629. [Google Scholar] [CrossRef]
- Gebert, M.S.; Torkelson, J.M. Synthesis, Characterization and Photophysical Properties of Benzil-Labeled Polymers for Studies of Diffusion-Limited Interactions by Phosphorescence Quenching. Polymer 1990, 31, 2402–2410. [Google Scholar] [CrossRef]
- Singh, A.K.; Palit, D.K. Excited-state dynamics and photophysics of 2,2′-furil. Chem. Phys. Lett. 2002, 357, 173–180. [Google Scholar] [CrossRef]
- Singh, A.K.; Palit, D.K.; Mittal, J.P. Conformational relaxation dynamics in the excited electronic states of benzil in solution. Chem. Phys. Lett. 2002, 360, 443–452. [Google Scholar] [CrossRef]
- Kundu, P.; Chattopadhyay, N. Photophysics of Anthril in Fluids and Glassy Matrixes. J. Phys. Chem. A 2018, 122, 5545–5554. [Google Scholar] [CrossRef]
- Harris, C.B.; Ippen, E.P.; Mourou, G.A.; Zewail, A.H. Ultrafast Phenomena VII. Femtosecond Laser Photolysis Studies on the Conformation Change of Benzil in Solutions. In Springer Series in Chemical Physics; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Ikeda, N.; Koshioka, M.; Masuhara, H.; Yoshihara, K. Picosecond Dynamics of Excited Singlet-States in Organic Microcrystals—Diffuse Reflectance Laser Photolysis Study. Chem. Phys. Lett. 1988, 150, 452–456. [Google Scholar] [CrossRef]
- Khara, D.C.; Samanta, A. Fluorescence, Phosphorescence, and Delayed Fluorescence of Benzil in Imidazolium Ionic Liquids. Aust. J. Chem. 2012, 65, 1291–1297. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Jana, B.; Bose, D.; Chattopadhyay, N. Multiple emissions of benzil at room temperature and 77 K and their assignments from ab initio quantum chemical calculations. J. Chem. Phys. 2011, 134, 044535. [Google Scholar] [CrossRef]
- Yamada, S.; Higashida, T.; Wang, Y.; Morita, M.; Hosokai, T.; Maduwantha, K.; Koswattage, K.R.; Konno, T. Development of fluorinated benzils and bisbenzils as room-temperature phosphorescent molecules. Beilstein J. Org. Chem. 2020, 16, 1154–1162. [Google Scholar] [CrossRef]
- Roy, D.S.; Bhattacharyya, K.; Bera, S.C.; Chowdhury, M. Conformational relaxation in the excited electronic states of benzil and naphthyl. Chem. Phys. Lett. 1980, 69, 134–140. [Google Scholar] [CrossRef]
- Hirata, Y. Photophysical and photochemical primary processes of diphenylacetylene derivatives and related compounds in liquid phase. Bull. Chem. Soc. Jpn. 1999, 72, 1647–1664. [Google Scholar] [CrossRef]
- Wahadoszamen, M.; Hamada, T.; Iimori, T.; Nakabayashi, T.; Ohta, N. External electric field effects on absorption, fluorescence, and phosphorescence spectra of diphenylpolyynes in a polymer film. J. Phys. Chem. A 2007, 111, 9544–9552. [Google Scholar] [CrossRef] [PubMed]
- Menning, S.; Kramer, M.; Coombs, B.A.; Rominger, F.; Beeby, A.; Dreuw, A.; Bunz, U.H. Twisted tethered tolanes: Unanticipated long-lived phosphorescence at 77 K. J. Am. Chem. Soc. 2013, 135, 2160–2163. [Google Scholar] [CrossRef]
- Agneeswari, R.; Tamilavan, V.; Hyun, M.H. Synthesis and Characterization of 1,2,4-Oxadiazole-Based Deep-Blue and Blue Color Emitting Polymers. Bull. Korean Chem. Soc. 2014, 35, 513–517. [Google Scholar] [CrossRef][Green Version]
- Kramer, M.; Bunz, U.H.; Dreuw, A. Comprehensive Look at the Photochemistry of Tolane. J. Phys. Chem. A 2017, 121, 946–953. [Google Scholar] [CrossRef]
- Tomin, V.I.; Wlodarkiewicz, A. Anti-Kasha behavior of DMABN dual fluorescence. JLum 2018, 198, 220–225. [Google Scholar] [CrossRef]
- Itoh, T. Fluorescence and Phosphorescence from Higher Excited States of Organic Molecules. Chem. Rev. 2012, 112, 4541–4568. [Google Scholar] [CrossRef]
- Zhou, Y.; Baryshnikov, G.; Li, X.; Zhu, M.; Ågren, H.; Zhu, L. Anti-Kasha’s Rule Emissive Switching Induced by Intermolecular H-Bonding. Chem. Mater. 2018, 30, 8008–8016. [Google Scholar] [CrossRef]
- Katoh, R.; Kotani, M.; Hirata, Y.; Okada, T. Triplet exciton formation in a benzophenone single crystal studied by picosecond time-resolved absorption spectroscopy. Chem. Phys. Lett. 1997, 264, 631–635. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maduwantha, K.; Yamada, S.; Koswattage, K.R.; Konno, T.; Hosokai, T. Excited-State Dynamics of Room-Temperature Phosphorescent Organic Materials Based on Monobenzil and Bisbenzil Frameworks. Materials 2020, 13, 3904. https://doi.org/10.3390/ma13173904
Maduwantha K, Yamada S, Koswattage KR, Konno T, Hosokai T. Excited-State Dynamics of Room-Temperature Phosphorescent Organic Materials Based on Monobenzil and Bisbenzil Frameworks. Materials. 2020; 13(17):3904. https://doi.org/10.3390/ma13173904
Chicago/Turabian StyleMaduwantha, Kaveendra, Shigeyuki Yamada, Kaveenga Rasika Koswattage, Tsutomu Konno, and Takuya Hosokai. 2020. "Excited-State Dynamics of Room-Temperature Phosphorescent Organic Materials Based on Monobenzil and Bisbenzil Frameworks" Materials 13, no. 17: 3904. https://doi.org/10.3390/ma13173904
APA StyleMaduwantha, K., Yamada, S., Koswattage, K. R., Konno, T., & Hosokai, T. (2020). Excited-State Dynamics of Room-Temperature Phosphorescent Organic Materials Based on Monobenzil and Bisbenzil Frameworks. Materials, 13(17), 3904. https://doi.org/10.3390/ma13173904






