Interface Structures and Electronic States of Epitaxial Tetraazanaphthacene on Single-Crystal Pentacene
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
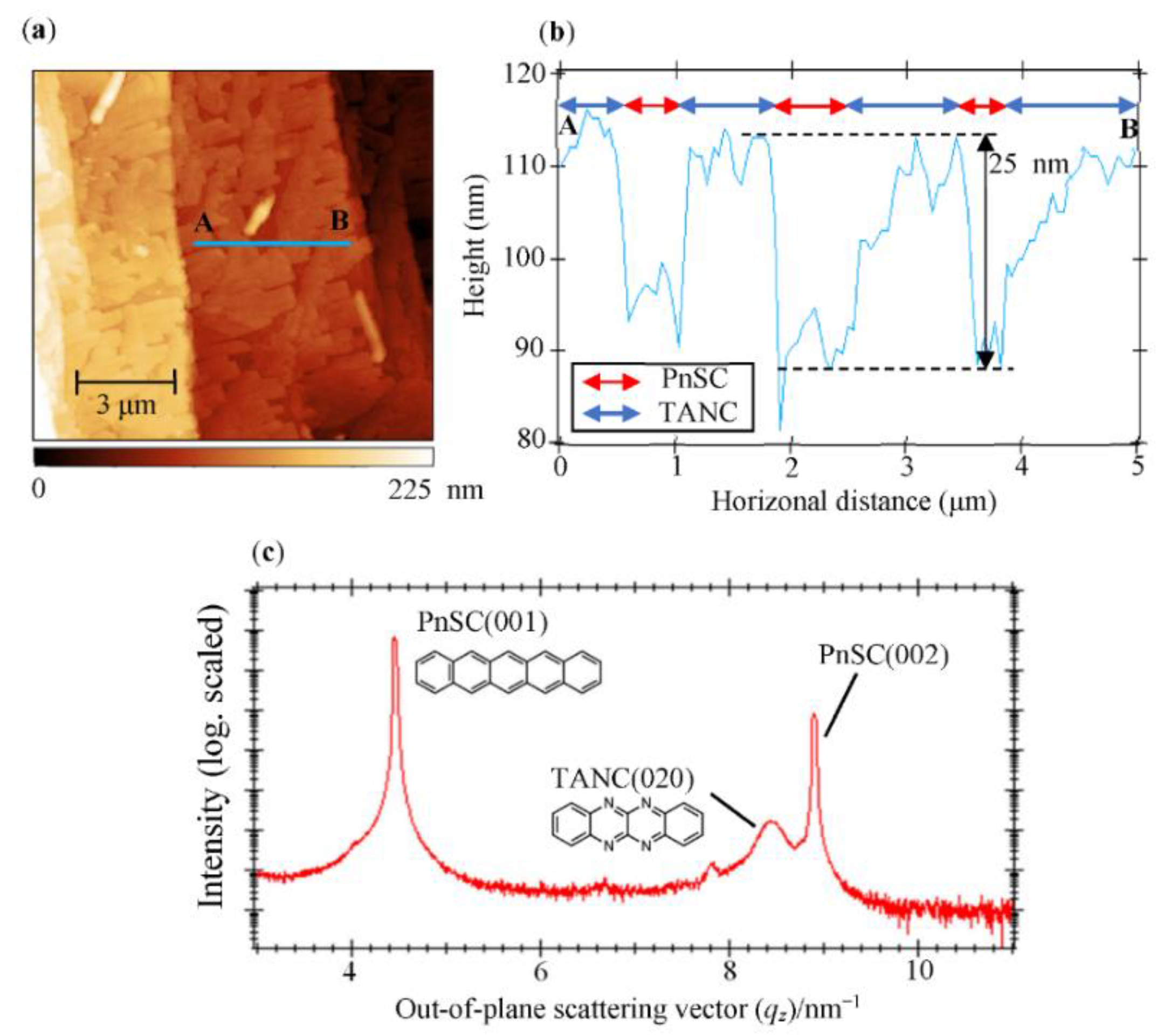
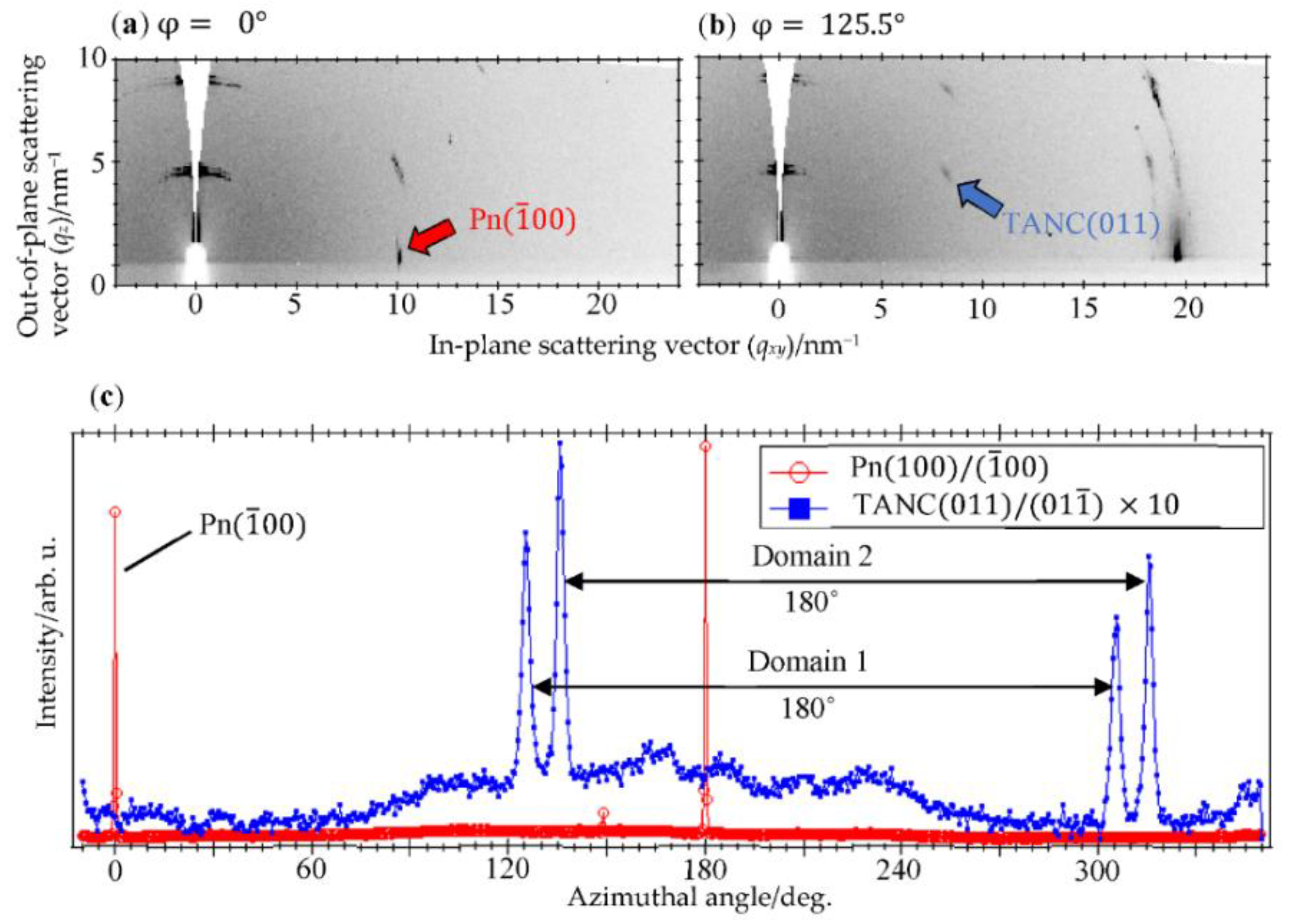
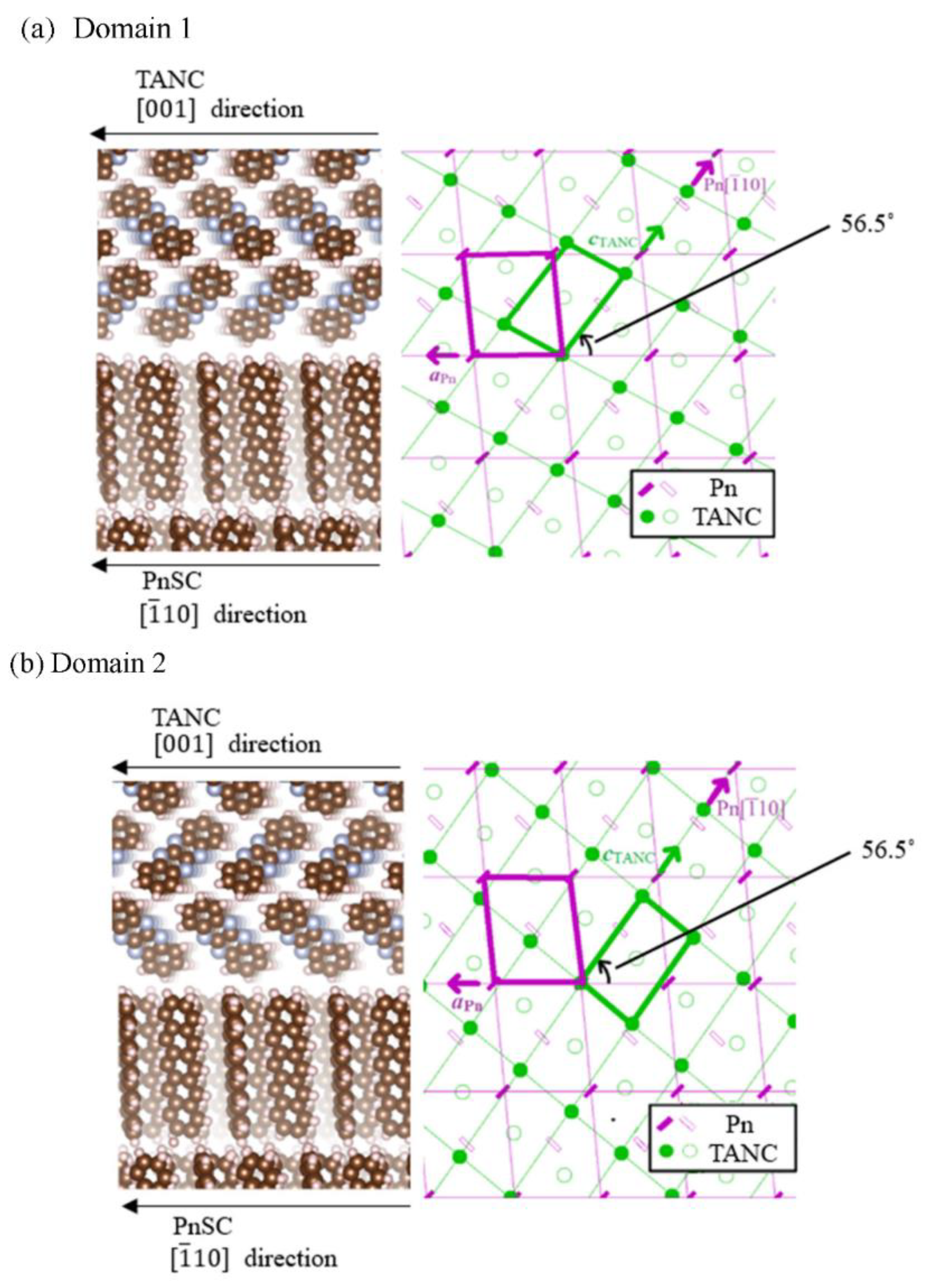
3.1. Crystallographic Analyses
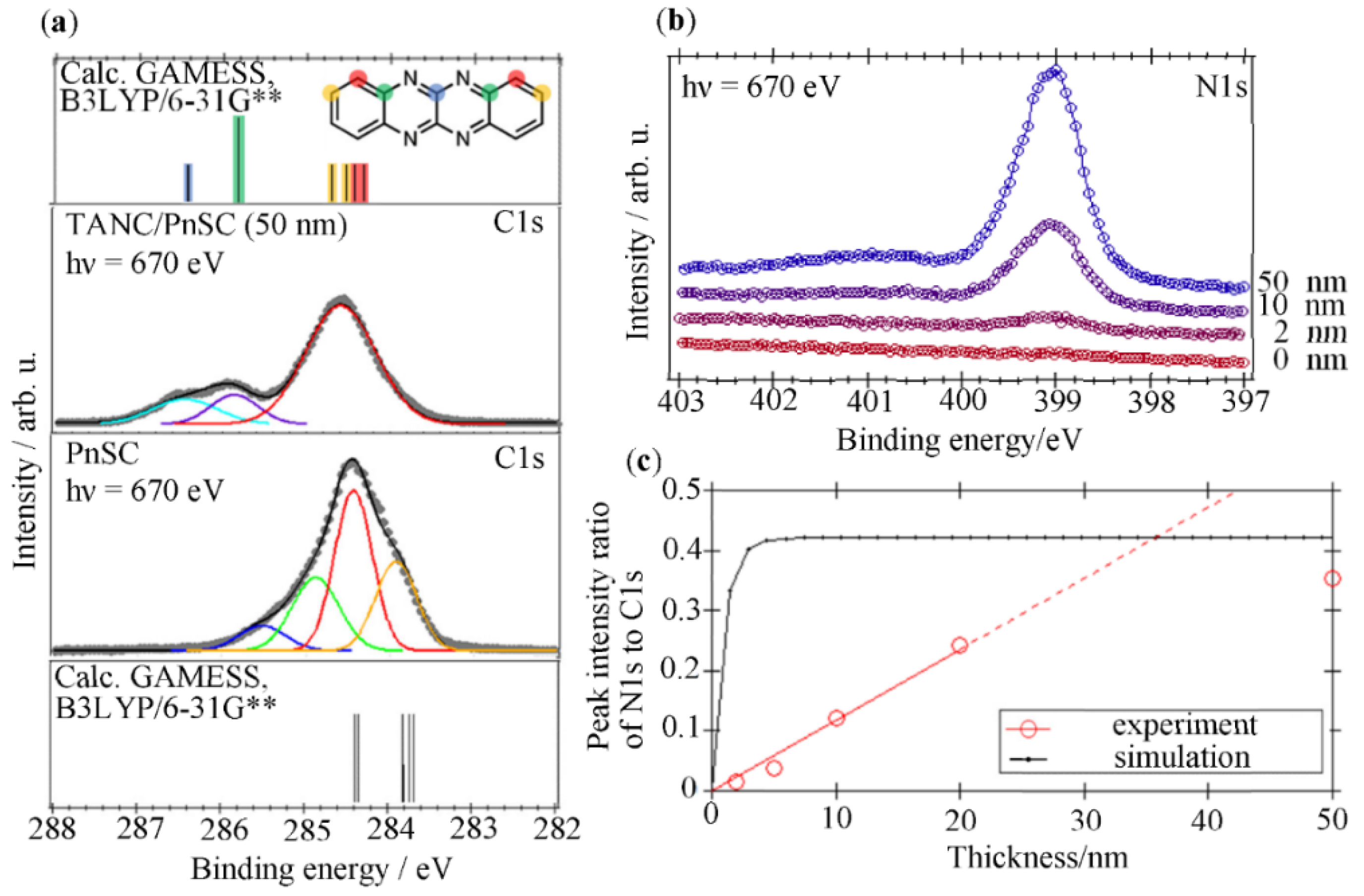
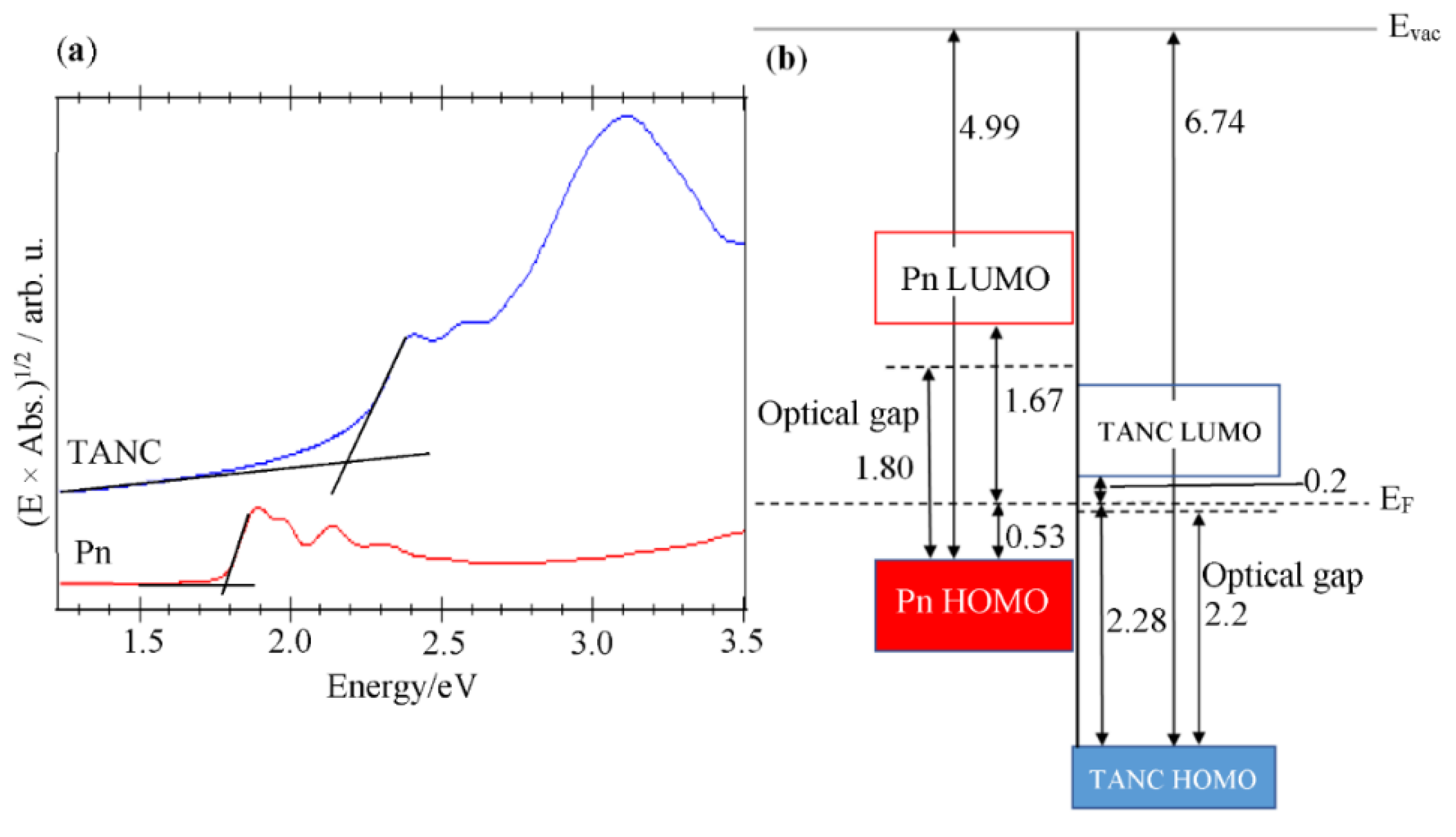
3.2. Electronic Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jurchescu, O.D.; Baas, J.; Palstra, T.T.M. Effect of impurities on the mobility of single crystal pentacene. Appl. Phys. Lett. 2004, 84, 3061–3063. [Google Scholar] [CrossRef]
- Nakayama, Y.; Mizuno, Y.; Hikasa, M.; Yamamoto, M.; Matsunami, M.; Ideta, S.; Tanaka, K.; Ishii, H.; Ueno, N. Single-Crystal Pentacene Valence-Band Disperion and Its Temperature Dependance. J. Phys. Chem. Lett. 2017, 8, 1259–1264. [Google Scholar] [CrossRef]
- Nakayama, Y.; Hikasa, M.; Moriya, N.; Meissner, M.; Yamaguchi, T.; Yoshida, K.; Murata, M.; Mase, K.; Ueba, T.; Kera, S. Anisotropic valence band dispersion of single crystal pentacene as measured by angle-resolved ultraviolet photoelectron spectroscopy. J. Mater. Res. 2018, 33, 3362–3370. [Google Scholar] [CrossRef]
- Rao, A.; Wilson, M.W.B.; Hodgkiss, J.M.; Albert-Seifried, S.; Bässler, H.; Friend, R.H. Exciton Fission and Charge Generation via Triplet Excitons in Pentacene/C60 Bilayers. J. Am. Chem. Soc. 2010, 132, 12698–12703. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.W.B.; Rao, A.; Ehrler, B.; Friend, R.H. Singlet Exciton Fission in Polycrystalline Pentacene: From Photophysics toward Devices. Acc. Chem. Res. 2013, 46, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, M.; Tsuruta, R.; Nakayama, Y.; Sasaki, M.; Hosokai, T.; Lee, S.; Fukumoto, K.; Yamada, Y. Exciton Dissociation and Electron Transfer at a Well-Defined Organic Interface of an Epitaxial C60 layer on a Pentacene Single Crystal. J. Phys. Chem. C 2020, 124, 13572–13579. [Google Scholar] [CrossRef]
- Tadokoro, M.; Yasuzuka, S.; Nakamura, M.; Shinoda, T.; Tatenuma, T.; Mitsumi, M.; Ozawa, Y.; Toriumi, K.; Yoshino, H.; Shiomi, D.; et al. A High-Conductivity Crystal Containing a Copper(I) Coordination Polymer Bridged by the Organic Acceptor TANC. Angew. Chem. Int. Ed. 2006, 45, 5144–5147. [Google Scholar] [CrossRef]
- Isoda, K.; Nakamura, M.; Tatenuma, T.; Ogata, H.; Sugaya, T.; Tadokoro, M. Synthesis and Characterization of Electron-accepting Nonsubstituted Tetraazaacene Derivatives. Chem. Lett. 2012, 41, 937–939. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Suzuki, T.; Kobayashi, M.; Gao, Y.; Fukai, Y.; Inoue, Y.; Sato, F.; Tokito, S. Perfluoropentacene: High-Performance p−n Junctions and Complementary Circuits with Pentacene. J. Am. Chem. Soc. 2004, 126, 8138–8140. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Prabhumirashi, P.L.; Nakayama, Y.; McGarry, K.; Geier, M.L.; Uragami, Y.; Mase, K.; Douglas, C.J.; Ishii, H.; Hersam, M.C.; et al. Utilizing Carbon Nanotube Electrodes to Improve Charge Injection and Transport in Bis(trifluoromethyl)-dimethyl-rubrene Ambipolar Single Crystal Transistors. ACS Nano 2013, 7, 10245–10256. [Google Scholar] [CrossRef]
- Kamebuchi, H.; Kobayashi, Y.; Mutoh, K.; Nakajima, S.; Enomoto, Y.; Tadokoro, M. Synthesis of Pyridine-fused 5,6,11,12-Tetraazanaphthacene as a Bis-bidentate Ligand and Electrochemistry of a Ruthenium(II) Dinuclear Complex. Chem. Lett. 2020, 49, 1367–1371. [Google Scholar] [CrossRef]
- Richards, G.J.; Hill, J.P.; Subbaiyan, N.K.; D’Souza, F.; Karr, P.A.; Elsegood, M.R.J.; Teat, S.J.; Mori, T.; Ariga, K. Pyrazinacenes: Aza Analogues of Acenes. J. Org. Chem. 2009, 74, 8914–8923. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, H.; Yan, D. Efficient organic photovoltaic cells with vertically ordered bulk heterojunctions. Nanotechnology 2013, 24, 484006. [Google Scholar] [CrossRef]
- Feenstra, J.; Eerden, M.V.; Lemmens, A.K.; Poel, W.D.; Kouwer, P.H.J.; Rowan, A.E.; Schermer, J.J. Muscovite mica as a growth template of PC61BM crystallites for organic photovoltaics. CrystEngComm 2017, 19, 4424–4436. [Google Scholar] [CrossRef]
- Nakayama, Y.; Mizuno, Y.; Hosokai, T.; Koganezawa, T.; Tsuruta, R.; Hinderhofer, A.; Gerlach, A.; Broch, K.; Belova, V.; Frank, H.; et al. Epitaxial Growth of an Organic p–n Heterojunction: C60 on Single-Crystal Pentacene. ACS Appl. Mater. Interfaces 2016, 8, 13499–13505. [Google Scholar] [CrossRef]
- Nakayama, Y.; Tsuruta, R.; Hinderhofer, A.; Mizuno, Y.; Broch, K.; Gerlach, A.; Koganezawa, T.; Hosokai, T.; Schreiber, F. Temperature Dependent Epitaxial Growth of C60 Overlayers on Single Crystal Pentacene. Adv. Mater. Interfaces 2018, 5, 1800084. [Google Scholar] [CrossRef]
- Tsuruta, R.; Mizuno, Y.; Hosokai, T.; Koganezawa, T.; Ishii, H.; Nakayama, Y. Crystallinity of the epitaxial heterojunction of C60 on single crystal pentacene. J. Cryst. Growth 2017, 468, 770–773. [Google Scholar] [CrossRef]
- Tsuruta, R.; Hosokai, T.; Yamanaka, S.; Yoshida, K.; Mizuno, Y.; Koganezawa, T.; Nakauama, Y. Evolution of crystallinity at a well-defined molecular interface of epitaxial C60 on the single crystal rubrene. J. Phys. Condens. Matter 2019, 31, 154001. [Google Scholar] [CrossRef]
- Nakayama, Y.; Tsuruta, Y.; Moriya, N.; Hikasa, M.; Meissner, M.; Yamaguchi, T.; Mizuno, Y.; Suzuki, T.; Koganezawa, T.; Hosokai, T.; et al. Widely Dispersed Intermolecular Valence Bands of Epitaxially Grown Perfluoropentacene on Pentacene Single Crystals. J. Phys. Chem. Lett. 2019, 10, 1312–1318. [Google Scholar] [CrossRef]
- Nakayama, Y.; Uragami, Y.; Yamamoto, M.; Yonezawa, K.; Mase, K.; Kera, S.; Ishii, H.; Ueno, N. High-resolution core-level photoemission measurements on the pentacene single crystal surface assisted by photoconduction. J. Phys. Condens. Matter 2016, 28, 094001. [Google Scholar] [CrossRef]
- Nakayama, Y.; Uragami, Y.; Yamamoto, M.; Machida, S.; Kinjo, H.; Mase, K.; Koswattage, K.R.; Ishii, H. Determination of the highest occupied molecular orbital energy of pentacene single crystals by ultraviolet photoelectron and photoelectron yield spectroscopies. Jpn. J. Appl. Phys. 2014, 53, 01AD03. [Google Scholar] [CrossRef]
- Sankarranarayanan, K.; Kavitha, C.; Mohan, M.L.N.M. Design, synthesis and characterization of hydrogen bonded thermotropic liquid crystals. Mol. Cryst. Liq. Cryst. 2017, 648, 35–52. [Google Scholar] [CrossRef]
- Toyoshima, A.; Kikuchi, T.; Tanaka, H.; Mase, K.; Amemiya, K.; Ozawa, K. Performance of PF BL-13A, a vacuum ultraviolet and soft X-ray undulator beamline for studying organic thin films adsorbed on surfaces. J. Phys. Conf. Ser. 2013, 425, 152019. [Google Scholar] [CrossRef]
- Machida, S.; Nakayama, Y.; Duhm, S.; Xin, Q.; Funakoshi, A.; Ogawa, N.; Kera, S.; Ueno, N.; Ishii, H. Highest-Occupied-Molecular-Orbital Band Dispersion of Rubrene Single Crystals as Observed by Angle-Resolved Ultraviolet Photoelectron Spectroscopy. Phys. Rev. Lett. 2010, 104, 156401. [Google Scholar] [CrossRef]
- Nakayama, Y.; Kera, S.; Ueno, N. Photoelectron spectroscopy on single crystals of organic semiconductors: Experimental electronic band structure for optoelectronic properties. J. Mater. Chem. C 2020, 8, 9090–9132. [Google Scholar] [CrossRef]
- Yamanaka, S.; Hayakawa, K.; Cojocaru, L.; Tsuruta, R.; Sato, T.; Mase, K.; Uchida, S.; Nakayama, Y. Electronic structures and chemical states of methylammonium lead triiodide thin films and the impact of annealing and moisture exposure. J. Appl. Phys. 2018, 123, 165501. [Google Scholar] [CrossRef]
- Powell, C.J. Recommended Auger parameters for 42 elemental solids. J. Electron Spectrosc. Relat. Phenom. 2012, 185, 1–3. [Google Scholar] [CrossRef]
- Itaka, K.; Yamashiro, M.; Yamaguchi, J.; Haemori, M.; Yaginuma, S.; Matsumoto, Y.; Kondo, M.; Koinuma, H. High-Mobility C60 Field-Effect Transistors Fabricated on Molecular- Wetting Controlled Substrates. Adv. Mater. 2006, 18, 1713–1716. [Google Scholar] [CrossRef]
- Salaneck, W.R. Intermolecular Relaxation Energies in Anthracene. Phys. Rev. Lett. 1978, 40, 60–63. [Google Scholar] [CrossRef]
- Harada, Y.; Ozaki, H.; Ohno, K. Selective Observation of Outermost Surface Layer during Epitaxial Growth by Penning-Ionization Electron Spectroscopy: Pentacene on Graphite. Phys. Rev. Lett. 1984, 52, 2269–2272. [Google Scholar] [CrossRef]
- Hino, S.; Kato, M.; Nakamura, M.; Tadokoro, M. Photoelectron Spectroscopy of a New Type Electron Acceptor, 5,6,11,12-Tetraazanaphthacene. Mol. Cryst. Liq. Cryst. 2006, 455, 205–209. [Google Scholar] [CrossRef]
- Yeh, J.J.; Lindau, I. Atomic subshell photoionization cross sections and asymmetry parameters: 1 ⩽ Z ⩽ 103. At. Data Nucl. Data Tables 1985, 32, 1–155. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. V. Data for 14 organic compounds over the 50–2000 eV range. Surf. Interface Anal. 1994, 21, 165–176. [Google Scholar] [CrossRef]
- Mattheus, C.C.; Dros, A.B.; Baas, J.; Meestma, A.; de Boer, J.L.; Palstra, T.T.M. Polymorphism in pentacene. Acta Cryst. Sect. C Cryst. Struct. Commun. 2001, C57, 939–941. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Han, W.; Yoshida, H.; Ueno, N.; Kera, S. Electron affinity of pentacene thin film studied by radiation-damage free inverse photoemission spectroscopy. Appl. Phys. Lett. 2013, 103, 123303. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nakayama, Y.; Uragami, Y.; Kinjo, Y.; Mizuno, Y.; Mase, K.; Koswattage, K.R.; Ishii, H. Electronic Structures of a Well-Defined Organic Hetero-Interface: C60 on Pentacene Single Crystal. e-J. Surf. Sci. Nanotechnol. 2015, 13, 59–64. [Google Scholar] [CrossRef]
- Yoo, S.; Domercq, B.; Kippelen, B. Efficient thin-film organic solar cells based on pentacene/C60 heterojunctions. Appl. Phys. Lett. 2004, 85, 5427–5429. [Google Scholar] [CrossRef]
- Kikuchi, M.; Hirota, M.; Kunawong, T.; Shinmura, Y.; Abe, M.; Sadamitsu, Y.; Moh, A.M.; Izawa, S.; Izaki, M.; Naito, H.; et al. Lateral Alternating Donor/Acceptor Multilayered Junction for Organic Solar Cells. ACS Appl. Energy Mater. 2019, 2, 2087–2093. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunjo, Y.; Kamebuchi, H.; Tsuruta, R.; Iwashita, M.; Takahashi, K.; Takeuchi, R.; Kanai, K.; Koganezawa, T.; Mase, K.; Tadokoro, M.; et al. Interface Structures and Electronic States of Epitaxial Tetraazanaphthacene on Single-Crystal Pentacene. Materials 2021, 14, 1088. https://doi.org/10.3390/ma14051088
Gunjo Y, Kamebuchi H, Tsuruta R, Iwashita M, Takahashi K, Takeuchi R, Kanai K, Koganezawa T, Mase K, Tadokoro M, et al. Interface Structures and Electronic States of Epitaxial Tetraazanaphthacene on Single-Crystal Pentacene. Materials. 2021; 14(5):1088. https://doi.org/10.3390/ma14051088
Chicago/Turabian StyleGunjo, Yuki, Hajime Kamebuchi, Ryohei Tsuruta, Masaki Iwashita, Kana Takahashi, Riku Takeuchi, Kaname Kanai, Tomoyuki Koganezawa, Kazuhiko Mase, Makoto Tadokoro, and et al. 2021. "Interface Structures and Electronic States of Epitaxial Tetraazanaphthacene on Single-Crystal Pentacene" Materials 14, no. 5: 1088. https://doi.org/10.3390/ma14051088
APA StyleGunjo, Y., Kamebuchi, H., Tsuruta, R., Iwashita, M., Takahashi, K., Takeuchi, R., Kanai, K., Koganezawa, T., Mase, K., Tadokoro, M., & Nakayama, Y. (2021). Interface Structures and Electronic States of Epitaxial Tetraazanaphthacene on Single-Crystal Pentacene. Materials, 14(5), 1088. https://doi.org/10.3390/ma14051088





