Effect of BMP-2 Adherent to Resorbable Sutures on Cartilage Repair: A Rat Model of Xyphoid Process
Abstract
1. Introduction
2. Materials and Methods
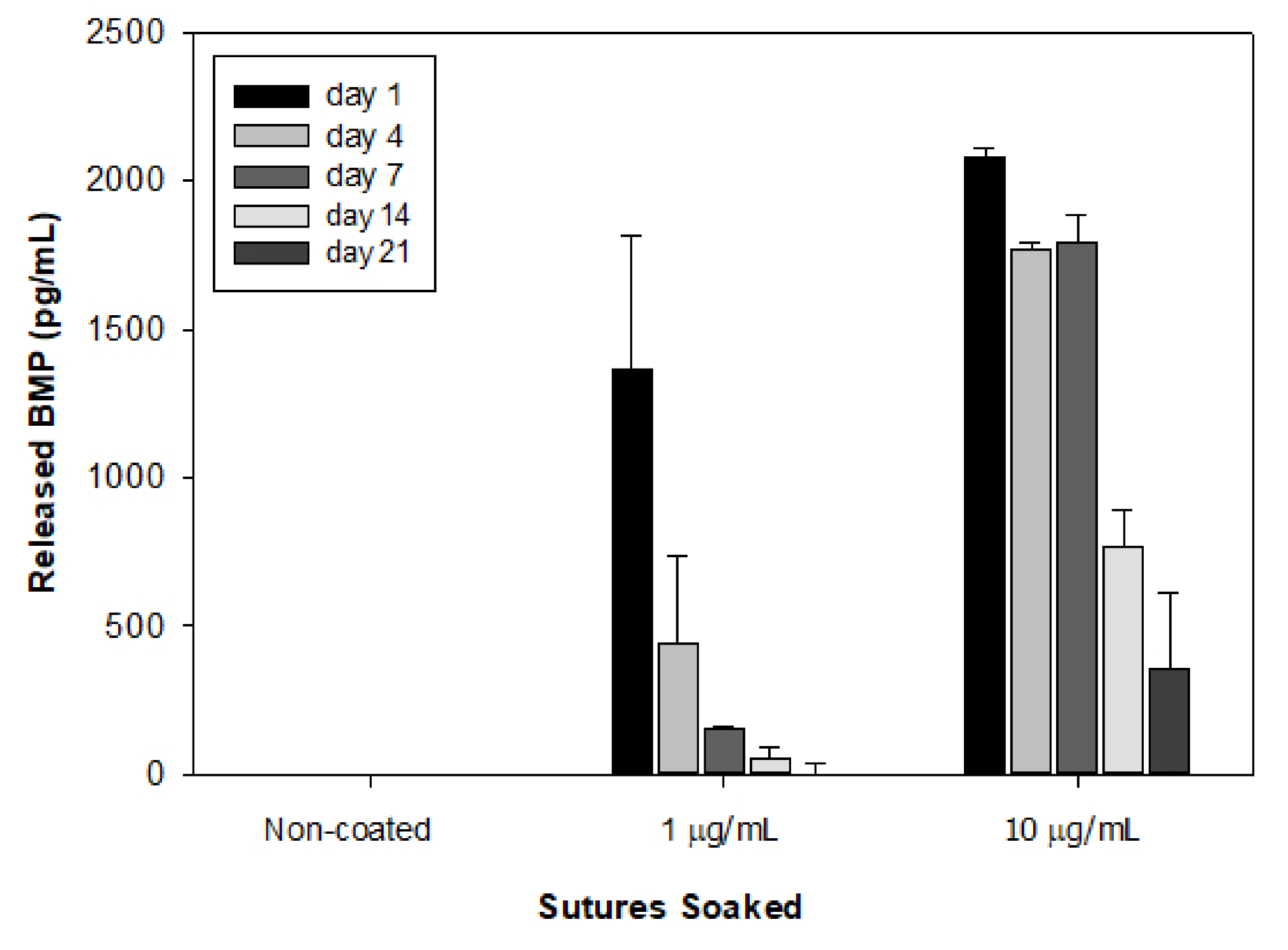
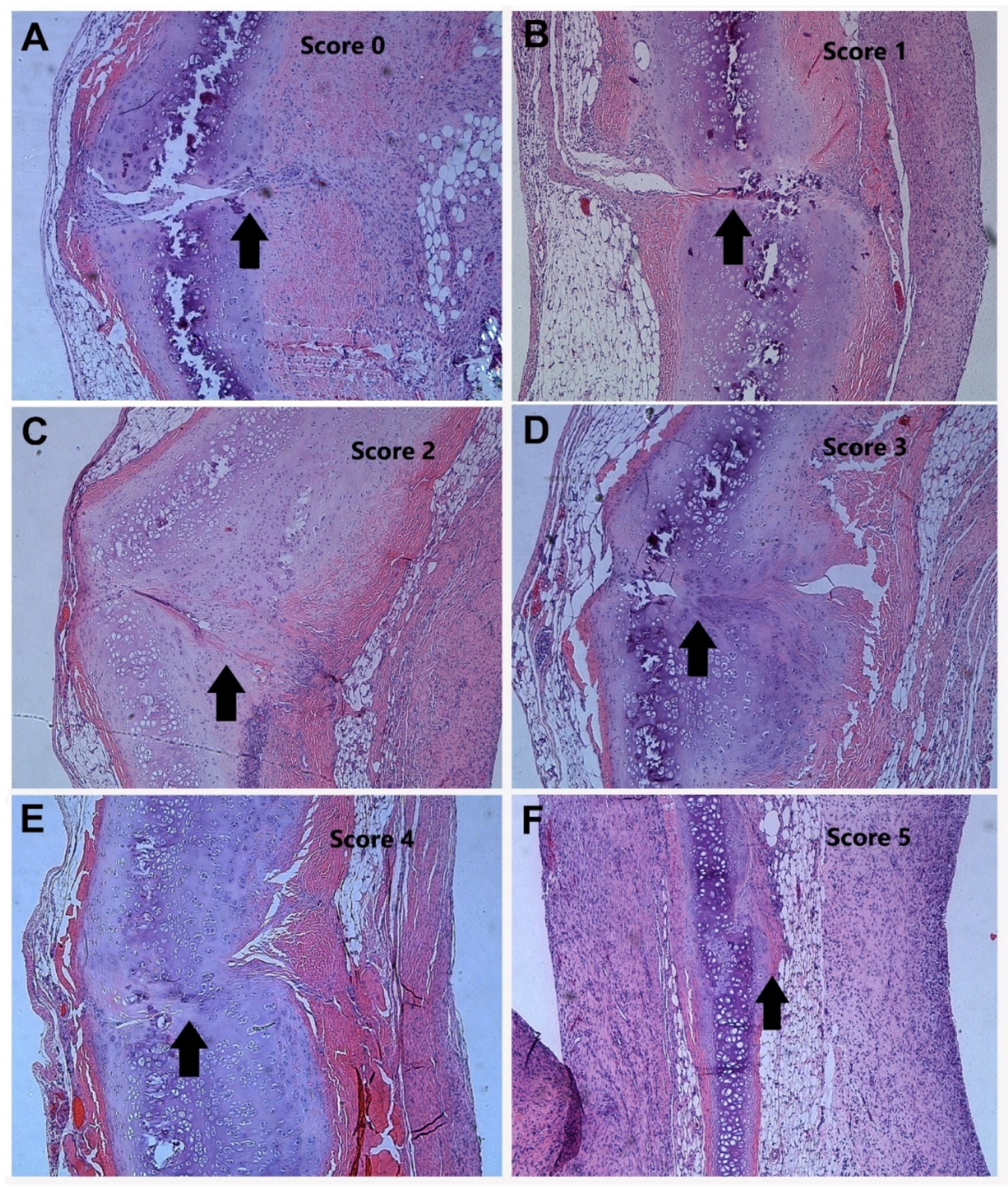
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giuliani, J.R.; Burns, T.C.; Svoboda, S.J.; Cameron, K.L.; Owens, B.D. Treatment of meniscal injuries in young athletes. J. Knee Surg. 2011, 24, 93–100. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Cook, J.L.; Carter, T.; Turner, A.S. Translational models for studying meniscal repair and replacement: What they can and cannot tell us. Tissue Eng. Part. B Rev. 2010, 16, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bedi, A.; Kelly, N.H.; Baad, M.; Fox, A.J.; Brophy, R.H.; Warren, R.F.; Maher, S.A. Dynamic contact mechanics of the medial meniscus as a function of radial tear, repair, and partial meniscectomy. J. Bone Joint Surg. Am. 2010, 92, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.E.; Arnoczky, S.P.; Warren, R.F. Meniscal repair. Clin. Sports Med. 1991, 10, 529–548. [Google Scholar] [CrossRef]
- Laible, C.; Stein, D.A.; Kiridly, D.N. Meniscal repair. J. Am. Acad. Orthop. Surg. 2013, 21, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Paxton, E.S.; Stock, M.V.; Brophy, R.H. Meniscal repair versus partial meniscectomy: A systematic review comparing reoperation rates and clinical outcomes. Arthroscopy 2011, 27, 1275–1288. [Google Scholar] [CrossRef]
- Gu, Y.L.; Wang, Y.B. Treatment of meniscal injury: A current concept review. Chin. J. Trauma. 2010, 13, 370–376. [Google Scholar]
- Scott, G.A.; Jolly, B.L.; Henning, C.E. Combined posterior incision and arthroscopic intra-articular repair of the meniscus. An examination of factors affecting healing. J. Bone Joint Surg. Am. 1986, 68, 847–861. [Google Scholar] [CrossRef]
- Cannon, W.D.; Vittori, J.M. The incidence of healing in arthroscopic meniscal repairs in anterior cruciate ligament-reconstructed knees versus stable knees. Am. J. Sports Med. 1992, 20, 176–781. [Google Scholar] [CrossRef]
- Ochi, M.; Uchio, Y.; Okuda, K.; Shu, N.; Yamaguchi, H.; Sakai, Y. Expression of cytokines after meniscal rasping to promote meniscal healing. Arthroscopy 2001, 17, 724–731. [Google Scholar] [CrossRef]
- Uchio, Y.; Ochi, M.; Adachi, N.; Kawasaki, K.; Iwasa, J. Results of rasping of meniscal tears with and without anterior cruciate ligament injury as evaluated by second-look arthroscopy. Arthroscopy 2003, 19, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yuan, M.; Meng, H.Y.; Wang, A.Y.; Guo, Q.Y.; Wang, Y.; Peng, J. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: A review. Osteoarthr. Cartil. 2013, 21, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Vayas, R.; Reyes, R.; Arnau, M.R.; Evora, C.; Delgado, A. Injectable Scaffold for Bone Marrow Stem Cells and Bone Morphogenetic Protein-2 to Repair Cartilage. Cartilage 2019. [Google Scholar] [CrossRef] [PubMed]
- Haversath, M.; Catelas, I.; Li, X.; Tassemeier, T.; Jager, M. PGE(2) and BMP-2 in bone and cartilage metabolism: 2 intertwining pathways. Can. J. Physiol. Pharmacol. 2012, 90, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Deng, J.; Yan, L.; Huang, W. Evaluation of the effects of the combination of BMP-2-modified BMSCs and PRP on cartilage defects. Exp. Ther. Med. 2018, 16, 4569–4577. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, E.; Thompson, E.M.; Matsiko, A.; O’Brien, F.J.; Lopez-Noriega, A. Long-term controlled delivery of rhBMP-2 from collagen-hydroxyapatite scaffolds for superior bone tissue regeneration. J. Control. Release 2015, 207, 112–119. [Google Scholar] [CrossRef]
- Ben-Amara, H.; Lee, J.W.; Kim, J.J.; Kang, Y.M.; Kang, E.J.; Koo, K.T. Influence of rhBMP-2 on Guided Bone Regeneration for Placement and Functional Loading of Dental Implants: A Radiographic and Histologic Study in Dogs. Int. J. Oral. Maxillofac. Implants 2017, 32, e265–e276. [Google Scholar] [CrossRef][Green Version]
- Guzman, R.C.; Saul, J.M.; Ellenburg, M.D.; Merrill, M.R.; Coan, H.B.; Smith, T.L.; Van-Dyke, M.E. Bone regeneration with BMP-2 delivered from keratose scaffolds. Biomaterials 2013, 34, 1644–1656. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Lippuner, K.; Keel, M.J.; Shintani, N. Novel organ-slice culturing system to simulate meniscal repair: Proof of concept using a synovium-based pool of meniscoprogenitor cells. J. Orthop. Res. 2016, 34, 1588–1596. [Google Scholar] [CrossRef]
- Zhang, S.; Matsushita, T.; Kuroda, R.; Nishida, K.; Matsuzaki, T.; Matsumoto, T.; Takayama, K.; Nagai, K.; Oka, S.; Tabata, Y.; et al. Local Administration of Simvastatin Stimulates Healing of an Avascular Meniscus in a Rabbit Model of a Meniscal Defect. Am. J. Sports Med. 2016, 44, 1735–1743. [Google Scholar] [CrossRef]
- Tessaro, I.; Giancamillo, A.; Benasciutti, E.; Nguyen, V.T.; Polito, U.; Mangiavini, L.; Peretti, G.M. Characterization of different in vitro culture conditions to induce a fibro-chondrogenic differentiation of swine adipose-derived stem cells. J. Biol. Regul. Homeost. Agents 2018, 32, 97–103. [Google Scholar] [PubMed]
- Gamer, L.W.; Pregizer, S.; Gamer, J.; Feigenson, M.; Ionescu, A.; Li, Q.; Han, L.; Rosen, V. The Role of Bmp2 in the Maturation and Maintenance of the Murine Knee Joint. J. Bone Miner. Res. 2018, 33, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.F.; Surke, C.; Stange, R.; Quandte, S.; Wildemann, B.; Raschke, M.J.; Schmidmaier, G. Local delivery of growth factors using coated suture material. Sci. World J. 2012, 2012, 109216. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Pufe, T.; Starke, C.; Fuchs, T.; Kopf, S.; Raschke, M.; Becker, R.; Tillmann, B. Locally applied angiogenic factors—A new therapeutic tool for meniscal repair. Ann. Anat. 2005, 187, 509–519. [Google Scholar] [CrossRef]
- Misak, H.E.; Asmatulu, R.; Gopu, J.S.; Man, K.P.; Zacharias, N.M.; Wooley, P.H.; Yang, S.Y. Albumin-based nanocomposite spheres for advanced drug delivery systems. Biotech. J. 2014, 9, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lazard, Z.W.; Heggeness, M.H.; Hipp, J.A.; Sonnet, C.; Fuentes, A.S.; Nistal, R.P.; Davis, A.R.; Olabisi, R.M.; West, J.L.; Olmsted-Davis, E.A. Cell-based gene therapy for repair of critical size defects in the rat fibula. J. Cell Biochem. 2011, 112, 1563–1571. [Google Scholar] [CrossRef]
- Sansanaphongpricha, K.; Sonthithai, P.; Kaewkong, P.; Thavornyutikarn, B.; Bamrungsap, S.; Kosorn, W.; Thinbanmai, T.; Saengkrit, N. Hyaluronic acid-coated gold nanorods enhancing BMP-2 peptide delivery for chondrogenesis. Nanotechnology 2020, 31, 435101. [Google Scholar] [CrossRef]
- Fu, H.L.; Diao, Z.Y.; Shao, L.; Yang, D.P. BMP-2 promotes chondrogenesis of rat adipose-derived stem cells by using a lentiviral system. Genet. Mol. Res. 2014, 13, 8620–8631. [Google Scholar] [CrossRef]
- Jin, E.J.; Lee, S.Y.; Choi, Y.A.; Jung, J.C.; Bang, O.S.; Kang, S.S. BMP-2-enhanced chondrogenesis involves p38 MAPK-mediated down-regulation of Wnt-7a pathway. Mol. Cells 2006, 22, 353–359. [Google Scholar]
- Kovermann, N.J.; Basoli, V.; Della-Bella, E.; Alini, M.; Lischer, C.; Schmal, H.; Kubosch, E.J.; Stoddart, M.J. BMP2 and TGF-beta Cooperate Differently during Synovial-Derived Stem-Cell Chondrogenesis in a Dexamethasone-Dependent Manner. Cells 2019, 8, 636. [Google Scholar] [CrossRef]
- Wu, C.; Jiao, H.; Lai, Y.; Zheng, W.; Chen, K.; Qu, H.; Deng, W.; Song, P.; Zhu, K.; Cao, H.; et al. Kindlin-2 controls TGF-beta signalling and Sox9 expression to regulate chondrogenesis. Nat. Commun. 2015, 6, 7531. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drummond, N.; W. Bruner, B.; Heggeness, M.H.; Dart, B.; Yang, S.-Y. Effect of BMP-2 Adherent to Resorbable Sutures on Cartilage Repair: A Rat Model of Xyphoid Process. Materials 2020, 13, 3764. https://doi.org/10.3390/ma13173764
Drummond N, W. Bruner B, Heggeness MH, Dart B, Yang S-Y. Effect of BMP-2 Adherent to Resorbable Sutures on Cartilage Repair: A Rat Model of Xyphoid Process. Materials. 2020; 13(17):3764. https://doi.org/10.3390/ma13173764
Chicago/Turabian StyleDrummond, Nathan, Bradley W. Bruner, Michael H. Heggeness, Bradley Dart, and Shang-You Yang. 2020. "Effect of BMP-2 Adherent to Resorbable Sutures on Cartilage Repair: A Rat Model of Xyphoid Process" Materials 13, no. 17: 3764. https://doi.org/10.3390/ma13173764
APA StyleDrummond, N., W. Bruner, B., Heggeness, M. H., Dart, B., & Yang, S.-Y. (2020). Effect of BMP-2 Adherent to Resorbable Sutures on Cartilage Repair: A Rat Model of Xyphoid Process. Materials, 13(17), 3764. https://doi.org/10.3390/ma13173764





