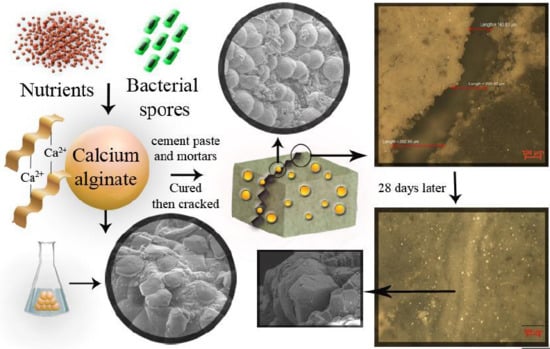
Biological Self-Healing of Cement Paste and Mortar by Non-Ureolytic Bacteria Encapsulated in Alginate Hydrogel Capsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Methods
2.2.1. Growth Media Preparation
2.2.2. Spore Suspension Preparation
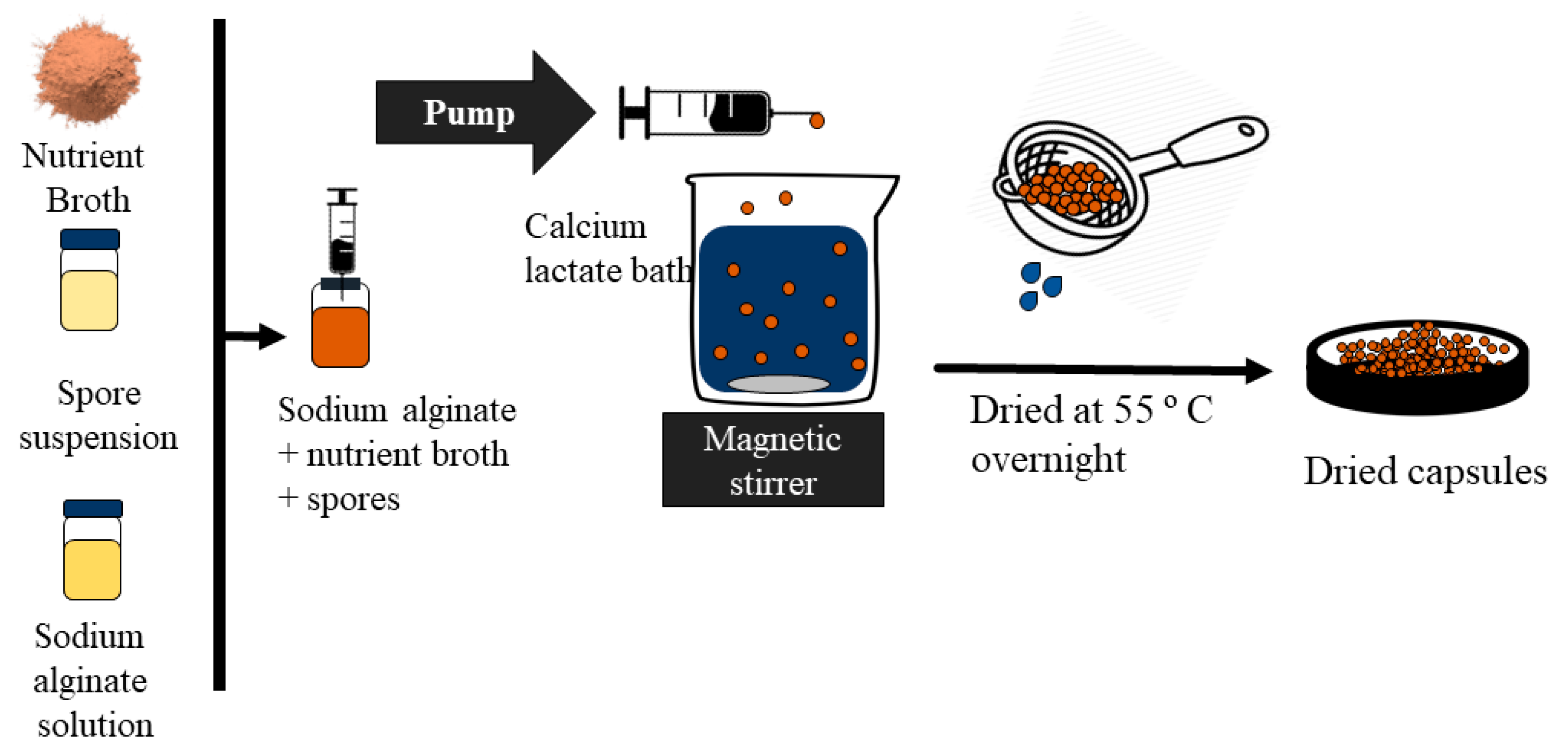
2.2.3. Encapsulation of Nutrients and Spores into the CaAlg
2.2.4. Test Solution Preparation
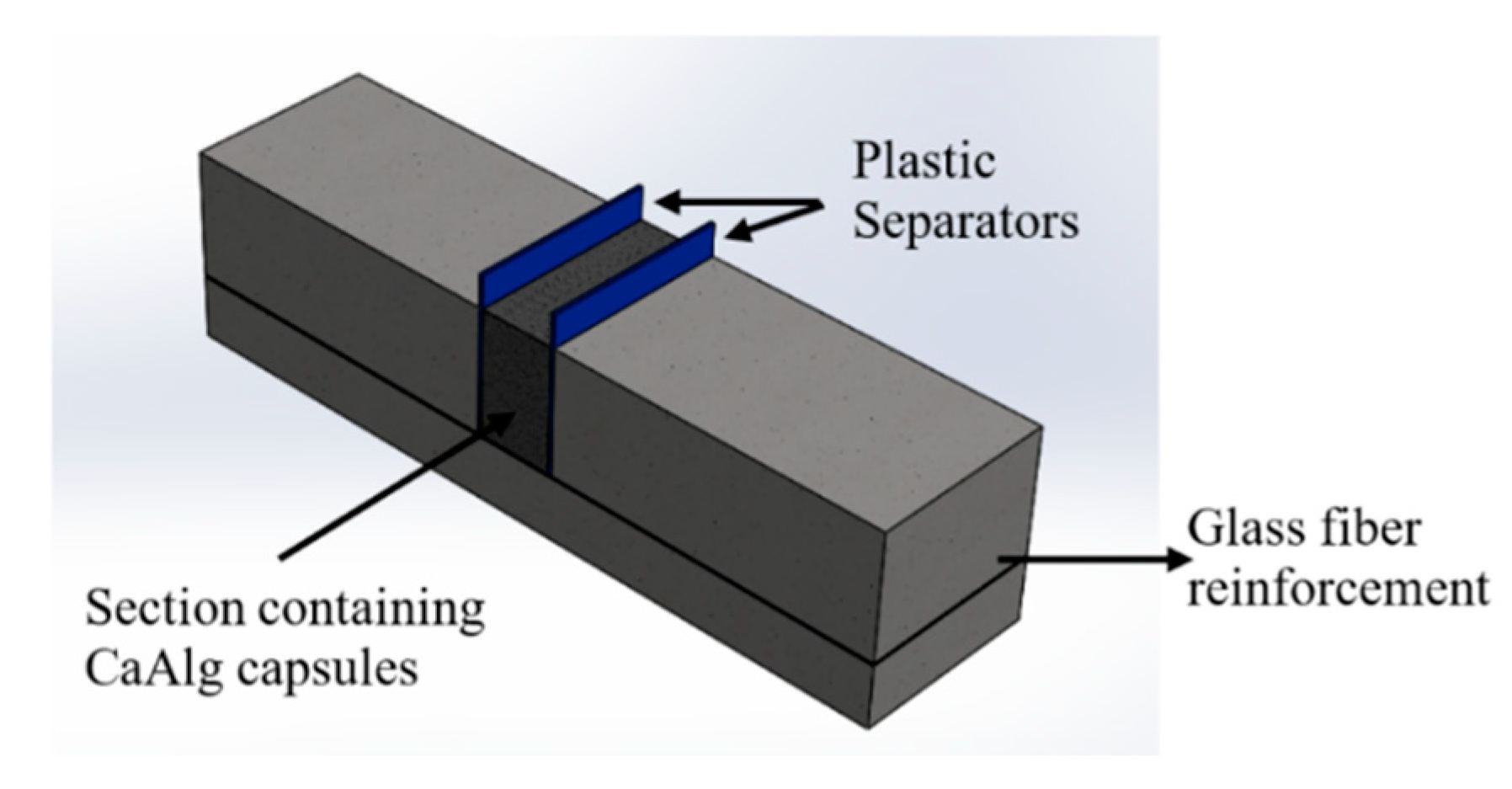
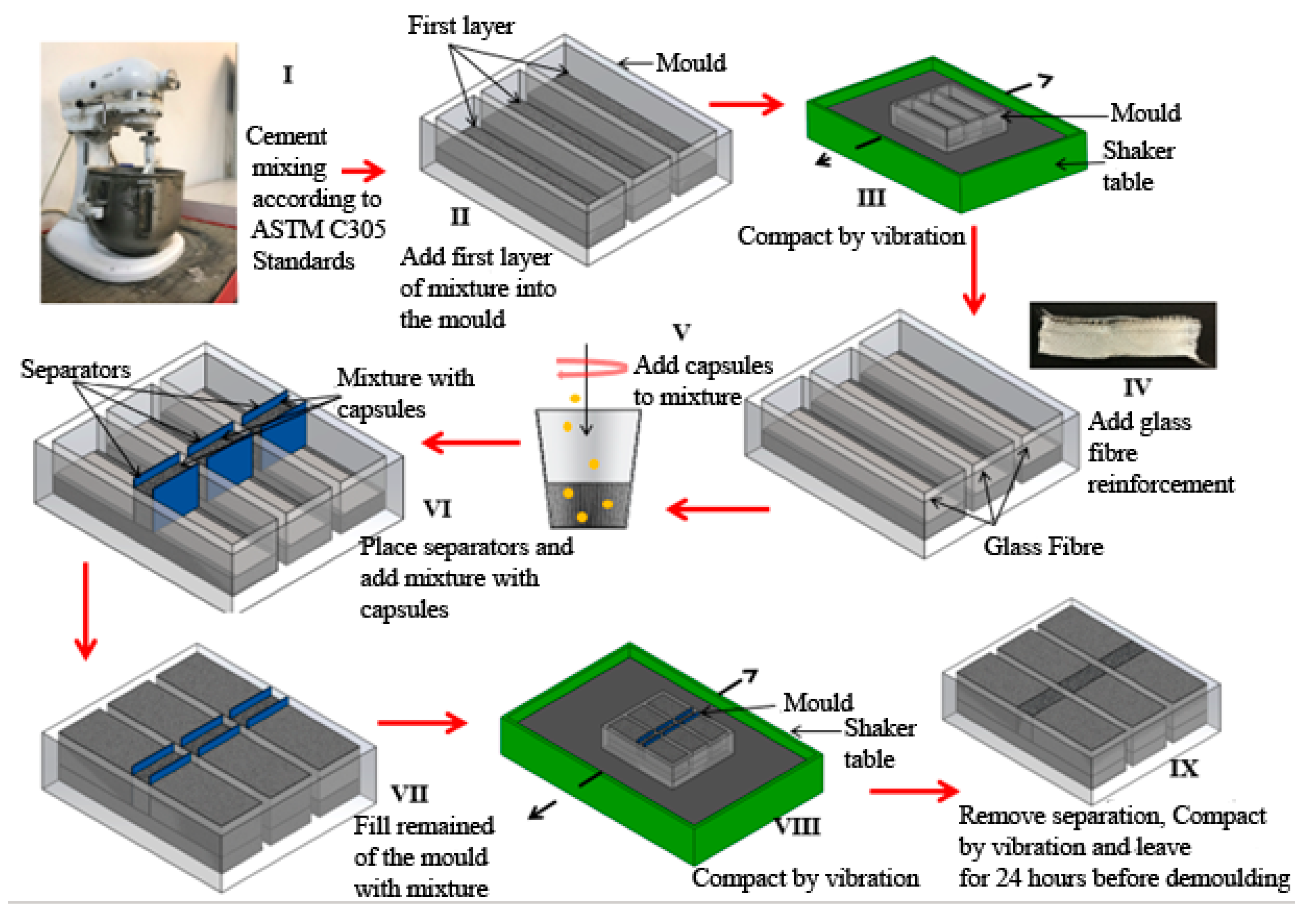
2.2.5. Preparation of Cement Paste and Mortars for Flexural Analysis
2.3. In Vitro Characterization Methods
2.3.1. Survival of Encapsulated Bacterial Spores
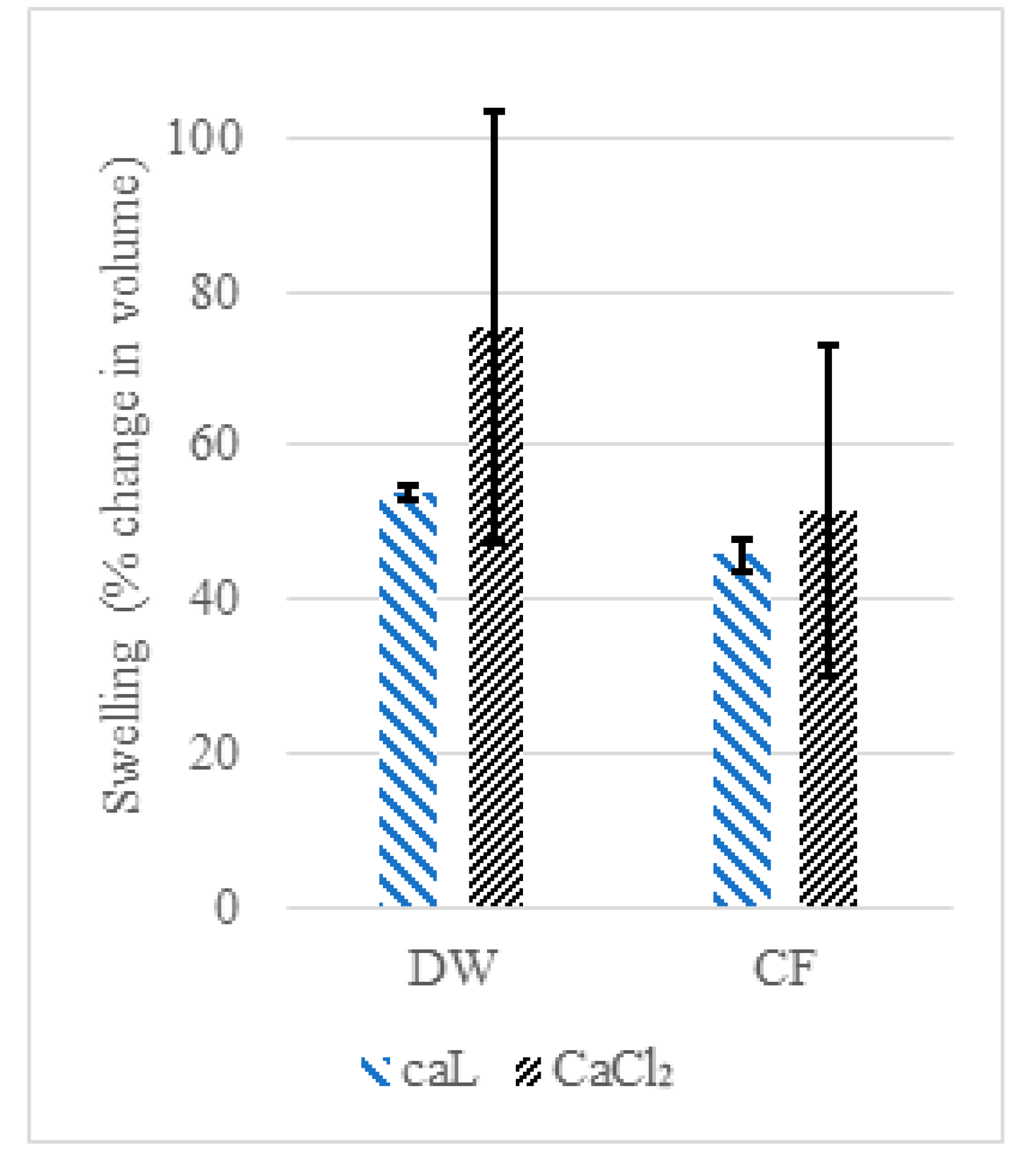
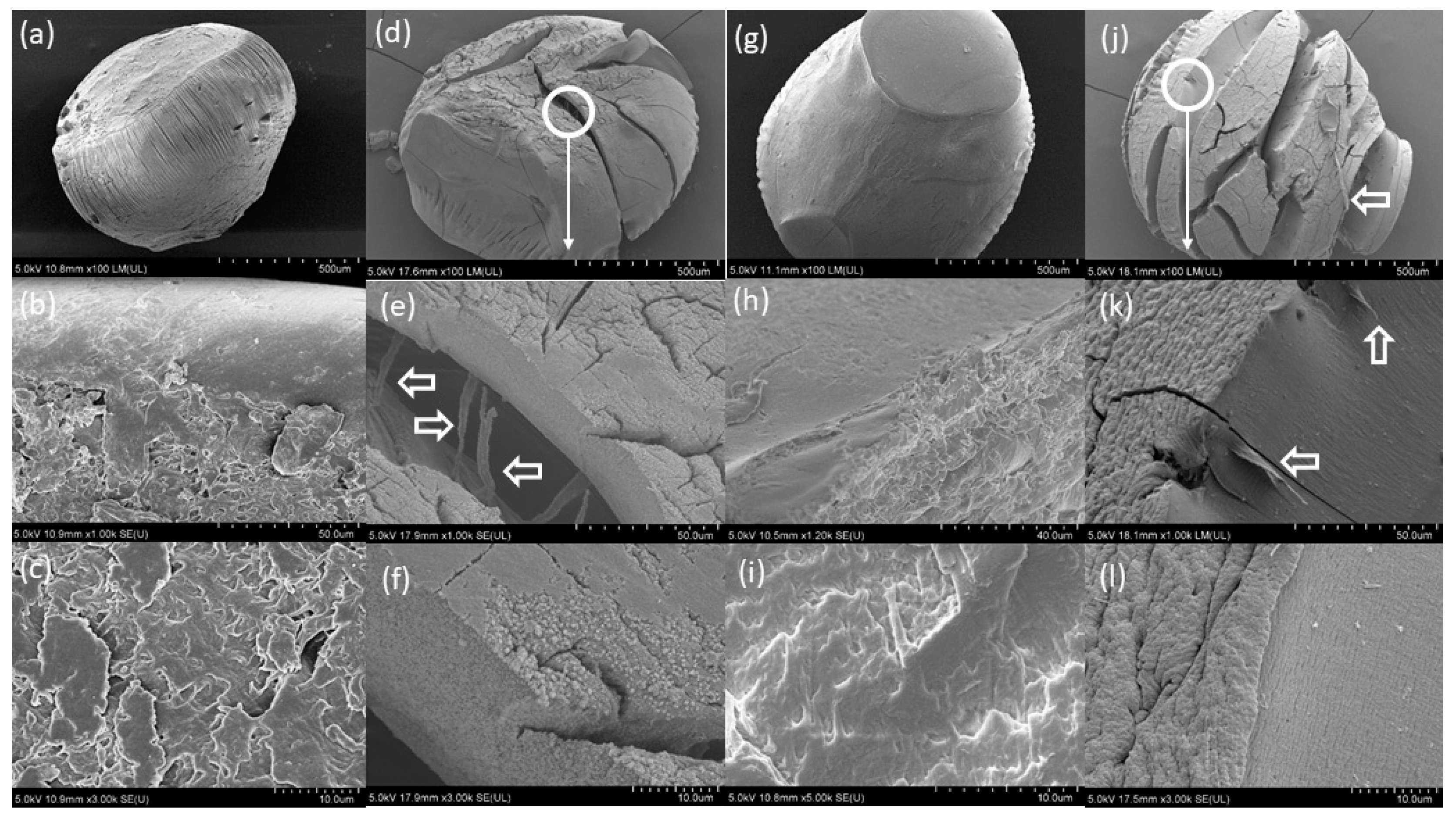
2.3.2. Swelling and Stability of CaAlg Capsules
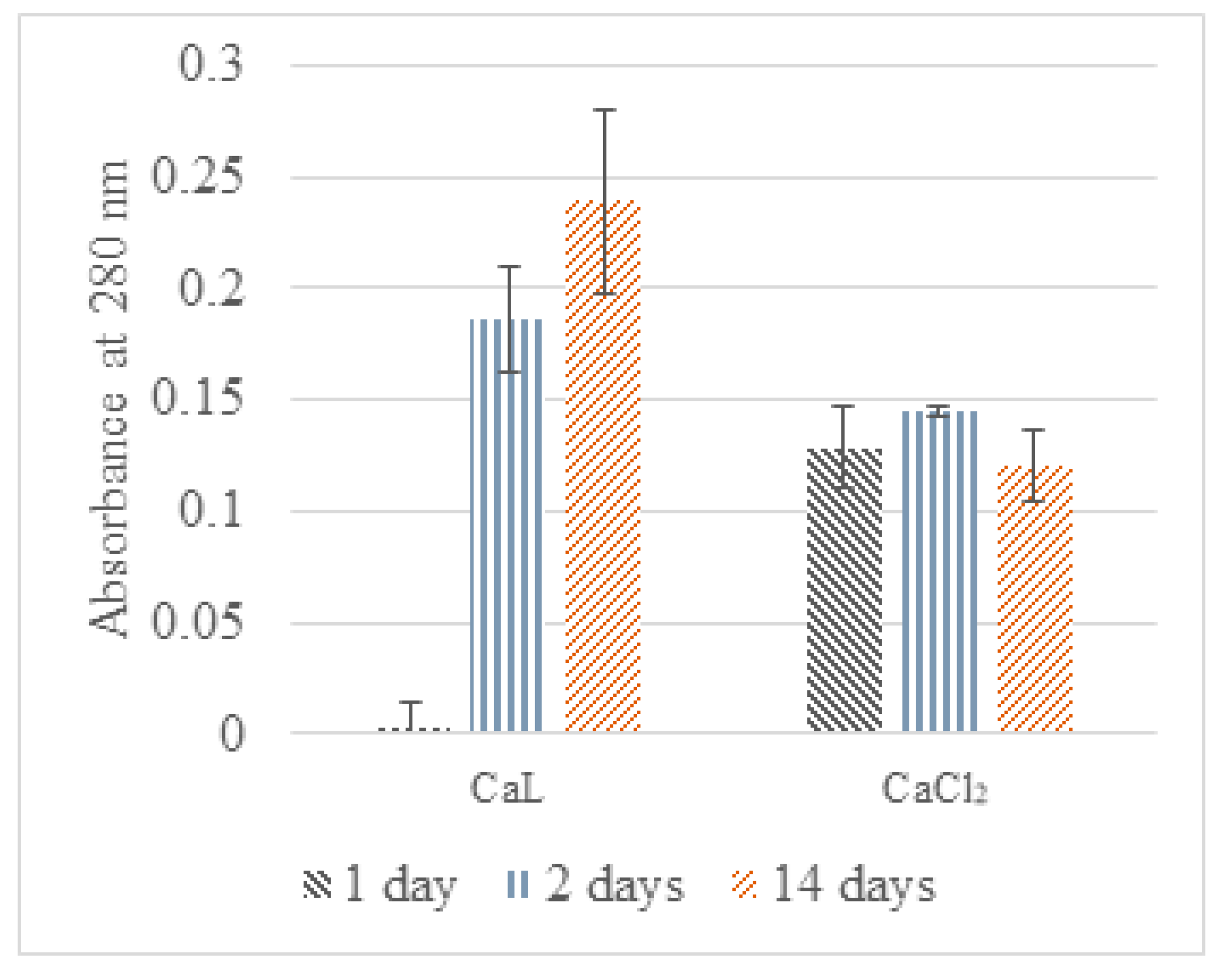
2.3.3. Nutrient Leaching in CaAlg Capsules
2.3.4. Spore Retention of CaAlg Capsules
2.3.5. CaCO3 Precipitation by Encapsulated Bacterial Spores
2.3.6. Statistical Analysis
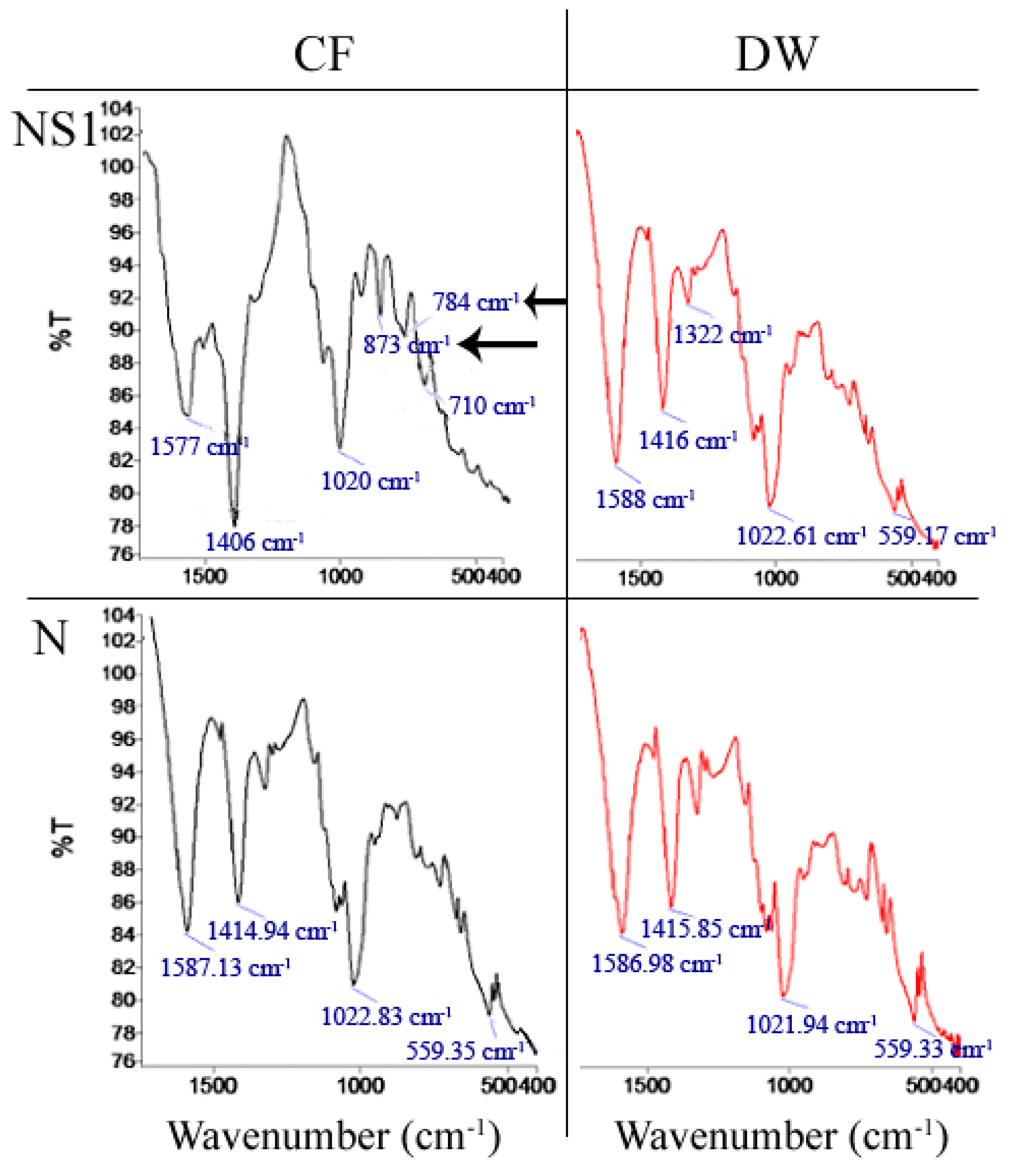
2.3.7. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
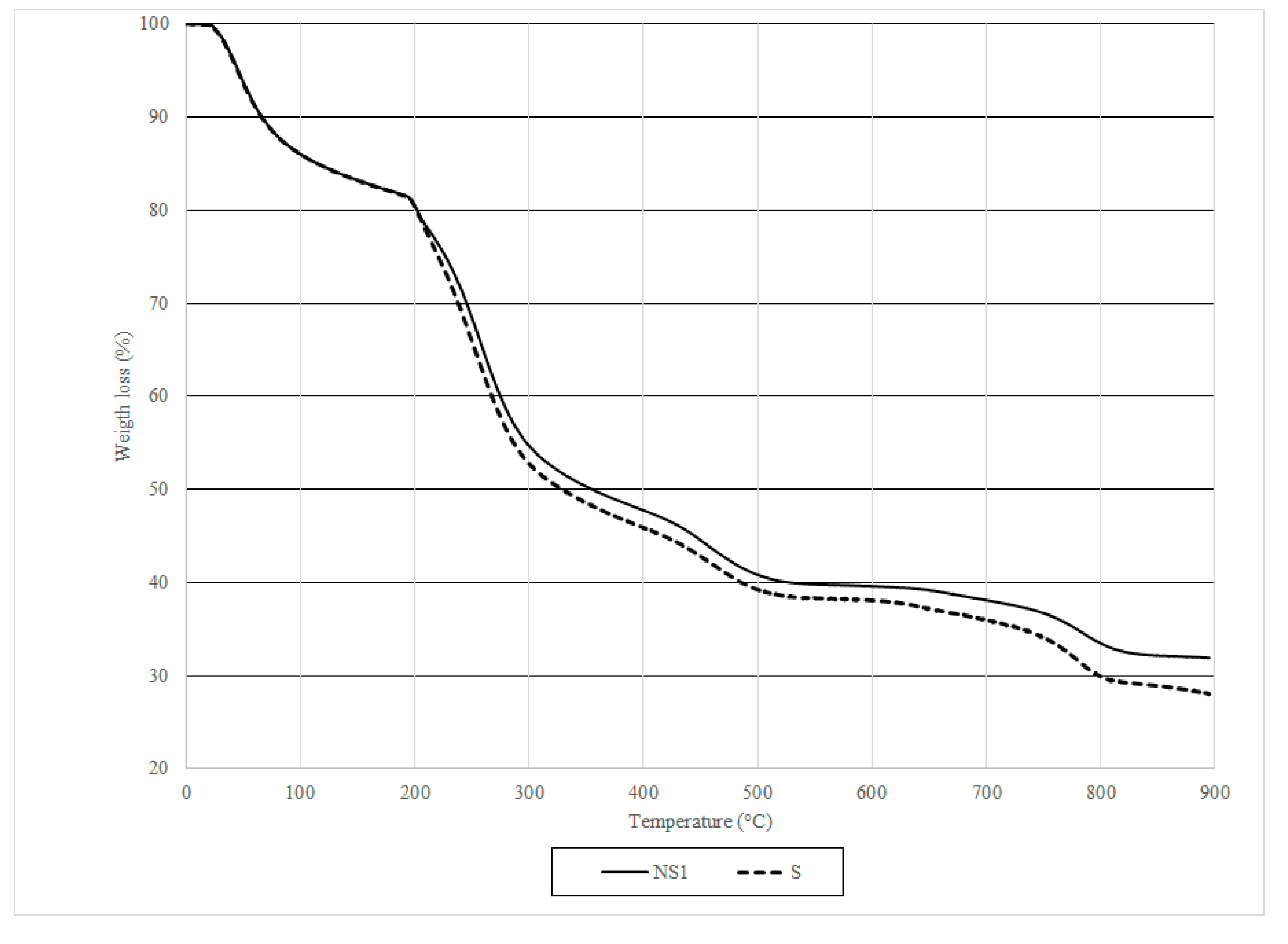
2.3.8. Thermogravimetric Analysis (TGA) of Capsules
2.4. In Situ Self-Healing Efficiency Assessment of Cement Paste and Mortars
2.4.1. Flexural Testing
2.4.2. Crack Width Measurements and Visualization of Self-Healing
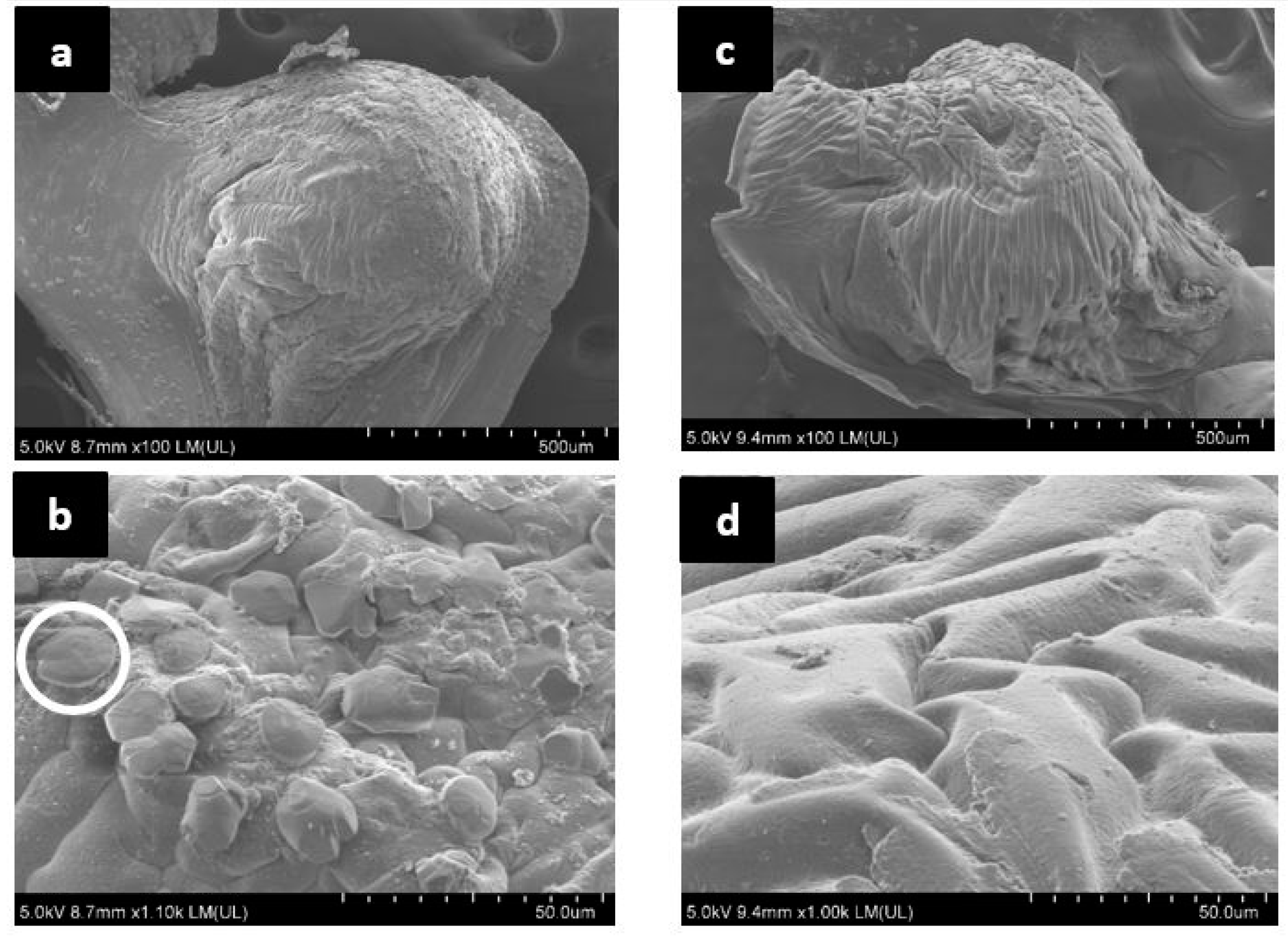
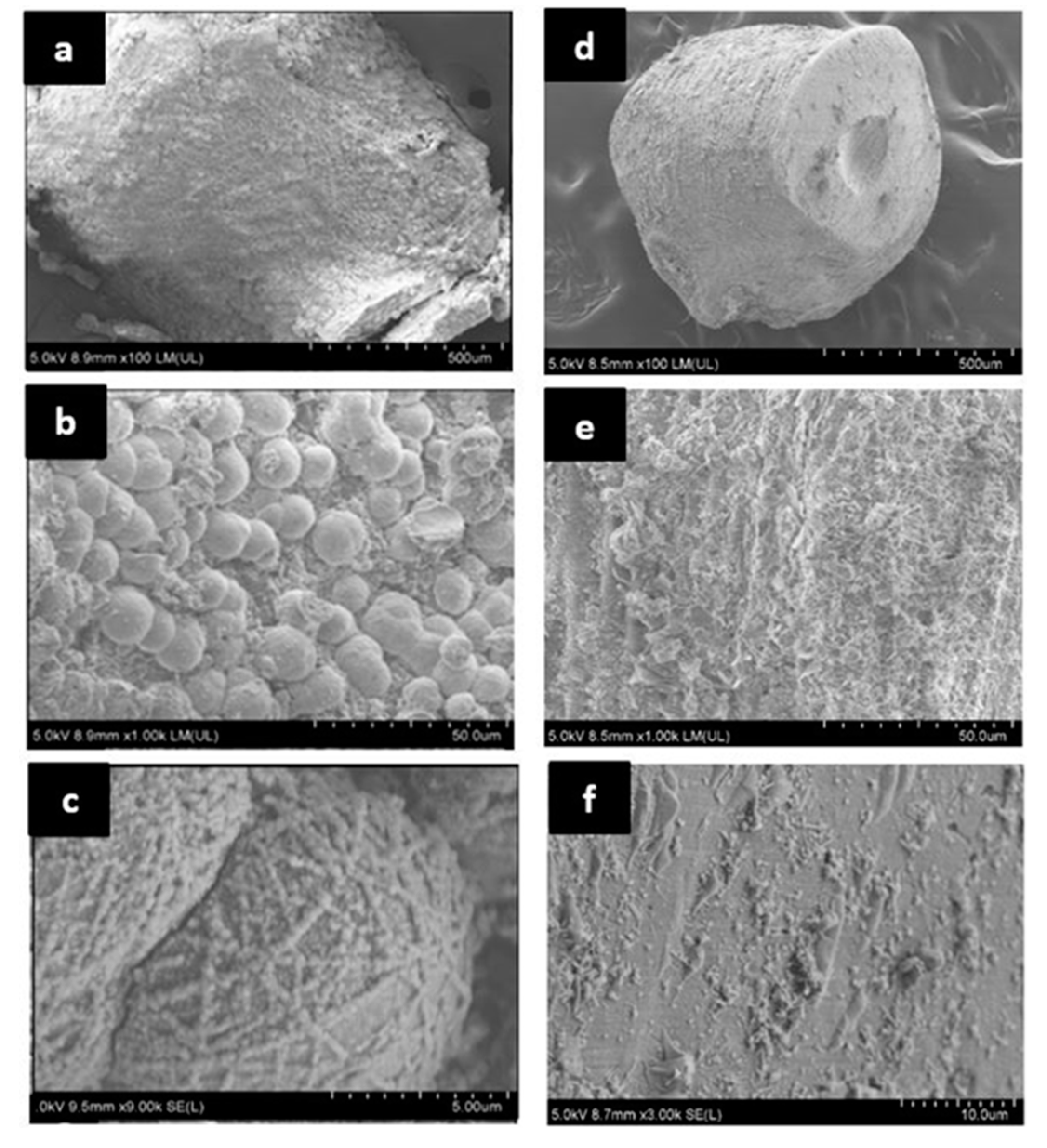
2.4.3. Morphological Analysis
3. Results and Discussion
3.1. Survival of Encapsulated Bacterial Spores
3.2. Swelling and Stability of the CaAlg Capsules
3.3. Spore Retention Ability of the CaAlg Capsules
3.4. Nutrient Retention of CaAlg Capsules
3.5. CaCO3 Precipitation by Encapsulated Spores
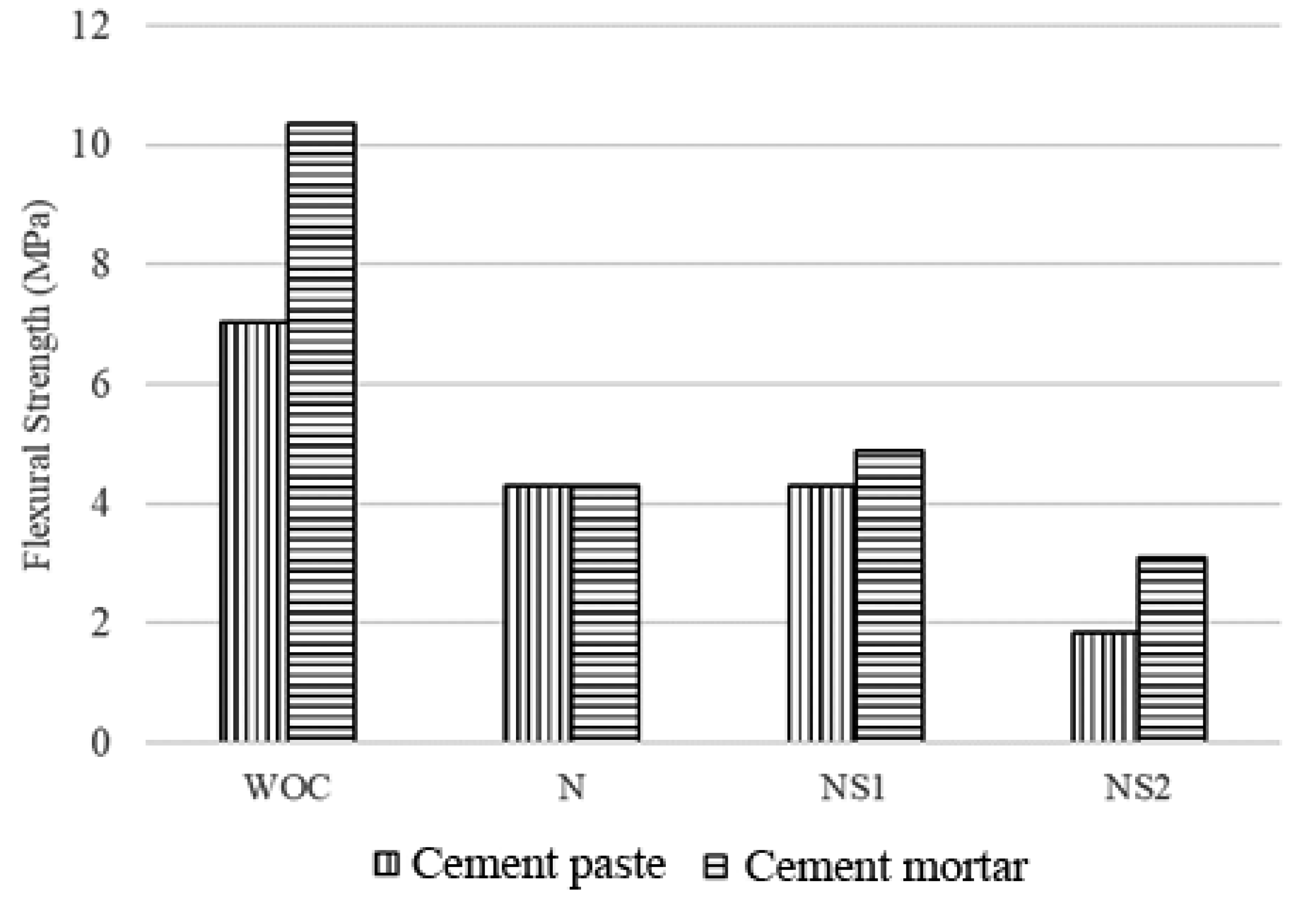
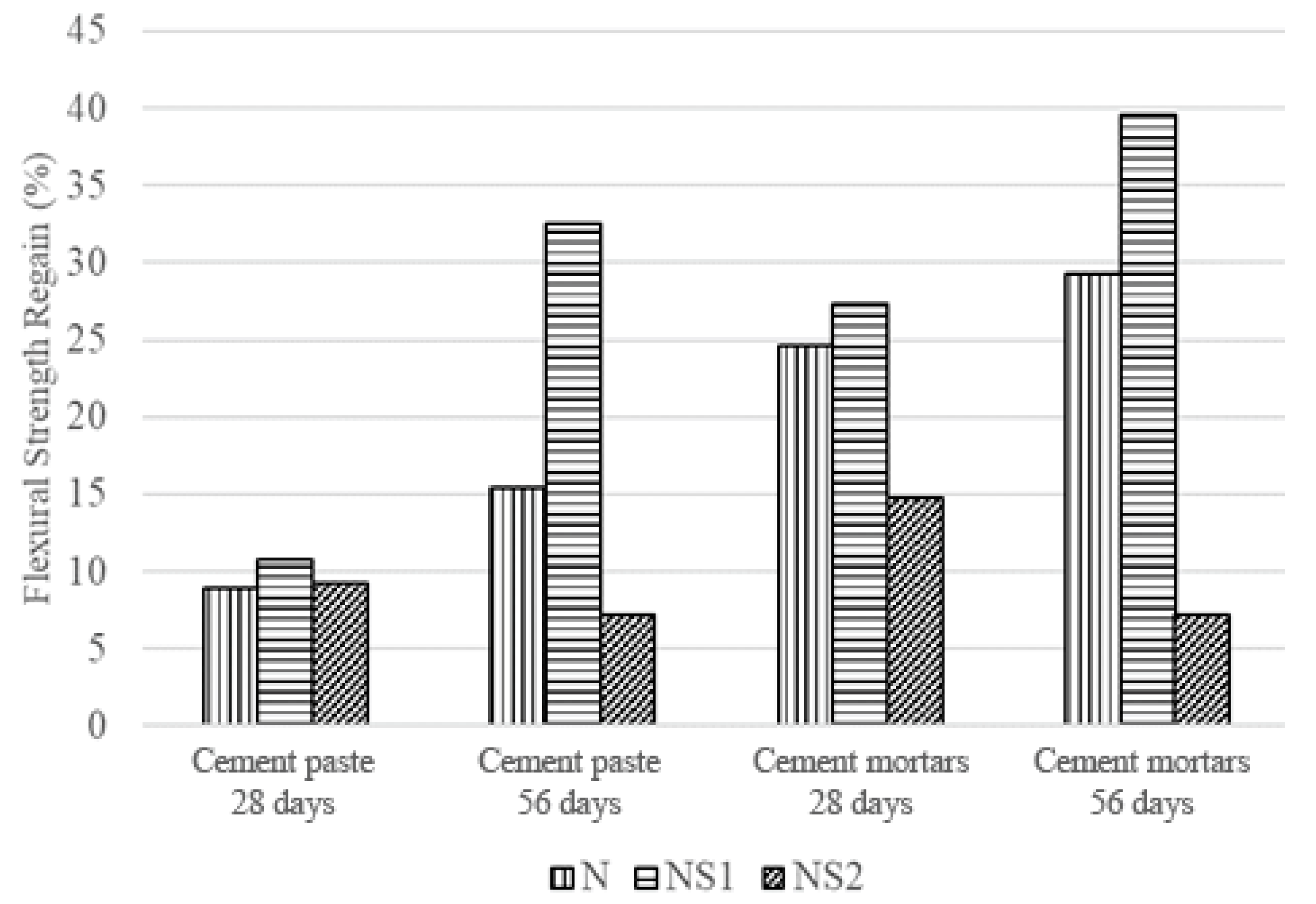
3.6. Flexural Strength Results of Cement Paste and Mortars
3.6.1. Flexural Strength of Cement Paste and Mortars before Self-Healing
3.6.2. Flexural Strength of Cement Paste and Mortars after Self-Healing
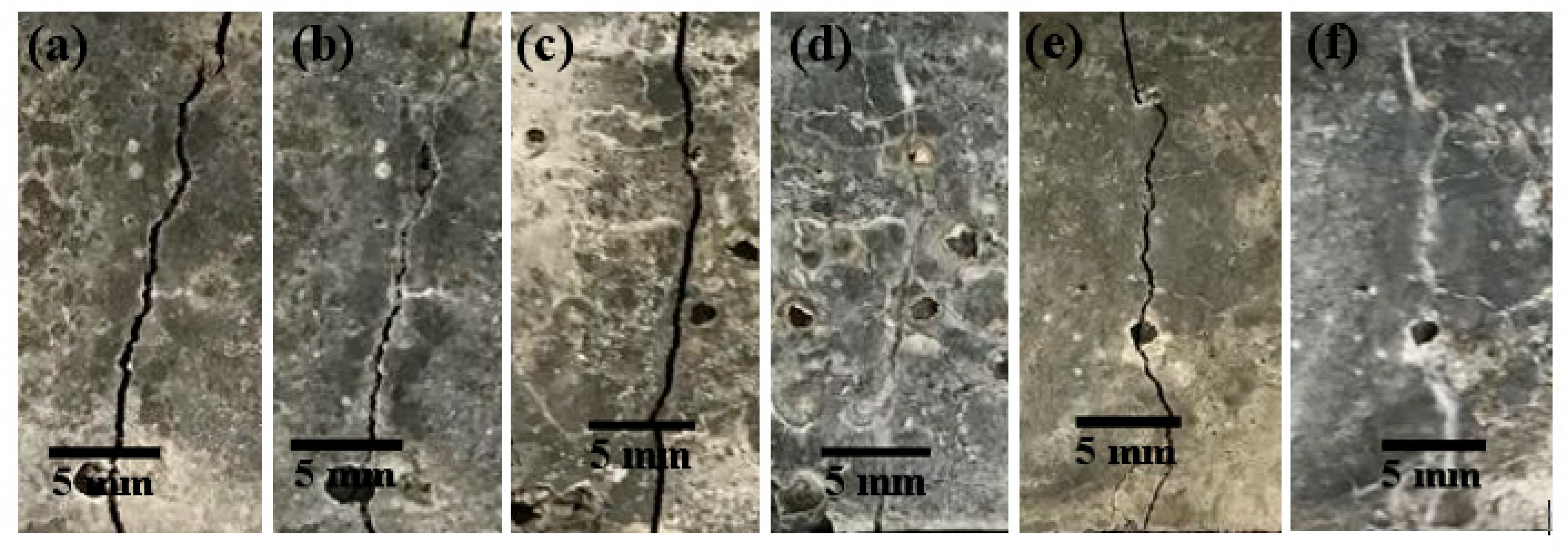
3.6.3. Visualization of Crack Healing
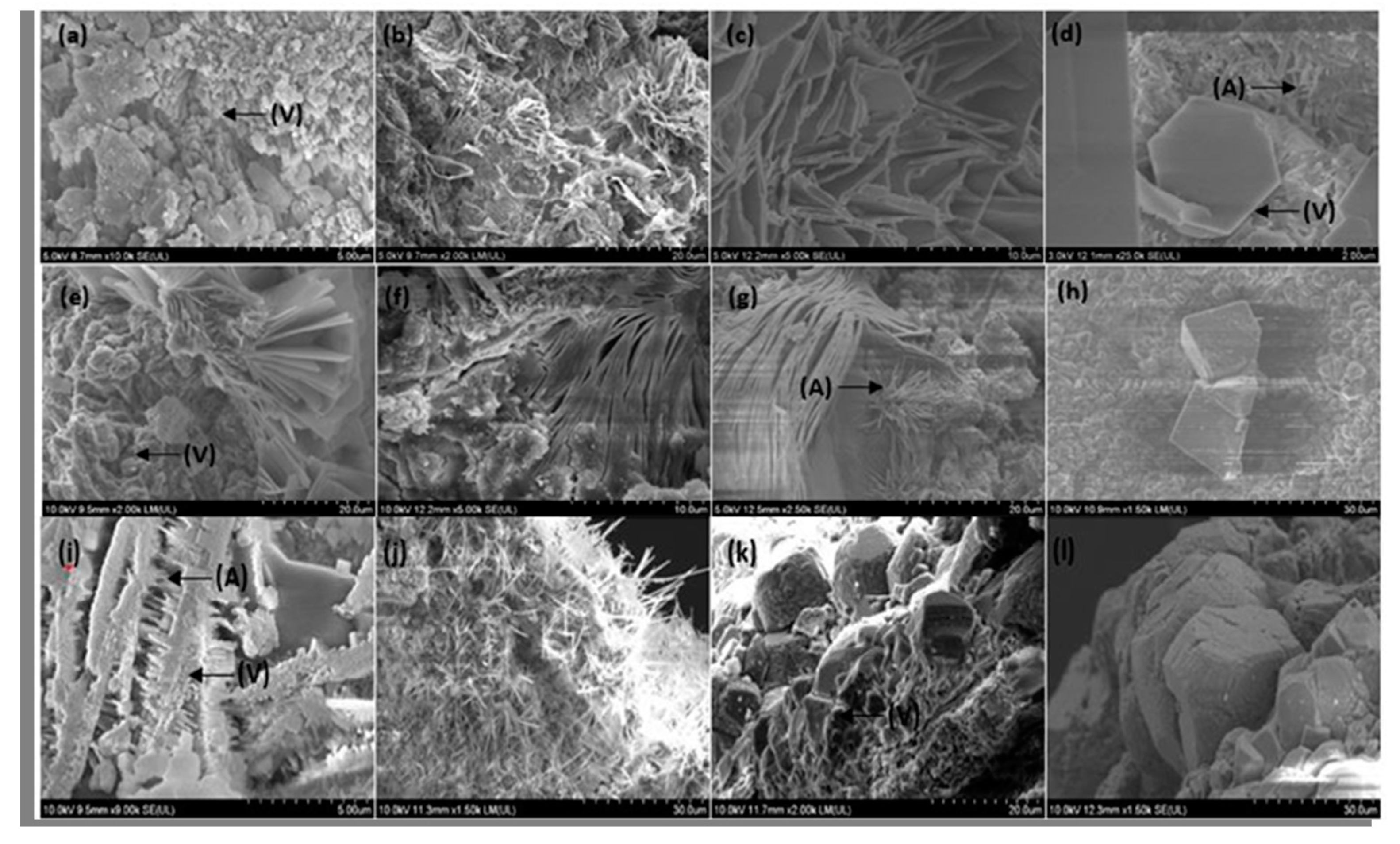
3.7. Morphological Analysis of Cracks after Self-Healing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joshi, S.; Goyal, S.; Mukherjee, A.; Reddy, M.S. Microbial healing of cracks in concrete: A review. J. Ind. Microbiol. Biotechnol. 2017, 44, 1511–1525. [Google Scholar] [CrossRef]
- Monteiro, P.J.M.; Miller, S.A.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698–699. [Google Scholar] [CrossRef]
- Basheer, P.; Chidiact, S.; Long, A.; Basheer, M. Predictive models for deterioration of concrete structures. Constr. Build. Mater. 1996, 10, 27–37. [Google Scholar] [CrossRef]
- Wang, J.Y.; Soens, H.; Verstraete, W.; De Belie, N. Self-healing concrete by use of microencapsulated bacterial spores. Cem. Concr. Res. 2014, 56, 139–152. [Google Scholar] [CrossRef]
- Wiktor, V.; Jonkers, H.M. Bacteria-based concrete: From concept to market. Smart Mater. Struct. 2016, 25, 84006. Available online: http://stacks.iop.org/0964-1726/25/i=8/a=084006 (accessed on 28 December 2017). [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Çopuroğlu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Yang, Y.; Lepech, M.D.; Yang, E.-H.; Li, V.C. Autogenous healing of engineered cementitious composites under wet–dry cycles. Cem. Concr. Res. 2009, 39, 382–390. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, Y.; Qian, S. Influence of bacterial incorporation on mechanical properties of engineered cementitious composites (ECC). Constr. Build. Mater. 2019, 196, 195–203. [Google Scholar] [CrossRef]
- Karimi, N.; Mostofinejad, D. Bacillus subtilis bacteria used in fiber reinforced concrete and their effects on concrete penetrability. Constr. Build. Mater. 2020, 230, 117051. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E. Innovative carboxylic acid waterproofing admixture for self-sealing watertight concretes. Constr. Build. Mater. 2018, 171, 817–824. [Google Scholar] [CrossRef]
- Haw, T.T.; Hart, F.; Rashidi, A.; Pasbakhsh, P. Sustainable cementitious composites reinforced with metakaolin and halloysite nanotubes for construction and building applications. Appl. Clay Sci. 2020, 188, 105533. [Google Scholar] [CrossRef]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of concrete using micro-organisms. ACI Mater. J. Am. Concr. Inst. 2001, 98, 3–9. [Google Scholar]
- Ghosh, P.; Mandal, S.; Chattopadhyay, B.; Pal, S. Use of microorganism to improve the strength of cement mortar. Cem. Concr. Res. 2005, 35, 1980–1983. [Google Scholar] [CrossRef]
- Jiang, L.; Jia, G.; Wang, Y.; Li, Z. Optimization of Sporulation and Germination Conditions of Functional Bacteria for Concrete Crack-Healing and Evaluation of their Repair Capacity. ACS Appl. Mater. Interfaces 2020, 12, 10938–10948. [Google Scholar] [CrossRef]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Ca-carbonates precipitation and limestone genesis—The microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Zhu, T.; Paulo, C.; Merroun, M.L.; Dittrich, M. Potential application of biomineralization by Synechococcus PCC8806 for concrete restoration. Ecol. Eng. 2015, 82, 459–468. [Google Scholar] [CrossRef]
- Lors, C.; Ducasse-Lapeyrusse, J.; Gagné, R.; Damidot, D. Microbiologically induced calcium carbonate precipitation to repair microcracks remaining after autogenous healing of mortars. Constr. Build. Mater. 2017, 141, 461–469. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Paegle, I.; Borisov, S.M.; Kjeldsen, K.U.; Røy, H.; Skibsted, J.; Koren, K. Optical Sensing of pH and O2 in the Evaluation of Bioactive Self-Healing Cement. ACS Omega 2019, 4, 20237–20243. [Google Scholar] [CrossRef]
- Maignien, L.; Depreiter, D.; Foubert, A.; Reveillaud, J.; De Mol, L.; Boeckx, P.; Blamart, D.; Henriet, J.-P.; Boon, N. Anaerobic oxidation of methane in a cold-water coral carbonate mound from the Gulf of Cadiz. Acta Diabetol. 2010, 100, 1413–1422. [Google Scholar] [CrossRef]
- DeJong, J.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Hamdan, N.; Kavazanjian, E.; Rittmann, B.E.; Karatas, I. Carbonate Mineral Precipitation for Soil Improvement Through Microbial Denitrification. Geomicrobiol. J. 2016, 34, 139–146. [Google Scholar] [CrossRef]
- Anbiu, P.; Kang, C.-H.; Shin, Y.; So, J.-S. Formations of calcium carbonate minerals by bacteria and its multiple applications. SpringerPlus 2016, 5, 250. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Berenjian, A. New insights into the role of pH and aeration in the bacterial production of calcium carbonate (CaCO3). Appl. Microbiol. Biotechnol. 2017, 101, 3131–3142. [Google Scholar] [CrossRef]
- Seifan, M.; Berenjian, A. Application of microbially induced calcium carbonate precipitation in designing bio self-healing concrete. World J. Microbiol. Biotechnol. 2018, 34, 168. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, A. (Dey) Review on microbial induced calcite precipitation mechanisms leading to bacterial selection for microbial concrete. Constr. Build. Mater. 2019, 225, 67–75. [Google Scholar] [CrossRef]
- Wang, J.; Snoeck, D.; Van Vlierberghe, S.; Verstraete, W.; De Belie, N. Application of hydrogel encapsulated carbonate precipitating bacteria for approaching a realistic self-healing in concrete. Constr. Build. Mater. 2014, 68, 110–119. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X. Self-healing of concrete cracks by use of bacteria-containing low alkali cementitious material. Constr. Build. Mater. 2018, 167, 1–14. [Google Scholar] [CrossRef]
- Gat, D.; Ronen, Z.; Tsesarsky, M. Long-term sustainability of microbial-induced CaCO3 precipitation in aqueous media. Chemosphere 2017, 184, 524–531. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yao, W.; Jiang, Z. Non-Ureolytic Bacterial Carbonate Precipitation as a Surface Treatment Strategy on Cementitious Materials. J. Mater. Civ. Eng. 2014, 26, 983–991. [Google Scholar] [CrossRef]
- Tziviloglou, E.; Wiktor, V.; Jonkers, H.; Schlangen, E. Selection of Nutrient Used in Biogenic Healing Agent for Cementitious Materials. Front. Mater. 2017, 4. [Google Scholar] [CrossRef]
- Sharma, T.; Alazhari, M.; Heath, A.; Paine, K.A.; Cooper, R. AlkaliphilicBacillusspecies show potential application in concrete crack repair by virtue of rapid spore production and germination then extracellular calcite formation. J. Appl. Microbiol. 2017, 122, 1233–1244. [Google Scholar] [CrossRef]
- Simó, G.; Fernández-Fernández, E.; Crespo, J.M.V.; Ruipérez, V.; Nogales, J.M.R. Research progress in coating techniques of alginate gel polymer for cell encapsulation. Carbohydr. Polym. 2017, 170, 1–14. [Google Scholar] [CrossRef]
- Tziviloglou, E.; Wiktor, V.; Jonkers, H.M.; Schlangen, E. Bacteria-based self-healing concrete to increase liquid tightness of cracks. Constr. Build. Mater. 2016, 122, 118–125. [Google Scholar] [CrossRef]
- Ding, F.; Wu, S.; Wang, S.; Xiong, Y.; Li, Y.; Li, B.; Deng, H.; Du, Y.; Xiao, L.; Shi, X. A dynamic and self-crosslinked polysaccharide hydrogel with autonomous self-healing ability. Soft Matter 2015, 11, 3971–3976. [Google Scholar] [CrossRef]
- Hia, I.L.; Chai, S.-P.; Pasbakhsh, P.; Chan, E.S. A novel repeated self-healing epoxy composite with alginate multicore microcapsules. J. Mater. Chem. A 2018, 6, 8470–8478. [Google Scholar] [CrossRef]
- Hia, I.L.; Vahedi, V.; Pasbakhsh, P. Self-healing polymer composites: Prospects, challenges, and applications. Polym. Rev. 2016, 56, 225–261. [Google Scholar] [CrossRef]
- Palin, D.; Wiktor, V.; Jonkers, H.M. A bacteria-based bead for possible self-healing marine concrete applications. Smart Mater. Struct. 2016, 25, 84008. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Meng, S.; Winters, H.; Liu, Y. Ultrafiltration behaviors of alginate blocks at various calcium concentrations. Water Res. 2015, 83, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Mignon, A.; Snoeck, D.; D’Halluin, K.; Balcaen, L.; Vanhaecke, F.; Dubruel, P.; Van Vlierberghe, S.; De Belie, N. Alginate biopolymers: Counteracting the impact of superabsorbent polymers on mortar strength. Constr. Build. Mater. 2016, 110, 169–174. [Google Scholar] [CrossRef]
- Wang, J.; Mignon, A.; Snoeck, D.; Wiktor, V.; Van Vliergerghe, S.; Boon, N.; De Belie, N. Application of modified-alginate encapsulated carbonate producing bacteria in concrete: A promising strategy for crack self-healing. Front. Microbiol. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Zhang, J.; Mai, B.; Cai, T.; Luo, J.; Wu, W.; Liu, B.; Han, N.; Xing, F.; Deng, X. Optimization of a Binary Concrete Crack Self-Healing System Containing Bacteria and Oxygen. Materials 2017, 10, 116. [Google Scholar] [CrossRef]
- Erşan, Y.C.; Verbruggen, H.; De Graeve, I.; Verstraete, W.; De Belie, N.; Boon, N. Nitrate reducing CaCO3 precipitating bacteria survive in mortar and inhibit steel corrosion. Cem. Concr. Res. 2016, 83, 19–30. [Google Scholar] [CrossRef]
- Erşan, Y.C.; Hernandez-Sanabria, E.; Boon, N.; De Belie, N. Enhanced crack closure performance of microbial mortar through nitrate reduction. Cem. Concr. Compos. 2016, 70, 159–170. [Google Scholar] [CrossRef]
- Sonmez, M.; Erşan, Y.C. Production of concrete compatible biogranules for self-healing concrete applications. MATEC Web Conf. 2019, 289, 1002. [Google Scholar] [CrossRef]
- Erşan, Y.C.; Van Tittelboom, K.; Boon, N.; De Belie, N. Nitrite producing bacteria inhibit reinforcement bar corrosion in cementitious materials. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Alazhari, M.; Sharma, T.; Heath, A.; Cooper, R.; Paine, K.A. Application of expanded perlite encapsulated bacteria and growth media for self-healing concrete. Constr. Build. Mater. 2018, 160, 610–619. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Hewitt, S.; Berenjian, A. The effect of cell immobilization by calcium alginate on bacterially induced calcium carbonate precipitation. Fermentation 2017, 3, 57. [Google Scholar] [CrossRef]
- Sierra-Beltrán, M.G.; Jonkers, H.M.; Schlangen, E. Characterization of sustainable bio-based mortar for concrete repair. Constr. Build. Mater. 2014, 67, 344–352. [Google Scholar] [CrossRef]
- Khaliq, W.; Ehsan, M.B. Crack healing in concrete using various bio influenced self-healing techniques. Constr. Build. Mater. 2016, 102, 349–357. [Google Scholar] [CrossRef]
- Mors, R.; Jonkers, H.M. Feasibility of lactate derivative based agent as additive for concrete for regain of crack water tightness by bacterial metabolism. Ind. Crop. Prod. 2017, 106, 97–104. [Google Scholar] [CrossRef]
- Janto, B.; Ahmed, A.; Ito, M.; Liu, J.; Hicks, D.B.; Pagni, S.; Fackelmayer, O.J.; Smith, T.-A.; Earl, J.; Elbourne, L.D.H.; et al. Genome of alkaliphilic Bacillus pseudofirmus OF4 reveals adaptations that support the ability to grow in an external pH range from 7.5 to 11.4. Environ. Microbiol. 2011, 13, 3289–3309. [Google Scholar] [CrossRef]
- Oniyama, E.; Wahlbeck, P. Application of transpiration theory to TGA data: Calcium carbonate and zinc chloride. Thermochim. Acta 1995, 250, 41–53. [Google Scholar] [CrossRef]
- Shoichet, M.S.; Li, R.H.; White, M.L.; Winn, S.R. Stability of hydrogels used in cell encapsulation: An in vitro comparison of alginate and agarose. Biotechnol. Bioeng. 1996, 50, 374–381. [Google Scholar] [CrossRef]
- Splittstoesser, D.F.; Farkas, D.F. Effect of Cations on Activation of Bacillus popilliae Spores1. J. Bacteriol. 1966, 92, 995–1001. [Google Scholar] [CrossRef]
- Lee, P.; Rogers, M.A. Effect of calcium source and exposure-time on basic caviar spherification using sodium alginate. Int. J. Gastron. Food Sci. 2012, 1, 96–100. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, X. Characterizing the degradation behavior of calcium alginate fibers wound dressings fabricated by needle-punching process. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Chiew, C.S.C.; Yeoh, H.K.; Pasbakhsh, P.; Krishnaiah, K.; Poh, P.E.; Tey, B.T.; Chan, E.-S. Halloysite/alginate nanocomposite beads: Kinetics, equilibrium and mechanism for lead adsorption. Appl. Clay Sci. 2016, 119, 301–310. [Google Scholar] [CrossRef]
- Hannoun, B.J.M.; Stephanopoulos, G. Diffusion coefficients of glucose and ethanol in cell-free and cell-occupied calcium alginate membranes. Biotechnol. Bioeng. 1986, 28, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Matsumura, M.; Veliky, I.A. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol. Bioeng. 1984, 26, 53–58. [Google Scholar] [CrossRef]
- Ulset, A.-S.T.; Mori, H.; Dalheim, M.Ø.; Hara, M.; Christensen, B.E. Influence of Amino Acids, Buffers, and pH on the γ-Irradiation-Induced Degradation of Alginates. Biomacromolecules 2014, 15, 4590–4597. [Google Scholar] [CrossRef]
- Hicks, M.; Gebicki, J.M. Rate constants for reaction of hydroxyl radicals with Tris, Tricine and Hepes buffers. FEBS Lett. 1986, 199, 92–94. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Matsumura, M.; Kataoka, H. Effective oxygenation of immobilized cells through reduction in bead diameters: A review. Process. Biochem. 1991, 26, 109–121. [Google Scholar] [CrossRef]
- Kozul, R.; Darwin, D. Effects of Aggregate Type, Size, and Content on Concrete Strength and Fracture Energy; University of Kansas Center for Research, Inc.: Lawrence, KS, USA, 1997. [Google Scholar]
- Mehta, P.K.; Monteiro, P.J.M. Concrete Microstructure, Properties and Materials; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Sahoo, K.K.; Sathyan, A.K.; Kumari, C.; Sarkar, P.; Davis, R. Investigation of cement mortar incorporatingBacillussphaericus. Int. J. Smart Nano Mater. 2016, 7, 91–105. [Google Scholar] [CrossRef]
- Olderøy, M.; Xie, M.; Strand, B.L.; Flaten, E.M.; Sikorski, P.; Andreassen, J.-P. Growth and nucleation of calcium carbonate vaterite crystals in presence of alginate. Cryst. Growth Des. 2009, 9, 5176–5183. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef]
- Wang, K.; Wen, Z.; Choi, S. Development of an Eco-Friendly, Cost-Effective Biogrout for Concrete Crack Repair; Iowa State University: Ames, IA, USA, 2016. [Google Scholar]
- Bentz, D.P.; Ardani, A.; Barrett, T.; Jones, S.Z.; Lootens, D.; Peltz, M.A.; Sato, T.; Stutzman, P.E.; Tanesi, J.; Weiss, W.J. Multi-scale investigation of the performance of limestone in concrete. Constr. Build. Mater. 2015, 75, 1–10. [Google Scholar] [CrossRef]

| Capsule | Capsule Description | Nutrient Broth (8 g/L) | Bacterial Spore (Cells/mL Na–Alginate Solution) |
|---|---|---|---|
| O | Capsules with no additives | - | - |
| S | Capsules containing only spores | - | 8.40 × 105 |
| N | Capsules containing only nutrients | √ | - |
| NS1 | Capsules containing nutrients and spores | √ | 8.40 × 105 |
| NS2 | Capsules containing nutrient and increased spores | √ | 7.88 × 108 |
| Sample Type | Capsule Type | Sections without Capsules | Section with Capsules | |||||
|---|---|---|---|---|---|---|---|---|
| Cement (wt %) | Water (wt %) | Sand (wt %) | Cement (wt %) | Water (wt %) | Sand (wt %) | Capsule (wt %) | ||
| WOC *-cement paste | - | 74.08 | 25.92 | - | - | - | - | - |
| N-cement paste | N | 74.07 | 25.93 | - | 72.37 | 25.28 | 0.00 | 2.34 |
| NS1-cement paste | NS1 | 74.07 | 25.93 | - | 72.37 | 25.28 | 0.00 | 2.34 |
| NS2-cement paste | NS2 | 74.07 | 25.93 | - | 72.37 | 25.28 | 0.00 | 2.34 |
| WOC-cement mortar | - | 42.55 | 14.89 | 42.55 | - | - | - | - |
| N-cement mortar | N | 42.56 | 14.89 | 42.56 | 41.47 | 14.53 | 41.47 | 2.53 |
| NS1-cement mortar | NS1 | 42.56 | 14.89 | 42.56 | 41.47 | 14.53 | 41.47 | 2.53 |
| NS2-cement mortar | NS2 | 42.56 | 14.89 | 42.56 | 41.47 | 14.53 | 41.47 | 2.53 |
| Capsule Type | Average Spore Concentration (Cells/mL) | Expected Values (Cells/mL) |
|---|---|---|
| S | 8.47 × 104 ± 1.84 × 104 | 7.39 × 104 |
| NS1 | 7.62 × 104 ± 2.21 × 104 | 7.39 × 104 |
| NS2 | 1.97 × 107 ± 5.65 × 106 | 6.93 × 107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahimizadeh, M.; Diane Abeyratne, A.; Mae, L.S.; Singh, R.K.R.; Pasbakhsh, P. Biological Self-Healing of Cement Paste and Mortar by Non-Ureolytic Bacteria Encapsulated in Alginate Hydrogel Capsules. Materials 2020, 13, 3711. https://doi.org/10.3390/ma13173711
Fahimizadeh M, Diane Abeyratne A, Mae LS, Singh RKR, Pasbakhsh P. Biological Self-Healing of Cement Paste and Mortar by Non-Ureolytic Bacteria Encapsulated in Alginate Hydrogel Capsules. Materials. 2020; 13(17):3711. https://doi.org/10.3390/ma13173711
Chicago/Turabian StyleFahimizadeh, Mohammad, Ayesha Diane Abeyratne, Lee Sui Mae, R. K. Raman Singh, and Pooria Pasbakhsh. 2020. "Biological Self-Healing of Cement Paste and Mortar by Non-Ureolytic Bacteria Encapsulated in Alginate Hydrogel Capsules" Materials 13, no. 17: 3711. https://doi.org/10.3390/ma13173711
APA StyleFahimizadeh, M., Diane Abeyratne, A., Mae, L. S., Singh, R. K. R., & Pasbakhsh, P. (2020). Biological Self-Healing of Cement Paste and Mortar by Non-Ureolytic Bacteria Encapsulated in Alginate Hydrogel Capsules. Materials, 13(17), 3711. https://doi.org/10.3390/ma13173711