Ceramization Mechanism of Ceramizable Silicone Rubber Composites with Nano Silica at Low Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
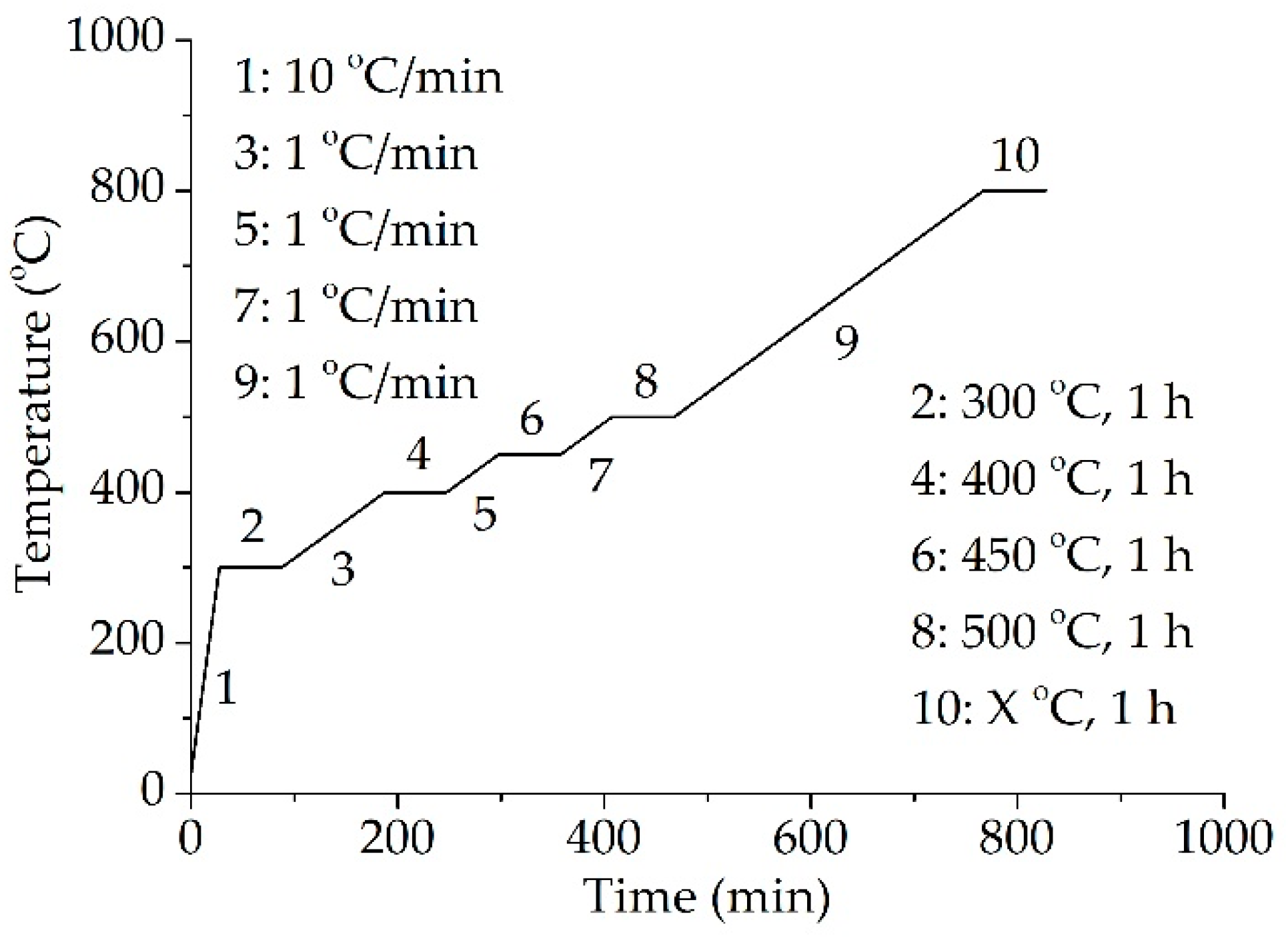
2.2. Preparation of Ceramizable Composites and Ceramic Samples
2.3. Characterization of Ceramizable Composites and Ceramic Samples
3. Results and Discussion
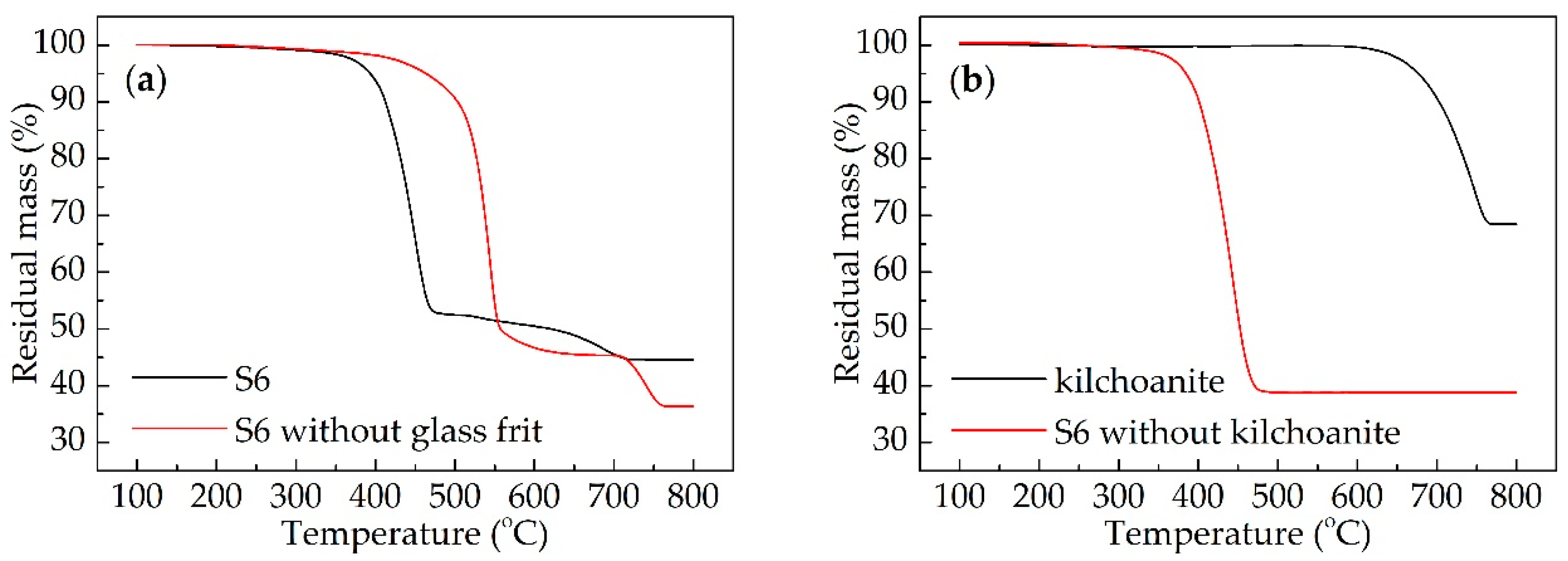
3.1. Thermogravimetric Analysis of Ceramizable Composites
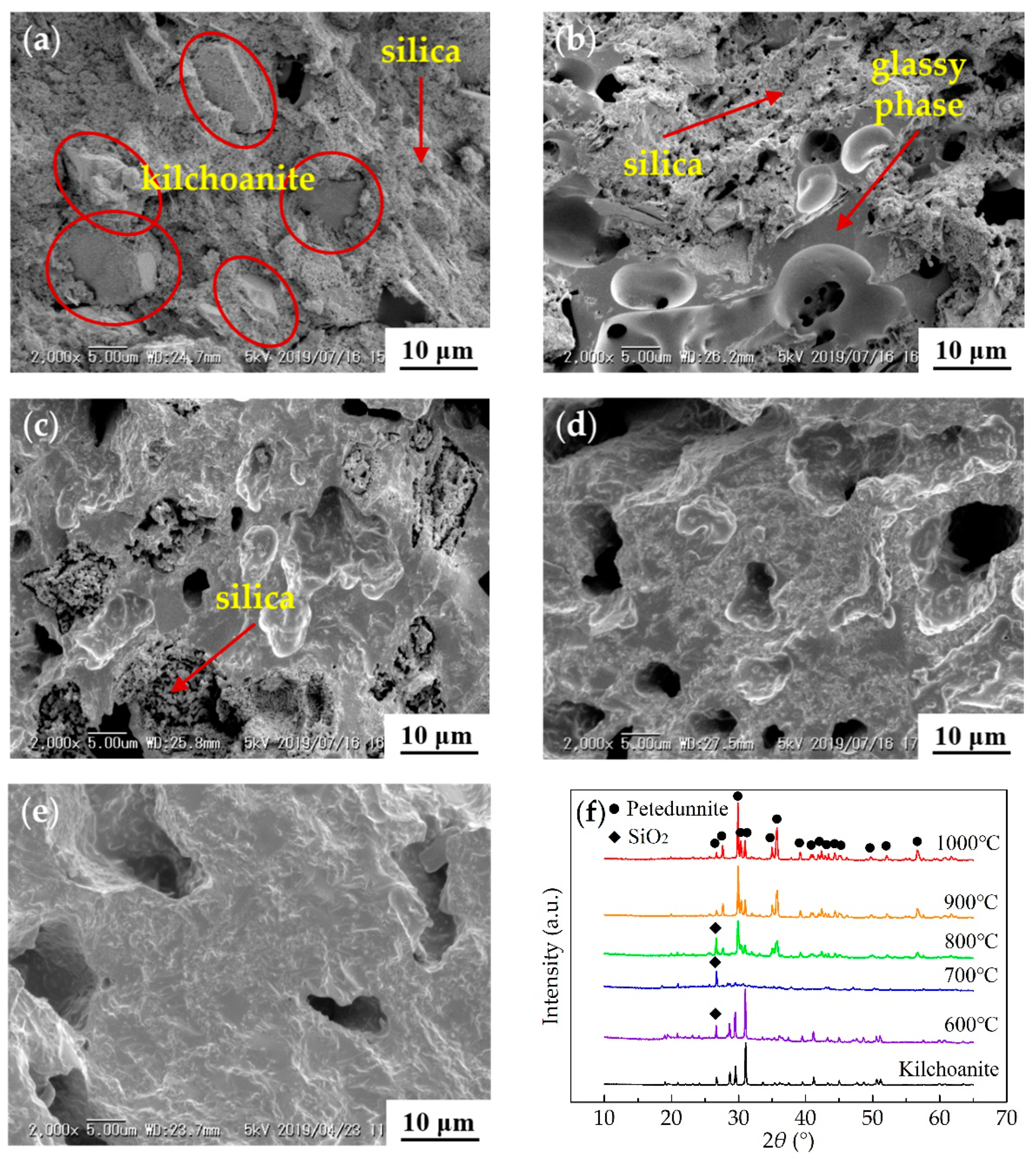
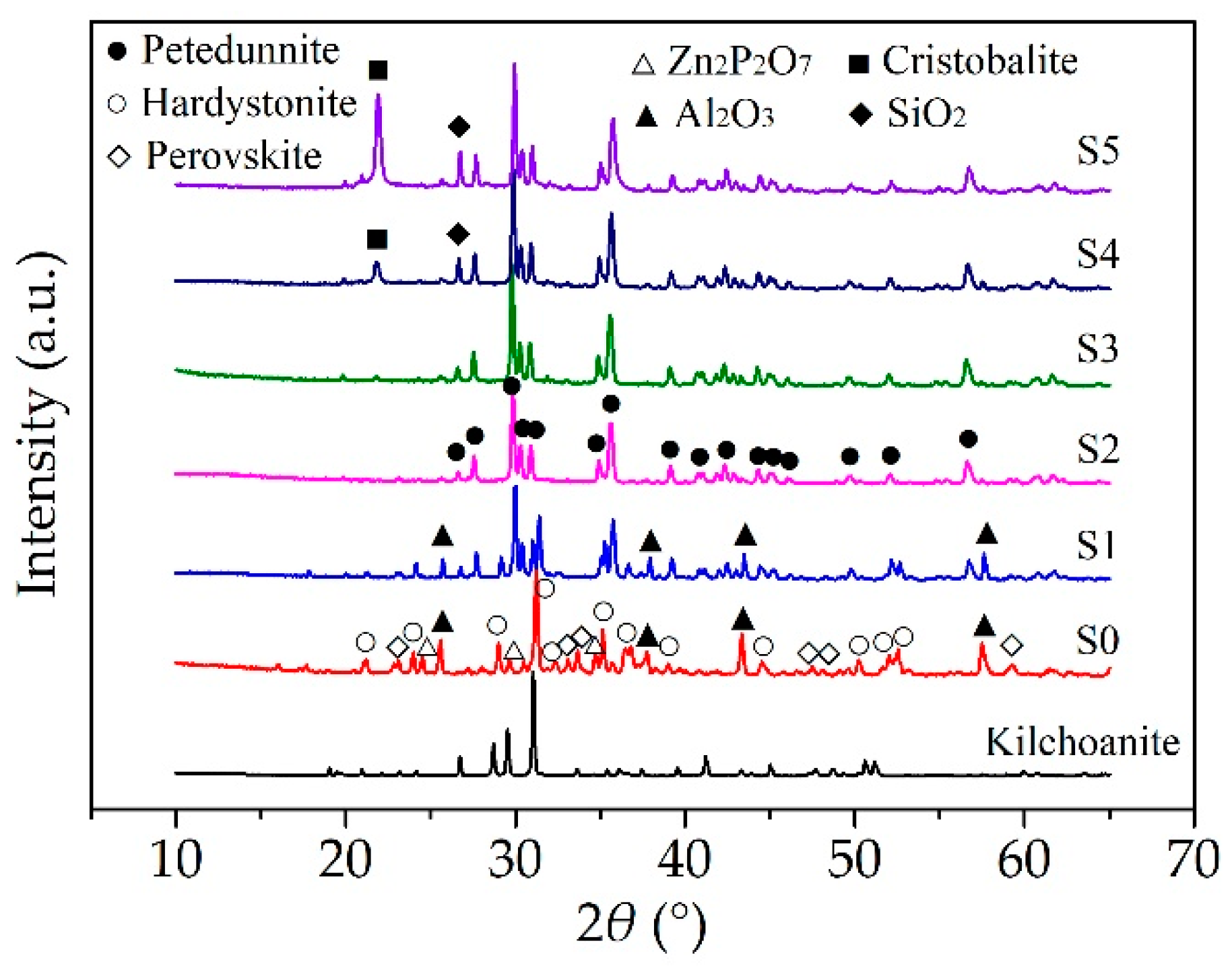
3.2. Ceramization Performance at Different Temperatures
3.3. Effect of Nano Silica on Ceramization
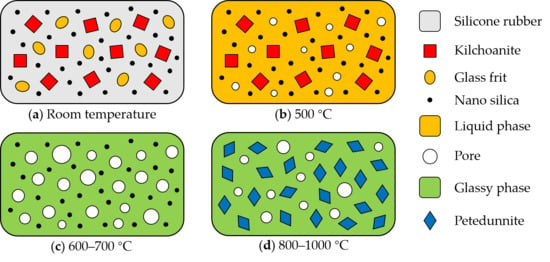
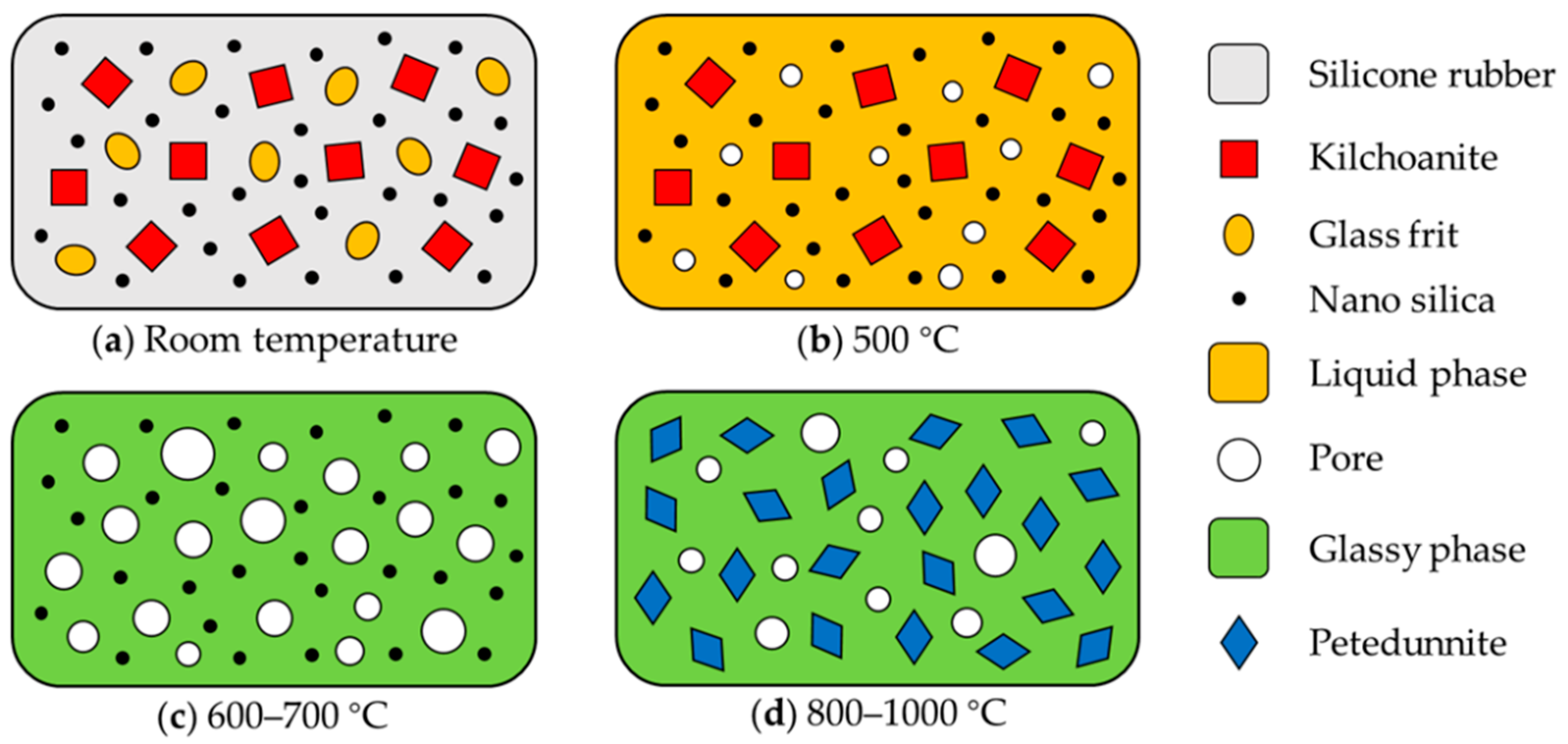
3.4. Ceramization Process and Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
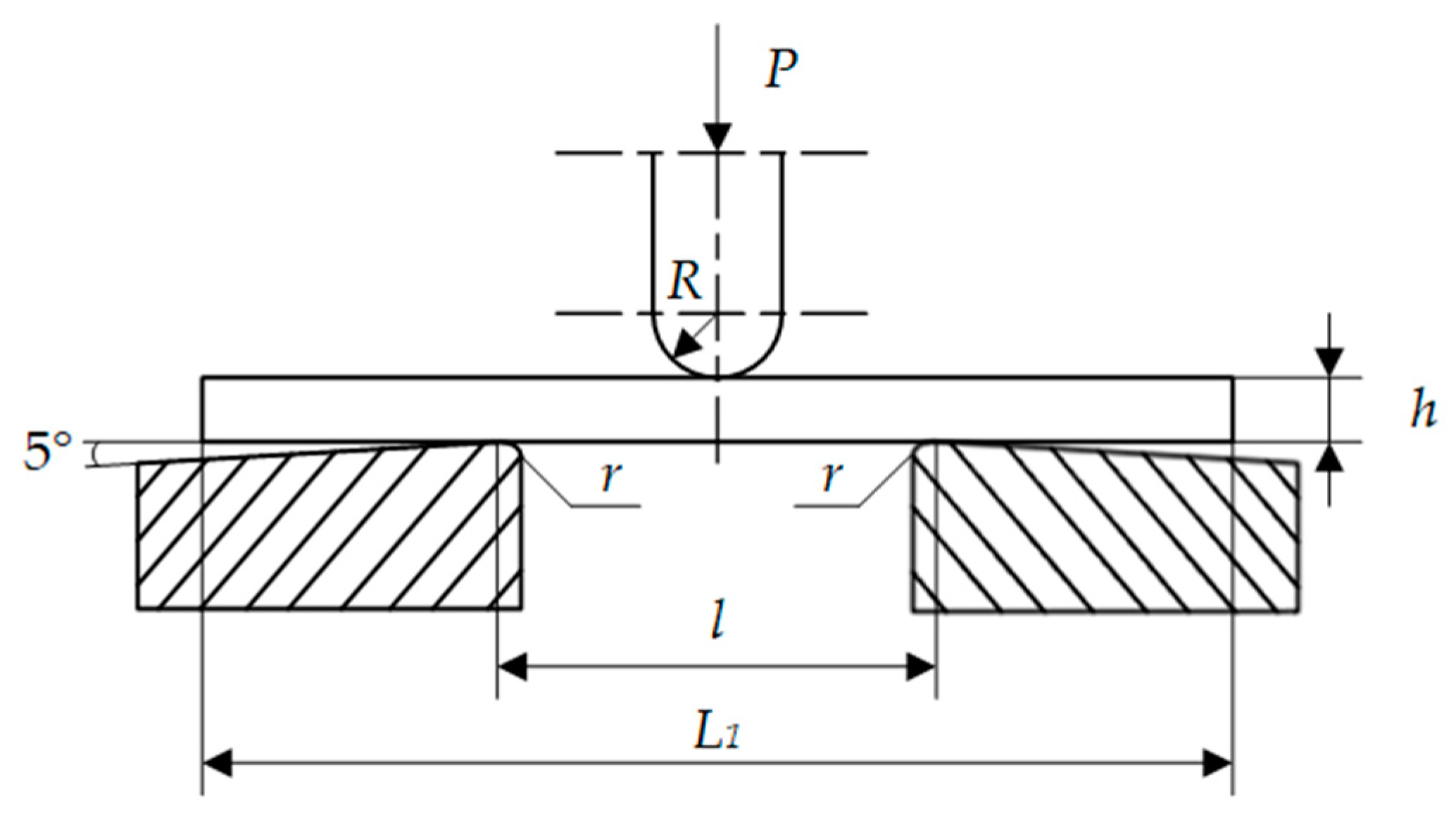
Appendix A. Three-Point Bending Test
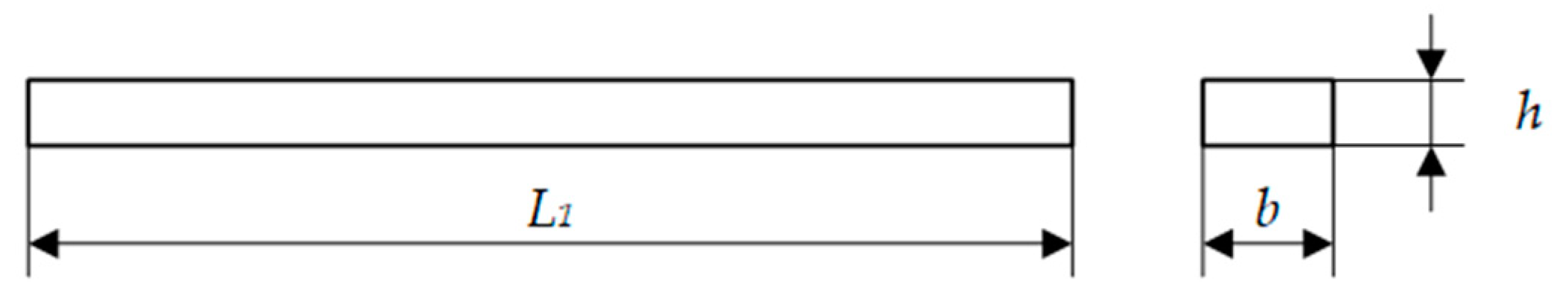
| Samples | Length L1 (mm) | Width b (mm) | Thickness h (mm) |
|---|---|---|---|
| S0 | 78 | 8.386 | 2.330 |
| S1 | 66 | 8.430 | 2.315 |
| S2 | 64 | 8.435 | 2.315 |
| S3 | 62 | 7.860 | 1.575 |
| S4 | 65 | 8.280 | 1.460 |
| S5 | 69 | 8.710 | 1.605 |
| S6 (600 °C) | 73 | 9.250 | 2.140 |
| S6 (700 °C) | 68 | 8.750 | 1.725 |
| S6 (800 °C) | 63 | 7.912 | 1.513 |
| S6 (900 °C) | 62 | 7.944 | 1.577 |
| S6 (1000 °C) | 61 | 7.780 | 1.424 |
References
- Hanu, L.G.; Simon, G.P.; Mansouri, J.; Burford, R.P.; Cheng, Y.B. Development of polymer-ceramic composites for improved fire resistance. J. Mater. Process. Technol. 2004, 153–154, 401–407. [Google Scholar] [CrossRef]
- Mansouri, J.; Burford, R.P.; Cheng, Y.B.; Hanu, L.G. Formation of strong ceramified ash from silicone-based compositions. J. Mater. Sci. 2005, 40, 5741–5749. [Google Scholar] [CrossRef]
- Mansouri, J.; Burford, R.P.; Cheng, Y.B. Pyrolysis behaviour of silicone-based ceramifying composites. Mater. Sci. Eng. A 2006, 425, 7–14. [Google Scholar] [CrossRef]
- Mansouri, J.; Wood, C.A.; Robert, K.; Cheng, Y.B.; Burford, R.P. Investigation of the ceramifying process of modified silicone-silicate compositions. J. Mater. Sci. 2007, 42, 6046–6055. [Google Scholar] [CrossRef]
- Pędzich, Z.; Dul, J. Optimisation of the ceramic phase for ceramizable silicone rubber based composites. Adv. Sci. Technol. 2010, 66, 162–167. [Google Scholar] [CrossRef]
- Hanu, L.G.; Simon, G.P.; Cheng, Y.B. Thermal stability and flammability of silicone polymer composites. Polym. Degrad. Stab. 2006, 91, 1373–1379. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, S.T.; Zou, H.W.; Liang, M. Thermal stability and ablation properties study of aluminum silicate ceramic fiber and acicular wollastonite filled silicone rubber composite. J. Appl. Polym. Sci. 2014, 131, 39700. [Google Scholar] [CrossRef]
- Pędzich, Z.; Bieliński, D.M.; Anyszka, R.; Lach, R.; Ziąbka, M. Ceramizable composites for fire resistant applications. Key Eng. Mater. 2014, 602–603, 290–295. [Google Scholar] [CrossRef]
- Imiela, M.; Anyszka, R.; Bieliński, D.M.; Lipińska, M.; Rybiński, P.; Syrek, B. Synergistic effect of mica, glass Frit, and melamine cyanurate for improving fire resistance of styrene-butadiene rubber composites destined for ceramizable coatings. Coatings 2019, 9, 170. [Google Scholar] [CrossRef]
- Hanu, L.G.; Simon, G.P.; Cheng, Y.B. Preferential orientation of muscovite in ceramifiable silicone composites. Mater. Sci. Eng. A 2005, 398, 180–187. [Google Scholar] [CrossRef]
- Park, E.S. Mechanical properties and processibilty of glass-fiber-, wollastonite-, and fluoro-rubber- reinforced silicone rubber composites. J. Appl. Polym. Sci. 2007, 105, 460–468. [Google Scholar] [CrossRef]
- Pędzich, Z.; Bukańska, A.; Bieliński, D.M.; Anyszka, R.; Dul, J.; Parys, G. Microstructure evolution of silicone rubber-based composites during ceramization in different conditions. Compos. Theory Pract. 2012, 12, 251–255. [Google Scholar]
- Bieliński, D.M.; Anyszka, R.; Pędzich, Z.; Parys, G.; Dul, J. Ceramizable silicone rubber composites. Influence of type of mineral filler on ceramization. Compos. Theory Pract. 2012, 12, 256–261. [Google Scholar]
- Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Szumera, M. Influence of surface-modified montmorillonites on properties of silicone rubber-based ceramizable composites. J. Therm. Anal. Calorim. 2015, 119, 111–121. [Google Scholar] [CrossRef]
- Wang, J.H.; Ji, C.T.; Yan, Y.T.; Zhao, D.; Shi, L.Y. Mechanical and ceramifiable properties of silicone rubber filled with different inorganic fillers. Polym. Degrad. Stab. 2015, 121, 149–156. [Google Scholar] [CrossRef]
- Imiela, M.; Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Zarzecka-Napierała, M.; Szumera, M. Effect of carbon fibers on thermal properties and mechanical strength of ceramizable composites based on silicone rubber. J. Therm. Anal. Calorim. 2016, 124, 197–203. [Google Scholar] [CrossRef]
- Guo, J.H.; Gao, W.; Wang, Y.; Liang, D.; Li, H.J.; Zhang, X. Effect of glass frit with low softening temperature on the properties, microstructure and formation mechanism of polysiloxane elastomer-based ceramizable composites. Polym. Degrad. Stab. 2017, 136, 71–79. [Google Scholar] [CrossRef]
- Lou, F.P.; Cheng, L.H.; Li, Q.Y.; Wei, T.; Guan, X.Y.; Guo, W.H. The combination of glass dust and glass fiber as fluxing agents for ceramifiable silicone rubber composites. RSC Adv. 2017, 7, 38805–38811. [Google Scholar] [CrossRef]
- Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Parys, G.; Rybiński, P.; Zarzecka-Napierała, M.; Imiela, M.; Gozdek, T.; Siciński, M.; Okraska, M.; et al. Effect of mineral filler additives on flammability, processing and use of silicone-based ceramifiable composites. Polym. Bull. 2018, 75, 1731–1751. [Google Scholar] [CrossRef]
- Pędzich, Z.; Bieliński, D.M.; Anyszka, R.; Zarzecka-Napierała, M. Influence of boron oxide on ceramization of silicone-basing composites. In Proceedings of the 12th Conference of the European Ceramic Society, Stockholm, Sweden, 19–23 June 2011. [Google Scholar]
- Rybiński, P.; Syrek, B.; Żukowski, W.; Bradło, D.; Imiela, M.; Anyszka, R.; Blume, A.; Verbouwe, W. Impact of basalt filler on thermal and mechanical properties, as well as fire hazard, of silicone rubber composites, including ceramizable composites. Materials 2019, 12, 2432. [Google Scholar] [CrossRef] [PubMed]
- Rybiński, P.; Syrek, B.; Żukowski, W.; Bradło, D. Impact of basalt filler and ceramizable additives on the toxicity of gaseous products emitted from thermal decomposition of silicone rubber composites. Materials 2019, 12, 3478. [Google Scholar] [CrossRef]
- Guo, J.H.; Zhang, Y.; Li, H.J.; Zhang, X. Effect of the sintering temperature on the microstructure, properties and formation mechanism of ceramic materials obtained from polysiloxane elastomer-based ceramizable composites. J. Alloys Compd. 2016, 678, 499–505. [Google Scholar] [CrossRef]
- Hu, S.; Chen, F.; Li, J.G.; Shen, Q.; Huang, Z.X.; Zhang, L.M. The ceramifying process and mechanical properties of silicone rubber/ammonium polyphosphate/ aluminium hydroxide/mica composites. Polym. Degrad. Stab. 2016, 126, 196–203. [Google Scholar] [CrossRef]
- Lou, F.P.; Yan, W.; Guo, W.H.; Wei, T.; Li, Q.Y. Preparation and properties of ceramifiable flame-retarded silicone rubber composites. J. Therm. Anal. Calorim. 2017, 130, 813–821. [Google Scholar] [CrossRef]
- Guo, J.H.; Chen, X.M.; Zhang, Y. Improving the mechanical and electrical properties of ceramizable silicone rubber/halloysite composites and their ceramic residues by incorporation of different borates. Polymers 2018, 10, 388. [Google Scholar] [CrossRef]
- Tang, H.C.; Li, P.H.; Kuang, G.W.; Liu, H.D.; Jin, H.Y.; Gao, N.K. Effect of glass powder content on properties of ceramizable silicone rubber. J. Chin. Ceram. Soc. 2020, 48, 1–7. [Google Scholar]
- Bieliński, D.M.; Anyszka, R.; Pędzich, Z.; Dul, J. Ceramizable silicone rubber-based composites. Int. J. Adv. Mater. Manuf. Charact. 2012, 1, 17–22. [Google Scholar]
- German, R.M.; Suri, P.; Park, S.J. Review: Liquid phase sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef]
- Zheng, Q.; Lim, L.C. Maps of spreading during the initial stage of liquid-phase sintering. Scr. Mater. 2010, 62, 544–547. [Google Scholar] [CrossRef]


| Components | ZnO | SiO2 | TiO2 | P2O5 | K2O | CaO | Others |
|---|---|---|---|---|---|---|---|
| Content (wt.%) | 43.58 | 27.64 | 10.86 | 7.56 | 7.38 | 2.34 | 0.64 |
| Samples | Silicone Rubber | Hydroxyl Silicone Oil | Nano Silica | Kilchoanite | Glass Frit | DCBP |
|---|---|---|---|---|---|---|
| S0 | 100 | 3 | 0 | 50 | 25 | 1.5 |
| S1 | 100 | 3 | 10 | 50 | 25 | 1.5 |
| S2 | 100 | 3 | 20 | 50 | 25 | 1.5 |
| S3 | 100 | 3 | 30 | 50 | 25 | 1.5 |
| S4 | 100 | 3 | 40 | 50 | 25 | 1.5 |
| S5 | 100 | 3 | 50 | 50 | 25 | 1.5 |
| S6 | 100 | 3 | 30 | 50 | 30 | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Jin, H.; Wei, S.; Liu, H.; Gao, N.; Shi, Z. Ceramization Mechanism of Ceramizable Silicone Rubber Composites with Nano Silica at Low Temperature. Materials 2020, 13, 3708. https://doi.org/10.3390/ma13173708
Li P, Jin H, Wei S, Liu H, Gao N, Shi Z. Ceramization Mechanism of Ceramizable Silicone Rubber Composites with Nano Silica at Low Temperature. Materials. 2020; 13(17):3708. https://doi.org/10.3390/ma13173708
Chicago/Turabian StyleLi, Penghu, Haiyun Jin, Shichao Wei, Huaidong Liu, Naikui Gao, and Zhongqi Shi. 2020. "Ceramization Mechanism of Ceramizable Silicone Rubber Composites with Nano Silica at Low Temperature" Materials 13, no. 17: 3708. https://doi.org/10.3390/ma13173708
APA StyleLi, P., Jin, H., Wei, S., Liu, H., Gao, N., & Shi, Z. (2020). Ceramization Mechanism of Ceramizable Silicone Rubber Composites with Nano Silica at Low Temperature. Materials, 13(17), 3708. https://doi.org/10.3390/ma13173708