Structural Characterization and Comparison of Monovalent Cation-Exchanged Zeolite-W
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Synchrotron X-ray Powder Diffraction
2.3. Structural Analysis by Rietveld Refinement
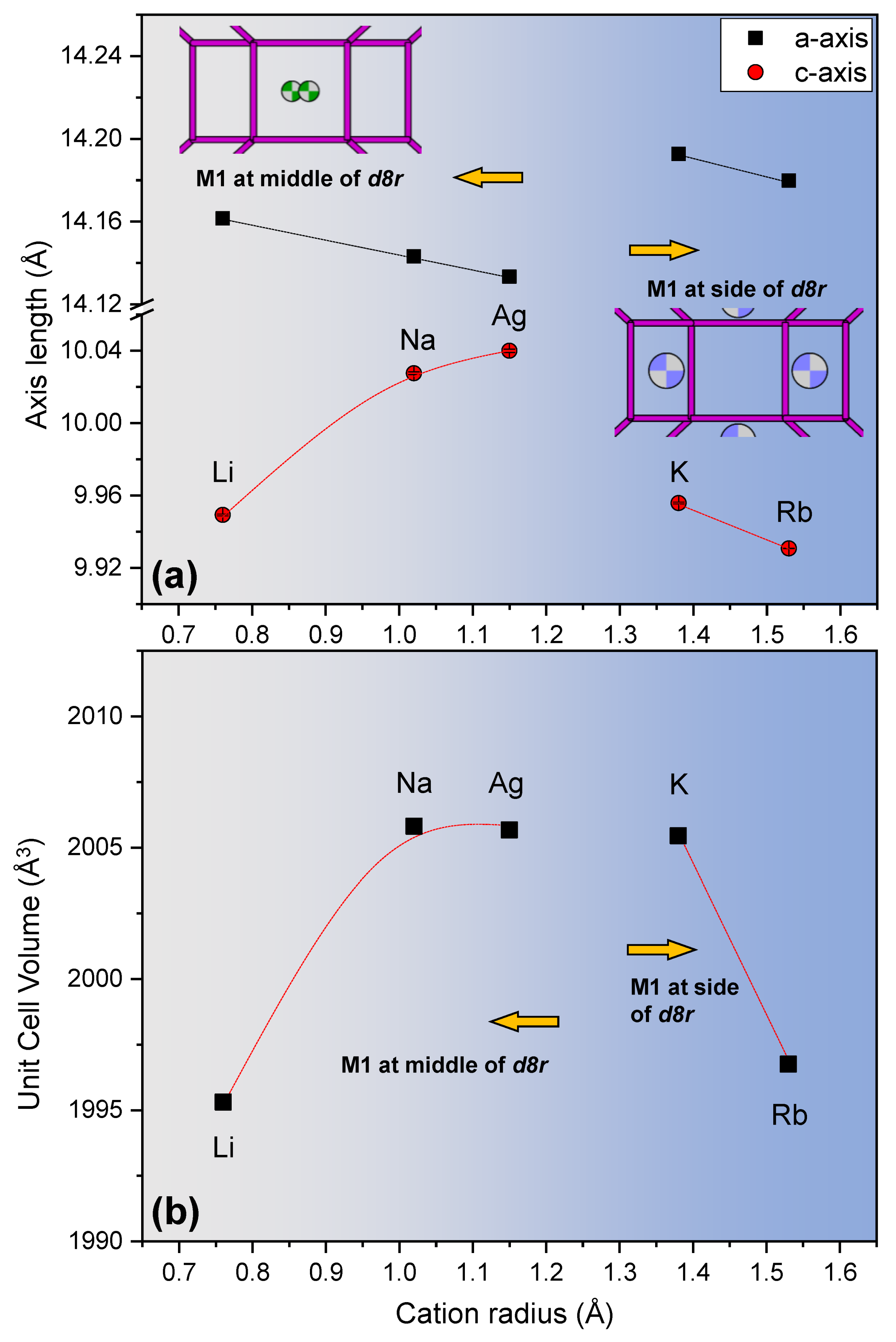
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, P.S.; Chen, G.F. General Introduction to Porous Materials. In Porous Materials; Liu, P.S., Chen, G.F., Eds.; Butterworth-Heinemann: Boston, UK, 2014. [Google Scholar]
- Tabacchi, G. Supramolecular Organization in Confined Nanospaces. ChemPhysChem 2018, 19, 1249–1297. [Google Scholar] [CrossRef] [PubMed]
- Calzaferri, G. Nanochannels: Hosts for the Supramolecular Organization of Molecules and Complexes. Langmuir 2012, 28, 6216–6231. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.S.; Chen, G.F. Characterization Methods: Basic Factors. In Porous Materials; Liu, P.S., Chen, G.F., Eds.; Butterworth-Heinemann: Boston, UK, 2014. [Google Scholar]
- Plank, C.J.; Rosinski, E.J.; Hawthorne, W.P. Acidic Crystalline Aluminosilicates. New Superactive, Superselective Cracking Catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1964, 3, 165–169. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves: Strcuture, Chemistry, and Use; John Wiley and Sons: New York, NY, USA, 1974. [Google Scholar]
- Beyer, H.; Jacobs, P.A.; Uytterhoeven, J.B. Redox behaviour of transition metal ions in zeolites. Part 2—Kinetic study of the reduction and reoxidation of silver-Y zeolites. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1976, 72, 674–685. [Google Scholar] [CrossRef]
- Jacobs, P.A.; Uytterhoeven, J.B.; Beyer, H.K. Redox behaviour of transition metal ions in zeolites. Part 6—Reversibility of the reduction reaction in silver zeolites. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1977, 73, 1755–1762. [Google Scholar] [CrossRef]
- Ackley, M.W. Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 2003, 61, 25–42. [Google Scholar] [CrossRef]
- Gatta, G.D.; Lee, Y. Zeolites at high pressure: A review. Miner. Mag. 2014, 78, 267–291. [Google Scholar] [CrossRef]
- Fee, J.P.H.; Murray, J.M.; Luney, S.R. Molecular sieves: An alternative method of carbon dioxide removal which does not generate compound A during simulated low-flow sevoflurane anaesthesia. Anaesthesia 1995, 50, 841–845. [Google Scholar] [CrossRef]
- Segawa, K.; Shimura, T. Effect of dealuminatation of mordenite catalyst for amination reaction of ethanoplamine. In A New Era of Catecholamines in the Laboratory and Clinic; Elsevier BV: Amsterdam, The Netherlands, 2000; Volume 130, pp. 2975–2980. [Google Scholar]
- Fujimoto, K.; Bischoff, S.; Omata, K.; Yagita, H. Hydrogen effects on nickel-catalyzed vapor-phase methanol carbonylation. J. Catal. 1992, 133, 370–382. [Google Scholar] [CrossRef]
- Altwasser, S.; Welker, C.; Traa, Y.; Weitkamp, J. Catalytic cracking of n-octane on small-pore zeolites. Microporous Mesoporous Mater. 2005, 83, 345–356. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H.; Baerlocher, C.; Burton, A.W.; Baur, W.H.; Broach, R.W.; Dorset, D.L.; Fischer, R.X.; Gies, H.; et al. Preface. In Atlas of Zeolite Framework Types; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007. [Google Scholar]
- International Zeolite Association. Available online: http://www.iza-structure.org/databases (accessed on 19 August 2020).
- Barrett, P.A.; Valencia, S.; Camblor, M.A. Synthesis of a merlinoite-type zeolite with an enhanced Si/Al ratioviapore filling with tetraethylammonium cations. J. Mater. Chem. 1998, 8, 2263–2268. [Google Scholar] [CrossRef]
- Quirin, J.C.; Yuen, L.; Zones, S.I. Merlinoite synthesis studies with and without organocations. J. Mater. Chem. 1997, 7, 2489–2494. [Google Scholar] [CrossRef]
- Bieniok, A.; Bornholdt, K.; Brendel, U.; Baur, W.H. Synthesis and crystal structure of zeolite W, resembling the mineral merlinoite. J. Mater. Chem. 1996, 6, 271. [Google Scholar] [CrossRef]
- Belhekar, A.; Chandwadkar, A.; Hegde, S. Physicochemical characterization of a synthetic merlinoite (Linde W-like) zeolite containing Na, K, and Sr cations. Zeolites 1995, 15, 535–539. [Google Scholar] [CrossRef]
- Chen, W.; Guo, Q.; Yang, C.; Hou, J. Preparation of novel functional MER zeolite membrane for potassium continuous extraction from seawater. J. Porous Mater. 2017, 25, 215–220. [Google Scholar] [CrossRef]
- Houlleberghs, M.; Breynaert, E.; Asselman, K.; Vaneeckhaute, E.; Radhakrishnan, S.; Anderson, M.W.; Taulelle, F.; Haouas, M.; Martens, J.A.; Kirschhock, C.E.A. Evolution of the crystal growth mechanism of zeolite W (MER) with temperature. Microporous Mesoporous Mater. 2019, 274, 379–384. [Google Scholar] [CrossRef]
- Itabashi, K.; Ikeda, T.; Matsumoto, A.; Kamioka, K.; Kato, M.; Tsutsumi, K. Syntheses and structural properties of four Rb-aluminosilicate zeolites. Microporous Mesoporous Mater. 2008, 114, 495–506. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld refinement of Debye–Scherrer synchrotron X-ray data from Al2O3. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- Larson, A.C.; VonDreele, R.B. GSAS: General Structure Analysis System Report LAUR; Los Alamos National Laboratory, LAUR: Los Alamos, NM, USA, 1986; pp. 86–748. [Google Scholar]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Dollase, W.A. Correction of intensities for preferred orientation in powder diffractometry: Application of the March model. J. Appl. Crystallogr. 1986, 19, 267–272. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Sect. B Struct. Sci. 1985, 41, 244–247. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seoung, D.; Kim, H.; Kim, P.; Song, C.; Lee, S.; Chae, S.; Lee, S.; Lee, H.; Lee, Y. Structural Characterization and Comparison of Monovalent Cation-Exchanged Zeolite-W. Materials 2020, 13, 3684. https://doi.org/10.3390/ma13173684
Seoung D, Kim H, Kim P, Song C, Lee S, Chae S, Lee S, Lee H, Lee Y. Structural Characterization and Comparison of Monovalent Cation-Exchanged Zeolite-W. Materials. 2020; 13(17):3684. https://doi.org/10.3390/ma13173684
Chicago/Turabian StyleSeoung, Donghoon, Hyeonsu Kim, Pyosang Kim, Chihyun Song, Suhyeong Lee, Sungmin Chae, Sihyun Lee, Hyunseung Lee, and Yongmoon Lee. 2020. "Structural Characterization and Comparison of Monovalent Cation-Exchanged Zeolite-W" Materials 13, no. 17: 3684. https://doi.org/10.3390/ma13173684
APA StyleSeoung, D., Kim, H., Kim, P., Song, C., Lee, S., Chae, S., Lee, S., Lee, H., & Lee, Y. (2020). Structural Characterization and Comparison of Monovalent Cation-Exchanged Zeolite-W. Materials, 13(17), 3684. https://doi.org/10.3390/ma13173684






