Effect of Mg Treatment on the Nucleation and Ostwald Growth of Inclusions in Fe-O-Al-Mg Melt
Abstract
1. Introduction
2. Experimental
Experimental Procedure
3. Measurement of Inclusions
4. Results and Discussion
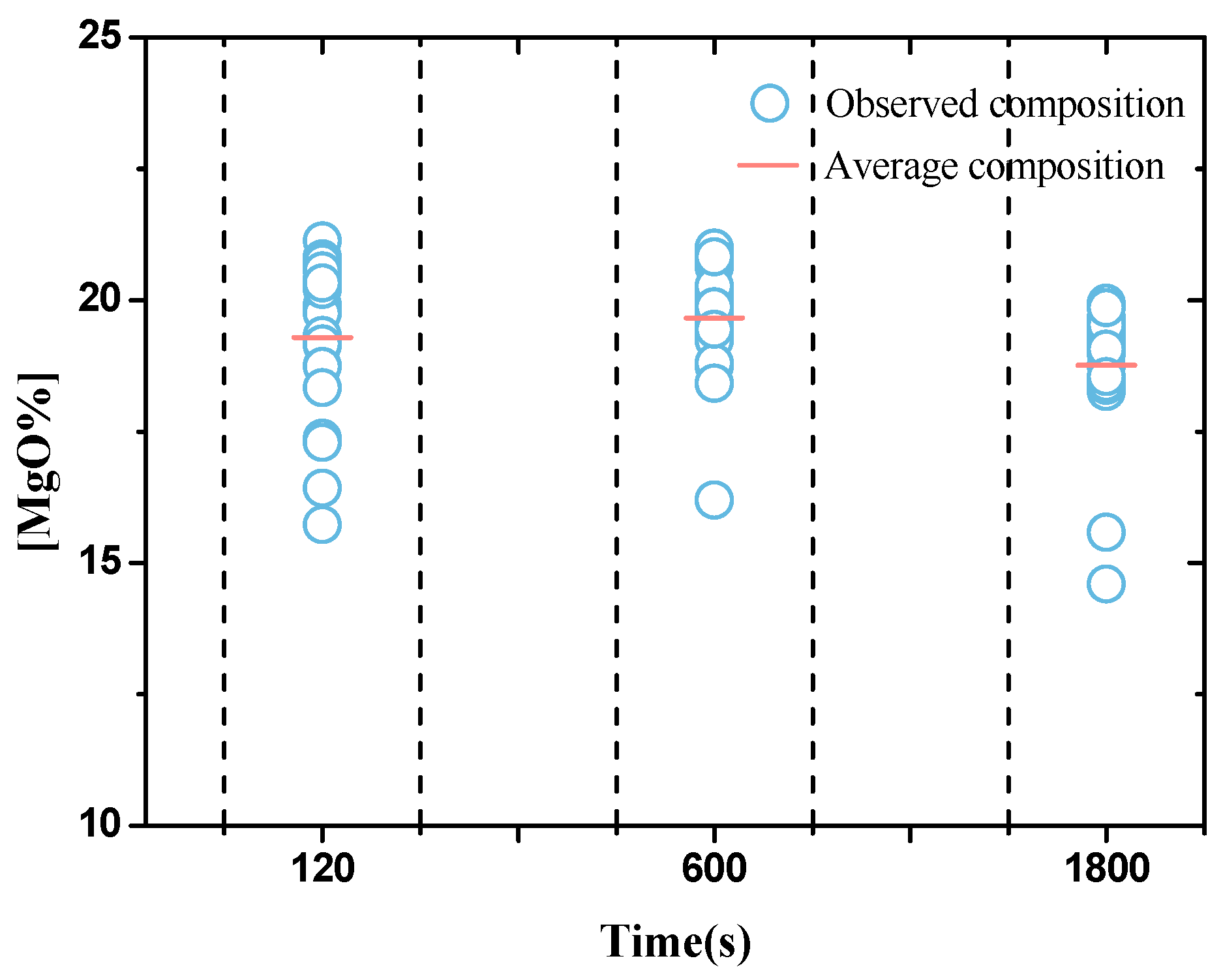
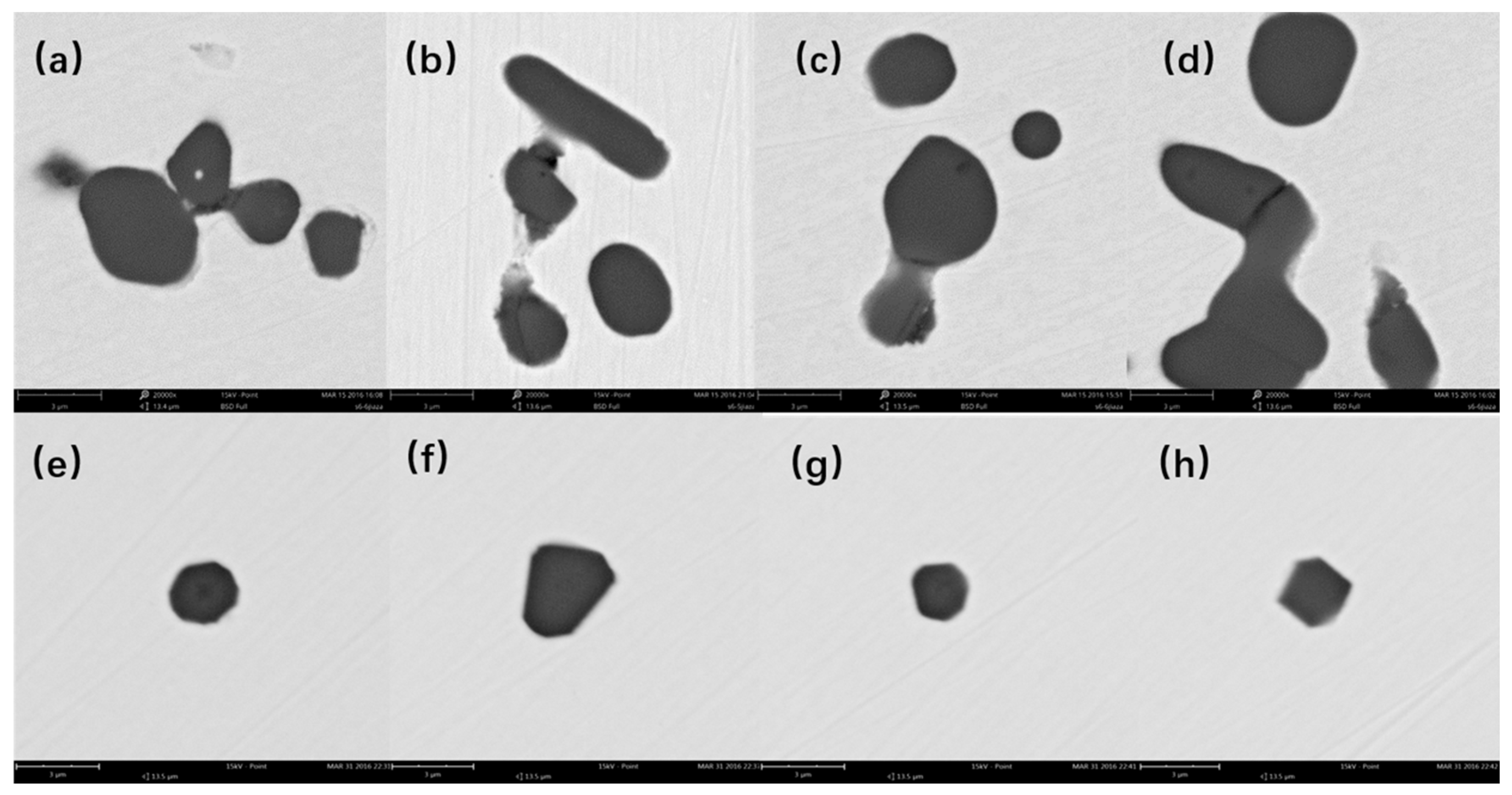
4.1. Composition and Morphologies of Inclusion
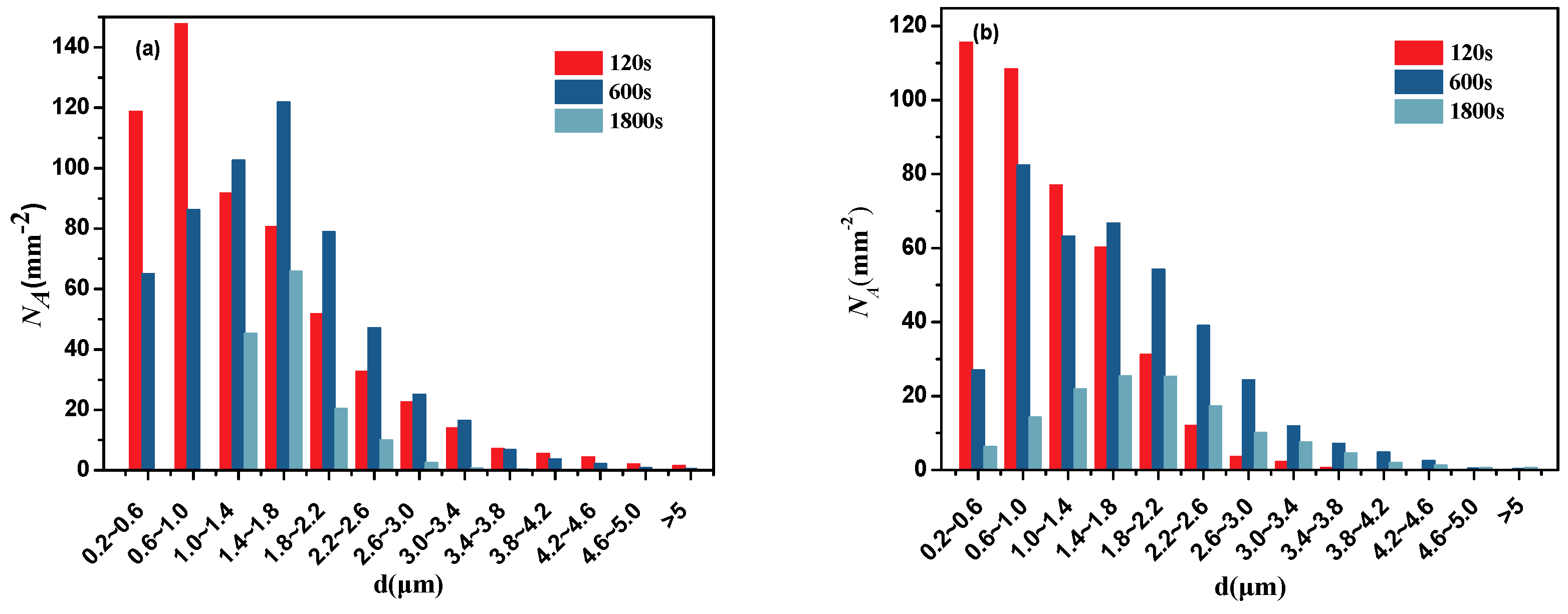
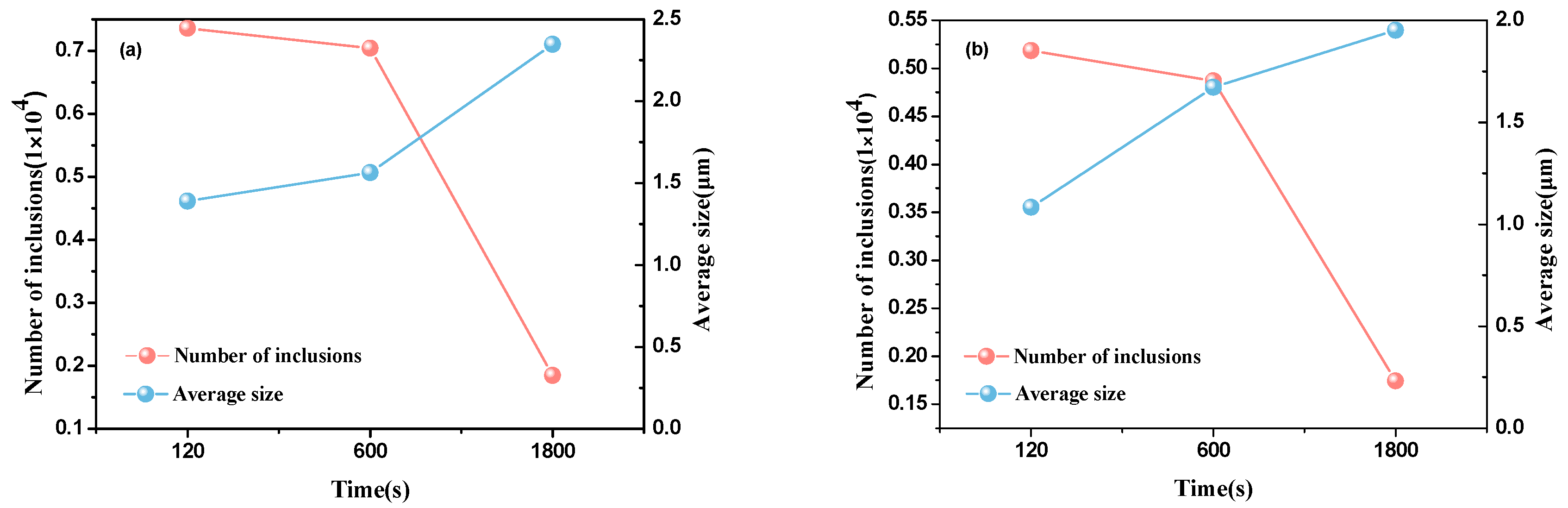
4.2. Characteristics of Inclusions
5. Calculation
5.1. Calculated Nucleation Rate and Critical Size of Nuclei
5.2. Ostwald Growth
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarma, D.S.; Karasev, A.V.; Jonsson, P.G. On the role of non-metallic inclusions in the nucleation of acicular ferrite in steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef]
- Ishikawa, F.; Takahashi, T.; Ochi, T. Intragranular ferrite nucleation in medium-carbon vanadium steels. Metall. Mater. Trans. A 1994, 25, 929–936. [Google Scholar] [CrossRef]
- Van Ende, M.-A.; Guo, M.; Proost, J.; Blanpain, B.; Wollants, P. Formation and morphology of Al2O3 inclusions at the onset of liquid Fe deoxidation by Al addition. ISIJ Int. 2011, 51, 27–34. [Google Scholar] [CrossRef]
- Braun, T.B.; Elliott, J.F.; Flemings, M.C. The clustering of alumina inclusions. Metall. Trans. B 1979, 10, 171–184. [Google Scholar] [CrossRef]
- Verma, N.; Pistorius, P.C.; Fruehan, R.J.; Potter, M.S.; Oltmann, H.G.; Pretorius, E.B. Calcium Modification of Spinel Inclusions in Aluminum-Killed Steel: Reaction Steps. Metall. Mater. Trans. B 2012, 43B, 830–840. [Google Scholar] [CrossRef]
- Verma, N.; Pistorius, P.C.; Fruehan, R.J.; Potter, M.S.; Lind, M.; Story, S. Transient Inclusion Evolution During Modification of Alumina Inclusions by Calcium in Liquid Steel: Part I. Background, Experimental Techniques and Analysis Methods. Metall. Mater. Trans. B 2011, 42B, 711–719. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.-B.; Kim, D.S. Inclusion control of ferritic stainless steel by aluminum deoxidation and calcium treatment. Metall. Mater. Trans. B 2005, 36, 67–73. [Google Scholar] [CrossRef]
- Saxena, S.K. Production of ultra-clean steels with better mechanical properties with magnesium treatment. In Proceedings of the Seventy Ninth Conference of the Steelmaking Division of the Iron and Steel Society, Pittsburgh, PA, USA, 24–27 March 1996; pp. 89–96. [Google Scholar]
- Miki, Y.; Thomas, B.G. Modeling of inclusion removal in a tundish. Metall. Mater. Trans. B 1999, 30B, 639–654. [Google Scholar] [CrossRef]
- Zhang, L.; Taniguchi, S.; Cai, K. Fluid flow and inclusion removal in continuous casting tundish. Metall. Mater. Trans. B 2000, 31B, 253–266. [Google Scholar] [CrossRef]
- Zhang, L.; Aoki, J.; Thomas, B.G. Inclusion removal by bubble flotation in a continuous casting mold. Metall. Mater. Trans. B 2006, 37B, 361–379. [Google Scholar] [CrossRef]
- Min, Y.; Li, X.; Yu, Z.; Liu, C.; Jiang, M. Characterization of the Acicular Ferrite in Al-Deoxidized Low-Carbon Steel Combined with Zr and Mg Additions. Steel Res. Int. 2016, 87, 1503–1510. [Google Scholar] [CrossRef]
- Lowe, J.H.; Mitchell, A. Clean Steel, Superclean steel. In Proceedings of the Institute of Materials, London, UK, 6–7 March 1995. [Google Scholar]
- Fu, J.; Yu, Y.G.; Wang, A.R.; Chen, B.P.; Sun, W.S. Inclusion Modification with Mg Treatment for 35CrNi3MoV Steel. J. Mater. Sci. Technol. 1998, 14, 53–56. [Google Scholar]
- Wang, L.; Yang, S.; Li, J.; Zhang, S.; Ju, J. Effect of Mg Addition on the Refinement and Homogenized Distribution of Inclusions in Steel with Different Al Contents. Metall. Mater. Trans. B 2017, 48, 805–818. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Yang, M. The Influencing Factor of MgAl2O4 on Heterogeneous Nucleation and Grain Refinement in Al Alloy Melts. Materials 2020, 13, 231. [Google Scholar] [CrossRef]
- Kimura, S.; Nakajima, K.; Mizoguchi, S. Behavior of alumina-magnesia complex inclusions and magnesia inclusions on the surface of molten low-carbon steels. Metall. Mater. Trans. B 2001, 32, 79–85. [Google Scholar] [CrossRef]
- Suito, H.; Ohta, H. Characteristics of Inclusion Size Distribution in Early Stage of Deoxidation. ISIJ Int. 2006, 46, 33–41. [Google Scholar] [CrossRef]
- Kluken, A.O.P.G. Mechanisms of inclusion formation in Al-Ti-Si-Mn deoxidized steel weld metals. Metall. Mater. Trans. A 1989, 20, 1335–1349. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamaguchi, R.; Murakami, K.; Nakada, M. Inclusion Growth during Solidificatio of Stainless Steel. ISIJ Int. 2001, 41, 247–256. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, J.; Wang, R.; Yang, Z. Effect of Mg Addition on Inhibiting Austenite Grain Growth in Heat Affected Zones of Ti-Bearing Low Carbon Steels. J. Iron Steel Res. Int. 2011, 18, 60–64. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Effects of dissolved oxygen and size distribution on particle coarsening of deoxidation product. ISIJ Int. 2006, 46, 42–49. [Google Scholar] [CrossRef]
- Baumgartner, J.; Dey, A.; Bomans, P.H.; Le Coadou, C.; Fratzl, P.; Sommerdijk, N.A.; Faivre, D. Nucleation and growth of magnetite from solution. Nat. Mater. 2013, 12, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.C.; Wang, Q.; Li, S.L.; Ai, X.G.; Fan, C.G. Evidence of multi-step nucleation leading to various crystallization pathways from an Fe-O-Al melt. Sci. Rep. 2014, 4, 50–82. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Characteristics of Particle Size Distribution of Deoxidation Products with Mg, Zr, Al, Ca, Si/Mn and Mg/Al in Fe-10mass%Ni Alloy. ISIJ Int. 2006, 46, 14–21. [Google Scholar] [CrossRef]
- Jung, I.H.; Decterov, S.A.; Pelton, A.D. Critical thermodynamic evaluation and optimization of the MgO-Al2O3, CaO-MgO- Al2O3, and MgO- Al2O3 -SiO2 Systems. J. Phase Equilibria Diffus. 2004, 25, 329–345. [Google Scholar] [CrossRef]
- Jimbo, I.; Cramb, A.W. Computer Aided Interfacial Measurements. ISIJ Int. 1992, 32, 26–35. [Google Scholar] [CrossRef]
- Keene, B.J. Review of data for the surface tension of iron and its binary alloys. Int. Mater. Rev. 1998, 33, 1–35v. [Google Scholar] [CrossRef]
- Poirier, D.R.; Yin, H.; Suzuki, M.; Emi, T. Interfacial Properties of Dilute Fe-O-S Melts on Alumina Substrates. ISIJ Int. 1998, 38, 229–238. [Google Scholar] [CrossRef]
- Zhao, L.; Sahajwalla, V. Interfacial phenomena during wetting of graphite/alumina mixtures by liquid iron. ISIJ Int. 2003, 43, 1–6. [Google Scholar] [CrossRef]
- Shibata, H.; Jiang, X.; Valdez, M.; Cramb, A.W. The contact angle between liquid iron and a single-crystal magnesium oxide substrate at 1873 K. Metall. Mater. Trans. B 2004, 35B, 179–181. [Google Scholar] [CrossRef]
- Humenik, M.; Kingery, W.D. Metal-Ceramic Interactions: III, Surface Tension and Wettability of Metal-Ceramic Systems. J. Am. Ceram. Soc. 1954, 37, 18–23. [Google Scholar] [CrossRef]
- Fang, C.M.; Parker, S.C.; de With, G. Atomistic simulation of the surface energy of spinel MgAl2O4. J. Am. Ceram. Soc. 2000, 83, 2082–2084. [Google Scholar] [CrossRef]
- Shibata, H.; Watanabe, Y.; Nakajima, K.; Kitamura, S.Y. Degree of Undercooling and Contact Angle of Pure Iron at 1933K on Single-crystal Al2O3, MgO, and MgAl2O4 under Argon Atmosphere with Controlled Oxygen Partial Pressure. ISIJ Int. 2009, 49, 985–991. [Google Scholar] [CrossRef][Green Version]
- Bruce, R.H. Science Ceramics; Stewart, G.H., Ed.; Academic Press: New York, NY, USA, 1965; Volume 2, pp. 359–367. [Google Scholar]
- Hino, M.; Ito, K. Thermodynamic Data for Steelmaking, 2nd ed.; Tohoku University Press: Tohoku, Japan, 2010; pp. 247–264. [Google Scholar]
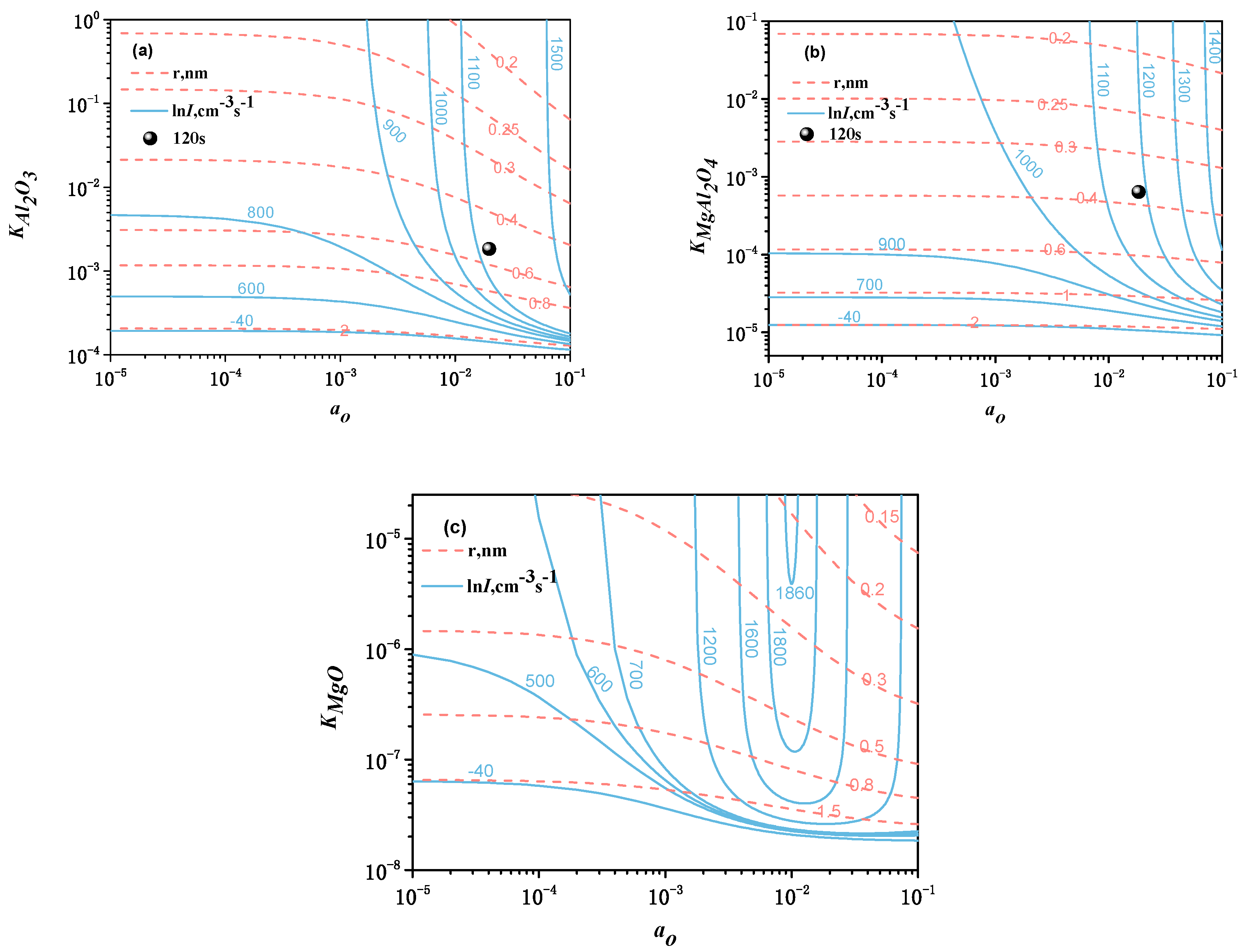
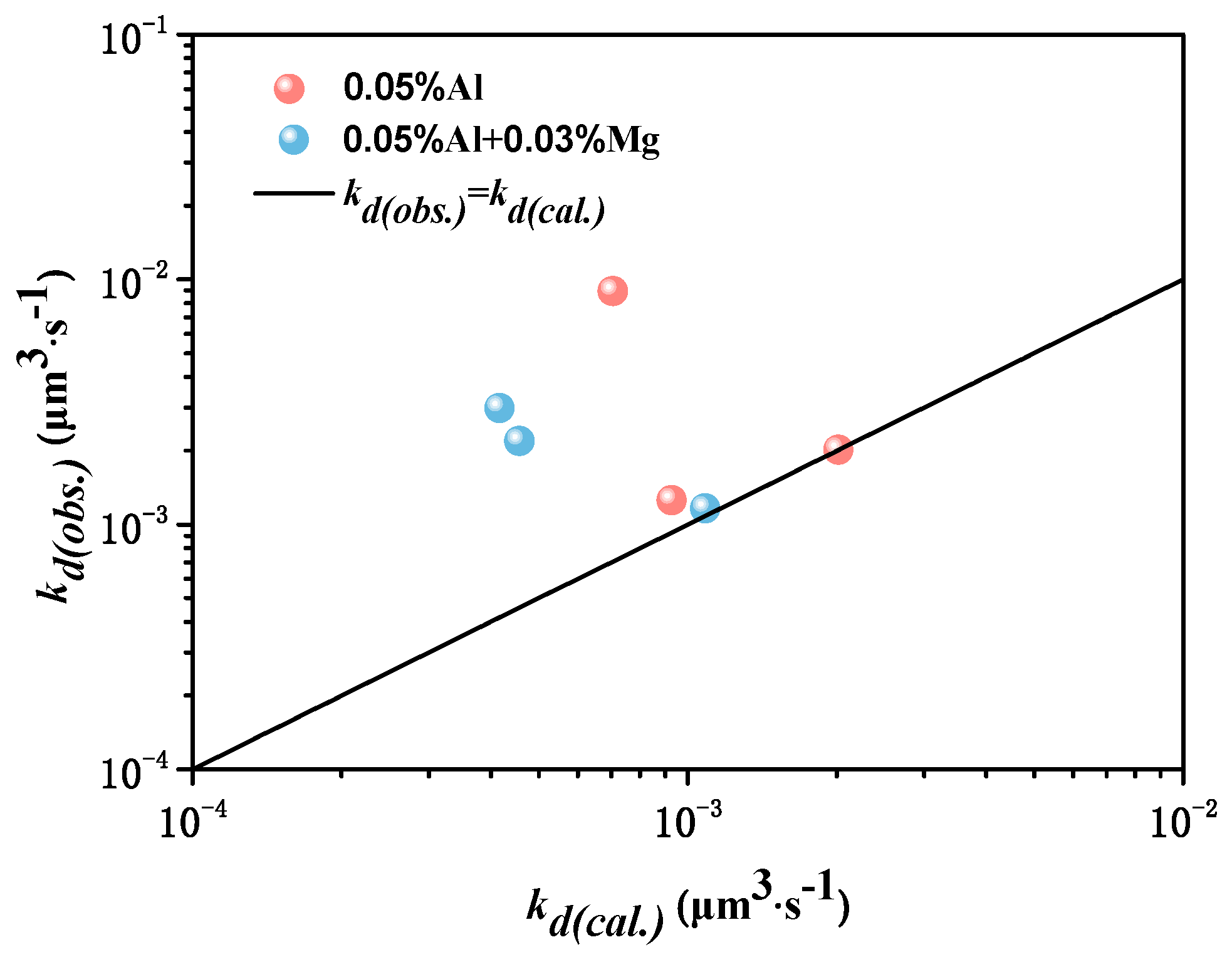
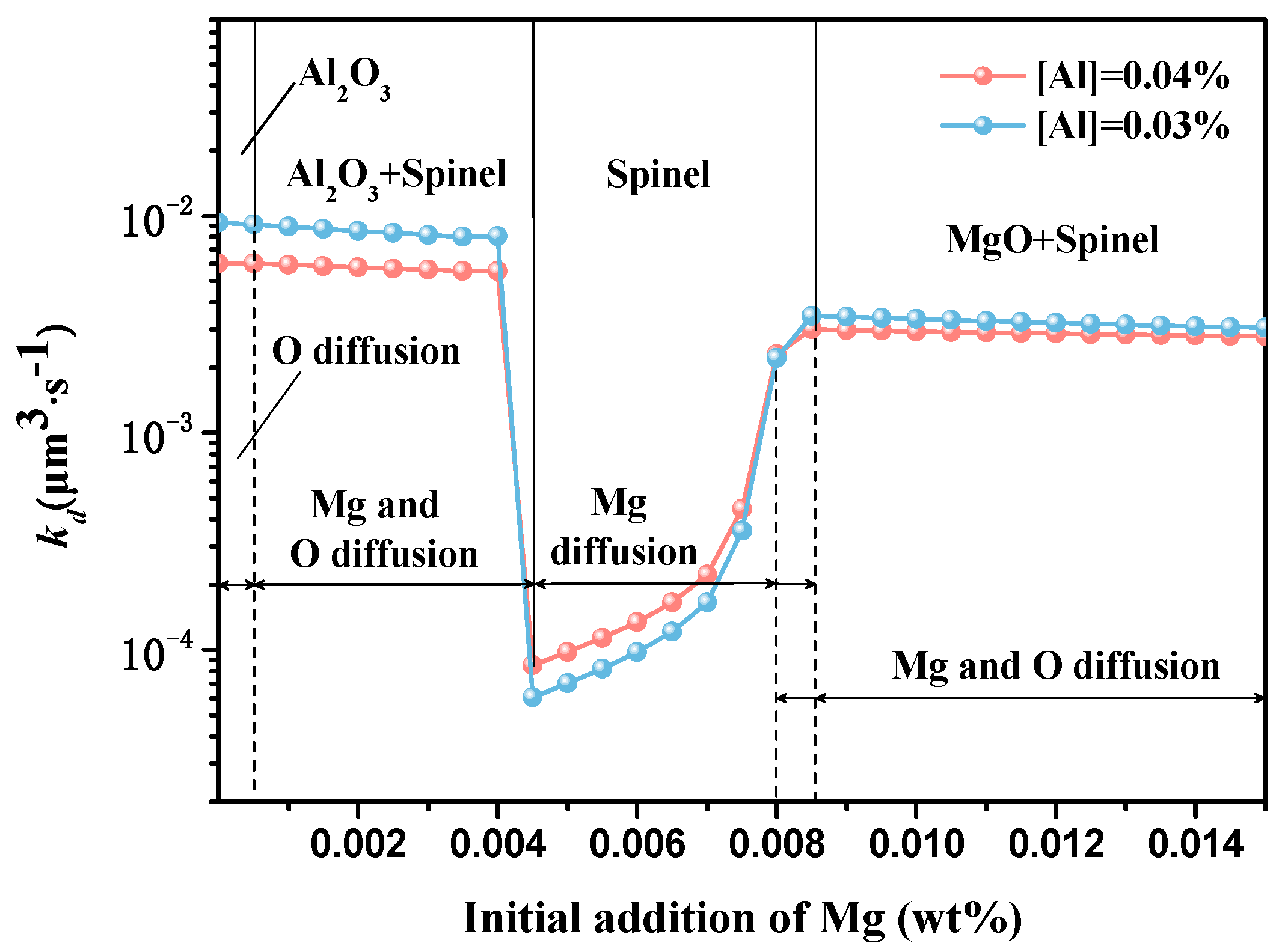
| Element | C | Si | Mn | P | S | Cr | Al | Cu | Ni | Ti | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.0016 | 0.0033 | 0.01 | 0.0053 | 0.0017 | 0.0107 | 0.003 | 0.0037 | 0.0038 | 0.001 | 0.0020 |
| Deoxidizer | Holding Time | [O] | [Al] | [Mg] | |||
|---|---|---|---|---|---|---|---|
| Total | Sol. | Sol. | Insol. | Sol. | Insol. | ||
| (Mass ppm) | (Mass ppm) | (Mass ppm) | (Mass ppm) | (Mass ppm) | (Mass ppm) | ||
| 0.05%Al | 120 s | 218 | 7.32 | 310 | 94 | - | - |
| 600 s | 90.9–92.8 | 3.6 | - | - | - | - | |
| 1800 s | 14.7–117 | 3.38 | 280 | 79 | - | - | |
| 0.05%Al+0.03%Mg | 120 s | 208 | 4.08 | 390 | 85 | 8–19 | - |
| 600 s | 162 | 3.44 | - | - | - | - | |
| 1800 s | 63 | 2.93 | 350 | 23 | 5 | - | |
| Element | C | Si | Mn | P | N | Al |
| 0.065[C pct] [27] | 0.026[Si pct] [28] | 0.05[Mn pct] [28] | 0.025[P pct] [28] | 5.585[N pct] [28] | 0.037[Al pct] [28] | |
| Element | Cr | Cu | Ni | S | O | |
| 0.008[Cr pct] [28] | 0.026[Cu pct] [28] | 0.002[Ni pct] [28] | 0.2ln(1+330[pct S]) [29] | 0.279 ln(1+140[]) [30] |
| Oxide | Θ (deg) | γSV (J/m2) | VO (m3/mol) |
|---|---|---|---|
| Al2O3 | 132 − 6.3ln(1 + 400[pctO])0.63ln(1 + 640[pctS]) [29] | 1.128 − 0.0001T [30] | 8.6 × 10−6 |
| MgO | 117 − 7.4ln(1 + 720aO) (−15 < logaO < 9) [31] | 0.86 [32] | 11 × 10−6 |
| MgAl2O4 | 105 [33] | 2.270 − 0.0006T [34,35] | 9.3 × 10−6 |
| Equation | logKeq |
|---|---|
| Al2O3(s) = 2[Al] + 3[O] | −12.57 = (−45300/T + 11.62) [36] |
| MgO(s) = [Mg] + [O] | −7.86 = (−38059/T + 12.45) [36] |
| MgAl2O4(s) = [Mg] + 2[Al] + 4[O] | −21.28 = (−84339/T + 23.75) [36] |
| C | Si | Mn | P | S | Cr | Cu | Ni | Ti | N | O | Al | Mg | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | −0.42 | −0.066 | −0.021 | 0.07 | −0.13 | −0.055 | −0.013 | 0.006 | −0.34 | −0.14 | −0.17 | −1.17 | −1.98 |
| Al | 0.091 | 0.056 | −0.004 | 0.033 | 0.035 | 0.012 | −0.013 | −0.017 | - | 0.015 | −1.98 | 0.043 | −0.13 |
| Mg | −0.31 | −0.088 | - | - | - | 0.047 | −0.012 | −0.64 | - | −3 | −0.12 | - |
| Holding Time | [O] | [Al] | [Mg] | aO | aAl | aMg |
|---|---|---|---|---|---|---|
| 120 s | 0.0218 | 0.0310 | 0 | 0.019783 | 0.028190 | 0 |
| 1800 s | 0.0046 | 0.0280 | 0 | 0.004237 | 0.027531 | 0 |
| 120 s | 0.0208 | 0.0390 | 0.0008−0.0019 | 0.018367 | 0.035641 | 0.001154 |
| 1800 s | 0.0063 | 0.0350 | 0.0005 | 0.005677 | 0.034167 | 0.000473 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, L.; Chen, C.; Li, J.; Li, X. Effect of Mg Treatment on the Nucleation and Ostwald Growth of Inclusions in Fe-O-Al-Mg Melt. Materials 2020, 13, 3355. https://doi.org/10.3390/ma13153355
Li Y, Wang L, Chen C, Li J, Li X. Effect of Mg Treatment on the Nucleation and Ostwald Growth of Inclusions in Fe-O-Al-Mg Melt. Materials. 2020; 13(15):3355. https://doi.org/10.3390/ma13153355
Chicago/Turabian StyleLi, Yutang, Linzhu Wang, Chaoyi Chen, Junqi Li, and Xiang Li. 2020. "Effect of Mg Treatment on the Nucleation and Ostwald Growth of Inclusions in Fe-O-Al-Mg Melt" Materials 13, no. 15: 3355. https://doi.org/10.3390/ma13153355
APA StyleLi, Y., Wang, L., Chen, C., Li, J., & Li, X. (2020). Effect of Mg Treatment on the Nucleation and Ostwald Growth of Inclusions in Fe-O-Al-Mg Melt. Materials, 13(15), 3355. https://doi.org/10.3390/ma13153355




