Comparative In Vitro Study of the Bond Strength of Composite to Carbon Fiber Versus Ceramic to Cobalt–Chromium Alloys Frameworks for Fixed Dental Prostheses
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Fabrication
2.1.1. Co–Cr Specimen Processing
2.1.2. CFRC Specimens Processing
2.2. Thermocycling
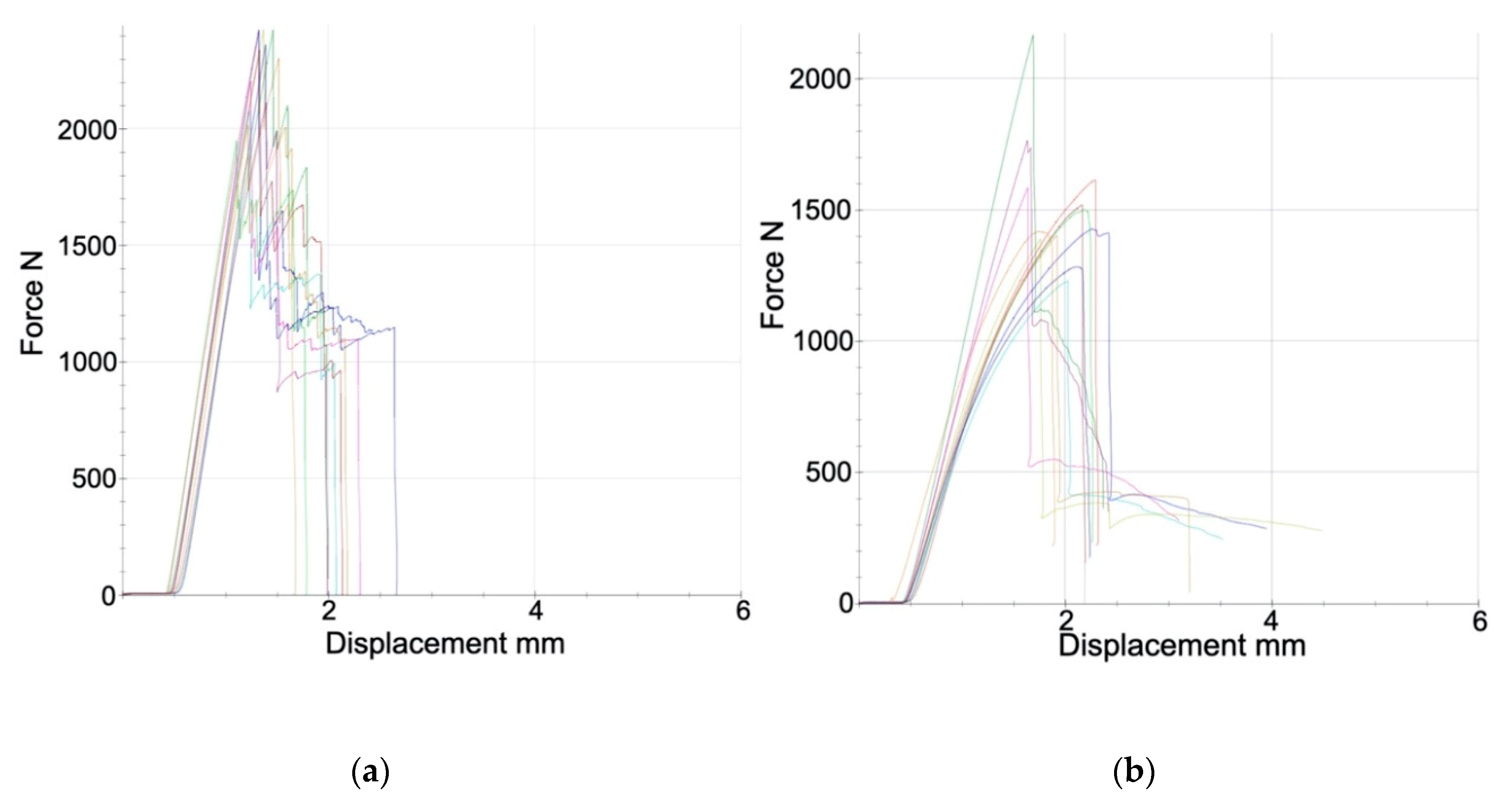
2.3. Mechanical Testing
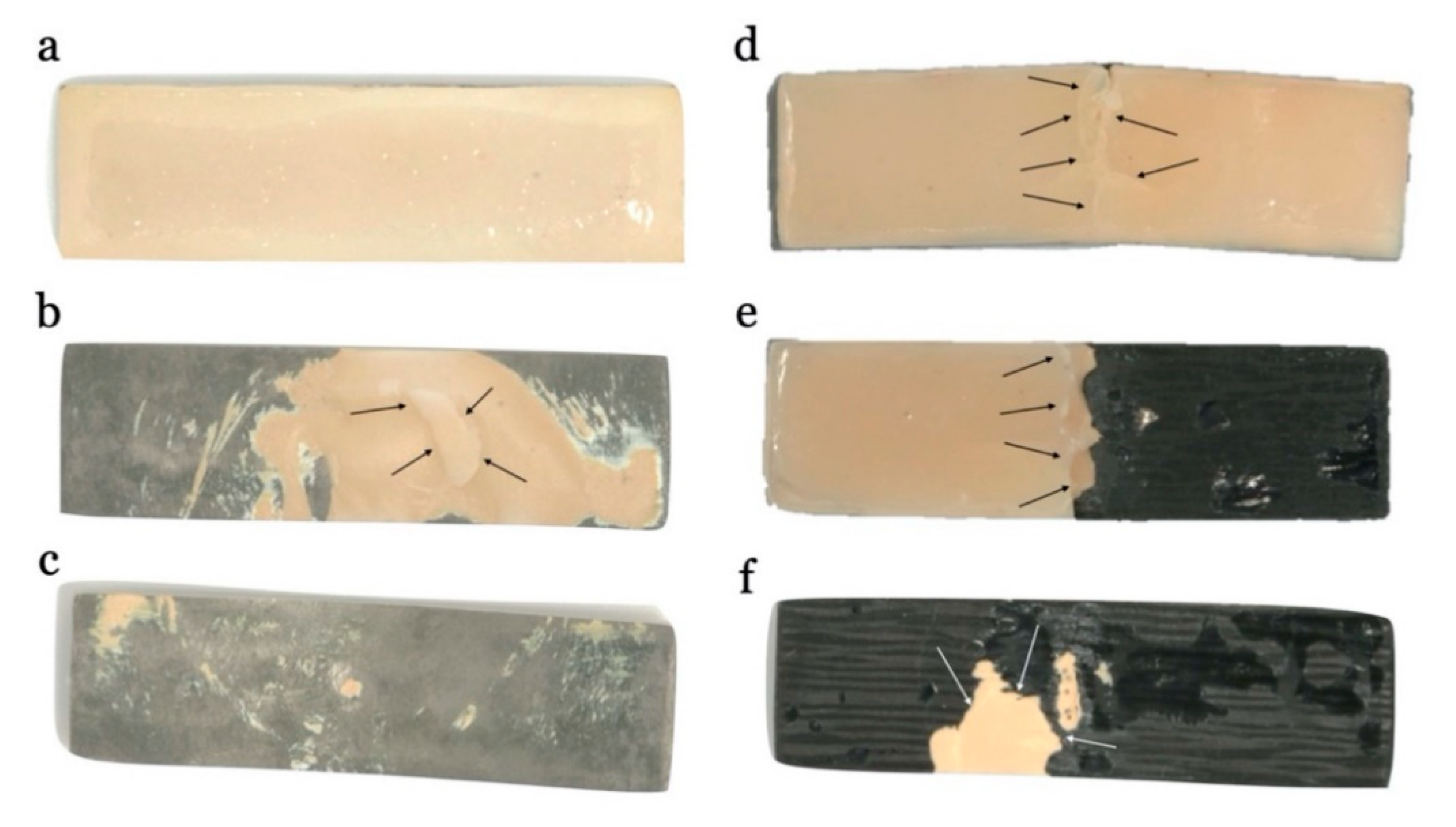
2.4. Failure Assessment
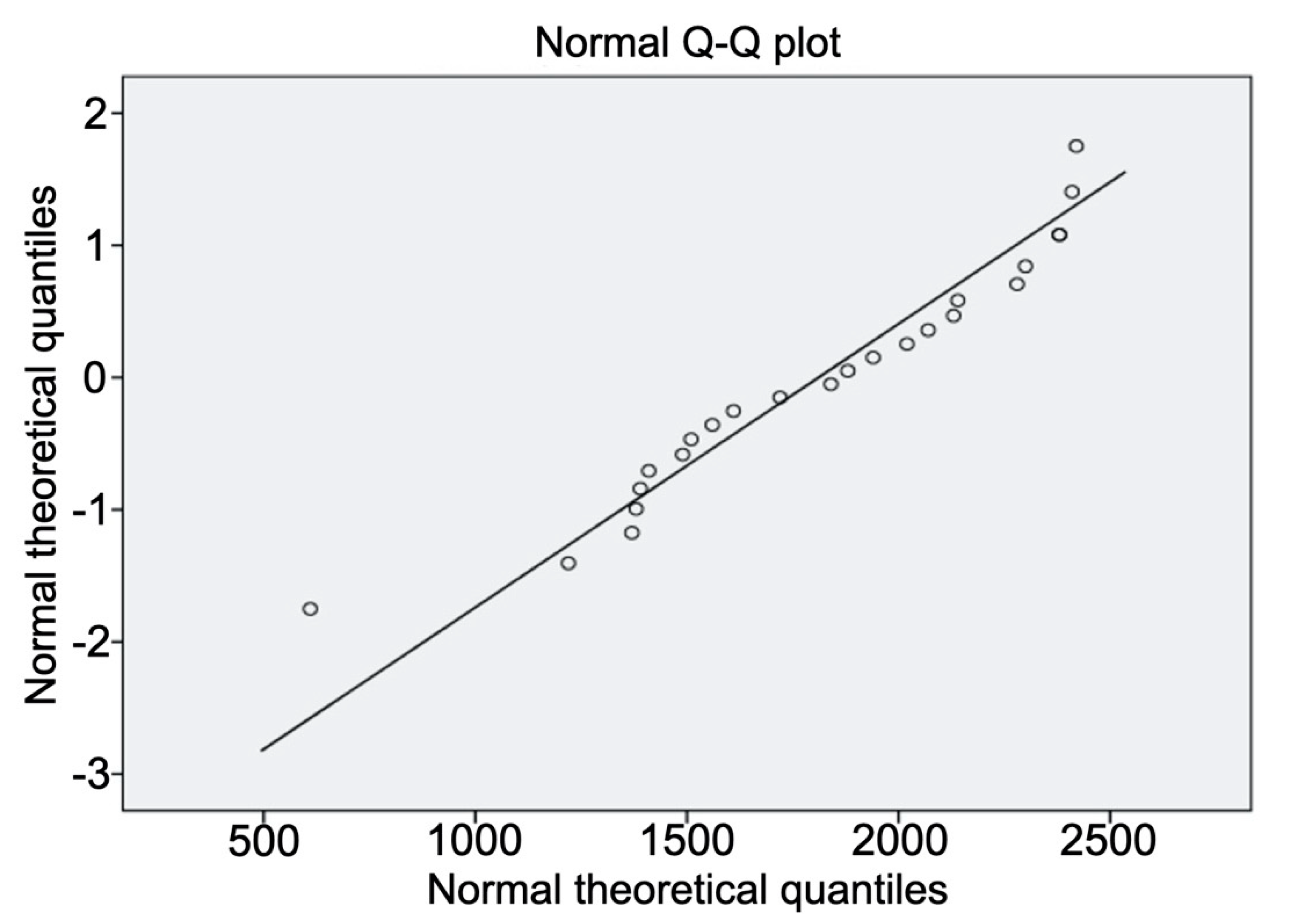
2.5. Data Analysis
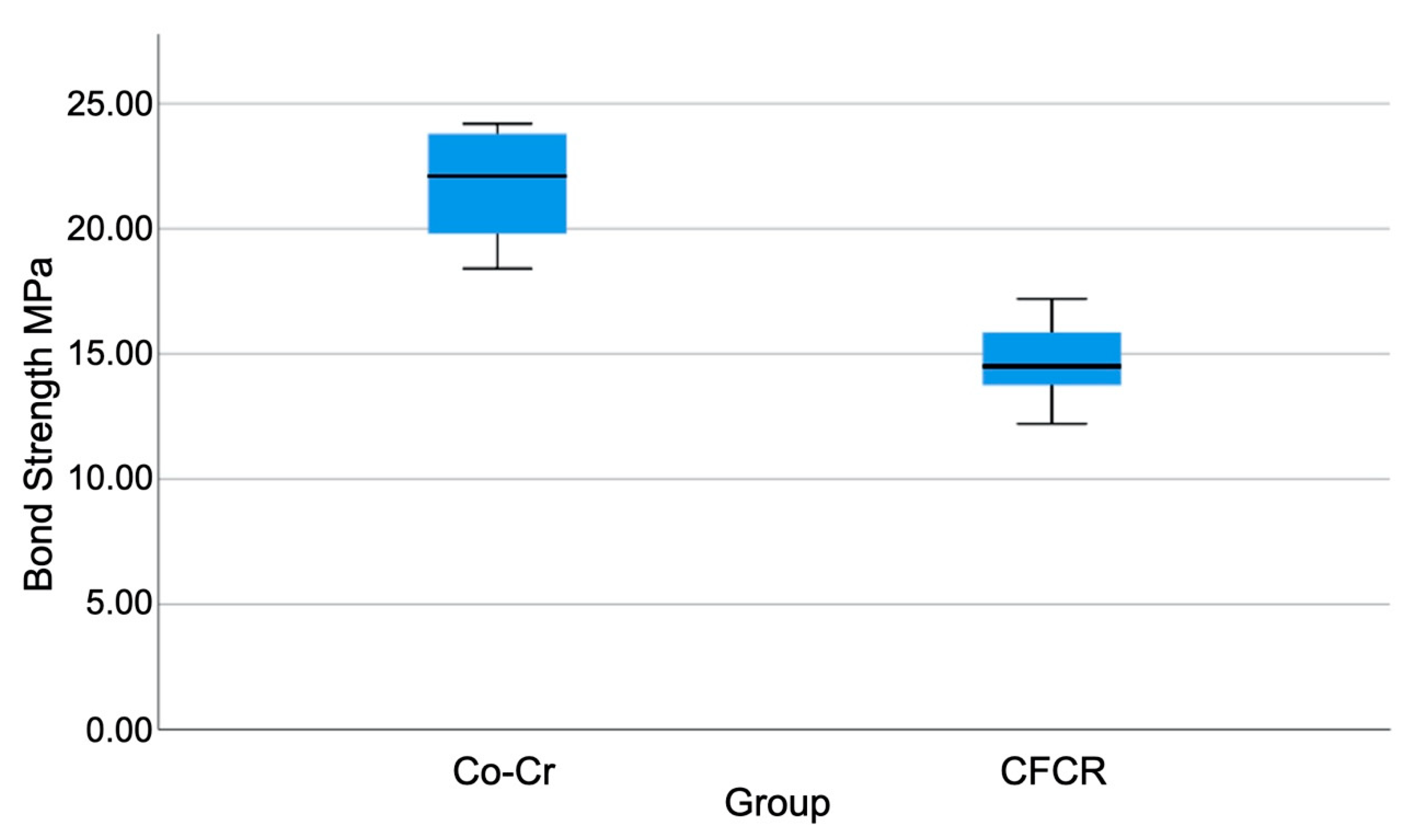
3. Results
4. Discussion
5. Conclusions
- The cobalt–chrome/ceramic group showed greater bonding strength compared to the carbon-fiber-reinforced composite.
- While in the carbon-fiber-reinforced composite group, most fractures were mixed or cohesive types, presenting a greater possibility of clinical repair, in the cobalt–chrome/ceramic group fractures, where most had no possibility of direct clinical repair.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Menini, M.; Pesce, P.; Pera, F.; Barberis, F.; Lagazzo, A.; Bertola, L. Biological and mechanical characterization of carbon fiber frameworks for dental implant applications. Mater. Sci. Eng. C 2017, 70, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Gao, S.; Mäder, E.; Sharma, H.; Wei, L.Y.; Bijwe, J. Carbon fiber surfaces and composite interphases. Compos. Sci. Technol. 2014, 102, 35–50. [Google Scholar] [CrossRef]
- Donnet, J.B.; Bansal, R.C. Carbon Fibers; Marcel Dekker: New York, NY, USA, 1990. [Google Scholar] [CrossRef]
- Chand, S. Review carbon fibers for composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- Rajzer, I.; Menaszek, E.; Bacakova, L.; Rom, M.; Blazewicz, M. In vitro and in vivo studies on biocompatibility of carbon fibres. J. Mater. Sci. Mater. Med. 2010, 21, 2611–2622. [Google Scholar] [CrossRef]
- Petersen, R. Carbon fiber biocompatibility for implants. Fibers 2016, 4, 1. [Google Scholar] [CrossRef]
- Schmitter, M.; Mueller, D.; Rues, S. In vitro chipping behaviour of all-ceramic crowns with a zirconia framework and feldspathic veneering: Comparison of CAD/CAM-produced veneer with manually layered veneer. J. Oral Rehabil. 2013, 40, 519–525. [Google Scholar] [CrossRef]
- Schmitter, M.; Mueller, D.; Rues, S. Chipping behaviour of all-ceramic crowns with zirconia framework and CAD/CAM manufactured veneer. J. Dent. 2012, 40, 154–162. [Google Scholar] [CrossRef]
- Pera, F.; Pesce, P.; Solimano, F.; Tealdo, T.; Pera, P.; Menini, M. Carbon fibre versus metal framework in full-arch immediate loading rehabilitations of the maxilla—a cohort clinical study. J. Oral Rehabil. 2017, 44, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Tealdo, T.; Menini, M.; Bevilacqua, M.; Pera, F.; Pesce, P.; Signori, A.; Pera, P. Inmediate versus delayed loading of dental implants in edentulous patients’ maxillae: A 6-year prospective study. Int. J. Prosthodont. 2014, 27, 207–214. [Google Scholar] [CrossRef]
- Hämmerle, C.H.; Wagner, D.; Brägger, U.; Lussi, A.; Karayiannis, A.; Joss, A. Threshold of tactile sensitivity perceived with dental endosseous implants and natural teeth. Clin. Oral Implants Res. 1995, 6, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Scurria, M.S.; Bader, J.D.; Shugars, D.A. Meta-analysis of fixed partial denture survival protheses and abutment. J. Prosthet. Dent. 1998, 79, 459–464. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Mokti, M.; Chen, C.J.; Chen, C.J.; Benic, G.I.; Gallucci, G.O.; Chronopoulos, V. Implant and prosthodontic survival rates with implant fixed complete dental prostheses in the edentulous mandible after at least 5 years: A systematic review. Clin. Implants Dent. Relat. Res. 2014, 16, 705–717. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Bordin, T.B.; Natto, Z.S.; Kim, Y.J.; El-Rafie, K.; Tsigarida, A.; Chochlidakis, K.; Weber, H.P. Double full-arch fixed implant-supported prostheses: Outcomes and complications after a mean follow-up of 5 years. J. Prosthodont. 2019, 28, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Ucar, Y.; Aksahin, Z.; Kurtoglu, C. Metal ceramic bond after multiple castings of base metal alloy. J. Prosthet. Dent. 2009, 102, 165–171. [Google Scholar] [CrossRef]
- Lee, D.; Lee, B.; Kim, S.; Lee, K. Shear bond strength of porcelain to a new millable alloy and a conventional castable alloy. J. Prosthet. Dent. 2015, 113, 329–335. [Google Scholar] [CrossRef]
- Nesse, H.; Ulstein, D.M.Å.; Vaage, M.M.; Øilo, M. Internal and marginal fit of cobalt-chromium fixed dental prostheses fabricated with 3 different techniques. J. Prosthet. Dent. 2015, 114, 686–692. [Google Scholar] [CrossRef]
- Ortorp, A.; Ascher, A.; Svanborg, P. A 5-year retrospective study of cobalt-chromium-based single crowns inserted in a private practice. Int. J. Prosthodont. 2012, 25, 480–483. [Google Scholar]
- Walton, T.R. A 10-year longitudinal study of fixed prosthodontics: Clinical characteristics and outcome of single-unit metal-ceramic crowns. Int. J. Prosthodont. 1999, 12, 519–526. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Asgeirsson, A.G.; Zwahlen, M.; Sailer, I. Improvements in implant dentistry over the last decade: Comparison of survival and complication rates in older and newer publications. Int. J. Oral Maxillofac. Implants 2014, 29, 308–324. [Google Scholar] [CrossRef]
- Brägger, U.; Aeschlimann, S.; Bürgin, W.; Hämmerle, C.H.; Lang, N.P. Biological and technical complications and failures with fixed partial dentures (FPD) on implants and teeth after four to five years of function. Clin. Oral Implants Res. 2001, 12, 26–34. [Google Scholar] [CrossRef]
- Brägger, U.; Karoussis, I.; Persson, R.; Pjetursson, B.E.; Salvi, G.; Lang, N. Technical and biological complications/failures with single crowns and fixed partial dentures on implants: A 10-year prospective cohort study. Clin. Oral Implants Res. 2005, 16, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Tan, K.; Lang, N.P.; Brägger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. Clin. Oral Implants Res. 2004, 15, 625–642. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Brägger, U.; Lang, N.P.; Zwahlen, M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs). Clin. Oral Implants Res. 2007, 18, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Kreissl, M.E.; Gerds, T.; Muche, R.; Heydecke, G.; Strub, J. Technical complications of implant-supported fixed partial dentures in partially edentulous cases after an average observation period of 5 years. Clin. Oral Implants Res. 2007, 18, 720–726. [Google Scholar] [CrossRef]
- Joias, R.M.; Tango, R.N.; Junho de Araujo, J.E.; Junho de Araujo, M.A.; Ferreira, G.S.; Paes-Junior, T.J. Shear bond strength of a ceramic to Co-Cr alloys. J. Prosthet. Dent. 2008, 99, 54–59. [Google Scholar] [CrossRef]
- Bader, J.D.; Rozier, R.G.; McFall, W.T.; Ramsey, D.L. Effect of crown margins on periodontal conditions in regularly attending patients. J. Prosthet. Dent. 1991, 65, 75–79. [Google Scholar] [CrossRef]
- Felton, D.A.; Kanoy, B.E.; Bayne, S.C.; Wirthman, G.P. Effect of in vivo crown margin discrepancies on periodontal health. J. Prosthet. Dent. 1991, 65, 357–364. [Google Scholar] [CrossRef]
- Hammad, I.A.; Talic, Y.F. Designs of bond strength tests for metal-ceramic complexes: Review of the literature. J. Prosthet. Dent. 1996, 75, 602–628. [Google Scholar] [CrossRef]
- Lawaf, S.; Nasermostofi, S.; Afradeh, M.; Azizi, A. Comparison of the bond strength of ceramics to Co-Cr alloys made by casting and selective laser melting. J. Adv. Prosthodont. 2017, 9, 52–56. [Google Scholar] [CrossRef]
- Segerström, S.; Ruyter, I.E. Mechanical and physical properties of carbon-graphite fiber-reinforced polymers intended for implant suprastructures. Dent. Mater. 2007, 23, 1150–1156. [Google Scholar] [CrossRef]
- Kul, E.; Aladag, L.; Duymus, Z.Y. Comparison of the metal-ceramic bond after recasting and after laser sintering. J. Prosthet. Dent. 2015, 114, 109–113. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization ISO 178:2010. Dentistry-Plastics-Determination of Flexural Properties; International Organization for Standardization: Geneva, Switzerland, 2010; Available online: https://www.iso.org/standard/45091.html (accessed on 15 July 2020).
- Scheftner Dental Alloys Web Page. Available online: https://scheftner.dental/starbond-easy-disc-en.html (accessed on 15 July 2020).
- International Organization for Standardization ISO TS 11405:2015. Dentistry-Testing of Adhesion to Tooth Structure; International Organization for Standardization: Geneva, Switzerland, 2015; Available online: https://www.iso.org/standard/62898.html (accessed on 15 July 2020).
- International Organization for Standardization ISO TS 14125:1998. Dentistry-Fibre-Reinforced Plastic Composites-Determination of Flexural Properties; International Organization for Standardization: Geneva, Switzerland, 1998; Available online: https://www.iso.org/standard/23637.html (accessed on 15 July 2020).
- Yan, X.; Xu, Y.; Wu, Y.; Lin, H. Effects of heat treatment on metal-ceramic combination of selective-laser-melted cobalt-chromium alloy. J. Prosthet. Dent. 2018, 120, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.; Liao, J.; Liu, L.; Ye, X.; Lin, S. Bond strengths of porcelain to cobalt-chromium alloys made by casting, milling, and selective laser melting. J. Prosthet. Dent. 2017, 118, 69–75. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Q.; Li, N.; Xu, S. Evaluation of metal-ceramic bond characteristics of three dental Co-Cr alloys prepared with different fabrication techniques. J. Prosthet. Dent. 2016, 116, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Xin, X.; Chen, J.; Wei, B. Metal-ceramic bond strength of Co-Cr alloy fabricated by selective laser melting. J. Dent. 2012, 40, 453–457. [Google Scholar] [CrossRef]
- Kaleli, N.; Saraç, D. Comparison of porcelain bond strength of different metal frameworks prepared by using conventional and recently introduced fabrication methods. J. Prosthet. Dent. 2017, 118, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Anusavice, K.J.; Dehoff, P.H.; Fairhurst, C.W. Comparative evaluation of ceramic-metal bond tests using finite element stress analysis. J. Dent. Res. 1980, 59, 608–613. [Google Scholar] [CrossRef]
- Della Bona, A.; Van Noort, R. Shear vs. tensile bond strength of resin composite bonded to ceramic. J. Dent. Res. 1995, 74, 1591–1596. [Google Scholar] [CrossRef]
- Papazoglou, E.; Brantley, W.A. Porcelain adherence vs force to failure for palladium-gallium alloys: A critique of metal-ceramic bond testing. Dent. Mater. 1998, 14, 112–119. [Google Scholar] [CrossRef]
- Sadeq, A.; Cai, Z.; Woody, R.D.; Miller, A.W. Effects of interfacial variables on ceramic adherence to cast and machined commercially pure titanium. J. Prosthet. Dent. 2003, 90, 10–17. [Google Scholar] [CrossRef]
- International Organization for Standardization ISO 9693-1. Dentistry- Compatibility Testing: Part 1: Metal-Ceramic Systems; International Organization for Standardization: Geneva, Switzerland, 2012; Available online: https://www.iso.org/standard/54946.html (accessed on 15 July 2020).
- Ren, X.; Zeng, L.; Wei, Z.; Xin, X.; Wei, B. Effects of multiple firings on metal-ceramic bond strength of Co-Cr alloy fabricated by selective laser melting. J. Prosthet. Dent. 2016, 115, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Ekren, O.; Ozkomur, A.; Ucar, Y. Effect of layered manufacturing techniques, alloy powders, and layer thickness on metal-ceramic bond strength. J. Prosthet. Dent. 2018, 119, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Nageshraj, R.; Rajagopal, M.P.; Madapathy, S.; Samuel, P.; Ramabhadran, N.; Mathew, J. An in vitro comparative evaluation of shear bond strengths of veneering porcelain and different core materials. J. Contemp. Dent. Pract. 2018, 19, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Pera, F.; Barberis, F.; Rosenberg, G.; Bagnascoa, F.; Pesce, P. Evaluation of adhesion between carbon fiber frameworks and esthetic veneering materials. Int. J. Prosthodont. 2018, 31, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, V.Z.; Ozcan, M.; Kimpara, E.T. Evaluation of interface characterization and adhesion of glass ceramics to commercially pure titanium and gold alloy after thermal- and mechanical-loading. Dent. Mater. 2009, 25, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, X.; Li, B.; Liao, J.; Zhuang, P.; Ye, J. Effect of oxidation heat treatment on the bond strength between a ceramic and cast and milled cobalt-chromium alloys. Eur. J. Oral Sci. 2015, 123, 297–304. [Google Scholar] [CrossRef]
- Serra-Prat, J.; Cano-Batalla, J.; Cabratosa-Termes, J.; Figueras-Àlvarez, O. Adhesion of dental porcelain to cast, milled, and laser-sintered cobalt-chromium alloys: Shear bond strength and sensitivity to thermocycling. J. Prosthet. Dent. 2014, 112, 600–605. [Google Scholar] [CrossRef]
- Akova, T.; Ucar, Y.; Tukay, A.; Balkaya, M.C.; Brantley, W.A. Comparison of the bond strength of laser-sintered and cast base metal dental alloys to porcelain. Dent. Mater. 2008, 24, 1400–1404. [Google Scholar] [CrossRef]
- Taufall, S.; Eichberger, M.; Schmidlin, P.R.; Stawarczyk, B. Fracture load and failure types of different veneered polyetheretherketone fixed dental prostheses. Clin. Oral Investig. 2016, 20, 2493–2500. [Google Scholar] [CrossRef]
- Cavalcanti, A.N.; De Lima, A.F.; Peris, A.R.; Mitsui, F.H.; Marchi, G.M. Effect of surface treatments and bonding agents on the bond strength of repaired composites. J. Esthet. Restor. Dent. 2007, 19, 90–98. [Google Scholar] [CrossRef]
- Jafarzadeh Kashi, T.S.; Erfan, M.; Rakhshan, V.; Aghabaigi, N.; Tabatabaei, F.S. An in vitro assessment of the effects of three surface treatments on repair bond strength of aged composites. Oper. Dent. 2011, 36, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Parolia, A.; Jain, A.; Kundabala, M.; Mohan, M.; De Moraes Porto, I.C. A comparative effect of various surface chemical treatments on the resin composite-composite repair bond strength. J. Indian Soc. Pedod. Prev. Dent. 2015, 33, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Kimyai, S.; Mohammadi, N.; Navimipour, E.J.; Rikhtegaran, S. Comparison of the effect of three mechanical surface treatments on the repair bond strength of a laboratory composite. Photomed. Laser Surg. 2010, 28, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Nassoohi, N.; Kazemi, H.; Sadaghiani, M.; Mansouri, M.; Rakhshan, V. Effects of three surface conditioning techniques on repair bond strength of nanohybrid and nanofilled composites. Dent. Res. J. 2015, 12, 554–561. [Google Scholar] [CrossRef]


| Component | Percentage (%) |
|---|---|
| Co | 61% |
| Cr | 27.5% |
| W | 8.5% |
| Si | 1.6% |
| C, Mn, Fe | <1% |
| Program | Preheating (°C) | Drying Time (min) | Heating Rate (°C/min) | Maximum Temp. (°C) | Total Time (min) |
|---|---|---|---|---|---|
| oxidation | 650 | 2 | 55 | 990 | 17 |
| metal bond | 550 | 3 | 80 | 980 | 15 |
| opaquer | 550 | 3 | 80 | 930 | 15 |
| body porcelain | 580 | 3 | 45 | 890 | 17 |
| glaze | 600 | 3 | 55 | 890 | 14 |
| Failure Type | Co–Cr | CFCR | X2 | p | R2 |
|---|---|---|---|---|---|
| Cohesive | 0.0% | 16.7% | 13.68 ** | 0.001 | 0.570 |
| Adhesive | 91.7% | 16.7% | |||
| Mixed | 8.3% | 66.7% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascos-Sanchez, R.; Molinero-Mourelle, P.; Ortega, R.; Agustin-Panadero, R.; Del Rio Highsmith, J.; Gomez-Polo, M. Comparative In Vitro Study of the Bond Strength of Composite to Carbon Fiber Versus Ceramic to Cobalt–Chromium Alloys Frameworks for Fixed Dental Prostheses. Materials 2020, 13, 3173. https://doi.org/10.3390/ma13143173
Cascos-Sanchez R, Molinero-Mourelle P, Ortega R, Agustin-Panadero R, Del Rio Highsmith J, Gomez-Polo M. Comparative In Vitro Study of the Bond Strength of Composite to Carbon Fiber Versus Ceramic to Cobalt–Chromium Alloys Frameworks for Fixed Dental Prostheses. Materials. 2020; 13(14):3173. https://doi.org/10.3390/ma13143173
Chicago/Turabian StyleCascos-Sanchez, Rocio, Pedro Molinero-Mourelle, Rocio Ortega, Ruben Agustin-Panadero, Jaime Del Rio Highsmith, and Miguel Gomez-Polo. 2020. "Comparative In Vitro Study of the Bond Strength of Composite to Carbon Fiber Versus Ceramic to Cobalt–Chromium Alloys Frameworks for Fixed Dental Prostheses" Materials 13, no. 14: 3173. https://doi.org/10.3390/ma13143173
APA StyleCascos-Sanchez, R., Molinero-Mourelle, P., Ortega, R., Agustin-Panadero, R., Del Rio Highsmith, J., & Gomez-Polo, M. (2020). Comparative In Vitro Study of the Bond Strength of Composite to Carbon Fiber Versus Ceramic to Cobalt–Chromium Alloys Frameworks for Fixed Dental Prostheses. Materials, 13(14), 3173. https://doi.org/10.3390/ma13143173






