Upgrading Sustainable Polyurethane Foam Based on Greener Polyols: Succinic-Based Polyol and Mannich-Based Polyol
Abstract
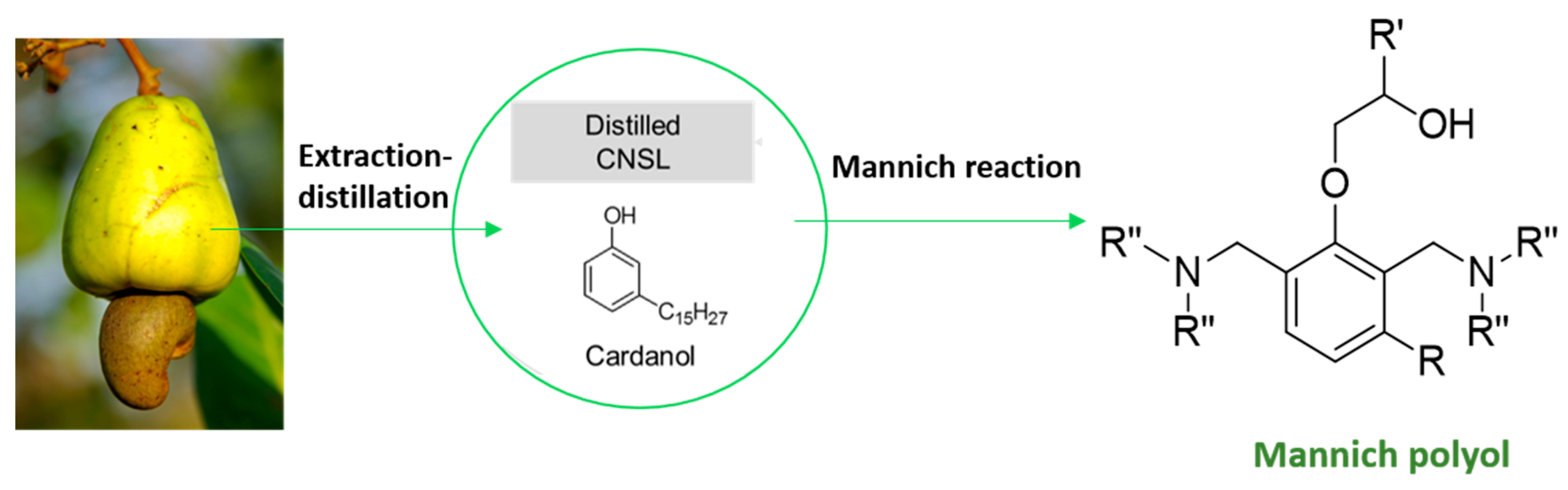
1. Introduction
2. Materials and Methods
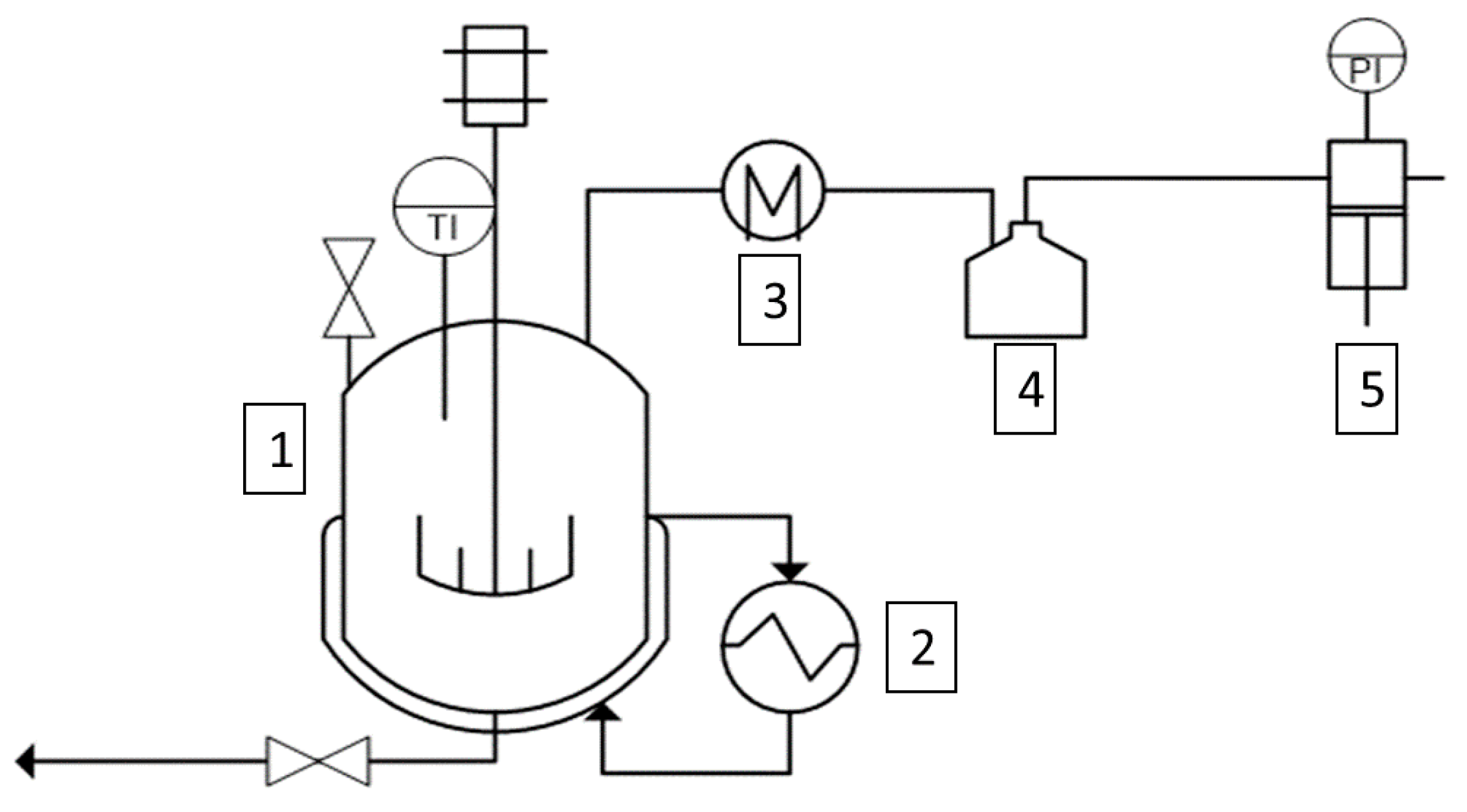
2.1. Procedure for the Synthesis of Succinic-Based Polyol
- (i)
- Esterification reaction: loading of the reactants followed by heating the system at the desired temperature value at atmospheric pressure, 1 h reaction time:

- (ii)
- Polycondensation reaction: adding TNB catalyst, stirring for 1 h under high vacuum.

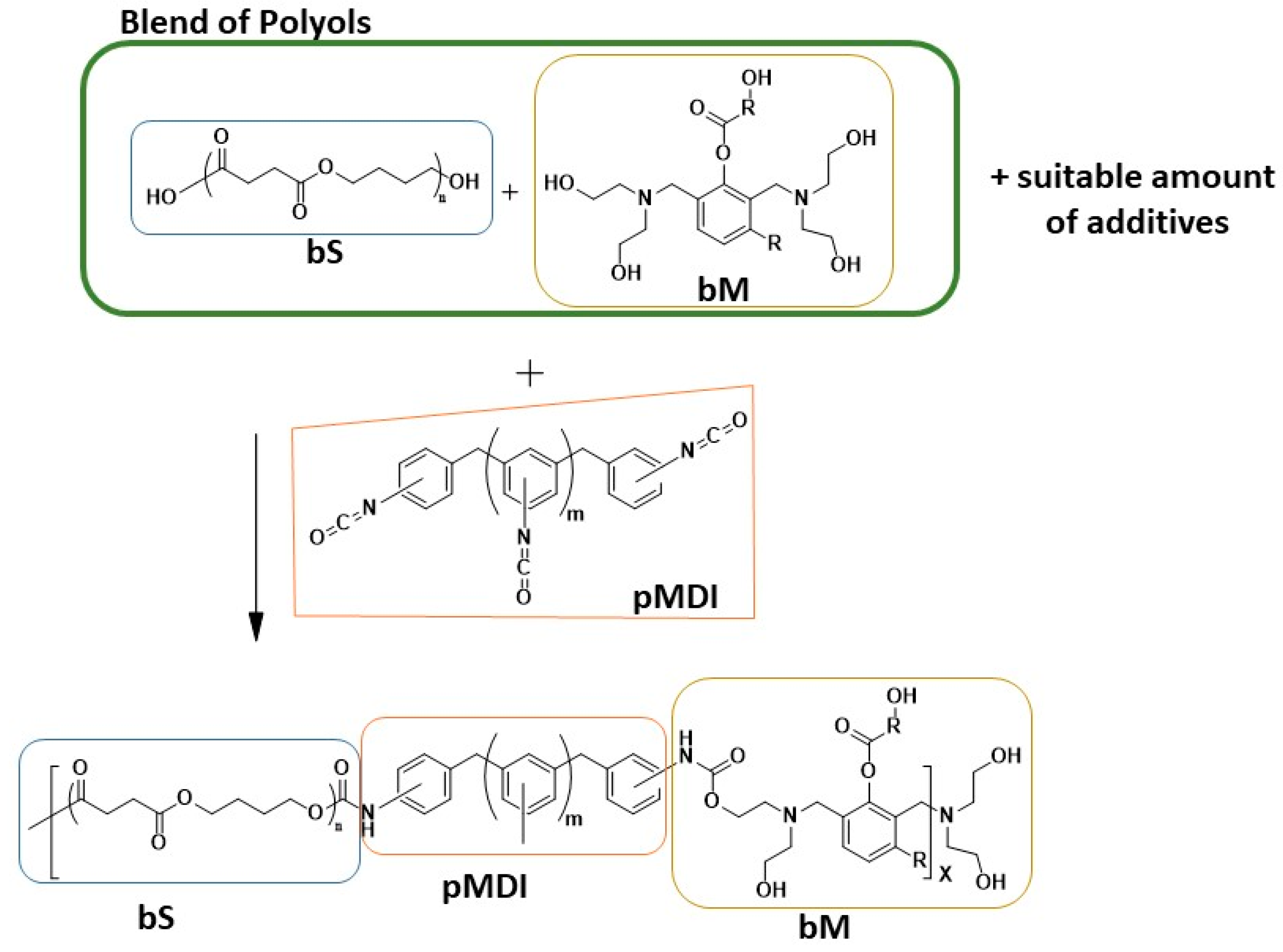
2.2. Polyurethane Foam Preparations
2.3. Characterizations of Polyols
2.4. Characterizations of Foams
2.4.1. Sustainability Index of the Developed Foams
3. Results
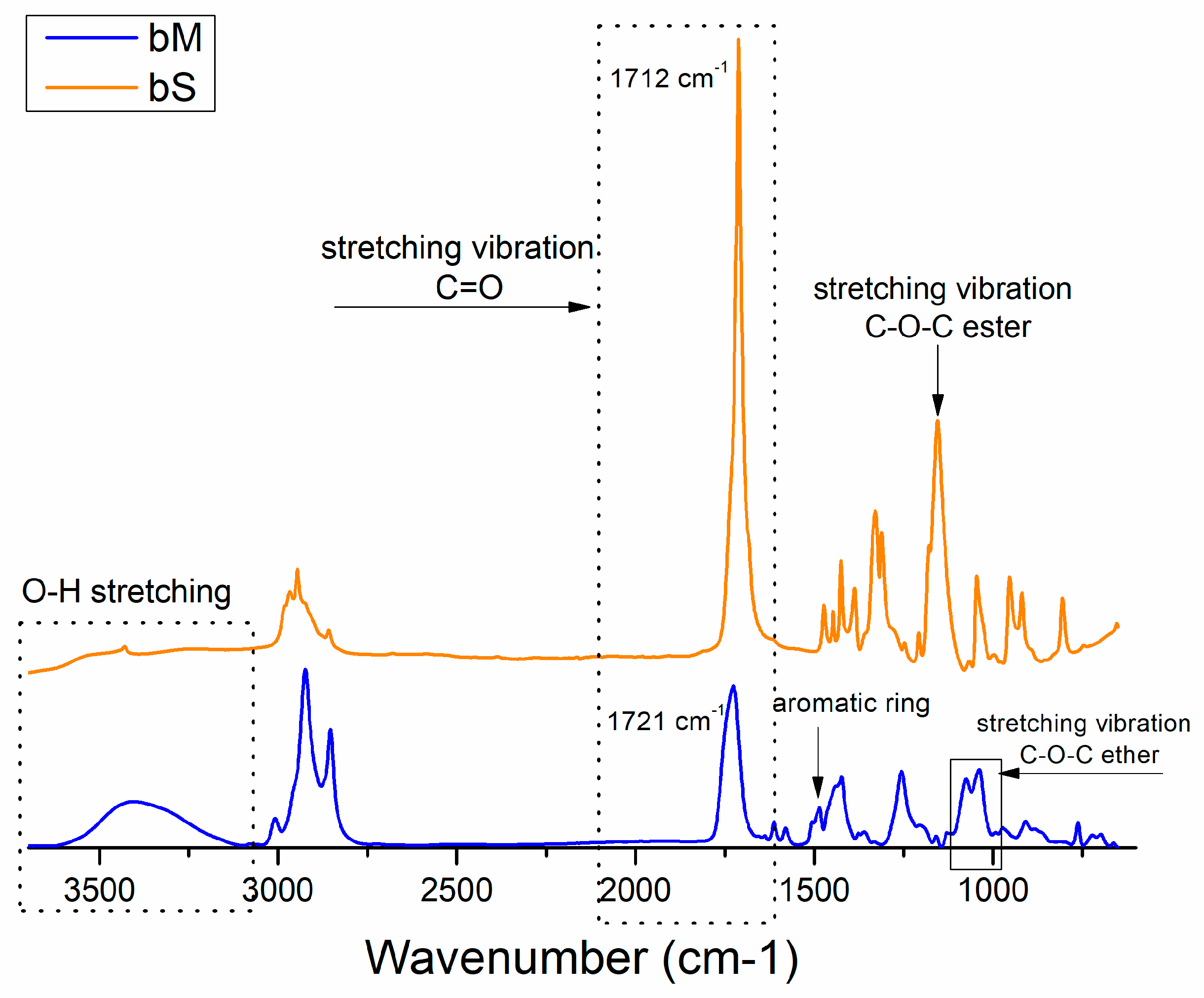
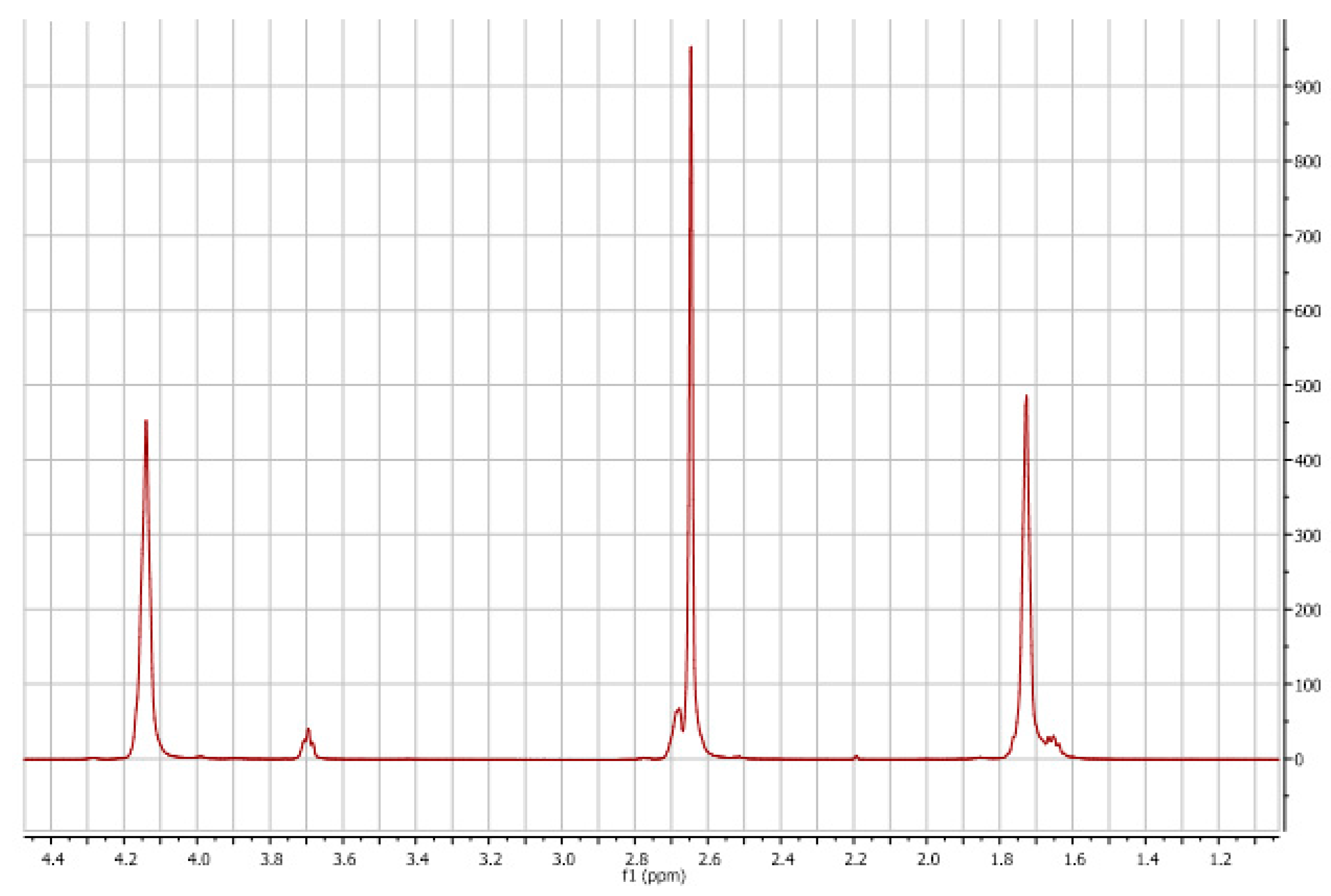
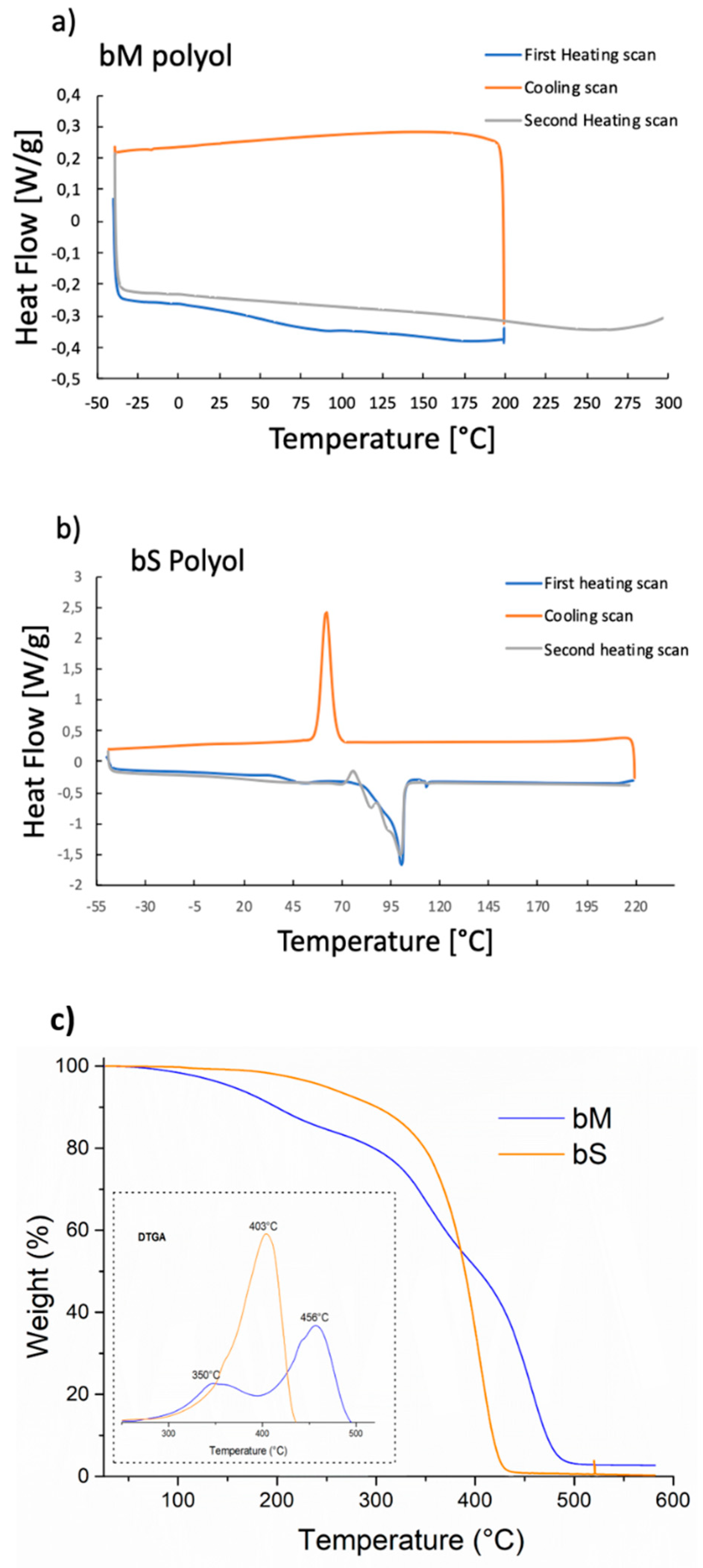
3.1. Polyols Characterizations
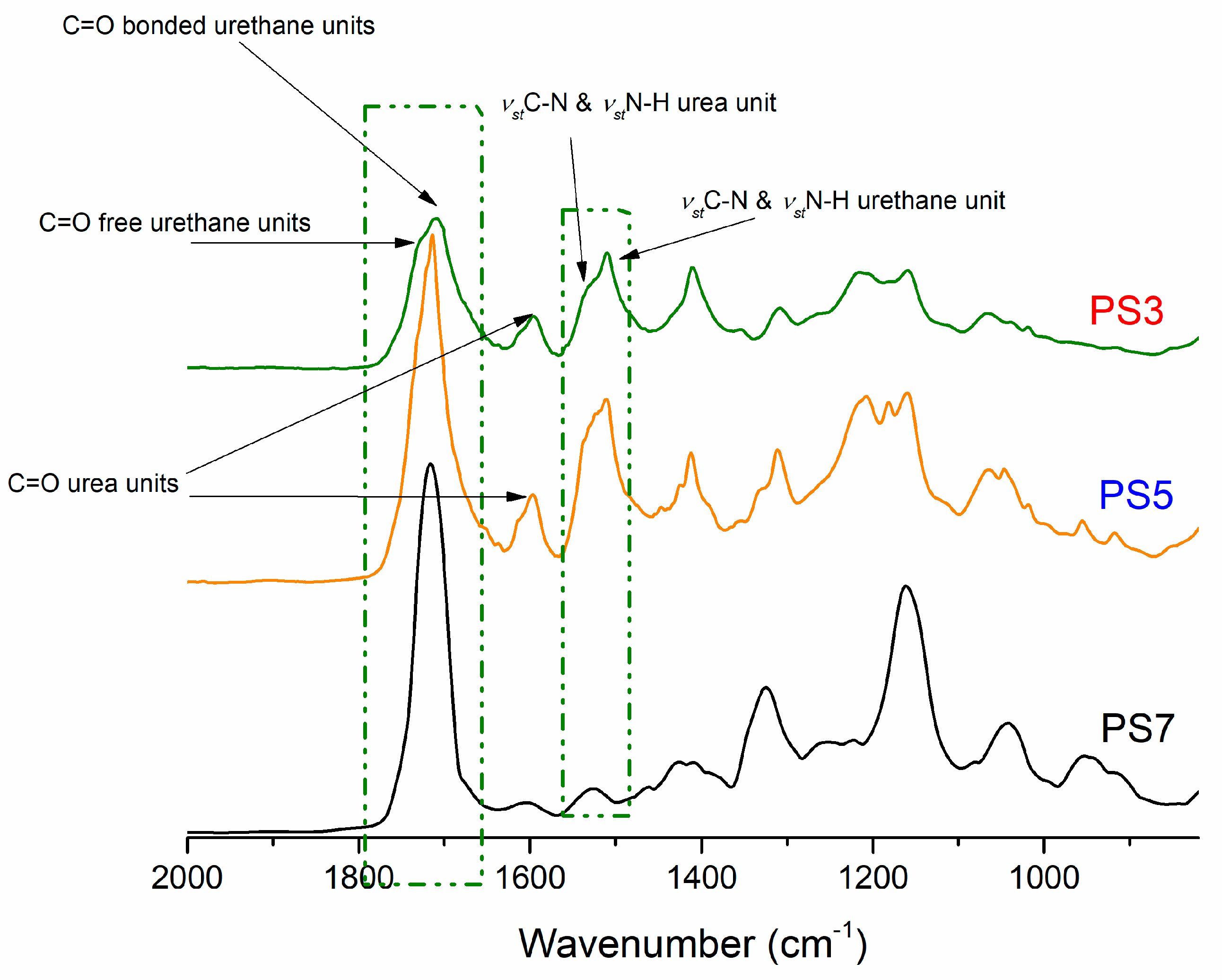
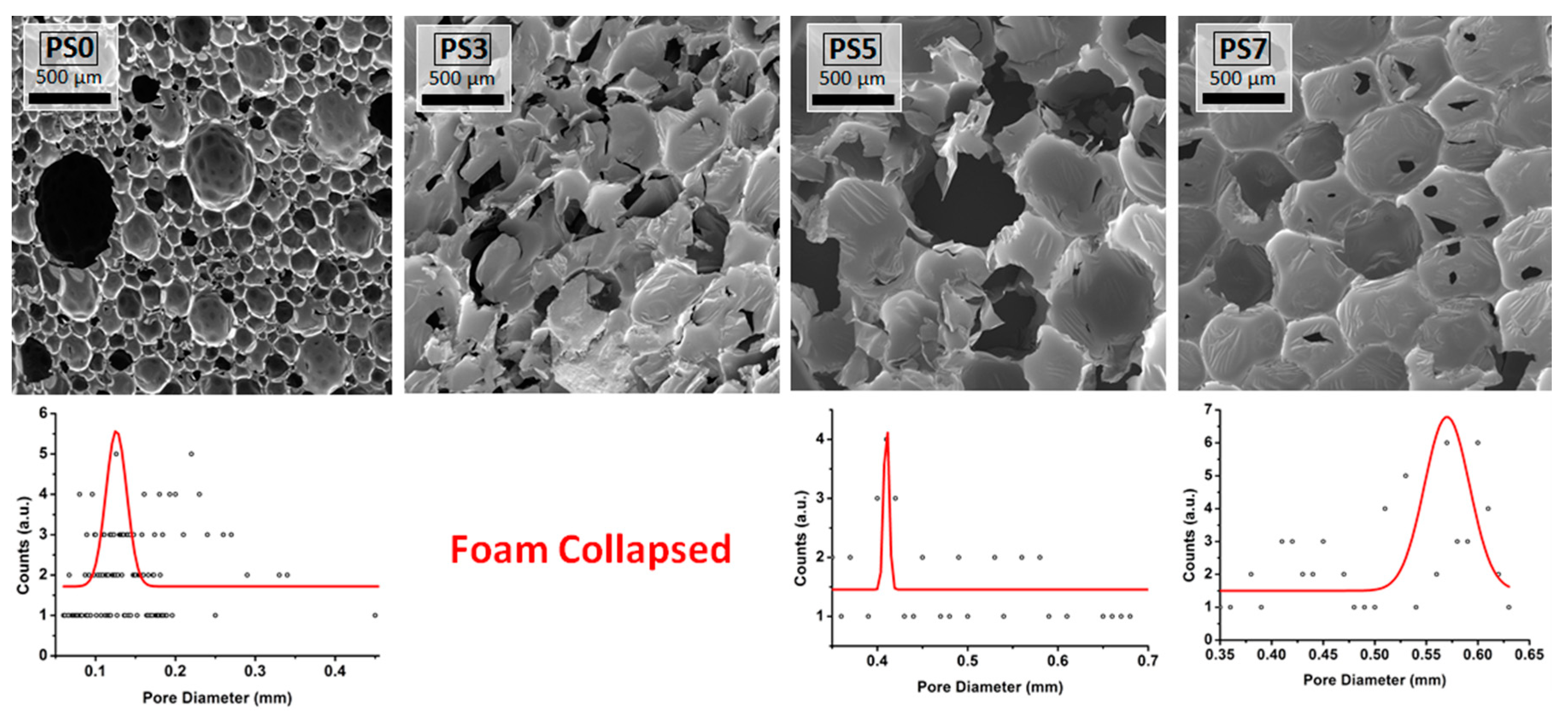
3.2. Polyurethane Foams Characterizations
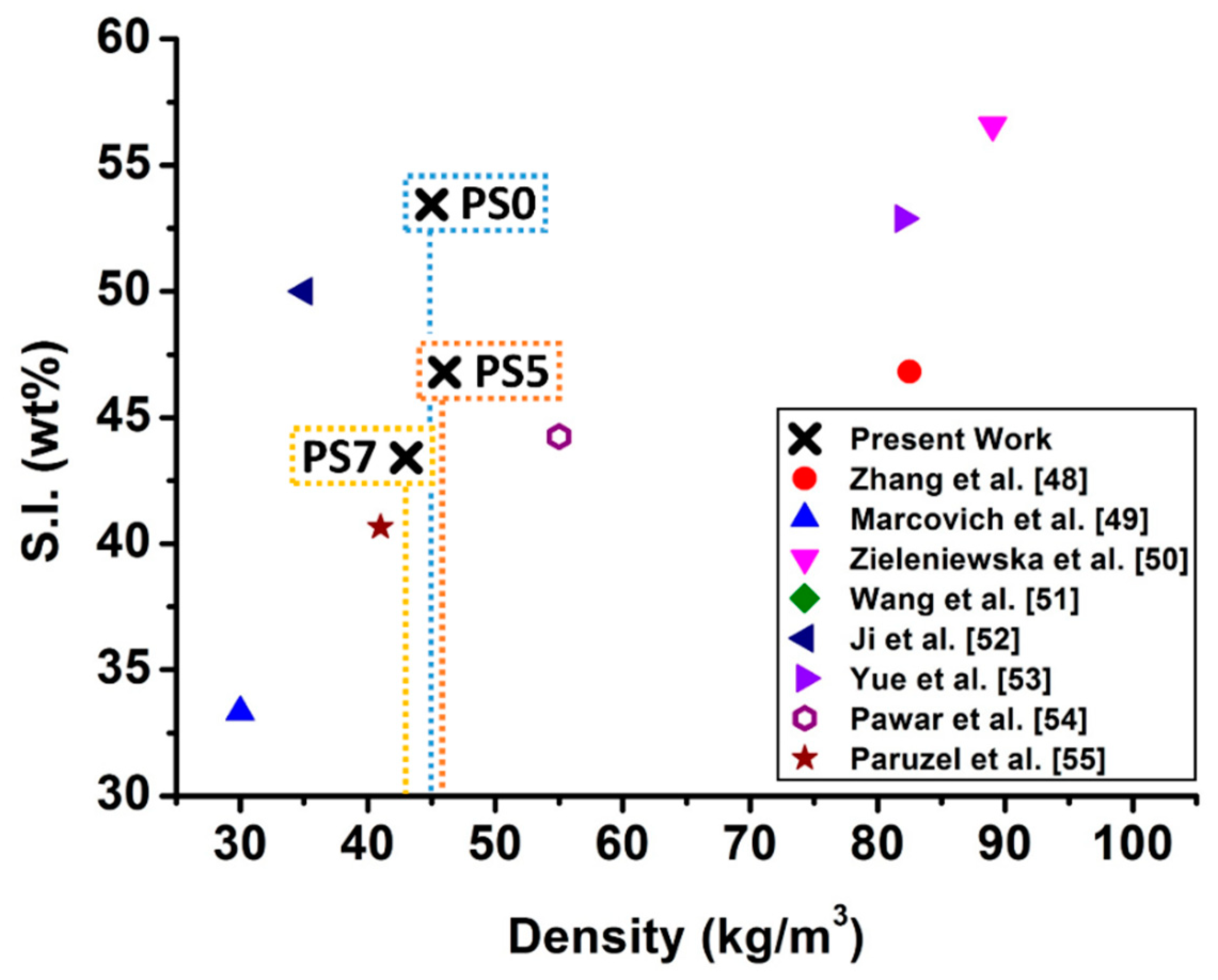
Sustainability Index of the Developed Foams
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Białkowska, A.; Mucha, K.; Przybyłek, M.; Bakar, M. Effect of hard segments content on the properties, structure and biodegradation of nonisocyanate polyurethane. Polym. Polym. Compos. 2018, 26, 423–430. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Bio-based routes to synthesize cyclic carbonates and polyamines precursors of non-isocyanate polyurethanes: A review. Eur. Polym. J. 2019, 118, 668–684. [Google Scholar] [CrossRef]
- Petrović, Z.S. Polyurethanes from Vegetable Oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Verdolotti, L.; Salerno, A.; Lamanna, R.; Nunziata, A.; Netti, P.; Iannace, S. A novel hybrid PU-alumina flexible foam with superior hydrophilicity and adsorption of carcinogenic compounds from tobacco smoke. Microporous Mesoporous Mater. 2012, 151, 79–87. [Google Scholar] [CrossRef]
- Oliviero, M.; Stanzione, M.; D’Auria, M.; Sorrentino, L.; Iannace, S.; Verdolotti, L. Vegetable Tannin as a Sustainable UV Stabilizer for Polyurethane Foams. Polymers 2019, 11, 480. [Google Scholar] [CrossRef]
- Verdolotti, L.; Di Maio, E.; Lavorgna, M.; Iannace, S. Hydration-induced reinforcement of rigid polyurethane–cement foams: Mechanical and functional properties. J. Mater. Sci. 2012, 47, 6948–6957. [Google Scholar] [CrossRef]
- De Luca Bossa, F.; Santillo, C.; Verdolotti, L.; Campaner, P.; Minigher, A.; Boggioni, L.; Losio, S.; Coccia, F.; Iannace, S.; Lama, G.C. Greener Nanocomposite Polyurethane Foam Based on Sustainable Polyol and Natural Fillers: Investigation of Chemico-Physical and Mechanical Properties. Materials 2020, 13, 211. [Google Scholar] [CrossRef]
- Stanzione, M.; Oliviero, M.; Cocca, M.; Errico, M.E.; Gentile, G.; Avella, M.; Lavorgna, M.; Buonocore, G.G.; Verdolotti, L. Tuning of polyurethane foam mechanical and thermal properties using ball-milled cellulose. Carbohydr. Polym. 2020, 231, 115772. [Google Scholar] [CrossRef]
- Verdolotti, L.; Di Maio, E.; Forte, G.; Lavorgna, M.; Iannace, S.A. Hydration-induced reinforcement of polyurethane-cement foams: Solvent resistance and mechanical properties. J. Mater. Sci. 2010, 45, 3388–3391. [Google Scholar] [CrossRef]
- Członka, S.; Bertino, M.F.; Kośny, J.; Strąkowska, A.; Masłowski, M.; Strzelec, K. Linseed oil as a natural modifier of rigid polyurethane foams. Ind. Crop. Prod. 2018, 115, 40–51. [Google Scholar] [CrossRef]
- Lochab, B.; Varma, I.K.; Bijwea, J. Sustainable Polymers Derived From Naturally Occurring Materials. AMPC 2012, 2, 221–225. [Google Scholar] [CrossRef]
- Chen, J.; Beaufort, M.d.L.; Gyurik, L.; Dorresteijn, J.; Otte, M.; Gebbink, R.J.M.K. Highly efficient epoxidation of vegetable oils catalyzed by a manganese complex with hydrogen peroxide and acetic acid. Green Chem. 2019, 21, 2436–2447. [Google Scholar] [CrossRef]
- Succinic Acid Market Size & Share, Industry Analysis Report, 2022. Available online: https://www.grandviewresearch.com/industry-analysis/succinic-acid-market (accessed on 1 July 2020).
- Stanzione, M.; Russo, V.; Oliviero, M.; Verdolotti, L.; Sorrentino, A.; Di Serio, M.; Tesser, R.; Iannace, S.; Lavorgna, M. Synthesis and characterization of sustainable polyurethane foams based on polyhydroxyls with different terminal groups. Polymer 2018, 149, 134–145. [Google Scholar] [CrossRef]
- Cimini, D.; Argenzio, O.; D’Ambrosio, S.; Lama, L.; Finore, I.; Finamore, R.; Pepe, O.; Faraco, V.; Schiraldi, C. Production of succinic acid from Basfia succiniciproducens up to the pilot scale from Arundo donax hydrolysate. Bioresour. Technol. 2016, 222, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Turco, R.; Di Serio, M.; Mazzocca, M.; Russo, V.; Tesser, R.; Vitiello, R.; Cimini, D.; Schiraldi, C. Production of succinic acid from arundo donax hydrolysate for bio-based polymers synthesis. DGMK Tagungsbericht 2017, 2, 83–88. [Google Scholar]
- Gandhi, T.S.; Patel, M.R.; Dholakiya, B.Z. Synthesis of cashew Mannich polyol via a three step continuous route and development of PU rigid foams with mechanical, thermal and fire studies. J. Polym. Eng. 2015, 35, 533–544. [Google Scholar] [CrossRef]
- Shrestha, M.L.; Ionescu, M.; Wan, X.; Bilić, N.; Petrović, Z.S.; Upshaw, T. Biobased Aromatic-Aliphatic Polyols from Cardanol by Thermal Thiol-Ene Reaction. J. Renew. Mater. 2018, 6, 87–101. [Google Scholar] [CrossRef]
- Rotaru, I.; Ionescu, M.; Donescu, D.; Vuluga, M.; Purcar, V. Synthesis of new aromatic Mannich polyols for rigid polyurethane foams. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2007, 69, 35–42. [Google Scholar]
- Gupta, R.K.; Ionescu, M.; Wan, X.; Radojcic, D.; Petroviƈ, Z.S. Synthesis of a Novel Limonene Based Mannich Polyol for Rigid Polyurethane Foams. J. Polym. Environ. 2015, 23, 261–268. [Google Scholar] [CrossRef]
- Furtwengler, P.; Avérous, L. Renewable polyols for advanced polyurethane foams from diverse biomass resources. Polym. Chem. 2018, 9, 4258–4287. [Google Scholar] [CrossRef]
- Pawar, M.S.; Kadam, A.S.; Singh, P.C.; Kusumkar, V.V.; Yemul, O.S. Rigid polyurethane foams from cottonseed oil using bio-based chain extenders: A renewable approach. Iran. Polym. J. 2016, 25, 59–68. [Google Scholar] [CrossRef]
- Nakamura, S.; Todoki, M.; Nakamura, K.; Kanetsuna, H. Thermal analysis of polymer samples by a round robin method: I. Reproducibility of melting, crystallization and glass transition temperatures. Thermochim. Acta 1988, 136, 163–178. [Google Scholar] [CrossRef]
- Verdolotti, L.; Lavorgna, M.; Di Maio, E.; Iannace, S. Hydration-induced reinforcement of rigid polyurethane–cement foams: The effect of the co-continuous morphology on the thermal-oxidative stability. Polym. Degrad. Stab. 2013, 98, 64–72. [Google Scholar] [CrossRef]
- Piscitelli, F.; Buonocore, G.G.; Lavorgna, M.; Verdolotti, L.; Pricl, S.; Gentile, G.; Mascia, L. Peculiarities in the structure—Properties relationship of epoxy-silica hybrids with highly organic siloxane domains. Polymer 2015, 63, 222–229. [Google Scholar] [CrossRef]
- Berardi, U.; Madzarevic, J. Microstructural analysis and blowing agent concentration in aged polyurethane and polyisocyanurate foams. Appl. Therm. Eng. 2020, 164, 114440. [Google Scholar] [CrossRef]
- Kairytė, A.; Kremensas, A.; Balčiūnas, G.; Członka, S.; Strąkowska, A. Closed Cell Rigid Polyurethane Foams Based on Low Functionality Polyols: Research of Dimensional Stability and Standardised Performance Properties. Materials 2020, 13, 1438. [Google Scholar] [CrossRef]
- Sheth, J.P.; Aneja, A.; Wilkes, G.L. Exploring long-range connectivity of the hard segment phase in model tri-segment oligomeric polyurethanes via lithium chloride. Polymer 2004, 45, 5979–5984. [Google Scholar] [CrossRef]
- Natesh, A.; Campaner, P. Novel cardanol based polyols and their use in rigid polyurethane foams. PU Mag. 2017, 14, 130–136. [Google Scholar]
- Kim, Y.M.; Natesh, A.; Lee, J.S.; Lee, D.E.; Campaner, P. Center for the Polyurethanes Industry’s Annual Polyurethanes Technical Conference. In Proceedings of the 2019 Polyurethanes Technical Conference, Orlando, FL, USA, 7–9 October 2019. [Google Scholar]
- Lopes, A.A.S.; Carneiro, E.A.; Rios, M.A.S.; Hiluy Filho, J.J.; Carioca, J.O.B.; Barros, G.G.; Mazzetto, S.E. Study of antioxidant property of a thiosphorated compound derived from cashew nut shell liquid in hydrogenated naphthenics oils. Braz. J. Chem. Eng. 2008, 25, 119–127. [Google Scholar] [CrossRef][Green Version]
- Lomonaco, D.; Cangane, F.Y.; Mazzetto, S.E. Thiophosphate esters of cashew nutshell liquid derivatives as new antioxidants for poly(methyl methacrylate). J. Therm. Anal. Calorim. 2011, 104, 1177–1183. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Papageorgiou, G.Z.; Achilias, D.S. Synthesis and comparative biodegradability studies of three poly(alkylene succinate)s. Polym. Degrad. Stab. 2006, 91, 31–43. [Google Scholar] [CrossRef]
- Kayalvizhi, M.; Vakees, E.; Suresh, J.; Arun, A. Synthesis and Characterization of Poly (urethane-urea) based on Functionalized Polystyrene and MDI. IOSR J. Appl. Chem. 2014, 7, 41–51. [Google Scholar] [CrossRef]
- Ionita, D.; Gaina, C.; Cristea, M.; Banabic, D. Tailoring the hard domain cohesiveness in polyurethanes by interplay between the functionality and the content of chain extender. RSC Adv. 2015, 5, 76852–76861. [Google Scholar] [CrossRef]
- Digar, M.; Hung, S.L.; Wang, H.L.; Wen, T.C.; Gopalan, A. Study of ionic conductivity and microstructure of a cross-linked polyurethane acrylate electrolyte. Polymer 2002, 43, 681–691. [Google Scholar] [CrossRef]
- Galzerano, B.; Capasso, I.; Verdolotti, L.; Lavorgna, M.; Vollaro, P.; Caputo, D.; Iannace, S.; Liguori, B. Design of sustainable porous materials based on 3D-structured silica exoskeletons, Diatomite: Chemico-physical and functional properties. Mater. Des. 2018, 145, 196–204. [Google Scholar] [CrossRef]
- Rotaru, I.; Ionescu, M.; Donescu, D.; Capitanu, S.; Vuluga, M. bis-Mannich Polyether Polyols with Aromatic Structures. Mater. Plast. 2009, 46, 21. [Google Scholar]
- Maleki, H.; Durães, L.; Portugal, A. Synthesis of lightweight polymer-reinforced silica aerogels with improved mechanical and thermal insulation properties for space applications. Microporous Mesoporous Mater. 2014, 197, 116–129. [Google Scholar] [CrossRef]
- Cha, J.; Seo, J.; Kim, S. Building materials thermal conductivity measurement and correlation with heat flow meter, laser flash analysis and TCi. J. Therm. Anal. Calorim. 2012, 109, 295–300. [Google Scholar] [CrossRef]
- Skochdopole, R.E. The Thermal Conductivity of foamed plastics. Chem. Eng. Prog. 1961, 57, 55–59. [Google Scholar]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1999; ISBN 978-0-521-49911-8. [Google Scholar]
- Shutov, F.A. Cellular Structure and Properties of Foamed Polymers. In Handbook of Polymeric Foams and Foam Technology, 2nd ed.; Klempner, D., Sendijarevic, V., Eds.; Carl Hanser Verlag Publishers: Munich, Germany, 2004; Volume 3, pp. 17–71. [Google Scholar]
- Tseng, C.; Yamaguchi, M.; Ohmori, T. Thermal conductivity of polyurethane foams from room temperature to 20 K. Cryogenics 1997, 37, 305–312. [Google Scholar] [CrossRef]
- Venkatesan, G.; Jin, G.-P.; Chyu, M.-C.; Zheng, J.-X.; Chu, T.-Y. Measurement of thermophysical properties of polyurethane foam insulation during transient heating. Int. J. Therm. Sci. 2001, 40, 133–144. [Google Scholar] [CrossRef]
- Hilyard, N.C.; Cunningham, A. Low Density Cellular Plastics: Physical Basis of Behaviour; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-94-011-1256-7. [Google Scholar]
- Singh, I.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent Advancement in Plant Oil Derived Polyol-Based Polyurethane Foam for Future Perspective: A Review. Eur. J. Lipid Sci. Technol. 2020, 122, 1900225. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, Y.; Chen, W.; Han, D.; Lin, X.; Xu, G.; Zhang, Q. Open-Cell Rigid Polyurethane Foams from Peanut Shell-Derived Polyols Prepared under Different Post-Processing Conditions. Polymers 2019, 11, 1392. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Kurańska, M.; Prociak, A.; Malewska, E.; Bujok, S. The effect of different palm oil-based bio-polyols on foaming process and selected properties of porous polyurethanes. Polym. Int. 2017, 66, 1522–1529. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Kurańska, M.; Prociak, A.; Szczepkowski, L.; Krzyżowska, M.; Ryszkowska, J. Preparation and characterisation of rigid polyurethane foams using a rapeseed oil-based polyol. Ind. Crop. Prod. 2015, 74, 887–897. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Y.; Xie, Y.; Qiao, K.; Sun, Y.; Yue, L. Synthesis of bio-castor oil polyurethane flexible foams and the influence of biotic component on their performance. J. Polym. Res. 2015, 22, 145. [Google Scholar] [CrossRef]
- Ji, D.; Fang, Z.; He, W.; Zhang, K.; Luo, Z.; Wang, T.; Guo, K. Synthesis of Soy-Polyols Using a Continuous Microflow System and Preparation of Soy-based Polyurethane Rigid Foams. ACS Sustain. Chem. Eng. 2015, 3, 1197–1204. [Google Scholar] [CrossRef]
- Yue, D.; Oribayo, O.L.; Rempel, G.; Pan, Q. Liquefaction of waste pine wood and its application in the synthesis of a flame retardant polyurethane foam. RSC Adv. 2017, 7, 30334–30344. [Google Scholar] [CrossRef]
- Paruzel, A.; Michałowski, S.; Hodan, J.; Horák, P.; Prociak, A.; Beneš, H. Rigid Polyurethane Foam Fabrication Using Medium Chain Glycerides of Coconut Oil and Plastics from End-of-Life Vehicles. ACS Sustain. Chem. Eng. 2017, 5, 6237–6246. [Google Scholar] [CrossRef]

| Sample | T(°C) | xTNB (wt%/SA) | SA/B (mol/mol) |
|---|---|---|---|
| bS | 150 | 0.26 | 1:1 |
| Sample | bM (wt%) | bS (wt%) | P-MDI (wt%) | KAc (wt%) * | PM40 (wt%) * | L5111 (wt%) * | H2O (wt%) * |
|---|---|---|---|---|---|---|---|
| PS0 | 53.5 | - | 45 | 0.27 | 0.27 | 0.53 | 0.53 |
| PS0 | 30 | 13 | 44 | 0.30 | 0.30 | 0.50 | 0.43 |
| PS5 | 23.4 | 23.4 | 51.2 | 0.60 | 0.47 | 0.47 | 0.47 |
| PS7 | 13 | 30 | 54 | 0.52 | 0.52 | 0.6 | 0.43 |
| Sample | RCOOH (%) | IOH (mg KOH/g) | H2O Content (wt%) | Mw * (g/mol) | Viscosity (cPs)@25 °C | Tg (°C) | Tm (°C) |
|---|---|---|---|---|---|---|---|
| bS | 93 | 430 | 0.01 | 5292 | - | 54.6 | 103 |
| bM | - | 245 | ≤0.1 | - | 3500–7500 | 75 | - |
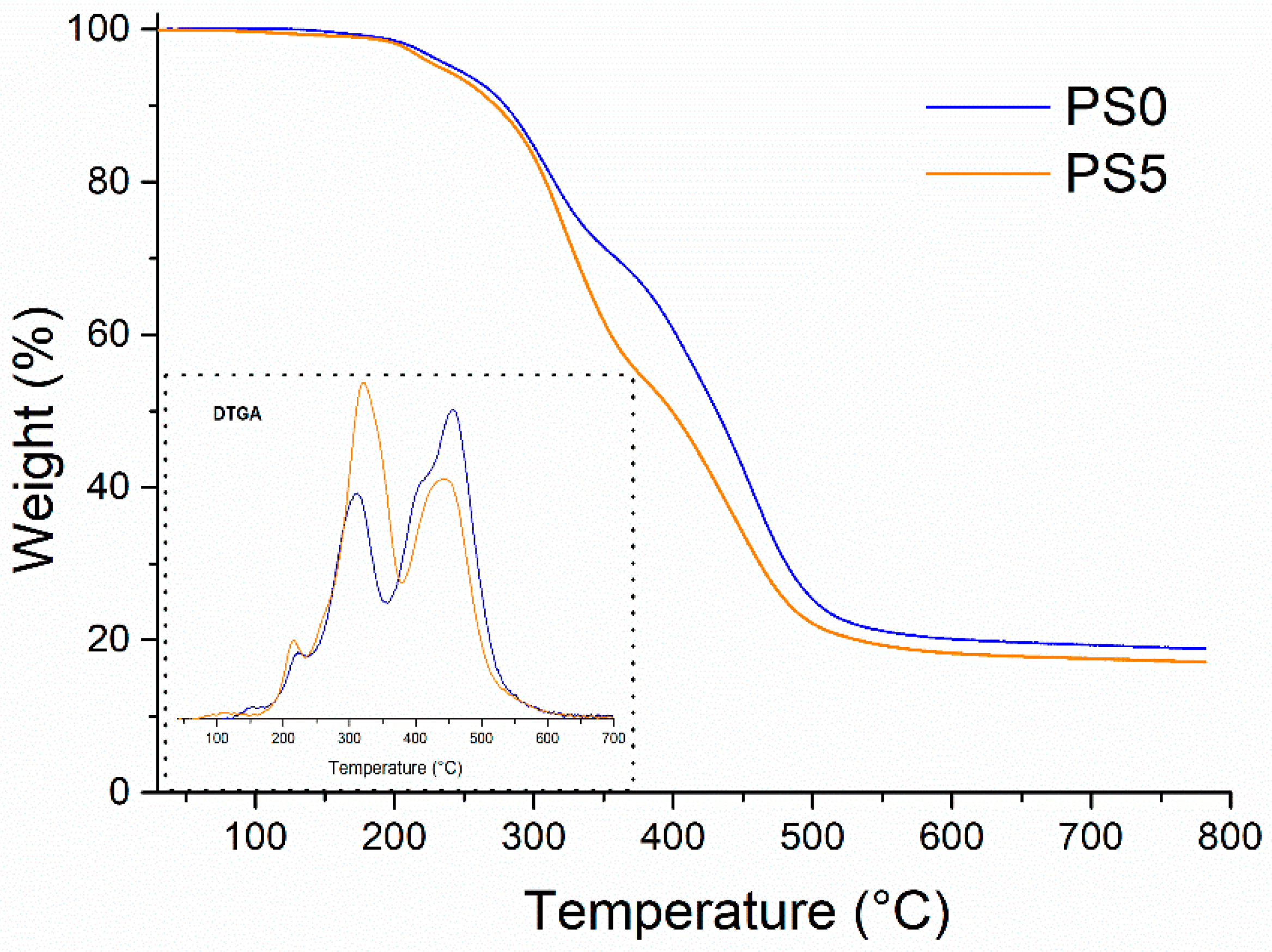
| Sample | Foam Density (kg/m3) | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | Char (wt%) | λ (W/m·K) |
|---|---|---|---|---|---|---|
| PS0 | 45 | 222 | 318 | 416 | 19 | 0.031 |
| PS3 | - | - | - | - | - | - |
| PS5 | 46 | 215 | 315 | 415 | 16 | 0.035 |
| PS7 | 43 | 206 | 310 | 400 | 14 | 0.029 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Luca Bossa, F.; Verdolotti, L.; Russo, V.; Campaner, P.; Minigher, A.; Lama, G.C.; Boggioni, L.; Tesser, R.; Lavorgna, M. Upgrading Sustainable Polyurethane Foam Based on Greener Polyols: Succinic-Based Polyol and Mannich-Based Polyol. Materials 2020, 13, 3170. https://doi.org/10.3390/ma13143170
de Luca Bossa F, Verdolotti L, Russo V, Campaner P, Minigher A, Lama GC, Boggioni L, Tesser R, Lavorgna M. Upgrading Sustainable Polyurethane Foam Based on Greener Polyols: Succinic-Based Polyol and Mannich-Based Polyol. Materials. 2020; 13(14):3170. https://doi.org/10.3390/ma13143170
Chicago/Turabian Stylede Luca Bossa, Ferdinando, Letizia Verdolotti, Vincenzo Russo, Pietro Campaner, Andrea Minigher, Giuseppe Cesare Lama, Laura Boggioni, Riccardo Tesser, and Marino Lavorgna. 2020. "Upgrading Sustainable Polyurethane Foam Based on Greener Polyols: Succinic-Based Polyol and Mannich-Based Polyol" Materials 13, no. 14: 3170. https://doi.org/10.3390/ma13143170
APA Stylede Luca Bossa, F., Verdolotti, L., Russo, V., Campaner, P., Minigher, A., Lama, G. C., Boggioni, L., Tesser, R., & Lavorgna, M. (2020). Upgrading Sustainable Polyurethane Foam Based on Greener Polyols: Succinic-Based Polyol and Mannich-Based Polyol. Materials, 13(14), 3170. https://doi.org/10.3390/ma13143170









