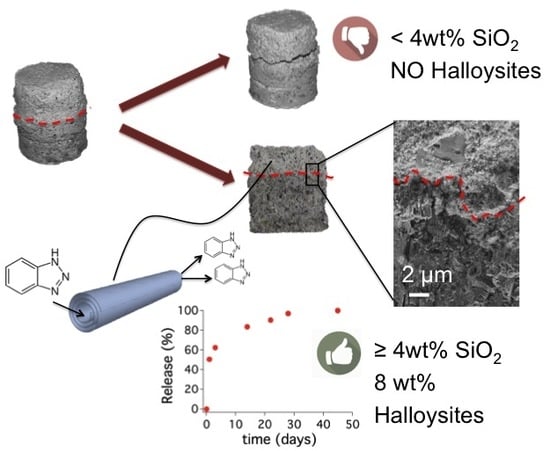
Halloysite Nanotubes as Nano-Carriers of Corrosion Inhibitors in Cement Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Loading of Halloysites
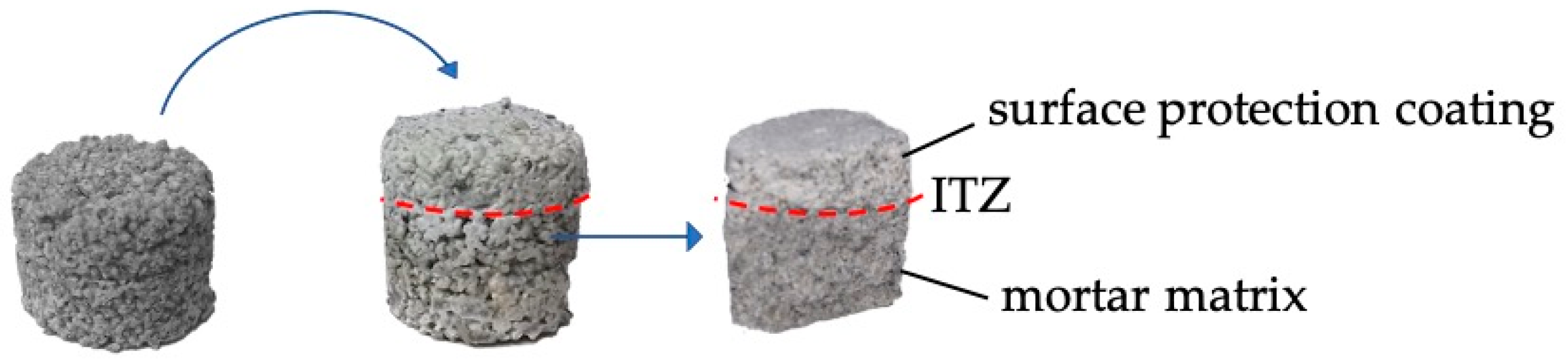
2.3. Preparation of the Samples
2.4. Methods
2.4.1. Characterization Techniques
2.4.2. Kinetics of Release
3. Results
3.1. Characterization of the Nanotubes
3.2. Characterization of the Surface-Protection Cementitious Coating
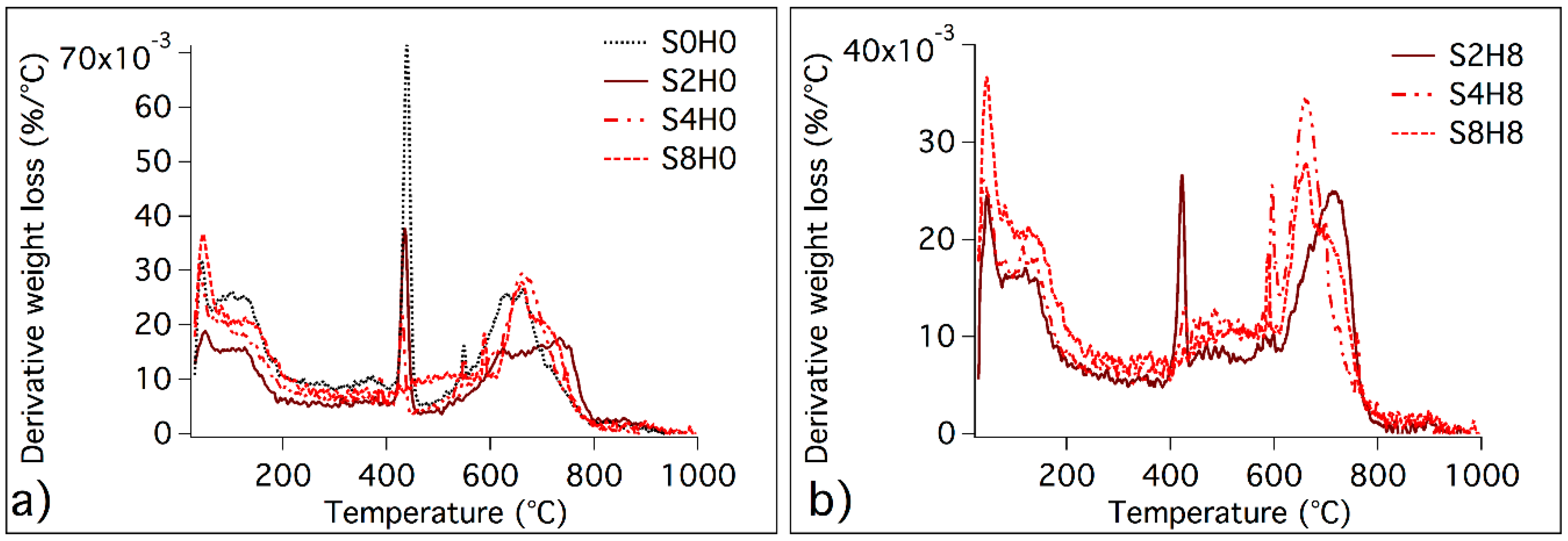
3.2.1. Thermogravimetric Analysis
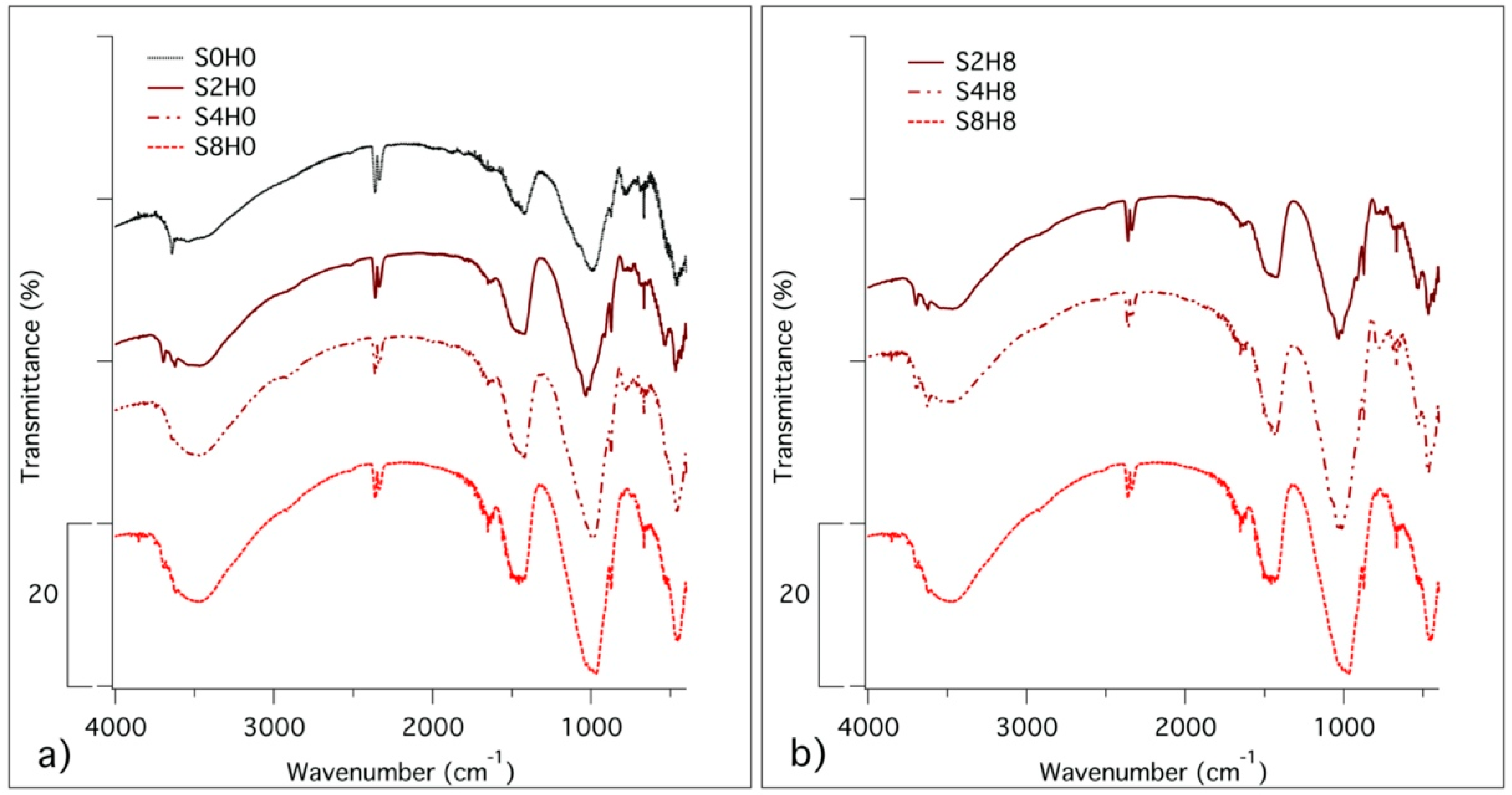
3.2.2. Fourier-Transform Infrared Spectroscopy
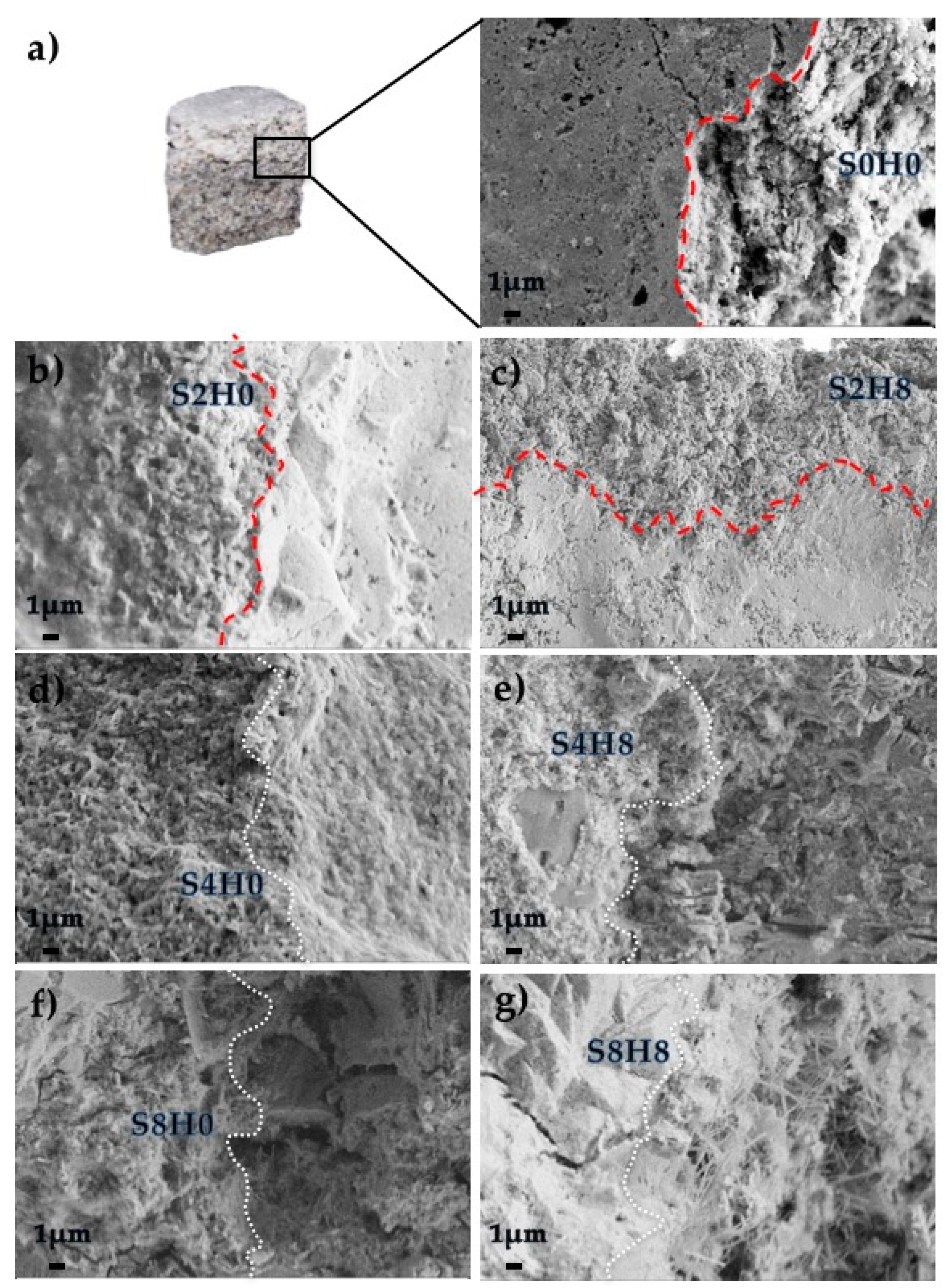
3.2.3. Scanning Electron Microscopy of the Interfacial Transition Zone
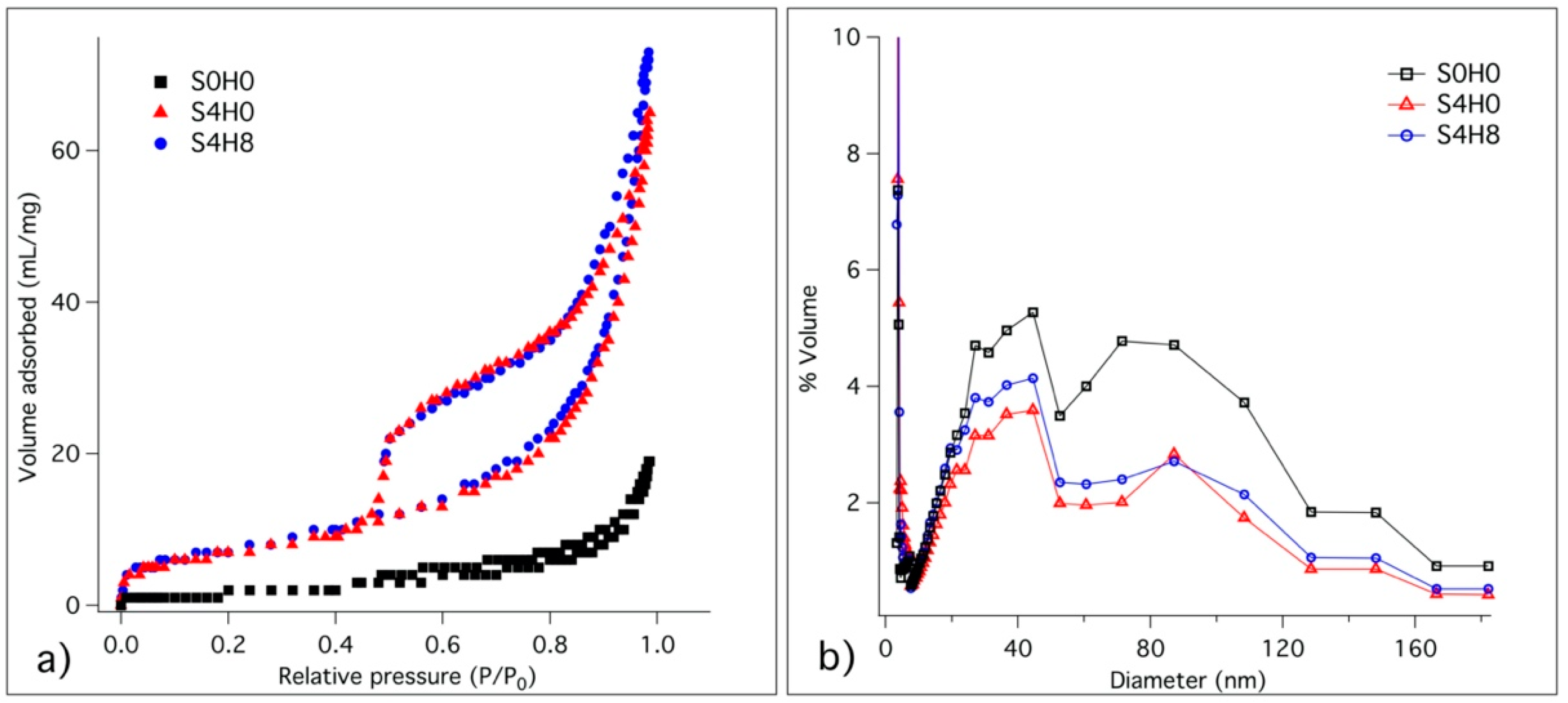
3.2.4. Specific Surface Area and Pore Size Distribution
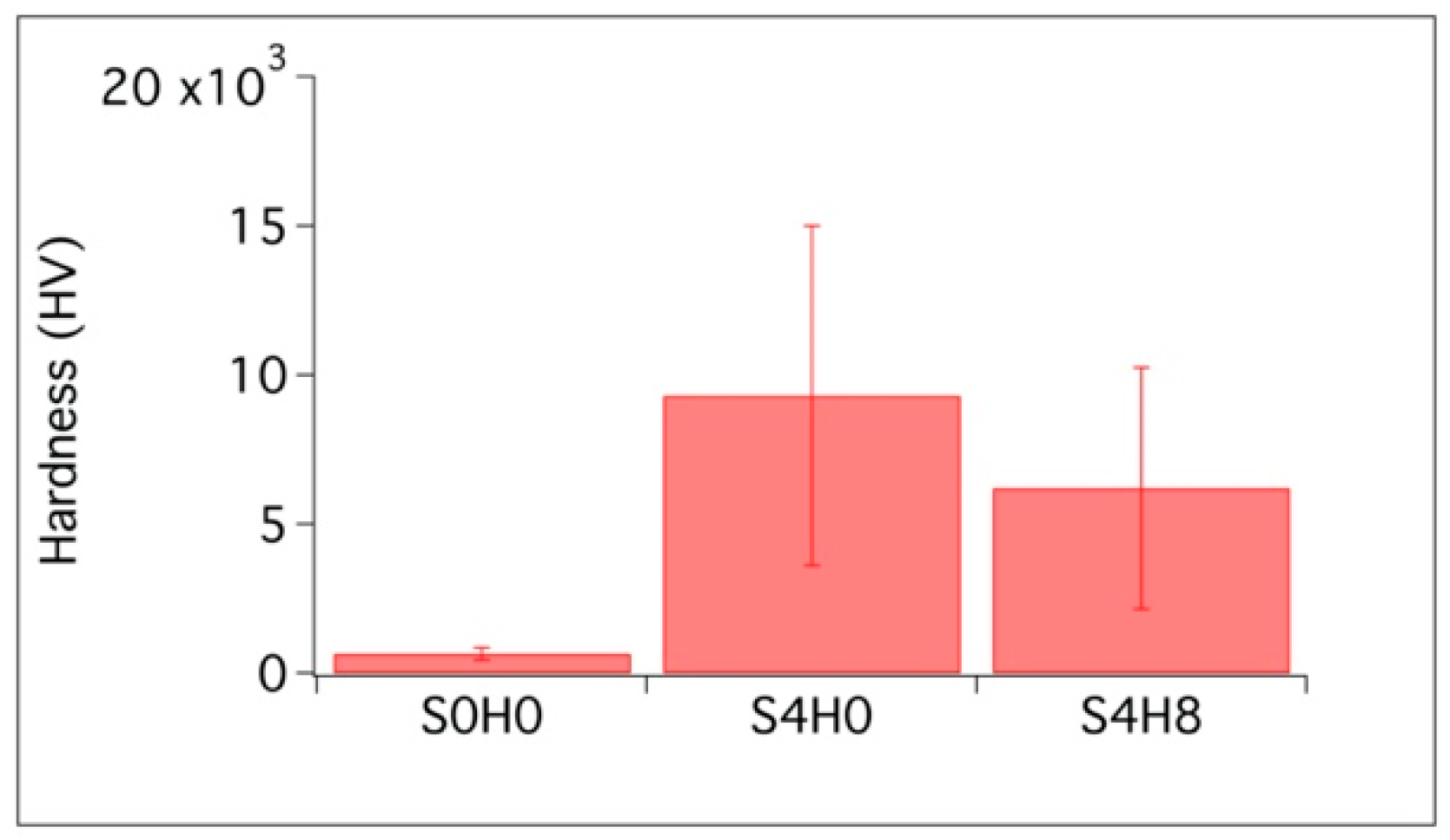
3.2.5. Vickers
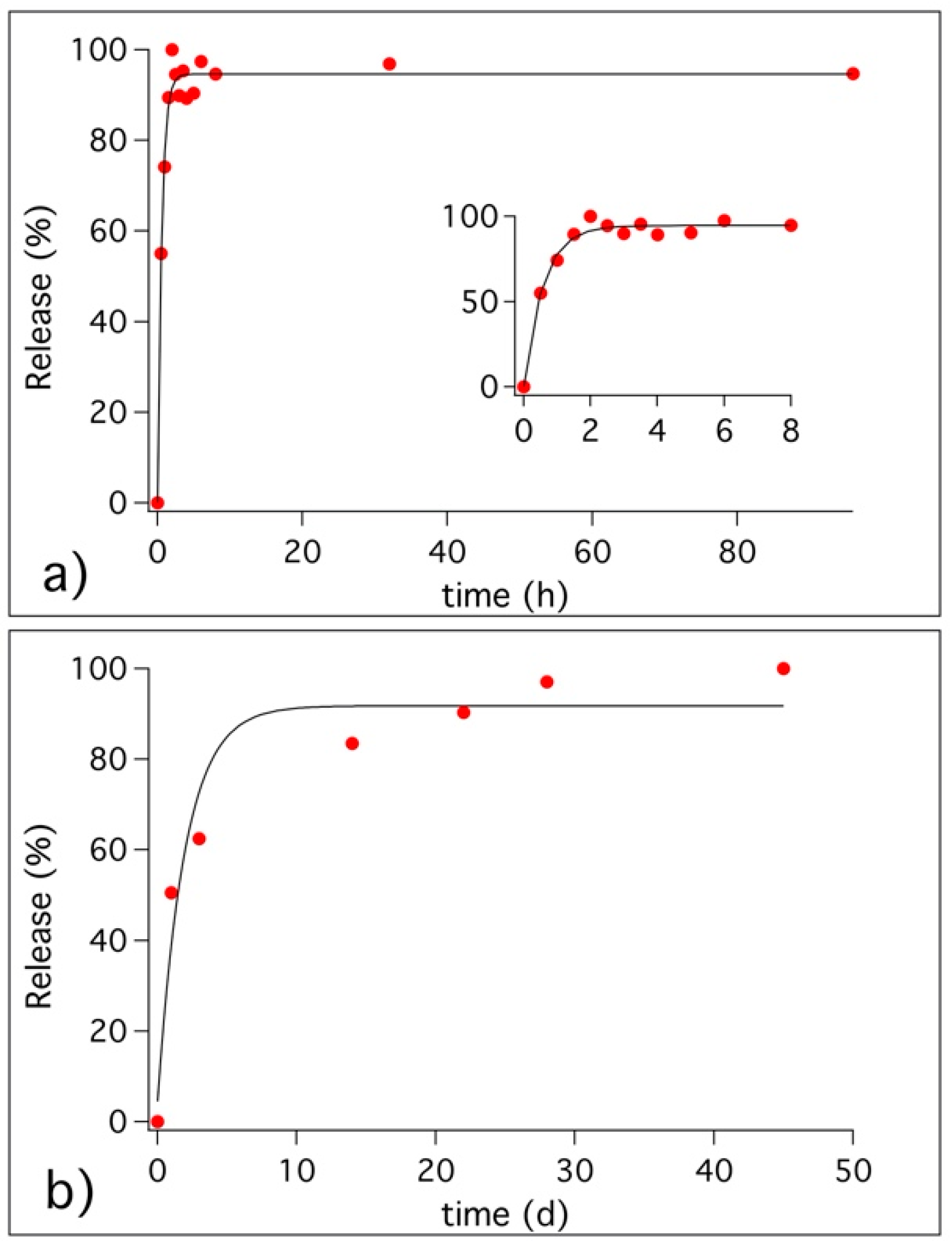
3.3. Release of Benzotriazole in Simulated Pore Solution and in Cementitious Coating
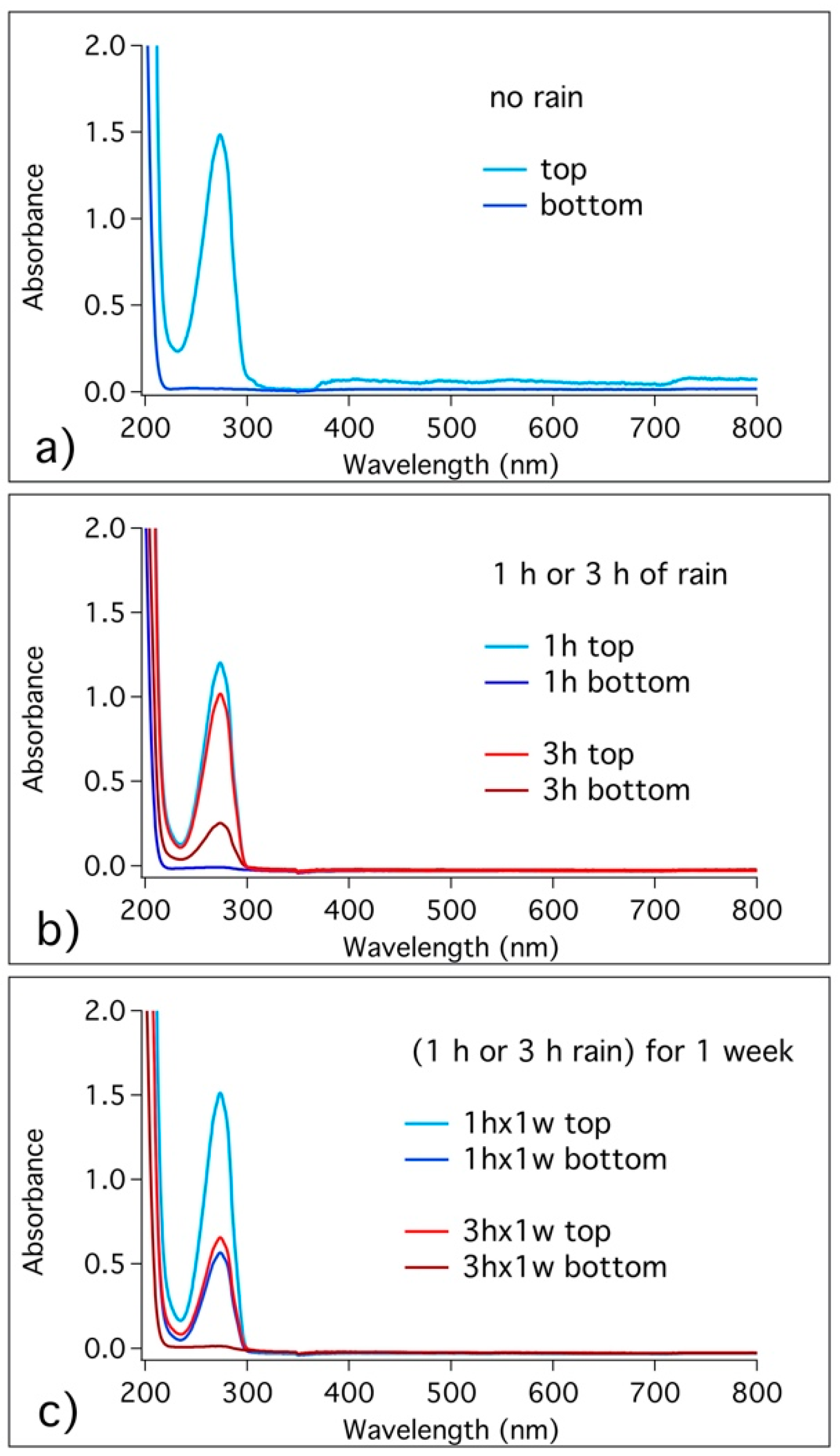
3.4. Migration of Benzotriazole in the Cement Matrix
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Costa, A.; Appleton, J. Chloride penetration into concrete in marine environment—Part I: Main parameters affecting chloride penetration. Mat. Struct. 1999, 32, 252–259. [Google Scholar] [CrossRef]
- Basheer, L.; Kropp, J.; Cleland, D.J. Assessment of the durability of concrete from its permeation properties: A review. Constr. Build. Mater. 2001, 15, 93–103. [Google Scholar] [CrossRef]
- Wittmann, F.H.; Wittmann, A.D.A.; Wang, P.G. Capillary absorption of integral water repellent and surface impregnated concrete. Restor. Build. Monum. 2014, 20, 281–290. [Google Scholar] [CrossRef]
- Muhammad, N.Z.; Keyvanfar, A.; Majid, M.Z.A.; Shafaghat, A.; Mirza, J. Waterproof performance of concrete: A critical review on implemented approaches. Constr. Build. Mater. 2015, 101, 80–90. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Ou, J. Hydrophobic/Superhydrophobic Concrete. In Smart and Multifunctional Concrete Toward Sustainable Infrastructures; Springer Singapore: Singapore, 2017; pp. 339–357. ISBN 978-981-10-4348-2. [Google Scholar]
- De Vries, J.; Polder, R.B. Hydrophobic treatment of concrete. Constr. Build. Mater. 1997, 11, 259–265. [Google Scholar] [CrossRef]
- Teng, L.W.; Huang, R.; Zou, S.Y.; Hsu, H.M. Protection Effectiveness of Concrete Surface Treating Materials. AMR 2013, 834–836, 749–754. [Google Scholar] [CrossRef]
- Liu, J.; Miao, C.; Chen, C.; Liu, J.; Cui, G. Effect and mechanism of controlled permeable formwork on concrete water adsorption. Constr. Build. Mater. 2013, 39, 129–133. [Google Scholar] [CrossRef]
- Muzenski, S.; Flores-Vivian, I.; Sobolev, K. Hydrophobic engineered cementitious composites for highway applications. Cem. Concr. Compos. 2015, 57, 68–74. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Muzenski, S.W.; Flores-Vivian, I.; Sobolev, K. The Development of Hydrophobic and Superhydrophobic Cementitious Composites. In Proceedings of the 4th International Conference on the Durability of Concrete Structures, West Lafayette, IN, USA, 24–26 July 2014. [Google Scholar]
- Tonelli, M.; Peppou-Chapman, S.; Ridi, F.; Neto, C. Effect of Pore Size, Lubricant Viscosity, and Distribution on the Slippery Properties of Infused Cement Surfaces. J. Phys. Chem. C 2019, 123, 2987–2995. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, H.; Shen, W.; Xu, G.; Ma, B.; Ji, X. Nano-silica and silica fume modified cement mortar used as Surface Protection Material to enhance the impermeability. Cem. Concr. Compos. 2018, 92, 7–17. [Google Scholar] [CrossRef]
- Faustino, P.; Brás, A.; Ripper, T. Corrosion inhibitors’ effect on design service life of RC structures. Constr. Build. Mater. 2014, 53, 360–369. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Brenna, A.; Bolzoni, F.; Berra, M.; Pastore, T.; Ormellese, M. Effect of polymer modified cementitious coatings on water and chloride permeability in concrete. Constr. Build. Mater. 2013, 49, 720–728. [Google Scholar] [CrossRef]
- Ormellese, M.; Berra, M.; Bolzoni, F.; Pastore, T. Corrosion inhibitors for chlorides induced corrosion in reinforced concrete structures. Cem. Concr. Res. 2006, 36, 536–547. [Google Scholar] [CrossRef]
- Balonis, M.; Sant, G.; Burkan Isgor, O. Mitigating steel corrosion in reinforced concrete using functional coatings, corrosion inhibitors, and atomistic simulations. Cem. Concr. Compos. 2019, 101, 15–23. [Google Scholar] [CrossRef]
- Ibrahim, M.; Al-Gahtani, A.S.; Maslehuddin, M.; Almusallam, A.A. Effectiveness of concrete surface treatmentmaterials in reducing chloride-induced reinforcement corrosion. Constr. Build. Mater. 1997, 11, 443–451. [Google Scholar] [CrossRef]
- James, A.; Bazarchi, E.; Chiniforush, A.A.; Panjebashi Aghdam, P.; Hosseini, M.R.; Akbarnezhad, A.; Martek, I.; Ghodoosi, F. Rebar corrosion detection, protection, and rehabilitation of reinforced concrete structures in coastal environments: A review. Constr. Build. Mater. 2019, 224, 1026–1039. [Google Scholar] [CrossRef]
- Seneviratne, A.M.G.; Sergi, G.; Page, C.L. Performance characteristics of surface coatings applied to concrete for control of reinforcement corrosion. Constr. Build. Mater. 2000, 14, 55–59. [Google Scholar] [CrossRef]
- Jin, M.; Jiang, L.; Tao, D.; Bai, S. Characterization of Ag/AgCl electrode manufactured by immersion in sodium hypochloride acid for monitoring chloride content in concrete. Constr. Build. Mater. 2016, 122, 310–319. [Google Scholar] [CrossRef]
- Saraswathy, V.; Muralidharan, S.; Kalyanasundaram, R.M.; Thangavel, K.; Srinivasan, S. Evaluation of a composite corrosion-inhibiting admixture and its performance in concrete under macrocell corrosion conditions. Cem. Concr. Res. 2001, 31, 789–794. [Google Scholar] [CrossRef]
- Xiong, C.; Li, W.; Jin, Z.; Gao, X.; Wang, W.; Tian, H.; Han, P.; Song, L.; Jiang, L. Preparation of phytic acid conversion coating and corrosion protection performances for steel in chlorinated simulated concrete pore solution. Corros. Sci. 2018, 139, 275–288. [Google Scholar] [CrossRef]
- Królikowski, A.; Kuziak, J. Impedance study on calcium nitrite as a penetrating corrosion inhibitor for steel in concrete. Electrochim. Acta 2011, 56, 7845–7853. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Singh, J.K.; Yang, H.-M.; Lee, H.-S.; Ismail, M.A. Evaluation of corrosion resistance properties of N, N′-Dimethyl ethanolamine corrosion inhibitor in saturated Ca(OH)2 solution with different concentrations of chloride ions by electrochemical experiments. Constr. Build. Mater. 2016, 114, 223–231. [Google Scholar] [CrossRef]
- Abd El Haleem, S.M.; Abd El Wanees, S.; Bahgat, A. Environmental factors affecting the corrosion behaviour of reinforcing steel. VI. Benzotriazole and its derivatives as corrosion inhibitors of steel. Corros. Sci. 2014, 87, 321–333. [Google Scholar] [CrossRef]
- Monticelli, C.; Frignani, A.; Trabanelli, G. A study on corrosion inhibitors for concrete application. Cem. Concr. Res. 2000, 30, 635–642. [Google Scholar] [CrossRef]
- Yang, H.; Li, W.; Liu, X.; Liu, A.; Hang, P.; Ding, R.; Li, T.; Zhang, Y.; Wang, W.; Xiong, C. Preparation of corrosion inhibitor loaded zeolites and corrosion resistance of carbon steel in simulated concrete pore solution. Constr. Build. Mater. 2019, 225, 90–98. [Google Scholar] [CrossRef]
- Abdullayev, E.; Price, R.; Shchukin, D.; Lvov, Y. Halloysite Tubes as Nanocontainers for Anticorrosion Coating with Benzotriazole. ACS Appl. Mater. Interfaces 2009, 1, 1437–1443. [Google Scholar] [CrossRef]
- Sheban, M.; Abu-Dalo, M.; Ababneh, A.; Andreescu, S. Effect of benzotriazole derivatives on the corrosion of steel in simulated concrete pore solutions. Anti Corros. Methods Mater. 2007, 54, 135–147. [Google Scholar] [CrossRef]
- Tian, H.; Li, W.; Liu, A.; Gao, X.; Han, P.; Ding, S.-J.; Yang, C.; Wang, D. Controlled delivery of multi-substituted triazole by metal-organic framework for efficient inhibition of mild steel corrosion in neutral chloride solution. Corros. Sci. 2018, 131, 1–6. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.S.; Möhwald, H. Layer-by-Layer Assembled Nanocontainers for Self-Healing Corrosion Protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Möhwald, H. Smart nanocontainers as depot media for feedback active coatings. Chem. Commun. 2011, 47, 8730. [Google Scholar] [CrossRef]
- Farzadnia, N.; Abang Ali, A.A.; Demirboga, R.; Anwar, M.P. Effect of halloysite nanoclay on mechanical properties, thermal behavior and microstructure of cement mortars. Cem. Concr. Res. 2013, 48, 97–104. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite Clay Minerals–A Review; Cambridge University Press: Cambridge, UK, 2005; Volume 40, pp. 383–426. [Google Scholar]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Kamble, R.; Ghag, M.; Gaikawad, S.; Panda, B.K. Halloysite Nanotubes and Applications: A Review. J. Adv. Sci. Res. 2012, 3, 25–29. [Google Scholar]
- Prashantha, K.; Lacrampe, M.-F.; Krawczak, P. Highly dispersed polyamide-11/halloysite nanocomposites: Thermal, rheological, optical, dielectric, and mechanical properties. J. Appl. Polym. Sci. 2013, 130, 313–321. [Google Scholar] [CrossRef]
- Rawtani, D.; Agrawal, Y.K. Multifarious applications of halloysite nanotubes: A review. Rev. Adv. Mater. Sci. 2012, 30, 282–295. [Google Scholar]
- Abdullayev, E.; Lvov, Y. Halloysite clay nanotubes as a ceramic “skeleton” for functional biopolymer composites with sustained drug release. J. Mater. Chem. B 2013, 1, 2894. [Google Scholar] [CrossRef]
- Scarfato, P.; Avallone, E.; Incarnato, L.; Di Maio, L. Development and evaluation of halloysite nanotube-based carrier for biocide activity in construction materials protection. Appl. Clay Sci. 2016, 132–133, 336–342. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Churchman, G.J. Natural Mineral Nanotubes: Properties and Applications; CRC Press: Cleveland, OH, USA, 2015. [Google Scholar]
- Allalou, S.; Kheribet, R.; Benmounah, A. Effects of calcined halloysite nano-clay on the mechanical properties and microstructure of low-clinker cement mortar. Case Stud. Constr. Mater. 2019, 10, e00213. [Google Scholar] [CrossRef]
- Liu, H.; Jin, J.; Yu, Y.; Liu, H.; Liu, S.; Shen, J.; Xia, X.; Ji, H. Influence of halloysite nanotube on hydration products and mechanical properties of oil well cement slurries with nano-silica. Constr. Build. Mater. 2020, 247, 118545. [Google Scholar] [CrossRef]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Farshad, F.F.; Pesacreta, T.C.; Garber, J.D.; Bikki, S.R. A comparison of surface roughness of pipes as measured by two profilometers and atomic force microscopy. Scanning 2001, 23, 241–248. [Google Scholar] [CrossRef]
- Glossary of Meteorology. “Rain”. American Meteorological Society. Available online: https://web.archive.org/web/20100725142506/http://amsglossary.allenpress.com/glossary/search?id=rain1 (accessed on 31 March 2020).
- Tonelli, M.; Martini, F.; Calucci, L.; Geppi, M.; Borsacchi, S.; Ridi, F. Traditional Portland cement and MgO-based cement: A promising combination? Phys. Chem. Earth Parts A/B/C 2017, 99, 158–167. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; T. Telford: London, UK, 1997; ISBN 978-0-7277-2592-9. [Google Scholar]
- Klimesch, D.S.; Ray, A. DTA-TGA evaluations of the CaO–Al2O3–SiO2–H2O system treated hydrothermally. Thermochim. Acta 1999, 334, 115–122. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Martini, F.; Borsacchi, S.; Geppi, M.; Tonelli, M.; Ridi, F.; Calucci, L. Monitoring the hydration of MgO-based cement and its mixtures with Portland cement by 1 H NMR relaxometry. Microporous Mesoporous Mater. 2017, 3. [Google Scholar] [CrossRef]
- Ridi, F.; Fratini, E.; Baglioni, P. Fractal Structure Evolution during Cement Hydration by Differential Scanning Calorimetry: Effect of Organic Additives. J. Phys. Chem. C 2013, 117, 25478–25487. [Google Scholar] [CrossRef]
- Tironi, A.; Cravero, F.; Scian, A.N.; Irassar, E.F. Pozzolanic activity of calcined halloysite-rich kaolinitic clays. Appl. Clay Sci. 2017, 147, 11–18. [Google Scholar] [CrossRef]

| Name | SiO2 (wt%) 1 | HNT (wt%) 1 | s/c | w/c |
|---|---|---|---|---|
| S0H0 | 0 | 0 | 3 | 0.5 |
| S2H0 | 2 | 0 | 3 | 1.2 |
| S2H8 | 2 | 8 | 3 | 1.2 |
| S4H0 | 4 | 0 | 3 | 1.8 |
| S4H8 | 4 | 8 | 3 | 1.8 |
| S8H0 | 8 | 0 | 3 | 2.4 |
| S4H8 | 8 | 8 | 3 | 2.4 |
| Sample | BET Surface Area (m2/g) | Total Pore Volume (mL/g) |
|---|---|---|
| S0H0 | 7 ± 1 | 0.03 ± 0.01 |
| S4H0 | 27 ± 1 | 0.10 ± 0.01 |
| S4H8 | 29 ± 1 | 0.11 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonelli, M.; Baglioni, P.; Ridi, F. Halloysite Nanotubes as Nano-Carriers of Corrosion Inhibitors in Cement Formulations. Materials 2020, 13, 3150. https://doi.org/10.3390/ma13143150
Tonelli M, Baglioni P, Ridi F. Halloysite Nanotubes as Nano-Carriers of Corrosion Inhibitors in Cement Formulations. Materials. 2020; 13(14):3150. https://doi.org/10.3390/ma13143150
Chicago/Turabian StyleTonelli, Monica, Piero Baglioni, and Francesca Ridi. 2020. "Halloysite Nanotubes as Nano-Carriers of Corrosion Inhibitors in Cement Formulations" Materials 13, no. 14: 3150. https://doi.org/10.3390/ma13143150
APA StyleTonelli, M., Baglioni, P., & Ridi, F. (2020). Halloysite Nanotubes as Nano-Carriers of Corrosion Inhibitors in Cement Formulations. Materials, 13(14), 3150. https://doi.org/10.3390/ma13143150