Oxidation of Al-Co Alloys at High Temperatures
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Alloy Microstructure and Constitution before Oxidation
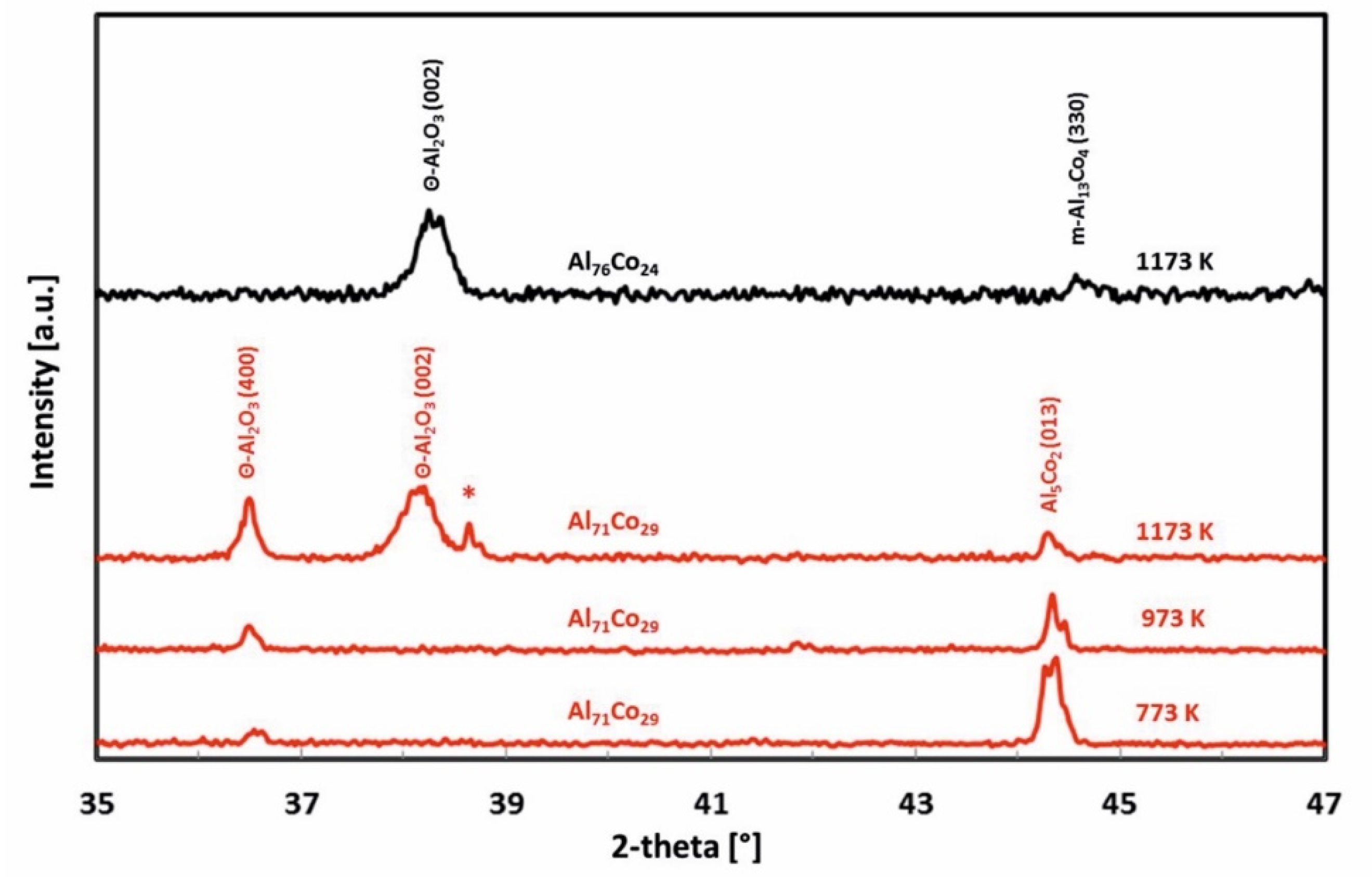
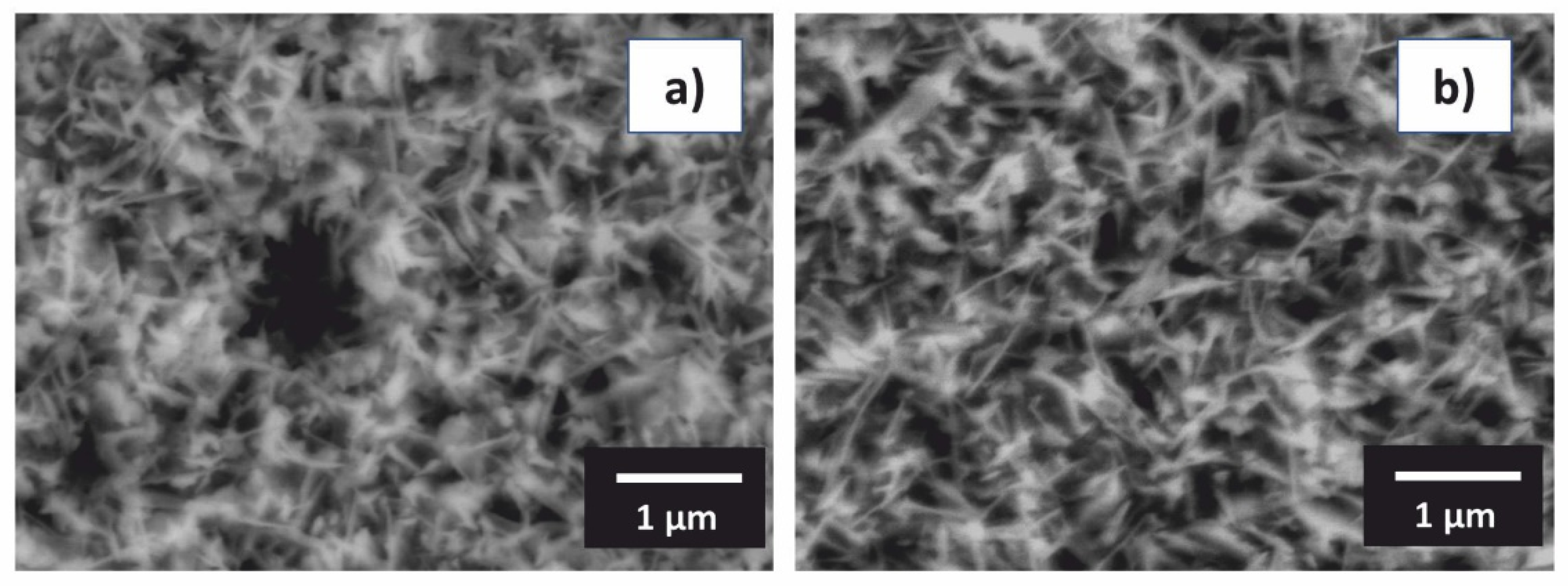
3.2. Oxidation Behavior
4. Conclusions
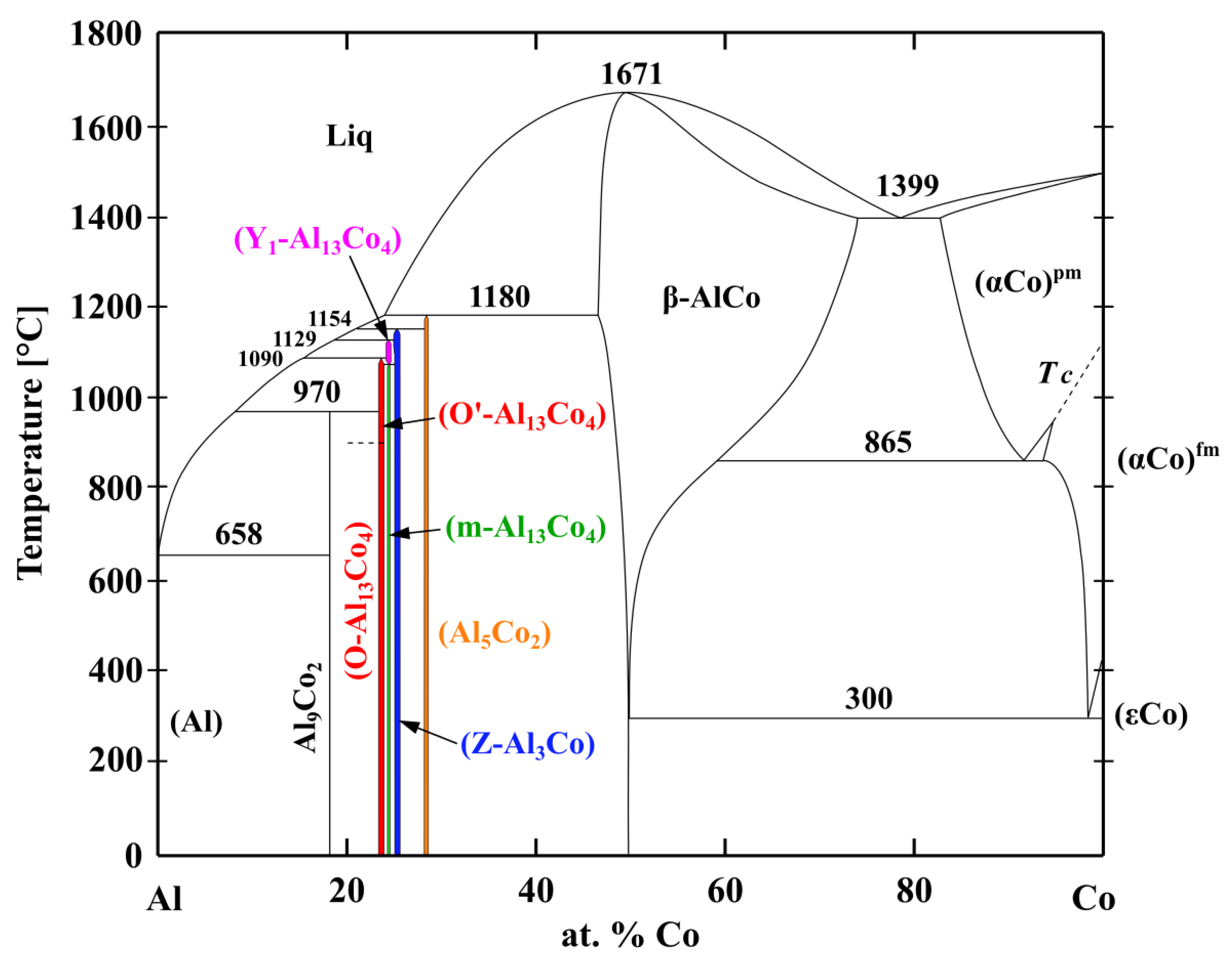
- The alloys were composed of different microstructure constituents. The Al76Co24 alloy was composed of Al9Co2, m-Al13Co4 and Z-Al3Co. The Al71Co29 alloy consisted of Z-Al3Co, Al5Co2 and β-AlCo. The precipitation sequences of the constituents were explained based on the equilibrium Al-Co phase diagram and rapid solidification processes taking place during casting.
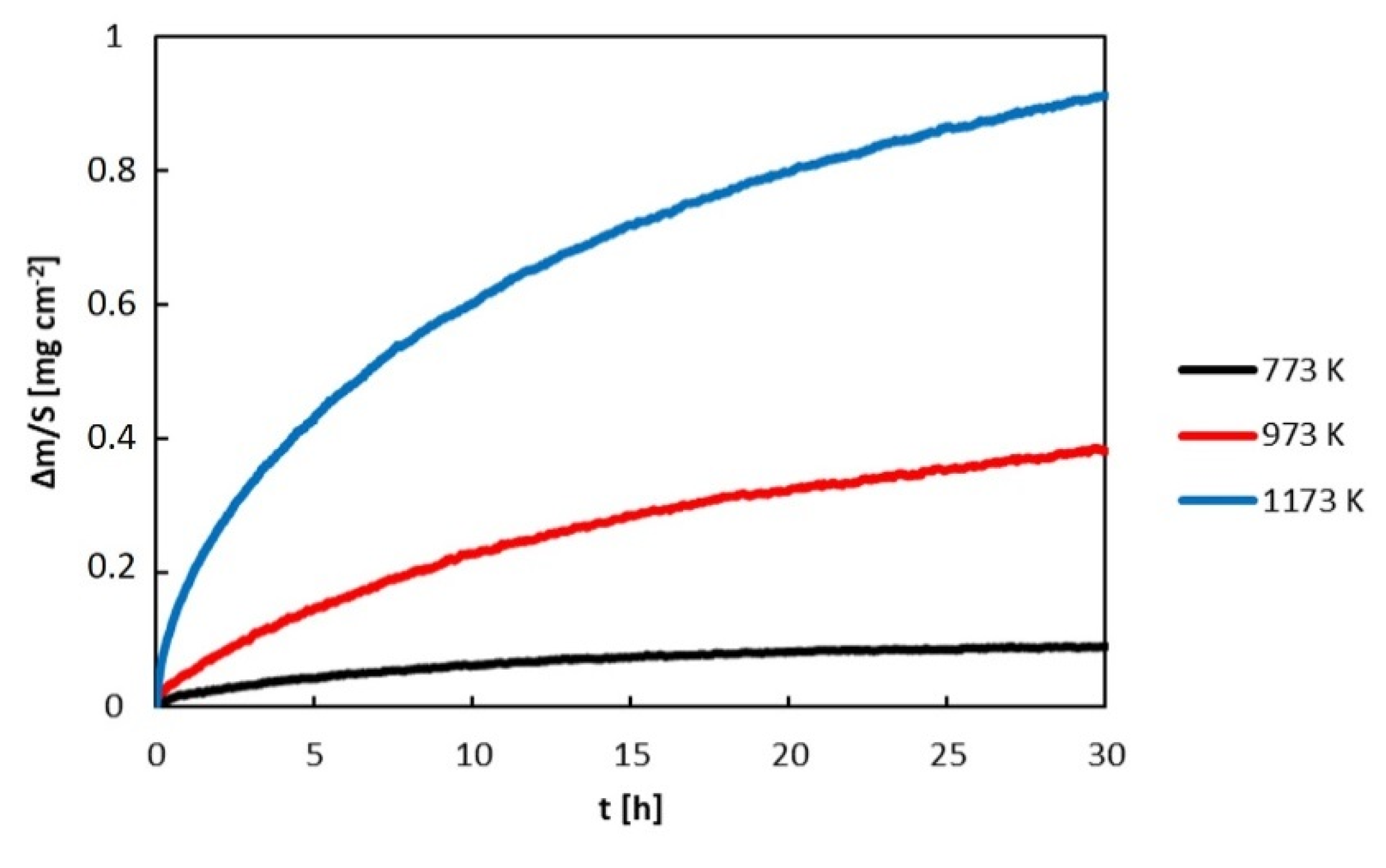
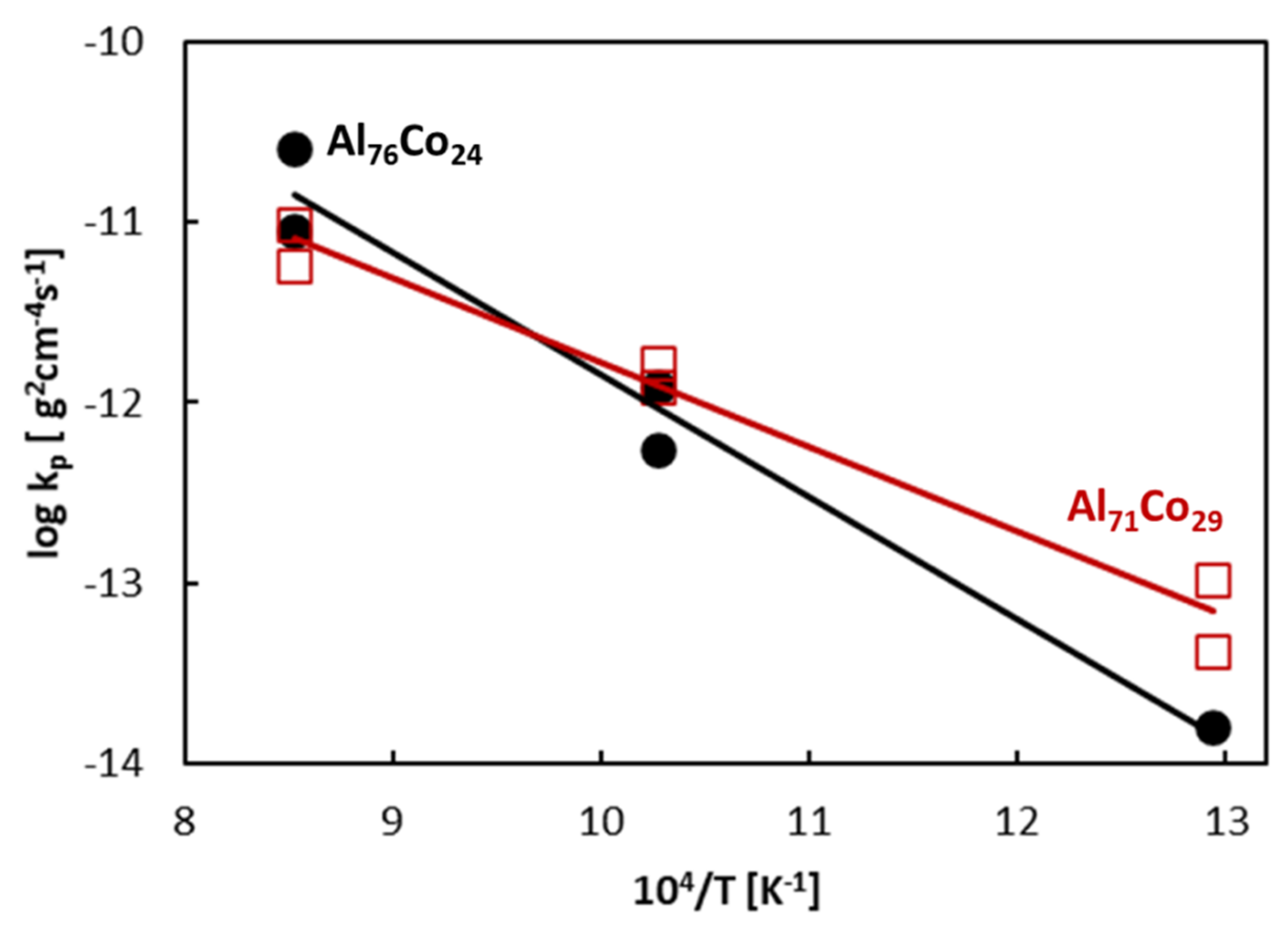
- During oxidation in air, aluminum in the alloys was selectively oxidized and a protective alumina scale was formed on the alloy surfaces. The oxidation kinetics followed a parabolic rate law. The rate constants of the alloys were between 1.63 × 10−14 and 8.83 × 10−12 g cm−4 s−1, depending on the annealing temperature. The activation energies of oxidation were 90 kJ mol−1 for the Al71Co29 alloy and 123 kJ mol−1 for the Al76Co24 alloy, respectively.
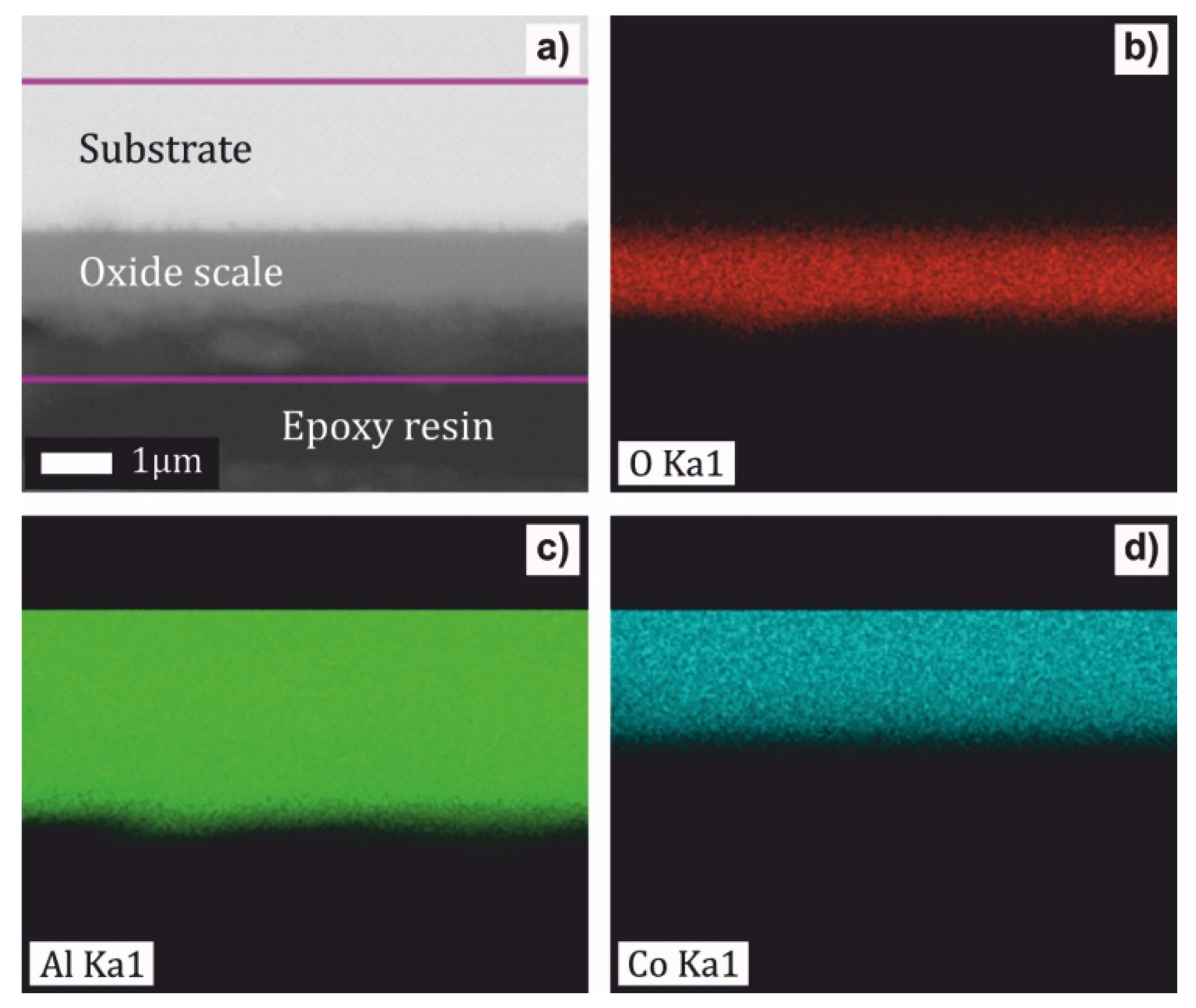
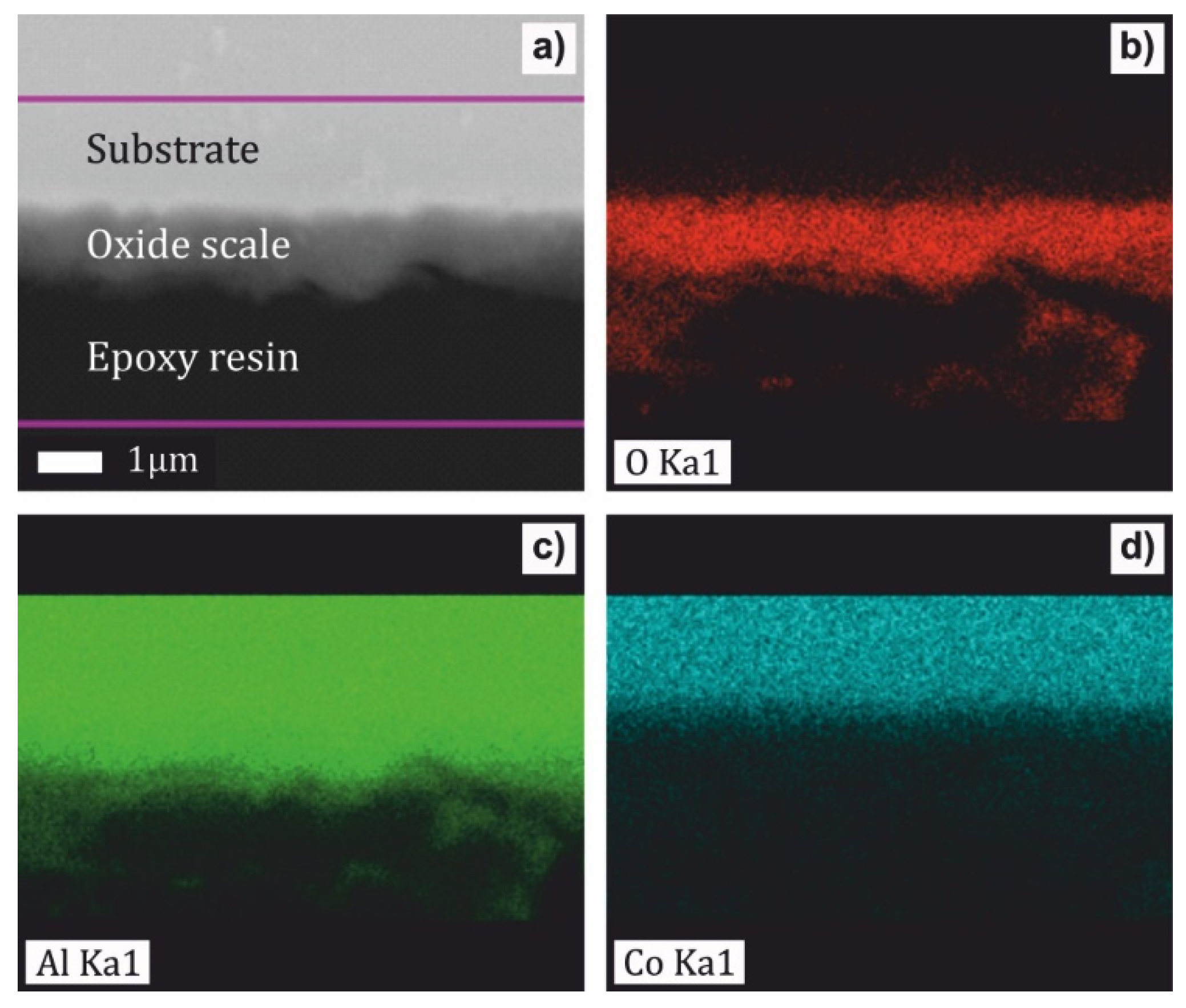
- The scale of the Al76Co24 alloy was adherent to the substrate and had a wave-like morphology. At 1173 K, a preferential orientation of θ-Al2O3 in (002) crystallographic plane was found. The scale on the Al71Co29 alloy was more uniform and spallation was observed locally on the dendritic β-AlCo.
- The oxidation kinetics of the Al71Co29 and Al76Co24 alloys was comparable to previously studied Al24Co76 and Al32Co68 alloys where a continuous alumina scale had been formed. The increased Al concentration contributes to the alloy’s corrosion resistance. The continuous Al2O3 scale forms a barrier to cobalt diffusion, and it hinders the nucleation and growth of cobalt oxides.
Author Contributions
Funding
Conflicts of Interest
References
- Sato, J.; Omori, T.; Oikawa, K.; Ohnuma, I.; Kainuma, R.; Ishida, K. Cobalt-Base High-Temperature Alloys. Science 2006, 312, 90–91. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, W.; Dong, C. Co-Al-W-based superalloys: A summary of current knowledge. Cailiao Daobao/Mater. Rep. 2020, 34, 3157–3164. [Google Scholar] [CrossRef]
- Pollock, T.M.; Dibbern, J.; Tsunekane, M.; Zhu, J.; Suzuki, A. New Co-based γ-γ′ high-temperature alloys. JoM 2010, 62, 58–63. [Google Scholar] [CrossRef]
- Betteridge, W.; Shaw, S.W.K. Development of superalloys. Mater. Sci. Technol. 1987, 3, 682–694. [Google Scholar] [CrossRef]
- Coutsouradis, D.; Davin, A.; Lamberigts, M. Cobalt-based superalloys for applications in gas turbines. Mater. Sci. Eng. 1987, 88, 11–19. [Google Scholar] [CrossRef]
- Klarstrom, D.L. Wrought cobalt- base superalloys. J. Mater. Eng. Perform. 1993, 2, 523–530. [Google Scholar] [CrossRef]
- Moffat, J.P.; Whitfield, T.; Christofidou, K.A.; Pickering, E.; Jones, N.; Stone, H.J. The Effect of Heat Treatment on the Oxidation Resistance of Cobalt-Based Superalloys. Metals 2020, 10, 248. [Google Scholar] [CrossRef]
- Liu, L.; Wu, S.-S.; Dong, Y.; Lü, S. Effects of alloyed Mn on oxidation behaviour of a Co-Ni-Cr-Fe alloy between 1050 and 1250 °C. Corros. Sci. 2016, 104, 236–247. [Google Scholar] [CrossRef]
- Holcomb, G.R. Steam Oxidation and Chromia Evaporation in Ultrasupercritical Steam Boilers and Turbines. J. Electrochem. Soc. 2009, 156, C292–C297. [Google Scholar] [CrossRef]
- Forsik, S.A.J.; Rosas, A.O.P.; Wang, T.; Colombo, G.A.; Zhou, N.; Kernion, S.J.; Epler, M.E. High-Temperature Oxidation Behavior of a Novel Co-Base Superalloy. Met. Mater. Trans. A 2018, 49, 4058–4069. [Google Scholar] [CrossRef]
- Klein, L.; Bauer, A.; Neumeier, S.; Göken, M.; Virtanen, S. High temperature oxidation of γ/γ′-strengthened Co-base superalloys. Corros. Sci. 2011, 53, 2027–2034. [Google Scholar] [CrossRef]
- Yan, H.-Y.; Vorontsov, V.; Dye, D. Effect of alloying on the oxidation behaviour of Co-Al-W superalloys. Corros. Sci. 2014, 83, 382–395. [Google Scholar] [CrossRef]
- Goward, G.W.; Cannon, L.W. Pack Cementation Coatings for Superalloys: A Review of History, Theory, and Practice. J. Eng. Gas Turbines Power 1988, 110, 150–154. [Google Scholar] [CrossRef]
- Goward, G. Protective coatings—Purpose, role, and design. Mater. Sci. Technol. 1986, 2, 194–200. [Google Scholar] [CrossRef]
- Goward, G. Progress in coatings for gas turbine airfoils. Surf. Coat. Technol. 1998, 108, 73–79. [Google Scholar] [CrossRef]
- Streiff, R.; Boone, D.H. Corrosion resistant modified aluminide coatings. J. Mater. Eng. 1988, 10, 15–26. [Google Scholar] [CrossRef]
- Meier, G.; Pettit, F. High-temperature corrosion of alumina-forming coatings for superalloys. Surf. Coat. Technol. 1989, 39, 1–17. [Google Scholar] [CrossRef]
- Liu, P.; Liang, K.; Gu, S. High-temperature oxidation behavior of aluminide coatings on a new cobalt-base superalloy in air. Corros. Sci. 2001, 43, 1217–1226. [Google Scholar] [CrossRef]
- Rahman, A.; Jayaganthan, R.; Chandra, R.; Ambardar, R. Microstructural Characterization and Cyclic Hot Corrosion Behaviour of Sputtered Co-Al Nanostructured Coatings on Superalloy. Oxid. Met. 2011, 76, 307–330. [Google Scholar] [CrossRef]
- Rahman, A.; Jayaganthan, R.; Chandra, R.; Ambardar, R. High temperature degradation behavior of sputtered nanostructured Co-Al coatings on superalloy. Appl. Surf. Sci. 2013, 265, 10–23. [Google Scholar] [CrossRef]
- Rahman, A.; Chawla, V.; Jayaganthan, R.; Chandra, R.; Ambardar, R. Degradation behaviour of sputtered Co-Al coatings on superalloy. Mater. Chem. Phys. 2013, 138, 49–62. [Google Scholar] [CrossRef]
- Jiang, J.; Zhou, T.; Shao, W.; Zhou, C. Interdiffusion behavior and lifetime prediction of Co-Al coating on Ni-based superalloy. J. Alloys Compd. 2019, 786, 920–929. [Google Scholar] [CrossRef]
- Stein, F.; He, C.; Dupin, N. Melting behaviour and homogeneity range of B2 CoAl and updated thermodynamic description of the Al-Co system. Intermetallics 2013, 39, 58–68. [Google Scholar] [CrossRef]
- Priputen, P.; Palcut, M.; Babinec, M.; Mišík, J.; Černičková, I.; Janovec, J. Correlation between Microstructure and Corrosion Behavior of Near-Equilibrium Al-Co Alloys in Various Environments. J. Mater. Eng. Perform. 2017, 26, 3970–3976. [Google Scholar] [CrossRef]
- Grushko, B.; Wittenberg, R.; Bickmann, K.; Freiburg, C. The constitution of aluminum-cobalt alloys between Al5Co2 and Al9Co2. J. Alloys Compd. 1996, 233, 279–287. [Google Scholar] [CrossRef]
- Priputen, P.; Kusy, M.; Drienovský, M.; Janičkovič, D.; Čička, R.; Černičková, I.; Janovec, J. Experimental reinvestigation of Al-Co phase diagram in vicinity of Al13Co4 family of phases. J. Alloys Compd. 2015, 647, 486–497. [Google Scholar] [CrossRef]
- Cooper, M.J. The electron distribution in the phases CoAl and NiAl. Philos. Mag. 1963, 8, 811–821. [Google Scholar] [CrossRef]
- Burkhardt, U.; Ellner, M.; Grin, Y.; Baumgartner, B. Powder diffraction refinement of the Co2Al5 structure. Powder Diffr. 1998, 13, 159–162. [Google Scholar] [CrossRef]
- Ma, X.L.; Li, X.Z.; Kuo, K.H. A family of τ-inflated monoclinic Al13Co4 phases. Acta Crystallogr. Sect. B Struct. Sci. 1995, 51, 36–43. [Google Scholar] [CrossRef]
- Freiburg, C.; Grushko, B.; Wittenberg, R.; Reichert, W. Once More about Monoclinic Al13Co4. Mater. Sci. Forum 1996, 228, 583–586. [Google Scholar] [CrossRef]
- Grin, J.; Burkhardt, U.; Ellner, M.; Peters, K. Crystal structure of orthorhombic Co4Al13. J. Alloys Compd. 1994, 206, 243–247. [Google Scholar] [CrossRef]
- Fleischer, F.; Weber, T.; Jung, D.; Steurer, W. o’-Al13Co4, a new quasicrystal approximant. J. Alloys Compd. 2010, 500, 153–160. [Google Scholar] [CrossRef]
- Sugiyama, K.; Genba, M.; Hiraga, K.; Waseda, Y. The structure of Y-Al13-xCo4 (x = 0.8) analyzed by single crystal X-ray diffraction coupled with anomalous X-ray scattering. J. Alloys Compd. 2010, 494, 98–101. [Google Scholar] [CrossRef]
- Boström, M.; Rosner, H.; Prots, Y.; Burkhardt, U.; Grin, Y. The Co2Al9 Structure Type Revisited. Z. Anorg. Allg. Chem. 2005, 631, 534–541. [Google Scholar] [CrossRef]
- Heggen, M.; Deng, D.; Feuerbacher, M. Plastic deformation properties of the orthorhombic complex metallic alloy phase Al13Co4. Intermetallics 2007, 15, 1425–1431. [Google Scholar] [CrossRef]
- Samuel, F.H. A study of the microstructure, mechanical properties and failure behaviour of Al-Li-Co melt-spun ribbons: Effect of Al9Co2 phase particle precipitation. J. Mater. Sci. 1986, 21, 3097–3107. [Google Scholar] [CrossRef]
- Lekatou, A.G.; Sfikas, A.; Karantzalis, A.E. The influence of the fabrication route on the microstructure and surface degradation properties of Al reinforced by Al 9 Co 2. Mater. Chem. Phys. 2017, 200, 33–49. [Google Scholar] [CrossRef]
- Wolf, W.; Schulz, R.; Savoie, S.; Bolfarini, C.; Kiminami, C.S.; Botta, W. Structural, mechanical and thermal characterization of an Al-Co-Fe-Cr alloy for wear and thermal barrier coating applications. Surf. Coat. Technol. 2017, 319, 241–248. [Google Scholar] [CrossRef]
- Hagel, W.C. The oxidation of iron, nickel and cobalt-base alloys containing aluminum. Corrosion 1965, 21, 316–326. [Google Scholar] [CrossRef]
- Young, D.J. Oxidation of Pure Metals. In High Temperature Oxidation and Corrosion of Metals, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 3; pp. 85–144. [Google Scholar] [CrossRef]
- Warde, M.; Herinx, M.; Ledieu, J.; Loli, L.S.; Fournée, V.; Gille, P.; Le Moal, S.; Barthés-Labrousse, M.-G. Adsorption of O2 and C2H (n = 2, 4, 6) on the Al9Co2(0 0 1) and o-Al13Co4(1 0 0) complex metallic alloy surfaces. Appl. Surf. Sci. 2015, 357, 1666–1675. [Google Scholar] [CrossRef]
- Villaseca, S.A.; Loli, L.N.S.; Ledieu, J.; Fournée, V.; Gille, P.; Dubois, J.-M.; Gaudry, É. Oxygen adsorption on the Al9Co2(001) surface: First-principles and STM study. J. Phys. Condens. Matter 2013, 25, 355003. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.-M. Properties- and applications of quasicrystals and complex metallic alloys. Chem. Soc. Rev. 2012, 41, 6760. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W.; Bolfarini, C.; Kiminami, C.S.; Botta, W. Designing new quasicrystalline compositions in Al-based alloys. J. Alloys Compd. 2020, 823, 153765. [Google Scholar] [CrossRef]
- Steurer, W. Twenty years of structure research on quasicrystals. Part I. Pentagonal, octagonal, decagonal and dodecagonal quasicrystals. Z. Krist. Cryst. Mater. 2004, 219, 391–446. [Google Scholar] [CrossRef]
- Barber, E.M. Chemical Bonding and Physical Properties in Quasicrystals and Their Related Approximant Phases: Known Facts and Current Perspectives. Appl. Sci. 2019, 9, 2132. [Google Scholar] [CrossRef]
- Rabson, D. Toward theories of friction and adhesion on quasicrystals. Prog. Surf. Sci. 2012, 87, 253–271. [Google Scholar] [CrossRef]
- Balbyshev, V.; King, D.; Khramov, A.; Kasten, L.; Donley, M. Investigation of quaternary Al-based quasicrystal thin films for corrosion protection. Thin Solid Films 2004, 447, 558–563. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Dubiel, B.; Wendler, B. AlCuFe(Cr) and AlCoFeCr coatings for improvement of elevated temperature oxidation resistance of a near-α titanium alloy. Mater. Charact. 2013, 83, 161–169. [Google Scholar] [CrossRef]
- Parsamehr, H.; Chang, S.-Y.; Lai, C.-H. Mechanical and surface properties of aluminum-copper-iron quasicrystal thin films. J. Alloy Compd. 2018, 732, 952–957. [Google Scholar] [CrossRef]
- Parsamehr, H.; Chen, T.-S.; Wang, D.-S.; Leu, M.-S.; Han, I.; Xi, Z.; Tsai, A.-P.; Shahani, A.J.; Lai, C.-H. Thermal spray coating of Al-Cu-Fe quasicrystals: Dynamic observations and surface properties. Materialia 2019, 8, 100432. [Google Scholar] [CrossRef]
- Chatelier, C.; Garreau, Y.; Piccolo, L.; Vlad, A.; Resta, A.; Ledieu, J.; Fournée, V.; De Weerd, M.-C.; Picca, F.-E.; De Boissieu, M.; et al. From the Surface Structure to Catalytic Properties of Al5Co2(2): A Study Combining Experimental and Theoretical Approaches. J. Phys. Chem. C 2020, 124, 4552–4562. [Google Scholar] [CrossRef]
- Piccolo, L.; Chatelier, C.; De Weerd, M.-C.; Morfin, F.; Ledieu, J.; Fournée, V.; Gille, P.; Gaudry, E. Catalytic properties of Al13TM4 complex intermetallics: Influence of the transition metal and the surface orientation on butadiene hydrogenation. Sci. Technol. Adv. Mater. 2019, 20, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.; Ledieu, J.; Fournée, V.; Gaudry, E. Semihydrogenation of Acetylene on Al5Co2 Surfaces. J. Phys. Chem. C 2017, 121, 4958–4969. [Google Scholar] [CrossRef]
- Soler, L.; Macanás, J.; Muñoz, M.; Casado, J. Aluminum and aluminum alloys as sources of hydrogen for fuel cell applications. J. Power Sources 2007, 169, 144–149. [Google Scholar] [CrossRef]
- Lekatou, A.G.; Sfikas, A.; Karantzalis, A.E.; Sioulas, D. Microstructure and corrosion performance of Al-32%Co alloys. Corros. Sci. 2012, 63, 193–209. [Google Scholar] [CrossRef]
- Palcut, M.; Priputen, P.; Kusý, M.; Janovec, J. Corrosion behaviour of Al-29at%Co alloy in aqueous NaCl. Corros. Sci. 2013, 75, 461–466. [Google Scholar] [CrossRef]
- Palcut, M.; Priputen, P.; Šalgó, K.; Janovec, J. Phase constitution and corrosion resistance of Al-Co alloys. Mater. Chem. Phys. 2015, 166, 95–104. [Google Scholar] [CrossRef]
- Zhang, H.H.; Xiang, J.; Wang, W.; Shi, Y.-J. The Oxidation of Co-5Al Alloys in 1 Atm of Pure O2 at 700 and 800 °C. Adv. Mater. Res. 2011, 295, 319–322. [Google Scholar] [CrossRef]
- Zhang, H.H.; Xiang, J.H.; Xu, X.C.; Wang, C. Comparison of Oxidation Behavior of Binary Co-10X (x = Al, Si, Cr) Alloys at 973 and 1073K. Appl. Mech. Mater. 2013, 395, 238–242. [Google Scholar] [CrossRef]
- Irving, G.N.; Stringer, J.; Whittle, D.P. The high-temperature oxidation resistance of Co-Al alloys. Oxid. Met. 1975, 9, 427–440. [Google Scholar] [CrossRef]
- Agliozzo, S.; Brunello, E.; Klein, H.; Mancini, L.; Hartwig, J.; Baruchel, J.; Gastaldi, J. Extended investigation of porosity in quasicrystals by synchrotron X-ray phase contrast radiography—I: In icosahedral AlPdMn grains. J. Cryst. Growth 2005, 281, 623–638. [Google Scholar] [CrossRef]
- Brunello, E.; Agliozzo, S.; Klein, H.; Mancini, L.; Härtwig, J.; Baruchel, J.; Gastaldi, J. Extended investigation of porosity in quasicrystals by synchrotron X-ray phase contrast radiography: Part II, in grains of other alloys and structures than AlPdMn. J. Cryst. Growth 2005, 282, 228–235. [Google Scholar] [CrossRef]
- Prescott, R.; Graham, M.J. The oxidation of iron-aluminum alloys. Oxid. Met. 1992, 38, 73–87. [Google Scholar] [CrossRef]
- Chevalier, S. Formation and growth of protective alumina scales. In Developments in High Temperature Corrosion and Protection of Materials, 1st ed.; Woodhead Publishing: Abington, Cambridge, UK, 2008; Chapter 10; pp. 290–328. [Google Scholar] [CrossRef]
- Wehner, B.I.; Köster, U. Microstructural Evolution of Alumina Layers on an Al-Cu-Fe Quasicrystal during High-Temperature Oxidation. Oxid. Met. 2000, 54, 445–456. [Google Scholar] [CrossRef]
- Tolpygo, V.; Clarke, D.R. Microstructural evidence for counter-diffusion of aluminum and oxygen during the growth of alumina scales. Mater. High Temp. 2003, 20, 261–271. [Google Scholar] [CrossRef]
- Dan’Ko, A.J.; Rom, M.A.; Sidelnikova, N.S.; Nizhankovskiy, S.V.; Budnikov, A.T.; Grin’, L.A.; Kaltaev, K.S.-O. Transformation of the corundum structure upon high-temperature reduction. Crystallogr. Rep. 2008, 53, 1112–1118. [Google Scholar] [CrossRef]
- Trunov, M.A.; Schoenitz, M.; Zhu, X.; Dreizin, E.L. Effect of polymorphic phase transformations in Al2O3 film on oxidation kinetics of aluminum powders. Combust. Flame 2005, 140, 310–318. [Google Scholar] [CrossRef]
- Zhou, R.S.; Snyder, R.L. Structures and transformation mechanisms of the η, γ and θ transition aluminas. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 617–630. [Google Scholar] [CrossRef]
- Kovarik, L.; Bowden, M.; Shi, D.; Washton, N.M.; Andersen, A.; Hu, J.Z.; Lee, J.; Szanyi, J.; Kwak, J.-H.; Peden, C.H.F. Unraveling the Origin of Structural Disorder in High Temperature Transition Al2O3: Structure of θ-Al2O3. Chem. Mater. 2015, 27, 7042–7049. [Google Scholar] [CrossRef]
- Jbara, A.S.; Othaman, Z.; Saeed, M. Structural, morphological and optical investigations of θ-Al2O3 ultrafine powder. J. Alloys Compd. 2017, 718, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Bronsveld, P.; De Hosson, J.T.M.; Djuričić, B.; McGarry, D.; Pickering, S. Twinning in θ Alumina Investigated with High Resolution Transmission Electron Microscopy. J. Eur. Ceram. Soc. 1998, 18, 299–304. [Google Scholar] [CrossRef]
- Kovarik, L.; Bowden, M.; Andersen, A.; Jaegers, N.R.; Washton, N.; Szanyi, J. Quantification of High Temperature Transitions and Their Phase Transformations. Available online: https://chemrxiv.org/articles/preprint/Quantification_of_High_Temperature_Transition_Al2O3_and_Their_Phase_Transformations/12584783 (accessed on 4 July 2020).
- Boumaza, A.; Favaro, L.; Ledion, J.; Sattonnay, G.; Brubach, J.; Berthet, P.; Huntz, A.; Roy, P.; Tétot, R. Transition alumina phases induced by heat treatment of boehmite: An X-ray diffraction and infrared spectroscopy study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- McCafferty, E. High-Temperature Gaseous Oxidation. In Introduction to Corrosion Science; Springer: New York, NY, USA, 2009; pp. 453–476. [Google Scholar] [CrossRef]
- Ledieu, J.; Gaudry, E.; Fournée, V. Surfaces of Al-based complex metallic alloys: Atomic structure, thin film growth and reactivity. Sci. Technol. Adv. Mater. 2014, 15, 34802. [Google Scholar] [CrossRef] [PubMed]
- Korte-Kerzel, S.; Schnabel, V.; Clegg, W.; Heggen, M. Room temperature plasticity in m-Al13Co4 studied by microcompression and high resolution scanning transmission electron microscopy. Scr. Mater. 2018, 146, 327–330. [Google Scholar] [CrossRef]
- Wang, W.; Yang, W.; Liu, Z.; Lin, Y.; Zhou, S.; Qian, H.; Wang, H.; Lin, Z.; Li, G. Epitaxial growth and characterization of high-quality aluminum films on sapphire substrates by molecular beam epitaxy. CrystEngComm 2014, 16, 7626–7632. [Google Scholar] [CrossRef]
- Alessandri, M.; Piagge, R.; Caniatti, M.; Del Vitto, A.; Wiemer, C.; Pavia, G.; Alberici, S.; Bellandi, E.; Nale, A. Structural and Chemical Investigation of Annealed Al2O3 Films for Interpoly Dielectric Application in Flash Memories. ECS Trans. 2006, 3, 183–192. [Google Scholar] [CrossRef]
- Queraltó, A.; De La Mata, M.; Arbiol, J.; Obradors, X.; Puig, T. Disentangling Epitaxial Growth Mechanisms of Solution Derived Functional Oxide Thin Films. Adv. Mater. Interfaces 2016, 3, 1600392. [Google Scholar] [CrossRef]
- Mattox, D.M. Atomistic Film Growth and Some Growth-Related Film Properties. In Handbook of Physical Vapor Deposition (PVD) Processing, 2nd ed.; Elsevier: Oxford, UK, 2010; pp. 333–398. [Google Scholar] [CrossRef]
- Meier, M.; Ledieu, J.; De Weerd, M.-C.; Huang, Y.-T.; Abreu, G.J.P.; Pussi, K.; Diehl, R.; Mazet, T.; Fournée, V.; Gaudry, E. Interplay between bulk atomic clusters and surface structure in complex intermetallic compounds: The case study of the Al5Co2(001) surface. Phys. Rev. B 2015, 91, 085414. [Google Scholar] [CrossRef]
- Anand, K.; Fournée, V.; Prevot, G.; Ledieu, J.; Gaudry, E. Nonwetting Behavior of Al-Co Quasicrystalline Approximants Owing to Their Unique Electronic Structures. ACS Appl. Mater. Interfaces 2020, 12, 15793–15801. [Google Scholar] [CrossRef]
- Gulbransen, E.A.; Wysong, W.S. Thin Oxide Films on Aluminum. J. Phys. Chem. 1947, 51, 1087–1103. [Google Scholar] [CrossRef]
- Smeltzer W, W. Oxidation of An Aluminum-3 Per Cent Magnesium Alloy in the Temperature Range 200–550 °C. J. Electrochem. Soc. 1958, 105, 67–71. [Google Scholar] [CrossRef]
- Sabat, K.C.; Paramguru, R.K.; Pradhan, S.; Mishra, B.K. Reduction of Cobalt Oxide (Co3O4) by Low Temperature Hydrogen Plasma. Plasma Chem. Plasma Process. 2014, 35, 387–399. [Google Scholar] [CrossRef]
- Chattopadhyay, B.; Wood, G.C. The transient oxidation of alloys. Oxid. Met. 1970, 2, 373–399. [Google Scholar] [CrossRef]
- Stott, F.H. Influence of alloy additions on oxidation. Mater. Sci. Technol. 1989, 5, 734–740. [Google Scholar] [CrossRef]
- Wehner, B.; Köster, U.; Rüdiger, A.; Pieper, C.; Sordelet, D. Oxidation of Al–Cu–Fe and Al–Pd–Mn quasicrystals. Mater. Sci. Eng. A 2000, 294, 830–833. [Google Scholar] [CrossRef]
- Prud’Homme, N.; Ribot, P.; Herinx, M.; Gille, P.; Bauer, B.; De Weerd, M.-C.; Barthés-Labrousse, M.-G. High temperature oxidation of AlCrFe complex metallic alloys. Corros. Sci. 2014, 89, 118–126. [Google Scholar] [CrossRef]
- Irving, G.N.; Stringer, J.; Whittle, D.P. The Oxidation Behavior of Co-Cr-Al Alloys at 1000 °C. Corrosion 1977, 33, 56–60. [Google Scholar] [CrossRef]
- Wood, G.C.; Stott, F.H. The influence of aluminum additions on the oxidation of Co-Cr alloys at 1000 and 1200 °C. Oxid. Met. 1971, 3, 365–398. [Google Scholar] [CrossRef]
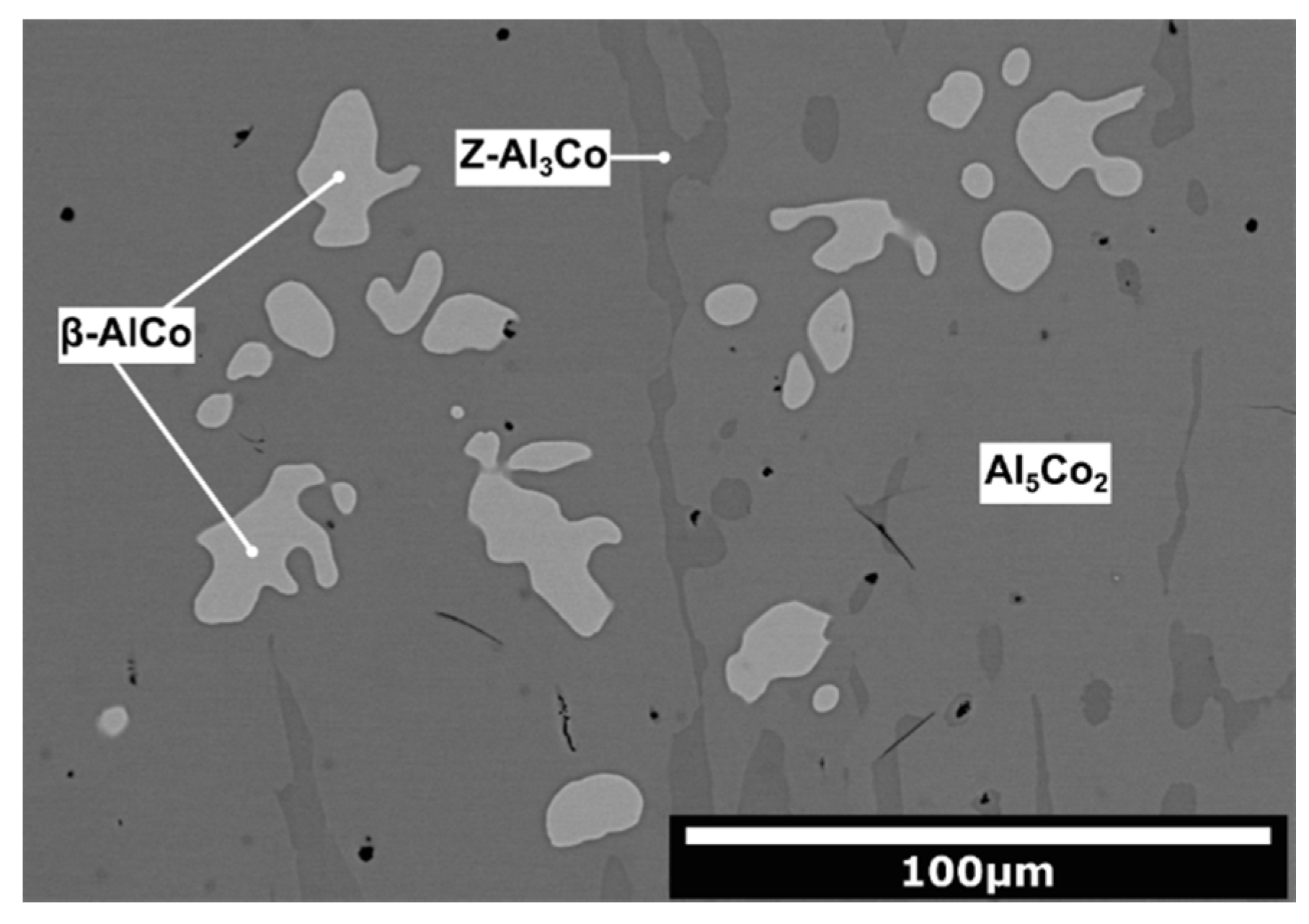



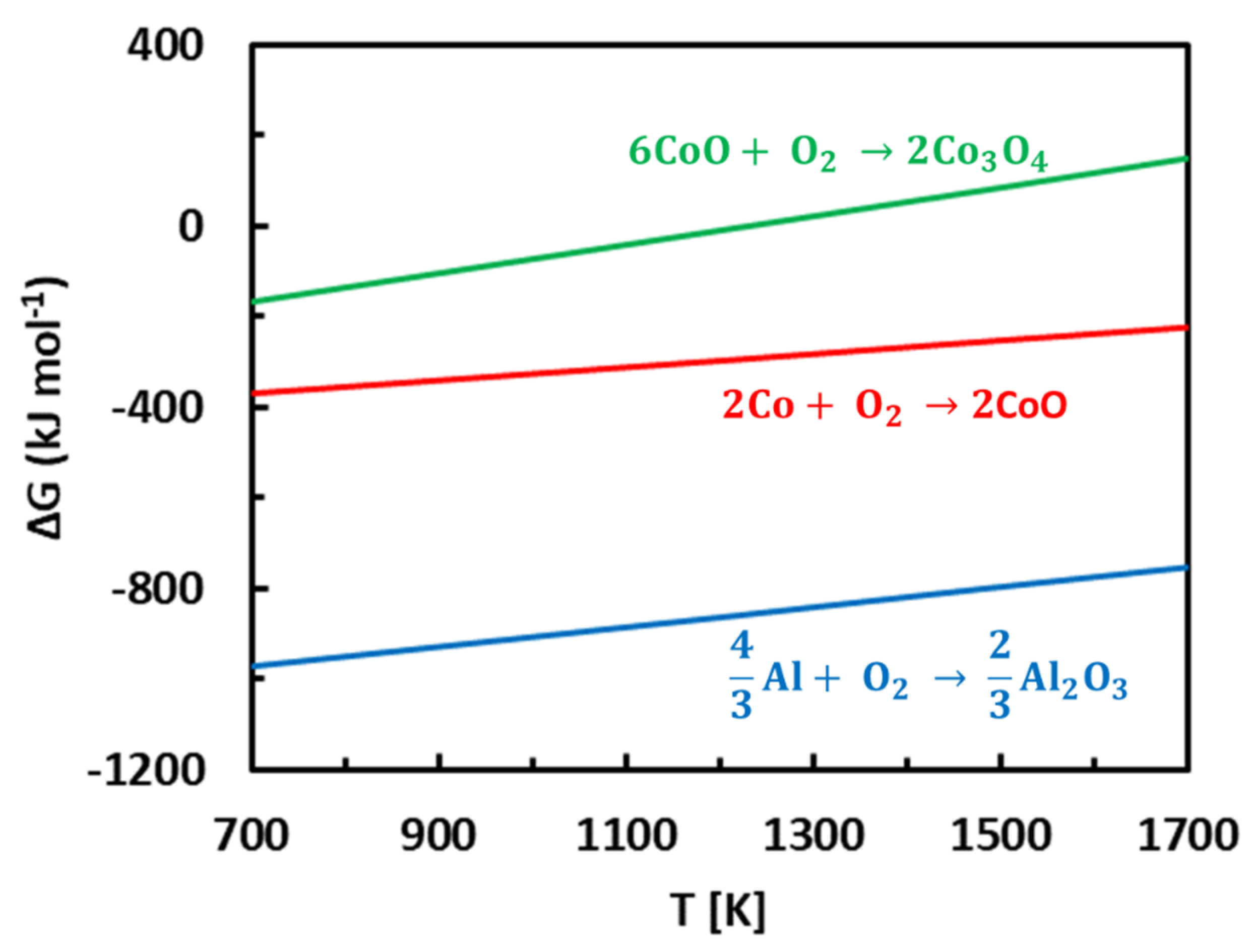
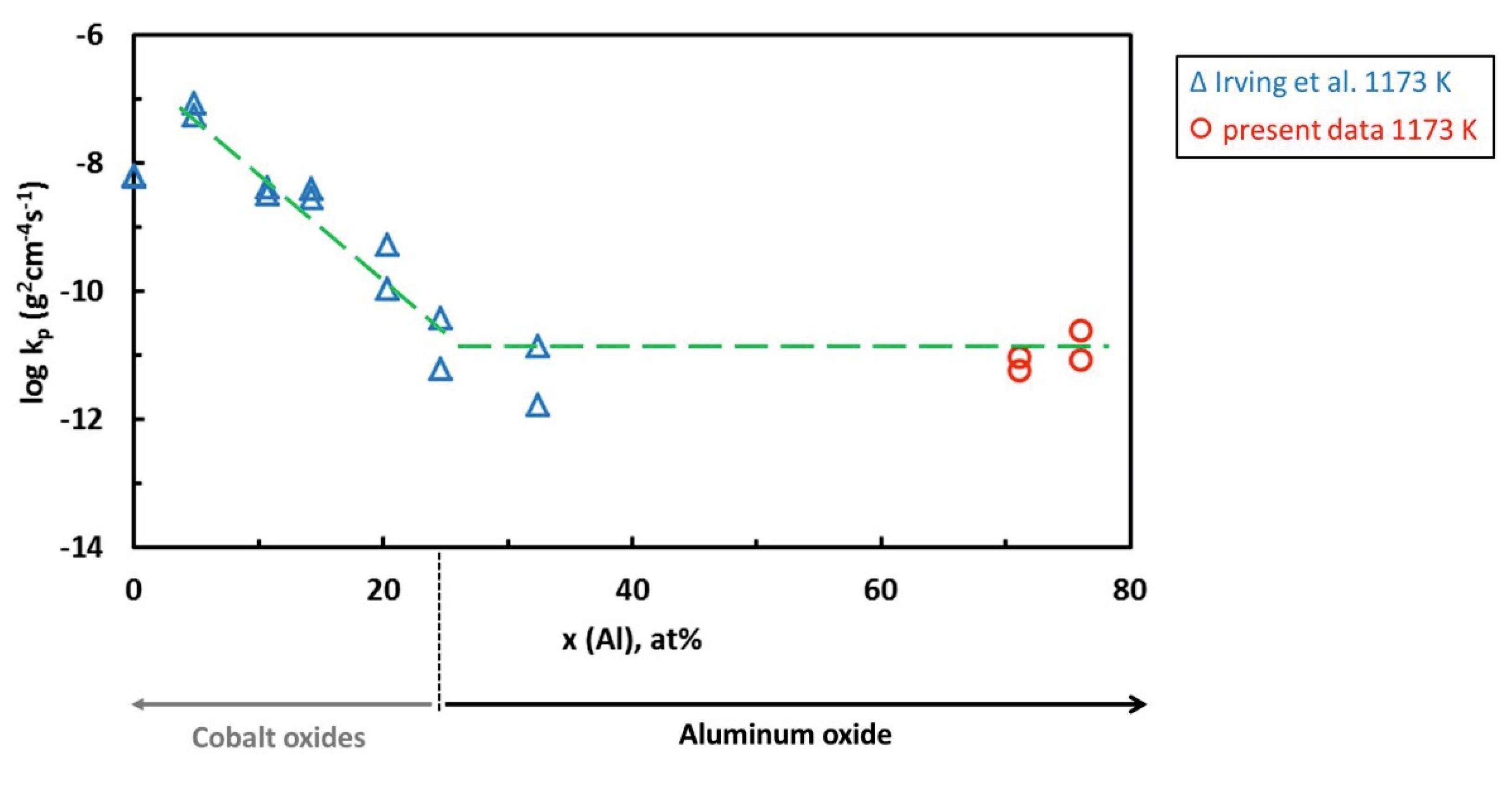
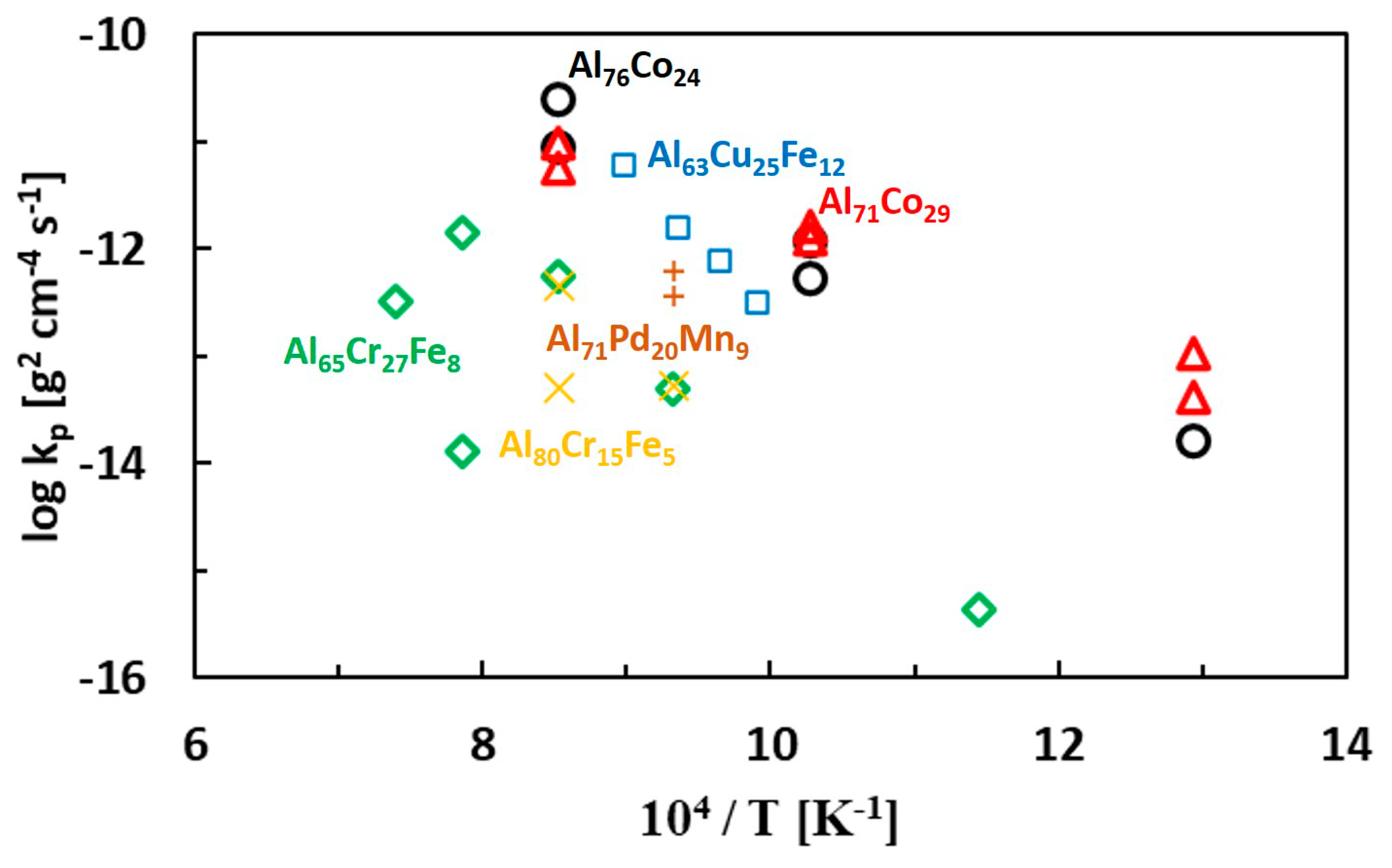
| Al71Co29 Alloy | ||||
| Microstructure Constituent | Phase Identified [57] | Al [at.%] | Co [at.%] | Volume Fraction [%] |
| Light-grey | β-AlCo | 55.2 ± 0.9 | 44.8 ± 0.9 | 8 |
| Medium-grey | Al5Co2 | 71.9 ± 0.4 | 28.1 ± 0.4 | 86 |
| Dark-grey | Z-Al3Co | 74.6 ± 0.4 | 25.4 ± 0.4 | 6 |
| Al76Co24 Alloy | ||||
| Microstructure Constituent | Phase Identified [58] | Al [at.%] | Co [at.%] | Volume Fraction [%] |
| Light-grey | Z-Al3Co | 74.4 ± 0.1 | 25.6 ± 0.1 | 5 |
| Medium-grey | m-Al13Co4 | 75.2 ± 0.2 | 24.8 ± 0.2 | 83 |
| Dark-grey | Al9Co2 | 81.5 ± 0.1 | 18.5 ± 0.1 | 12 |
| kp [g2 cm−4 s−1] | ||
| Al71Co29 Alloy | ||
| 773 K | 973 K | 1173 K |
| 1.04 × 10−13 (0–15 h) | 1.64 × 10−12 (0–15 h) | 9.71 × 10−12 (0–15 h) |
| 4.20 × 10−14 (20–30 h) | 1.23 × 10−12 (15–30 h) | 5.81 × 10−12 (15–30 h) |
| Al76Co24 Alloy | ||
| 773 K | 973 K | 1173 K |
| 1.63 × 10−14 | 1.21 × 10−12 (0–15 h) | 2.54 × 10−11 (0–10 h) |
| 5.43 × 10−13 (15–30 h) | 8.83 × 10−12 (20–30 h) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šulhánek, P.; Drienovský, M.; Černičková, I.; Ďuriška, L.; Skaudžius, R.; Gerhátová, Ž.; Palcut, M. Oxidation of Al-Co Alloys at High Temperatures. Materials 2020, 13, 3152. https://doi.org/10.3390/ma13143152
Šulhánek P, Drienovský M, Černičková I, Ďuriška L, Skaudžius R, Gerhátová Ž, Palcut M. Oxidation of Al-Co Alloys at High Temperatures. Materials. 2020; 13(14):3152. https://doi.org/10.3390/ma13143152
Chicago/Turabian StyleŠulhánek, Patrik, Marián Drienovský, Ivona Černičková, Libor Ďuriška, Ramūnas Skaudžius, Žaneta Gerhátová, and Marián Palcut. 2020. "Oxidation of Al-Co Alloys at High Temperatures" Materials 13, no. 14: 3152. https://doi.org/10.3390/ma13143152
APA StyleŠulhánek, P., Drienovský, M., Černičková, I., Ďuriška, L., Skaudžius, R., Gerhátová, Ž., & Palcut, M. (2020). Oxidation of Al-Co Alloys at High Temperatures. Materials, 13(14), 3152. https://doi.org/10.3390/ma13143152