Formulation of Bicelles Based on Lecithin-Nonionic Surfactant Mixtures
Abstract
1. Introduction
2. Materials and Methods
- XC: ChEO10 weight fraction in the SL-ChEO10 mixture
- WS: Weight fraction of the SL-ChEO10 mixture in the system
3. Results
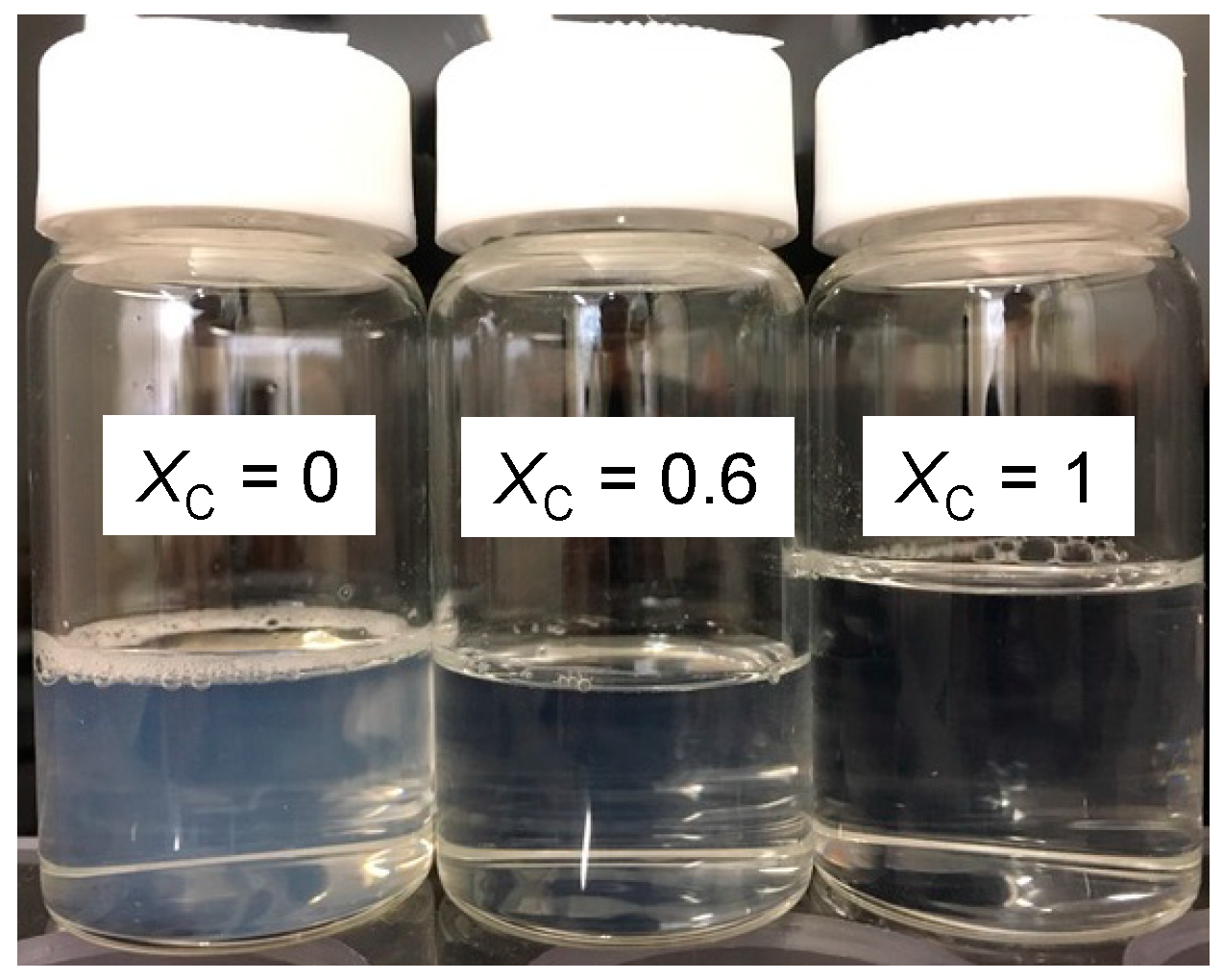
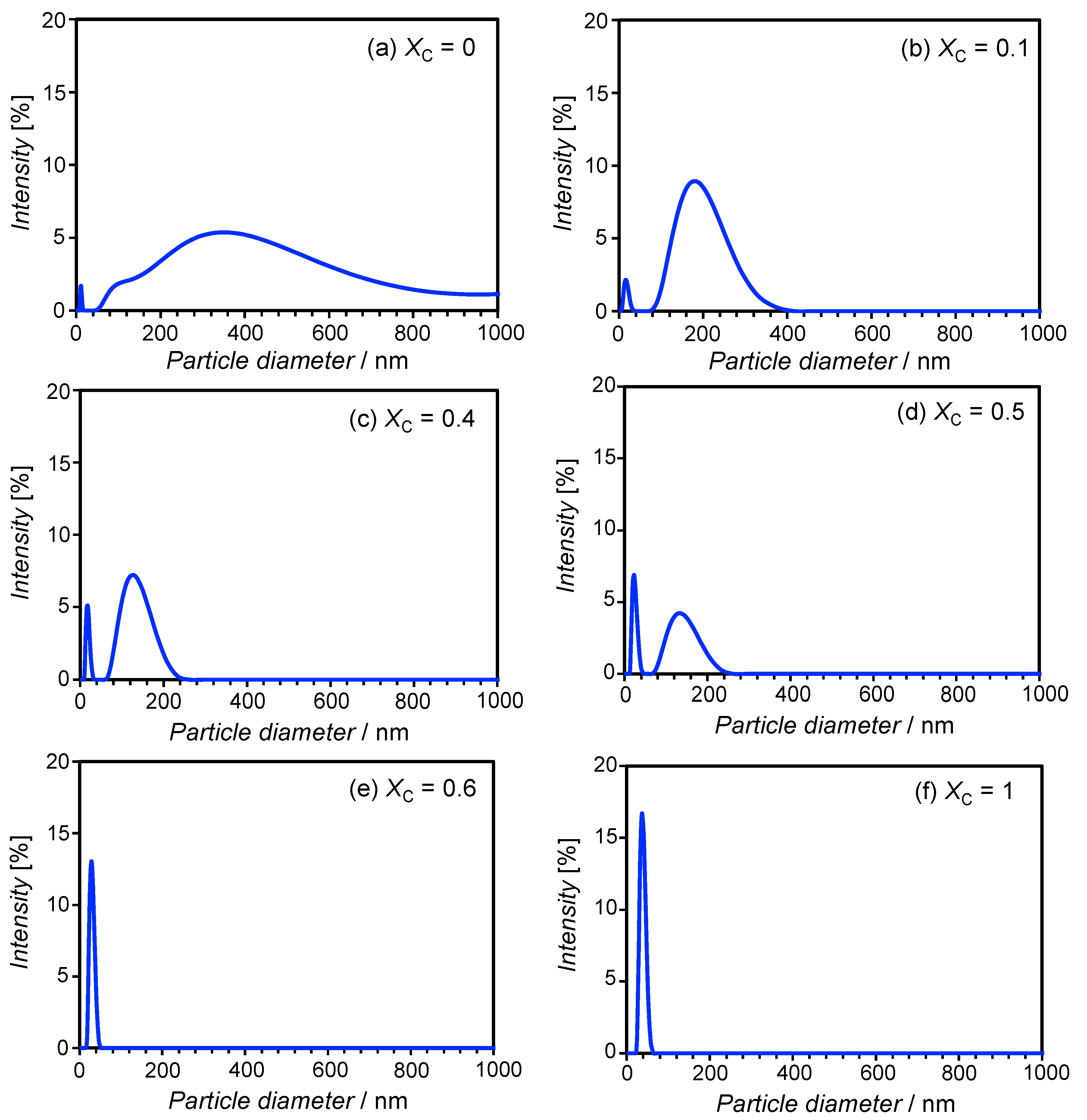
3.1. Particle Size in the Aqueous SL-ChEO10 System
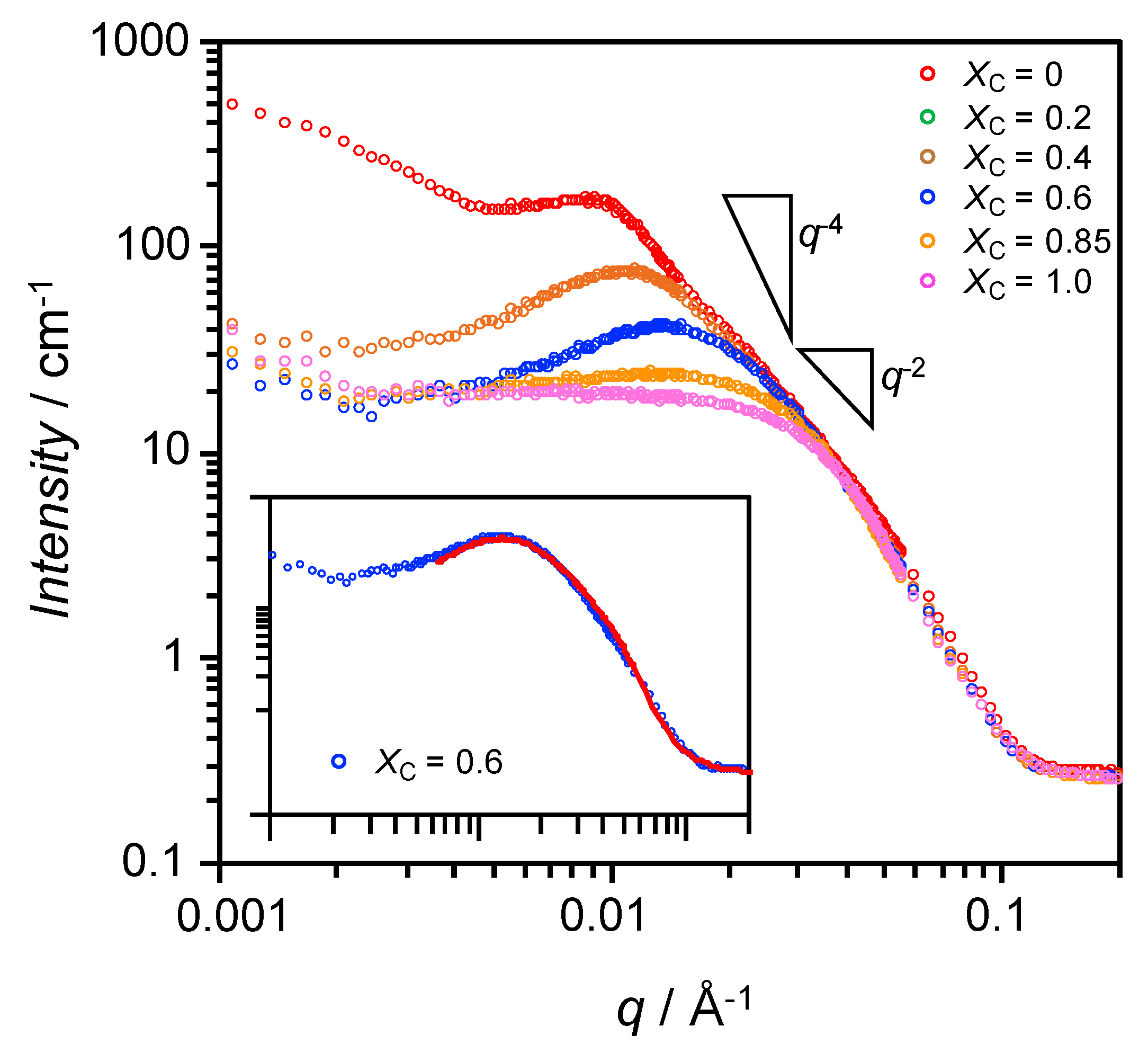
3.2. Characterization of Bicelles by SANS
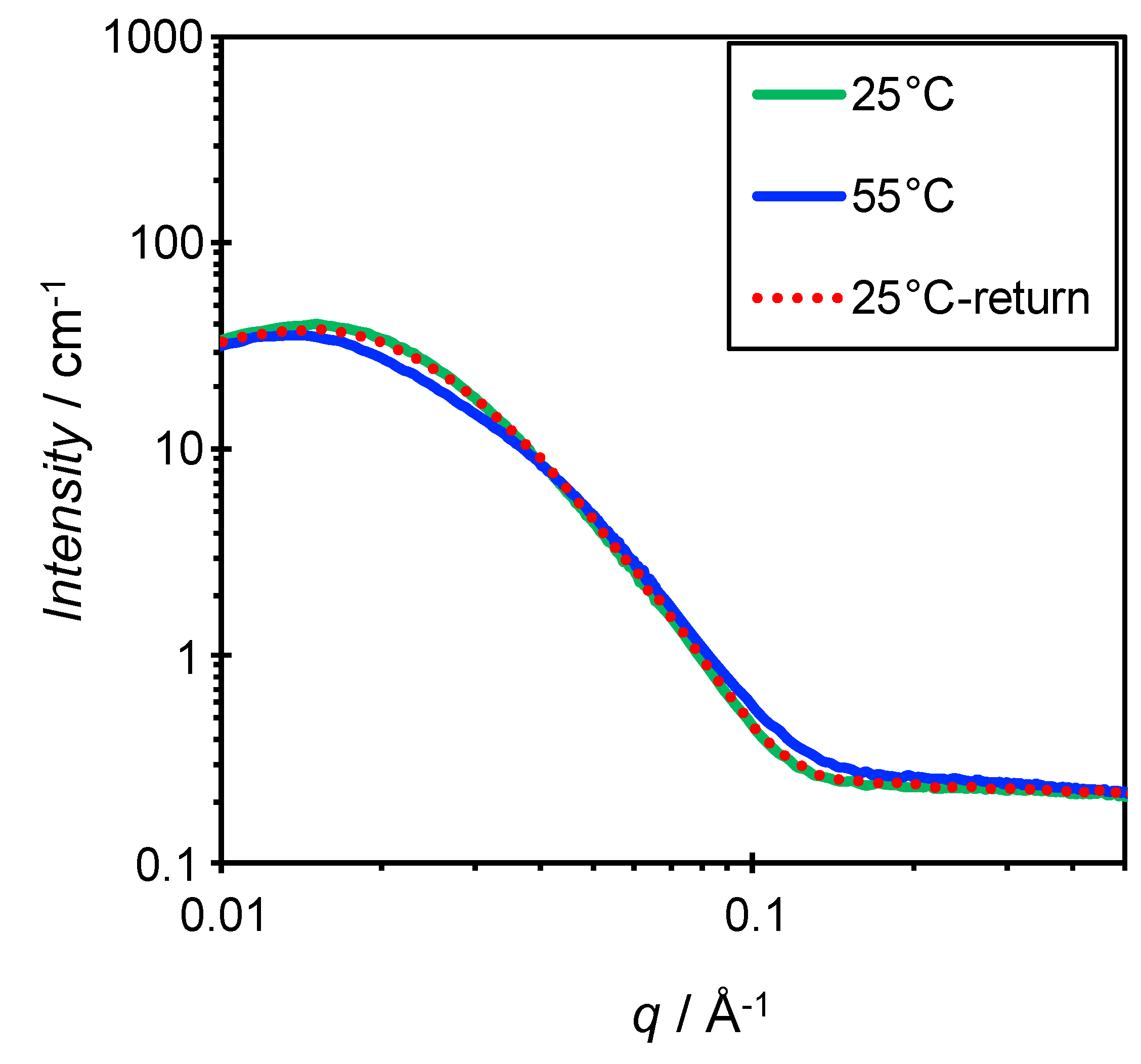
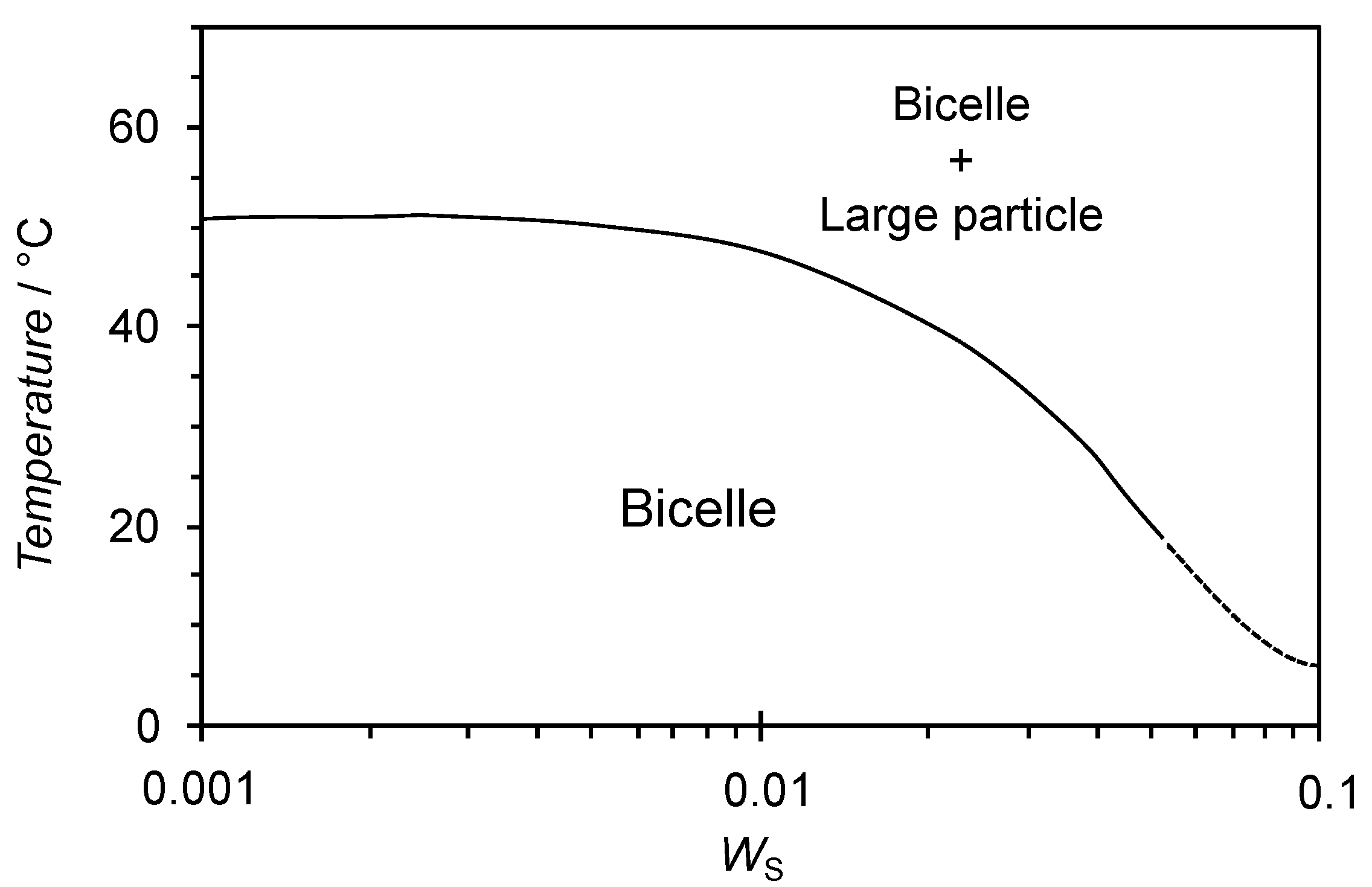
3.3. Effect of Temperature and Concentration on Structure of Bicelles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.d.; Kouhi, M.d.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications Nanoscale. Res. Lett. 2013, 8, 102. [Google Scholar]
- Lila, A.S.A.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kasamaki, M. Enhanced Delivery of Retinoic Acid to Skin by Cationic Liposomes. Chem. Pharm. Bull. 2006, 54, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, K.; Hayashi, H. Kikuchi Thermal Stability of Synthetic Lipid Bicelles Encompassed by Siloxane Surfaces as Organic-Inorganic Hybrid Nanodiscs. J. Chem. Lett. 2012, 41, 1223–1225. [Google Scholar] [CrossRef]
- Wu, H.; Su, K.; Guan, X.; Sublette, M.; Stark, R. Assessing the Size, Stability, and Utility of Isotropically Tumbling Bicelle Systems for Structural Biology. Biochim. Biophys. Acta 2010, 1798, 482–488. [Google Scholar] [CrossRef]
- Björnerås, J.; Nilsson, M.; Mäler, L. Analysing DHPC/DMPC bicelles by diffusion NMR and multivariate decomposition. Biochim. Biophys. Acta 2015, 1848, 2910–2917. [Google Scholar] [CrossRef]
- Glover, K.J.; Whiles, J.A.; Wu, G.H.; Yu, N.J.; Deems, R.; Struppe, J.O.; Stark, R.E.; Komives, E.A.; Vold, R.R. Structural Evaluation of Phospholipid Bicelles for Solution-State Studies of Membrane-Associated Biomolecules. Biophys. J. 2001, 81, 2163–2171. [Google Scholar] [CrossRef]
- Rodríguez, G.; Cócera, M.; Rubio, L.; Alonso, C.; Pons, R.; Sandt, C.; Dumas, P.; López-Iglesias, C.; de la Maza, A.; López, O. Bicellar systems to modify the phase behaviour of skin stratum corneum lipids. Phys. Chem. Chem. Phys. 2012, 14, 14523–14533. [Google Scholar] [CrossRef]
- Viseu, M.I.; Correia, R.F.; Fernandes, A.C. Time evolution of the thermotropic behavior of spontaneous liposomes and disks of the DMPC–DTAC aqueous system. J. Colloid Interface Sci. 2010, 351, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Watanabe; Aramaki, Y.; Kadomatsu, K.; Tanaka, Y.; Konno, K. YPreparation of Bicelles Using the Semi-spontaneous. Method Chem. Lett. 2016, 45, 558–560. [Google Scholar] [CrossRef]
- Ogunsola, O.A.; Kraeling, M.E.; Zhong, S.; Pochan, D.J.; Bronaugh, R.L.; Raghavan, S.R. Structural analysis of “flexible” liposome formulations: New insights into the skin-penetrating ability of soft nanostructures. Soft Matter 2012, 8, 10226–10232. [Google Scholar] [CrossRef]
- Lindholm, L.; Ariöz, C.; Jawurek, M.; Liebau, J.; Mäler, L.; Wieslander, Å.; Ballmoos, C.V.; Barth, A. Effect of lipid bilayer properties on the photocycle of green proteorhodopsin. Biochim. Biophys. Acta 2015, 1847, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Bennett, B.C.; Hong, W.-X.; Fu, Y.; Baker, K.A.; Marcoux, J.; Robinson, C.V.; Ward, A.B.; Halpert, J.R.; Stevens, R.C.; et al. Steroid-based facial amphiphiles for stabilization and crystallization of membrane proteins. Proc. Natl. Acad. Sci. USA 2013, 110, E1203–E1211. [Google Scholar] [CrossRef]
- Dürr, U.H.N.; Gildenberg, M.; Ramamoorthy, A. The Magic of Bicelles Lights up Membrane Protein Structure. Chem. Rev. 2012, 112, 6054–6074. [Google Scholar] [CrossRef]
- Yang, P.; Lin, T.; Lin, T.; Yang, C.; Hu, Y.; Jeng, U. Packing DNA with disc-shaped bicelles. Soft Matter 2013, 9, 11542–11548. [Google Scholar] [CrossRef]
- Yang, P.-W.; Lin, T.-L.; Hu, Y.; Jeng, U.-S. Formation of divalent ion mediated anionic disc bicelle–DNA complexes. Soft Matter 2014, 10, 2313–2319. [Google Scholar] [CrossRef]
- Fernández, E.; Rodríguez, G.; Cócera, M.; Barbosa-Barros, L.; Alonso, C.; López-Iglesias, C.; Jawhari, T.; de la Maza, A.; López, O. Advanced lipid systems containing b-carotene: Stability under UV-vis radiation and application on porcine skin in vitro. Phys. Chem. Chem. Phys. 2015, 17, 18710–18721. [Google Scholar] [CrossRef]
- Saleem, Q.; Zhang, Z.; Petretic, A.; Gradinaru, C.C.; Macdonald, P.M. Single Lipid Bilayer Deposition on Polymer Surfaces Using Bicelles. Biomacromolecules 2015, 16, 1032–1039. [Google Scholar] [CrossRef]
- Garcia, R.M.; Song, Y.; Dorin, R.M.; Wang, H.; Moreno, A.M.; Jiang, Y.-B.; Tian, Y.; Qiu, Y.; Medforth, C.J.; Coker, E.N.; et al. Templated growth of platinum nanowheels using the inhomogeneous reaction environment of bicelles. Phys. Chem. Chem. Phys. 2011, 13, 4846–4852. [Google Scholar] [CrossRef]
- Holland, L.A.; Leigh, A.M. Bilayered phospholipid micelles and capillary electrophoresis: A new additive for electrokinetic chromatography. Electrophoresis 2003, 24, 2935–2939. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Barros, L.; Barba, C.; Cocera, M. Effect of bicellar systems on skin properties. Int. J. Pharm. 2008, 352, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Alonso, C.; Rodriguez, G.; Cocera, M.; Barbosa-Barros, L.; Coderch, L.; de la Maza, A.; Parra, J.L.; Lopez, O. Bicellar systems as vehicle for the treatment of impaired skin. Eur. J. Pharm. Biopharm. 2014, 86, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-C.; Huang, Z.-L.; Yang, Y.-M.; Chang, C.-H.; Chou, T.-H. Enhancement of catansome formation by means of cosolvent effect: Semi-spontaneous preparation method. Colloids. Surfaces A 2007, 302, 599–697. [Google Scholar] [CrossRef]
- Li, M.; Morales, H.H.; Katsaras, J.; Kucĕrka, N.; Yang, Y.; Macdonald, P.M.; Nieh, M.-P. Morphological Characterization of DMPC/CHAPSO Bicellar Mixtures: A Combined SANS and NMR Study. Langmuir 2013, 29, 15943–15957. [Google Scholar] [CrossRef]
- Wood, K.; Mata, J.P.; Garvey, C.J.; Wu, C.-M.; Hamilton, W.A.; Abbeywick, P.; Bartlett, D.; Bartsch, F.; Baxter, P.; Booth, N.; et al. QUOKKA the pinhole small-angle neutron scattering instrument at the OPAL Research Reactor Australia: Design performance operation and scientific highlights. J. Appl. Crystallogr. 2018, 51, 294–314. [Google Scholar] [CrossRef]
- Kline, S.R. ‘Quokka’—The small-angle neutron scattering instrument at OPAL. J. Appl. Crystallogr. 2006, 39, 895–900. [Google Scholar] [CrossRef]
- Livsey, I. Reduction and analysis of SANS and USANS data using IGOR Pro. J. Chem. Soc. Fraday Trans. 1987, 83, 1445–1452. [Google Scholar] [CrossRef]
- SasView for Small Angle Scattering Analysis. Available online: http://www.sasview.org/ (accessed on 27 February 2020).
- Sato, T.; Hossain, M.d.K.; Acharya, D.P.; Glatter, O.; Chiba, A.; Kunieda, H. Phase Behavior and Self-Organized Structures in Water/Poly(oxyethylene) Cholesteryl Ether Systems. J. Phys. Chem. B 2004, 108, 12927–12939. [Google Scholar] [CrossRef]
- Danino, D.; Abezgauz, L.; Portnaya, I.; Dan, N. From Discs to Ribbons Networks: The Second Critical Micelle Concentration in Nonionic Sterol Solutions. J. Phys. Chem. Lett. 2016, 7, 1434–1439. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Yang, Y.; Xia, Y.; Nieh, M.-P. The effects of temperature, salinity, concentration and PEGylated lipid on the spontaneous nanostructures of bicellar mixtures. Biochim. Biophys. Acta 2014, 1838, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, K.; Iwata, C.; Mata, J.; Maehara, T.; Aburano, D.; Sakanishi, Y.; Kitao, K. One-step formulation of nonionic surfactant bicelles (NSBs) by a double-tailed polyglycerol-type nonionic surfactant. Phys. Chem. Chem. Phys. 2017, 19, 23802–23808. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.S.; Ketner, A.M.; Raghavan, S.R. Self-Assembly of Surfactant Vesicles that Transform into Viscoelastic Wormlike Micelles upon Heating. J. Am. Chem. Soc. 2006, 128, 6669–6675. [Google Scholar] [CrossRef] [PubMed]
- Hayter, J.B.; Penfold, J. An analytic structure factor for macroion solutions. Mol. Phys. 1981, 42, 109–118. [Google Scholar] [CrossRef]
- Hansen, J.P.; Hayter, J.B. A rescaled MSA structure factor for dilute charged colloidal dispersions. Mol. Phys. 1982, 46, 651–656. [Google Scholar] [CrossRef]
- Tanford, C. Micelle shape and size. J. Phys. Chem. 1972, 76, 3020–3024. [Google Scholar] [CrossRef]
- Carnie, S.; Israelachvili, J.N.; Pailthorpe, B.A. Lipid packing and transbilayer asymmetries of mixed lipid vesicles. Biochim. Biophys. Acta Biomembr. 1979, 554, 340–357. [Google Scholar] [CrossRef]
- Ipsen, J.H.; Karlström, G.; Mouritsen, O.G.; Wennerström, H.; Zuckermann, M.J. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta 1987, 905, 162–172. [Google Scholar] [CrossRef]
- Aramaki, K.; Yamada, J.; Tsukijima, Y.; Maehara, T.; Aburano, D.; Sakanishi, Y.; Kitao, K. Formation of bilayer membrane and niosomes by double-tailed polyglyceryl-type nonionic surfactant. Langmuir 2015, 31, 10664–10671. [Google Scholar] [CrossRef]
- Shapiro, R.A.; Brindley, A.J.; Martin, R.W. Thermal Stabilization of DMPC/DHPC Bicelles by Addition of Cholesterol Sulfate. J. Am. Chem. Soc. 2015, 132, 11406–11407. [Google Scholar] [CrossRef][Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aramaki, K.; Adachi, K.; Maeda, M.; Mata, J.; Kamimoto-Kuroki, J.; Tsukamoto, D.; Konno, Y. Formulation of Bicelles Based on Lecithin-Nonionic Surfactant Mixtures. Materials 2020, 13, 3066. https://doi.org/10.3390/ma13143066
Aramaki K, Adachi K, Maeda M, Mata J, Kamimoto-Kuroki J, Tsukamoto D, Konno Y. Formulation of Bicelles Based on Lecithin-Nonionic Surfactant Mixtures. Materials. 2020; 13(14):3066. https://doi.org/10.3390/ma13143066
Chicago/Turabian StyleAramaki, Kenji, Keita Adachi, Miho Maeda, Jitendra Mata, Junko Kamimoto-Kuroki, Daisuke Tsukamoto, and Yoshikazu Konno. 2020. "Formulation of Bicelles Based on Lecithin-Nonionic Surfactant Mixtures" Materials 13, no. 14: 3066. https://doi.org/10.3390/ma13143066
APA StyleAramaki, K., Adachi, K., Maeda, M., Mata, J., Kamimoto-Kuroki, J., Tsukamoto, D., & Konno, Y. (2020). Formulation of Bicelles Based on Lecithin-Nonionic Surfactant Mixtures. Materials, 13(14), 3066. https://doi.org/10.3390/ma13143066




