New Oral Surgery Materials for Bone Reconstruction—A Comparison of Five Bone Substitute Materials for Dentoalveolar Augmentation
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Pattern | Entropy | LngREmph | Entr/ LngREmph | Pattern | Entropy | LngREmph | Entr/ LngREmph |
 | 1.79 | 39.5 | 0.045 |  | 2.62 | 4.5 | 0.581 |
 | 1.84 | 21.0 | 0.087 |  | 2.79 | 2.6 | 1.069 |
 | 2.03 | 6.3 | 0.320 |  | 2.80 | 2.1 | 1.341 |
 | 1.92 | 35.2 | 0.054 |  | 2.41 | 5.6 | 0.429 |
 | 2.55 | 5.9 | 0.432 |  | 2.73 | 2.6 | 1.046 |
 | 2.55 | 3.7 | 0.689 |  | 2.77 | 2.1 | 1.327 |
References
- Klein, C.P.A.T.; Drissen, A.A.; Degroot, K. Biodegradation behaviour of various calcium phosphate material in bone tissue. J. Biomed. Mater. Res. 1983, 17, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Bröseler, F.; Tietmann, C.; Hinz, A.-K.; Jepsen, S. Long-term results of periodontal regenerative therapy: A retrospective practice-based cohort study. J. Clin. Periodontol. 2017, 44, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Piątowska, D. Radiographic eavaluation of the results pf periapical lesion healing after endodontic versus surgical treatment. Dent. Med. Probl. 2006, 43, 181–189. [Google Scholar]
- Hallman, M.; Mordenfeld, A.; Strandkvist, T. Bone replacement following dental trauma prior to implant surgery—Present status. Dent. Traumatol. 2009, 25, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Kołaciński, M.; Kozakiewicz, M.; Materka, A. Textural entropy as a potential feature for quantitative assessment of jaw bone healing process. Arch. Med. Sci. 2015, 11, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, B.; Shamsoddin, E.; Golabgiran, M. Biomaterial selection for bone augmentation in implant dentistry: A systematic review. J. Adv. Pharm. Technol. Res. 2019, 10, 46–50. [Google Scholar] [CrossRef]
- Uchida, A.; Nade, S.M.L.; McCartney, E.R.; Ching, W. The use of ceramics for bone replacement. J. Bone Joint. Surg. (Br.) 1984, 66, 269–275. [Google Scholar] [CrossRef]
- Shimazaki, K.; Mooney, V. Comparative study of porous hydroxyapatite and tricalcium phosphate as bone substitute. J. Orthop. Res. 1985, 3, 301–310. [Google Scholar] [CrossRef]
- Verheij, J.; Geraets, W.; Van Der Stelt, P.; Horner, K.; Lindh, C.; Nicopoulou-Karayianni, K.; Jacobs, R.; Marjanovic, E.; Adams, J.; Devlin, H. Prediction of osteoporosis with dental radiographs and age. Dentomaxillofacial Radiol. 2009, 38, 431–437. [Google Scholar] [CrossRef]
- Ranjanomennahary, P.; Ghalila, S.S.; Malouche, D.; Marchadier, A.; Rachidi, M.; Benhamou, C.; Chappard, C. Comparison of radiograph-based texture analysis and bone mineral density with three-dimensional microarchitecture of trabecular bone. Med Phys. 2010, 38, 420. [Google Scholar] [CrossRef]
- Apostol, L.; Boudousq, V.; Basset, O.; Odet, C.; Yot, S.; Tabary, J.; Dinten, J.-M.; Boller, E.; Kotzki, P.-O.; Peyrin, F. Relevance of 2D radiographic texture analysis for the assessment of 3D bone micro-architecture. Med. Phys. 2006, 33, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Haralick, R.; Shanmugam, K.; Dinstein, I. Textural features for image classification. IEEE Trans. Syst. Man Cybern. 1973, 3, 610–621. [Google Scholar] [CrossRef]
- Haralick, R. Statistical and structural approaches to texture. Proc. IEEE 1979, 67, 786–804. [Google Scholar] [CrossRef]
- Materka, A.; Strzelecki, M. Texture analysis methods—A review, COST B11 report. In Proceedings of the MC Meeting and Workshop, Brussels, Belgium, June 1998; Technical University of Lodz: Lodz, Poland, 1998. [Google Scholar]
- Materka, A.; Strzelecki, M.; Lerski, R.; Schad, L. Feature evaluation of texture test objects for magnetic resonance imaging. In Proceedings of the Workshop on Texture Analysis and Machine Vision, Oulu, Finland, 14–15 June 1999; pp. 13–19. [Google Scholar]
- Mucciardi, A.N.; Gose, E.E. A comparison of seven techniques for choosing subsets of patter recognition properties. IEEE Trans. Comput. 1971, c-20, 1023–1031. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Bogusiak, K.; Hanclik, M.; Denkowski, M.; Arkuszewski, P. Noise in subtraction images made from pairs of intraoral radiographs: A comparison between four methods of geometric alignment. Dentomaxillofacial Radiol. 2008, 37, 40–46. [Google Scholar] [CrossRef]
- Szczypiński, P.; Strzelecki, M.; Materka, A.; Klepaczko, A. MaZda—A software package for image texture analysis. Comput. Methods Prog. Biomed. 2009, 94, 66–76. [Google Scholar] [CrossRef]
- Lee, J.-S.; Cha, J.-K.; Kim, C.-S. Alveolar ridge regeneration of damaged extraction sockets using deproteinized porcine versus bovine bone minerals: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2018, 20, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, J.; Sheng, M.; Qi, W.; Jin, J.; He, F. Facial alveolar bone alterations and gray value changes based on cone beam computed tomography around maxillary anterior implants: A clinical retrospective study of 1–3 years. Clin. Oral Implant. Res. 2020, 31, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Lee, J.-K.; Choi, S.-H.; Kim, Y.-K.; Kim, B.; Lee, J.-H. Maxillary Sinus Augmentation with Calcium Phosphate Double-Coated Anorganic Bovine Bone. Implant. Dent. 2019, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Junior, O.F.; Munhoz, E.A.; Segantin, J.D.F.; Gonçales, E.S.; De Carvalho, P.S.P. Tomographic late evaluation of xenogeneic bone grafts in sockets of impacted third molars. J. Appl. Oral Sci. 2018, 26, 20170396. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Marciniak-Hoffman, A.; Denkowski, M. Long-term comparison of application of two beta-tricalcium phosphates in oral surgery. Dent. Med. Probl. 2009, 46, 384–388. [Google Scholar]
- Kozakiewicz, M.; Marciniak-Hoffman, A.; Olszycki, M. Comparative analysis of 3 bone substitute materials based on co-occurance matrix. Dent. Med. Probl. 2010, 47, 23–29. [Google Scholar]
- Mol, A. Imaging methods in periodontology. Periodontology 2000 2004, 34, 34–48. [Google Scholar] [CrossRef]
- Choi, J.-W.; Han, W.-J.; Kim, E.-K. Image enhancement of digital periapical radiographs according to diagnostic tasks. Imaging Sci. Dent. 2014, 44, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Tirrell, B.C.; Miles, D.A.; Brown, C.E.; Legan, J.J. Interpretation of chemically created lesions using direct digital imaging. J. Endod. 1996, 22, 74–78. [Google Scholar] [CrossRef]
- Sullivanjr, J.; Di Fiore, P.M.; Koerber, A.; E Sullivan, J. RadioVisiography in the Detection of Periapical Lesions. J. Endod. 2000, 26, 32–35. [Google Scholar] [CrossRef]
- Kullendorff, B.; Nilsson, M. Diagnostic accuracy of direct digital dental radiography for the detection of periapical bone lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1996, 82, 585–589. [Google Scholar] [CrossRef]
- Geraets, W. Comparison of two methods for measuring orientation. Bone 1998, 23, 383–388. [Google Scholar] [CrossRef]
- Zaman, M.U.; Nakamoto, T.; Tanimoto, K. A retrospective study of digital subtraction technique to detect sclerotic changes in alveolar bone on intraoral radiographs of bisphosphonate-treated patients. Dentomaxillofacial Radiol. 2013, 42, 20130242. [Google Scholar] [CrossRef]
- Bodek, K.H.; Michalska, M.; Bodek, A.; Department of Applied Pharmacy, Faculty of Pharmacy, Medical University of Lodz, Poland. Evaluation of the use of microcrystalline chitosan and collagen membranes as carriers for the platelet derived growth factor (PDGF-BB) in the presence of amoxicillin. Curr. Issues Pharm. Med. Sci. 2013, 26, 176–182. [Google Scholar] [CrossRef]
- Michalska, M.; Kozakiewicz, M.; Bodek, A.; Bodek, K.H. Estimation of the use of fibrin and collage membranes as carriers for platelet-derived growth factor-BB (PDGF-BB) in the presence of amoxicillin. Indian J. Biochem. Biophys. 2015, 52, 196–202. [Google Scholar] [PubMed]
- Cicciù, M.; Cervino, G.; Herford, A.S.; Fama’, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Meyer, U.; Meyer, T.; Jones, D.B. No mechanical role for vinculin in strain transduction in primary bovine osteoblasts. Biochem. Cell Biol. 1997, 75, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Büchter, A.; Wiesmann, H.P.; Joos, U.; Jones, D.B. Basic reactions of osteoblasts on structured material surfaces. Eur. Cell Mater. 2005, 9, 39–49. [Google Scholar] [CrossRef]
- Matlaga, B.F.; Yasenchak, L.P.; Salthouse, T.N. Tissue response to implanted polymers: The significance of sample shape. J. Biomed. Mater. Res. 1976, 10, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Misiek, D.J.; Kent, J.N.; Carr, R.F. Soft tissue responses to hydroxylapatite particles of different shapes. J. Oral Maxillofac. Surg. 1984, 42, 150–160. [Google Scholar] [CrossRef]
- Hench, L.; Wilson, J. Surface-active biomaterials. Sciences 1984, 226, 630–636. [Google Scholar] [CrossRef]
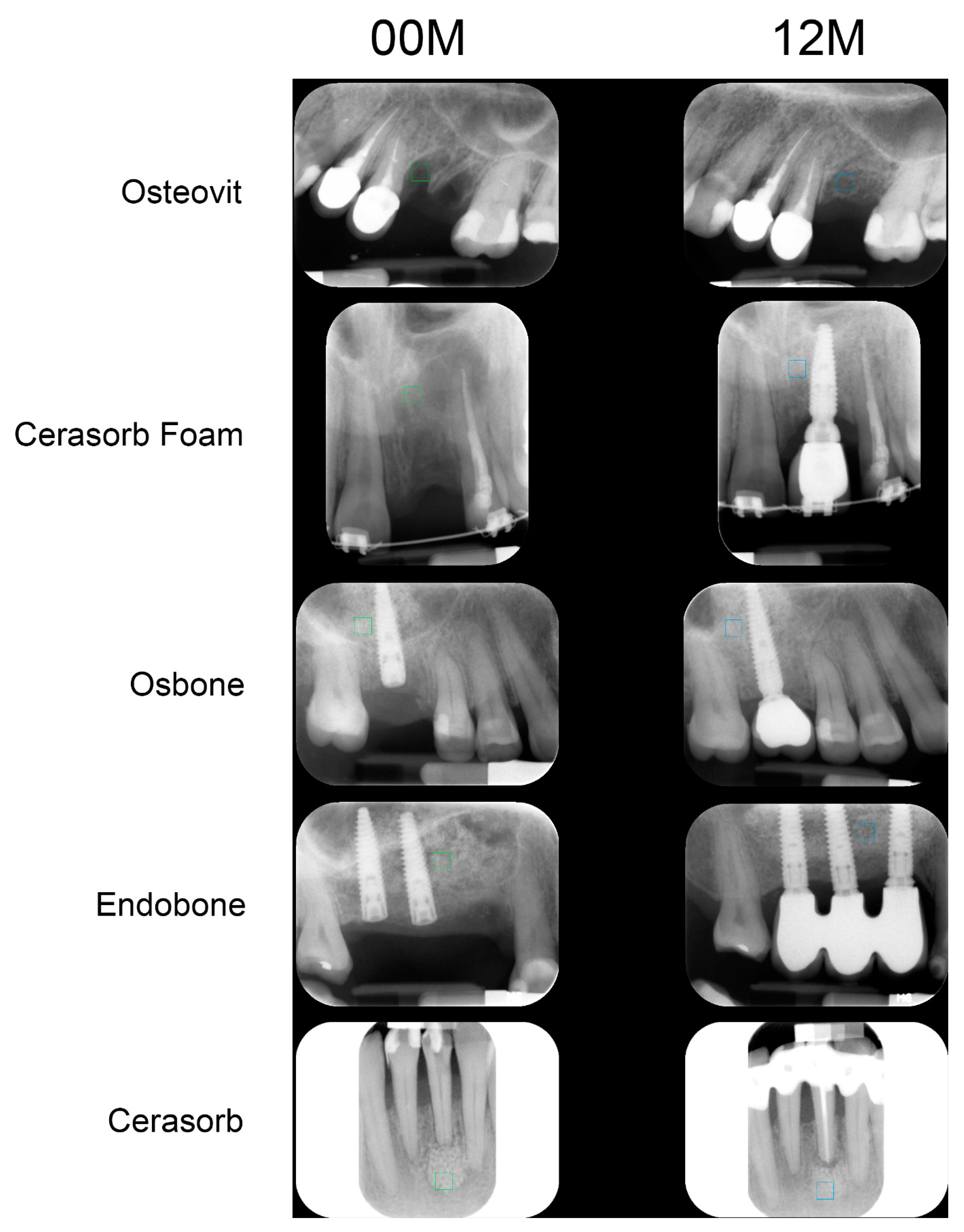
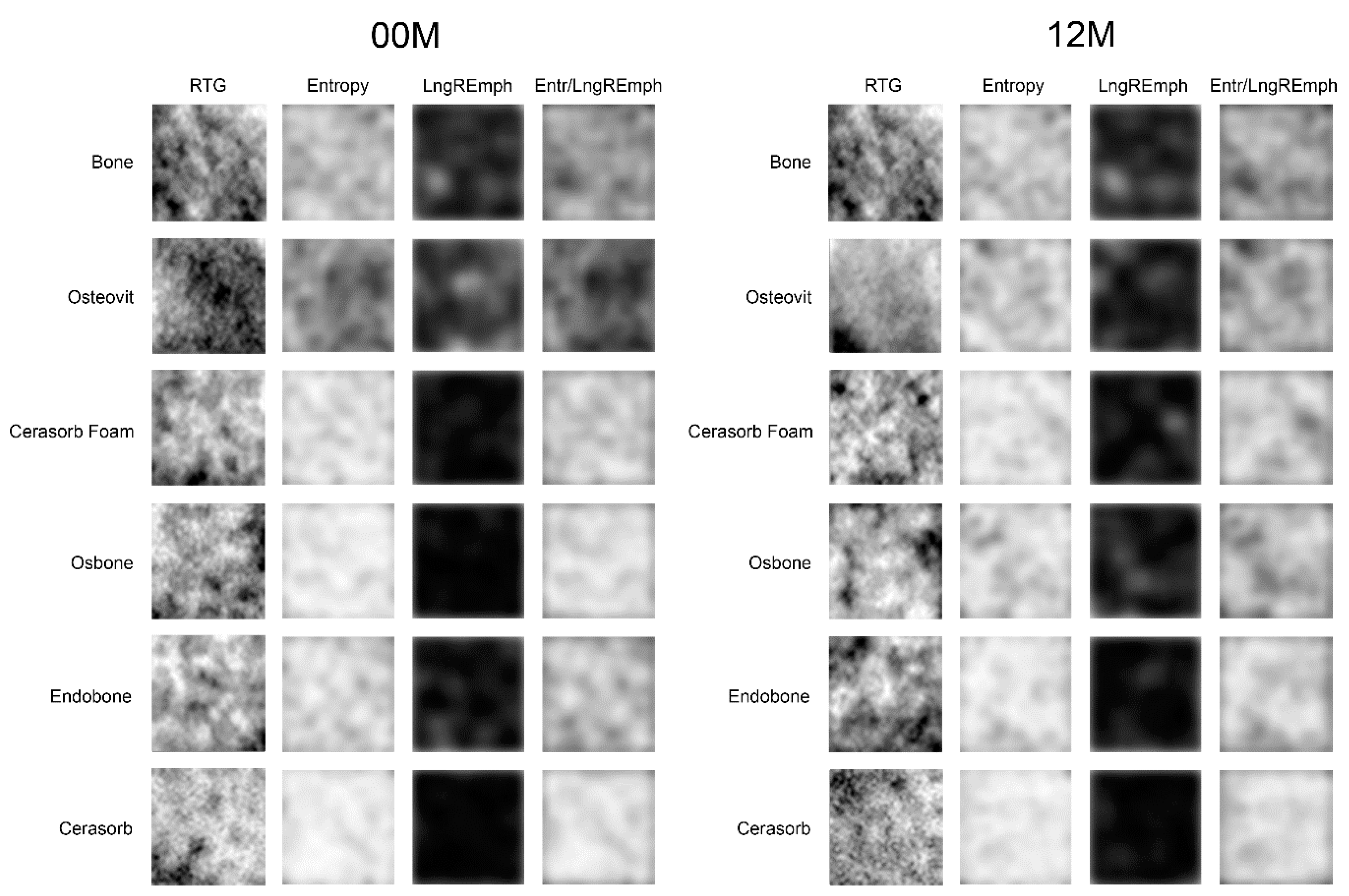
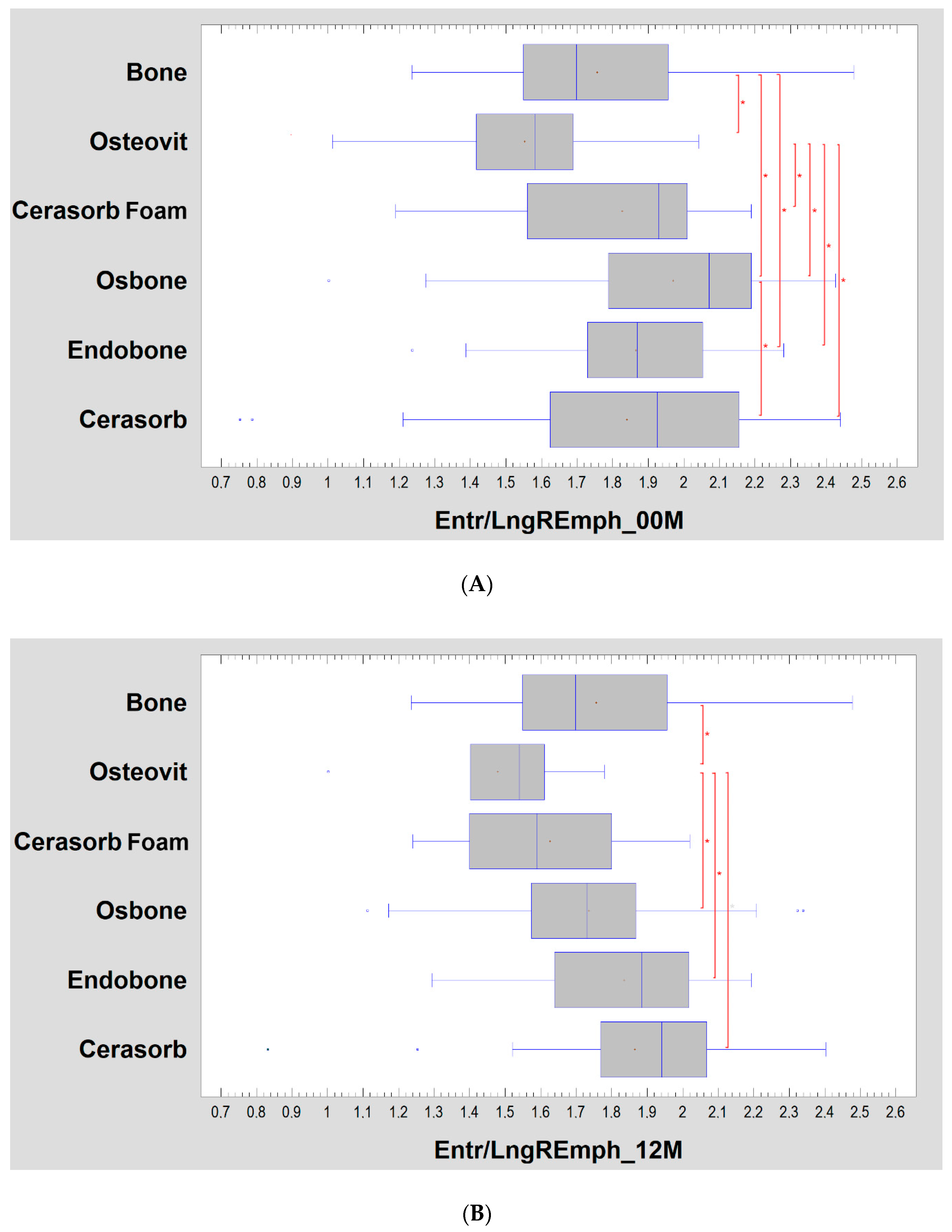

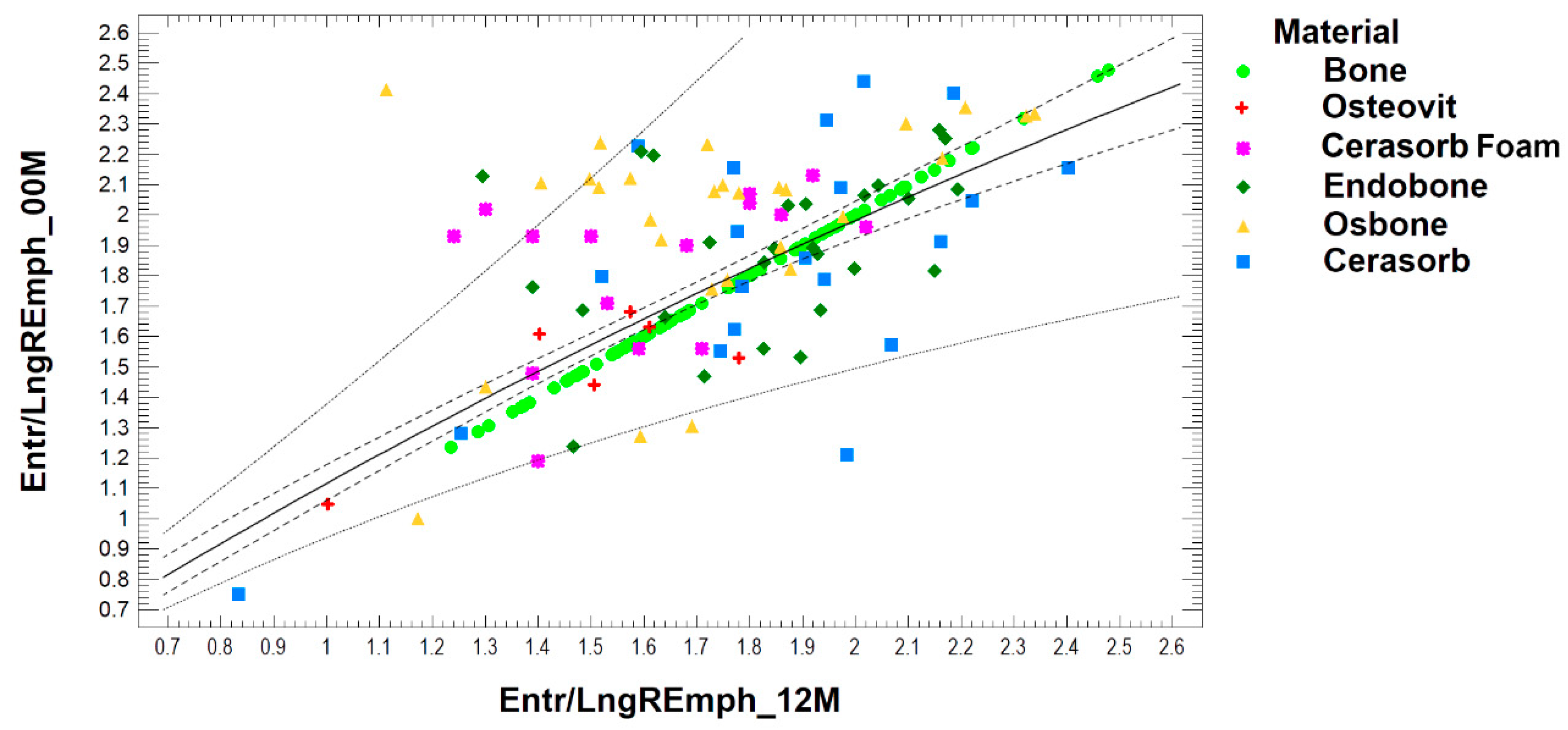

| Material | 00M | 12M | Significance |
|---|---|---|---|
| Bone | 1.76 ± 0.28 | 1.76 ± 0.28 | n.s. |
| Osteovit | 1.55 ± 0.26 | 1.48 ± 0.26 | n.s. |
| Cerasorb Foam | 1.82 ± 0.27 | 1.63 ± 0.24 | p < 0.05 |
| Osbone | 1.97 ± 0.31 | 1.74 ± 0.30 | p < 0.01 |
| Endobone | 1.86 ± 0.25 | 1.84 ± 0.25 | n.s. |
| Cerasorb | 1.84 ± 0.41 | 1.86 ± 0.36 | n.s. |
| Material | Entropy | LngREmph | ||
|---|---|---|---|---|
| 00M | 12M | 00M | 12M | |
| Bone | 2.70 ± 0.18 | 2.70 ± 0.18 | 1.56 ± 0.21 | 1.56 ± 0.21 |
| Osteovit | 2.67 ± 0.14 | 2.67 ± 0.10 | 1.76 ± 0.28 | 1.86 ± 0.36 |
| Cerasorb Foam | 2.79 ± 0.18 | 2.67 ± 0.20 | 1.54 ± 0.19 | 1.65 ± 0.27 |
| Osbone | 2.86 ± 0.12 | 2.80 ± 0.12 | 1.49 ± 0.24 | 1.65 ± 0.25 |
| Endobone | 2.88 ± 0.10 | 2.86 ± 0.09 | 1.57 ± 0.19 | 1.58 ± 0.19 |
| Cerasorb | 2.78 ± 0.18 | 2.72 ± 0.18 | 1.60 ± 0.47 | 1.52 ± 0.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozakiewicz, M.; Wach, T. New Oral Surgery Materials for Bone Reconstruction—A Comparison of Five Bone Substitute Materials for Dentoalveolar Augmentation. Materials 2020, 13, 2935. https://doi.org/10.3390/ma13132935
Kozakiewicz M, Wach T. New Oral Surgery Materials for Bone Reconstruction—A Comparison of Five Bone Substitute Materials for Dentoalveolar Augmentation. Materials. 2020; 13(13):2935. https://doi.org/10.3390/ma13132935
Chicago/Turabian StyleKozakiewicz, Marcin, and Tomasz Wach. 2020. "New Oral Surgery Materials for Bone Reconstruction—A Comparison of Five Bone Substitute Materials for Dentoalveolar Augmentation" Materials 13, no. 13: 2935. https://doi.org/10.3390/ma13132935
APA StyleKozakiewicz, M., & Wach, T. (2020). New Oral Surgery Materials for Bone Reconstruction—A Comparison of Five Bone Substitute Materials for Dentoalveolar Augmentation. Materials, 13(13), 2935. https://doi.org/10.3390/ma13132935






