Effect of Etching Procedures on the Adhesion of Biofilm-Coated Dentin
Abstract
1. Introduction
2. Materials and Methods
2.1. Dentin Disc Preparation
2.2. Human Saliva Collection and Pre-Coating of Dentin Slices
2.3. Biofilm Formation
2.4. Group Assignment and Surface Treatment
- (1)
- Group C (control): no biofilm formation;
- (2)
- Group BF: biofilm formation and no surface treatment;
- (3)
- Group BF-E: biofilm formation and treatment with etching using 37% phosphoric acid (3M ESPE, St. Paul, MN, USA) gel for 15 s and rinsing with distilled water for 30 s;
- (4)
- Group BF-EC: biofilm formation and treatment with etching using 37% phosphoric acid gel for 15 s, soaking in chlorhexidine for 5 min after drying, and rinsing with distilled water for 30 s;
- (5)
- Group BF-RE: biofilm formation and prophylaxis using a rubber cup and plain pumice for 30 s, followed by etching using 37% phosphoric acid gel for 15 s, and rinsing with distilled water for 30 s.
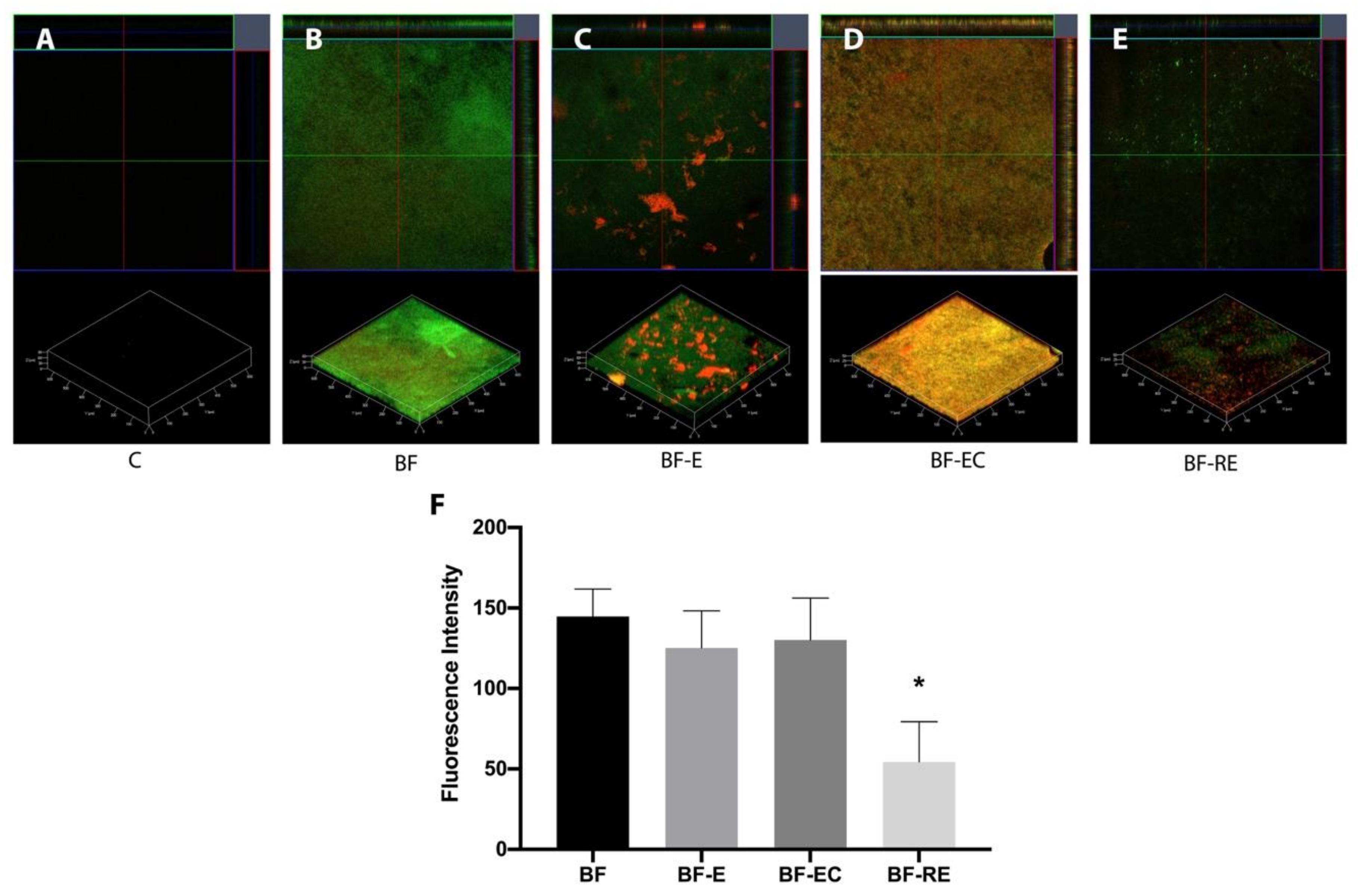
2.5. Evaluation of Biofilm with Confocal Laser Scanning Microscopy
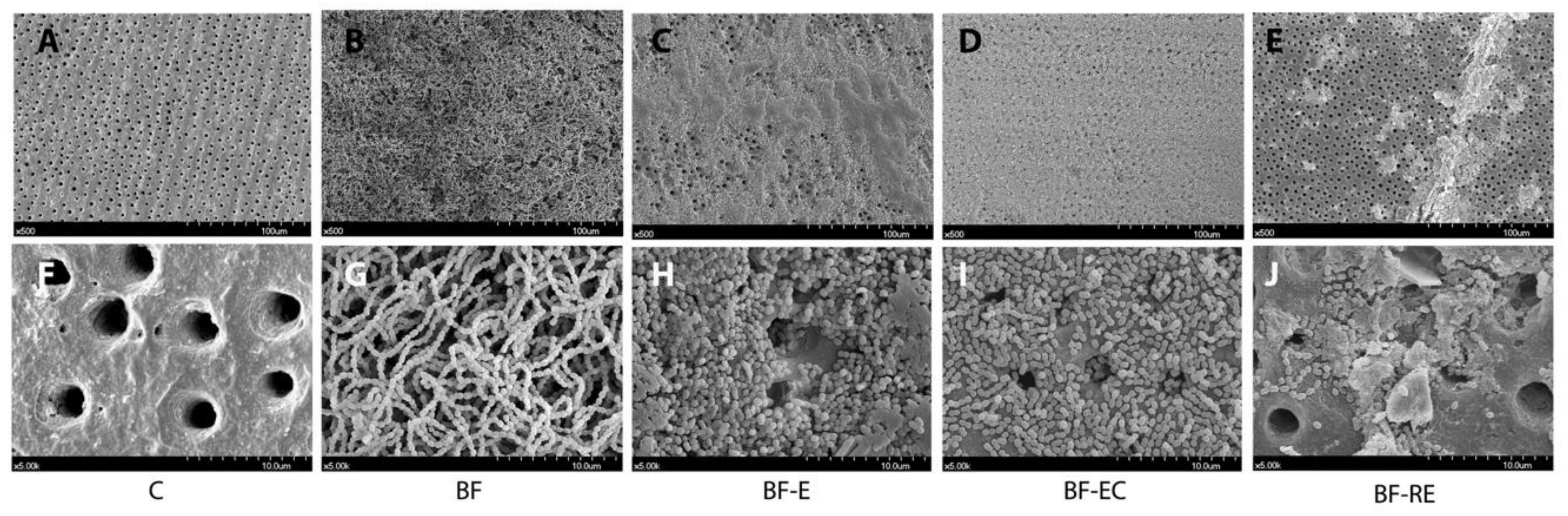
2.6. Evaluation of Biofilm with Scanning Electron Microscopy
2.7. Specimen Preparation for Bond Strength Test
2.8. Group Assignment and Surface Treatment for the Bond Strength Test
- (1)
- Group C-E (control): no biofilm formation and treatment with etching using 37% phosphoric acid solution for 15 s and rinsing with distilled water for 30 s;
- (2)
- Group BF-E: biofilm formation and treatment with etching using 37% phosphoric acid solution for 15 s and rinsing with distilled water for 30 s;
- (3)
- Group BF-EC: biofilm formation and treatment with etching using 37% phosphoric acid solution for 15 s, soaking into chlorhexidine for 5 min after drying, and rinsing with distilled water for 30 s;
- (4)
- Group BF-RE: Biofilm formation and treatment with rubbing using rubber cup and plain pumice for 30 s, etching using 37% phosphoric acid solution for 15 s, and rinsing with distilled water for 30 s.
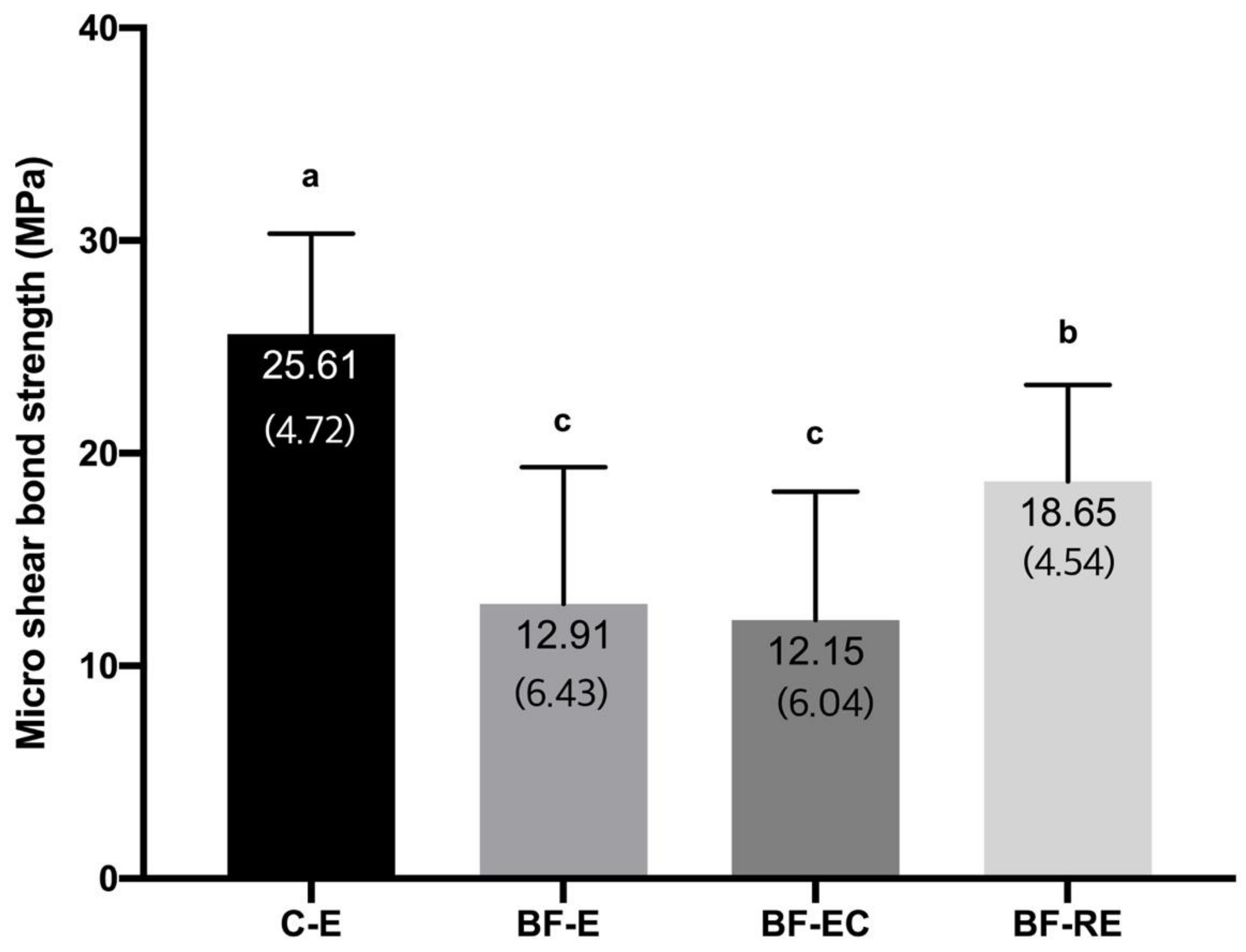
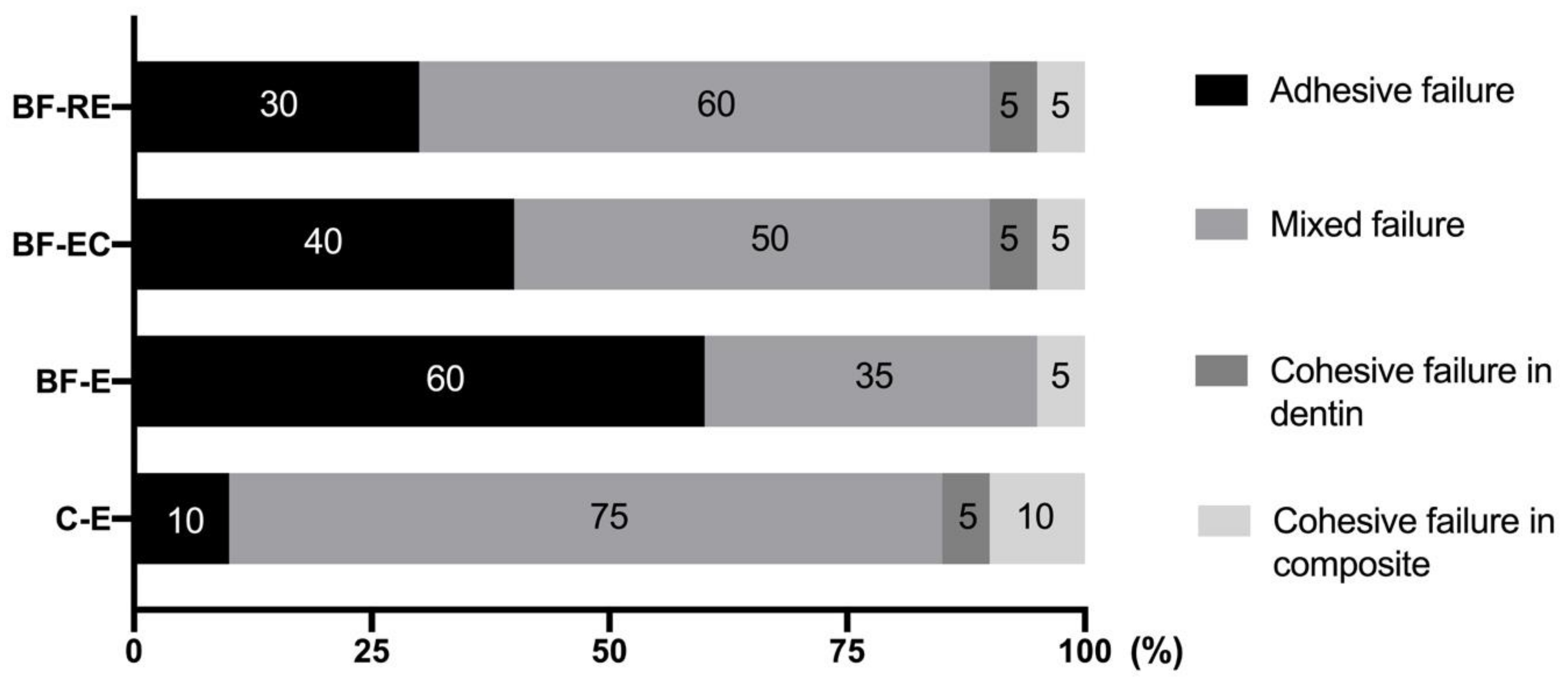
2.9. Micro-Shear Bond Strength Test and Failure Mode Observation
2.10. Statistical Analysis
3. Results
3.1. Evaluation of Remaining Biofilm on Dentin Surface after Surface Treatment
3.2. Evaluation of Bond Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scannapieco, F.A. Saliva-bacterium interactions in oral microbial ecology. Crit. Rev. Oral Biol. Med. 1994, 5, 203–248. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of dental prosthesis and restorative materials interface on oral biofilms. Int. J. Mol. Sci. 2018, 19, 3157. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque as a microbial biofilm. Caries Res. 2004, 38, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Sbordone, L.; Bortolaia, C. Oral microbial biofilms and plaque-related diseases: Microbial communities and their role in the shift from oral health to disease. Clin. Oral Investig. 2003, 7, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32, 7–15. [Google Scholar] [CrossRef]
- Beyth, N.; Bahir, R.; Matalon, S.; Domb, A.J.; Weiss, E.I. Streptococcus mutans biofilm changes surface-topography of resin composites. Dent. Mater. 2008, 24, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Carlén, A.; Nikdel, K.; Wennerberg, A.; Holmberg, K.; Olsson, J. Surface characteristics and in vitro biofilm formation on glass ionomer and composite resin. Biomaterials 2001, 22, 481–487. [Google Scholar] [CrossRef]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef]
- Collins, C.J.; Bryant, R.W.; Hodge, K.-L.V. A clinical evaluation of posterior composite resin restorations: 8-Year findings. J. Dent. 1998, 26, 311–317. [Google Scholar] [CrossRef]
- Øilo, M.; Bakken, V. Biofilm and dental biomaterials. Materials 2015, 8, 2887–2900. [Google Scholar] [CrossRef]
- Li, Y.; Carrera, C.; Chen, R.; Li, J.; Lenton, P.; Rudney, J.D.; Jones, R.S.; Aparicio, C.; Fok, A. Degradation in the dentin-composite interface subjected to multi-species biofilm challenges. Acta Biomater. 2014, 10, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.J.; Al-Ahmad, A.; Follo, M.; Spitzmüller, B.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Visualization of initial bacterial colonization on dentine and enamel in situ. J. Microbiol. Methods 2010, 81, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar]
- Gultz, J.; Kaim, J.; Scherer, W. Treating enamel surfaces with a prepared pumice prophy paste prior to bonding. Gen. Dent. 1999, 47, 200–201. [Google Scholar]
- Abreu, L.G.; Paiva, S.M.; Pretti, H.; Lages, E.M.; Junior, J.B.; Ferreira, R.A. Comparative study of the effect of acid etching on enamel surface roughness between pumiced and non-pumiced teeth. J. Int. Oral Health 2015, 7, 1–6. [Google Scholar]
- Fitzgerald, I.; Bradley, G.T.; Bosio, J.A.; Hefti, A.F.; Berzins, D.W. Bonding with self-etching primers--pumice or pre-etch? An in vitro study. Eur. J. Orthod. 2011, 34, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Lill, D.J.; Lindauer, S.J.; Tüfekçi, E.; Shroff, B. Importance of pumice prophylaxis for bonding with self-etch primer. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Lindauer, S.J.; Browning, H.; Shroff, B.; Marshall, F.; Anderson, R.H.B.; Moon, P.C. Effect of pumice prophylaxis on the bond strength of orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 1997, 111, 599–605. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Giannini, M.; Makishi, P.; Ayres, A.P.A.; Vermelho, P.M.; Fronza, B.M.; Nikaido, T.; Tagami, J. Self-etch adhesive systems: A literature review. Braz. Dent. J. 2015, 26, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Weiser, F.; Behr, M. Self-adhesive resin cements: A clinical review. J. Prosthodont. 2014, 24, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Erickson, R.L.; Barkmeier, W.W.; Latta, M.A. The role of etching in bonding to enamel: A comparison of self-etching and etch-and-rinse adhesive systems. Dent. Mater. 2009, 25, 1459–1467. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Flausino, J.S.; Soares, P.B.F.; Carvalho, V.F.; Magalhães, D.; da Silva, W.M.; Costa, H.L.; Soares, C.J. Biofilm formation on different materials for tooth restoration: Analysis of surface characteristics. J. Mater. Sci. 2014, 49, 6820–6829. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- Staudt, C.; Horn, H.; Hempel, D.C.; Neu, T.R. Volumetric measurements of bacterial cells and extracellular polymeric substance glycoconjugates in biofilms. Biotechnol. Bioeng. 2004, 88, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef]
- Nakabayashi, N.; Nakamura, M.; Yasuda, N. Hybrid layer as a dentin-bonding mechanism. J. Esthet. Dent. 1991, 3, 133–138. [Google Scholar] [CrossRef]
- Fernández, C.E.; Tenuta, L.M.A.; Cury, J.A. Validation of a cariogenic biofilm model to evaluate the effect of fluoride on enamel and root dentine demineralization. PLoS ONE 2016, 11, e0146478. [Google Scholar] [CrossRef]
- Fonseca, R.B.; Martins, L.R.; Quagliatto, P.S.; Soares, C.J. Influence of provisional cements on ultimate bond strength of indirect composite restorations to dentin. J. Adhes. Dent. 2005, 7, 225–230. [Google Scholar] [PubMed]
- Chaiyabutr, Y.; Kois, J.C. The effects of tooth preparation cleansing protocols on the bond strength of self-adhesive resin luting cement to contaminated dentin. Oper. Dent. 2008, 33, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Bapoo, H.; Rizkalla, A.; Santos, G. Effect of dentin-cleaning techniques on the shear bond strength of self-adhesive resin luting cement to dentin. Oper. Dent. 2011, 36, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Pereira, J.; Souza, S.; Menezes, M.; Armstrong, S. The effect of prophylaxis method on microtensile bond strength of indirect restorations to dentin. Oper. Dent. 2012, 37, 602–609. [Google Scholar] [CrossRef]
- Hebling, J.; Pashley, D.H.; Tjäderhane, L.; Tay, F.R. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J. Dent. Res. 2005, 84, 741–746. [Google Scholar] [CrossRef]
- Matthijs, S.; Adriaens, P.A. Chlorhexidine varnishes: A review. J. Clin. Periodontol. 2002, 29, 1–8. [Google Scholar] [CrossRef]
- Greenstein, G.; Berman, C.; Joffin, R. Chlorhexidine-an adjunct to periodontal therapy. J. Periodontol. 1986, 57, 370–377. [Google Scholar] [CrossRef]
- Grasso, C.A.; Caluori, D.M.; Goldstein, G.R.; Hittelman, E. In vivo evaluation of three cleansing techniques for prepared abutment teeth. J. Prosthet. Dent. 2002, 88, 437–441. [Google Scholar] [CrossRef]
- Hiraishi, N.; Yiu, C.K.Y.; King, N.M.; Tay, F.R. Effect of 2% chlorhexidine on dentin microtensile bond strengths and nanoleakage of luting cements. J. Dent. 2009, 37, 440–448. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, B.-K.; Lee, C.-H.; Kim, A.R.; Han, S.H.; Kim, H.-J.; Antonson, S.A.; Kim, S.-Y. Effect of Etching Procedures on the Adhesion of Biofilm-Coated Dentin. Materials 2020, 13, 2762. https://doi.org/10.3390/ma13122762
Jeon B-K, Lee C-H, Kim AR, Han SH, Kim H-J, Antonson SA, Kim S-Y. Effect of Etching Procedures on the Adhesion of Biofilm-Coated Dentin. Materials. 2020; 13(12):2762. https://doi.org/10.3390/ma13122762
Chicago/Turabian StyleJeon, Bo-Kyung, Chang-Ha Lee, A Reum Kim, Seung Hyun Han, Hyun-Jung Kim, Sibel A. Antonson, and Sun-Young Kim. 2020. "Effect of Etching Procedures on the Adhesion of Biofilm-Coated Dentin" Materials 13, no. 12: 2762. https://doi.org/10.3390/ma13122762
APA StyleJeon, B.-K., Lee, C.-H., Kim, A. R., Han, S. H., Kim, H.-J., Antonson, S. A., & Kim, S.-Y. (2020). Effect of Etching Procedures on the Adhesion of Biofilm-Coated Dentin. Materials, 13(12), 2762. https://doi.org/10.3390/ma13122762






