Performance Evaluation and Optimization of Dyes Removal using Rice Bran-Based Magnetic Composite Adsorbent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
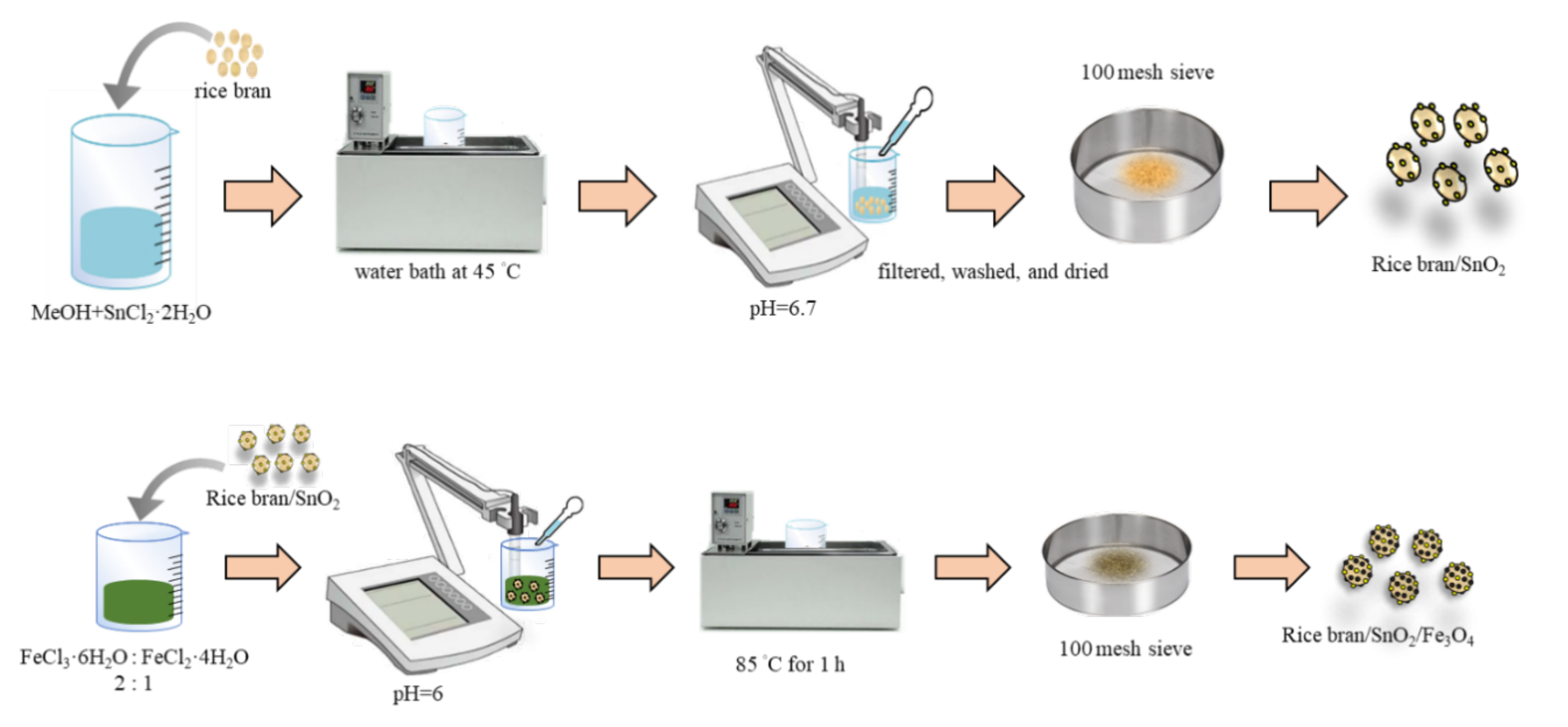
2.2. Synthesis of Rice Bran/SnO2/Fe3O4 Composite
2.3. Composite Adsorbent Characterizations
2.4. Batch Adsorption Experiments
2.5. Experimental Optimization of Adsorption
3. Results and Discussion
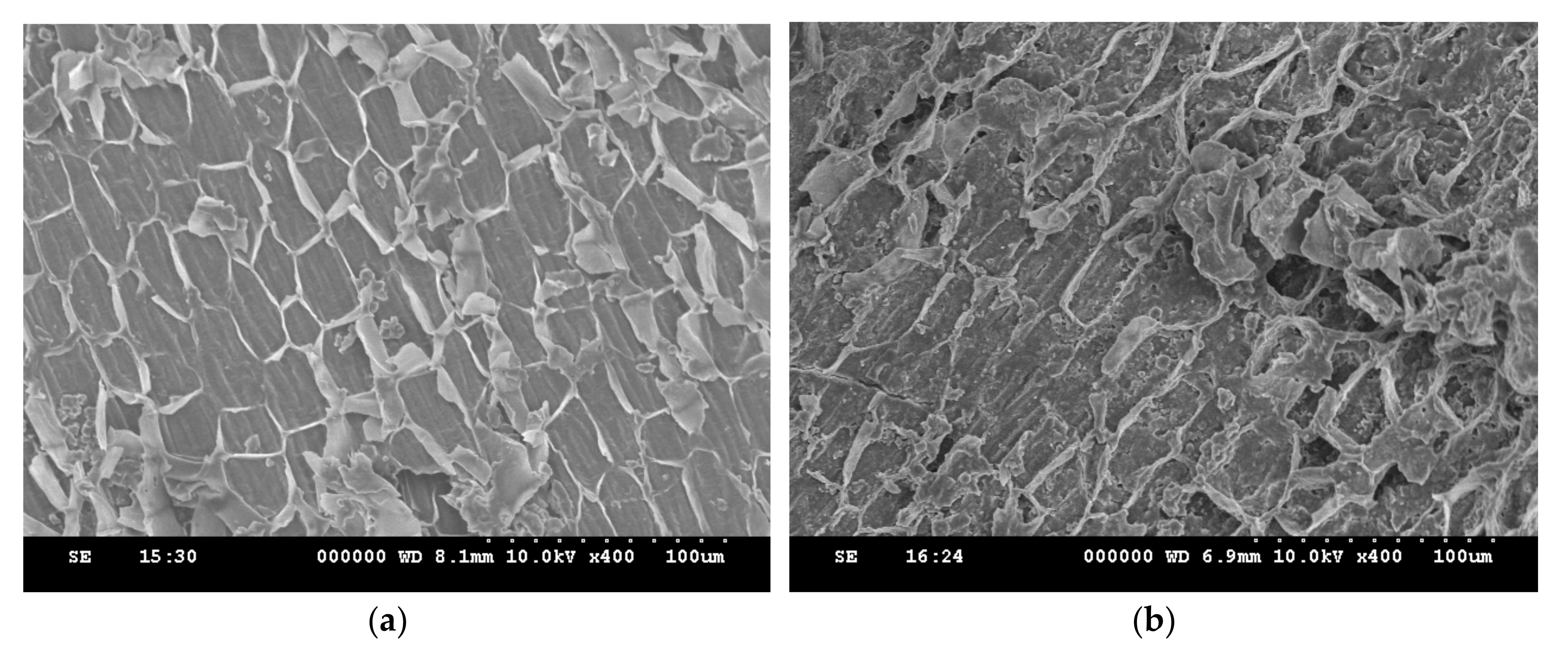
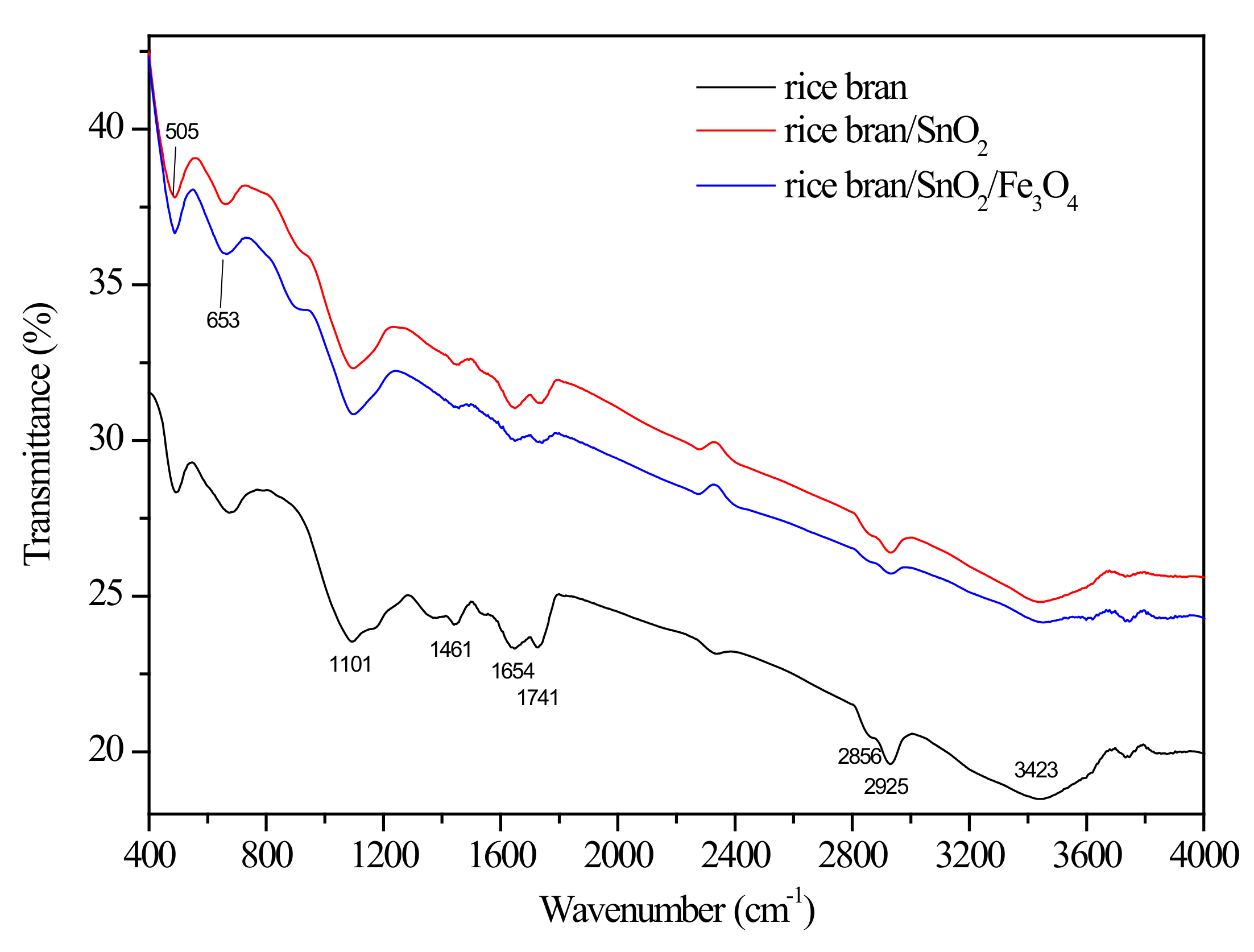
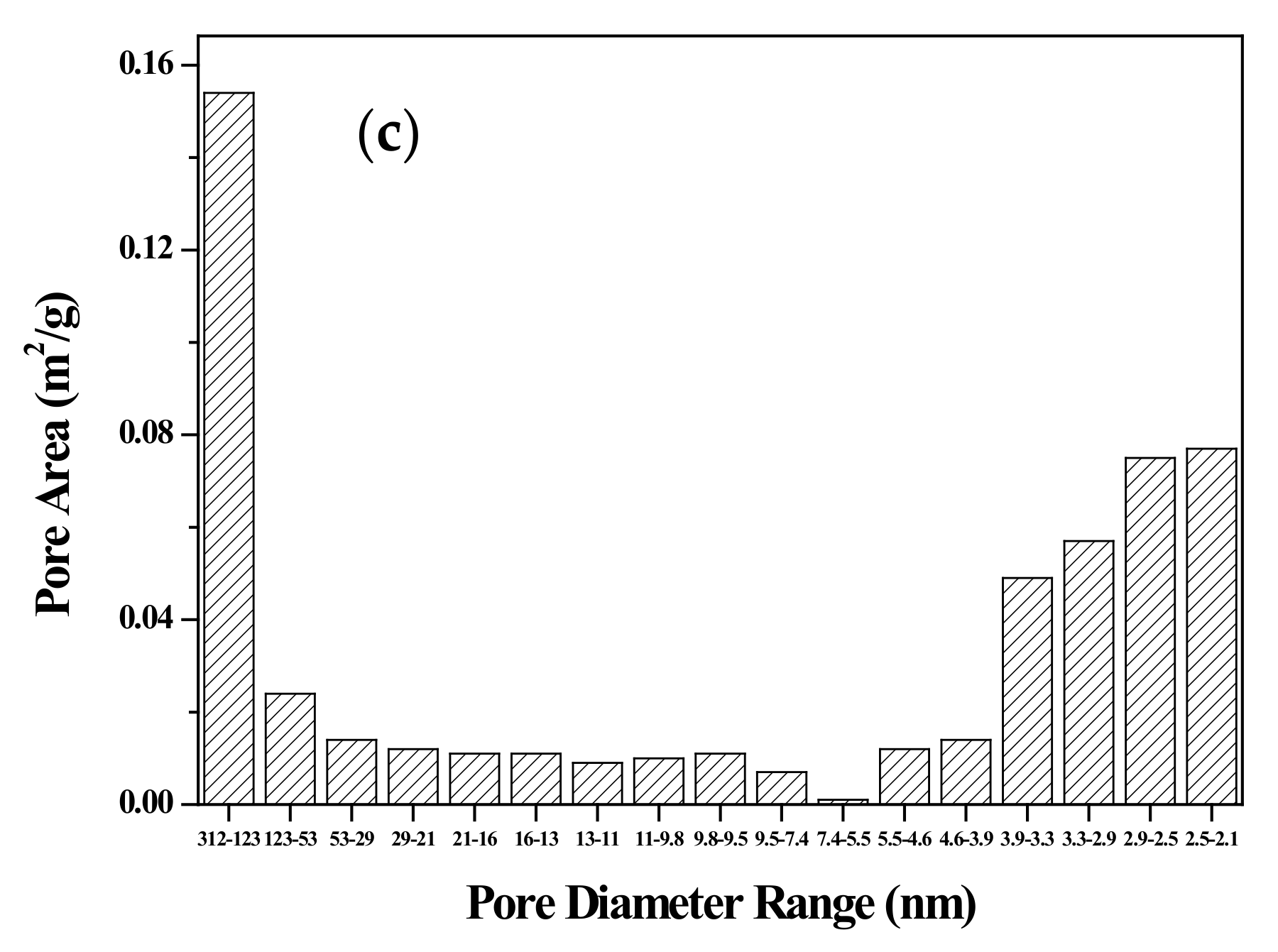
3.1. Characterization of Adsorbent
3.2. Effect of the Operation Condition
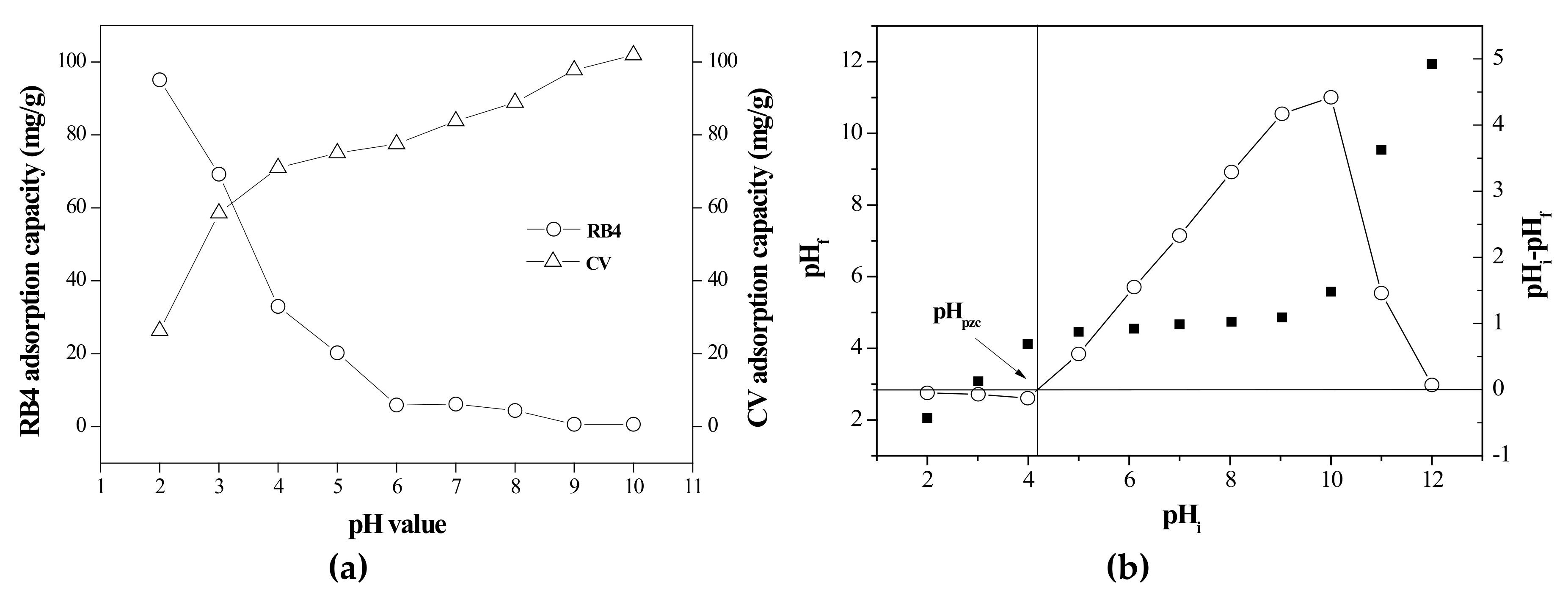
3.2.1. Effect of pH
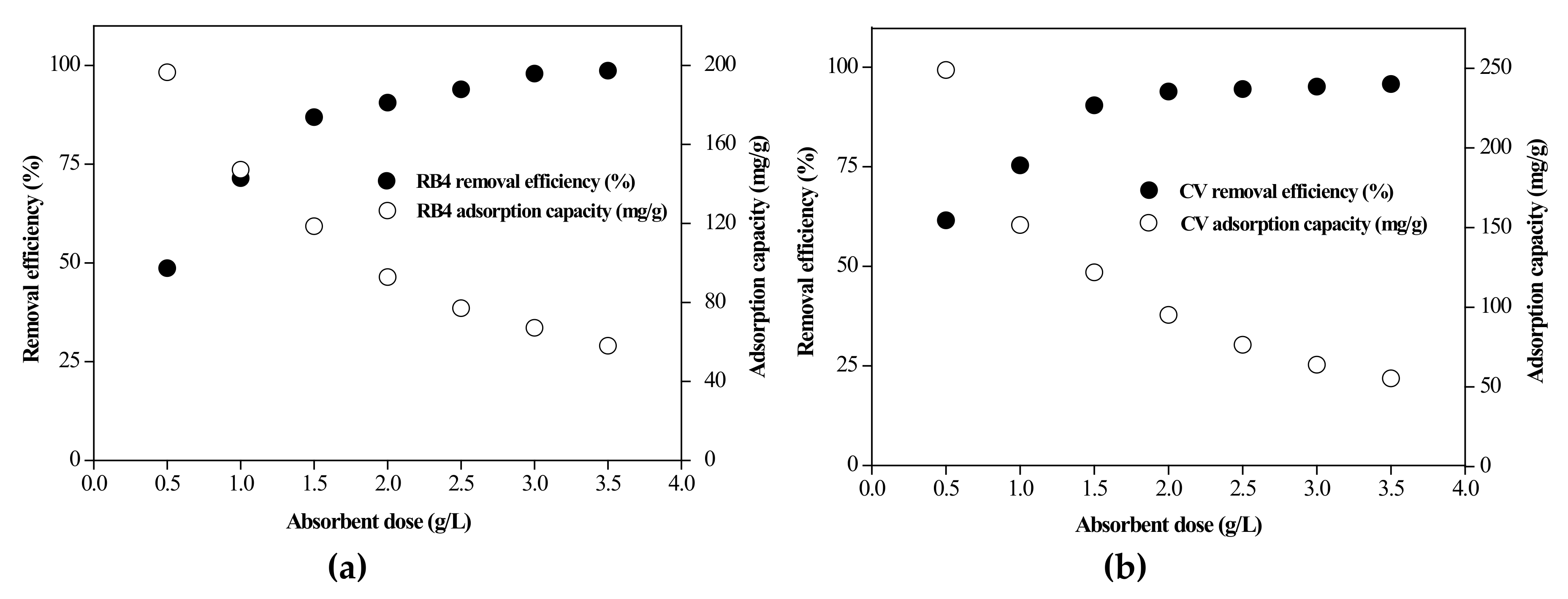
3.2.2. Effect of Adsorbent Dose
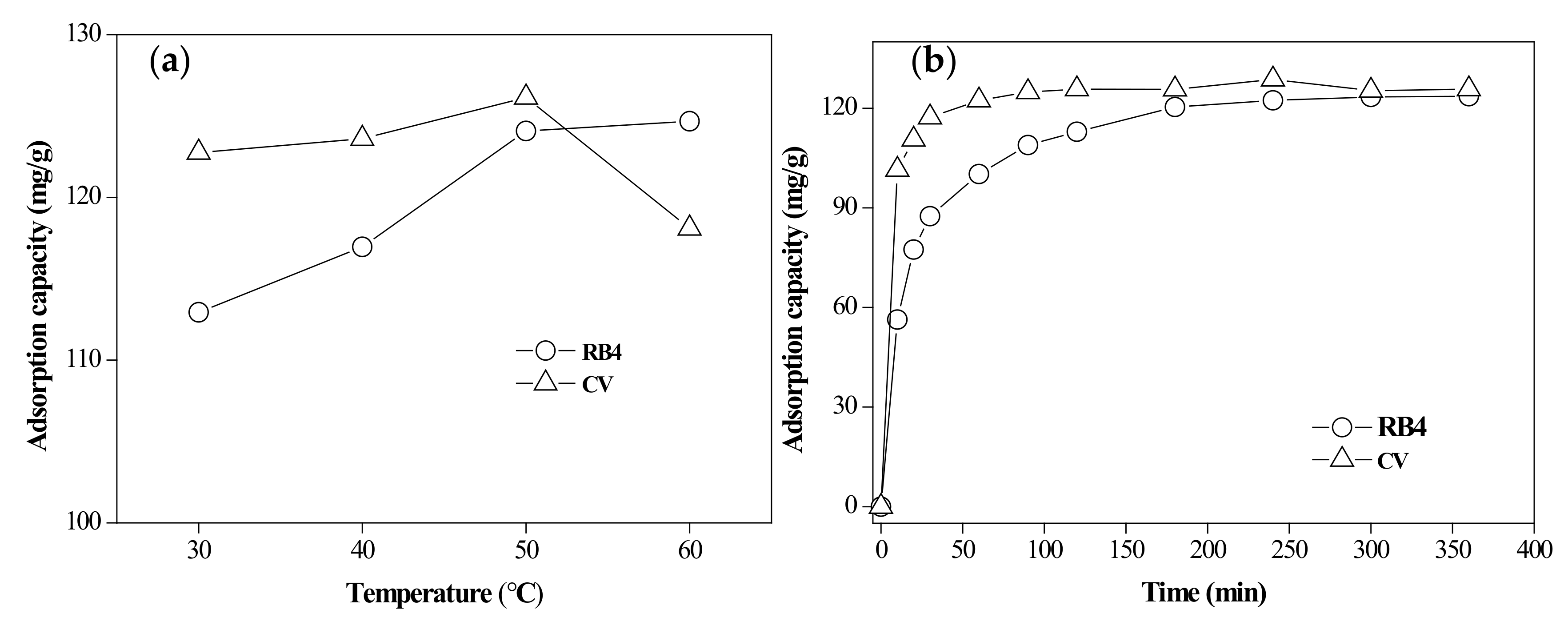
3.2.3. Effect of Temperature
3.3. Adsorption Kinetics and Isotherm Modeling
3.3.1. Adsorption Kinetics
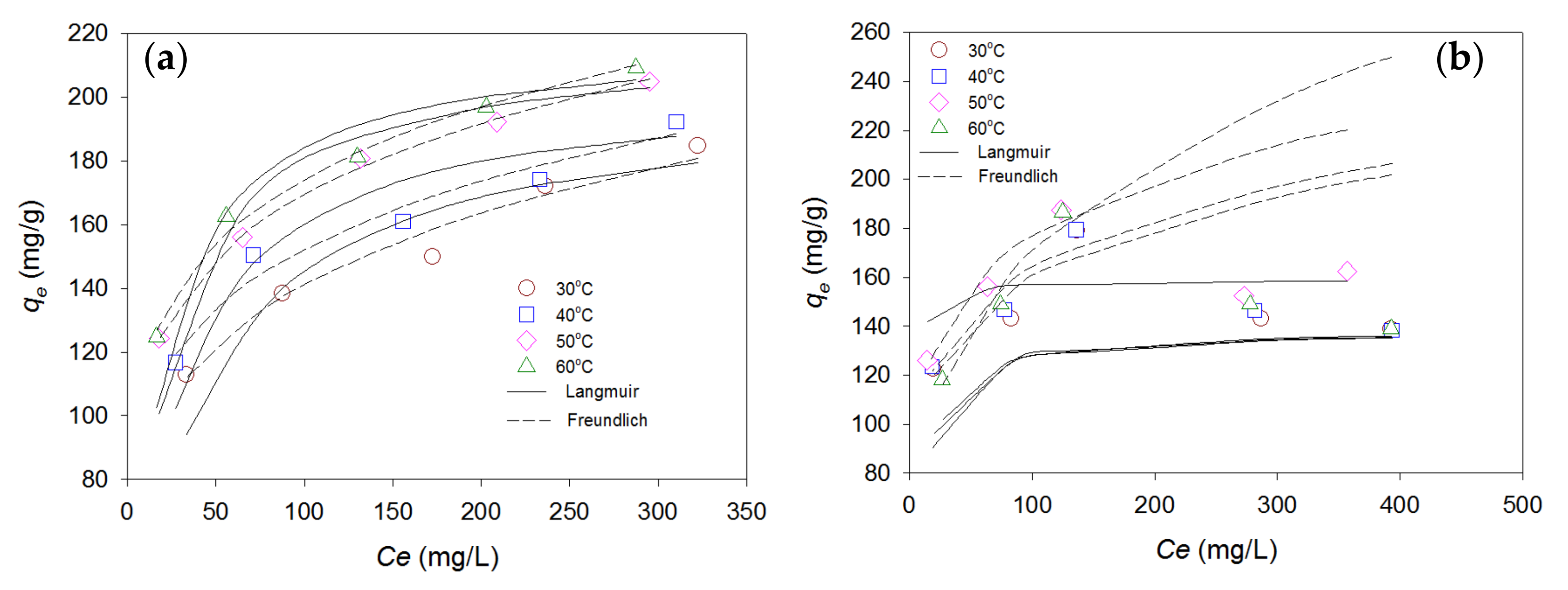
3.3.2. Isotherm Modeling
3.4. Optimal Adsorption and Interactive
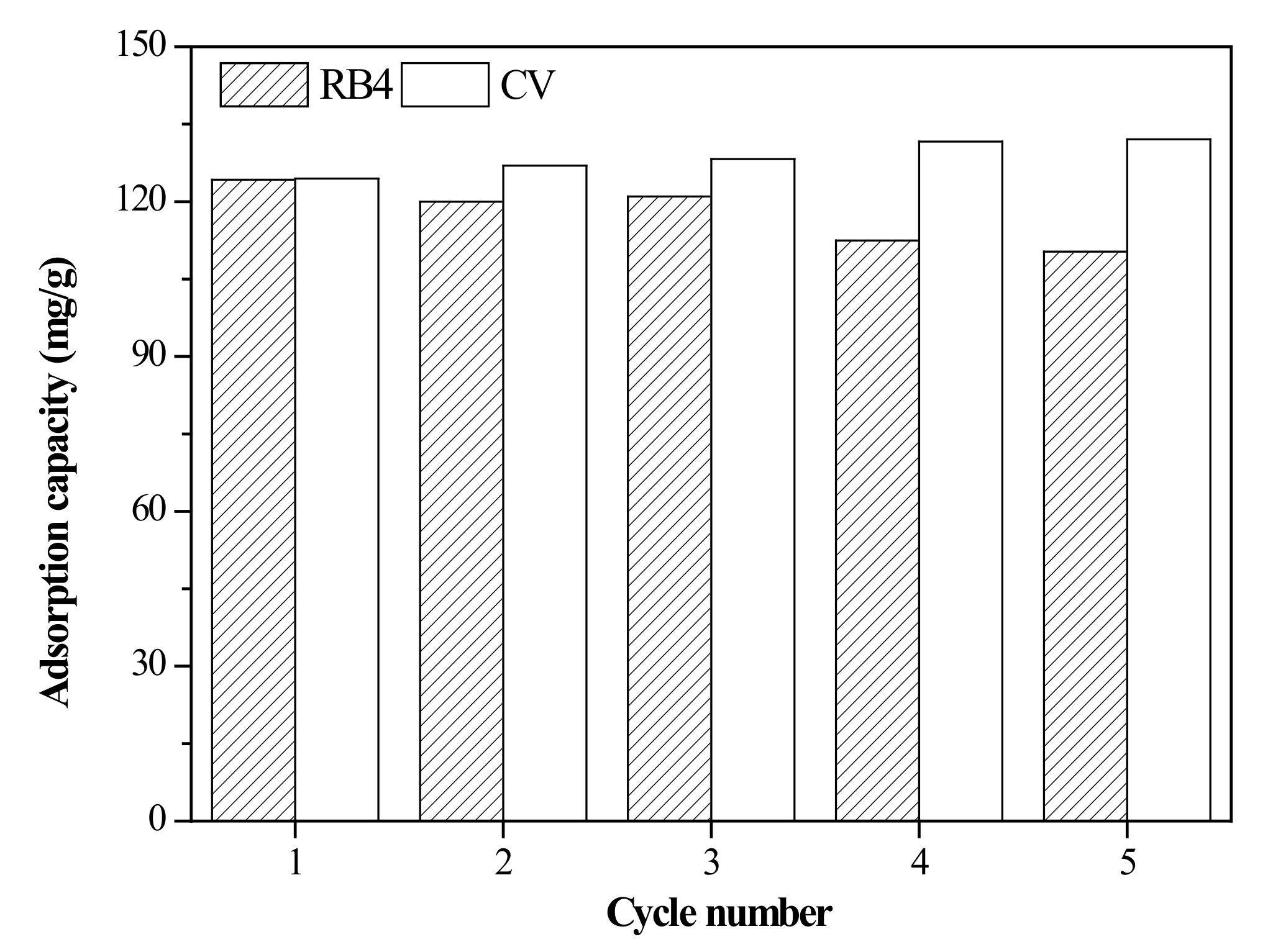
3.5. Regeneration of the Adsorbent
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Angelova, R.; Baldikova, E.; Pospiskova, K.; Maderova, Z.; Safarikova, M.; Safarik, I. Magnetically modified sargassum horneri biomass as an adsorbent for organic dye removal. J. Clean. Prod. 2016, 137, 189–194. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sangeetha, D. Recyling of agricultural solid waste, coir pith: Removal of anions, heavy metals, organics and dyes from water by adsorption onto ZnCl2 activated coir pith carbon. J. Hazard. Mater. 2006, B135, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.; Chamjangali, M.A.; Bagherian, G.; Goudarzi, N. Application of linear and non-linear methods for modeling removal efficiency of textile dyes from aqueous solutions using magnetic Fe3O4 impregnated onto walnut shell. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 171, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Soudi, M.; Li, L.; Zhu, Z.H. Coal ash conversion into effective adsorbents for removal of heavy metals and dyes from wastewater. J. Hazard. Mater. 2006, B133, 243–251. [Google Scholar] [CrossRef]
- Belhouchat, N.; Zaghouane-Boudiaf, H.; Viseras, C. Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Appl. Clay Sci. 2017, 135, 9–15. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Bagheri, A.R.; Ghaedi, M.; Asfaram, A.; Hajati, S.; Ghaedi, A.M.; Bazrafshan, A.; Rahimi, M.R. Modeling and optimization of simultaneous removal of ternary dyes onto copper sulfide nanoparticles loaded on activated carbon using second-derivative spectrophotometry. J. Taiwan Inst. Chem. Eng. 2016, 65, 212–224. [Google Scholar] [CrossRef]
- Debnath, S.; Ballav, N.; Maity, A.; Pillay, K. Competitive adsorption of ternary dye mixture using pine cone powder modified with β-cyclodextrin. J. Mol. Liq. 2017, 225, 679–688. [Google Scholar] [CrossRef]
- Guo, J.Z.; Li, B.; Liu, L.; Lv, K. Removal of methylene blue from aqueous solutions by chemically modified bamboo. Chemosphere 2014, 111, 225–231. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, C.; Saha, P.D. Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Kim, D.S. Adsorption of anionic azo dye congo red from aqueous solution by cationic modified orange peel powder. J. Mol. Liq. 2016, 220, 540–548. [Google Scholar] [CrossRef]
- Hong, G.B.; Wang, Y.K. Synthesis of low-cost adsorbent from rice bran for the removal of reactive dye based on the response surface methodology. Appl. Surf. Sci. 2017, 423, 800–809. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Cheng, Z. Removal of organic pollutants from aqueous solution using agricultural wastes: A review. J. Mol. Liq. 2015, 212, 739–762. [Google Scholar] [CrossRef]
- Grag, V.K.C.; Amita, M.; Kumar, R.; Gupta, R. Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: A timber industry waste. Dyes Pigm. 2004, 63, 243–250. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Jabeen, A.; Iqbal, M.; Noreen, S.; Naseem, Z. Adsorptive behavior of rice bran-based composites for malachite green dye: Isotherm, kinetic and thermodynamic studies. J. Mol. Liq. 2017, 237, 322–333. [Google Scholar] [CrossRef]
- Hong, G.B.; Jiang, C.J. Biosynthesis of SnO2 nanoparticles based on response surface methodology and the study of their dye removal. J. Nanosci. Nanotechnol. 2018, 18, 5020–5025. [Google Scholar] [CrossRef]
- Hemmati, M.; Asghari, A.; Ghaedi, M.; Rajabi, M. Chemometric assisted sonochemical dyes adsorption in ternary solutions onto Cu nanowires loaded on activated carbon. J. Taiwan Inst. Chem. Eng. 2017, 76, 115–125. [Google Scholar] [CrossRef]
- Hoa, N.V.; Quyen, T.T.H.; Trung, T.S. One-step facile synthesis of mesoporous graphene/Fe3O4/chitosan nanocomposite and its adsorption capacity for a textile dye. J. Water Process Eng. 2016, 9, 170–178. [Google Scholar]
- Alvand, M.; Shemirani, F. Preconcentration of trace cadmium ion using magnetic graphene nanoparticles as an efficient adsorbent. Microchim. Acta 2014, 181, 181–188. [Google Scholar] [CrossRef]
- Moeinpour, F.; Kamyab, S.; Akhgar, M.R. NiFe2O4 magnetic nanoparticles as an adsorbent for cadmium removal from aqueous solution. J. Water Chem. Technol. 2017, 39, 281–288. [Google Scholar] [CrossRef]
- Bao, X.; Qiang, Z.; Chang, J.H.; Ben, W.; Qu, J. Synthesis of carbon-coated magnetic nanocomposite (Fe3O4@C) and its application for sulfonamide antibiotics removal from water. J. Environ. Sci. 2014, 26, 962–969. [Google Scholar] [CrossRef]
- Yarazavi, M.; Noroozian, E. A novel sorbent based on carbon nanotube/amino-functionalized sol-gel for the headspace solid-phase microextraction of α-bisabolol from medicinal plant samples using experimental design. J. Sep. Sci. 2018, 41, 2229–2236. [Google Scholar] [CrossRef] [PubMed]
- Guvenc, S.Y.; Okut, Y.; Ozak, M.; Haktanir, B.; Bilgili, M.S. Process optimization via response surface methodology in the treatment of metal working industry wastewater with electrocoagulation. Water Sci. Technol. 2017, 75, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.T.; Makwana, A.R.; Ahammed, M.M. The use of response surface methodology for modelling and analysis of water and wastewater treatment processes: A review. Water Sci. Technol. 2014, 69, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, G.K.; Erkan, H.S.; Sekman, E.; Top, S.; Karaman, H.; Bilgili, M.S.; Engin, G.O. Modeling and optimizing Fenton and electro-Fenton processes for dairy wastewater treatment using response surface methodology. Int. J. Environ. Sci. Technol. 2019, 16, 2343–2358. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, M.; Zhou, J.; Wang, J. Optimization of biochar preparation from the stem of eichhornia crassipes using response surface methodology on adsorption of Cd2+. Sci. Rep. 2019, 9, 17538. [Google Scholar] [CrossRef]
- Kakoi, B.; Kaluli, J.W.; Ndiba, P.; Thiongo, G. Optimization of maerua decumbent bio-coagulant in paint industry wastewater treatment with response surface methodology. J. Clean. Prod. 2017, 164, 1124–1134. [Google Scholar] [CrossRef]
- Cao, R.; Fan, M.; Hu, J.; Ruan, W.; Xiong, K.; Wei, X. Optimizing low-concentration mercury removal from aqueous solutions by reduced graphene oxide-supported Fe3O4 composites with the aid of an artificial neural network and genetic algorithm. Materials 2017, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for development of functional food-a review. Bioact. Carbohydr. Dietary Fibre. 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Maity, D.; Agrawal, D.C. Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J. Magn. Magn. Mater. 2007, 308, 46–55. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mishra, R.; Saha, P.; Kushwaha, P. Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 2011, 265, 159–168. [Google Scholar] [CrossRef]
- Shojaei, S.; Ardakani, M.A.H.; Sodaiezadeh, H.; Jafari, M.; Afzali, S.F. Simultaneous optimization of parameters influencing organic mulch test using response surface methodology. Sci. Rep. 2020, 10, 6717. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Naik, N. Gamma-oryzanol from rice bran oil-a review. J. Sci. Ind. Res. 2004, 63, 569–578. [Google Scholar]
- Sahu, J.N.; Acharya, J.; Meikap, B.C. Optimization of production conditions for activated carbons from Tamarind wood by zinc chloride using response surface methodology. Bioresour. Technol. 2010, 101, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.Q.; He, J.; Xu, F.; Sun, R.C. Fractionation and physico-chemical analysis of degraded lignins from the black liquor of eucalyptus pellita KP-AQ pulping. Polym. Degrad. Stabil. 2009, 94, 1142–1150. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Hou, B.; Wang, Y.; Hao, C.; Wu, J. Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ahmaruzzaman, M.; Sinha, T. A novel approach for the synthesis of SnO2 nanoparticles and its application as a catalyst in the reduction and photodegradation of organic compounds. Spectrochim. Acta A 2015, 136, 751–760. [Google Scholar] [CrossRef]
- Kumar, P.S.; Kumar, P.S.; Ramalingam, S.; Senthamarai, C.; Niranjanaa, M.; Vijayalakshmi, P.; Sivanesan, S. Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 2010, 261, 52–60. [Google Scholar] [CrossRef]
- Rashid, A.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Fungal biomass composite with bentonite efficiency for nickel and zinc adsorption: A mechanistic study. Ecol. Eng. 2016, 91, 459–471. [Google Scholar] [CrossRef]
- Cinar, S.; Kaynar, U.H.; Aydemir, T.; Kaynar, S.C.; Ayvacikli, M. An efficient removal of RB5 from aqueous solution by adsorption onto nano-ZnO/chitosan composite beads. Int. J. Biol. Macromol. 2017, 96, 459–465. [Google Scholar] [CrossRef]
- Hamzeh, Y.; Ashori, A.; Azadeh, E.; Abdulkhani, A. Removal of acid orange 7 and remazol black 5 reactive dyes from aqueous solutions using a novel biosorbent. Mater. Sci. Eng. 2012, C32, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Saha, P. Sea shell powder as a new adsorbent to remove basic green 4 (malachite green) from aqueous solutions: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2010, 164, 168–177. [Google Scholar] [CrossRef]
- Nadeem, R.; Manzoor, Q.; Iqbal, M.; Nisar, J. Biosorption of Pb (II) onto immobilized and native mangifera indica waste biomass. J. Ind. Eng. Chem. 2016, 35, 184–195. [Google Scholar] [CrossRef]
- Trivedi, H.C.; Patel, V.M.; Patel, R.D. Adsorption of cellulose triacetate on calcium silicate. Eur. Polym. J. 1973, 9, 525–531. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Proceed. Am. Soc. Civil. Eng. 1963, 89, 31–59. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Ong, S.T.; Lee, C.K.; Zainal, Z. Removal of basic and reactive dyes using ethylenediamine modified rice hull. Bioresour. Technol. 2007, 98, 2792–2799. [Google Scholar] [CrossRef]
- Parab, H.; Sudersanan, M.; Shenoy, N.; Pathare, T.; Vaze, B. Use of agroindustrial wastes for removal of basic dyes from aqueous solutions. Clean (Weinh) 2009, 37, 963–969. [Google Scholar]
- Pavan, F.A.; Camacho, E.S.; Lima, E.C.; Dotto, G.L.; Branco, V.T.A.; Dias, S.L.P. Formosa papaya seed powder (FPSP): Preparation, characterization and application as an alternative adsorbent for the removal of crystal violet from aqueous phase. J. Environ. Chem. Eng. 2014, 2, 230–238. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Ahmadi, M. Adsorption and kinetic studies of seven different organic dyes onto magnetite nanoparticles loaded tea waste and removal of them from wastewater samples. Spectrochim. Acta A 2012, 99, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Shuy, W.Z.; Ang, H.M.; Wang, S. Preparation of bioadsorbents foreffective adsorption of a reactive dye in aqueous solution. Asia Pac. J. Chem. Eng. 2010, 5, 563–569. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Ramirez-Montoya, L.A.; Bonilla-Petriciolet, A.; Hernandez-Montoya, V.; Montes-Moran, M.A.; Ramirez-Lopez, E.M. Sorption mechanism of anionic dyes on pecannut shells (Carya illinoinensis) using batch and continuous systems. Ind. Crops. Prod. 2013, 48, 89–97. [Google Scholar] [CrossRef]
- Tanyildizi, M.S. Modeling of adsorption isotherms and kinetics of reactive dye from aqueous solution by peanut hull. Chem. Eng. J. 2011, 168, 1234–1240. [Google Scholar] [CrossRef]
- Fakhri, A. Application of response surface methodology to optimize the process variables for fluoride ion removal using maghemite nanoparticles. J. Saudi Chem. Soc. 2014, 18, 340–347. [Google Scholar] [CrossRef]
- Mourabet, M.; Rhilassi, A.E.; Boujaady, H.E.; Bennani-Ziatni, M.; Hamri, R.E.; Taitai, A. Removal of fluoride from aqueous solution by adsorption on hydroxyapatite (HAp) using response surface methodology. J. Saudi Chem. Soc. 2015, 19, 603–615. [Google Scholar] [CrossRef]
- Zhao, B.; Xiao, W.; Shang, Y.; Zhu, H.; Han, R. Adsorption of light green anionic dye using cationic surfactant-modified peanut husk in batch mode. Arab. J. Chem. 2017, 10, 3595–3602. [Google Scholar] [CrossRef]


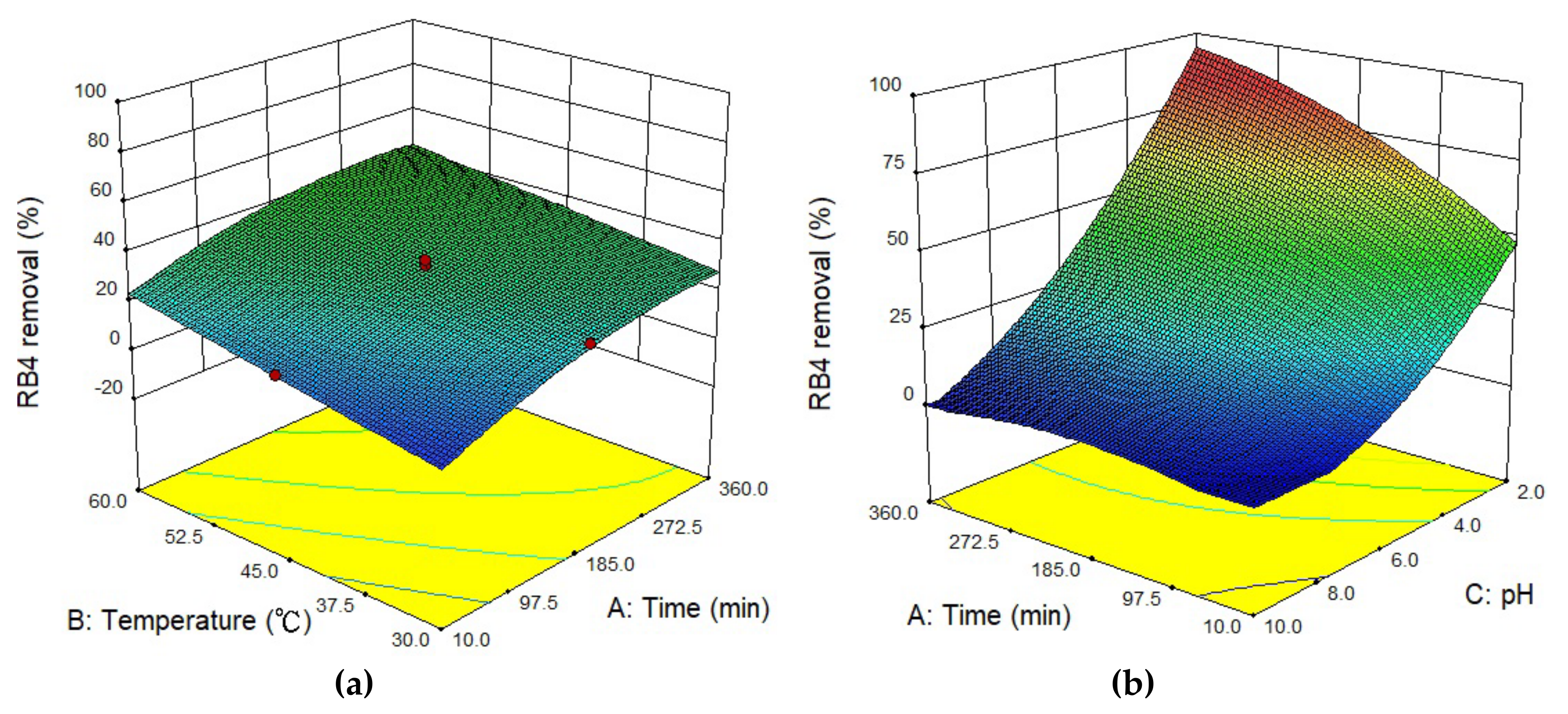

| Run | Independent Variables | Responses (RB4 Removal) (%) | Remark | |||
|---|---|---|---|---|---|---|
| A: Contact Times | B: Temperature | C: pH | Predicted | Experimental | ||
| 1 | 185 (0) | 60 (1.68) | 6 (0) | 40.95 | 36.36 | axial point |
| 2 | 360 (1.68) | 45 (0) | 6 (0) | 36.72 | 33.58 | axial point |
| 3 | 81 (−1) | 54 (1) | 3.6 (−1) | 56.24 | 56.33 | level point |
| 4 | 185 (0) | 30 (−1.68) | 6 (0) | 21.87 | 23.66 | axial point |
| 5 | 290 (1) | 54 (1) | 3.6 (−1) | 77.48 | 80.28 | level point |
| 6 | 185 (0) | 45 (0) | 10 (1.68) | 3.68 | 0.13 | axial point |
| 7 | 185 (0) | 45 (0) | 2 (−1.68) | 84.61 | 85.41 | axial point |
| 8 | 185 (0) | 45 (0) | 6 (0) | 31.41 | 30.21 | center point |
| 9 | 185 (0) | 45 (0) | 6 (0) | 31.41 | 35.32 | center point |
| 10 | 290 (1) | 54 (1) | 8.4 (1) | 14.38 | 17.7 | level point |
| 11 | 185 (0) | 45 (0) | 6 (0) | 31.41 | 30.01 | center point |
| 12 | 81 (−1) | 36 (−1) | 8.4 (1) | 3.82 | 3.00 | level point |
| 13 | 290 (1) | 36 (−1) | 3.6 (−1) | 59.96 | 58.82 | level point |
| 14 | 10 (−1.68) | 45 (0) | 6 (0) | 13.00 | 13.33 | axial point |
| 15 | 81 (−1) | 54 (1) | 8.4 (1) | 9.02 | 12.14 | level point |
| 16 | 185 (0) | 45 (0) | 6 (0) | 31.41 | 32.79 | center point |
| 17 | 81 (−1) | 36 (−1) | 3.6 (−1) | 37.08 | 35.75 | level point |
| 18 | 185 (0) | 45 (0) | 6 (0) | 31.41 | 31.4 | center point |
| 19 | 290 (1) | 36 (−1) | 8.4 (1) | 10.82 | 12.73 | level point |
| 20 | 185 (0) | 45 (0) | 6 (0) | 31.41 | 29.21 | center point |
| Sample | BET Surface Area (m2/g) | Average Pore Volume (cm3/g) |
|---|---|---|
| rice bran | 0.588 | 0.0058 |
| rice bran/SnO2 | 0.699 | 0.0028 |
| rice bran/SnO2/Fe3O4 | 0.746 | 0.0028 |
| Dye | RB4 | CV |
|---|---|---|
| qexp (mg/g) | 123.58 | 125.71 |
| PFOM | ||
| k1 (1/min) | 0.0089 | 18.8 |
| qe (mg/g) | 59.1 | 0.007 |
| R2 | 0.888 | 0.487 |
| PSOM | ||
| k2 (g/mg·g) | 6.62 × 10−4 | 4.99 × 10−3 |
| qe (mg/g) | 127.55 | 126.58 |
| R2 | 0.999 | 0.999 |
| IPDM (stage 1) | ||
| kid (g/mg·min0.5) | 17.41 | 26.09 |
| C | 0.2966 | 4.3046 |
| R2 | 0.999 | 0.909 |
| IPDM (stage 2) | ||
| kid (g/mg·min0.5) | 4.74 | 1.92 |
| C | 62.46 | 106.96 |
| R2 | 0.976 | 0.972 |
| Dye | RB4 | CV | ||||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 30 | 40 | 50 | 60 | 30 | 40 | 50 | 60 |
| Langmuir | ||||||||
| qm (mg/g) | 200.40 | 204.08 | 217.39 | 218.82 | 138.31 | 138.50 | 159.24 | 139.28 |
| KL (L/g) | 0.027 | 0.037 | 0.048 | 0.054 | 0.117 | 0.103 | 0.567 | 0.103 |
| R2 | 0.988 | 0.992 | 0.997 | 0.997 | 0.993 | 0.992 | 0.993 | 0.990 |
| Freundlich | ||||||||
| KF (mg/g) (L/mg)1/n | 53.48 | 64.37 | 74.11 | 77.43 | 71.78 | 73.79 | 77.80 | 45.81 |
| n | 4.74 | 5.34 | 5.57 | 5.67 | 5.78 | 5.81 | 5.65 | 3.52 |
| R2 | 0.974 | 0.965 | 0.998 | 0.992 | 0.859 | 0.917 | 0.971 | 0.968 |
| Adsorbent | Dye | Concentration (mg/L) | pH | Adsorbent Dose (g/L) | T (K) | qm (mg/g) | Reference |
|---|---|---|---|---|---|---|---|
| rice husk | CV | 50 | 8 | 1 | 303 | 44.87 | [10] |
| Sawdust | CV | 100 | - | 4 | 300 | 37.83 | [49] |
| papaya seed | CV | 50 | 8 | 12 | 298 | 85.99 | [50] |
| tea waste | CV | 20 | 10 | 1 | 298 | 113.64 | [51] |
| rice bran/SnO2/Fe3O4 | CV | 200 | 9 | 1.5 | 323 | 159.24 | This study |
| Barley straw | RB4 | 100 | 3 | 2 | 298 | 31.5 | [52] |
| Pecan shells | RB4 | 1000 | 6.5 | 10 | 303 | 5.0 | [53] |
| peanut hull | RB4 | 50–350 | 0.5 | 1 | 333 | 55.55 | [54] |
| rice bran | RB4 | 200 | 2 | 1.5 | 323 | 78.0 | This study |
| rice bran/Fe3O4 | RB4 | 200 | 2 | 1.5 | 303 | 185.19 | Prior study [12] |
| rice bran/SnO2/Fe3O4 | RB4 | 200 | 2 | 1.5 | 333 | 218.82 | This study |
| Source | RB4 Removal (%) | |||||
|---|---|---|---|---|---|---|
| SS | DF | MS | F Value | p-Value | ||
| Model | 9677.16 | 9 | 1075.24 | 98.84 | <0.0001 | significant |
| A | 679.99 | 1 | 679.99 | 62.50 | <0.0001 | significant |
| B | 439.90 | 1 | 439.90 | 40.44 | <0.0001 | significant |
| C | 7927.37 | 1 | 7927.37 | 728.69 | <0.0001 | significant |
| AB | 1.35 | 1 | 1.35 | 0.12 | 0.7317 | not significant |
| AC | 125.85 | 1 | 125.85 | 11.57 | 0.0068 | significant |
| BC | 97.51 | 1 | 97.51 | 8.96 | 0.0135 | significant |
| A2 | 77.40 | 1 | 77.40 | 7.11 | 0.0236 | significant |
| B2 | 7.831× 10−9 | 1 | 7.831× 10−9 | 7.198 × 10−10 | 1.0000 | not significant |
| C2 | 293.30 | 1 | 293.30 | 26.96 | 0.0004 | significant |
| Residual | 108.79 | 10 | 10.88 | |||
| Lack of Fit | 83.40 | 5 | 16.68 | 3.28 | 0.1089 | not significant |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.M.; Hong, G.B.; Wang, Y.K. Performance Evaluation and Optimization of Dyes Removal using Rice Bran-Based Magnetic Composite Adsorbent. Materials 2020, 13, 2764. https://doi.org/10.3390/ma13122764
Ma CM, Hong GB, Wang YK. Performance Evaluation and Optimization of Dyes Removal using Rice Bran-Based Magnetic Composite Adsorbent. Materials. 2020; 13(12):2764. https://doi.org/10.3390/ma13122764
Chicago/Turabian StyleMa, Chih Ming, Gui Bing Hong, and Yi Kai Wang. 2020. "Performance Evaluation and Optimization of Dyes Removal using Rice Bran-Based Magnetic Composite Adsorbent" Materials 13, no. 12: 2764. https://doi.org/10.3390/ma13122764
APA StyleMa, C. M., Hong, G. B., & Wang, Y. K. (2020). Performance Evaluation and Optimization of Dyes Removal using Rice Bran-Based Magnetic Composite Adsorbent. Materials, 13(12), 2764. https://doi.org/10.3390/ma13122764





