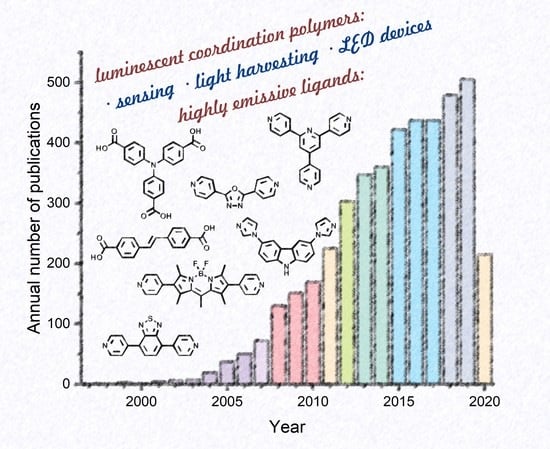
Coordination Polymers Based on Highly Emissive Ligands: Synthesis and Functional Properties
Abstract
1. Introduction
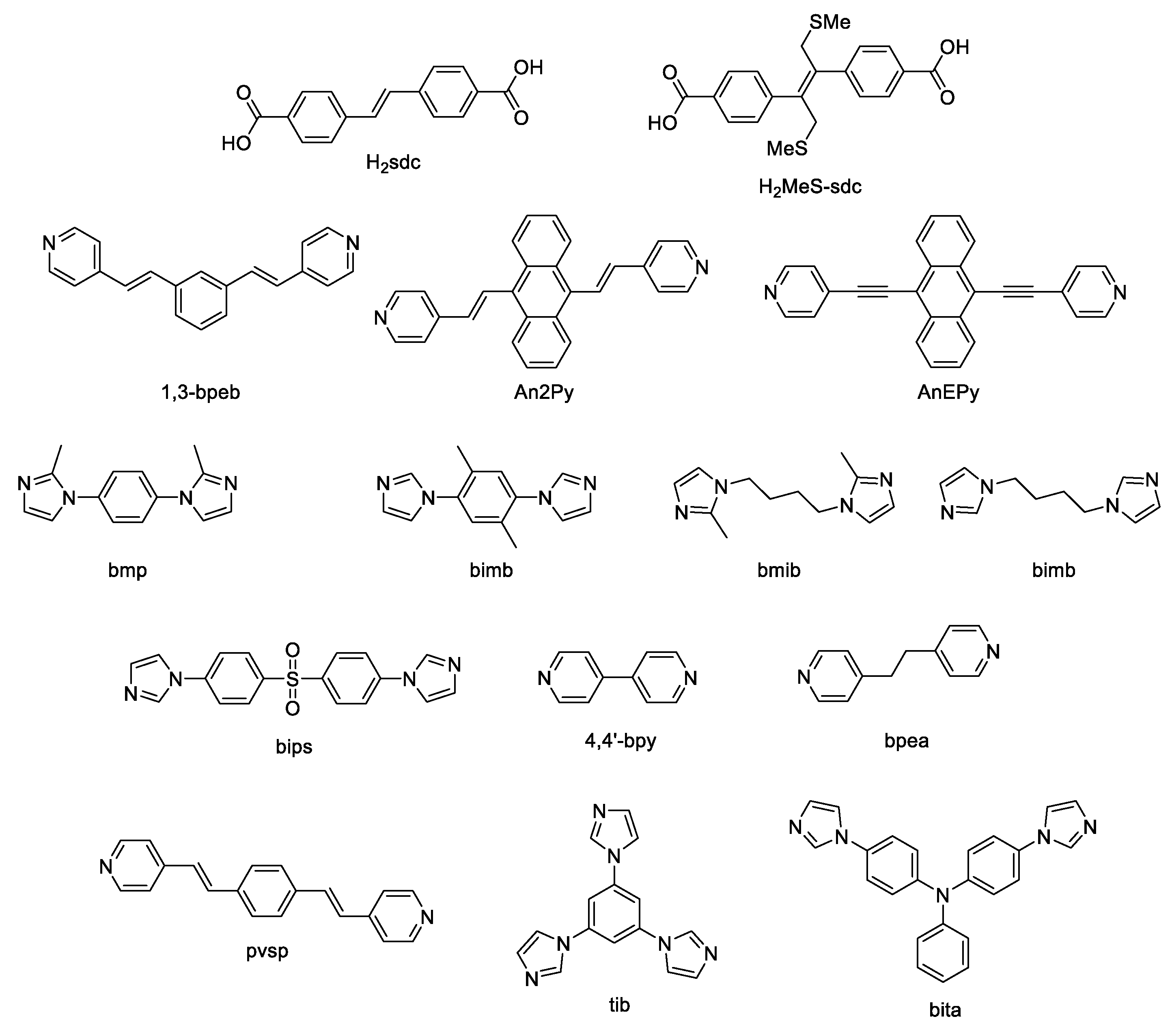
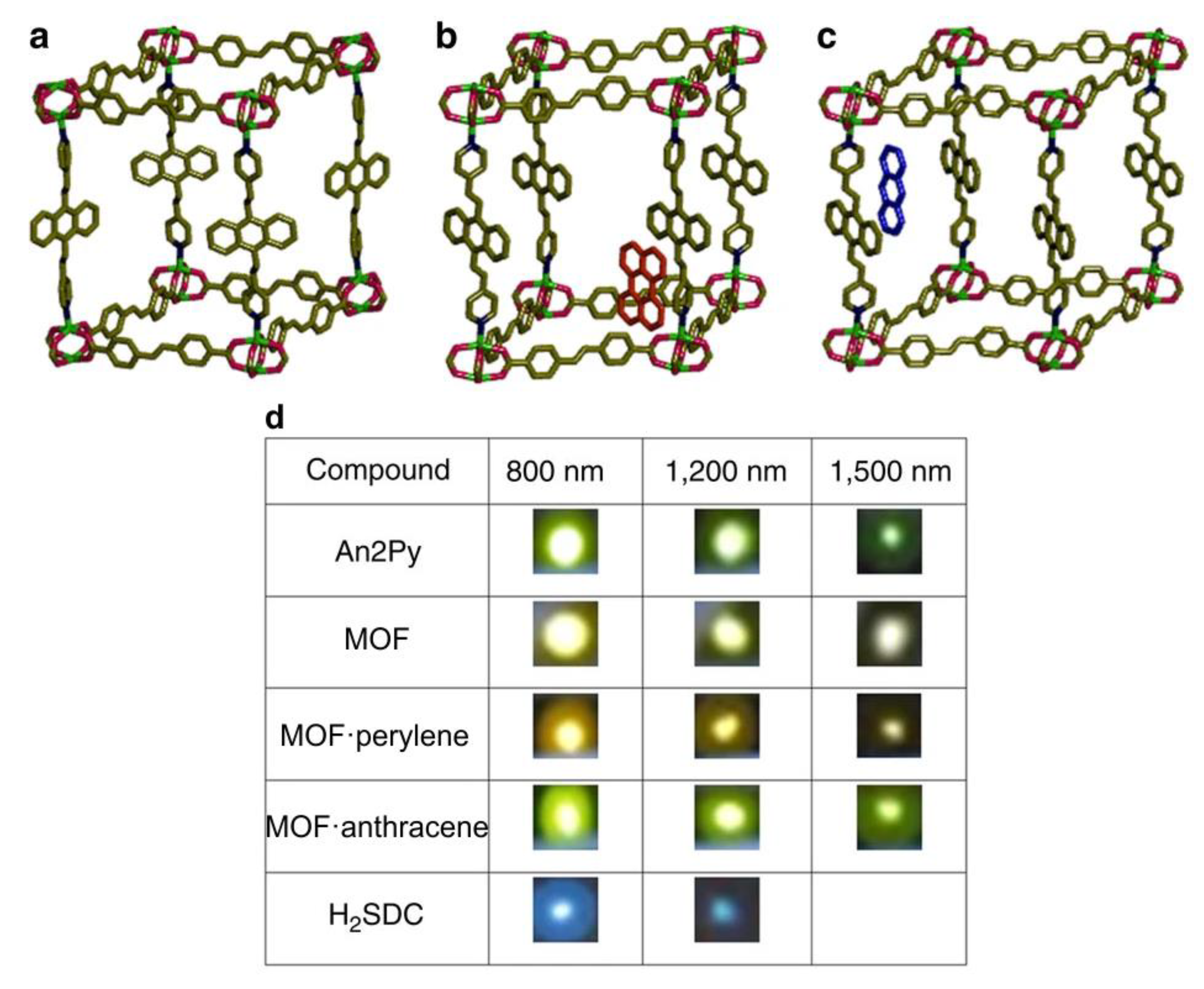
2. Coordination Polymers Based on 4,4′-Stilbenedicarboxylic Acid
3. Coordination Polymers Based on 1,3,4-Oxadiazole Derivatives
4. Coordination Polymers Based on Sulfur Heterocyclic Derivatives
4.1. Thiazole Derivatives
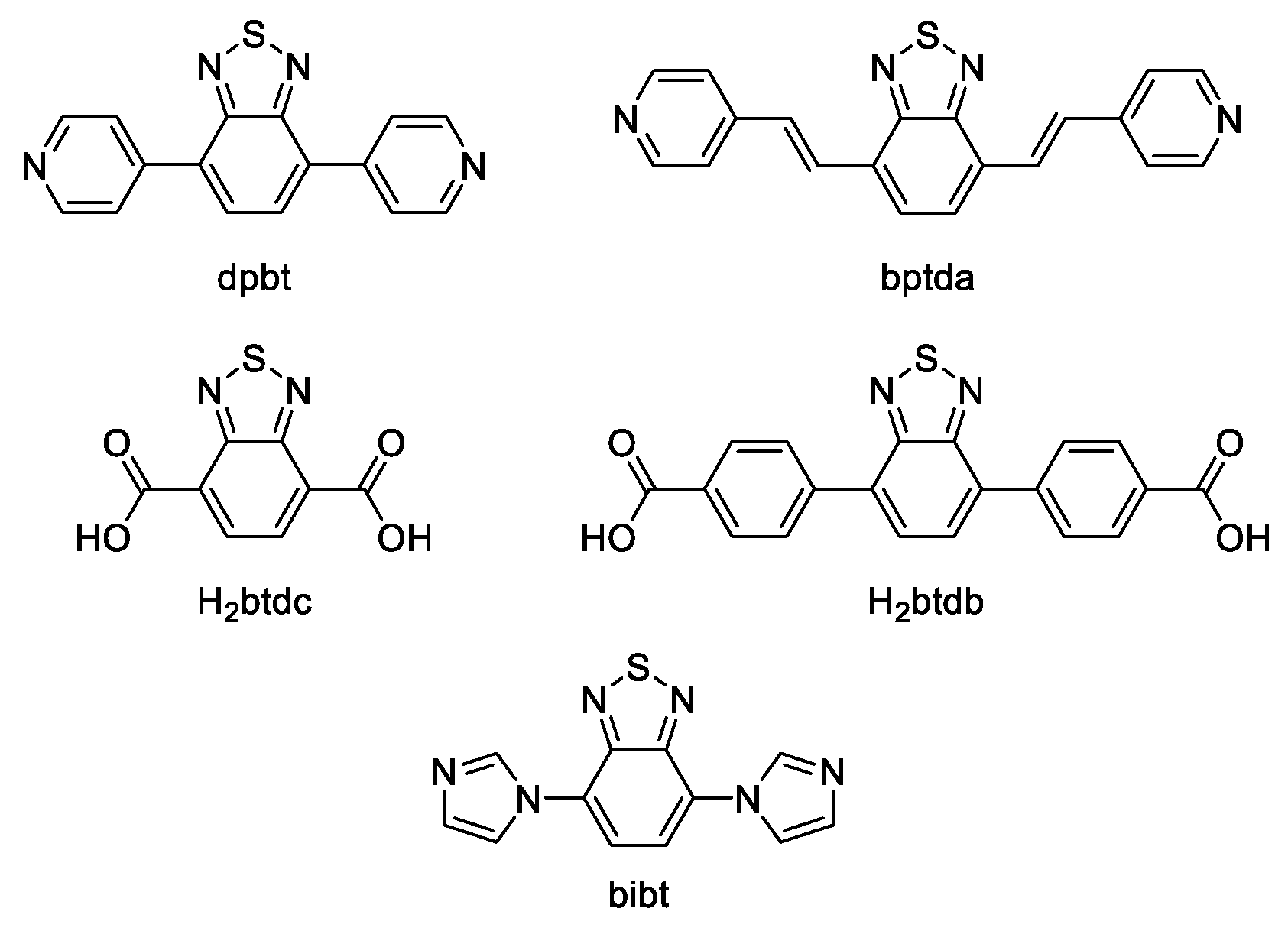
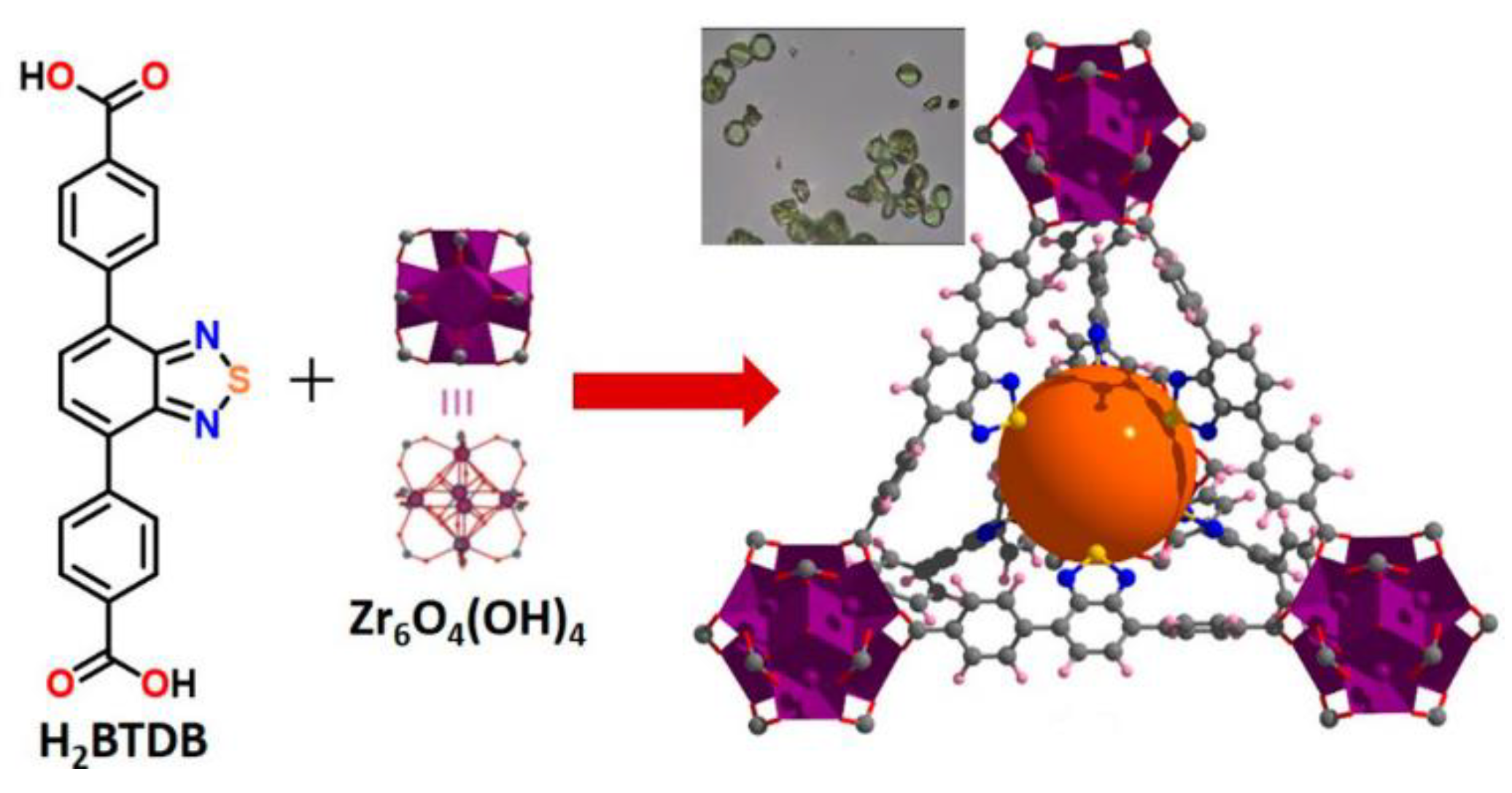
4.2. Derivatives of 2,1,3-Benzothiadiazole
5. Coordination Polymers Based on Nitrogen Heterocycles
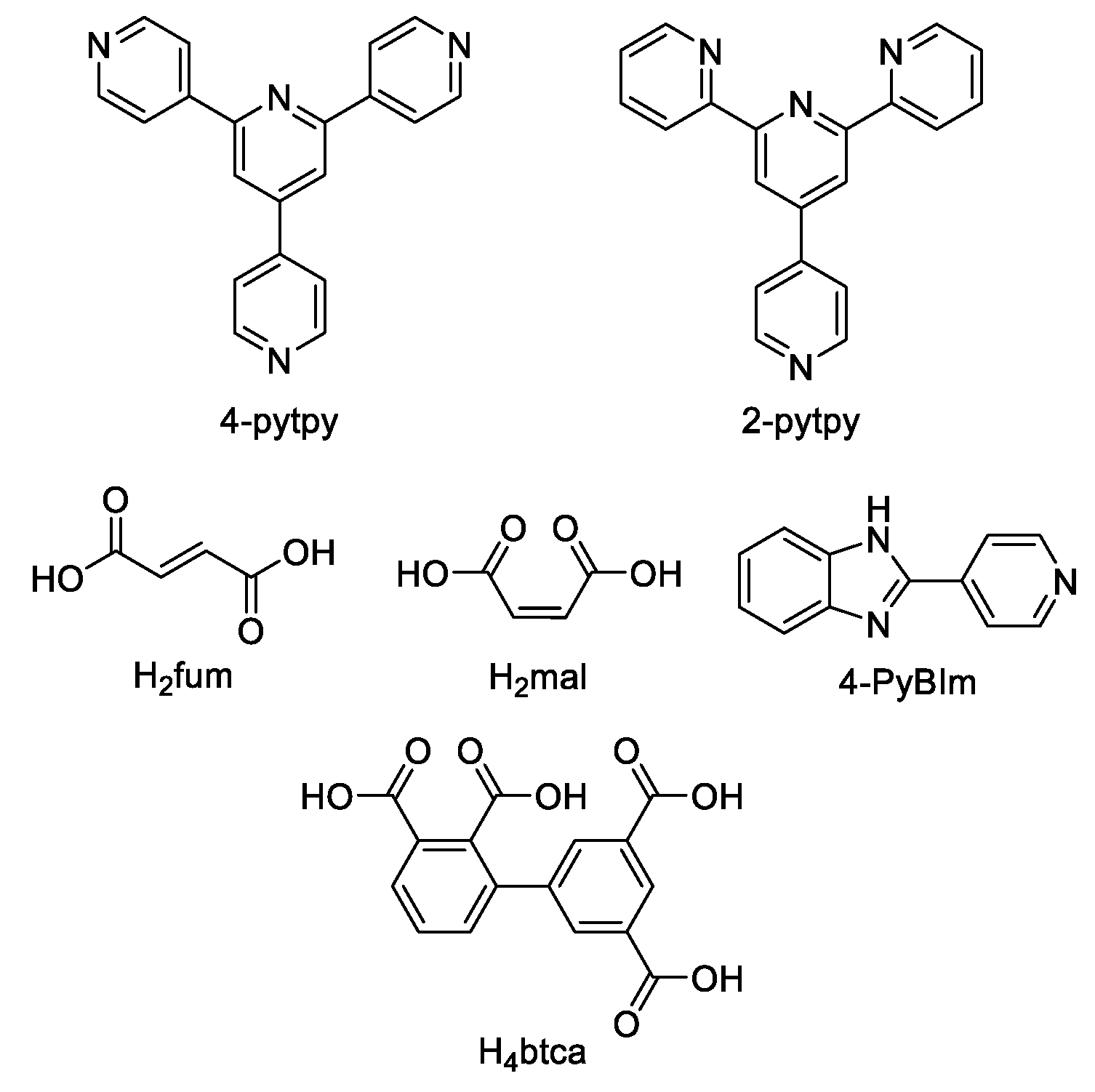
5.1. Terpyridine Derivatives
5.2. Carbazole Derivatives
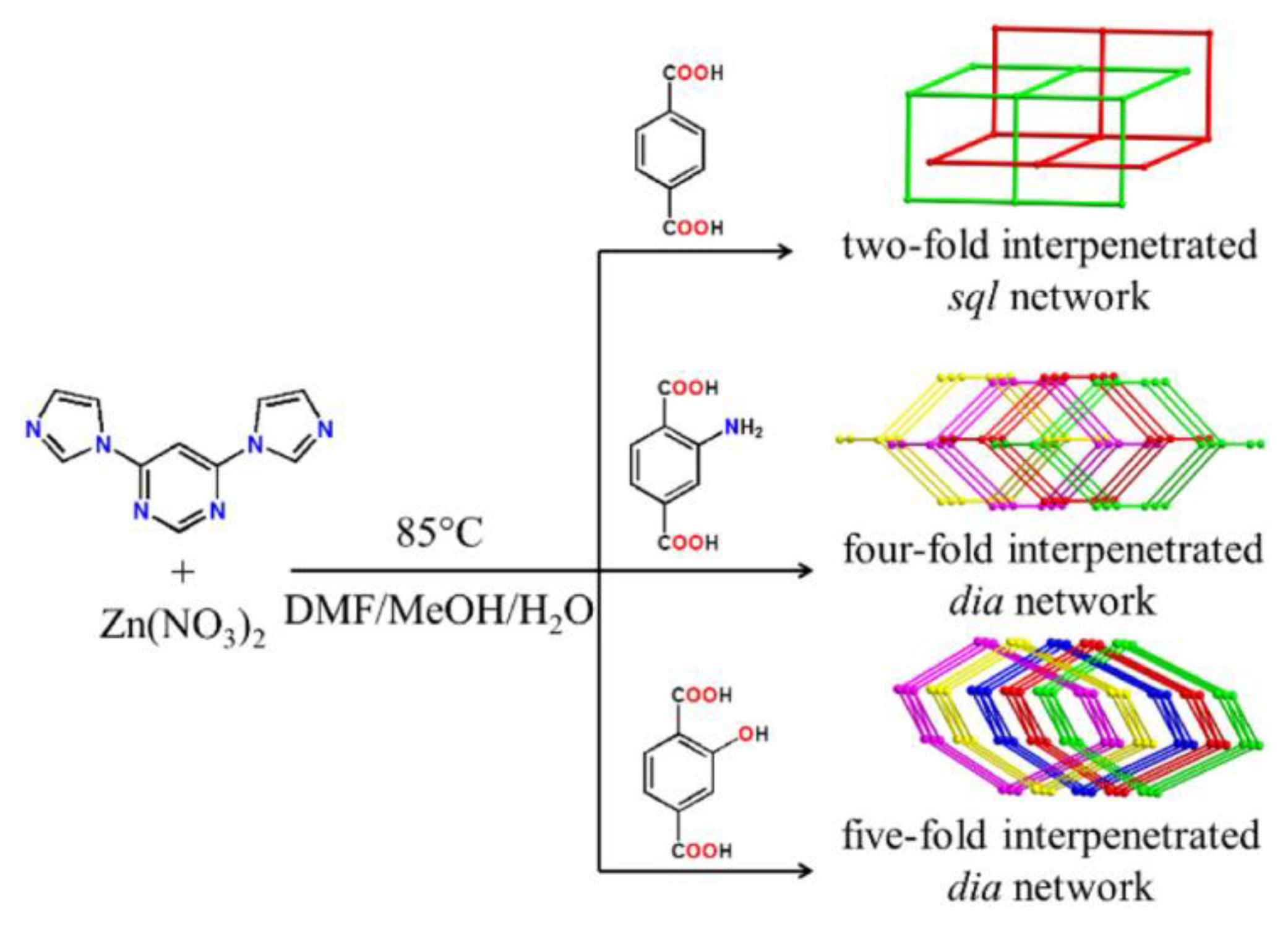
5.3. Bis(imidazol-1-yl)arenes
5.4. Bis(1,2,4-triazol-1-yl)arenes
5.5. Benzimidazole Derivatives
5.6. Other N-heterocyclic Derivatives
6. Ligands with BODIPY-Type Fluorophores
7. Naphthalene Diimide Derivatives
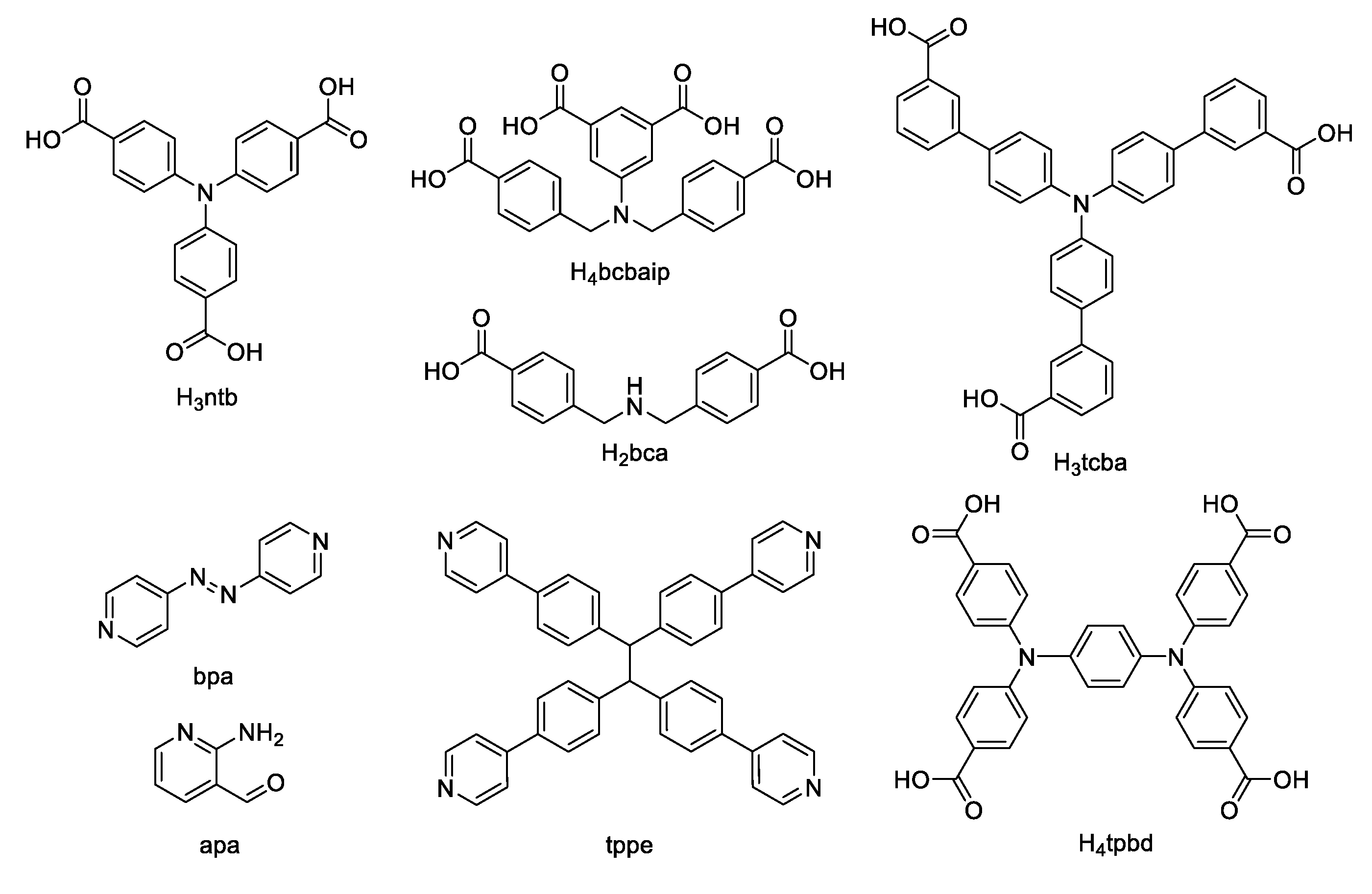
8. Derivatives of 4,4′,4′′-Nitrilotribenzoic Acid
9. Ligands Based on Highly Emissive Ruthenium(II) and Iridium(III) Complexes
10. Porphyrin Derivatives
10.1. Tetrakis(4-Carboxyphenyl)porphyrin
10.2. Other Porphyrin Derivatives with the Carboxylic Groups
10.3. Porphyrins Functionalized with Nitrogen-Containing Heterocycles
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Öhrström, L. Let’s talk about MOFs—Topology and terminology of metal-organic frameworks and why we need them. Crystals 2015, 5, 154–162. [Google Scholar] [CrossRef]
- Kraft, A. On the Discovery and History of Prussian Blue. Bull. Hist. Chem. 2008, 32, 61–67. [Google Scholar]
- Keggin, J.F.; Miles, F.D. Structures and formulæ of the prussian blues and related compounds. Nature 1936, 137, 577–578. [Google Scholar] [CrossRef]
- Grandjean, F.; Samain, L.; Long, G.J. Characterization and utilization of Prussian blue and its pigments. Dalt. Trans. 2016, 45, 18018–18044. [Google Scholar] [CrossRef] [PubMed]
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Barsukova, M.O.; Sapchenko, S.A.; Dybtsev, D.N.; Fedin, V.P. Scandium-organic frameworks: Progress and prospects. Russ. Chem. Rev. 2018, 87, 1139–1167. [Google Scholar] [CrossRef]
- Peh, S.B.; Karmakar, A.; Zhao, D. Multiscale Design of Flexible Metal-Organic Frameworks. Trends Chem. 2020, 2, 199–213. [Google Scholar] [CrossRef]
- Gu, J.; Wen, M.; Liang, X.; Shi, Z.; Kirillova, M.; Kirillov, A. Multifunctional Aromatic Carboxylic Acids as Versatile Building Blocks for Hydrothermal Design of Coordination Polymers. Crystals 2018, 8, 83. [Google Scholar] [CrossRef]
- Griffin, S.L.; Champness, N.R. A periodic table of metal-organic frameworks. Coord. Chem. Rev. 2020, 414, 213295. [Google Scholar] [CrossRef]
- Ali Akbar Razavi, S.; Morsali, A. Linker functionalized metal-organic frameworks. Coord. Chem. Rev. 2019, 399, 213023. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Mosca, N.; Tosi, G.; Drozdov, A. Application of metal—Organic frameworks. Polym. Int. 2017, 66, 731–744. [Google Scholar] [CrossRef]
- Tsivadze, A.Y.; Aksyutin, O.E.; Ishkov, A.G.; Knyazeva, M.K.; Solovtsova, O.V.; Men’shchikov, I.E.; Fomkin, A.A.; Shkolin, A.V.; Khozina, E.V.; Grachev, V.A. Metal-organic framework structures: Adsorbents for natural gas storage. Russ. Chem. Rev. 2019, 88, 925–978. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Lin, R.-B.; Xiang, S.; Zhou, W.; Chen, B. Microporous Metal-Organic Framework Materials for Gas Separation. Chem 2020, 6, 337–363. [Google Scholar] [CrossRef]
- Barnett, B.R.; Gonzalez, M.I.; Long, J.R. Recent Progress Towards Light Hydrocarbon Separations Using Metal–Organic Frameworks. Trends Chem. 2019, 1, 159–171. [Google Scholar] [CrossRef]
- Iacomi, P.; Formalik, F.; Marreiros, J.; Shang, J.; Rogacka, J.; Mohmeyer, A.; Behrens, P.; Ameloot, R.; Kuchta, B.; Llewellyn, P.L. Role of structural defects in the adsorption and separation of C3 hydrocarbons in Zr-fumarate-MOF (MOF-801). Chem. Mater. 2019, 31, 8413–8423. [Google Scholar] [CrossRef]
- Bao, Z.; Chang, G.; Xing, H.; Krishna, R.; Ren, Q.; Chen, B. Potential of microporous metal-organic frameworks for separation of hydrocarbon mixtures. Energy Env. Sci. 2016, 9, 3612–3641. [Google Scholar] [CrossRef]
- Mukherjee, S.; Desai, A.V.; Ghosh, S.K. Potential of metal–organic frameworks for adsorptive separation of industrially and environmentally relevant liquid mixtures. Coord. Chem. Rev. 2018, 367, 82–126. [Google Scholar] [CrossRef]
- Pei, J.; Shao, K.; Zhang, L.; Wen, H.-M.; Li, B.; Qian, G. Current Status of Microporous Metal–Organic Frameworks for Hydrocarbon Separations. Top. Curr. Chem. 2019, 377, 33. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Yuan, X.; Zhang, J.; Chew, J.W. Metal-organic framework membranes for wastewater treatment and water regeneration. Coord. Chem. Rev. 2020, 404, 213116. [Google Scholar] [CrossRef]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Xu, J.; Bu, X.-H. Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2019, 378, 17–31. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Lu, Y.; Chen, K.; Zhao, Y.; Wang, P.; Sun, W.-Y. Metal–organic frameworks with catalytic centers: From synthesis to catalytic application. Coord. Chem. Rev. 2019, 378, 262–280. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.-B.; Cao, R. Metal–organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates. Coord. Chem. Rev. 2019, 378, 32–65. [Google Scholar] [CrossRef]
- Aljammal, N.; Jabbour, C.; Thybaut, J.W.; Demeestere, K.; Verpoort, F.; Heynderickx, P.M. Metal-organic frameworks as catalysts for sugar conversion into platform chemicals: State-of-the-art and prospects. Coord. Chem. Rev. 2019, 401, 213064. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Zhou, H.-C. Synthesis of MOFs for heterogeneous catalysis via linker design. Polyhedron 2018, 154, 189–201. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; García, H. Metal–Organic Frameworks as Multifunctional Solid Catalysts. Trends Chem. 2020, 2, 454–466. [Google Scholar] [CrossRef]
- Wang, D.-G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404, 213093. [Google Scholar] [CrossRef]
- Xie, X.-X.; Yang, Y.-C.; Dou, B.-H.; Li, Z.-F.; Li, G. Proton conductive carboxylate-based metal–organic frameworks. Coord. Chem. Rev. 2020, 403, 213100. [Google Scholar] [CrossRef]
- Potapov, A.S.; Nudnova, E.A.; Khlebnikov, A.I.; Ogorodnikov, V.D.; Petrenko, T.V. Synthesis, crystal structure and electrocatalytic activity of discrete and polymeric copper(II) complexes with bitopic bis(pyrazol-1-yl) methane ligands. Inorg. Chem. Commun. 2015, 53, 72–75. [Google Scholar] [CrossRef]
- Kempahanumakkagari, S.; Vellingiri, K.; Deep, A.; Kwon, E.E.; Bolan, N.; Kim, K.-H. Metal–organic framework composites as electrocatalysts for electrochemical sensing applications. Coord. Chem. Rev. 2018, 357, 105–129. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.-L.; Li, D. Biological metal–organic frameworks: Structures, host–guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Cui, J.; Ren, S.; Sun, B.; Jia, S. Optimization protocols and improved strategies for metal-organic frameworks for immobilizing enzymes: Current development and future challenges. Coord. Chem. Rev. 2018, 370, 22–41. [Google Scholar] [CrossRef]
- Chowdhury, M.A. Metal-organic-frameworks for biomedical applications in drug delivery, and as MRI contrast agents. J. Biomed. Mater. Res. Part A 2017, 105, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Young, A.P.; Tsung, C.K. Integration of Biomolecules with Metal–Organic Frameworks. Small 2017, 13, 1–14. [Google Scholar] [CrossRef]
- Marchenko, R.D.; Lysova, A.A.; Samsonenko, D.G.; Dybtsev, D.N.; Potapov, A.S. Synthesis, structural diversity, luminescent properties and antibacterial effects of cadmium(II) and silver(I) coordination compounds with bis(1,2,3-benzotriazol-1-yl)alkanes. Polyhedron 2020, 177, 114330. [Google Scholar] [CrossRef]
- Banerjee, S.; Lollar, C.T.; Xiao, Z.; Fang, Y.; Zhou, H.-C. Biomedical Integration of Metal–Organic Frameworks. Trends Chem. 2020, 2, 467–479. [Google Scholar] [CrossRef]
- Kaur, H.; Sundriyal, S.; Pachauri, V.; Ingebrandt, S.; Kim, K.-H.; Sharma, A.L.; Deep, A. Luminescent metal-organic frameworks and their composites: Potential future materials for organic light emitting displays. Coord. Chem. Rev. 2019, 401, 213077. [Google Scholar] [CrossRef]
- Zak, P.P.; Lapina, V.A.; Pavich, T.A.; Trofimov, A.V.; Trofimova, N.N.; Tsaplev, Y.B. Luminescent materials for modern light sources. Russ. Chem. Rev. 2017, 86, 831–844. [Google Scholar] [CrossRef]
- Ahmad, S.; Liu, J.; Ji, W.; Sun, L. Metal–Organic Framework Thin Film-Based Dye Sensitized Solar Cells with Enhanced Photocurrent. Materials 2018, 11, 1868. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Dhas, N.; Deshmukh, P.; Caro, C.; Patil, P.; Luisa García-Martín, M.; Padya, B.; Nikam, A.; Mehta, T.; Mutalik, S. Heterogeneous surface architectured metal-organic frameworks for cancer therapy, imaging, and biosensing: A state-of-the-art review. Coord. Chem. Rev. 2020, 409, 213212. [Google Scholar] [CrossRef]
- Liao, Z.; Xia, T.; Yu, E.; Cui, Y. Luminescent Metal–Organic Framework Thin Films: From Preparation to Biomedical Sensing Applications. Crystals 2018, 8, 338. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Ebrahim, F.M.; Stylianou, K.C. Photoluminescent, upconversion luminescent and nonlinear optical metal-organic frameworks: From fundamental photophysics to potential applications. Coord. Chem. Rev. 2018, 377, 259–306. [Google Scholar] [CrossRef]
- Medishetty, R.; Nalla, V.; Nemec, L.; Henke, S.; Mayer, D.; Sun, H.; Reuter, K.; Fischer, R.A. A New Class of Lasing Materials: Intrinsic Stimulated Emission from Nonlinear Optically Active Metal–Organic Frameworks. Adv. Mater. 2017, 29, 1605637. [Google Scholar] [CrossRef]
- Zaręba, J.K.; Nyk, M.; Janczak, J.; Samoć, M. Three-Photon Absorption of Coordination Polymer Transforms UV-to-VIS Thermometry into NIR-to-VIS Thermometry. ACS Appl. Mater. Interfaces 2019, 11, 10435–10441. [Google Scholar] [CrossRef]
- Medishetty, R.; Zaręba, J.K.; Mayer, D.; Samoć, M.; Fischer, R.A. Nonlinear optical properties, upconversion and lasing in metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 4976–5004. [Google Scholar] [CrossRef]
- Medishetty, R.; Nemec, L.; Nalla, V.; Henke, S.; Samoć, M.; Reuter, K.; Fischer, R.A. Multi-Photon Absorption in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2017, 56, 14743–14748. [Google Scholar] [CrossRef]
- Zaręba, J.K.; Białek, M.J.; Janczak, J.; Nyk, M.; Zoń, J.; Samoć, M. Beyond Single-Wavelength SHG Measurements: Spectrally-Resolved SHG Studies of Tetraphosphonate Ester Coordination Polymers. Inorg. Chem. 2015, 54, 10568–10575. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Morsali, A. Metal ion detection using luminescent-MOFs: Principles, strategies and roadmap. Coord. Chem. Rev. 2020, 415, 213299. [Google Scholar] [CrossRef]
- Pamei, M.; Puzari, A. Luminescent transition metal–organic frameworks: An emerging sensor for detecting biologically essential metal ions. Nano-Struct. Nano-Objects 2019, 19, 100364. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhao, Y.; Zhang, S.; Li, S.; Jia, L.; Du, L.; Zhao, Q. Three Novel Zn-Based Coordination Polymers: Synthesis, Structure, and Effective Detection of Al3+ and S2− Ions. Molecules 2020, 25, 382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, A.-S.; Zou, B.; Zhang, H.-X.; Yan, K.-L.; Lin, Y. Advances of metal–organic frameworks for gas sensing. Polyhedron 2018, 154, 83–97. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.-H.; Rarotra, S.; Ge, L.; Lisak, G. The advanced sensing systems for NOx based on metal-organic frameworks: Applications and future opportunities. Trac. Trends Anal. Chem. 2020, 122, 115730. [Google Scholar] [CrossRef]
- Li, Y. Temperature and humidity sensors based on luminescent metal-organic frameworks. Polyhedron 2020, 179, 114413. [Google Scholar] [CrossRef]
- Lin, R.-B.; Liu, S.-Y.; Ye, J.-W.; Li, X.-Y.; Zhang, J.-P. Photoluminescent Metal-Organic Frameworks for Gas Sensing. Adv. Sci. 2016, 3, 1500434. [Google Scholar] [CrossRef]
- Kustov, L.M.; Isaeva, V.I.; Přech, J.; Bisht, K.K. Metal-organic frameworks as materials for applications in sensors. Mendeleev Commun. 2019, 29, 361–368. [Google Scholar] [CrossRef]
- Semitut, E.Y.; Sukhikh, T.S.; Filatov, E.Y.; Anosova, G.A.; Ryadun, A.A.; Kovalenko, K.A.; Potapov, A.S. Synthesis, Crystal Structure, and Luminescent Properties of Novel Zinc Metal–Organic Frameworks Based on 1,3-Bis(1,2,4-triazol-1-yl)propane. Cryst. Growth Des. 2017, 17, 5559–5567. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F. Luminescent metal-organic frameworks as potential sensory materials for various environmental toxic agents. Coord. Chem. Rev. 2019, 401, 213065. [Google Scholar] [CrossRef]
- Roales, J.; Moscoso, G.F.; Gámez, F.; Lopes-Costa, T.; Sousaraei, A.; Casado, S.; Castro-Smirnov, R.J.; Cabanillas-Gonzalez, J.; Almeida, J.; Queirós, C.; et al. Preparation of Luminescent Metal-Organic Framework Films by Soft-Imprinting for 2,4-Dinitrotoluene Sensing. Materials 2017, 10, 992. [Google Scholar] [CrossRef]
- Kukkar, D.; Vellingiri, K.; Kim, K.-H.; Deep, A. Recent progress in biological and chemical sensing by luminescent metal-organic frameworks. Sens. Actuators B Chem. 2018, 273, 1346–1370. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.-Y.; Cheng, C.; Shao, Z.-S.; Wang, H.-S. Strategies to fabricate metal–organic framework (MOF)-based luminescent sensing platforms. J. Mater. Chem. C 2019, 7, 10743–10763. [Google Scholar] [CrossRef]
- Wang, P.-L.; Xie, L.-H.; Joseph, E.A.; Li, J.-R.; Su, X.-O.; Zhou, H.-C. Metal–Organic Frameworks for Food Safety. Chem. Rev. 2019, 119, 10638–10690. [Google Scholar] [CrossRef] [PubMed]
- Petrochenkova, N.V.; Mirochnik, A.G.; Kulikov, A.P.; Karasev, V.E. Structure and fluorescence properties of coordination polymers of europium(III) with pyromellitic acid. Russ. J. Coord. Chem. Khimiya 1997, 23, 817–819. [Google Scholar]
- Hao, Y.; Chen, S.; Zhou, Y.; Zhang, Y.; Xu, M. Recent Progress in Metal–Organic Framework (MOF) Based Luminescent Chemodosimeters. Nanomaterials 2019, 9, 974. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H.C. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, Y.; Song, W.-J. Zeolitic imidazolate frameworks for use in electrochemical and optical chemical sensing and biosensing: A review. Microchim. Acta 2020, 187, 234. [Google Scholar] [CrossRef]
- Yao, C.-X.; Zhao, N.; Liu, J.-C.; Chen, L.-J.; Liu, J.-M.; Fang, G.-Z.; Wang, S. Recent progress on Luminescent Metal-Organic Framework-Involved Hybrid Materials for Rapid Determination of Contaminants in Environment and Food. Polymers 2020, 12, 691. [Google Scholar] [CrossRef]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Voort, P.; Zhao, S.-N.; Wang, G.; Poelman, D.; Voort, P. Van Der Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Luo, Z.-D.; Pan, Y.; Kumar Singh, A.; Trivedi, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Wang, K.; Ma, Y.; Tang, H. Lanthanide Coordination Polymers as Luminescent Sensors for the Selective and Recyclable Detection of Acetone. Crystals 2017, 7, 199. [Google Scholar] [CrossRef]
- Marakulin, A.V.; Lysova, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazarenko, V.A.; Dybtsev, D.N.; Fedin, V.P. New one-, two-, and three-dimensional metal-organic frameworks based on magnesium(II): Synthesis and structure. Russ. Chem. Bull. 2020, 69, 360–368. [Google Scholar] [CrossRef]
- Xiao, F.; Zhang, J.; Gan, J.; Tang, Y.; Cui, Y.; Yu, Y.; Qian, G. Controlled dye release from a metal–organic framework: A new luminescent sensor for water. RSC Adv. 2020, 10, 2722–2726. [Google Scholar] [CrossRef]
- Asha, P.; Mandal, S. Series of Mn(II)/Mg(II)/Zn(II) Coordination Polymers with Azo/Alkene Functionalized Ligands. Cryst. Growth Des. 2018, 18, 4937–4944. [Google Scholar]
- Bauer, C.A.; Timofeeva, T.V.; Settersten, T.B.; Patterson, B.D.; Liu, V.H.; Simmons, B.A.; Allendorf, M.D. Influence of connectivity and porosity on ligand-based luminescence in zinc metal-organic frameworks. J. Am. Chem. Soc. 2007, 129, 7136–7144. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.A.; Jones, S.C.; Kinnibrugh, T.L.; Tongwa, P.; Farrell, R.A.; Vakil, A.; Timofeeva, T.V.; Khrustalev, V.N.; Allendorf, M.D. Homo- and heterometallic luminescent 2-D stilbene metal-organic frameworks. Dalt. Trans. 2014, 43, 2925–2935. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Guo, Y.; Wang, Y.; Xuan, X. Ligand geometry-driven formation of Zn(II) MOFs based on 1,3-bis[2-(4-pyridyl)ethenyl] benzene and aromatic dicarboxylic acids. Inorg. Chim. Acta 2018, 471, 50–56. [Google Scholar] [CrossRef]
- Quah, H.S.; Chen, W.; Schreyer, M.K.; Yang, H.; Wong, M.W.; Ji, W.; Vittal, J.J. Multiphoton harvesting metal–organic frameworks. Nat. Commun. 2015, 6, 7954. [Google Scholar] [CrossRef]
- Quah, H.S.; Nalla, V.; Zheng, K.; Lee, C.A.; Liu, X.; Vittal, J.J. Tuning Two-Photon Absorption Cross Section in Metal Organic Frameworks. Chem. Mater. 2017, 29, 7424–7430. [Google Scholar] [CrossRef]
- Deng, X. Solvothermal Syntheses, Crystal Structures, and Luminescent Properties of Two Coordination Polymers with Different Interpenetrated Diamond Topology. Z. Anorg. Allg. Chem. 2015, 641, 1269–1273. [Google Scholar] [CrossRef]
- Deng, M.; Tai, S.; Zhang, W.; Wang, Y.; Zhu, J.; Zhang, J.; Ling, Y.; Zhou, Y. A self-catenated rob-type porous coordination polymer constructed from triazolate and carboxylate ligands: Fluorescence response to the reversible phase transformation. CrystEngComm 2015, 17, 6023–6029. [Google Scholar] [CrossRef]
- Fan, C.-B.; Meng, X.-M.; Fan, Y.-H.; Cui, L.-S.; Zhang, X.; Bi, C.-F. Synthesis, crystal structures and photoluminescent properties of two Cd(II)/Zn(II) coordination polymers with rarely 8-/2-fold interpenetrated based on 4,4′-stilbenedicarboxylic acid and imidazole ligands. J. Coord. Chem. 2017, 70, 734–745. [Google Scholar] [CrossRef]
- Fan, C.B.; Meng, X.M.; Fan, Y.H.; Zong, Z.A.; Zhang, X.Y.; Bi, C.F. Two 3D ZnII metal-organic frameworks with 3- and 8-fold interpenetration: Syntheses, structures, photodegradation, and photoluminescent properties. Aust. J. Chem. 2017, 70, 314–321. [Google Scholar] [CrossRef]
- Guo, H.; Yan, Y.; Wang, N.; Guo, X.; Zheng, G.; Qi, Y. A series of entangled coordination polymers assembled by a V-shaped bisimidazole ligand and various dicarboxylic acids: Synthesis, characterization and luminescence properties. CrystEngComm 2015, 17, 6512–6526. [Google Scholar] [CrossRef]
- Barsukova, M.O.; Sapchenko, S.A.; Kovalenko, K.A.; Samsonenko, D.G.; Potapov, A.S.; Dybtsev, D.N.; Fedin, V.P. Exploring the multifunctionality in metal-organic framework materials: How do the stilbenedicarboxylate and imidazolyl ligands tune the characteristics of coordination polymers? New J. Chem. 2018, 42, 6408–6415. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, Z.; Liu, W.; Zhou, L.; Chen, L.; Li, J. Two blue-light excitable yellow-emitting LMOF phosphors constructed by triangular tri(4-pyridylphenyl)amine. Dalt. Trans. 2017, 46, 956–961. [Google Scholar] [CrossRef]
- Cheng, A.L.; Ma, Y.; Sun, Q.; Gao, E.Q. Layered and pillar-layered metal-organic frameworks based on pinwheel trinuclear zinc-carboxylate clusters. CrystEngComm 2011, 13, 2721–2726. [Google Scholar] [CrossRef]
- Wang, H.Y.; Gao, S.; Huo, L.H.; Ng, S.W.; Zhao, J.G. Three interpenetrated frameworks assembly from a long multicarboxylate ligand and transition metal. Cryst. Growth Des. 2008, 8, 665–670. [Google Scholar] [CrossRef]
- Guo, X.; Yan, Y.; Guo, H.; Qi, Y.; Liu, C. Seven entangled coordination polymers assembled from triphenylamine-based bisimidazole and dicarboxylates: Interpenetration, self-penetration and mixed entanglement. CrystEngComm 2016, 18, 2546–2558. [Google Scholar] [CrossRef]
- Huang, K.L.; Liu, X.; Liang, G.M. Second-ligand-dependent multi-fold interpenetrated architectures of two 3-D metal(II)-organic coordination polymers. Inorg. Chim. Acta 2009, 362, 1565–1570. [Google Scholar] [CrossRef]
- Liu, S.; Guo, M.; Guo, H.; Sun, Y.; Guo, X. Structural diversity of three entangled metal-organic frameworks from a bisimidazole ligand and dicarboxylic acids with varied length. Inorg. Chem. Commun. 2017, 86, 187–191. [Google Scholar] [CrossRef]
- Huang, K.L.; He, Y.T.; Huang, M. [Cd(SDC)(H2O)]: A 3-D hybrid open framework with an interpenetrated (4,4) topology and double-strand helicates (SDC=4,4′-stilbenedicarboxylate). J. Coord. Chem. 2008, 61, 2735–2742. [Google Scholar] [CrossRef]
- Hu, Y.J.; Yang, J.; Liu, Y.Y.; Song, S.; Ma, J.F. A Family of Capsule-Based Coordination Polymers Constructed from a New Tetrakis(1,2,4-triazol-ylmethyl)resorcin[4]arene Cavitand and Varied Dicarboxylates for Selective Metal-Ion Exchange and Luminescent Properties. Cryst. Growth Des. 2015, 15, 3822–3831. [Google Scholar] [CrossRef]
- Xiao, Y.J.; Liu, F.H.; Zhao, L.; Su, Z.M. An unprecedented stable self-penetrating metal-organic framework with solvent-dependent luminescence properties. Inorg. Chem. Commun. 2015, 59, 32–35. [Google Scholar] [CrossRef]
- Wang, X.P.; Han, L.L.; Lin, S.J.; Li, X.Y.; Mei, K.; Sun, D. Synthesis, structure and photoluminescence of three 2D Cd(II) coordination polymers based on varied dicarboxylate ligand. J. Coord. Chem. 2016, 69, 286–294. [Google Scholar] [CrossRef]
- Wang, X.L.; Qin, C.; Wang, E.B.; Xu, L.; Su, Z.M.; Hu, C.W. Interlocked and interdigitated architectures from self-assembly of long flexible ligands and cadmium salts. Angew. Chem. Int. Ed. 2004, 43, 5036–5040. [Google Scholar] [CrossRef]
- Golafale, S.T.; Ingram, C.W.; Bacsa, J.; Steiner, A.; Solntsev, K.M. Synthesis, structure, and photoluminescence properties of lanthanide based metal organic frameworks and a cadmium coordination polymer derived from 2,2′-diamino-trans 4,4′-stilbenedicarboxylate. Inorg. Chim. Acta 2018, 478, 243–249. [Google Scholar] [CrossRef]
- Mathis, S.R.; Golafale, S.T.; Bacsa, J.; Steiner, A.; Ingram, C.W.; Doty, F.P.; Auden, E.; Hattar, K. Mesoporous stilbene-based lanthanide metal organic frameworks: Synthesis, photoluminescence and radioluminescence characteristics. Dalt. Trans. 2017, 46, 491–500. [Google Scholar] [CrossRef]
- Li, Y.; Song, D. Syntheses, structures and luminescent properties of decorated lanthanide metal-organic frameworks of (E)-4,4′-(ethene-1,2-diyl)dibenzoic acids. CrystEngComm 2011, 13, 1821–1830. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Yue, D.; Cui, Y.; Yang, Y.; Li, B.; Qian, G. Solvent-Triggered Reversible Phase Changes in Two Manganese-Based Metal-Organic Frameworks and Associated Sensing Events. Chem. A Eur. J. 2018, 24, 13231–13237. [Google Scholar] [CrossRef]
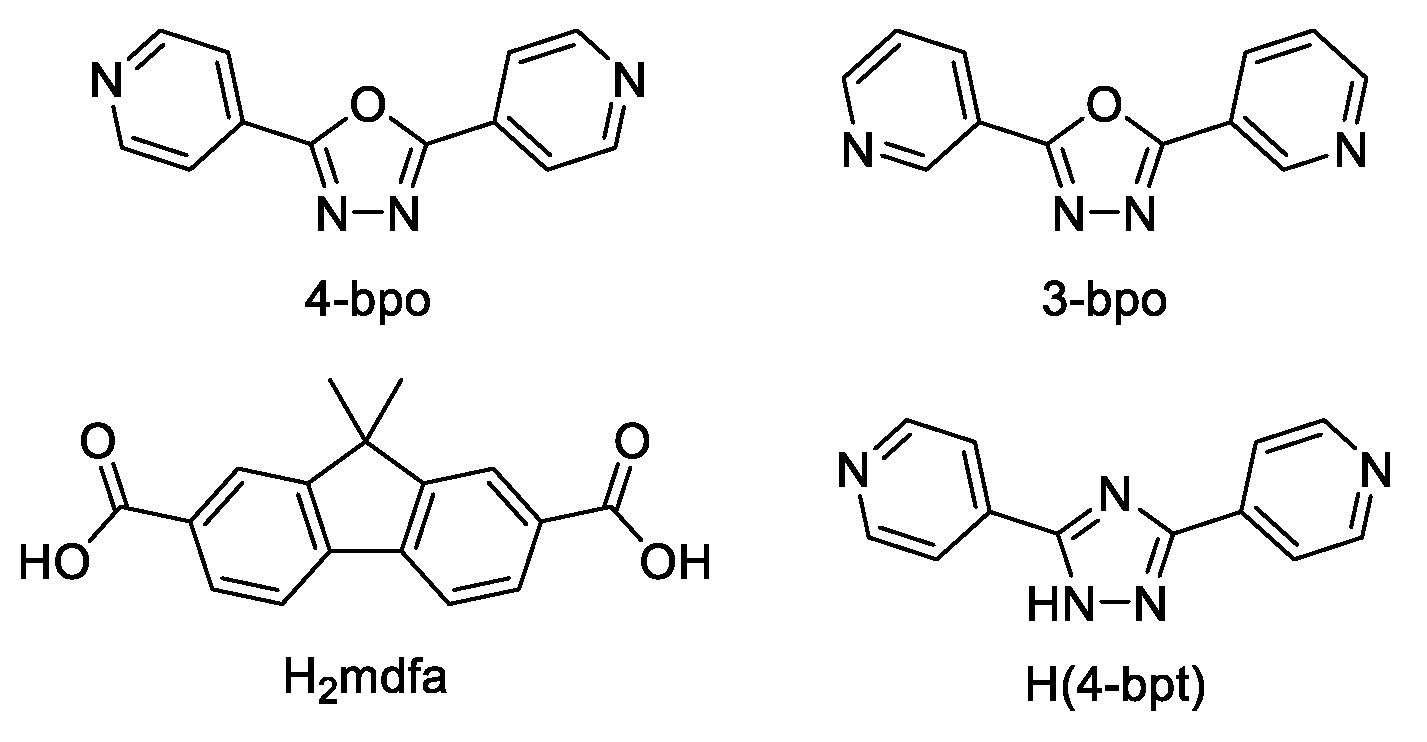
- Glomb, T.; Szymankiewicz, K.; Świątek, P. Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole. Molecules 2018, 23, 3361. [Google Scholar] [CrossRef] [PubMed]
- Paun, A.; Hadade, N.D.; Paraschivescu, C.C.; Matache, M. 1,3,4-Oxadiazoles as luminescent materials for organic light emitting diodes via cross-coupling reactions. J. Mater. Chem. C 2016, 4, 8596–8610. [Google Scholar] [CrossRef]
- Salassa, G.; Terenzi, A. Metal Complexes of Oxadiazole Ligands: An Overview. Int. J. Mol. Sci. 2019, 20, 3483. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.D.; Qiu, D.F.; Guo, X.M.; Zheng, G.L.; Wang, X.; Dang, S.; Zhang, H.J. Entangled metal-organic frameworks modulated by N-donor ligands of different conformations. CrystEngComm 2009, 11, 2425–2430. [Google Scholar] [CrossRef]
- Du, M.; Wang, Q.; Li, C.P.; Zhao, X.J.; Ribas, J. Coordination assemblies of CoII/CuII/Zn II/CdII with 2,5-bipyridyl-1,3,4-oxadiazole and dicyanamide anion: Structural diversification and properties. Cryst. Growth Des. 2010, 10, 3285–3296. [Google Scholar] [CrossRef]
- Li, C.P.; Chen, J.; Du, M. Structural diversification and metal-directed assembly of coordination architectures based on tetrabromoterephthalic acid and a bent dipyridyl tecton 2,5-bis(4-pyridyl)-1,3,4-oxadiazole. CrystEngComm 2010, 12, 4392–4402. [Google Scholar] [CrossRef]
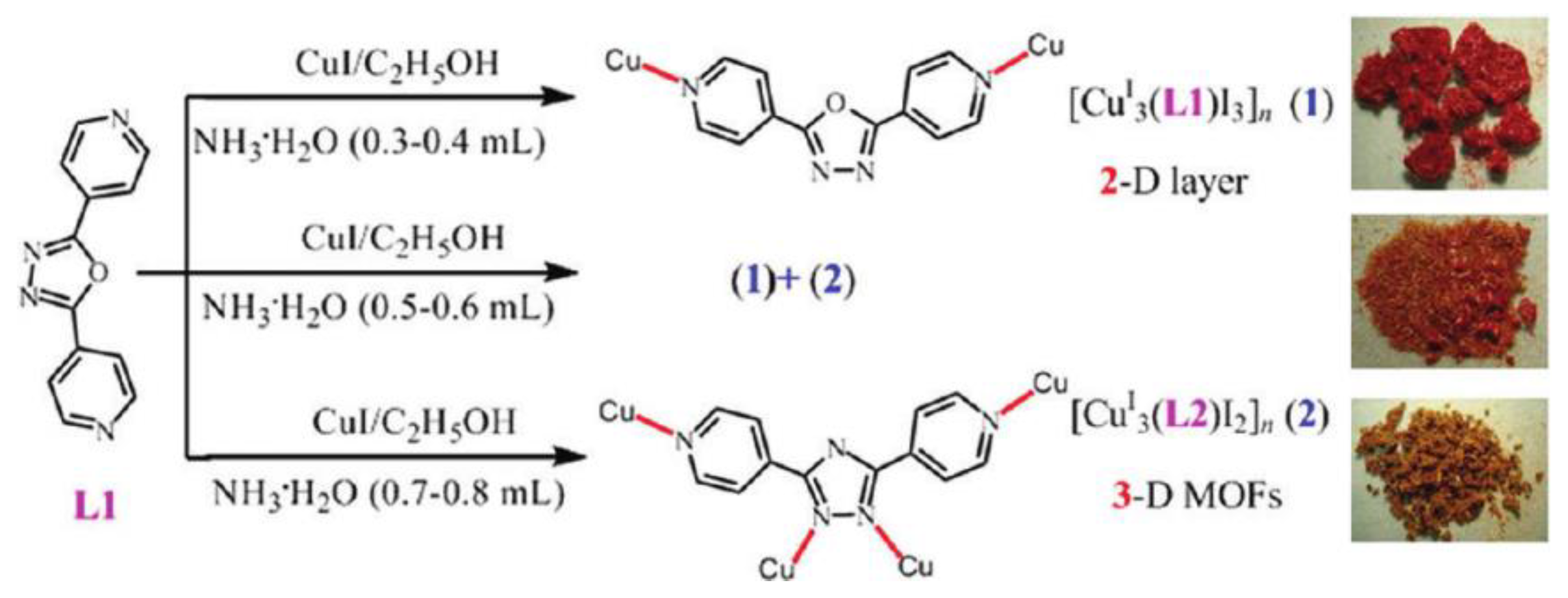
- Fang, Z.L.; He, J.G.; Zhang, Q.S.; Zhang, Q.K.; Wu, X.Y.; Yu, R.M.; Lu, C.Z. PH-controlled construction of Cu(I) coordination polymers: In situ transformation of ligand, network topologies, and luminescence and UV-vis-NIR absorption properties. Inorg. Chem. 2011, 50, 11403–11411. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.Y.; Li, C.P. Cadmium(II) and zinc(II) coordination polymers with mixed building blocks of benzenedicarboxyl and 2,5-bipyridyl-1,3,4-oxadiazole: Syntheses, crystal structures, and properties. Inorg. Chim. Acta 2011, 378, 206–212. [Google Scholar] [CrossRef]
- Schramm, S.; Weiß, D. Fluorescent heterocycles: Recent trends and new developments. Adv. Heterocycl. Chem. 2019, 128, 103–179. [Google Scholar]
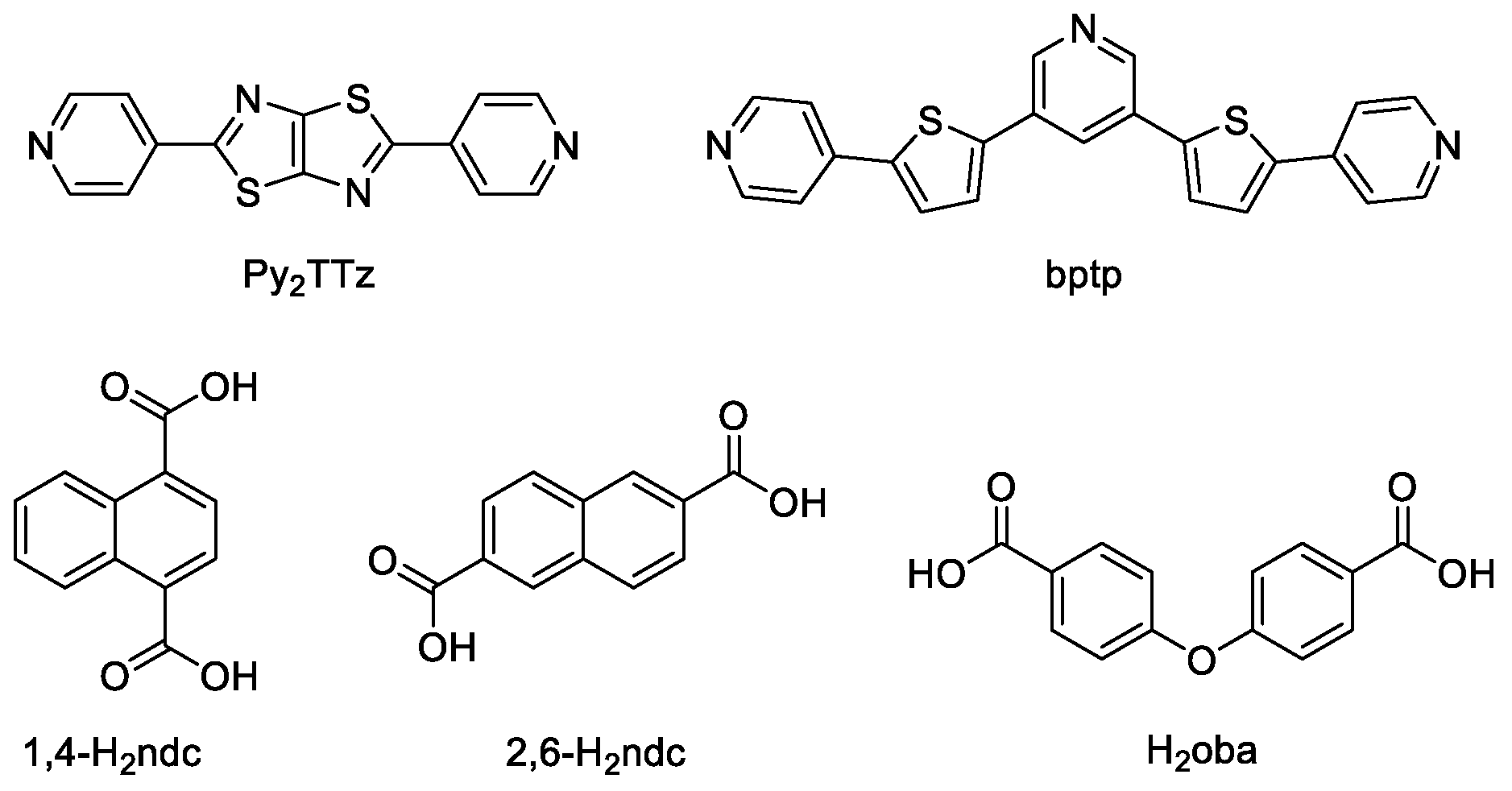
- Zhai, Z.W.; Yang, S.H.; Luo, P.; Li, L.K.; Du, C.X.; Zang, S.Q. Dicarboxylate-Induced Structural Diversity of Luminescent Zn(II)/Cd(II) Metal–Organic Frameworks Based on the 2,5-Bis(4-pyridyl)thiazolo[5,4-d]thiazole Ligand. Eur. J. Inorg. Chem. 2019, 2019, 2725–2734. [Google Scholar] [CrossRef]
- Han, L.J.; Yan, W.; Chen, S.G.; Shi, Z.Z.; Zheng, H.G. Exploring the Detection of Metal Ions by Tailoring the Coordination Mode of V-Shaped Thienylpyridyl Ligand in Three MOFs. Inorg. Chem. 2017, 56, 2936–2940. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Yan, W.; Gao, X.; Shi, Z.; Wang, T.; Zheng, H. Syntheses, Characterization, and Luminescence Properties of Four Metal-Organic Frameworks Based on a Linear-Shaped Rigid Pyridine Ligand. Cryst. Growth Des. 2016, 16, 2496–2503. [Google Scholar] [CrossRef]
- Shen, K.; Ju, Z.; Qin, L.; Wang, T.; Zheng, H. Two stable 3D porous metal-organic frameworks with high selectivity for detection of PA and metal ions. Dye. Pigment. 2017, 136, 515–521. [Google Scholar] [CrossRef]
- Song, W.C.; Liang, L.; Cui, X.Z.; Wang, X.G.; Yang, E.C.; Zhao, X.J. Assembly of ZnII-coordination polymers constructed from benzothiadiazole functionalized bipyridines and V-shaped dicarboxylic acids: Topology variety, photochemical and visible-light-driven photocatalytic properties. CrystEngComm 2018, 20, 668–678. [Google Scholar] [CrossRef]
- Cheng, Q.; Han, X.; Tong, Y.; Huang, C.; Ding, J.; Hou, H. Two 3D Cd(II) Metal-Organic Frameworks Linked by Benzothiadiazole Dicarboxylates: Fantastic S@Cd6 Cage, Benzothiadiazole Antidimmer, and Dual Emission. Inorg. Chem. 2017, 56, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Mallick, A.; El-Zohry, A.M.; Shekhah, O.; Yin, J.; Jia, J.; Aggarwal, H.; Emwas, A.H.; Mohammed, O.F.; Eddaoudi, M. Unprecedented Ultralow Detection Limit of Amines using a Thiadiazole-Functionalized Zr(IV)-Based Metal-Organic Framework. J. Am. Chem. Soc. 2019, 141, 7245–7249. [Google Scholar] [CrossRef]
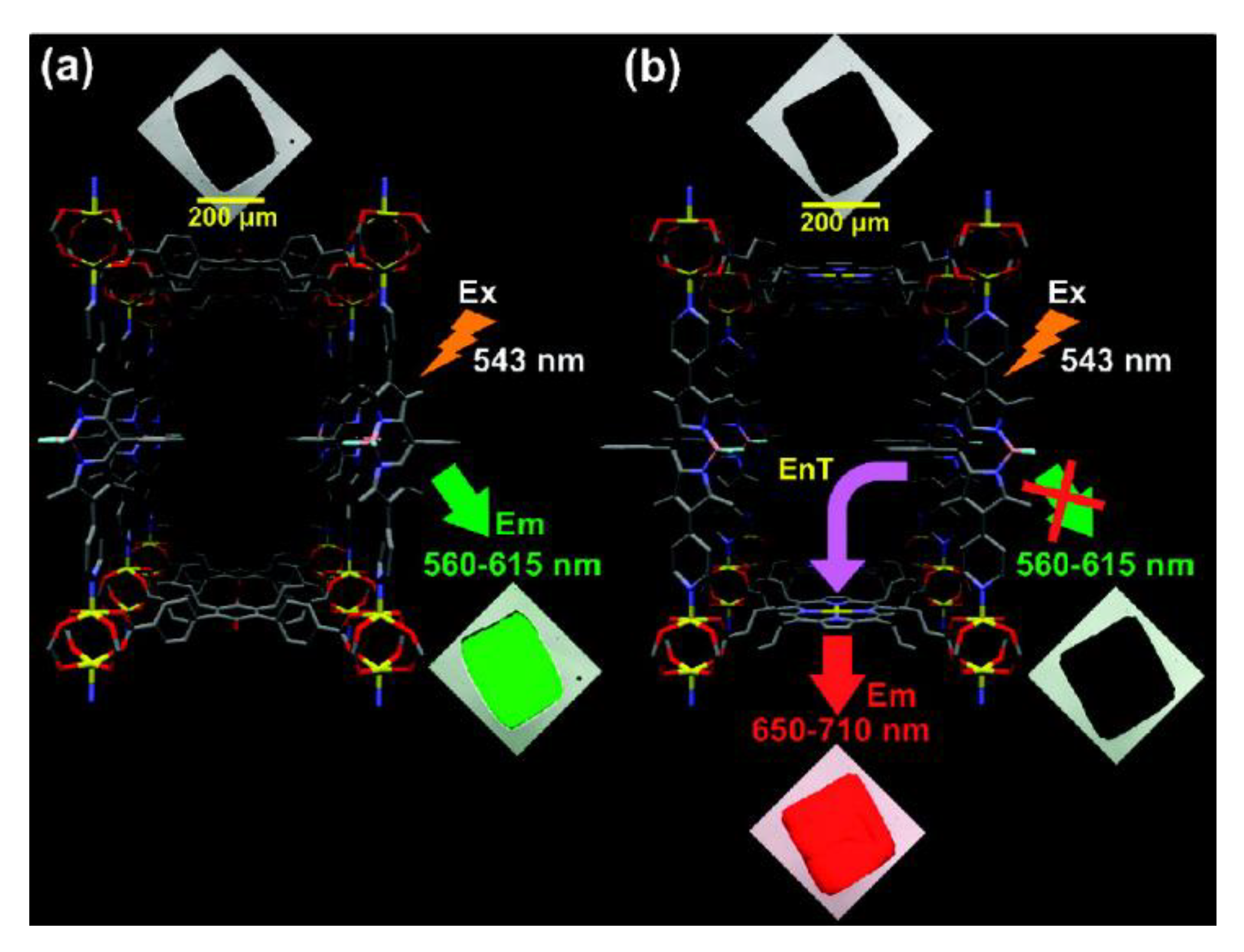
- Jia, J.; Gutiérrez-Arzaluz, L.; Shekhah, O.; Alsadun, N.; Czaban-Jóźwiak, J.; Zhou, S.; Bakr, O.M.; Mohammed, O.F.; Eddaoudi, M. Access to Highly Efficient Energy Transfer in Metal–Organic Frameworks via Mixed Linkers Approach. J. Am. Chem. Soc. 2020, 142, 8580–8584. [Google Scholar] [CrossRef]
- Wei, N.; Zhang, Y.R.; Han, Z.B. Thiadiazole-functional porous metal-organic framework as luminescent probe for Cd2+. CrystEngComm 2013, 15, 8883–8886. [Google Scholar] [CrossRef]
- Tian, X.M.; Yao, S.L.; Qiu, C.Q.; Zheng, T.F.; Chen, Y.Q.; Huang, H.; Chen, J.L.; Liu, S.J.; Wen, H.R. Turn-On Luminescent Sensor toward Fe3+, Cr3+, and Al3+ Based on a Co(II) Metal-Organic Framework with Open Functional Sites. Inorg. Chem. 2020, 59, 2803–2810. [Google Scholar] [CrossRef]
- An, D.L.; Chen, Y.Q.; Tian, Y. Assembly of cadmium(II)-terpyridine coordination frameworks by different multicarboxylate ancillary ligands. Z. Anorg. Allg. Chem. 2014, 640, 1776–1781. [Google Scholar] [CrossRef]
- Song, J.; Wang, B.C.; Hu, H.M.; Gou, L.; Wu, Q.R.; Yang, X.L.; Shangguan, Y.Q.; Dong, F.X.; Xue, G.L. In situ hydrothermal syntheses, crystal structures and luminescent properties of two novel zinc(II) coordination polymers based on tetrapyridyl ligand. Inorg. Chim. Acta 2011, 366, 134–140. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.; Liu, H.; Liu, Z. A series of 1D-to-3D zinc coordination polymers based on the rigid aromatic multicarboxylate ligands and different N-donor ligands: Synthesis, structures, and photoluminescent properties. Polyhedron 2016, 109, 1–6. [Google Scholar] [CrossRef]
- Cheng, H.J.; Tang, H.X.; Shen, Y.L.; Xia, N.N.; Yin, W.Y.; Zhu, W.; Tang, X.Y.; Ma, Y.S.; Yuan, R.X. Carboxylate ligands induced structural diversity of zinc(II) coordination polymers based on 3,6-bis(imidazol-1-yl)carbazole: Syntheses, structures and photocatalytic properties. J. Solid State Chem. 2015, 232, 200–206. [Google Scholar] [CrossRef]
- Cheng, H.J.; Tang, X.Y.; Yuan, R.X.; Lang, J.P. Structural diversity of Zn(II) coordination polymers based on bis-imidazolyl ligands and 5-R-1,3-benzenedicarboxylate and their photocatalytic properties. CrystEngComm 2016, 18, 4851–4862. [Google Scholar] [CrossRef]
- Cheng, H.J.; Lu, Y.F.; Li, C.; Shu, Y.; Ma, J.; Li, W.-h.; Yuan, R.X. Two Cd(II) coordination polymers based on 3,6-bis(imidazol-1-yl)carbazole: Syntheses, structures and photocatalytic properties. Inorg. Chem. Commun. 2016, 73, 12–15. [Google Scholar] [CrossRef]
- Du, J.L.; Gao, J.P.; Li, C.P.; Zhang, X.Y.; Hou, J.X.; Jing, X.; Mu, Y.J.; Li, L.J. A stable 3D Cd(II) metal-organic framework for highly sensitive detection of Cu2+ ions and nitroaromatic explosives. RSC Adv. 2017, 7, 49618–49625. [Google Scholar] [CrossRef]
- Hou, J.X.; Gao, J.P.; Liu, J.; Jing, X.; Li, L.J.; Du, J.L. Highly selective and sensitive detection of Pb2+ and UO22+ ions based on a carboxyl-functionalized Zn(II)-MOF platform. Dye Pigment 2019, 160, 159–164. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Zhao, L.; Wang, X.L.; Shao, K.Z.; Su, Z.M. Luminescent Metal-Organic Frameworks with Anthracene Chromophores: Small-Molecule Sensing and Highly Selective Sensing for Nitro Explosives. Cryst. Growth Des. 2016, 16, 4374–4382. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L.; Wang, Y.; Zhai, L.; Wang, X.; Niu, X.; Hu, T. Luminescent and magnetic properties of two novel coordination polymers based on N-heterocyclic carboxylic acid. J. Solid State Chem. 2019, 277, 356–362. [Google Scholar] [CrossRef]
- Fan, Y.; Li, H.; Ji, Z.; Liu, J.; Wu, M. Syntheses, structures and photoluminescence of three Zn(II) coordination polymers based on N-containing heterocyclic ligand and varied auxiliary ligands. Inorg. Chem. Commun. 2019, 102, 229–232. [Google Scholar] [CrossRef]
- Vasylevskyi, S.I.; Regeta, K.; Ruggi, A.; Petoud, S.; Piguet, C.; Fromm, K.M. Cis- and trans-9,10-di(1H-imidazol-1-yl)-anthracene based coordination polymers of ZnII and CdII: Synthesis, crystal structures and luminescence properties. Dalt. Trans. 2018, 47, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.Y.; Liu, G.Z.; Ma, L.F.; Zhang, X.; Wang, L.Y. Structural diversity and fluorescence regulation of three ZnII coordination polymers assembled from mixed ligands tectons. Aust. J. Chem. 2015, 68, 758–765. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, L.; Feng, J.; Wu, J.; Zhang, C. Two luminescent Zn(II)/Cd(II) metal-organic frameworks as rare multifunctional sensors. New J. Chem. 2017, 41, 8107–8117. [Google Scholar] [CrossRef]
- Jin, J.C.; Wu, J.; Liu, W.C.; Ma, A.Q.; Liu, J.Q.; Singh, A.; Kumar, A. A new Zn(II) metal-organic framework having 3D CdSO4 topology as luminescent sensor and photocatalyst for degradation of organic dyes. New J. Chem. 2018, 42, 2767–2775. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, L.; Liu, Z.Y.; Wang, X.G.; Ding, B.; Yin, L.; Zhou, B.B.; Li, M.S.; Wang, J.X.; Zhao, X.J. An Ideal Detector Composed of Two-Dimensional Cd(II)-Triazole Frameworks for Nitro-Compound Explosives and Potassium Dichromate. Chem. A Eur. J. 2015, 21, 14171–14178. [Google Scholar] [CrossRef]
- Wang, X.X.; Li, Z.X.; Yu, B.; Van Hecke, K.; Cui, G.H. Syntheses, structures, and luminescence properties of two copper(II) complexes constructed by rigid bis(triazole) and nitrogen-containing carboxylic acid ligands. Bull. Korean Chem. Soc. 2015, 36, 1848–1853. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhang, S.; Van Hecke, K.; Cui, G.H.; Liu, Y.G. Synthesis, structures and luminescence properties of two mixed nickel(II) coordination polymers bearing a rigid bis-triazole linker. Transit. Met. Chem. 2015, 40, 565–571. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.Q.; Dong, G.Y.; Cui, G.H. Copper(I) and Silver(I) Coordination Polymers Based on Rigid Bis(triazole) Ligand: Syntheses, Structures, and Catalytic Properties. Z. Anorg. Allg. Chem. 2016, 642, 36–40. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, G.Y.; Yu, B.; Van Hecke, K.; Cui, G.H. In situ construction of four copper(I) cyanide coordination polymers: Crystal structures, fluorescence and catalytic properties. Transit. Met. Chem. 2015, 40, 907–916. [Google Scholar] [CrossRef]
- Zong, Z.; Fan, Y.; Xu, C.; Zuo, S.; Yan, F.; Meng, X.; Zhang, X.; Bi, C. A penetrating metal-organic framework based on N-heterocyclic carboxylic acid with sensing properties toward Cr(VI)/Fe(III) and nitrobenzene. J. Coord. Chem. 2018, 71, 2691–2701. [Google Scholar] [CrossRef]
- Li, F.F.; Zhu, M.L.; Lu, L.P. A luminescent Cd(II)-based metal−organic framework for detection of Fe(III) ions in aqueous solution. J. Solid State Chem. 2018, 261, 31–36. [Google Scholar] [CrossRef]
- Wang, J.H.; Tang, G.M.; Qin, T.X.; Wang, Y.T.; Cui, Y.Z.; Ng, S.W. Structural and luminescent properties of a series of Cd(II) pyridyl benzimidazole complexes that exhibit extended three-dimensional hydrogen bonded networks. J. Coord. Chem. 2017, 70, 1168–1189. [Google Scholar] [CrossRef]
- Liang, J.; Liang, J.; Zhai, L.; Wu, H.; Niu, X.; Zhang, J.; Hu, T. Three new coordination polymers based on an N-heterocyclic carboxylic acid: Structural diversity, luminescent properties and gas adsorption. J. Solid State Chem. 2019, 280, 120916. [Google Scholar] [CrossRef]
- Dai, H.-Y.; Tang, Y.-Y.; Wang, C.-J.; Chen, S.; Tong, Y.; Zhang, Z.B. Preparation, structure and luminescent characterization of a series of metal–organic frameworks based on flexible ligands with nitrogen heterocycles and carboxyl. J. Solid State Chem. 2017, 256, 130–140. [Google Scholar] [CrossRef]
- Han, L.; Zhou, J.; Li, X.; Sun, C.Y.; Zhao, L.; Zhang, Y.T.; Zhu, M.; Wang, X.L.; Su, Z.M. Recognition of harmful fused aromatic hydrocarbons via a metal-organic framework with hydrophobic pores. Inorg. Chem. Commun. 2017, 86, 200–203. [Google Scholar] [CrossRef]
- Zhao, S.; Xiao, J.; Zheng, T.; Liu, M.; Wu, H.; Liu, Z. Highly Selective and Sensitive Detection of PO43- Ions in Aqueous Solution by a Luminescent Terbium Metal-Organic Framework. ACS Omega 2019, 4, 16378–16384. [Google Scholar] [CrossRef]
- Li, X.; Chi, X.L.; Xu, Y.C.; Chen, Y.; Yang, Q.; Zeng, X.S.; Xu, H.L.; Xiao, D.R. Three novel 3D pillared-layer molybdenum-oxide-based inorganic–organic hybrids constructed by tetranuclear Zn4/Co4/Mo4 metal clusters. Inorg. Chim. Acta 2016, 445, 160–166. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Li, Q.; Li, S.; Yi, Z.; Xu, Z.; Wang, Y. Highly sensitive and selective fluorescent probe for Fe3+ and hazardous phenol compounds based on a water-stable Zn-based metal-organic framework in aqueous media. RSC Adv. 2017, 7, 50035–50039. [Google Scholar] [CrossRef]
- Liu, J.Q.; Wu, J.; Li, F.M.; Liu, W.C.; Li, B.H.; Wang, J.; Li, Q.L.; Yadav, R.; Kumar, A. Luminescent sensing from a new Zn(II) metal-organic framework. RSC Adv. 2016, 6, 31161–31166. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, C.; Cen, P.; Jin, X.; Liang, C.; Yang, J.; Liu, X. Stable Ln-MOFs as multi-responsive photoluminescence sensors for the sensitive sensing of Fe3+, Cr2O72−, and nitrofuran. CrystEngComm 2020, 22, 1695–1704. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Li, M.; Huang, C.; Xu, H.; Hou, H.W.; Fan, Y.T. Three Cd(II) coordination polymers based on rigid 2,6-bis(3-(pyrid-3-yl)-1,2,4-triazolyl)pyridine ligand: Syntheses, crystal structures, and photocatalytic properties. Polyhedron 2014, 83, 197–204. [Google Scholar] [CrossRef]
- Lee, L.W.; Chi, H.Y.; Kao, Y.C.; Hung, T.H.; Chiou, D.S.; Lee, G.H.; Peng, S.M.; Kang, D.Y.; Wang, C.M.; Lu, K.L. Zinc(II)–Organic Framework Films with Thermochromic and Solvatochromic Applications. Chem. A Eur. J. 2020, 26, 4204–4208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, L.; Liu, Y.; Li, M.; Li, G.; Meng, X. Two luminescent transition-metal-organic frameworks with a predesigned ligand as highly sensitive and selective iron(III) sensors. New J. Chem. 2018, 42, 6839–6847. [Google Scholar] [CrossRef]
- Chen, Z.; Gallo, G.; Sawant, V.A.; Zhang, T.; Zhu, M.; Liang, L.; Chanthapally, A.; Bolla, G.; Quah, H.S.; Liu, X.; et al. Giant Enhancement of Second Harmonic Generation Accompanied by the Structural Transformation of 7-Fold to 8-Fold Interpenetrated Metal–Organic Frameworks (MOFs). Angew. Chem. Int. Ed. 2020, 59, 833–838. [Google Scholar] [CrossRef]
- Li, G.; Li, J.; Otsuka, Y.; Zhang, S.; Takahashi, M.; Yamada, K. A BODIPY-Based Fluorogenic Probe for Specific Imaging of Lipid Droplets. Materials 2020, 13, 677. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Recent advances in the application of BODIPY in bioimaging and chemosensing. J. Mater. Chem. C 2019, 7, 11361–11405. [Google Scholar] [CrossRef]
- Wang, L.; Ding, H.; Ran, X.; Tang, H.; Cao, D. Recent progress on reaction-based BODIPY probes for anion detection. Dye Pigment 2020, 172, 107857. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Z.; Yan, M.; Wang, X. Recent Progress of BODIPY Dyes With Aggregation-Induced Emission. Front. Chem. 2019, 7, 712. [Google Scholar] [CrossRef]
- Baudron, S.A. Luminescent metal-organic frameworks based on dipyrromethene metal complexes and BODIPYs. CrystEngComm 2016, 18, 4671–4680. [Google Scholar] [CrossRef]
- Zhou, L.; Xue, Y.S.; Xu, Y.; Zhang, J.; Du, H. Bin Two photoluminescent metal-organic frameworks based on a BODIPY-derived bipyridine ligand. CrystEngComm 2013, 15, 7315–7320. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, M.; Zhang, J.; Ma, J.; Wu, P.; Liu, W.; Wen, L. A noble-metal-free photocatalyst system obtained using BODIPY-based MOFs for highly efficient visible-light-driven H2 evolution. J. Mater. Chem. A 2019, 7, 20742–20749. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Ma, J.; Yang, H.; Zhang, J.; Lv, K.; Wen, L.; Peng, T. A novel BODIPY-based MOF photocatalyst for efficient visible-light-driven hydrogen evolution. J. Mater. Chem. A 2019, 7, 10439–10445. [Google Scholar] [CrossRef]
- Li, M.; Yao, Y.; Ding, J.; Liu, L.; Qin, J.; Zhao, Y.; Hou, H.; Fan, Y. Spectroscopic and crystallographic investigations of novel Bodipy-derived metal-organic frameworks. Inorg. Chem. 2015, 54, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Farha, O.K.; Hong, B.J.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Light-harvesting metal-organic frameworks (MOFs): Efficient strut-to-strut energy transfer in bodipy and porphyrin-based MOFs. J. Am. Chem. Soc. 2011, 133, 15858–15861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Baudron, S.A.; Hosseini, M.W. Solvent and anion effects on the organization of a luminescent [2 + 2] BODIPY/Ag(i) metallamacrocycle in the crystalline state. CrystEngComm 2017, 19, 4393–4400. [Google Scholar] [CrossRef]
- Ye, Y.; Lin, R.-B.; Cui, H.; Alsalme, A.; Zhou, W.; Yildirim, T.; Zhang, Z.; Xiang, S.; Chen, B. A microporous metal–organic framework with naphthalene diimide groups for high methane storage. Dalt. Trans. 2020, 49, 3658–3661. [Google Scholar] [CrossRef]
- Kumari, N.; Naqvi, S.; Ahuja, M.; Bhardwaj, K.; Kumar, R. Facile synthesis of naphthalene diimide (NDI) derivatives: Aggregation-induced emission, photophysical and transport properties. J. Mater. Sci. Mater. Electron. 2020, 31, 4310–4322. [Google Scholar] [CrossRef]
- Ren, G.; Gao, L.; Wang, X.; Fan, L.; Hu, T. Highly fluorescent selectivity of two Cd(II) coordination networks with active sites for nitroaromatic compounds and Fe3+ and Hg2+. Inorg. Chim. Acta 2018, 471, 746–753. [Google Scholar] [CrossRef]
- Wei, F.; Ye, Y.; Huang, W.; Lin, Q.; Li, Z.; Liu, L.; Chen, S.; Zhang, Z.; Xiang, S. A naphthalene diimide-based MOF with mog net featuring photochromic behaviors and high stability. Inorg. Chem. Commun. 2018, 93, 105–109. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Xiang, F.; Lin, Q.; Ye, Y.; Zhang, J.; Chen, S.; Zhang, Z.; Xiang, S. Photochromic naphthalene diimide Cd-MOFs based on different second dicarboxylic acid ligands. CrystEngComm 2018, 20, 7567–7573. [Google Scholar] [CrossRef]
- Wang, M.L.; Fu, C.; Li, L.; Zhang, H. A 2D photochromic zinc-based metal–organic framework with naphthalene diimide-type chromophore. Inorg. Chem. Commun. 2018, 94, 142–145. [Google Scholar] [CrossRef]
- Li, G.-B.; Liu, J.-M.; Cai, Y.-P.; Su, C.-Y. Structural Diversity of a Series of Mn(II), Cd(II), and Co(II) Complexes with Pyridine Donor Diimide Ligands. Cryst. Growth Des. 2011, 11, 2763–2772. [Google Scholar] [CrossRef]
- Liu, J.-J.; Xia, S.-B.; Liu, D.; Hou, J.; Suo, H.; Cheng, F.-X. Multifunctional naphthalene diimide-based coordination polymers: Photochromism and solventchromism. Dye Pigment 2020, 177, 108269. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, W.N.; Yu, G.J.; Xu, L.P.; Han, L. A zinc-organic coordination polymer of glycine-functionalized naphthalenediimide ligand. Inorg. Chem. Commun. 2013, 34, 47–50. [Google Scholar] [CrossRef]
- Xu, H.L.; Zeng, X.S.; Li, J.; Xu, Y.C.; Qiu, H.J.; Xiao, D.R. The impact of metal ions on photoinduced electron-transfer properties: Four photochromic metal-organic frameworks based on a naphthalenediimide chromophore. CrystEngComm 2018, 20, 2430–2439. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, G.S.; Wang, H.Y.; Li, L.; Fu, J.W.; Sun, Y.N.; Zhang, H. Different photochromic properties induced by lone pair-π interactions with varying strengths in two stereocontrolled self-assembly isomeric coordination polymers. CrystEngComm 2018, 20, 6821–6827. [Google Scholar] [CrossRef]
- Fu, C.; Wang, H.Y.; Zhang, G.S.; Li, L.; Sun, Y.N.; Fu, J.W.; Zhang, H. The influence of the secondary building linker geometry on the photochromism of naphthalenediimide-based metal-organic frameworks. CrystEngComm 2018, 20, 4849–4856. [Google Scholar] [CrossRef]
- Liao, J.Z.; Zhang, H.L.; Wang, S.S.; Yong, J.P.; Wu, X.Y.; Yu, R.; Lu, C.Z. Multifunctional radical-doped polyoxometalate-based host-guest material: Photochromism and photocatalytic activity. Inorg. Chem. 2015, 54, 4345–4350. [Google Scholar] [CrossRef]
- Liu, J.J.; Guan, Y.F.; Chen, Y.; Lin, M.J.; Huang, C.C.; Dai, W.X. The impact of lone pair-π interactions on photochromic properties in 1-D naphthalene diimide coordination networks. Dalt. Trans. 2015, 44, 17312–17317. [Google Scholar] [CrossRef]
- Liu, J.J.; Dong, Y.; Chen, L.Z.; Wang, L.; Xia, S.B.; Huang, C.C. A two-dimensional CdII coordination polymer based on naphthalenediimide: Synthesis, crystal structure and photochromic properties. Acta Cryst. Sect. C Struct. Chem. 2018, 74, 94–99. [Google Scholar] [CrossRef]
- Shang, X.; Song, I.; Jung, G.Y.; Choi, W.; Ohtsu, H.; Lee, J.H.; Koo, J.Y.; Liu, B.; Ahn, J.; Kawano, M.; et al. Chiral self-sorted multifunctional supramolecular biocoordination polymers and their applications in sensors. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-J.; Yan, C.; Pan, M.; Wang, Z.; Deng, H.-Y.; He, J.-R.; Yang, Q.-Y.; Fu, L.; Xu, X.-F.; Su, C.-Y. Structural Conformation and Optical and Electrochemical Properties of Imidazolyl-Substituted Naphthalenediimide and Its HgII, CdII, and CuII Halide Complexes. Eur. J. Inorg. Chem. 2012, 2012, 1171–1179. [Google Scholar] [CrossRef]
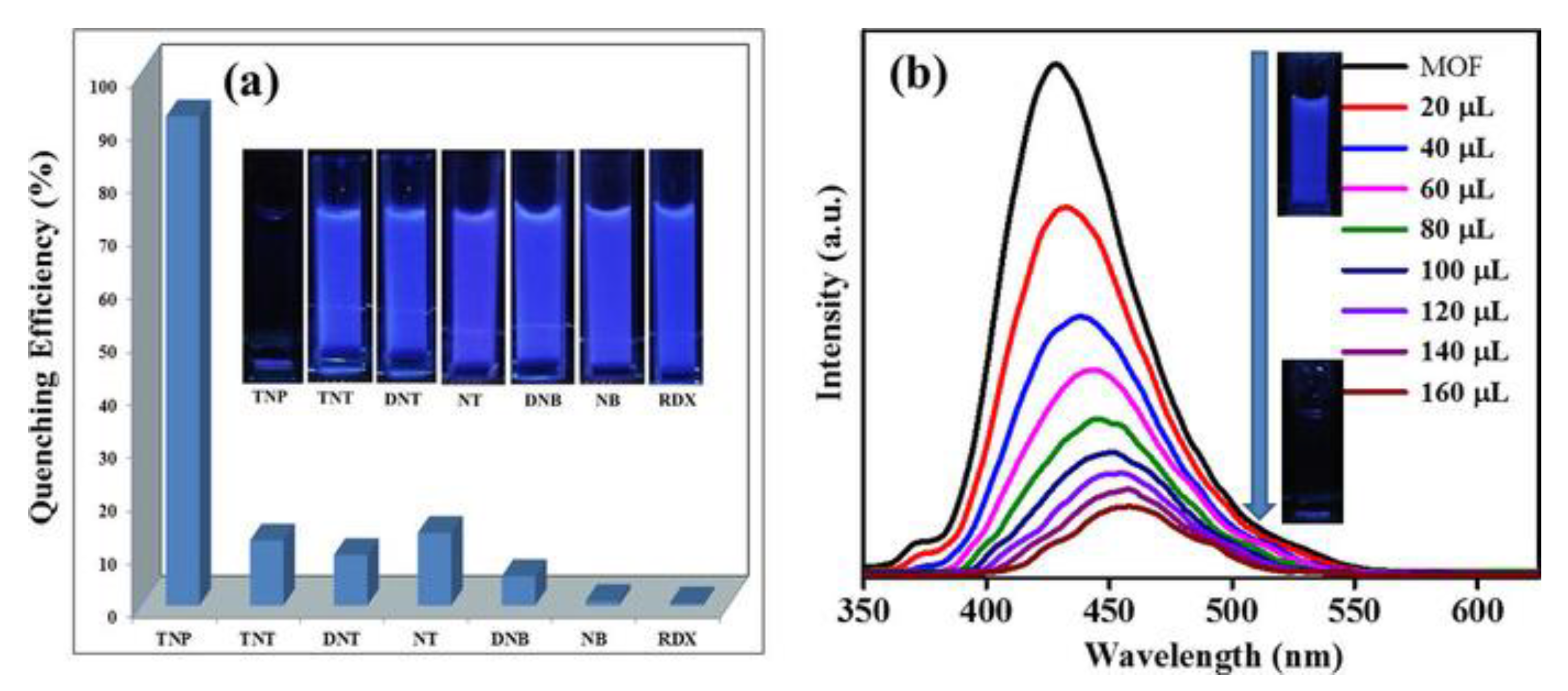
- Singh Dhankhar, S.; Sharma, N.; Kumar, S.; Dhilip Kumar, T.J.; Nagaraja, C.M. Rational Design of a Bifunctional, Two-Fold Interpenetrated ZnII-Metal–Organic Framework for Selective Adsorption of CO2 and Efficient Aqueous Phase Sensing of 2,4,6-Trinitrophenol. Chem. A Eur. J. 2017, 23, 16204–16212. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xu, L.-P.; Qin, L.; Zhao, W.-N.; Yan, X.-Z.; Yu, L. Syntheses, Crystal Structures, and Physical Properties of Two Noninterpenetrated Pillar-Layered Metal–Organic Frameworks Based on N,N′-Di(4-pyridyl)-1,4,5,8-naphthalenetetracarboxydiimide Pillar. Cryst. Growth Des. 2013, 13, 4260–4267. [Google Scholar] [CrossRef]
- Xie, Y.X.; Zhao, W.N.; Li, G.C.; Liu, P.F.; Han, L. A Naphthalenediimide-Based Metal-Organic Framework and Thin Film Exhibiting Photochromic and Electrochromic Properties. Inorg. Chem. 2016, 55, 549–551. [Google Scholar] [CrossRef]
- Takashima, Y.; Martínez, V.M.; Furukawa, S.; Kondo, M.; Shimomura, S.; Uehara, H.; Nakahama, M.; Sugimoto, K.; Kitagawa, S. Molecular decoding using luminescence from an entangled porous framework. Nat. Commun. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Deng, H.Y.; He, J.R.; Pan, M.; Li, L.; Su, C.Y. Synergistic metal and anion effects on the formation of coordination assemblies from a N,N′-bis(3-pyridylmethyl)naphthalene diimide ligand. CrystEngComm 2009, 11, 909–917. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Qin, C.; Wang, X.L.; Shao, K.Z.; Su, Z.M. A highly electrical conducting, 3D supermolecular Ag(I) coordination polymer material with luminescent properties. Inorg. Chem. Commun. 2016, 70, 31–34. [Google Scholar] [CrossRef]
- Mallick, A.; Garai, B.; Addicoat, M.A.; Petkov, P.S.; Heine, T.; Banerjee, R. Solid state organic amine detection in a photochromic porous metal organic framework. Chem. Sci. 2015, 6, 1420–1425. [Google Scholar] [CrossRef]
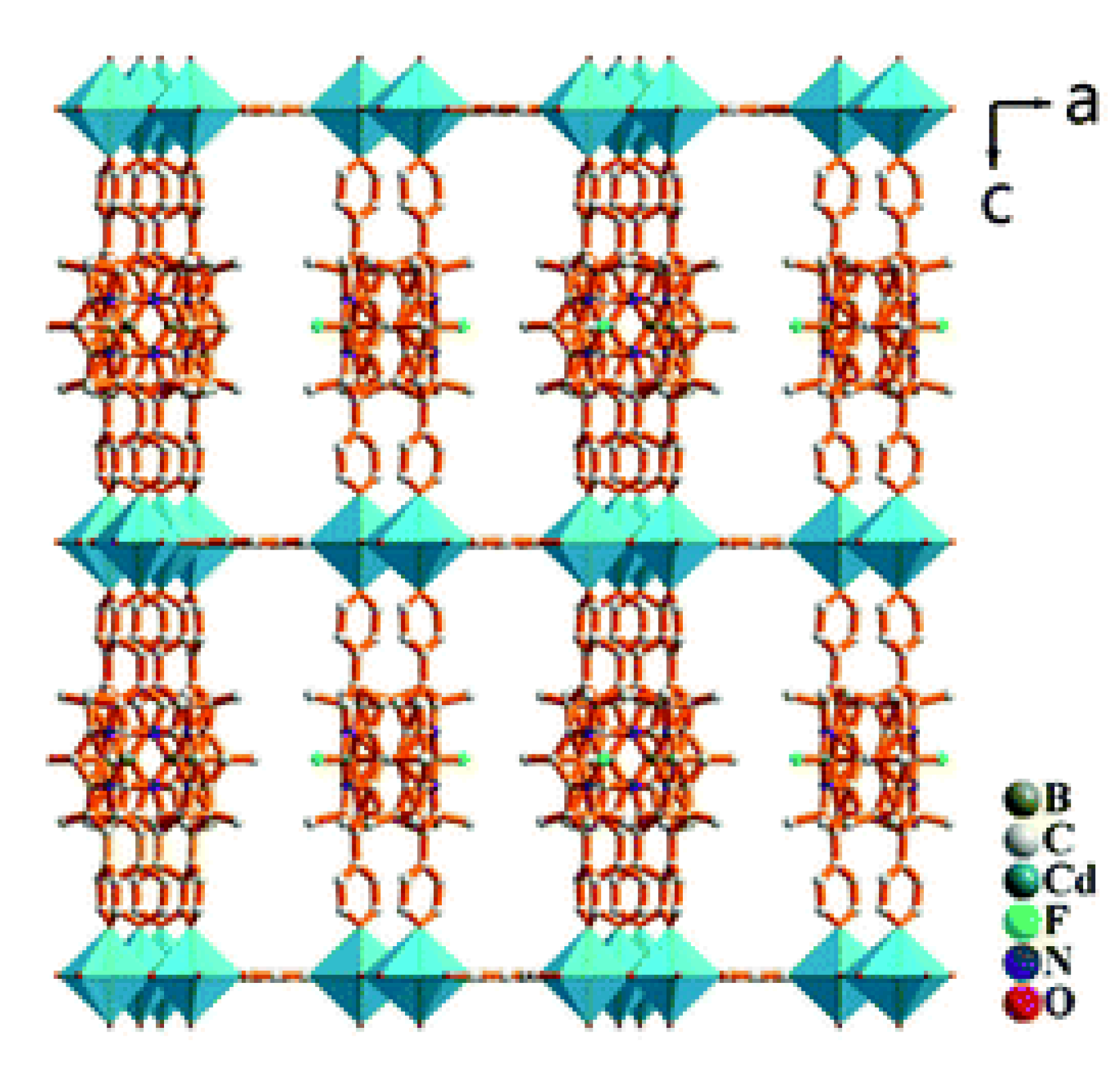
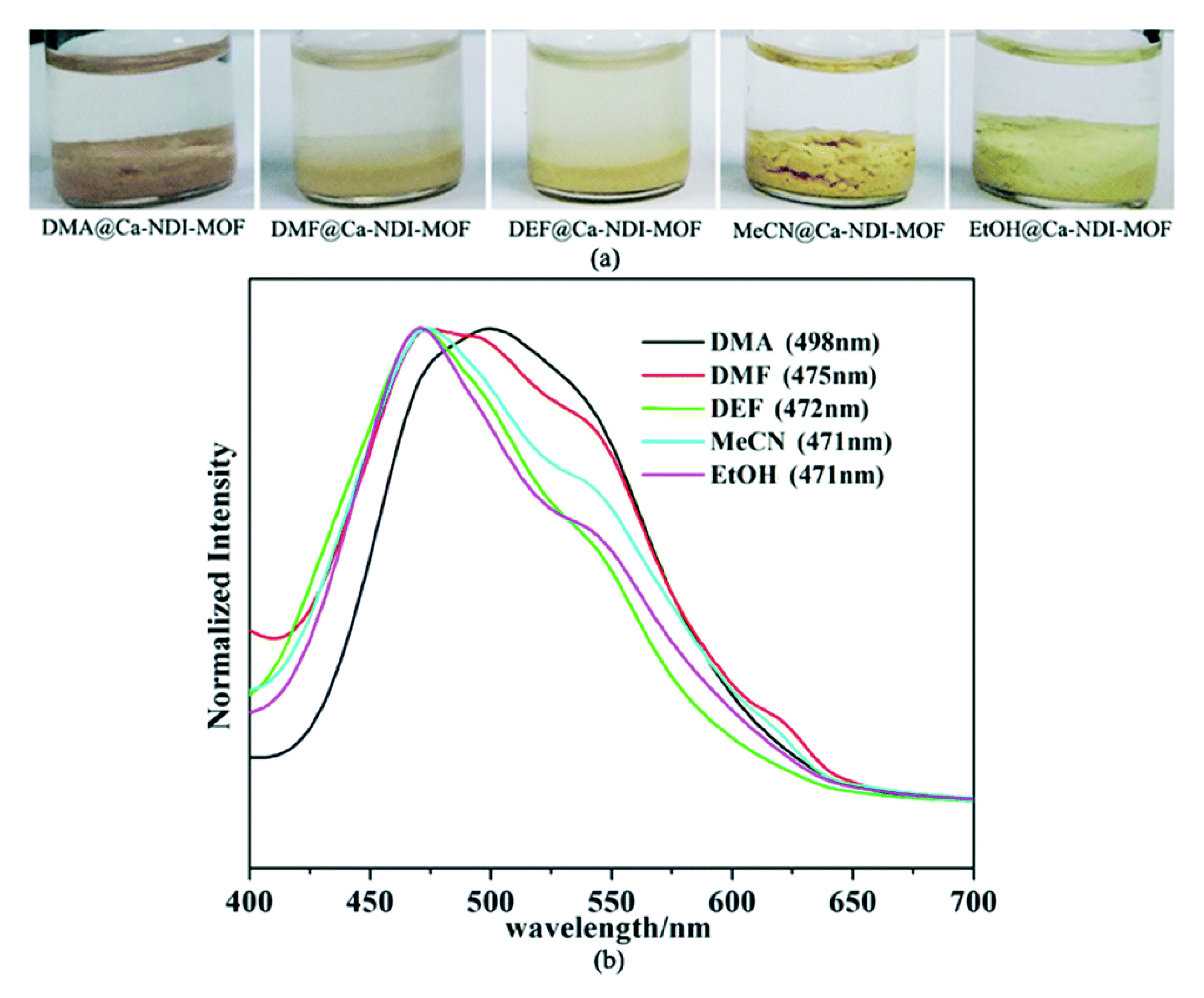
- Han, L.; Qin, L.; Xu, L.; Zhou, Y.; Sun, J.; Zou, X. A novel photochromic calcium-based metal-organic framework derived from a naphthalene diimide chromophore. Chem. Commun. 2013, 49, 406–408. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, H. Fine-Tuning Aromatic Stacking and Single-Crystal Photoluminescence Through Coordination Chemistry. Eur. J. Org. Chem. 2019, 2019, 1778–1783. [Google Scholar] [CrossRef]
- Cui, L.T.; Niu, Y.F.; Han, J.; Zhao, X.L. Ancillary ligand-assisted assembly of C3-symmetric 4,4′,4″-nitrilotribenzoic acid with divalent Zn2+ ions: Syntheses, topological structures, and photoluminescence properties. J. Solid State Chem. 2015, 227, 155–164. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, S.; Qu, X.; Yang, Q.; Liu, X.; Wei, Q.; Xie, G.; Chen, S. Synthesis, crystal structure and photoluminescence property of Eu/Tb MOFs with mixed polycarboxylate ligands. J. Solid State Chem. 2015, 231, 223–229. [Google Scholar] [CrossRef]
- Hu, X.L.; Qin, C.; Wang, X.L.; Shao, K.Z.; Su, Z.M. Cluster-based metal-organic frameworks as sensitive and selective luminescent probes for sensing nitro explosives. New J. Chem. 2015, 39, 7858–7862. [Google Scholar] [CrossRef]
- Venkateswarulu, M.; Pramanik, A.; Koner, R.R. Novel metal-organic framework with tunable fluorescence property: Supramolecular signaling platform for polynitrophenolics. Dalt. Trans. 2015, 44, 6348–6352. [Google Scholar] [CrossRef]
- Zhang, M.; Han, J.; Wu, H.; Wei, Q.; Xie, G.; Chen, S.; Gao, S. Tb-MOF: A naked-eye and regenerable fluorescent probe for selective and quantitative detection of Fe3+ and Al3+ ions. RSC Adv. 2016, 6, 94622–94628. [Google Scholar] [CrossRef]
- Wen, L.; Wang, X.; Shi, H.; Lv, K.; Wang, C. A multifunctional cadmium-organic framework comprising tricarboxytriphenyl amine: Selective gas adsorption, liquid-phase separation and luminescence sensing. RSC Adv. 2016, 6, 1388–1394. [Google Scholar] [CrossRef]
- Niu, Y.F.; Cui, L.T.; Han, J.; Zhao, X.L. Solvent-mediated secondary building units (SBUs) diversification in a series of MnII-based metal-organic frameworks (MOFs). J. Solid State Chem. 2016, 241, 18–25. [Google Scholar] [CrossRef]
- Wu, X.X.; Fu, H.R.; Han, M.L.; Zhou, Z.; Ma, L.F. Tetraphenylethylene Immobilized Metal-Organic Frameworks: Highly Sensitive Fluorescent Sensor for the Detection of Cr2O72− and Nitroaromatic Explosives. Cryst. Growth Des. 2017, 17, 6041–6048. [Google Scholar] [CrossRef]
- Xia, L.; Ni, J.; Wu, P.; Ma, J.; Bao, L.; Shi, Y.; Wang, J. Photoactive metal-organic framework as a bifunctional material for 4-hydroxy-4′-nitrobiphenyl detection and photodegradation of methylene blue. Dalt. Trans. 2018, 47, 16551–16557. [Google Scholar] [CrossRef]
- Fu, H.R.; Zhao, Y.; Xie, T.; Han, M.L.; Ma, L.F.; Zang, S.Q. Stable dye-encapsulated indium-organic framework as dual-emitting sensor for the detection of Hg2+/Cr2O72− and a wide range of nitro-compounds. J. Mater. Chem. C 2018, 6, 6440–6448. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, M.; Su, J.; Xu, S.; Hu, L.; Liu, H.; Zhang, Q.; Zhang, J.; Wu, J.; Tian, Y. Three coordination polymers based on a star-like geometry 4,4’,4’’ -nitrilotribenzoic acid ligand and their framework dependent luminescent properties. J. Solid State Chem. 2018, 258, 328–334. [Google Scholar] [CrossRef]
- Zhong, F.; Li, C.; Xie, Y.; Xu, H.; Gao, J. Titanium metal-organic framework nanorods for highly sensitive nitroaromatic explosives detection and nanomolar sensing of Fe3+. J. Solid State Chem. 2019, 278, 120892. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.J.; Cui, L.L.; Hu, X.L.; Wang, X.L.; Su, Z.M. A 3D pillared-layer metal-organic framework with fluorescence property for detection of nitroaromatic explosives. New J. Chem. 2019, 43, 963–969. [Google Scholar] [CrossRef]
- Liu, Y.; Huangfu, M.; Wu, P.; Jiang, M.; Zhao, X.; Liang, L.; Xie, L.; Bai, J.; Wang, J. Post-imparting Brønsted acidity into an amino-functionalized MOF as a bifunctional luminescent turn-ON sensor for the detection of aluminum ions and lysine. Dalt. Trans. 2019, 48, 13834–13840. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, X.; Liu, L.; Zhai, H.; Meng, Q.; Shi, Z.; Tai, X. Two d10 luminescent metal-organic frameworks as dual functional luminescent sensors for (Fe3+,Cu2+) and 2,4,6-trinitrophenol (TNP) with high selectivity and sensitivity. RSC Adv. 2020, 10, 4817–4824. [Google Scholar] [CrossRef]
- Sun, L.; Xing, H.; Liang, Z.; Yu, J.; Xu, R. A 4 + 4 strategy for synthesis of zeolitic metal-organic frameworks: An indium-MOF with SOD topology as a light-harvesting antenna. Chem. Commun. 2013, 49, 11155–11157. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, W.; Zou, L.; Feng, J.; Gu, C.; Li, B.; Batten, S.R.; Yadav, R.; Kumar, A. Luminescent sensing of a new 8-connected topological metal-organic framework. Inorg. Chem. Commun. 2016, 70, 160–163. [Google Scholar] [CrossRef]
- Wang, X.; Yan, P.; Li, Y.; An, G.; Yao, X.; Li, G. Highly Efficient White-Light Emission and UV-Visible/NIR Luminescence Sensing of Lanthanide Metal-Organic Frameworks. Cryst. Growth Des. 2017, 17, 2178–2185. [Google Scholar] [CrossRef]
- Lu, L.; Wang, J.; Xie, B.; Liu, J.Q.; Yadav, R.; Singh, A.; Kumar, A. Fluorescence sensing of nitro-aromatics by Zn(ii) and Cd(ii) based coordination polymers having the 5-[bis(4-carboxybenzyl)-amino]isophthalic acid ligand. New J. Chem. 2017, 41, 3537–3542. [Google Scholar] [CrossRef]
- Ren, G.; Li, Z.; Li, M.; Liang, Z.; Yang, W.; Qiu, P.; Pan, Q.; Zhu, G. A hexanuclear cluster based metal-organic framework for Fe3+ sensing. Inorg. Chem. Commun. 2018, 91, 108–111. [Google Scholar] [CrossRef]
- Qiao, Y.; Ma, Y.; Jiang, W.; Wang, X.; Guan, W.; Che, G.; Li, W.; Qin, F. A series of metal-organic frameworks constructed by a rigid-flexible 5-(bis(4-carboxybenzyl)amino)isophthalic acid: Syntheses, crystal structures and physical properties. CrystEngComm 2018, 20, 7782–7794. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.B.; Tao, X.M.; Su, J.; Ning, P.F.; Xu, X.F.; Zhou, Y.; Gu, W.; Liu, X. Novel single component tri-rare-earth emitting MOF for warm white light LEDs. Dalt. Trans. 2018, 47, 8427–8433. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, W.; Lv, R.; Zhai, A.; Li, X.L.; Gu, W.; Liu, X. Dual-Function Mixed-Lanthanide Metal-Organic Framework for Ratiometric Water Detection in Bioethanol and Temperature Sensing. Anal. Chem. 2019, 91, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Du, K.; Chen, L.Q.; Xu, N.N.; Liu, X.C.; Wang, F.C.; Zhang, W.; Ding, X.D. A fluorescent channel-type Eu(III)-organic framework for selective detection of Fe3+ ion and protective effect against Parkinson disease by increasing mitochondrial complex activity. J. Mol. Struct. 2020, 1203, 127439. [Google Scholar] [CrossRef]
- Feng, L.; Ren, G.; Ding, S.; Wang, F.; Yang, W.; Liang, Z.; Pan, Q. A lithium-organic framework as a fluorescent sensor for detecting aluminum (III) ion. Appl. Organomet. Chem. 2019, 33, 5044. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Li, B.; Liu, G.; Liu, X. Construction of functional coordination polymers derived from designed flexible bis(4-carboxybenzyl)amine. CrystEngComm 2019, 21, 1231–1241. [Google Scholar] [CrossRef]
- Xu, W.; Chen, H.; Xia, Z.; Ren, C.; Han, J.; Sun, W.; Wei, Q.; Xie, G.; Chen, S. A Robust TbIII-MOF for Ultrasensitive Detection of Trinitrophenol: Matched Channel Dimensions and Strong Host-Guest Interactions. Inorg. Chem. 2019, 58, 8198–8207. [Google Scholar] [CrossRef]
- Mayer, D.C.; Manzi, A.; Medishetty, R.; Winkler, B.; Schneider, C.; Kieslich, G.; Pöthig, A.; Feldmann, J.; Fischer, R.A. Controlling Multiphoton Absorption Efficiency by Chromophore Packing in Metal–Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 11594–11602. [Google Scholar] [CrossRef]
- Xie, Z.; Ma, L.; DeKrafft, K.E.; Jin, A.; Lin, W. Porous phosphorescent coordination polymers for oxygen sensing. J. Am. Chem. Soc. 2010, 132, 922–923. [Google Scholar] [CrossRef]
- Barrett, S.M.; Wang, C.; Lin, W. Oxygen sensing via phosphorescence quenching of doped metal-organic frameworks. J. Mater. Chem. 2012, 22, 10329–10334. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Xu, L.; Chen, Z.N.; Luo, J. Highly sensitized near-infrared luminescence in Ir-Ln heteronuclear coordination polymers via light-harvesting antenna of Ir(III) unit. J. Mater. Chem. C 2014, 2, 1698–1703. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Xu, Y.; Zhao, S.; Sun, Z.; Luo, J. Dynamic Entangled Framework Based on an Iridium-Organic Unit Showing Reversible Luminescence Turn-On Sensing. Inorg. Chem. 2015, 54, 8872–8874. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Lin, C.Y.; Lee, G.H.; Ho, M.L. Four new lead(ii)-iridium(iii) heterobimetallic coordination frameworks: Synthesis, structures, luminescence and oxygen-sensing properties. CrystEngComm 2015, 17, 2129–2140. [Google Scholar] [CrossRef]
- Xu, Y.; Li, L.; Zhang, S.; Zhao, S.; Luo, J. Three Highly Fluorescent Iridium(III) Unit Based Coordination Polymers: Coordinated Solvent-Dependent Photoluminescence. Cryst. Growth Des. 2016, 16, 406–411. [Google Scholar] [CrossRef]
- Rota Martir, D.; Rajamalli, P.; Cordes, D.B.; Slawin, A.M.Z.; Zysman-Colman, E. Marigold Flower-Like Assemblies of Phosphorescent Iridium–Silver Coordination Polymers. Macromol. Rapid Commun. 2018, 39, 1800501. [Google Scholar] [CrossRef]
- Fan, K.; Bao, S.S.; Nie, W.X.; Liao, C.H.; Zheng, L.M. Iridium(III)-Based Metal-Organic Frameworks as Multiresponsive Luminescent Sensors for Fe3+, Cr2O72−, and ATP2− in Aqueous Media. Inorg. Chem. 2018, 57, 1079–1089. [Google Scholar] [CrossRef]
- Wang, C.; Lin, W. Diffusion-controlled luminescence quenching in metal-organic frameworks. J. Am. Chem. Soc. 2011, 133, 4232–4235. [Google Scholar] [CrossRef]
- Liu, D.; Huxford, R.C.; Lin, W. Phosphorescent nanoscale coordination polymers as contrast agents for optical imaging. Angew. Chem. Int. Ed. 2011, 50, 3696–3700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Han, L.; Li, L.; Cheng, J.; Yuan, D.; Luo, J. A highly symmetric metal-organic framework based on a propeller-like Ru-organic metalloligand for photocatalysis and explosives detection. Cryst. Growth Des. 2013, 13, 5466–5472. [Google Scholar] [CrossRef]
- Kobayashi, A.; Ohba, T.; Saitoh, E.; Suzuki, Y.; Noro, S.I.; Chang, H.C.; Kato, M. Flexible coordination polymers composed of luminescent ruthenium(II) metalloligands: Importance of the position of the coordination site in metalloligands. Inorg. Chem. 2014, 53, 2910–2921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, L.; Zhao, S.; Sun, Z.; Hong, M.; Luo, J. Hierarchical metal-organic framework nanoflowers for effective CO2 transformation driven by visible light. J. Mater. Chem. A 2015, 3, 15764–15768. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Zhao, S.; Sun, Z.; Luo, J. Construction of Interpenetrated Ruthenium Metal-Organic Frameworks as Stable Photocatalysts for CO2 Reduction. Inorg. Chem. 2015, 54, 8375–8379. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Kobayashi, A.; Saitoh, E.; Nagao, Y.; Yoshida, M.; Kato, M. Visualization of Ion Conductivity: Vapochromic Luminescence of an Ion-Conductive Ruthenium(II) Metalloligand-Based Porous Coordination Polymer. Inorg. Chem. 2015, 54, 11058–11060. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yin, X.B.; He, X.W.; Zhang, Y.K. Electrochemistry and electrochemiluminescence from a redox-active metal-organic framework. Biosens. Bioelectron. 2015, 68, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Polapally, M.; Chen, B.; Kinyon, J.; Yan, B.; Dalal, N. Self-assembled hybrid solid of luminescent Ru(II)/Cu(II) polypyridyl metal-organic framework. Inorg. Chem. Commun. 2017, 84, 93–95. [Google Scholar] [CrossRef]
- Watanabe, A.; Kobayashi, A.; Saitoh, E.; Nagao, Y.; Omagari, S.; Nakanishi, T.; Hasegawa, Y.; Sameera, W.M.C.; Yoshida, M.; Kato, M. Development of Ion-Conductive and Vapoluminescent Porous Coordination Polymers Composed of Ruthenium(II) Metalloligand. Inorg. Chem. 2017, 56, 3005–3013. [Google Scholar] [CrossRef]
- Kobayashi, A.; Shimizu, K.; Watanabe, A.; Nagao, Y.; Yoshimura, N.; Yoshida, M.; Kato, M. Two-Step Vapochromic Luminescence of Proton-Conductive Coordination Polymers Composed of Ru(II)-Metalloligands and Lanthanide Cations. Inorg. Chem. 2019, 58, 2413–2421. [Google Scholar] [CrossRef]
- Beyene, B.B.; Hung, C.-H. Recent progress on metalloporphyrin-based hydrogen evolution catalysis. Coord. Chem. Rev. 2020, 410, 213234. [Google Scholar] [CrossRef]
- Orzechowska, S.; Mazurek, A.; Świsłocka, R.; Lewandowski, W. Electronic Nose: Recent Developments in Gas Sensing and Molecular Mechanisms of Graphene Detection and Other Materials. Materials 2020, 13, 80. [Google Scholar] [CrossRef]
- Moura, M.M.N.; Faustino, A.F.M.; Neves, G.P.M.S.M. Tetrapyrrolic Macrocycles: Synthesis, Functionalization and Applications 2018. Molecules 2020, 25, 433. [Google Scholar] [CrossRef] [PubMed]
- Tsolekile, N.; Nelana, S.; Oluwafemi, S.O. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.-Y.; Chrzanowski, M.; Ma, S. Metal–metalloporphyrin frameworks: A resurging class of functional materials. Chem. Soc. Rev. 2014, 43, 5841–5866. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Kim, S.-J.; Kim, Y. Porphyrinic metal–organic frameworks from custom-designed porphyrins. CrystEngComm 2016, 18, 345–368. [Google Scholar] [CrossRef]
- Younis, S.A.; Lim, D.-K.; Kim, K.-H.; Deep, A. Metalloporphyrinic metal-organic frameworks: Controlled synthesis for catalytic applications in environmental and biological media. Adv. Colloid Interface Sci. 2020, 277, 102108. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.-Y.; Joseph, E.; Zhou, H.-C. Catalytic Porphyrin Framework Compounds. Trends Chem. 2020. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Zhuang, Y.H.; Shan, D.; Su, G.F.; Cosnier, S.; Zhang, X.J. Zirconium-based porphyrinic metal-organic framework (PCN-222): Enhanced photoelectrochemical response and its application for label-free phosphoprotein detection. Anal. Chem. 2016, 88, 11207–11212. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Jiang, H.L. Porphyrinic Metal-Organic Framework Catalyzed Heck-Reaction: Fluorescence “turn-On” Sensing of Cu(II) Ion. Chem. Mater. 2016, 28, 6698–6704. [Google Scholar] [CrossRef]
- Deibert, B.J.; Li, J. A distinct reversible colorimetric and fluorescent low pH response on a water-stable zirconium-porphyrin metal-organic framework. Chem. Commun. 2014, 50, 9636–9639. [Google Scholar] [CrossRef]
- Ye, Y.; Huang, C.; Yang, J.; Li, Y.; Zhuang, Q.; Gu, J. Highly selective and rapid detection of pentachlorophenol in aqueous solution with metalloporphyrinic MOFs. Microporous Mesoporous Mater. 2019, 284, 36–42. [Google Scholar] [CrossRef]
- Morris, W.; Volosskiy, B.; Demir, S.; Gándara, F.; McGrier, P.L.; Furukawa, H.; Cascio, D.; Stoddart, J.F.; Yaghi, O.M. Synthesis, structure, and metalation of two new highly porous zirconium metal-organic frameworks. Inorg. Chem. 2012, 51, 6443–6445. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shen, S.; Lin, R.; Bai, Y.; Liu, H. Rapid and specific luminescence sensing of Cu(II) ions with a porphyrinic metal-organic framework. Chem. Commun. 2017, 53, 9986–9989. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Z.; Hu, K.; Li, Y.; Feng, J.; Shi, J.; Gu, J. Rapid and Specific Aqueous-Phase Detection of Nitroaromatic Explosives with Inherent Porphyrin Recognition Sites in Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2015, 7, 11956–11964. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Feng, D.; Wang, K.; Gu, Z.Y.; Wei, Z.; Chen, Y.P.; Zhou, H.C. An exceptionally stable, porphyrinic Zr metal-organic framework exhibiting pH-dependent fluorescence. J. Am. Chem. Soc. 2013, 135, 13934–13938. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fukuzumi, S. Change in supramolecular networks through in situ esterification of porphyrins. Eur. J. Inorg. Chem. 2009, 5494–5505. [Google Scholar] [CrossRef]
- Zou, C.; Xie, M.H.; Kong, G.Q.; Wu, C. De Five porphyrin-core-dependent metal-organic frameworks and framework-dependent fluorescent properties. CrystEngComm 2012, 14, 4850–4856. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, L.; Zhang, Z.; Sun, J.; Zhang, Y.; Jiang, J. Synthesis, crystal structures, and fluorescence properties of porphyrin alkaline earth MOFs. Inorg. Chem. Commun. 2018, 95, 36–39. [Google Scholar] [CrossRef]
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A water-stable porphyrin-based metal-organic framework active for visible-light photocatalysis. Angew. Chem. Int. Ed. 2012, 51, 7440–7444. [Google Scholar] [CrossRef]
- Spoerke, E.D.; Small, L.J.; Foster, M.E.; Wheeler, J.; Ullman, A.M.; Stavila, V.; Rodriguez, M.; Allendorf, M.D. MOF-Sensitized Solar Cells Enabled by a Pillared Porphyrin Framework. J. Phys. Chem. C 2017, 121, 4816–4824. [Google Scholar] [CrossRef]
- Johnson, J.A.; Luo, J.; Zhang, X.; Chen, Y.-S.; Morton, M.D.; Echeverría, E.; Torres, F.E.; Zhang, J. Porphyrin-Metalation-Mediated Tuning of Photoredox Catalytic Properties in Metal–Organic Frameworks. ACS Catal. 2015, 5, 5283–5291. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Zou, M.Z.; Zhang, M.; Wang, X.S.; Zeng, X.; Cong, H.; Zhang, X.Z. π-Extended Benzoporphyrin-Based Metal-Organic Framework for Inhibition of Tumor Metastasis. ACS Nano 2018, 12, 4630–4640. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; He, C.; Lin, W. A Chlorin-Based Nanoscale Metal-Organic Framework for Photodynamic Therapy of Colon Cancers. J. Am. Chem. Soc. 2015, 137, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Demel, J.; Kubát, P.; Millange, F.; Marrot, J.; Císařová, I.; Lang, K. Lanthanide-porphyrin hybrids: From layered structures to metal-organic frameworks with photophysical properties. Inorg. Chem. 2013, 52, 2779–2786. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, E.B.; Shachter, A.M. Coordination Oligomers and a Coordination Polymer of Zinc Tetraarylporphyrins. Inorg. Chem. 1991, 30, 3763–3769. [Google Scholar] [CrossRef]
- Pan, L.; Kelly, S.; Huang, X.; Li, J. Unique 2D metalloporphyrin networks constructed from iron(ii) and meso-tetra(4-pyridyl)porphyrin. Chem. Commun. 2002, 2, 2334–2335. [Google Scholar] [CrossRef]
- Son, H.J.; Jin, S.; Patwardhan, S.; Wezenberg, S.J.; Jeong, N.C.; So, M.; Wilmer, C.E.; Sarjeant, A.A.; Schatz, G.C.; Snurr, R.Q.; et al. Light-harvesting and ultrafast energy migration in porphyrin-based metal-organic frameworks. J. Am. Chem. Soc. 2013, 135, 862–869. [Google Scholar] [CrossRef]
- Liu, D.; Liu, T.F.; Chen, Y.P.; Zou, L.; Feng, D.; Wang, K.; Zhang, Q.; Yuan, S.; Zhong, C.; Zhou, H.C. A Reversible Crystallinity-Preserving Phase Transition in Metal-Organic Frameworks: Discovery, Mechanistic Studies, and Potential Applications. J. Am. Chem. Soc. 2015, 137, 7740–7746. [Google Scholar] [CrossRef]
- Deria, P.; Yu, J.; Balaraman, R.P.; Mashni, J.; White, S.N. Topology-dependent emissive properties of zirconium-based porphyrin MOFs. Chem. Commun. 2016, 52, 13031–13034. [Google Scholar] [CrossRef]
- Feng, D.; Chung, W.C.; Wei, Z.; Gu, Z.Y.; Jiang, H.L.; Chen, Y.P.; Darensbourg, D.J.; Zhou, H.C. Construction of ultrastable porphyrin Zr metal-organic frameworks through linker elimination. J. Am. Chem. Soc. 2013, 135, 17105–17110. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Li, Y.; Zhuang, Q.; Zhao, W.; Gu, J. Porphyrinic MOFs for reversible fluorescent and colorimetric sensing of mercury(II) ions in aqueous phase. RSC Adv. 2016, 6, 69807–69814. [Google Scholar] [CrossRef]
- Guo, L.; Wang, M.; Cao, D. A Novel Zr-MOF as Fluorescence Turn-On Probe for Real-Time Detecting H2S Gas and Fingerprint Identification. Small 2018, 14, 1703822. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; He, C.; Lin, W. Nanoscale metal-organic framework for highly effective photodynamic therapy of resistant head and neck cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Son, H.J.; Farha, O.K.; Wiederrecht, G.P.; Hupp, J.T. Energy transfer from quantum dots to metal-organic frameworks for enhanced light harvesting. J. Am. Chem. Soc. 2013, 135, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Zha, Q.; Ding, C.; Rui, X.; Xie, Y. A novel porphyrin-based ligand containing four 4,4′-dipyridylamine moieties: Syntheses, structures, and luminescent properties of Mn(II), Cu(II), Zn(II), and Cd(II) coordination polymers. Cryst. Growth Des. 2013, 13, 4583–4590. [Google Scholar] [CrossRef]



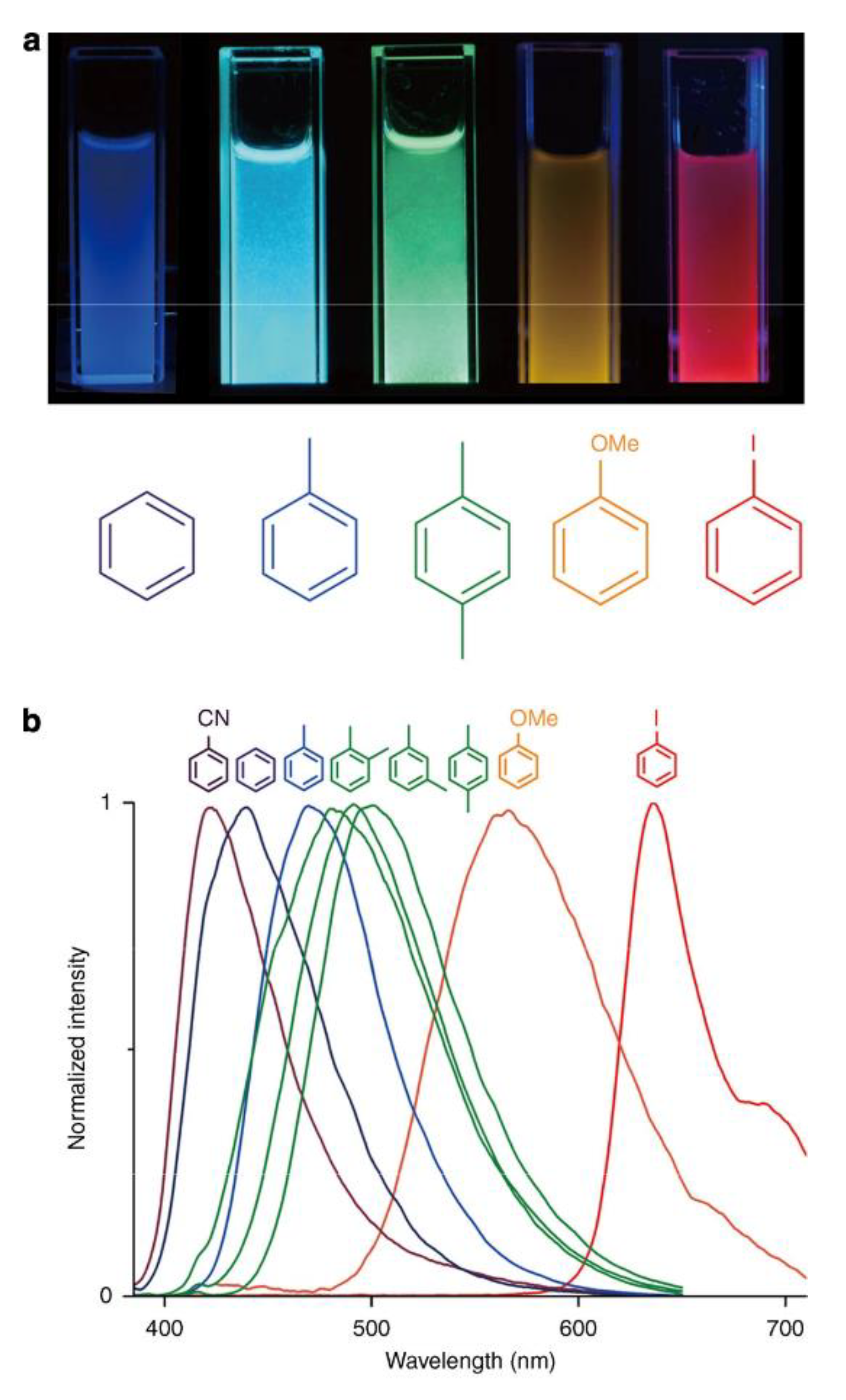

| Formula | λex, nm | λem, nm | QY, % | Reference |
|---|---|---|---|---|
| {[Zn4O(sdc)3(dmf)]·CHCl3}n | 350 | 441 | – | [75] |
| [Zn3(sdc)3(dmf)2]n | 325 | 390 | – | [75] |
| {[Zn2(sdc)2(AnEPy)]·2DMA·1.5H2O}n | – | 560 | 20.8 | [79] |
| {[Zn(sdc) (bmp)]·H2O}n | 328 | 398 | – | [80] |
| {[Ni(sdc)(bimb)]·DMF}n | 303 | 393 | – | [80] |
| {[Zn2(dmtrz)2(sdc)]·6H2O}n/ MAC-11 | 250 | 418–453 | – | [81] |
| [Cd(sdc)(bmib)]n | 250 | 399 | – | [82] |
| {[Zn(sdc)(tib)]·0.5H2O}n | 250 | 410 | – | [82] |
| {[Zn(sdc)(bmib)]·0.4H2O}n | 245 | 384 | – | [83] |
| {[Zn(sdc)(bibd)]·DMF}n | 240 | 401 | – | [83] |
| {[Zn2(sdc)2(bips)2]·5H2O}n | 337 | 446 | – | [84] |
| {[Zn(sdc)(bimb)]·DMF}n | 390 | 455 | 82.0 | [85] |
| {[Zn4(tppa)2(sdc)3(NO3)2]·4DMF·2MeCN}n | 365 | 525 | 20.6 | [86] |
| [Zn3(sdc)3(4,4′-bpy)]n | 350 | 487 | – | [87] |
| [Zn3(sdc)3(py)2]n | 350 | 460 | – | [87] |
| {[Zn3(sdc)3(bpea)] 3H2O}n | 350 | 469 | – | [87] |
| [Zn(sdc)(H2O)]n | 387 | 435, 459 | – | [88] |
| [Zn(sdc)(bita)]n | 367 | 470 | – | [89] |
| [Zn2(sdc)2(pvsp)]n | 425 | 484 | – | [90] |
| {[Zn(sdc)(4,4′-bpy)]·2DMF}n | 417 | 505 | – | [90] |
| {[Zn4(sdc)4(beips)2]∙14DMF}n | 406 | 437, 464 | – | [91] |
| [Cd(sdc)(H2O)]n | 392 | 460 | – | [92] |
| {[Cd(ttr4a)(sdc)]·1.5H2O}n | 367 | 447 | – | [93] |
| {[Cd3(sdc)2(trz)2(H2O)2]·DMF}n | 320 | 443 | 37.8 | [94] |
| [Cd3(sdc)3(dma)]n | – | 450 | – | [95] |
| {[Cd3(sdc)(phen)3(OH)3(H2O)]·0.5sdc·4H2O}n | 386 | 489 | – | [96] |
| {[Yb(sdcNH2)(HCOO)(H2O)]·2DEF}n | 382 | 528 | – | [97] |
| {[Tm(sdcNH2)(HCOO)(H2O)]·2DEF}n | 345 | 518 | – | [97] |
| [Cd2(sdcNH2)(NO3)2(dmf)4]n | 351, 376 | 475, 495 | – | [97] |
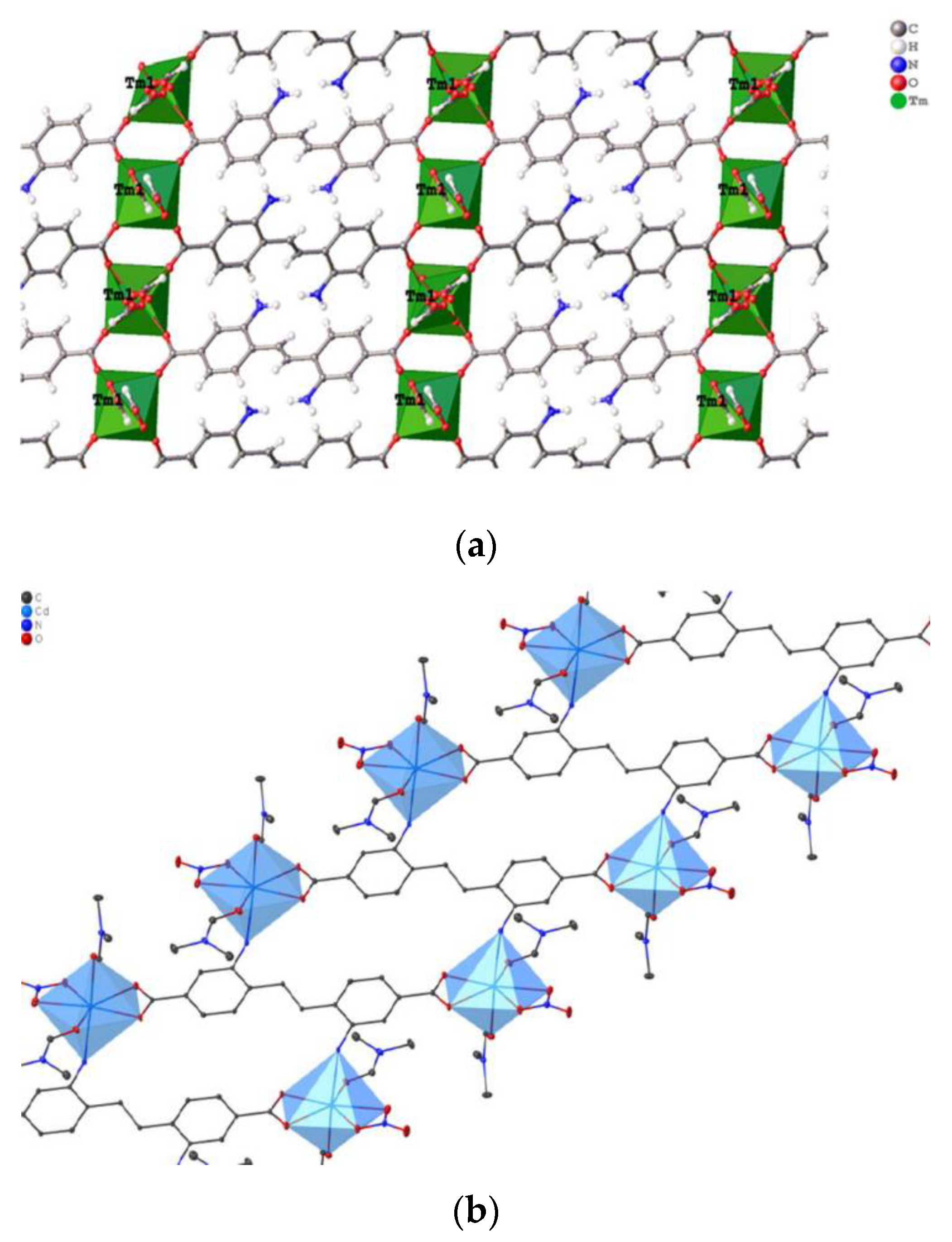
| [Tm3O5(sdc)4]n | 341 | 475 | – | [98] |
| {[Tb2(sdc)3(dmso)4]·DMSO}n | – | 447 | – | [99] |
| {[Eu2(sdc)3(dmso)4]·DMSO}n | – | 447 | – | [99] |
| [Eu2(MeS-sdc)3(dmf)2(H2O)2]n | 393, 464, 535, 370 | 579, 591, 615, 696 | – | [99] |
| [Tb2(MeS-sdc)3(dmf)2(H2O)2]n | 400, 487 | 542, 584, 620, 600 | – | [99] |
| [Mn3(sdc)3(H2O)2]n | 347 | 447 | – | [100] |
| [Mn(sdc)(H2O)2]n | 347 | 466 | – | [100] |
| H2sdc (free ligand) | 250 | 381 | – | [82] |
| Formula | λex, nm | λem, nm | λem, nm (ligand) | Reference |
|---|---|---|---|---|
| {[Zn2(Py2TTz)(2-CH3-bdc)2]∙2DMF∙3H2O}n | 413 | 485 | 439, 452 | [110] |
| {[Cd2(Py2TTz)2(2-CH3-bdc)2]·2DMF·2EtOH}n | 422 | 506 | 439, 452 | [110] |
| {[Zn(Py2TTz)0.5(2,6-ndc)]·DMF·EtOH}n | 424 | 463 | 439, 452 | [110] |
| {[Zn(Py2TTz)(1,4-ndc)]·DMF·H2O}n | 434 | 508 | 439, 452 | [110] |
| {[Zn0.5(Py2TTz)0.5(2,5-di-CH3-bdc)0.5]·DMF}n | 423 | 464 | 439, 452 | [110] |
| {[Cd2(Py2TTz)2(2,6-ndc)2]∙3DMF∙4H2O}n | 424 | 519 | 439, 452 | [110] |
| {[Zn2(Py2TTz)2(5-CH3-bdc)2]·3DMF·3H2O}n | 404 | 497 | 439, 452 | [110] |
| {[Cd2(Py2TTz)2(5-CH3-bdc)2]·3DMF·3H2O}n | 424 | 515 | 439, 452 | [110] |
| [Zn2(oba)2(bptp)]n | 396 | 431 | 474 | [111] |
| [Ni(oba)2(bptp)2(H2O)2]n | 408 | 487 | 474 | [111] |
| [Cd2(oba)2(bptp)(H2O)]n | 391 | 489 | 474 | [111] |
| {[Cd(dpbt)(bdc)]·2H2O}n | 356 | 464 | 465 | [112] |
| {[Zn2(dpbt)2(ipa)2]·2DMA}n | 375 | 513 | 465 | [112] |
| {[Zn4(dpbt)2(bdc)4]∙H2O∙2dpbt}n | 364 | 478 | 465 | [113] |
| {[Cd2(dpbt)2(bdc)2]∙DMA}n | 372 | 514 | 465 | [113] |
| {[Zn2(bptda)(oba)2]·2.75DMF}n | 367 | 550 | 560 | [114] |
| {[Zn4(bptda)3(oba)4]·2H2O}n | 367 | 550 | 560 | [114] |
| [Zn2(bptda)(sdba)2]n | 367 | 520 | 560 | [114] |
| {[S@Cd6(btdc)6]∙9H2O}n | 370 | 450 | 470 | [115] |
| {[S@Cd6(btdc)6]∙6H2O}n | 370 | 450 | 470 | [115] |
| Zr-btdb-fcu-MOF | 365 | 501 | 480 | [116] |
| Zr-btdb-fcu-MOF | 450 | 530 | 480 | [117] |
| {[Zn(btdb)(DMA)]∙H2O}n | 370 | 494 | 480 | [118] |
| {[Co3(bibt)3(btc)2(H2O)2]·solvents}n | 394 | 396530 1 | 540 | [119] |
| Formula | Ligands | λex, nm | λem, nm | λem, nm (ligand) | Ref. |
|---|---|---|---|---|---|
| {[Cd(4-pytpy)(1,4-ndc)]∙1.5H2O}n |  | 310 | 523 | 380 | [120] |
| {[Cd(4-pytpy)(2,5-tdc)]∙H2O}n |  | 310 | 526 | 380 | [120] |
| [Cd2(4-pytpy)(sdba)2]n |  | 310 | 373 | 380 | [120] |
| {[Zn6(4-pytpy)3(mal)4]∙5H2O}n | 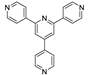 | 310 | 385 | 375 | [121] |
| {[Zn(2-pytpy)(fum)]∙H2O}n | 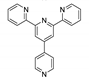 | 310 | 383 | 375 | [121] |
| {[Zn(H2btca)2(bpt)]∙H2O}n | 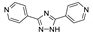 | 396 | 446, 575 | 477 | [122] |
| [Zn2(btca)(4-PyBIm)(H2O)]n | 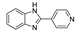 | 366 | 460 | 414, 516 | [122] |
| {[Zn1.5(Hbtca)(pytpy)]∙H2O}n | 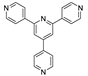 | 420 | 466 | 375 | [122] |
| [Zn(bdc)(3,6-bimcz)]n | 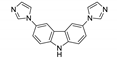 | 339 | 401 | 440 | [123] |
| {[Zn(p-pda)(3,6-bimcz)]∙1.5H2O}n |  | 319 | 403 | 440 | [123] |
| {[Zn(bpda)(3,6-bimcz)]∙0.25H2O}n | 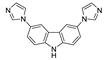 | 428 | 528 | 440 | [123] |
| {[Zn(ipa)(abimb)]∙H2O}n |  | 350 | 407 | 426 | [124] |
| {[Zn(Br-ipa)(abimb)]2∙0.5H2O}n | 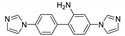 | 369 | 419 | 426 | [124] |
| [Zn(ipa)(3,6-bimcz)]n | 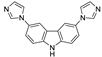 | 263 | 401 | 440 | [124] |
| [Zn(Br-ipa)(3,6-bimcz)]n | 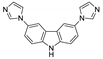 | 263 | 398 | 440 | [124] |
| [Zn(ipa)(nbimb)]n | 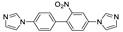 | 515 | 627 | 431 | [124] |
| [Zn(Br-ipa)(nbimb)]n |  | 482 | 591 | 431 | [124] |
| {[Zn(ipa)(bimpa)]∙2H2O}n | 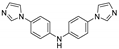 | 365 | 416 | 405 | [124] |
| {[Zn(Br-ipa)(bimpa)]∙2H2O}n | 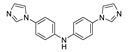 | 379 | 451 | 405 | [124] |
| [Cd(NH2bdc)(bmcz)]n | 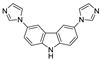 | 313 | 440 | 440 | [125] |
| [Cd(1,4-ndc)(bmcz)]n | 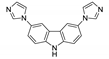 | 318 | 429 | 440 | [125] |
| {[Cd3(cpczdc)2(H2O)5]∙4H2O}n |  | 366 | 420 | 420 | [126] |
| {[Zn(Hcpczdc)(4,4′-bpy)0.5(H2O)]·2H2O}n | 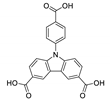 | 352, 297 | 512, 433 1 | 420 | [127] |
| {[Cd(1,4-ndc)(dia)]∙MeOH}n |  | 380 | 428 | 474 | [128] |
| {[Cd(bpdc)(dia)0.5]∙DMF}n | 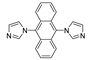 | 370 | 434 | 474 | [128] |
| {[Cd(bpdc)(dia)]∙DEF}n | 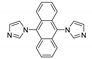 | 338 | 425 | 474 | [128] |
| {[Cd(tzmb)(1,4-bimb)0.5]∙2.5H2O}n | 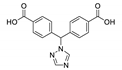 | 280 | 461 | 461 | [129] |
| {[Zn(dipm)(bdc)]∙CH3OH∙H2O}n | 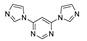 | 365 | 475 | 475 | [130] |
| {[Zn(dipm)(NH2bdc)]∙2H2O}n |  | 365 | 525 | 475 | [130] |
| [Zn(dipm)(OHbdc)]n | 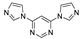 | 365 | 465 | 475 | [130] |
| {[Zn(dia)2](ClO4)2∙xMeOH⋅yDCM}n | 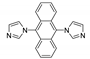 | 350 | 450 2 | 433 | [131] |
| {[Zn(dia)2](BF4)2∙xMeOH⋅yDCM}n | 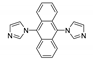 | 350 | 450 3 | 433 | [131] |
| {[Zn(dia)2](p-Tos)2∙xMeOH⋅yDCM}n | 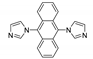 | 350 | 460 4 | 433 | [131] |
| {[Cd(dia)2(p-Tos)2]⋅m(DCM)}n | 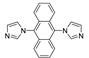 | 350 | 460 5 | 433 | [131] |
| {[Cd(dia)2(p-Tos)2]⋅xMeOH⋅yDioxane}n | 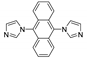 | 350 | 465 6 | 433 | [131] |
| {[Zn(dia)2(CF3CO2)2]⋅2Dioxane}n | 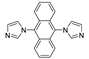 | 350 | 450 7 | 433 | [131] |
| {[Zn2(phda)2(4-bpo)2]∙2H2O}n | 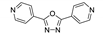 | 316 | 383, 471 | 477 | [132] |
| [Zn(phda)(4-bpt)]n | 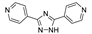 | 310 | 371 | – | [132] |
| [Zn(phda)(bib)]n∙H2O | 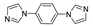 | 340 305 | 403 377 | 408 | [132] |
| {[Zn2(1,4-ndc)2(3-abpt)]∙2DMF}n | 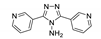 | 343 | 419 | 423 | [133] |
| {[Cd(1,4-ndc)(3-abit)]∙H2O}n | 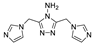 | 350 | 428 | 430 | [133] |
| {[Zn(btrbdc)]∙2.7DMF}n |  | 380 | 432 | 380 | [134] |
| [Cd(btb)(H2O)2(HCOOH)(ipa)2]n | 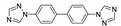 | 350, 360 | 550,450 | 450 | [135] |
| [Cu(btb)(pydc)(H2O)]n | 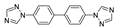 | 360 | 410 | 439 | [136] |
| [Cu(btb)0.5(nph)(H2O)]n | 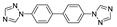 | 210 | 333 | 439 | [136] |
| [Ni3(btb)4(nbta)2(H2O)4]n | 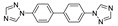 | 230 | 418 | 439 | [137] |
| [Cu2(btb)(CN)2]n | 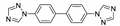 | 300 | 403 | 439 | [138] |
| [Ag2(btb)(muco)]n |  | 360 | 388 | 439 | [138] |
| [Cu(btb)(CN)]n |  | 280 | 382 | 441 | [139] |
| [Cu2(bdmbib)(CN)2]n | 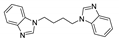 | 280 | 377 | 368 | [139] |
| [Cu2(bmbip)(CN)2]n | 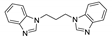 | 280 | 396 | 349 | [139] |
| [Cd(tzmb)(bbibp)]n | 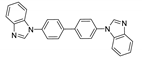 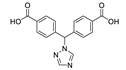 | 285 | 360 | 400, 420 | [140] |
| [Cd(Hcbic)]n | 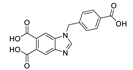 | 320 | 377 | 407 | [141] |
| [CdI2(3-PyBim)](H2O)3] | 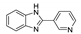 | 280 | 404 | 417, 540 | [142] |
| [Cd(SO4)(3-PyBim)(H2O)4] | 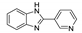 | 280 | 413 | 417, 540 | [142] |
| [CdCl2(4-PyBim)2(H2O)2] | 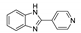 | 280 | 451 | 411, 516 | [142] |
| [CdBr2(4-PyBim)2(H2O)2] | 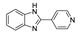 | 280 | 444, 540 | 411, 516 | [142] |
| [CdI2(4-PyBim)2(H2O)2] | 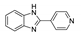 | 280 | 435 | 411, 516 | [142] |
| {[Zn2(tzmb)2(bim)]∙2DMA∙EtOH}n |  | 350 | 445 | 430–470 | [143] |
| {[Zn2(tzmb)2(4,4′-bipb)∙2DMA⋅EtOH]}n | 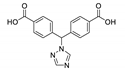 | 275 | 450 | 430–470 | [143] |
| {[Cd(tzmb)(bbibp)]∙DMA∙EtOH}n | 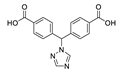 | 290 | 405 | 430–470 | [143] |
| [Zn(pypymba)2]n | 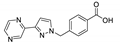 | 380 | 417 | 431 | [144] |
| [Co(pypymba)2]n | 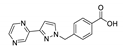 | 288 | 436 | 431 | [144] |
| [Cd(pypymba)2]n | 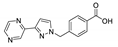 | 398 | 506 | 431 | [144] |
| [Cd(Hpypymba)Cl2]n | 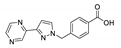 | 344 | 380 | 431 | [144] |
| {[Cd(pypymba)Cl]∙EtOH∙H2O}n | 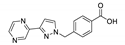 | 366 | 423 | 431 | [144] |
| [Cd(pypyaa)Cl]n | 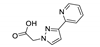 | 325 | 378 | 472 | [144] |
| {[Cd2(pyznpy)2Cl2H2O]∙H2O}n | 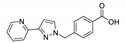 | 355 | 405 | 480 | [144] |
| {[Cd(atbdc)]·DMF}n | 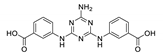 | 338 | 397 | – | [145] |
| [Tb(Hpia)(pia)(H2O)2]n | 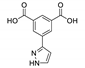 | 254 | 493, 456, 589, 624 | 393 | [146] |
| [Zn2(tyty)(µ3-OH)(MoO4)(H2O)]n | 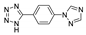 | 300 | 409 | 370 | [147] |
| {[Zn(dptmia)]∙2H2O·0.5DMF}n | 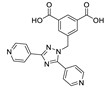 | 350 | 430 | 440 | [148] |
| {(Me2NH2)[Zn2(dcpp)(OAc)]∙0.5DMF}n | 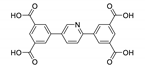 | 320 | 428 | 375 | [149] |
| {[Eu4(pta)5(Hpta)2(H2O)4]·9H2O}n | 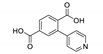 | 366 | 581, 593, 613, 653 | 425 | [150] |
| {[Tb(Hpta)(C2O4)]·3H2O}n | 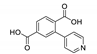 | 376 | 490, 546, 585, 622 | 425 | [150] |
| {[Cd2(bptp)2(H2O)4]∙3H2O}n | 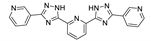 | 363 | 410 | 523 | [151] |
| {[Cd2(Hbptp)(btc)(H2O)]∙4H2O}n |  | 396 | 464 | 523 | [151] |
| {[Cd2(H2bptp)(btec)]∙H2O}n | 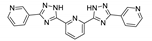 | 284 | 456 | 523 | [151] |
| {[Zn2(Htpim)(3,4-pydc)2]∙4DMF·4H2O}n | 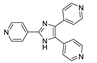 | 380 | 423, 466, 515 | – | [152] |
| [Cd(p-CNPhHIDC)(4,4′-bipy)0.5]n | 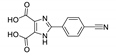 | 380 | 441 | 489 | [153] |
| [Zn(p-CNPhHIDC)(4,4′-bipy)]n | 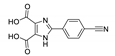 | 370 | 457 | 489 | [153] |
| {[Zn(pvb)2]⋅DMF}n | 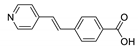 | 340 | 460 8 | 460 | [154] |
| [Zn(pvb)2]n | 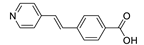 | 340 | 478 9 | 460 | [154] |
| Formula | Ligand | λex, nm | λem, nm | ΦPL, % | λem, nm (ligand) | Ref. |
|---|---|---|---|---|---|---|
| {[Zn3(L1)2(btc)2(H2O)]·guests}n | 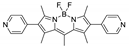 | 512 | 540 | – | 618 660 sh. | [160] |
| {[Cd(L1)(bdc)]·guests}n | 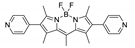 | 512 | 540 | – | 618 660 sh. | [160] |
| [Cd2(L2)2(bpdc)2]n/CCNU-11 | 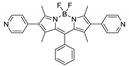 | 440 | 620 | – | 540 | [161] |
| [Cd2(L2)2(sdb)2]n/CCNU-12 | 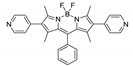 | 440 | 620 | – | 540 | [161] |
| {[Zn2(L2)2(bpdc)2]·H2O}n | 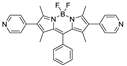 | 440 | 600 | – | 540 | [162] |
| {[Zn2(L3)2(bpp)]·2H2O·2EtOH}n |  | 450 | 577 | 0.82 | 577 | [163] |
| {[Cd2(L3)2(bpp)]·2H2O·EtOH}n | 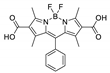 | 450 | 602 | 0.67 | 577 | [163] |
| {[Cd2(L3)(bpe)3(NO3)2]··2H2O·DMF·EtOH}n | 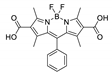 | 450 | 574 | 1.7 | 577 | [163] |
| {[Cd(L3)(bpe)0.5(dmf)(H2O)]}n |  | 450 | 596 | 0.99 | 577 | [163] |
| {[Cd(L3)(bpe)0.5] 1.5H2O·DMF}n | 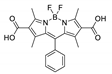 | 450 | 573 | 0.76 | 577 | [163] |
| [Zn2(dbtcpb)(L2)] |  | 520 | 596 | – | 540 | [164] |
| [Zn(Zntpcp)(L2)] |  | 520 | 667 | – | 540 | [164] |
| {[Ag2(L4)2(MeOH)](AsF6)2}n | 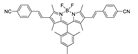 | 530 | 750 790 | – | – | [165] |
| Formula | Ligand | λex, nm | λem, nm | ΦPL, % | Ref. |
|---|---|---|---|---|---|
| {[Cd(bipatc)0.5(bib)(H2O)]·3.5H2O}n |  | – | 325 | – | [168] |
| {[Cd2(bipatc)(bibp)(H2O)]·H2O}n |  | – | 350 | – | [168] |
| {[Zn(bipatc)0.5(4,4′-bpy)(H2O)]·3.5H2O}n | 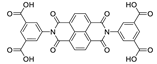 | 350 | 550 | – | [169] |
| [Cd(bdc)(PzNDI)]n | 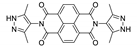 | 345 | 570 | – | [170] |
| {[Cd(2,6-ndc)(PzNDI)]·2H2O}n | 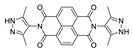 | 345 | 577 | – | [170] |
| {[Zn(2,6-ndc)(isondi)(H2O)2]·2H2O}n | 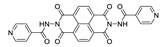 | – | 660 | 0.02 | [171] |
| {[Cd(L6)I2]}n |  | 300 | 437 | – | [172] |
| {[Cd(dpmni)(NCS)2]·2.5H2O}n | 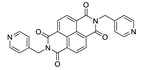 | 465 | 575 | – | [172] |
| [Zn2(dpmni)(tdc)2]n | 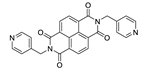 | 485 | – | – | [173] |
| [Zn(GlyNDI)(dmf)2]n | 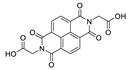 | 320 | 458 | – | [174] |
| {[Zn2(bindi)(dma)2]·2DMA}n | 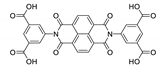 | 280 | 585 | – | [175] |
| {[Cd(H4bindi)(H2bindi)]·4DMF}n | 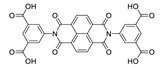 | 280 | 585 | – | [175] |
| {[Ca2(Hbindi)Cl(dma)3]·DMA}n | 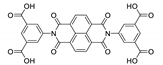 | 280 | 499 | – | [175] |
| {[Ba4(bindi)2(dmf)7]·7DMF} | 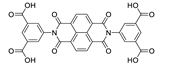 | 280 | 467 | – | [175] |
| [Cd(dpmni)(ipa)]n | 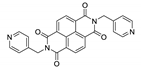 | 380 | 424, 452, 475 | – | [176] |
| [Cd(dpmni)(bdc)]n | 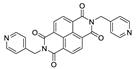 | 350 | 484 | – | [176] |
| [Cd(isondi)(2,6-ndc)(H2O)2]n | 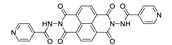 | 380 | 628, 403 | – | [177] |
| [Cd(isondi)(ipa)(dmf)]n | 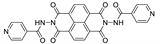 | 375 | 418, 438 | – | [177] |
| [Cd2(isondi)2(bdc)0.5(HCOO)2]n | 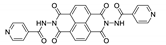 | 390 | 534, 570, 612 | – | [177] |
| [C88H100F4W24N16O96P2Zn3]n | 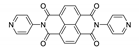 | 350 | 450 | – | [178] |
| [Zn(dpndi)Cl2]n |  | 490 | 595 | – | [179] |
| [Zn(dpndi)Br2]n |  | 490 | 595 | – | [179] |
| [Zn(dpndi)I2]n |  | 490 | 595 | – | [179] |
| {[Cd(dpmni)2(ClO4)2]·4CHCl3}n |  | 340 | 467 | – | [180] |
| [Zn(AlaNDI)]n | 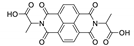 | 360 | 475 | – | [181] |
| {[Cd2(3-imntd)2I4]·2H2O}n |  | 309 | 410, 590 | – | [182] |
| [Zn2(NH2bdc)2(dpndi)]n | 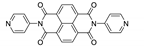 | 330 | 430 | – | [183] |
| {[Zn2(hfipbb)2(dpndi)]⋅8DMF}n | 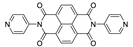 | 360 | 415 | – | [184] |
| [Zn(tzndi)(dmf)2]n/NBU-3 |  | 322 | 468 | – | [185] |
| [Zn2(bdc)2(dpndi)]n⋅benzonitrile | 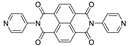 | 370 | 421 | 1 | [186] |
| [Zn2(bdc)2(dpndi)]n⋅benzene | 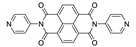 | 370 | 439 | 5 | [186] |
| [Zn2(bdc)2(dpndi)]n⋅toluene | 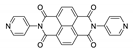 | 370 | 476 | 22 | [186] |
| [Zn2(bdc)2(dpndi)]n⋅o-xylene | 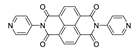 | 370 | 496 | 5 | [186] |
| [Zn2(bdc)2(dpndi)]n⋅m-xylene | 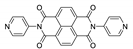 | 370 | 503 | 9 | [186] |
| [Zn2(bdc)2(dpndi)]n⋅p-xylene |  | 370 | 518 | 19 | [186] |
| [Zn2(bdc)2(dpndi)]n⋅anisole | 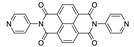 | 370 | 592 | 1 | [186] |
| [Zn2(bdc)2(dpndi)]n⋅iodobenzene | 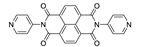 | 370 | 640 | 3 | [186] |
| [Cd(3-pmntd)2(NO3)2]n | 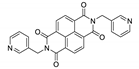 | 447 | 557 | – | [187] |
| [Ag(2,6-ndc)0.5(dpndi)0.5(H2O)]n | 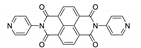 | 320 | 370 | – | [188] |
| [Mg2(bindi)2(dmf)2(H2O)]n | 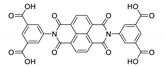 | 515 | 570 | – | [189] |
| {[Ca2(bindi)(dmf)4]·2DMF}n | 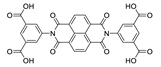 | 505 | 575 | – | [190] |
| [Li2(GABA-NDI)]n | 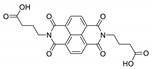 | 407 | 437 | 3 | [191] |
| [Na(HGABA-NDI)]n | 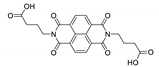 | 407 | 471 | 1 | [191] |
| [K(HGABA-NDI)]n | 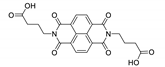 | 407 | 455 | 5 | [191] |
| [Ca(GABA-NDI)(MeOH)(dmf)]n | 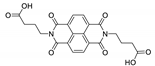 | 407 | 456 | 1 | [191] |
| [Ca(GABA-NDI)(MeOH)]n | 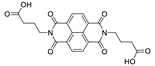 | 407 | 466 | 1 | [191] |
| [Mg(GABA-NDI)(MeOH)2]n | 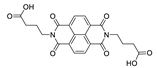 | 407 | 471 | 6 | [191] |
| [Cd(GABA-NDI)(MeOH)2]n |  | 407 | 468 | 1 | [191] |
| [Mn(GABA-NDI)(MeOH)2]n |  | 407 | 457 | 3 | [191] |
| [Ni(GABA-NDI)(MeOH)2]n | 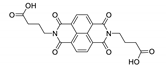 | 407 | 467 | 3 | [191] |
| [Co(GABA-NDI)(EtOH)2]n |  | 407 | 463 | 1 | [191] |
| [Zn(GABA-NDI)(dmf)]n | 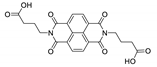 | 407 | 438 | 2 | [191] |
| [La2(GABA-NDI)3(dmf)]n | 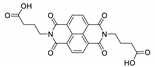 | 407 | 453 | 6 | [191] |
| [Eu2(GABA-NDI)3(dmf)]n | 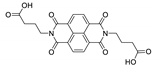 | 407 | 453 | 1 | [191] |
| [Tb2(GABA-NDI)3(dmf)]n | 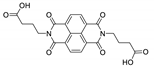 | 407 | 453 | 5 | [191] |
| [Sm2(GABA-NDI)3(dmf)]n | 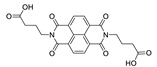 | 407 | 452 | 1 | [191] |
| [Gd2(GABA-NDI)3(dmf)]n |  | 407 | 460 | 1 | [191] |
| H4bindi (free ligand) | 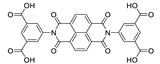 | – | no emission | – | [190] |
| L6 (free ligand) | 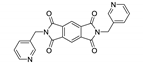 | 300 | 425 | – | [172] |
| DPNDI (free ligand) |  | – | 375 | – | [188] |
| GlyNDI (free ligand) | 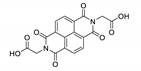 | 320 | 430 457 525 | – | [174] |
| ISONDI (free ligand) | 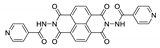 | 380 | 519 549 584 | – | [177] |
| DPMNI (free ligand) |  | 350 | 484 | – | [176] |
| H2AlaNDI (in DCM) | 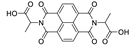 | 360 | 475 | – | [181] |
| 3-IMNTD (free ligand) | 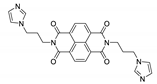 | 375 | 390, 410 | – | [182] |
| H2tzndi (free ligand) | 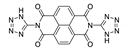 | 328 | 470 | – | [185] |
| H2PzNDI (free ligand) | 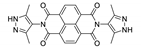 | 345 | 590 | – | [170] |
| Na2GABA-NDI (in water) | 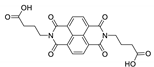 | 360 | 395 420 | – | [191] |
| Formula | λex, nm | λem, nm | ΦPL, % | Reference |
|---|---|---|---|---|
| {(Me2NH2)2[Zn2(ntb)2(py)2]·DMF·2H2O}n | 360 | 458 | – | [192] |
| {[Zn2(ntb)(H2ntb)(bipy)]·1.5H2O·Guest}n | 342 | 524 | – | [192] |
| {(Me2NH2)2[Zn2(ntb)2(bipy)]·2DMF}n | 351 | 490 | – | [192] |
| [Eu(ntb)(ndc)(H2O)]n | 402 | 593 614 698 | 0.06 | [193] |
| [Tb(ntb)(ndc)(H2O)]n | 374 | 488 544 589 620 | 11 | [193] |
| {[Cd2.5Na(ntb)2(dmf)4]·3DMF}n | 370 | 445 | – | [194] |
| [Cd5(ntb)4(H2O)2]n | 352 | 417 | – | [195] |
| {[Tb3(ntb)2(dma)0.5(OH)3(H2O)0.5]·3H2O}n | 395 | 497 545 587 620 | – | [196] |
| {[Cd3(ntb)2(bimb)]·6DMA}n | 360 | 470 | – | [197] |
| [Mn3(ntb)(HCOO)3(def)3]n | 320 | 421 | – | [198] |
| {(Me2NH2)2[Mn5(ntb)4(H2O)2]·4DMF·2H2O}n | 362 | 453 | – | [198] |
| {[Mn3(ntb)2(py)4(H2O)]·H2O}n | 351 | 453 | – | [198] |
| {[Zn3(tppe)0.5(tnb)2]·4DMA·7H2O}n | – | 475 | – | [199] |
| {[Cd1.5(ntb)(bpa)0.5(EtOH)]·1.5EtOH}n | 350 | 450 | – | [200] |
| {(Me2NH2)[In(tnb)4/3]·2DMF·3H2O}n | – | 478 | – | [201] |
| [Zn(μ2-Htnb)(phen)]n | – | 513 | 5 | [202] |
| {[Cd(μ3-Htnb)(phen)]·2H2O}n | – | 515 | <1 | [202] |
| {[Ti6(μ3-O)6(μ2-OH)6(tnb)2]·H2O·2DMF}n | – | ≈460 | – | [203] |
| {(Me2NH2)2[Cd3(ntb)2(bdc)]}·4DMF}n | 311 | 440 | – | [204] |
| Cd-TCOOH | 350 | 450 | – | [205] |
| {[Cd3(ntb)2(2,2′-bipy)2]·3DMF·2EtOH·H2O}n | – | 532 | – | [206] |
| {[Zn3(ntb)2(2,2′-bipy)2(dmf)2]·2DMF·2EtOH·H2O}n | – | 528 | – | [206] |
| {(Et2NH2)[In(bcbaip)]·4DEF·4EtOH}n | 381 | 450 | – | [207] |
| {[Cd3(H2O)(bcbaip)2][Cd(im)3(H2O)2]·3H2O}n | 300 | 410 | – | [208] |
| {[La2(bcbaip)2(dma)2]·nH2O}n | 398 | 428 | 3.0 | [209] |
| {[Pr2(bcbaip)2(dma)2]·nH2O}n | 420 | 1227 | 1.4 | [209] |
| {[Nd2(bcbaip)2(dma)2]·nH2O}n | 382 | 1054 | 3.5 | [209] |
| {[Sm2(bcbaip)2(dma)2]·nH2O}n | 417 | 642 | 2.7 | [209] |
| {[Eu2(bcbaip)2(dma)2]·nH2O}n | 398 | 614 | 21.8 | [209] |
| {[Gd2(bcbaip)2(dma)2]·nH2O}n | 385 | 490 | 2.4 | [209] |
| {[Tb2(bcbaip)2(dma)2]·nH2O}n | 379 | 542 | 20.6 | [209] |
| {[Tm2(bcbaip)2(dma)2]·nH2O}n | 380 | 421 | 2.3 | [209] |
| [Zn2(bcbaip)]n | 325 | 416 | – | [210] |
| {[Cd3(bcbaip)2(H2O)][Na2(μ2-H2O)(H2O)7]}n | 325 | 411 | – | [210] |
| {[Zn2(bcbaip)(phen)2(H2O)]·H2O}n | 325 | 520 | – | [210] |
| [ZnLi2(bcbaip)(dmf)(H2O)]n | 368 | 420 | 10.8 | [211] |
| [Zn2(bcbaip)]n | 370 | 520 | – | [212] |
| {[Cd(H2bcbaip)(H2O)2]·H2O}n | 335 | 405 450 510 | – | [212] |
| {[Pb2(bcbaip)(H2O)]·3H2O·DMF}n | 368 | 491 | – | [212] |
| {(Me2NH2)[Sm2(bcbaip)2]·H2O}n | 370 | 410 563 600 645 | – | [213] |
| {(Me2NH2)[Tb2(bcbaip)2]·H2O}n | 370 | 489 545 585 620 | – | [213] |
| {(Me2NH2)[Dy2(bcbaip)2]·H2O}n | 370 | 410 480 573 662 | – | [213] |
| {(Me2NH2)[Eu2(bcbaip)2]·H2O}n | 370 | 591 614 | – | [214] |
| {(Me2NH2)2[Ln2(bcbaip)2]·DMA}n | 395 | 594 618 | – | [215] |
| [Li4(bcbaip)(dmf)2]n | – | 402 | – | [216] |
| {[Zn2(bca)2(o-bimb)2]·(H2O)2}n | 325 | 405 | – | [217] |
| {[Zn(bca)(m-bib)]·H2O}n | 325 | 407 | – | [217] |
| {[Gd(tcba)(H2O)2]2·DMF}n | 380 | 462 | – | [218] |
| {[Tb(tcba)(H2O)2]2·DMF}n | 380 | 491 545 587 623 | – | [218] |
| {[Eu(tcba)(H2O)2]2·DMF}n | 380 | 480 593 614 653 689 | – | [218] |
| [Zn2(tpbd)(dma)2]n | 400 | 520 | 24 | [219] |
| [Cd2(tpbd)(H2O)4]n | 400 | 521 | 52 | [219] |
| H3ntb (free ligand) | 333 | 433 | – | [192] |
| H4bcbaip (free ligand) | 396 | 424 | – | [209] |
| H2bca (free ligand) | – | 310 | – | [217] |
| H3tcba (free ligand) | 380 | 462 | – | [218] |
| H4tpbd (free ligand) | 372 | 573 | 19 | [219] |
| Formula | λex, nm | λem, nm | ΦPL, % | λem, nm (ligand) | Ref. |
|---|---|---|---|---|---|
| {[Zn4(μ4-O)(Ir(3-cppy)3)2]·6DMF·H2O}n | 385 | 538 | – | 538 | [220] |
| {[Zn3(Ir(4-cppy)3)2(DEF)2(H2O)2]·DEF·H2O}n | 400 | 565 | – | 565 | [220] |
| {[Zn3(Ir(4-cppy)3)2(DMF)(H2O)3]·2DMF·3H2O}n | 385 | 538 | – | 565 | [220] |
| {Zr6(µ3-OH)4(µ3-OH)4(bpdc)6-x[Ir(ppy)2(5,5′-dcbpy)]x}n (x = 0.058) | 420 | 593 | – | – | [221] |
| {Zr6(µ3-OH)4(µ3-OH)4(bpdc)6-x[Ir(ppy)2(dcppy)]x}n (x = 0.029) | 420 | 599 | – | – | [221] |
| {Zr6(µ3-OH)4(µ3-OH)4(bpdc)6-x[Ru(2,2′-bpy)2(5,5′-dcbpy)]x}n(x = 0.179) | 420 | 614 | – | – | [221] |
| {[Yb[Ir(ppy)2(5,5′-dcbpy)]2(OH)]·H2O}n | 500 | 980 | 1 | 575 | [222] |
| {[Er[Ir(ppy)2(5,5′-dcbpy)]2(OH)]·H2O}n | 500 | 1535 | 0.15 | 575 | [222] |
| {[Nd[Ir(ppy)2(5,5′-dcbpy)]2(OH)]·H2O}n | 500 | 880 1056 1326 | 2.8 | 575 | [222] |
| {[Zn[Ir(ppy)2(5,5′-dcbpy)]2]·4H2O}n | 500 | 620 | 0.46 | – | [223] |
| {[Pb2[Ir(ppy)2(4,4′-dcbpy)]4(dmf)2](ClO4)·2DMF·13H2O}n | 405 | 610 | 0.13 | 613 | [224] |
| {[Pb[Ir(ppy)2(4,4′-dcbpy)]2(H2O)](ClO4)·3Me2CO·3H2O}n | 405 | 631 | 0.15 | 613 | [224] |
| {[Pb[Ir(ppy)2(4,4′-dcbpy)]2(H2O)]·3Me2CO·3H2O·CH3CN}n | 405 | 630 | 0.17 | 613 | [224] |
| {[Pb4[Ir(ppy)2(4,4′-dcbpy)]4I4(dmf)2]·10H2O}n | 405 | 602 | 0.05 | 613 | [224] |
| {[Mg[Ir(ppy)2(4,4′-dcbpy)]2(H2O)2]·3.5H2O}n | 468 | 544 | 14.6 | 575 | [225] |
| {[Mg[Ir(ppy)2(4,4′-dcbpy)]2(dmf)2]·3.5H2O}n | 468 | 554 | 18.1 | 575 | [225] |
| {[Mg((Ir(ppy)2(4,4′-dcbpy))2(def)(H2O)]·3H2O}n | 468 | 570 | 2.4 | 575 | [225] |
| {[Ag(rac-[Ir(mesppy)2(qpy)])]PF6}n | 360 | 646 | 15 | 614 | [226] |
| {[Ag(Λ-[Ir(mesppy)2(qpy)])]PF6}n | 360 | 646 | 14 | 614 | [226] |
| {[Ag(Δ-[Ir(mesppy)2(qpy)])]PF6}n | 360 | 646 | 15 | 614 | [226] |
| {[Cd3[Ir(ppyCOO)3]2(dmf)2(H2O)4]·6H2O·2DMF}n | 380 | 519 | – | 488 510 | [227] |
| {[Zn2(bpdc)2[Ru(2,2′-bpy)2(5,5′-dcbpy)]0.5]·9DMF·9H2O}n | 452 | 627 | – | – | [228] |
| {Zn[Ru(2,2′-bpy)2(5,5′-dcbpy)]}n | 475 | 635 | 2.1 | – | [229] |
| {(Me2NH2)[In[Ru(4,4′-dcbpy)3]]·6H2O}n | 400 | 657 | 2.8 | 633 | [230] |
| {[Mg2[Ru(4,4′-dcbpy)3]]·13H2O}n | 590 | 681 | 2.7 | 633 | [231] |
| {[Sr4[Ru(4,4′-dcbpy)3]2]·18H2O}n | 590 | 683 | 2.4 | 633 | [231] |
| {[Mg2[Ru(5,5′-dcbpy)3]]·19H2O}n | 590 | 662 | – | 668 | [231] |
| {[Cd2[Ru(5,5′-dcbpy)3]]·12H2O}n | 500 | 688 | – | 668 | [231] |
| {[Cd2[Ru(4,4′-dcbpy)3]]·12H2O}n | 490 | 650 | – | 630 | [232] |
| {(Me2NH2)2[Cd3[Ru(5,5′-dcbpy)3]2}n | 490 | – | – | – | [233] |
| {[La1.75(OH)1.25[Ru(4,4′-dcbpy)3]]·16H2O}n | 573 | 691 | – | – | [234] |
| {[Zn[Ru(4,4′-dcbpy)2(2,2′-bpy)]]·2DMF·4H2O}n | – | 671 | – | 689 | [235] |
| {[Ru(4,4′-H2dcbpy)Cu(4,4′-dcbpy)(4,4′-Hdcbpy)2(H2O)]·5H2O}n | 440 | 605 | – | 622 | [236] |
| {[Ce7(OH)5[Ru(4,4′-dcbpy)3]4]·4H2O}n | 400 | 684 | – | – | [237] |
| {[Nd7(OH)5[Ru(4,4′-dcbpy)3]4]·4H2O}n | 420 | 884 1054 1336 | – | – | [237] |
| Formula | Ligand | λex, nm | λem, nm | λem, nm (ligand) | Ref. |
|---|---|---|---|---|---|
| [Zr6(μ3-OH)8(OH)8(H2tcpp)2]n/PCN-222 |  | 420 | 652 718 | – | [247,248] |
| PCN-222 | 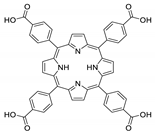 | – | 645 760 | – | [249] |
| PCN-222(Zn) | 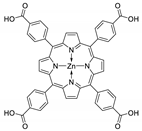 | 512 | 651 | – | [250] |
| [Zr6O4(OH)4(H2tcpp)3]n | 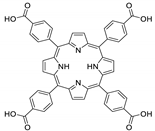 | 512 | 651 | – | [251] |
| [Zr6O4(OH)4(H2tcpp)3]n/MOF-525 |  | 449 | 629 | – | [252] |
| [Zr6(OH)8(H2tcpp)]n/PCN-224 | 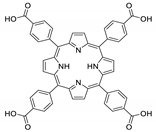 | 590 | 651 705 sh. | – | [253] |
| [Zr6(μ3-O)4(μ3-OH)4(OH)4(H2O)4(H2tcpp)2]n/PCN-225 |  | 415 | 725 | 725 714 656 | [254] |
| [Zn(Me4tcpp)]n | 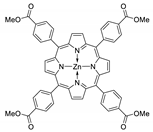 | 589 | 650 706 sh. | – | [255] |
| {[Pb2(H2tcpp)]∙4DMF∙H2O}n | 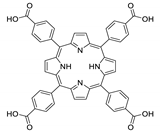 | 290 | 403 436 467 | – | [256] |
| {[Pb2(Cutcpp)(dmf)(H2O)]∙1.5DMF∙2H2O}n | 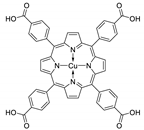 | 290 | 390 460 | 415 475 | [256] |
| {Pb2(Cotcpp)(H2O)(dmf)]∙1.5DMF}n | 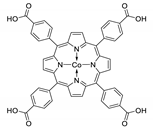 | 290 | 400 465 | 450 | [256] |
| {[Pb2(Nitcpp)(dmf)(H2O)]∙1.5DMF∙2H2O}n | 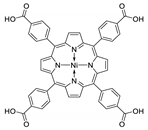 | 290 | 390 460 | 400 | [256] |
| {[Pb2(VOtcpp)(H2O)2]∙4DMF}n | 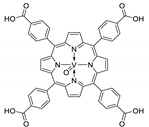 | 290 | 405 470 | 450 | [256] |
| [Sr2(H2tcpp)(dmf)4]n | 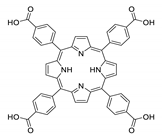 | 420 | 662 711 sh. | – | [257] |
| [Ba2(H2tcpp)(dmf)4]n | 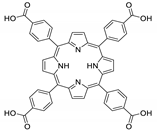 | 420 | 662 711 sh. | – | [257] |
| {[Al2(OH)2(H2tcpp)]·3DMF·2H2O}n | 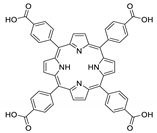 | 415 | 660 | – | [258] |
| {[Al2(OH)2(Zntcpp)]·3DMF·2H2O}n | 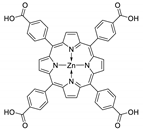 | 425 | 620 660 | – | [258] |
| [Zn2(Zntcpp)(4,4′-bpy)1.5]n/PPF-4 | 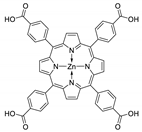 | 590 | 618 633 664 | – | [259] |
| UNLPF-10a | 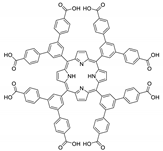 | 400 | 656 714 | 655 705 | [260] |
| UNLPF-10b |  | 400 | 606 648 | 655 705 | [260] |
| UNLPF-11 | 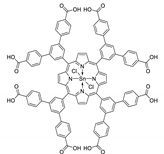 | 400 | 607 648 | – | [260] |
| UNLPF-12 | 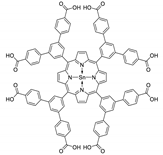 | 400 | 593 642 | – | [260] |
| TBP-MOF | 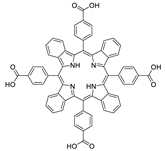 | – | 705 780 | 705 780 | [261] |
| [Hf6(μ3-O)4(μ3-OH)4(dbc)6]n/DBC−UiO | 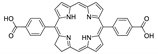 | 408 | 641 | 641 | [262] |
| {[Eu2(OH)4.7(H2tpps)0.33]∙2H2O}n/LEuH-TPPS |  | 420 | 660 716 | 642 703 | [263] |
| {[Tb2(OH)4.7(H2tpps)0.33]∙2H2O}n/LTbH-TPPS | 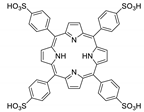 | 420 | 660 716 | 642 703 | [263] |
| {[Eu6(μ6-O)(μ3-OH)8(H2O)14(H2tpps)2]∙12H2O}n/MOF-Eu-TPPS | 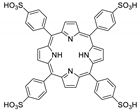 | 420 | 670 733 | 642 703 | [263] |
| {[Eu2(OH)4.7(Pdtpps)0.33]∙2H2O}n/LEuH-PdTPPS | 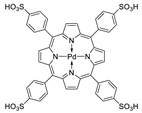 | 420 | 614 715 780 | 606 700 765 | [263] |
| {[Tb2(OH)4.7(Pdtpps)0.33]∙2H2O}n/LTbH-PdTPPS | 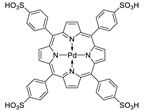 | 420 | 614 715 780 | – | [263] |
| ZnMPyTPP |  | 410 | 603 649 | – | [264] |
| [Fe(tpyp)]n | 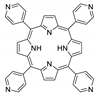 | 410 | 668 716 | – | [265] |
| [Zn2(tcpb)(FZnP)]n/F-MOF | 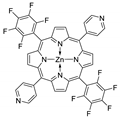 | 446 | 680 | 608 670 | [266] |
| PCN-526 | 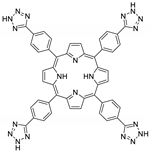 | 323 | 407 | – | [267] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, A.; Matveevskaya, V.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination Polymers Based on Highly Emissive Ligands: Synthesis and Functional Properties. Materials 2020, 13, 2699. https://doi.org/10.3390/ma13122699
Kuznetsova A, Matveevskaya V, Pavlov D, Yakunenkov A, Potapov A. Coordination Polymers Based on Highly Emissive Ligands: Synthesis and Functional Properties. Materials. 2020; 13(12):2699. https://doi.org/10.3390/ma13122699
Chicago/Turabian StyleKuznetsova, Anastasia, Vladislava Matveevskaya, Dmitry Pavlov, Andrei Yakunenkov, and Andrei Potapov. 2020. "Coordination Polymers Based on Highly Emissive Ligands: Synthesis and Functional Properties" Materials 13, no. 12: 2699. https://doi.org/10.3390/ma13122699
APA StyleKuznetsova, A., Matveevskaya, V., Pavlov, D., Yakunenkov, A., & Potapov, A. (2020). Coordination Polymers Based on Highly Emissive Ligands: Synthesis and Functional Properties. Materials, 13(12), 2699. https://doi.org/10.3390/ma13122699