Abstract
Five trans-1,4-cyclohexanedicarboxylate (chdc2−) metal–organic frameworks of transition metals were synthesized in aqueous systems. A careful control of pH, reaction temperature and solvent composition were shown to direct the crystallization of a particular compound. Isostructural [Co(H2O)4(chdc)]n (1) and [Fe(H2O)4(chdc)]n (2) consist of one-dimensional hydrogen-bonded chains. Compounds [Cd(H2O)(chdc)]n∙0.5nCH3CN (3), [Mn4(H2O)3(chdc)4]n (4) and [Mn2(Hchdc)2(chdc)]n (5) possess three-dimensional framework structures. The compounds 1, 4 and 5 were further characterized by magnetochemical analysis, which reveals paramagnetic nature of these compounds. A presence of antiferromagnetic exchange at low temperatures is observed for 5 while the antiferromagnetic coupling in 4 is rather strong, even at ambient conditions. The thermal decompositions of 1, 4 and 5 were investigated and the obtained metal oxide (cubic Co3O4 and MnO) samples were analyzed by X-ray diffraction and scanning electron microscopy.
1. Introduction
Metal–organic frameworks (MOFs) are one of the most fascinating family of solid-state materials because of their highly tunable compositions, structures and functional properties [1]. Lying on the crossing of fundamental inorganic/organic chemistry and development of novel materials, MOFs have become one of the most attractive research fields during the past two decades [2,3,4]. Typically, aromatic carboxylate ligands are used to construct porous coordination frameworks due to high rigidity of such linkers. For example, terephthalate (bdc2−) linker is reported in more than 2500 examples of metal–organic coordination polymers (CSD May 2019). On the other hand, its aliphatic counterpart, that is trans-1,4-cyclohexanedicarboxylate (chdc2−), is far less explored despite appropriate rigidity and availability. Among other reasons, a synthesis of such aliphatic-based MOFs is generally more challenging and requires a careful customization of reaction parameters, such as temperature, crystallization time, solvent composition, template role, coordination modulators, acidity/basicity of the reaction medium, etc. [5,6,7,8], which are often hard to rationalize. More efforts are required to prepare new aliphatic-based MOFs, however, the successful syntheses could lead to unusual crystal structures as a result of hydrophobic interactions between aliphatic moieties and their unique effect on the crystal packing [9,10,11]. An increased conformational lability of organic blocks provides ligand-driven structural transitions in the corresponding porous networks, including reversible breathing phenomena [12,13,14,15,16]. Also, a lower thermal stability of the aliphatic linkers allows for the introduction of defects into regular MOF structures [17], as well as synthesis of various metal oxide nanoparticles [18], and nanoporous carbon materials [19,20], etc.
According to the literature, only one cobalt [21], two cadmium [22,23] and one mixed-valence Fe(II/III) [24] MOF with the single bridge ligand trans-1,4-cyclohexanedicarboxylate have been prepared before. Recently, we also reported the modulation of crystallization of chdc-based MOFs by urotropine in organic solvents [25]. Motivated by a lack of development of aliphatic-based MOFs, we investigated the water-based reaction systems of trans-1,4-cyclohexanedicarboxylic acid (H2chdc) and transition metal cations (Co2+, Fe2+, Cd2+, Mn2+). The weak organic bases (1,4-diazabicyclo[2.2.2]octane, urotropine) were used to modulate and buffer the acidity of the reaction mixture for the optimal crystal growth conditions. Magnetic properties in the temperature range of 2–300 K were investigated. A controlled thermolysis of MOFs results in a formation of nanostructured metal oxides, the composition, structure and morphology of which were analyzed and discussed.
2. Materials and Methods
2.1. Materials
Trans-1,4-cyclohexanedicarboxylic acid (H2chdc, >97.0%) and 1,4-diazabicyclo[2.2.2]octane (DABCO, >98.0%) were purchased from TCI (Tokyo, Japan). Mn(ClO4)2∙6H2O (>99.0%) and urotropine (>99.0%) were purchased from Sigma Aldrich (St. Louis, MO, USA). HClO4 65% water solution (reagent grade) and Fe powder (high-purity grade) were purchased from Reachem (Moscow, Russia). All reagents were used as purchased without further purification. Distilled water was used in all experiments.
2.2. Characterization Techniques
Infrared (IR) spectra in KBr pellets in the range 4000−400 cm−1 were recorded on a Bruker Scimitar FTS 2000 spectrometer (Billerica, MA, USA). Thermogravimetric (TG) analysis in the temperature range 30−600 °C was carried out using a Netzsch TG 209 F1 Iris instrument (Selb, Germany). The experiments were performed under He or Ar flow (80 cm3 min−1) at a 10 K min−1 heating rate. Elemental analysis was made on a EuroVector EA3000 analyzer (Pavia, Italy). Powder X-ray diffraction (PXRD) analysis was performed at room temperature on a Shimadzu XRD-7000 diffractometer (Cu-Kα radiation, λ = 1.54178 Å, Kyoto, Japan). pH of the solutions was measured by Anion 4100 pH meter (Novosibirsk, Russia). SEM images were made on TM-3000 Scanning Electron Microscope (Osaka, Japan). Typical conditions for the measurements were the following: 20.0 kV accelerating voltage, high vacuum pumping.
Investigation of magnetic properties of the compound 1 was performed on a Faraday balance (sensitivity ~3∙× 10−7 g). Measurements of the magnetic susceptibility were performed in the field of 9.7 kOe, the stabilization accuracy of the field strength was 2%. During measurements, the samples were placed in an inert helium atmosphere at the pressure of 5 torr. The magnetic susceptibility of the polycrystalline samples 4 and 5 was measured with a Quantum Design MPMSXL SQUID magnetometer (San Diego, CA, USA) in the temperature range 2–300 K with magnetic field of up to 5 kOe. None of the complexes exhibited any field dependence of molar magnetization at low temperatures. Diamagnetic corrections were made using the Pascal constants. The effective magnetic moment was calculated as µeff(T) = [(3k/NAµB2)χT]1/2 ≈ (8χT)1/2.
2.3. Synthesis
2.3.1. Synthesis of [Co(H2O)4(chdc)]n (1)
100 mg (0.34 mmol) of Co(NO3)2∙6H2O, 56 mg (0.33 mmol) of H2chdc and 24 mg (0.17 mmol) of urotropine were dissolved in 8.00 mL of H2O in a 10 mL glass flask and heated at 80 °C for 18 h. After the cooling at the room temperature, the crimson crystals were filtered off, washed with water and dried in air. Yield: 66 mg (67%). IR (KBr, cm−1): 3433 (br. m, νO-H), 3276 (m), 2957 (m, νC-H), 2947 (m, νC-H), 2933 (m, νC-H), 2865 (m, νC-H), 2241 (w), 1552 (s, νCOOas), 1406 (s, νCOOs), 1363 (m), 1328 (m), 1292 (m), 1227 (w), 1213 (w), 1090 (w), 1048(w), 974 (m), 916 (m), 888 (w), 778 (s), 713 (m), 544 (m), 500 (m). Elemental analysis data calculated for [Co(H2O)4(chdc)]: C 31.9%, H 6.0%. Found: C 31.3%, H 5.8%.
2.3.2. Synthesis of [Fe(H2O)4(chdc)]n (2)
10 mg (0.18 mmol) of Fe powder were dispersed in 1.00 mL of H2O and 0.035 mL of 65% (0.56 mmol) HClO4 were added. After dissolution of iron, 28 mg (0.16 mmol) of H2chdc and 24 mg (0.17 mmol) of urotropine were added to the mixture in the glass ampoule. Then the ampoule was soldered, then sonicated for 10 min and heated at 80 °C for 18 h. After cooling at room temperature, the ampoule was opened and the colorless single crystals were selected for single crystal X-ray diffraction (SCXRD) analysis.
2.3.3. Synthesis of [Cd(H2O)(chdc)]n∙0.5nCH3CN (3)
350 mg of Cd(NO3)2∙4H2O (1.13 mmol) were dissolved in 15.00 mL of acetonitrile. 250 mg (1.45 mmol) of H2chdc and 150 mg (1.34 mmol) of DABCO were dissolved in 15.00 mL of water. Then, the obtained solutions were mixed in a 50 mL Teflon vessel and heated at 100 °C for 24 h. After the cooling at the room temperature, the white precipitate was filtered off, washed with acetonitrile and dried in air. Yield: 192 mg (52%). IR (KBr, cm−1): 3534 (m, νO-H), 3389 (br. m, νO-H), 2936 (m, νC-H), 2857 (m, νC-H), 2257 (w), 2051 (w), 1643 (m), 1579 (s, νCOOas), 1450 (m), 1422 (s, νCOOs), 1386 (m), 1359 (m), 1328 (m), 1288 (m), 1270 (m), 1212 (m), 1043 (w), 975 (w), 928 (m), 902 (w), 887 (w), 809 (w), 790 (m), 758 (w), 738 (w), 691 (w), 675 (w), 537 (w), 470 (m). Elemental analysis data calculated for [Cd(H2O)(chdc)]∙0.5CH3CN: C 33.4%, H 4.2%, N 2.2%. Found: C 33.3%, H 4.0%, N 2.2%.
2.3.4. Synthesis of [Mn4(H2O)3(chdc)4]n (4)
300 mg (0.83 mmol) of Mn(ClO4)2∙6H2O, 120 mg (0.70 mmol) of H2chdc and 120 mg (0.86 mmol) of urotropine were dispersed in 5.00 mL of H2O in a 10 mL glass flask and sonicated for 5 min. Then the flask was heated at 80 °C for 18 h. After the cooling at the room temperature, the white precipitate was filtered off, washed several times with water and dried in air. Yield: 85 mg (51%). IR (KBr, cm−1): 3615 (w, νO-H), 3572 (w, νO-H), 3348 (br. m, νO-H), 2932 (m, νC-H), 2856 (m, νC-H), 1623 (m), 1562 (s, νCOOas), 1448 (m), 1417 (s, νCOOs), 1388 (m), 1358 (m), 1327 (m), 1281 (m), 1210 (m), 1144 (m), 1109 (m), 1049 (w), 977 (w), 927 (m), 901 (m), 886 (w), 812 (w), 778 (m), 745 (m), 709 (m), 685 (m), 590 (w), 555 (m), 532 (m), 479 (s), 397 (m). Elemental analysis data calculated for [Mn4(H2O)3(chdc)4]: C 40.3%, H 4.8%. Found: C 40.6%, H 5.0%.
2.3.5. Synthesis of [Mn2(Hchdc)2(chdc)]n (5)
300 mg (0.83 mmol) of Mn(ClO4)2∙6H2O, 280 mg (1.63 mmol) of H2chdc and 120 mg (0.86 mmol) of urotropine were dispersed in 5.00 mL of H2O and 0.025 mL of 65% (0.40 mmol) HClO4 was added. Then the mixture was sonicated for 5 min and heated at 120 °C for 30 min in a screwed-cap glass vial to allow a partial evaporation of the reaction solution (ca. to 4 mL total volume). The crystallized white precipitate was hot-filtered and washed quickly with H2O to avoid the formation of the compound 4 impurity. After washing, the product was dried in air. Yield: 87 mg (34%). IR (KBr, cm−1): 3448 (br. w, νO-H), 2928 (s, νC-H), 2856 (s, νC-H), 2659 (w), 2550 (w), 1672 (s), 1586 (s, νCOOas), 1540 (m), 1439 (m), 1409 (s, νCOOs), 1383 (m), 1362 (m), 1330 (m), 1273 (m), 1246 (m), 1214 (m), 1134 (w), 1110 (m), 1039 (w), 1024 (w), 977 (w), 953 (w), 916 (m), 882 (w), 801 (w), 770 (w), 741 (m), 722 (w), 711 (w), 682 (w), 543 (w), 529 (m), 472 (m), 404 (m). Elemental analysis data calculated for [Mn2(Hchdc)2(chdc)]: C 46.3%, H 5.1%. Found: C 46.4%, H 5.2%.
2.3.6. Synthesis of Oxides
50–100 mg of 1, 4 or 5 were placed in an open porcelain crucible and heated with 4 °C∙min−1 rate up to 600 °C in an oven, kept at 600 °C for 2 h and cooled to the room temperature with 4 °C∙min−1 cooling rate.
2.4. X-ray Crystallography
Diffraction data for single-crystals of 1–5 were obtained at 130 K on an automated Agilent Xcalibur diffractometer (Santa Clara, CA, USA) equipped with an area AtlasS2 detector (graphite monochromator, λ(MoKα) = 0.71073 Å, ω-scans). Integration, absorption correction, and determination of unit cell parameters were performed using the CrysAlisPro 1.171.38.46 program package [26]. The structures were solved by dual space algorithm (SHELXT [27]) and refined by the full-matrix least squares technique (SHELXL [28]) in the anisotropic approximation (except hydrogen atoms). Positions of hydrogen atoms of organic ligands were calculated geometrically and refined in the riding model. A crystal structure of 5 is solved in two alternatives: orthorhombic Fdd2 (5o) and monoclinic P21 (5m). The crystallographic data and details of the structure refinements are summarized in Table A1. Cambridge Crystallographic Data Center (CCDC) numbers 1973662-1973668 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center at https://www.ccdc.cam.ac.uk/structures/.
3. Results and Discussion
3.1. Synthesis
Compound [Co(H2O)4(chdc)]n (1) was obtained with 67% yield by heating (80 °C) of an aqueous solution of stoichiometric amount of cobalt (II) nitrate and trans-1,4-cyclohexanedicarboxylic acid in the presence of urotropine. The phase purity of 1 was confirmed by PXRD (Figure S1). The crystallization takes place in a mildly acidic conditions (pHstart = 4.5, pHfinal = 5.0). In comparison, other cobalt-chdc compounds [Co5(OH)8(chdc)]n∙4nH2O reported earlier by Kurmoo et al. [21], was synthesized in hydrothermal conditions (170 °C) using an excess of NaOH to create strongly alkaline medium, which apparently facilitates a formation of hydroxyl-rich polynuclear {Co5(OH)8}n2+ building blocks. In moderately acidic synthetic conditions used herein, only mononuclear aqua-complexes were obtained. It should be noted that carrying out the synthesis of 1 without urotropine results in a recrystallization of H2chdc (See Appendix A, Figure A1 and Figure A2) after the cooling. On the contrary, an elevation of the basicity of the reaction system by either increasing the amount of urotropine (two times) or by a replacement of urotropine to the stronger base (NaOH) only lead to the formation of the unknown white powder. Details of the optimized synthetic methods for all compounds are summarized in Table 1.

Table 1.
Synthetic details for 1–5.
Compound [Fe(H2O)4(chdc)]n (2) was obtained by heating (80 °C) of aqueous solution of H2chdc, urotropine and Fe(ClO4)2, which was synthesized in situ by the dissolution of Fe in the solution of perchloric acid. Despite our attempts to avoid the oxidation of iron(II), the crystalline precipitate of 2 was always contaminated by hydrated ferric(III) oxide; therefore, the chemical composition and structure were established by a single-crystal X-ray analysis only. Urotropine is not included in the final coordination polymer, but is a necessary component of the reaction mixture, apparently as a pH modulator for the proper crystallization process. Remarkably, 2 is the first structurally characterized example of iron(II) 1,4-cychohexanedicarboxyalte reported in the literature, besides of one mixed-valence Fe(II/III) compound [Fe2O(chdc)1.5]n [24]. 2 is isostructural to 1 and to [Ni(H2O)4(chdc)], which was reported by Kurmoo et al. [29] and Chen et al. [7].
Compound [Cd(H2O)(chdc)]n∙0.5nCH3CN (3) was synthesized in the solvothermal conditions by heating (100 °C) of the cadmium(II) nitrate, H2chdc and DABCO in the mixture of water and acetonitrile. The phase purity of 3 was confirmed by PXRD (Figure S2). The starting pH of the solution (pHstart = 4.8) is only slightly changed during the reaction (pHfinal = 4.5). The synthesis of one-dimensional compound [Cd(H2O)2(chdc)]n, reported by Thirumurugan et al. [23], was carried out in water from cadmium acetate and H2chdc with piperidine as the acidity modulator, while [Cd2(DMF)(chdc)2]n, reported by Yoon and co-workers [22], was obtained from the organic medium (N,N-dimethylformamide). In our case, both water/acetonitrile mixture and DABCO appeared to be necessary for synthesis 3, since the reaction in pure solvents did not result in any MOF precipitation except for recrystallization of H2chdc. Using one equivalent of NaOH instead of DABCO led to the formation of single crystals of the known compound [Cd(H2O)2(chdc)]. Oddly enough, no precipitate was formed if replacing 1,4-diazabicyclo[2.2.2]octane (DABCO, pKb = 5.2) [30] to urotropine (pKb = 9.5); therefore, in addition to being a very specific pH modulator, DABCO molecules probably act as an intermediate template facilitating the crystallization of 3. The starting pH (pHstart = 4.8) is quite similar to the reported pH = 5…6 in the synthesis of [Cd(H2O)2(chdc)]n [23], but the role of DABCO in our case is likely to fix pH at certain values to complement the templation effect.
New Mn-based MOFs [Mn4(H2O)3(chdc)4]n (4) and [Mn2(Hchdc)2(chdc)]n (5) were obtained in aqueous medium from the manganese(II) perchlorate, H2chdc and urotropine. Due to the similar chemical compositions, the simultaneous precipitation of these two compounds is hard to avoid. After optimization of the reaction conditions, the pure phase of 4 was obtained at lower acidity (pHstart = 4.9; pHfinal = 5.2) while the compound 5 was isolated at higher acidity of the reaction solution (pHstart = 4.6; pHfinal = 4.9) due to (i) greater molar ratio of chdc:Mn and (ii) additional presence of strong HClO4 acid. Such conditions are consistent with a more “acidic” nature of 5, which contains a partially protonated Hchdc− moieties, compared with 4, where only fully deprotonated chdc2− ligands are present. Importantly, the concentration of the urotropine in the synthesis of both 4 and 5 should not be lowered to avoid the formation of H2chdc crystals. The phase purity of the obtained 4 and 5 samples was confirmed by PXRD (Figures S3 and S4).
3.2. Structure Descriptions and Infrared Spectroscopy
An asymmetric unit of [Co(H2O)4(chdc)]n (1) contains one Co atom, which has an octahedral coordination environment of four aqua ligands and two O atoms of two COO-groups, which are situated in trans positions (Figure 1). Co–OH2 distances are 2.0947(9) Å and 2.1168(10) Å, and Co–OCO distance is 2.0993(9) Å. The metal centers are bound by bidentate (e,e)-1,4-chdc ligands to form polymeric chains, which are packed into a dense crystal structure. It seems that both hydrogen bonding (interchain OCOO–Oaqua distances are 2.762 Å for O(1)···O(12), 2.868 Å for O(1)···O(11), and 2.889 Å for O(2)···O(11)) and hydrophobic interactions between cyclohexane rings play an important role in the formation of the dense phase. The smallest interlayer HCH...HCH distance is calculated to be 2.320 Å, which indicates a close linker-to-linker packing in 1. There is also an intramolecular hydrogen bond between aqua ligand and O atom of the COO-group (O(2)···O(12) distance is 2.652 Å). 1 is isostructural to [Ni(H2O)4(chdc)]n [7,29]. The synthesis of [Co(μ-H2O)(H2O)2(cis-chdc)]n containing a cis-isomer of 1,4-cyclohexanedicarboxylate ligand is also reported [31].
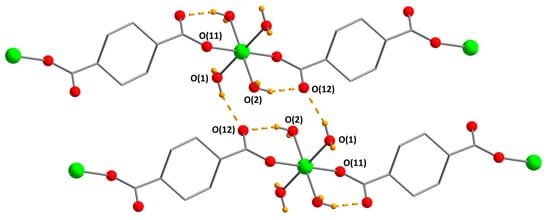
Figure 1.
Packing of polymeric chains in 1 (CCDC 1973662). Metal atoms are green, O atoms are red, H atoms of aqua-ligands are orange. Hydrogen bonds are shown with dashed lines. H atoms of chdc2− ligands are omitted.
Compound [Fe(H2O)4(chdc)]n (2) is isostructural to 1. Fe–OH2 distances are 2.1292(12) Å and 2.1524(12) Å, and Fe–OCO distance is 2.1076(11) Å. Interchain OCOO···Oaqua distances are 2.753, 2.887, and 2.902 Å. The intramolecular OCOO···Oaqua distance is 2.665 Å. Compound 2 is the first example of iron(II) cyclohexanedicarboxylate reported in the literature.
The asymmetric unit of the structure [Cd(H2O)(chdc)]n·0.5nCH3CN (3) contains two Cd atoms, two trans-1,4-cyclohexanedicarboxylate ligands and two molecules of coordinated water. Cd(1) has a distorted octahedral coordination environment of five carboxylate O atoms and one aqua ligand. Cd(2) has less distorted octahedral coordination environment, which also consists of five carboxylate O atoms and one aqua ligand. Cd–O distances are in range of 2.2224(16)–2.3860(17) Å. Cd(1) and Cd(2) are interconnected via three bridging COO-groups to form a binuclear unit {Cd2(μ-RCOO-κ1,κ1)2(μ-RCOO-κ1)}. The binuclear units are interconnected to form waved polymeric chains parallel to the c axis (Figure 2a). The chains are interconnected by (e,e)-1,4-cyclohexanedicarboxylate ligands in six directions to form a 3D porous network (Figure 2b). There are isolated cages in structure 3 (two cages per unit cell). The void volume in 3 is 14% (PLATON [32]) and the voids are occupied by the guest CH3CN molecules (Figure S5).
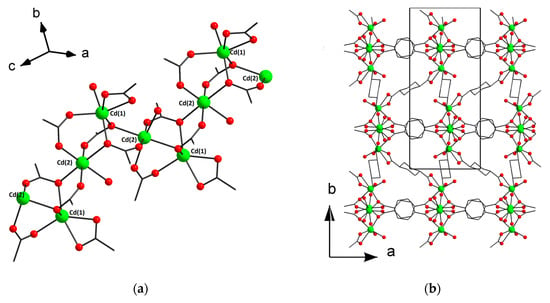
Figure 2.
Fragment of polymeric chain (a) and 3D metal–organic framework (b) in 3 (CCDC 1973664). Hydrogen atoms and guest CH3CN molecules are omitted for clarity.
The asymmetric unit of [Mn4(H2O)3(chdc)4]n (4) contains four Mn atoms, four trans-1,4-cyclohexanedicarboxylate ligands and three molecules of coordinated water. Mn(1) has a distorted octahedral coordination environment containing six O atoms of five COO-groups. Mn(2) and Mn(3) have less distorted octahedral coordination environment, which contains four O atoms of four COO-groups, one bridging and one terminal H2O molecule. Mn(4) has a slightly distorted square-pyramidal coordination environment containing five O atoms of five COO-groups. For octahedral Mn centers, the Mn–O distances are in the range of 2.0884(14)–2.3142(15) Å for water molecules and nonchelate COO groups. One Mn–OCOO,chelate distance is 2.3572(13) Å. For square-pyramidal Mn(4) center, Mn–O distances are 1.993(6)–2.219(7) Å and there is a long Mn(4)...O contact of 2.8047 Å. Mn atoms are interconnected via bridging carboxylate groups to form a decanuclear ring incorporating an endo-chdc2− ligand (Figure 3a). Translating along the a and b axis, the decanuclear rings tile (004) plane to form polymeric layer with hexagonal topology (Figure 3b). Such a structure is unique for metal–organic frameworks, although there are reported examples of manganese or iron MOFs, which are constructed of endotemplated “honeycombs” with a different size or metal rings [10,11,33,34]. The layers alternate along the c axis, interconnecting by bridging chdc2− ligands to form 3D metal–organic framework (see also Figures S6 and S7).
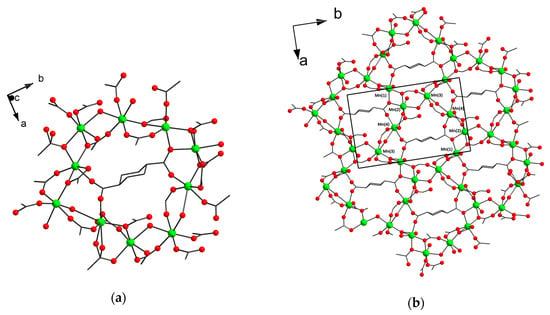
Figure 3.
Decanuclear Mn(II) wheel with endo-chdc2− ligand in 4 (CCDC 1973665) (a). Framework structure of 4 along the c axis (b).
The crystal structure of the compound [Mn2(Hchdc)2(chdc)]n (5) could be solved in two alternatives: orthorhombic Fdd2 (5o) and monoclinic P21 (5m). Higher symmetry variant 5o has less independent atoms, and demonstrates lower R1 value. However, in the structure 5o, the chdc2− ligand is disordered over two orientations around a two-fold rotary axis, whereas lower symmetry variant 5m demonstrates no disordering in the structure, but is characterized by a higher R1 value. Since the topology of framework 5 is the same in both cases, a further description of the crystal structure is given for the higher symmetry variant 5o.
The asymmetric unit of 5 contains one Mn(II) cation, which has an octahedral coordination environment consisting of six O atoms of six COO-groups (Figure 4a). Mn–O distances are in range 2.083(8)–2.211(3) Å for oxygen atoms of bridging COO groups and 2.325(4) Å for oxygen atom of monocoordinated carboxylate. Mn atoms are bridged through COO-groups to form polymeric chain running along the c axis. The chains are interconnected by (e,e)-1,4-cyclohexanedicarboxylate ligands to form a 3D metal–organic framework (Figure 4b). One of the 1,4-cyclohexanedicarboxylates is monoprotonated. The structure is densely packed and has no free solvent accessible volume.
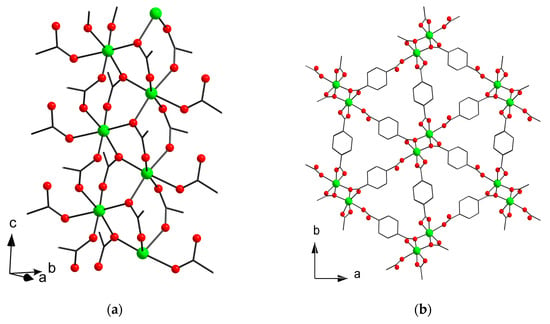
Figure 4.
Fragment of polymeric chain in (a) and metal–organic framework (b) in 5o (CCDC 1973666). Hydrogen atoms are omitted. Only one of possible orientations of chdc2− ligand is shown.
Infrared spectra of the compounds 1, 3–5 are typical for metal–carboxylate complexes. All spectra contain O–H stretching bands in the 3348–3448 cm−1 region, cyclohexane ring C(sp3)–H stretching bands in the 2856–2957 cm−1 region, COO antisymetric and symmetric stretching bands at 1552–1586 cm−1 and 1406–1422 cm−1, respectively. For 5, the O–H band at 3448 cm−1 is very broad and weak and can be attributed to the presence of protonated COOH groups.
3.3. Magnetochemical Analysis
Temperature dependencies of the effective magnetic moment (µeff) and inverse magnetic susceptibility for complex 1 are shown in Figure 5. The µeff value is 4.82 µB at 300 K and decreases to 4.69 µB at 80 K. The 1/χ dependence is linear in the temperature range of 80–300 K and obeys the Curie–Weiss law with the best fit parameters С = 2.99 K cm3 mol−1 and θ = −7 K. The values of the µeff at 300 K and Curie constant C are higher than the theoretical spin only for the values of 3.87 µB and 1.875 K·cm3/mol−1, respectively, which is typical for the Co(II) ion in the octahedral environment orbital contribution to the magnetic susceptibility [35,36]. Spin-orbit coupling causes decreasing of the µeff with lowering temperature.
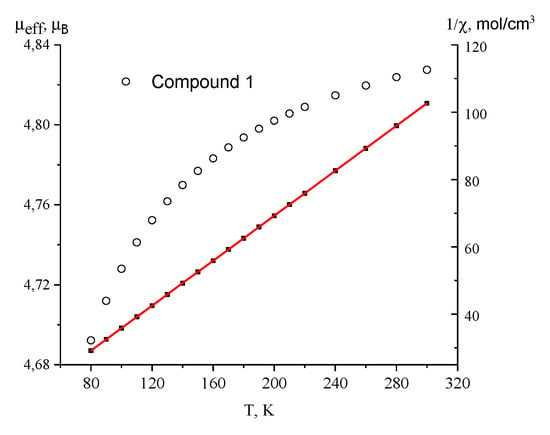
Figure 5.
Temperature dependencies of the µeff (○) and 1/χ (■) for complex 1. Solid line is a theoretical curve.
The µeff(T) and 1/χ(T) dependencies for 4 and 5 are presented in Figure 6. The µeff values at 300 K are 9.23 µB and 7.77 µB and decrease gradually with lowering temperature down to 1.28 µB and 3.4 µB at 2 K for complexes 4 and 5, respectively. The 1/χ(T) dependencies are linear in the temperature range 30–300 K and obey the Curie–Weiss law with optimal values of Curie constant C and Weiss constant θ equal to 15.25 K·cm3/mol and −126 K for 4 and 8.033 K·cm3/mol and −19.5 K for 5. The values of μeff at 300 K and the Curie constant C are lower than theoretical spin only ones 11.83 μB and 17.5 K·cm−1/mol for four noninteracting Mn (II) ions and 8.37 µB and 8.75 K·cm3/mol for two noninteracting Mn (II) ions for complexes 4 and 5, respectively. A decrease of µeff with lowering temperature and negative values of the Weiss constant θ indicate the presence of antiferromagnetic exchange interactions between the spins of Mn (II) ions. It should be noted that the low μeff value for 4 at 300 K (9.23 µB instead of 11.83 μB theoretical value) indicates that antiferromagnetic coupling is strong even at room temperature. This behavior is reasonable due to the presence of four crystallographically independent Mn(II) ions and several possible ways of magnetic exchange within the polymeric layer with unique decanuclear Mn(II) rings.
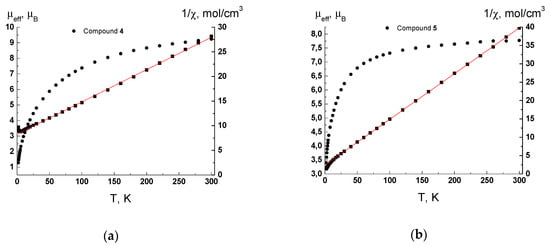
Figure 6.
Temperature dependences of the µeff (●) and 1/χ (■) for complexes 4 (a) and 5 (b). Solid lines are theoretical curves.
3.4. Thermal Stability and Thermolysis
Thermogravimetric analyses of the compounds 1, 3–5 were performed under He of Ar flow. Compound 1 shows weight loss ca. 24% occurring up to 120 °C, assigned to the full loss of coordinated water molecules (Figure S8). The first weight loss is followed by a plateau region ranging up to 460 °C with further heating leading to decomposition of the framework.
Compound 3 demonstrates weight loss ca. 12% at 160–180 °C, which corresponds to the loss of coordinated water and CH3CN guest molecules (Figure S9). This temperature is much higher than boiling point of acetonitrile (82 °С) and water, despite its coordinated nature. The reason for such pronounced thermal stability is isolated structure of pores (see the structure description in Section 2.2). The first weight loss in followed by a plateau region ranging up to 450 °C. Several attempts were performed to activate samples of 3 by heating at 80–100 °C in vacuum. It was shown that the crystallinity of activated samples is significantly reduced (Figure S10), and the samples do not show adsorption of N2 at 77 K and CO2 at 195 K.
Compound 4 demonstrates weight loss ca. 5% at 180–200 °C due to the loss of coordinated water molecules (Figure S11), and further decomposition at 530 °С. The thermal properties of 5 (Figure S12) are more complex and reveal multiple intermediate states due to consecutive liberation of organic ligands. The first weight loss at 250–270 °C (29%) leads to Mn2(chdc)2 intermediate (calculated—28%) apparently due to the escape of the H2chdc molecule. The second step at 330–340 °C (20%) leads to an unknown phase or a mixture, followed by pyrolysis, completed at 520 °C with the formation of MnO (21% of the residual sample mass vs. 23% in theory).
Due to the wide applicability of metal oxide nanoparticles and a number of advantages of MOF sources for pyrolysis [37,38,39,40,41,42], the compounds 1, 4, 5 were considered as the precursors for the synthesis of the corresponding oxides by thermolysis in air. The temperatures of the decomposition were chosen as 600 °C for all compounds, according to TG data. The thermolysis products were identified as cubic Co3O4 for 1 (Figure S13) and cubic MnO for 4–5 according to PXRD (Figure S14). The coherence scattering areas of crystalline oxide nanoparticles were estimated by the Scherrer equation. The corresponding numbers are 53.6(5) nm for Co3O4 derived from 1, 43.5(4) nm for MnO derived from 4 and 55.7(9) nm for MnO derived from 5. While the coherence scattering areas of MnO nanocrystallites obtained from 4 or 5 are quite similar, the macroscopic shape of the metal oxide aggregates is markedly different from each other. As revealed by scanning electron microscopy (SEM), the morphology of MnO aggregates is similar to that of the initial MOF crystals: rhomboidal blocks for 4 and sticks for 5, respectively (Figure 7). Such a morphological “memory effect” is not uncommon and could provide a convenient way for the control of the texture of the oxide phase on a macroscopic level, which, in turn, will affect certain functional properties of the bulk material, such as particle density, surface area, catalytic activity, and chemical reactivity.
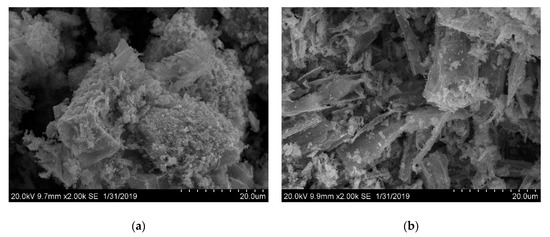
Figure 7.
SEM images of MnO sample obtained by the oxidative thermolysis of 4 (a) and 5 (b).
4. Conclusions
To summarize, five new metal–organic frameworks (MOFs) based on trans-1,4-cyclohexanedicarboxylate linker were synthesized and characterized by elemental analysis, IR spectroscopy, powder diffraction and X-ray single crystal analysis. Fine optimization of the reaction conditions, including the solvent composition, temperature and reaction mixture pH, was found to be crucial to achieve a phase pure product with high crystallinity.
Isostructural [Co(H2O)4(chdc)]n (1) and [Fe(H2O)4(chdc)]n (2) consist of one-dimensional hydrogen-bonded chains. Compounds [Cd(H2O)(chdc)]n∙0.5nCH3CN (3), [Mn4(H2O)3(chdc)4]n (4) and [Mn2(Hchdc)2(chdc)]n (5) possess three-dimensional framework structures.
Metal–organic frameworks with paramagnetic metal cations Co(II), Mn(II) were studied by magnetochemical and thermal analyses. For the compound [Co(H2O)4(chdc)]n (1) with chain-like structure, a typical decrease of the magnetic moment µeff was observed with lowering temperature due to a spin-orbit coupling. For the metal–organic frameworks with Mn(II), a presence of antiferromagnetic exchange was revealed, which is especially strong in [Mn4(H2O)3(chdc)4]n (4) even at room temperature due to different possible ways of magnetic exchange within the polymeric layer with unique decanuclear Mn(II) rings.
The thermolysis of the compounds based on Co(II) and Mn(II) in oxygen-containing atmosphere produces macroscopic aggregates made of nanosized oxide phases of cubic Co3O4 and MnO, respectively. These aggregates inherit the shape of the crystals of the initial MOFs, making possible a control of functional properties of the bulk material by varying the MOF precursor.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/13/2/486/s1, Figure S1: PXRD pattern of the synthesized sample of 1 (black) in comparison with the theoretical one (red), Figure S2: PXRD pattern of the synthesized sample of 3 (black) in comparison with the theoretical one (red), Figure S3: PXRD pattern of the synthesized sample of 4 (black) in comparison with the theoretical one (red), Figure S4: PXRD pattern of the synthesized sample of 5 (black) in comparison with the theoretical one (red), Figure S5: Location of guest CH3CN molecules in the cage of 3, Figure S6: Interconnection between AB layers into 3D framework in 4, Figure S7: The schematic illustration of AB-packing in 4, Figure S8: TG plot for the compound 1, Figure S9: TG plot for the compound 3, Figure S10: PXRD pattern of the activated sample of 3 (black) in comparison with the theoretical for 3 (red), Figure S11: TG plot for the compound 4, Figure S12: TG plot for the compound 5, Figure S13: PXRD pattern of the Co3O4 sample derived from 1 by the oxidative thermolysis, Figure S14: PXRD patterns of the MnO samples derived from 4 (red) and 5 (black) by the oxidative thermolysis, Figure S15: SEM image of Co3O4 sample obtained by the oxidative thermolysis of 1.
Author Contributions
D.N.D. and V.P.F. conceptualized and supervised. P.A.D. prepared the original manuscript. P.A.D., A.S.B., A.S.U., A.Y.A. and D.G.S. performed experimental work. V.P.F. acquired the funding. P.A.D., A.S.B., A.Y.A., D.G.S., D.N.D. and V.P.F. contributed to writing, editing and analysis. All the authors equally contributed in this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Russian Foundation for Basic Research, Project Number 18-29-04001.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. New Polymorph of (e,e)-H2chdc
The single crystals of trans-H2chdc (6) were obtained in all synthetic schemes, which were similar to the 1–5 methods, but carried out without the addition of the organic base (DABCO or urotropine). Moreover, similar crystals of 6 were obtained at recrystallization of H2chdc from water. This structure has not been reported early and is different to the known monoclinic crystal structure of trans-H2chdc [43]. Compound 6 crystallizes in the triclinic symmetry with P-1 space group. CCOO-OCOO distances are 1.2205(1) Å and 1.3195(2) Å. С–С bond lengths in the cyclohexane ring are in the range of 1.5261(3)–1.5348(3) Å H2chdc molecules adopt biequatroial (e,e) conformation. The COOH groups of neighboring H2chdc molecules contact with the formation of H-bonds in a typical for carboxylic acids manner resulting in the formation of parallel one-dimensional H-bonded chains that are packed along each other in AA manner to form a 3D dense structure. The reported earlier structure of trans-H2chdc adopts AB-packing. The rotation angle of carboxylic groups relative to the cyclohexane ring plane is also different in these to structures ~45° for 6 and ~15° for the previously reported trans-H2chdc phase.

Figure A1.
Packing of H2chdc molecules in 6 (CCDC 1973668) (a) and the previously reported H2chdc (b). Hydrogen bonds are shown in orange.

Figure A2.
Conformations of H2chdc molecule in 6 (a) and in previously reported polymorph (b).
The crystal structure of the less stable (a,a) conformer is not reported in literature. There is a reported SCXRD data to cis-isomer—(e,a)-H2chdc [44], which cannot pass to (e,e)-H2chdc by a conformational transition.
Appendix B. Crystallographic Data

Table A1.
Crystallographic data for 1–6.
Table A1.
Crystallographic data for 1–6.
| Compound Number | 1 | 2 | 3 | 4 | 5o | 5m | 6 |
|---|---|---|---|---|---|---|---|
| Chemical formula | C8H18CoO8 | C8H18FeO8 | C9H13.5CdN0.5O5 | C32H46Mn4O19 | C24H32Mn2O12 | C24H32Mn2O12 | C8H12O4 |
| Mr, g∙mol−1 | 301.15 | 298.07 | 321.10 | 954.45 | 622.37 | 622.37 | 172.18 |
| Crystal system, space group | Triclinic, P¯1 | Triclinic, P¯1 | Monoclinic, P21/c | Monoclinic, 21/c | Orthorhombic, Fdd2 | Monoclinic, P21 | Triclinic, P¯1 |
| Temperature (K) | 130 | 130 | 130 | 130 | 130 | 130 | 130 |
| a, b, c (Å) | 4.9320(4), 6.3130(5), 9.5216(7) | 4.9361(4), 6.3194(6), 9.5296(9) | 10.6697(5), 22.4618(11), 9.6558(5) | 10.9672(2), 16.7768(3), 19.6763(4) | 42.792(3), 23.7832(12), 4.8132(2) | 23.7915(19), 4.81470(19), 24.4802(18) | 5.2936(7), 5.6436(8), 7.2022(12) |
| α, β, γ (°) | 80.347(6), 79.008(6), 77.250(7) | 80.655(8), 79.183(8), 77.743(8) | 90.0000, 113.017(6), 90.0000 | 90.0000, 90.2332(18), 90.0000 | 90.0000, 90.0000, 90.0000 | 90.0000, 119.049(10), 90.0000 | 71.861(14), 78.609(13), 79.854(12) |
| V (Å3) | 281.39(4) | 282.99(5) | 2129.9(2) | 3620.29(13) | 4898.5(5) | 2451.4(4) | 198.92(5) |
| Z | 1 | 1 | 8 | 4 | 8 | 4 | 1 |
| μ (mm−1) | 1.55 | 1.36 | 2.05 | 1.45 | 1.10 | 1.10 | 0.12 |
| Crystal size (mm) | 0.50 × 0.31 × 0.15 | 0.23 × 0.07 × 0.07 | 0.58 × 0.47 × 0.16 | 0.55 × 0.30 × 0.22 | 0.29 × 0.05 × 0.05 | 0.29 × 0.05 × 0.05 | 0.13 × 0.08 × 0.07 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 4449, 1382, 1351 | 2138, 1302, 1220 | 10553, 4912, 4554 | 30543, 8653, 7694 | 5975, 2077, 1837 | 12172, 7973, 5190 | 1493, 918, 722 |
| Rint | 0.026 | 0.020 | 0.017 | 0.024 | 0.044 | 0.045 | 0.013 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.021, 0.057, 1.13 | 0.026, 0.061, 1.05 | 0.022, 0.048, 1.11 | 0.030, 0.069, 1.02 | 0.037, 0.074, 1.04 | 0.059, 0.105, 0.98 | 0.040, 0.089, 1.03 |
| No. of parameters | 91 | 91 | 293 | 533 | 220 | 698 | 57 |
| No. of restraints | 4 | 4 | 5 | 69 | 103 | 453 | 0 |
| Δρmax, Δρmin (e∙Å-3) | 0.36, −0.47 | 0.39, −0.30 | 0.63, −0.63 | 1.23, −1.26 | 0.44, −0.36 | 0.49, −0.57 | 0.28, −0.20 |
References
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to Metal−Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best practices for the synthesis, activation, and characterization of metal−organic frameworks. Chem. Mater. 2017, 29, 26–39. [Google Scholar] [CrossRef]
- Pascanu, V.; González Miera, G.; Ken Inge, A.; Martín-Matute, B. Metal–Organic Frameworks as Catalysts for Organic Synthesis: A Critical Perspective. J. Am. Chem. Soc. 2019, 141, 7223–7234. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Shi, W.; Cheng, P. Synthetic strategies for chiral metal-organic frameworks. Chin. Chem. Lett. 2017, 29, 819–822. [Google Scholar] [CrossRef]
- Sun, D.; Han, L.-L.; Yuan, S.; Deng, Y.-K.; Xu, M.-Z.; Sun, D.-F. Four New Cd(II) Coordination Polymers with Mixed Multidentate N-Donors and Biphenyl-Based Polycarboxylate Ligands: Syntheses, Structures, and Photoluminescent Properties. Cryst. Growth Des. 2013, 13, 377–385. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jung, D.Y. Conformation change of the cyclohexanedicarboxylate ligand toward 2D and 3D La(iii)-organic coordination networks. Chem. Commun. 2002, 8, 908–909. [Google Scholar]
- Chen, J.; Ohba, M.; Zhao, D.; Kaneko, W.; Kitagawa, S. Polynuclear core-based nickel 1,4-cyclohexanedicarboxylate coordination polymers as temperature-dependent hydrothermal reaction products. Cryst. Growth Des. 2006, 6, 664–668. [Google Scholar] [CrossRef]
- Chi-Duran, I.; Enríquez, J.; Manquian, C.; Wrighton-Araneda, K.; Cañon-Mancisidor, W.; Venegas-Yazigi, D.; Herrera, F.; Pratap Singh, D. pH-Controlled Assembly of 3D and 2D Zinc-Based Metal-Organic Frameworks with Tetrazole Ligands. ACS Omega 2018, 3, 801–807. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jung, D.Y. Hydrothermal Synthesis and Magnetic Behavior of a Novel Layered Coordination Polymer Generated from Manganese (II) Adipate. Inorg. Chem. 2000, 39, 1470–1475. [Google Scholar] [CrossRef]
- Zhou, G.J.; Richter, J.; Schnack, J.; Zheng, Y.Z. Hydrophobicity-Driven Self-Assembly of an Eighteen-Membered Honeycomb Lattice with Almost Classical Spins. Chem. Eur. J. 2016, 22, 14846–14850. [Google Scholar] [CrossRef]
- Martínez Casado, F.J.; Fabelo, O.; Rodríguez-Velamazan, J.A.; Ramos Riesco, M.; Rodríguez Cheda, J.A.; Labrador, A.; Rodríguez-Blanco, C.; Campo, J.; Sanchez-Alarcos, V.; Muller, H. Manganese(II) Butyrate-Based MOFs: Structures, Thermal and Magnetic Properties. Cryst. Growth Des. 2011, 11, 4080–4089. [Google Scholar] [CrossRef]
- Reinsch, H.; De Vos, D. Structures and properties of gallium-MOFs with MIL-53-topology based on aliphatic linker molecules. Microporous Mesoporous Mater. 2014, 200, 311–316. [Google Scholar] [CrossRef]
- Reinsch, H.; Pillai, R.S.; Siegel, R.; Senker, J.; Lieb, A.; Maurin, G.; Stock, N. Structure and properties of Al-MIL-53-ADP, a breathing MOF based on the aliphatic linker molecule adipic acid. Dalton Trans. 2016, 45, 4179–4186. [Google Scholar] [CrossRef]
- Bueken, B.; Vermoortele, F.; Vanpoucke, D.E.P.; Reinsch, H.; Tsou, C.-C.; Valvekens, P.; De Baerdemaeker, T.; Ameloot, R.; Kirschhock, C.E.A.; Van Speybroeck, V.; et al. A Flexible Photoactive Titanium Metal–Organic Framework Based on a [TiIV3(μ3-O)(O)2(COO)6] Cluster. Angew. Chem. Int. Ed. 2015, 54, 13912–13917. [Google Scholar] [CrossRef] [PubMed]
- Niekiel, F.; Lannoeye, J.; Reinsch, H.; Munn, A.S.; Heerwig, A.; Zizak, I.; Kaskel, S.; Walton, R.I.; De Vos, D.; Llewellyn, P.; et al. Conformation-controlled sorption properties and breathing of the aliphatic Al-MOF [Al(OH)(CDC)]. Inorg. Chem. 2014, 53, 4610–4620. [Google Scholar] [CrossRef] [PubMed]
- Bueken, B.; Vermoortele, F.; Cliffe, M.J.; Wharmby, M.T.; Foucher, D.; Wieme, J.; Vanduyfhuys, L.; Martineau, C.; Stock, N.; Taulelle, F.; et al. A Breathing Zirconium Metal-Organic Framework with Reversible Loss of Crystallinity by Correlated Nanodomain Formation. Chem. Eur. J. 2016, 22, 3264–3267. [Google Scholar] [CrossRef]
- Bueken, B.; Van Velthoven, N.; Krajnc, A.; Smolders, S.; Taulelle, F.; Mellot-Draznieks, C.; Mali, G.; Bennett, T.D.; De Vos, D. Tackling the Defect Conundrum in UiO-66: A Mixed-Linker Approach to Engineering Missing Linker Defects. Chem. Mater. 2017, 29, 10478–10486. [Google Scholar] [CrossRef]
- Kim, T.K.; Lee, K.J.; Cheon, J.Y.; Lee, J.H.; Joo, S.H.; Moon, H.R. Nanoporous Metal Oxides with Tunable and Nanocrystalline Frameworks via Conversion of Metal–Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 8940–8946. [Google Scholar] [CrossRef]
- Lim, S.; Suh, K.; Kim, Y.; Yoon, M.; Park, H.; Dybtsev, D.N.; Kim, K. Porous carbon materials with a controllable surface area synthesized from metal-organic frameworks. Chem. Commun. 2012, 48, 7447–7449. [Google Scholar] [CrossRef]
- Xi, K.; Cao, S.; Peng, X.; Ducati, C.; Kumar, V.; Cheetham, A.K. Carbon with hierarchical pores from carbonized metal–organic frameworks for lithium sulphur batteries. Chem. Commun. 2013, 49, 2192–2194. [Google Scholar] [CrossRef]
- Kurmoo, M.; Kumagai, H.; Hughes, S.M.; Kepert, C.J. Reversible guest exchange and ferrimagnetism (T(C) = 60.5 K) in a porous cobalt(II)-hydroxide layer structure pillared with trans-1,4-cyclohexanedicarboxylate. Inorg. Chem. 2003, 42, 6709–6722. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Sun, H.J.; Lee, D.H.; Park, G. Synthesis and Structure of a 3-D Metal-Organic Framework, [Cd2(1,4-cyclohexanedicarboxylate)2·DMF], Comprising Unusual Two Different Ligand Conformations. Bull. Korean Chem. Soc. 2012, 33, 3111–3114. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Avinash, M.B.; Rao, C.N.R. 1, 2-, 1, 3- and 1, 4-Cyclohexanedicarboxylates of Cd and Mn with chain and layered structures. Dalton Trans. 2006, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Xue, W.; Zhang, W.X.; Tong, M.L.; Chen, X.M.; Grandjean, F.; Long, G.J.; Ng, S.W.; Panissod, P.; Drillon, M. Spin-Frustrated Complex, [FeIIFeIII(trans-1,4-cyclohexanedicarboxylate)1.5]∞: Interplay between Single-Chain Magnetic Behavior and Magnetic Ordering. Inorg. Chem. 2009, 48, 2028–2042. [Google Scholar] [CrossRef]
- Demakov, P.A.; Sapchenko, S.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Coordination polymers based on zinc(II) and manganese(II) with 1,4-cyclohexanedicarboxylic acid. Russ. Chem. Bull. 2018, 67, 490–496. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro 1.171.38.46; Rigaku Oxford Diffraction: The Woodlands, TX, USA, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3. [Google Scholar]
- Kurmoo, M.; Kumagai, H.; Akita-Tanaka, M.; Inoue, K.; Takagi, S. Metal-organic frameworks from homometallic chains of nickel(II) and 1,4-cyclohexanedicarboxylate connectors: Ferrimagnet-ferromagnet transformation. Inorg. Chem. 2006, 45, 1627–1637. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Koyioni, M.; Michaelidou, S.S. Synthesis of [(4-Chloro-5H-1,2,3-dithiazol-5-ylidene)amino]azines. Molecules 2011, 16, 8992–9002. [Google Scholar] [CrossRef]
- Li, Z.Y.; Cao, Y.Q.; Zhang, X.M.; Xu, Y.L.; Cao, G.X.; Zhang, F.L.; Li, S.Z.; Zhanga, F.Q.; Zhai, B. Linking cobalt-water chains with cis-1,4-cyclohexanedicarboxylate bridges to form a 2D network exhibiting spin-canted antiferromagnetism. New J. Chem. 2017, 41, 457–461. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Liu, S.; Jeppson, P.; Sandstrom, J.; Caruso, A.N.; Schulz, D.L. Synthesis, structure and magnetic properties of {Mn5(OC(O)CH3)6(OC(O)C6H5)4}∞. Polyhedron 2007, 26, 2235–2242. [Google Scholar] [CrossRef]
- Liu, S.; Bremer, M.T.; Lovaasen, J.; Caruso, A.N.; O’Neill, K.; Simpson, L.; Parilla, P.A.; Heben, M.J.; Schulz, D.L. Structural and magnetic studies of two-dimensional solvent-free manganeseII complexes prepared via ligand exchange reaction under solvothermal conditions. Inorg. Chem. 2008, 47, 1568–1575. [Google Scholar] [CrossRef]
- Bushuev, M.B.; Virovets, A.V.; Garcia, Y.; Gieck, C.; Sheludyakova, L.A.; Ikorskii, V.N.; Tremel, W.; Gütlich, P.; Lavrenova, L.G. Mononuclear coordination compounds based on a novel chelating triazole ligand: 1-vinyl-3-acetylamino-1,2,4-triazole. Polyhedron 2002, 21, 797–804. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Bushuev, M.B.; Virovets, A.V.; Naumov, D.Y.; Sheludyakova, L.A.; Shvedenkov, Y.G. Coordination Compounds—Synthesis and Study of Cobalt(II), Nickel(II), and Copper(II) Nitrate Complexes with 3-Acetylamino-1,2,4-triazole. Russ. J. Inorg. Chem. 2000, 45, 1658–1663. [Google Scholar]
- Peng, L.; Zhang, J.; Xue, Z.; Han, B.; Li, J.; Yang, G. Large-pore mesoporous Mn3O4 crystals derived from metal–organic frameworks. Chem. Commun. 2013, 49, 11695–11697. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Low, Z.-X.; Feng, Y.; Leong, S.; Zhong, Z.; Yao, J.; Hapgood, K.; Wang, H. Direct conversion of two-dimensional ZIF-L film to porous ZnO nano-sheet film and its performance as photoanode in dye-sensitized solar cell. Microporous Mesoporous Mater. 2014, 194, 1–7. [Google Scholar] [CrossRef]
- Wu, R.; Qian, X.; Rui, X.; Liu, H.; Yadian, B.; Zhou, K.; Wei, J.; Yan, Q.; Feng, X.-Q.; Long, Y.; et al. Zeolitic Imidazolate Framework 67-Derived High Symmetric Porous Co3O4 Hollow Dodecahedra with Highly Enhanced Lithium Storage Capability. Small 2014, 10, 1932–1938. [Google Scholar] [CrossRef]
- Gan, X.; Zheng, R.; Liu, T.; Meng, J.; Chen, R.; Sun, X.; Sun, X. N-Doped Mesoporous In2O3 for Photocatalytic Oxygen Evolution from the In-based Metal–Organic Frameworks. Chem. Eur. J. 2017, 23, 7264–7271. [Google Scholar] [CrossRef]
- Xiao, J.; Diao, K.; Zheng, Z.; Cui, X. MOF-derived porous ZnO/Co3O4 nanocomposites for high performance acetone gas sensing. J. Mater. Sci. Mater. Electron. 2018, 29, 8535–8546. [Google Scholar] [CrossRef]
- Dong, X.; Su, Y.; Lu, T.; Zhang, L.; Wu, L.; Lv, Y. MOFs-derived dodecahedra porous Co3O4: An efficient cataluminescence sensing material for H2S. Sens. Actuators B 2018, 258, 349–357. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Strickler, P. Die Kristallstruktur von 1,4-trans-Cyclohexan-dicarbonsäure. Helv. Chim. Acta 1966, 49, 2505–2515. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Weng, J.B. Cis-Cyclohexane-1,4-dicarboxylic acid. Acta Crystallogr. 2009, E65, o1293. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).