Magnetic Nanomaterials as Contrast Agents for MRI
Abstract
1. Introduction
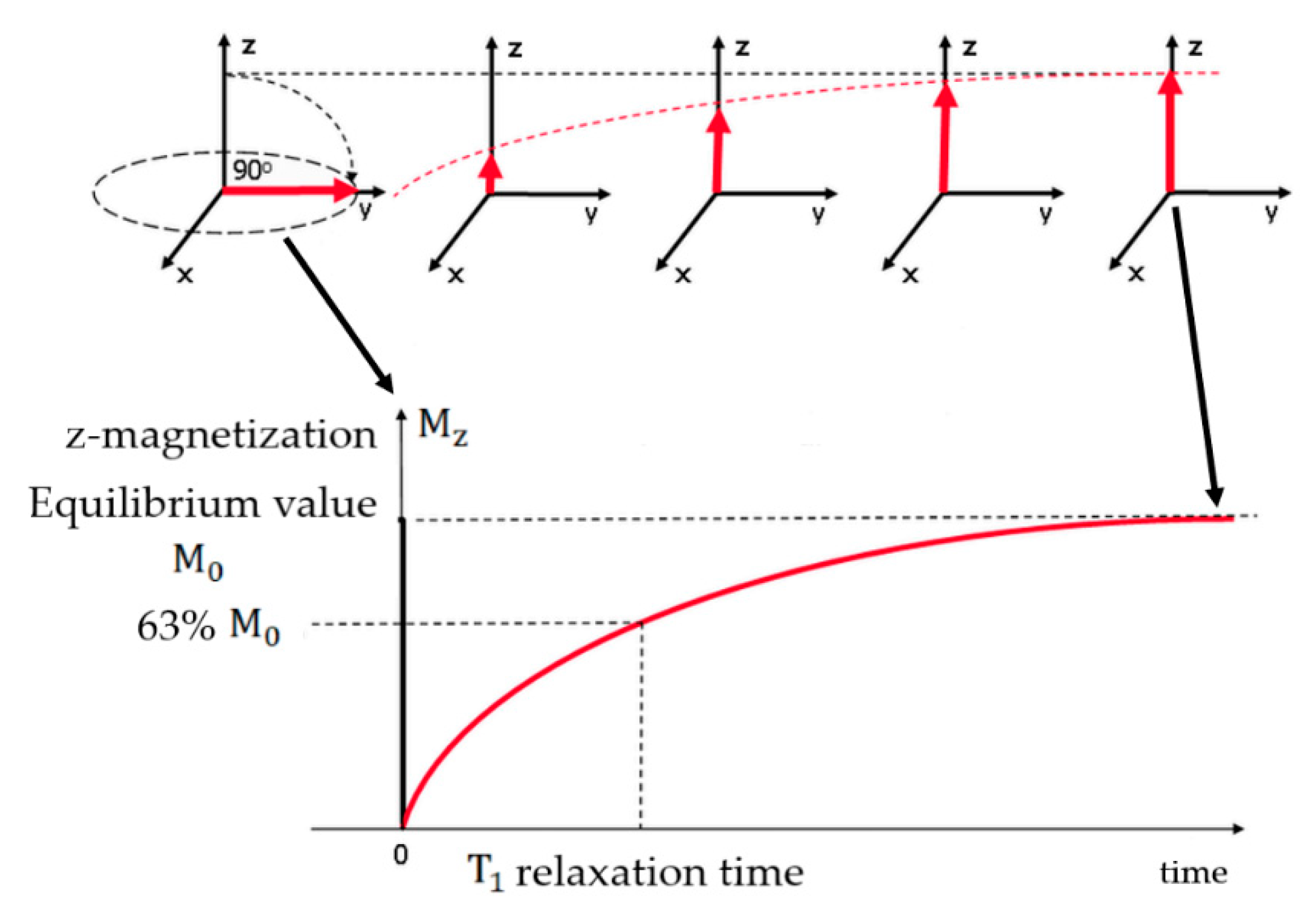
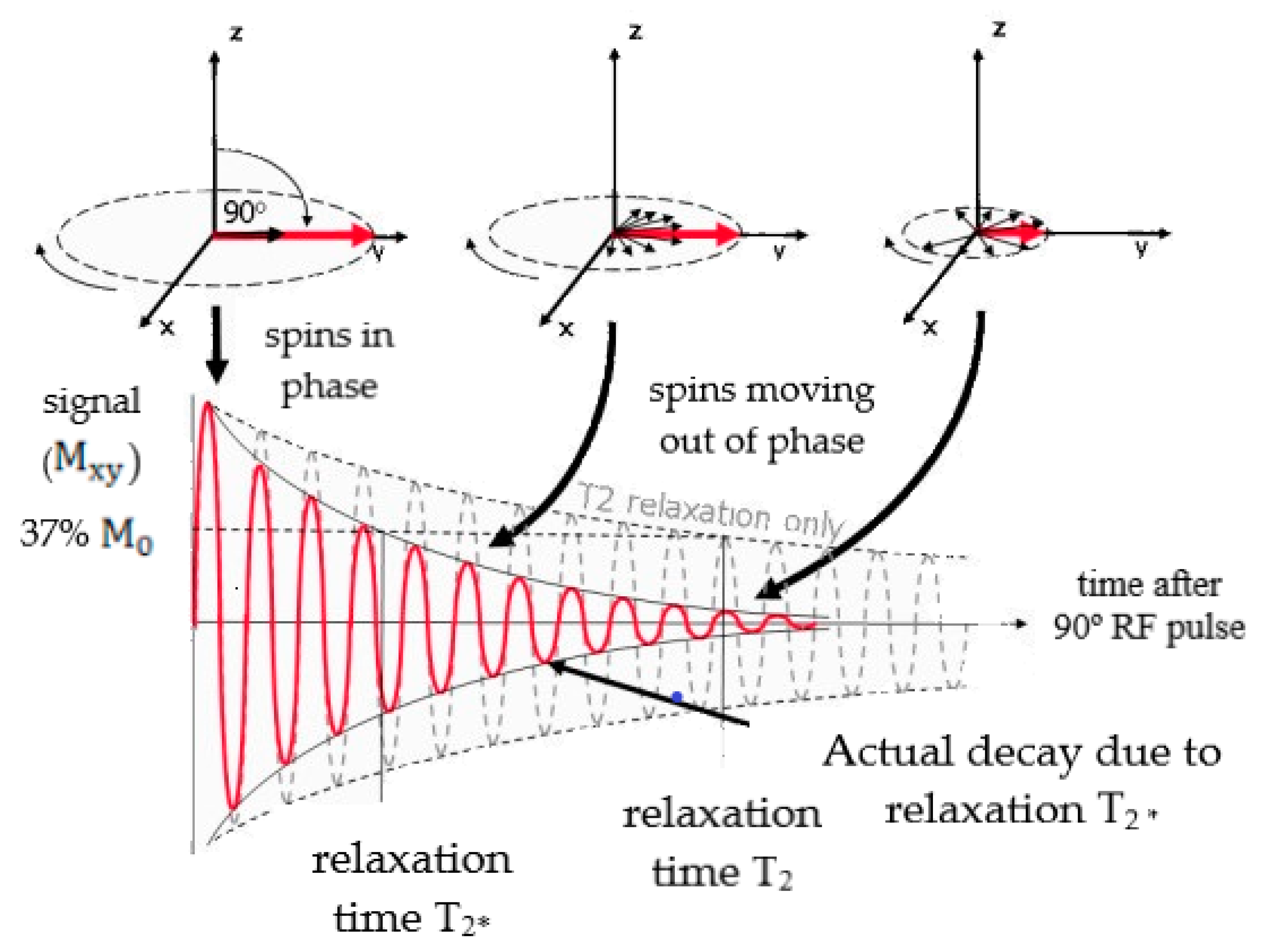
2. T1 and T2 Contrast Agents
2.1. T1 Contrast Agents
2.2. T2 Contrast Agents
3. Magnetic Properties
3.1. Paramagnetic Contrast Agents
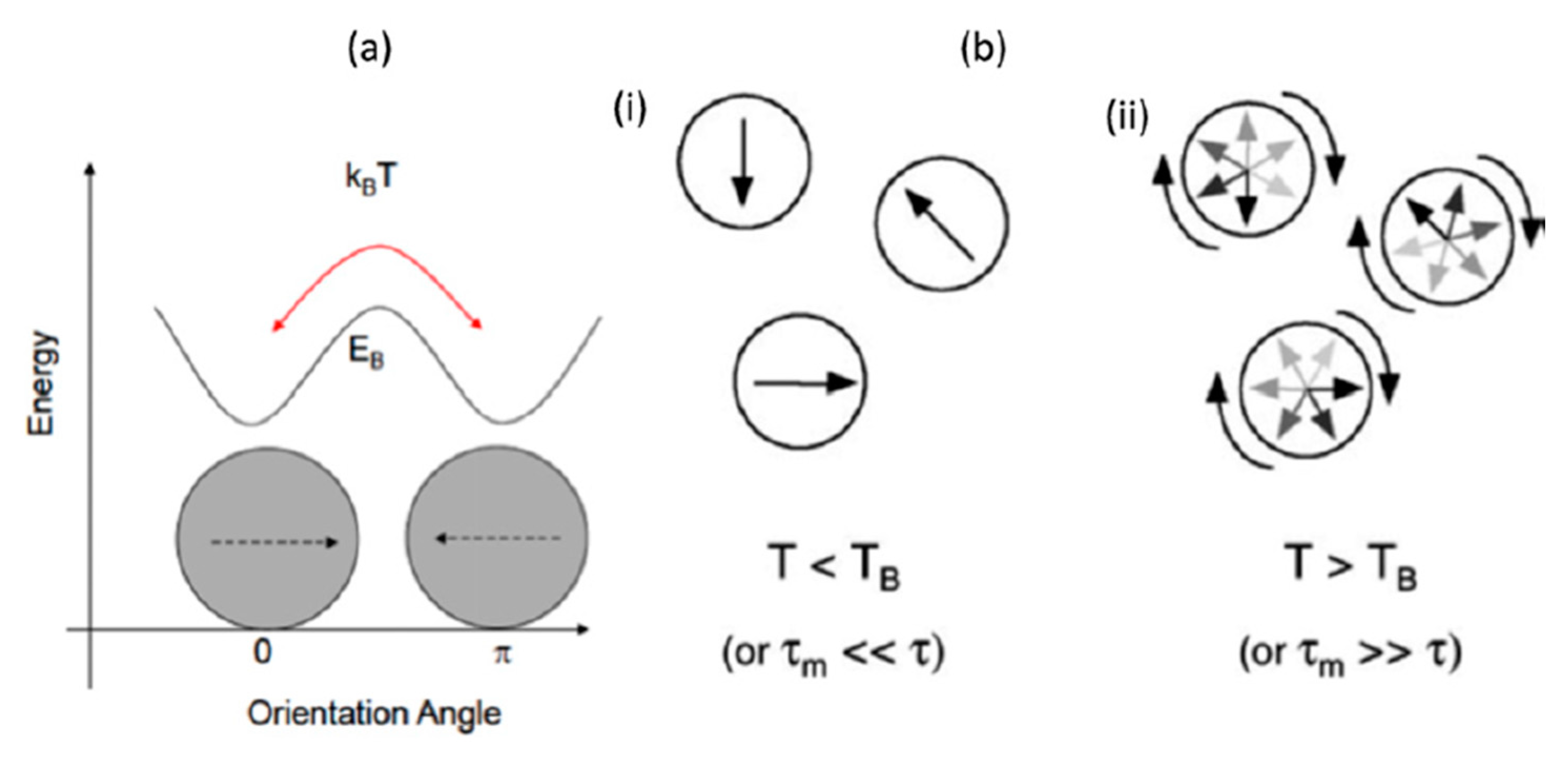
3.2. Superparamagnetic Nanoparticles
3.3. Synthetic Antiferromagnetic
3.4. High Aspect Ratio Nanowires
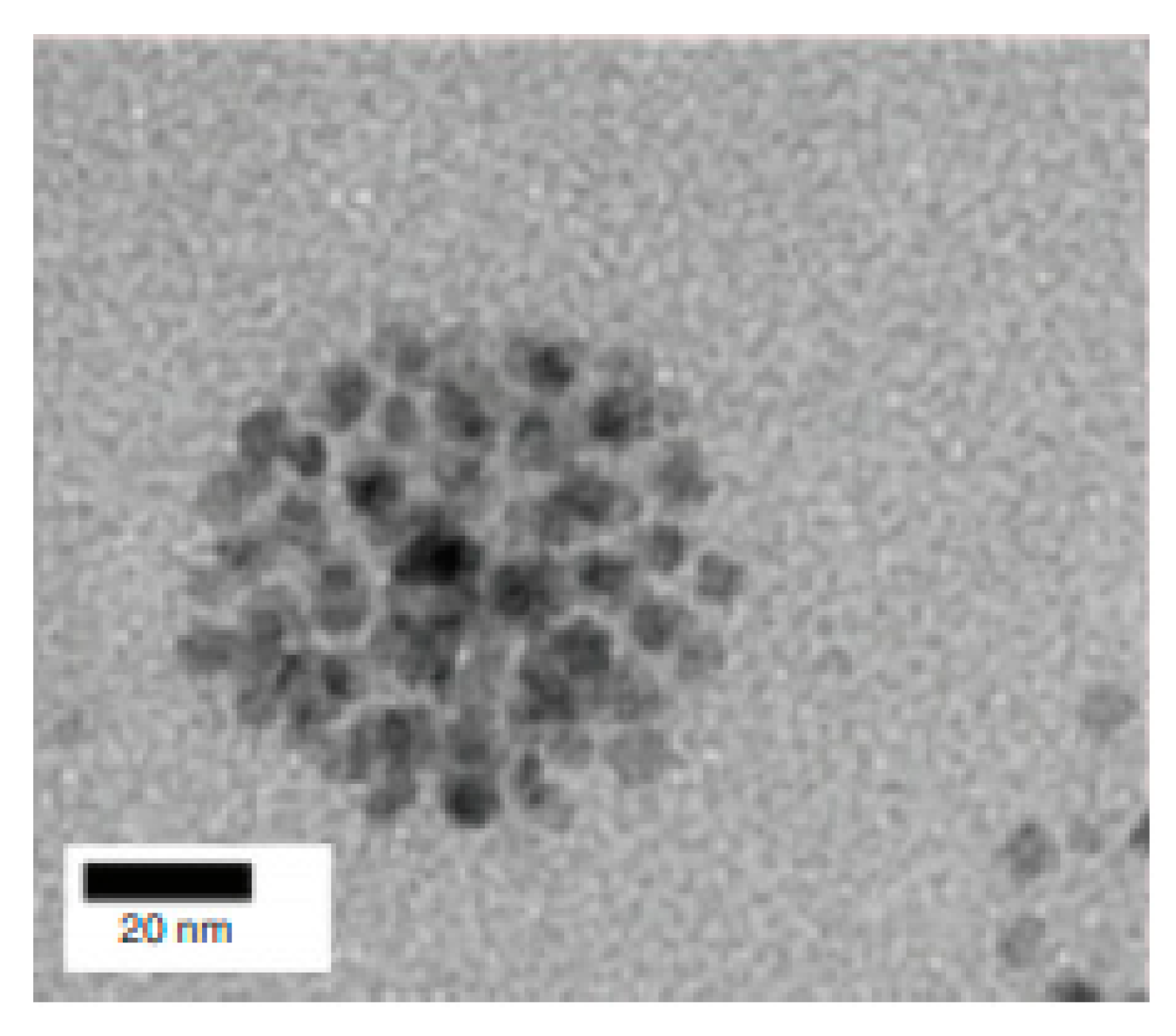
4. Iron Oxide Nanoparticles
5. Gd and Mn-based Nanomaterials
6. Synthetic Antiferromagnetic Nanostructures
7. High aspect Ratio Nanowires
8. Theragnosis Applications
9. Prospects and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuperman, V. Magnetic Resonance Imaging-Physical Principles and Applications; Academic Press: New York, NY, USA, 2000. [Google Scholar]
- Shokrollahi, H. Contrast agents for MRI. Mater. Sci. Eng. C 2013, 33, 4485–4497. [Google Scholar] [CrossRef] [PubMed]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Marckmann, P.; Skov, L.; Rossen, K.; Dupont, A.; Damholt, M.B.; Heaf, J.G.; Thomsen, H.S. Nephrogenic Systemic Fibrosis: Suspected Causative Role of Gadodiamide Used for Contrast-Enhanced Magnetic Resonance Imaging. J. Am. Soc. Nephrol. 2006, 17, 2359–2362. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Sherwood, J.A.; Suna, Z. Magnetic Iron Oxide Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging. J. Mater. Chem. C 2018, 6, 1280–1290. [Google Scholar] [CrossRef]
- Vuong, Q.L.; Gillis, P.; Gossuin, Y. Monte Carlo simulation and theory of proton NMR transverse relaxation induced by aggregation of magnetic particles used as MRI contrast agents. J. Magn. Reson. 2011, 212, 139–148. [Google Scholar] [CrossRef]
- Peixoto, L.; Magalhães, R.; Navas, D.; Moraes, S.; Redondo, C.; Morales, R.; Araújo, J.P.; Sousa, C.T. Magnetic nanostructures for emerging biomedical applications. Appl. Phys. Rev. 2020, 7, 011310. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Xu, C. Magnetic Nanoparticles for Biomedical Applications: From Diagnosis to Treatment to Regeneration. In Engineering in Translational Medicine; Springer: London, UK, 2014; pp. 567–583. [Google Scholar]
- Jeong, Y.; Hwang, H.S.; Na, K. Theranostics and contrast agents for magnetic resonance imaging. Biomater. Res. 2018, 22, 20. [Google Scholar] [CrossRef]
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-k. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef]
- Tromsdorf, U.I.; Bigall, N.C.; Kaul, M.G.; Bruns, O.T.; Nikolic, M.S.; Mollwitz, B.; Sperling, R.A.; Reimer, R.; Hohenberg, H.; Parak, W.J.; et al. Size and surface effects on the MRI relaxivity of manganese ferrite nanoparticle contrast agents. Nano Lett. 2007, 7, 2422–2427. [Google Scholar] [CrossRef]
- Van Geuns, R.-J.M.; Wielopolski, P.A.; de Bruin, H.G.; Rensing, B.J.; van Ooijen, P.M.A.; Hulshoff, M.; Oudkerk, M.; de Feyter, P.J. Basic Principles of Magnetic Resonance Imaging. Prog. Cariovascular Dis. 1999, 42, 149–156. [Google Scholar] [CrossRef]
- Ridgway, J.P. Cardiovascular magnetic resonance physics for clinicians: Part I. J. Cardiovasc. Magn. Reson. 2010, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Lee, G.H.; Chang, Y. Gadolinium as an MRI contrast agent. Future Med. Chem. 2018, 10, 639–661. [Google Scholar] [CrossRef] [PubMed]
- Pellico, J.; Ellis, C.M.; Davis, J.J. Nanoparticle-Based Paramagnetic Contrast Agents for Magnetic Resonance Imaging. Hindawi 2019, 1845637. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.; Semelka, R.C.; Ramalho, M.; Nunes, R.H.; AlObaidy, M.; Castillo, M. Gadolinium-Base Contrast Agent Accumulation and Toxicity: An Update. Am. J. Neuroradiol. 2016, 37, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Caravan, P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J. Magn. Reson. Imaging 2009, 30, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.-C.; Nikolaou, K.; et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016, 33, 1–28. [Google Scholar] [CrossRef]
- Hu, F.; Zhao, Y.S. Inorganic nanoparticle-based T1 and T1/T2 magnetic resonance contrast probes. Nanoscale 2012, 4, 6235–6243. [Google Scholar] [CrossRef]
- Park, J.Y.; Baek, M.J.; Choi, E.S.; Woo, S.; Kim, J.H.; Kim, T.J.; Jung, J.C.; Chae, K.S.; Chang, Y.; Lee, G.H. Paramagnetic ultrasmall gadolinium oxide nanoparticles as advanced T1 MRI contrast agent: Account for large longitudinal relaxivity, optimal particle diameter, and in vivo T1 MR images. ACS Nano 2009, 3, 3663–3669. [Google Scholar] [CrossRef]
- Na, H.B.; Lee, J.H.; An, K.; Park, Y.I.; Park, M.; Lee, I.S.; Nam, D.-H.; Kim, S.T.; Kim, S.-H.; Kim, S.-W.; et al. Development of a T1 Contrast Agent for Magnetic Resonance Imaging Using MnO Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 5397–5401. [Google Scholar] [CrossRef]
- Deka, K.; Guleria, A.; Kumar, D.; Biswas, J.; Lodha, S.; Kaushik, S.D.; Dasgupta, S.; Deb, P. Mesoporous 3D carbon framework encapsulated manganese oxide nanoparticles as biocompatible T1 MR imaging probe. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 229–236. [Google Scholar] [CrossRef]
- Singh, G.; McDonagh, B.H.; Hak, S.; Peddis, D.; Bandopadhyay, S.; Sandvig, I.; Sandvigef, A.; Glommbg, W.R. Synthesis of gadolinium oxide nanodisks and gadolinium doped iron oxide nanoparticles for MR contrast agents. J. Mater. Chem. B 2017, 5, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-Y.; Pellico, J.; Khrapitchev, A.A.; Sibson, N.R.; Davis, J.J. Dy-DOTA integrated mesoporous silica nanoparticles as promising ultrahigh field magnetic resonance imaging contrast agents. Nanoscale 2018, 10, 21041–21045. [Google Scholar] [CrossRef]
- Craciun, I.; Gunkel-Grabole, G.; Belluati, A.; Palivan, C.G.; Meier, W. Expanding the potential of MRI contrast agents through multifunctional polymeric nanocarriers. Nanomedicine 2017, 12, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, S.; Li, H.; Zhang, Y.; Xie, J.; Hwang, D.W.; Zhang, A.; Liu, M.; Zhou, X. Engineered Paramagnetic Graphene Quantum Dots with Enhanced Relaxivity for Tumor Imaging. Nano Lett. 2018, 19, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Litti, L.; Rivato, N.; Fracasso, G.; Bontempi, P.; Nicolato, E.; Marzola, P.; Venzo, A.; Colombatti, M.; Gobbo, M.; Meneghetti, M.A. SERRS/MRI multimodal contrast agent based on naked Au nanoparticles functionalized with a Gd(iii) loaded PEG polymer for tumor imaging and localized hyperthermia. Nanoscale 2018, 10, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Vangijzegem, T.; Stanicki, D.; Boutry, S.; Paternoster, Q.; Elst, L.V.; Muller, R.N.; Laurent, S. VSION as high field MRI T1 contrast agent: Evidence of their potential as positive contrast agent for magnetic resonance angiography. Nanotechnology 2018, 29, 265103. [Google Scholar] [CrossRef]
- Taboada, E.; Rodríguez, E.; Roig, A.; Oró, J.; Roch, A.; Muller, R.N. Relaxometric and Magnetic Characterization of Ultrasmall Iron Oxide Nanoparticles with High Magnetization. Evaluation as Potential T1 Magnetic Resonance Imaging Contrast Agents for Molecular Imaging. Langmuir 2007, 23, 4583–4588. [Google Scholar] [CrossRef]
- Tromsdorf, U.I.; Bruns, O.T.; Salmen, S.C.; Beisiegel, U.; Weller, H. A highly effective, nontoxic T1 MR contrast agent based on ultrasmall PEGylated iron oxide nanoparticles. Nano Lett. 2009, 9, 4434–4440. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef]
- Hu, F.; Jia, Q.; Li, Y.; Gao, M. Facile synthesis of ultrasmall PEGylated iron oxide nanoparticles for dual-contrast T1- and T2-weighted magnetic resonance imaging. Nanotechnology 2011, 22, 245604. [Google Scholar] [CrossRef]
- Hu, F.; MacRenaris, K.W.; Waters, E.A.; Liang, T.; Schultz-Sikma, E.A.; Eckermann, A.L.; Meade, T.J. Ultrasmall, Water-Soluble Magnetite Nanoparticles with High Relaxivity for Magnetic Resonance Imaging. J. Phys. Chem. 2009, 113, 20855–20860. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Guo, Z.; Guo, C.; Mao, Y.; Qin, Z.; Ye, D.; Zang, F.; Lou, Z.; Zhang, Z.; Li, M.; et al. Moderate cooling coprecipitation for extremely small iron oxide as a pH dependent T1-MRI contrast agent. Nanoscale 2020, 12, 5521–5532. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, T.; Ma, X.; Ren, W.; Zhou, Z.; Zhu, G.; Zhang, A.; Liu, Y.; Song, J.; Li, Z.; et al. Multifunctional Theranostic Nanoparticles Based on Exceedingly Small Magnetic Iron Oxide Nanoparticles for T1-Weighted Magnetic Resonance Imaging and Chemotherapy. ACS Nano 2017, 11, 10992–11004. [Google Scholar] [CrossRef] [PubMed]
- Macher, T.; Totenhagen, J.; Sherwood, J.; Qin, Y.; Gurler, D.; Bolding, M.S.; Bao, Y. Ultrathin Iron Oxide Nanowhiskers as Positive Contrast Agents for Magnetic Resonance Imaging. Adv. Funct. Mater. 2014, 25, 490–494. [Google Scholar] [CrossRef]
- Bao, Y.; Wen, T.; Samia, A.C.S.; Khandhar, A.; Krishnan, K.M. Magnetic Nanoparticles: Material Engineering and Emerging Applications in Lithography and Biomedicine. J. Mater. Sci. 2017, 51, 513–553. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.C.F.; Wang, Y.X.J. Mn–Fe Nanowires Towards Cell Labeling and Magnetic Resonance Imaging. In Nanowires: Science and Technology; IntechOpen: London, UK, 2010. [Google Scholar] [CrossRef][Green Version]
- Shore, D.; Pailloux, S.L.; Zhang, J.; Gage, T.; Flannigan, D.J.; Garwood, M.; Pierre, V.C.; Stadler, B.J.H. Electrodeposited Fe and Fe–Au nanowires as MRI contrast agents. Chem. Commun. 2016, 52, 12634–12637. [Google Scholar] [CrossRef]
- Sherwood, J. Shape Dependent Iron Oxide Nanoparticles for Simultaneous Imaging and Therapy. Ph.D. Thesis, The University of Alabama, Tuscaloosa, AL, USA, 2018. [Google Scholar]
- Li, Z.; Yi, P.W.; Sun, Q.; Lei, H.; Zhao, H.L.; Zhu, Z.H.; Smith, S.C.; Lan, M.B.; Lu, G.Q. (Ultrasmall Water-Soluble and Biocompatible Magnetic Iron Oxide Nanoparticles as Positive and Negative Dual Contrast Agents. Adv. Funct. Mater. 2012, 22, 2387–2393. [Google Scholar] [CrossRef]
- Sharma, V.K.; Alipour, A.; Soran-Erdem, Z.; Aykuta, Z.G.; Demir, H.V. Highly monodisperse low-magnetization magnetite nanocubes as simultaneous T1–T2 MRI contrast agents. Nanoscale 2015, 7, 10519–10526. [Google Scholar] [CrossRef]
- Huang, G.; Li, H.; Chen, J.; Zhao, Z.; Yang, L.; Chi, X.; Chen, Z.; Wangb, X.; Gao, J. Tunable T1 and T2 contrast abilities of manganese-engineered iron oxide nanoparticles through size control. Nanoscale 2014, 6, 10404. [Google Scholar] [CrossRef]
- Bailey, M.; Van Der Weegen, R.; Klemm, P.J.; Baker, S.L.; Helms, B.A. Stealth Rare Earth Oxide Nanodiscs for Magnetic Resonance Imaging. Adv. Healthc. Mater. 2012, 1, 437–442. [Google Scholar] [CrossRef]
- Corr, S.A.; Byrne, S.J.; Tekoriute, R.; Meledandri, C.J.; Brougham, D.F.; Lynch, M.; Kerskens, C.M.; O’Dwyer, L.; Gun’Ko, Y.K. Linear Assemblies of Magnetic Nanoparticles as MRI Contrast Agents. J. Am. Chem. Soc. 2008, 130, 4214–4215. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-K.; Liu, C.-L.; Chen, H.-C.; Chou, S.-W.; Tseng, W.-H.; Tseng, Y.-J.; Kang, C.-C.; Hsiao, J.-K.; Chou, P.-T. Antiferromagnetic Iron Nanocolloids: A New Generation in Vivo T1 MRI Contrast Agent. J. Am. Chem. Soc. 2013, 135, 18621–18628. [Google Scholar] [CrossRef] [PubMed]
- Neves, H.R.; A Bini, R.; Barbosa, J.H.O.; Salmon, C.E.G.; Varanda, L.C. Dextran-Coated Antiferromagnetic MnO Nanoparticles for a T1-MRI Contrast Agent with High Colloidal Stability. Part. Part. Syst. Charact. 2016, 33, 167–176. [Google Scholar] [CrossRef]
- Mangrum, W.; Hoang, Q.B.; Amrhein, T.J.; Duncan, S.M.; Maxfield, C.M.; Merkle, E.; Song, A.W. Duke Review of MRI Principles:Case Review Series E-Book; Elsevier Health Sciences: London, UK, 2018. [Google Scholar]
- Fogel, M.A. Principles and Practice of Cardiac Magnetic Resonance in Congenital Heart Disease: Form, Function and Flow; Wiley: New York, NY, USA, 2010. [Google Scholar]
- Lam, T.; Pouliot, P.; Avti, P.K.; Lesage, F.; Kakkar, A.K. Superparamagnetic iron oxide based nanoprobes for imaging and theranostics. Adv. Colloid Interface Sci. 2013, 199, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Hyeon, T. Magnetic Nanoparticles for Magnetic Resonance Imaging Contrast Agents. In Magnetic Nanoparticles in Biosensing and Medicine; Cambridge University Press: Cambridge, UK, 2019; pp. 228–250. [Google Scholar]
- Busquets, M.A.; Estelrich, J.; Sánchez-Martín, M.-J. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.-H.; Choi, J.-S.; Yun, S.; Kim, I.-S.; Song, H.-T.; Kim, Y.; Park, K.I.; Cheon, J. T1 and T2 Dual-Mode MRI Contrast Agent for Enhancing Accuracy by Engineered Nanomaterials. ACS Nano 2014, 8, 3393–3401. [Google Scholar] [CrossRef]
- Szpak, A.; Fiejdasz, S.; Prendota, W.; Strączek, T.; Kapusta, C.; Szmyd, J.S.; Nowakowska, M.; Zapotoczny, S. T1–T2 Dual-modal MRI contrast agents based on superparamagnetic iron oxide nanoparticles with surface attached gadolinium complexes. J. Nanoparticle Res. 2014, 16, 2678. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic Iron Oxide Nanoparticles as MRI contrast agents for Non-invasive Stem Cell Labeling and Tracking. Theranostics 2013, 3, 595–615. [Google Scholar] [CrossRef]
- Boutry, S.; Muller, R.; Laurent, S. Targeted Iron Oxide (Nano)particles Used as MRI Contrast Agent in Small Animal Models. Nanoparticles Biomed. Appl. 2018, 135–164. [Google Scholar] [CrossRef]
- Berry, C.C.; Curtis, A.S.G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R198–R206. [Google Scholar] [CrossRef]
- Michael, J.S.; Lee, B.-S.; Zhang, M.; Yu, J.S. Nanotechnology for Treatment of Glioblastoma Multiforme. J. Transl. Intern. Med. 2018, 6, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.; Luna, C.; Morales, M.; Sanz, R.; Serna, C.; Mijangos, C. Magnetic nanoparticles: Synthesis, ordering and properties. Phys. B Condens. Matter 2004, 354, 71–79. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Bran, C.; Rodríguez-Abreu, C.; Gallo, J.; Vazquez, M.; Rivas, J. A colloidally stable water dispersion of Ni nanowires as an efficient T2-MRI contrast agent. J. Mater. Chem. B 2017, 5, 3338–3347. [Google Scholar] [CrossRef]
- Van Roosbroeck, R.; Van Roy, W.; Stakenborg, T.; Trekker, J.; D’Hollander, A.; Dresselaers, T.; Himmelreich, U.; Lammertyn, J.; Lagae, L. Synthetic Antiferromagnetic Nanoparticles as Potential Contrast Agents in MRI. ACS Nano 2014, 8, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnol. 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- A Pankhurst, Q.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Xu, C.; Sun, S. Monodisperse magnetic nanoparticles for biomedical applications. Polym. Int. 2007, 56, 821–826. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-Lopez, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2017, 7, 1700845. [Google Scholar] [CrossRef]
- Plouffe, B.; Murthy, S.K.; Lewis, L.H. Fundamentals and application of magnetic particles in cell isolation and enrichment: A review. Rep. Prog. Phys. 2014, 78, 016601. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.L.; Hu, W.; Wilson, R.J.; Wang, S.X.; Sinclair, R. Preparation, structural and magnetic characterization of synthetc anti-ferromagnetic nanoparticles. Philos. Mag. 2008, 88, 4225. [Google Scholar] [CrossRef]
- Bruno, P. Theory of interlayer exchange interactions in magnetic multilayers. J. Phys. Condens. Matter 1999, 11, 9403–9419. [Google Scholar] [CrossRef]
- Bruno, P.; Chappert, C. Oscillatory Coupling between Ferromagnetic Layers Separated by a Nonmagnetic Metal Spacer. Phys. Rev. Lett. 1991, 67, 2592. [Google Scholar] [CrossRef]
- Dieny, B.; Gavigan, J.P.; Rebouillat, J.P. Magnetisation processes, hysteresis and finite-size effects in model multilayer systems of cubic or uniaxial anisotropy with antiferromagnetic coupling between adjacent ferromagnetic layers. J. Phys. Condens. Matter 1990, 2, 159–185. [Google Scholar] [CrossRef]
- Hu, W.; Wilson, R.J.; Earhart, C.M.; Koh, A.L.; Sinclair, R.; Wang, S.X. Synthetic antiferromagnetic nanoparticles with tunable susceptibilities. J. Appl. Phys. 2009, 105, 07B508. [Google Scholar] [CrossRef]
- Susano, M.; Proenca, M.P.; Moraes, S.; Sousa, C.T.; Araújo, J.P. Tuning the magnetic properties of multisegmented Ni/Cu electrodeposited nanowires with controllable Ni lengths. Nanotechnol. 2016, 27, 335301. [Google Scholar] [CrossRef]
- Arrott, A.; Heinrich, B.; Aharoni, A. Point singularities and magnetization reversal in ideally soft ferromagnetic cylinders. IEEE Trans. Magn. 1979, 15, 1228–1235. [Google Scholar] [CrossRef]
- Frei, E.H.; Shtrikman, S.; Treves, D. Critical Size and Nucleation Field of Ideal Ferromagnetic Particles. Phys. Rev. 1957, 106, 446–455. [Google Scholar] [CrossRef]
- Stoner, E.C.; Wohlfarth, E.P. A mechanism of magnetic hysteresis in heterogeneous alloys. IEEE Trans. Magn. 1991, 27, 3475–3518. [Google Scholar] [CrossRef]
- Fratila, R.M.M.; Rivera-Fernández, S.; De La Fuente, J.M. Shape matters: Synthesis and biomedical applications of high aspect ratio magnetic nanomaterials. Nanoscale 2015, 7, 8233–8260. [Google Scholar] [CrossRef] [PubMed]
- Issa, B.; Obaidat, I.M. Magnetic Nanoparticles as MRI Contrast Agents. Magnetic Resonance Imaging 2019, 467. [Google Scholar] [CrossRef][Green Version]
- Javed, Y.; Akhtar, K.; Anwar, H.; Jamil, Y. MRI based on iron oxide nanoparticles contrast agents: Effect of oxidation state and architecture. J. Nanoparticle Res. 2017, 19, 366. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M.N. anoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54. [Google Scholar]
- Association, I.R. Management. In Pharmaceutical Sciences: Breakthroughs in Research and Practice; IGI Global: Hershey, PA, USA, 2016. [Google Scholar]
- Hobson, N.J.; Weng, X.; Siow, B.; Veiga, C.; Ashford, M.; Thanh, N.T.; Schätzlein, A.G.; Uchegbu, I.F. Clustering superparamagnetic iron oxide nanoparticles produces organ-targeted high-contrast magnetic resonance images. Nanomedicine 2019, 14, 1135–1152. [Google Scholar] [CrossRef]
- Basly, B.; Felder-Flesch, D.; Perriat, P.; Billotey, C.; Taleb, J.; Pourroy, G.; Bégin-Colin, S. Dendronized iron oxide nanoparticles as contrast agents for MRI. Chem. Commun. 2010, 46, 985–987. [Google Scholar] [CrossRef]
- Xie, H.; Zhu, Y.; Jiang, W.; Zhou, Q.; Yang, H.; Gu, N.; Zhang, Y.; Xu, H.; Xu, H.; Yang, X. Lactoferrin-conjugated superparamagnetic iron oxide nanoparticles as a specific MRI contrast agent for detection of brain glioma in vivo. Biomaterials 2011, 32, 495–502. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.; Campbell, E.; Naumov, A. Multifunctional graphene oxide/iron oxide nanoparticles for magnetic targeted drug delivery dual magnetic resonance/fluorescence imaging and cancer sensing. PLoS ONE 2019, 14, e0217072. [Google Scholar] [CrossRef]
- Sulek, S.; Mammadov, B.; Mahcicek, D.I.; Sözeri, H.; Atalar, E.; Tekinay, A.B.; Guler, M.O. Peptide functionalized superparamagnetic iron oxide nanoparticles as MRI contrast agents. J. Mater. Chem. 2011, 21, 15157. [Google Scholar] [CrossRef]
- Ali, L.M.A.; Marzola, P.; Nicolato, E.; Fiorini, S.; Guillamón, M.D.L.H.; Piñol, R.; Gabilondo, L.; Millán, A.; Palacio, F. Polymer-coated superparamagnetic iron oxide nanoparticles as T2 contrast agent for MRI and their uptake in liver. Futur. Sci. OA 2019, 5, FSO235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Song, X.; Gu, X.; Sun, H.; Fu, C.; Meng, F. Synthesis of Superparamagnetic Iron Oxide Nanoparticles Modified with MPEG-PEI via Photochemistry as New MRI Contrast Agent. J. Nanomater. 2015, 2015, 417389. [Google Scholar] [CrossRef]
- Yue-Jian, C.; Juan, T.; Fei, X.; Jia-Bi, Z.; Ning, G.; Yi-Hua, Z.; Ye, D.; Liang, G. Synthesis, self-assembly, and characterization of PEG-coated iron oxide nanoparticles as potential MRI contrast agent. Drug Dev. Ind. Pharm. 2010, 36, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Liu, S.Z.; Louie, A.Y. Biological effects of MRI contrast agents: Gadolinium retention, potential mechanisms and a role for phosphorus. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170180. [Google Scholar] [CrossRef]
- Dai, Z. Advances in Nanotheranostics II: Cancer Theranostic Nanomedicine; Springer Series in Biomaterials Science and Engineering: London, UK, 2016. [Google Scholar]
- Wei, H.; Bruns, O.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wiśniowska, A.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef]
- Yin, X.; Russek, S.E.; Zabow, G.; Sun, F.; Mohapatra, J.; Keenan, K.E.; Boss, M.A.; Zeng, H.; Liu, J.P.; Viert, A.; et al. Large T1 contrast enhancement using superparamagnetic nanoparticles in ultra-low field MRI. Sci. Rep. 2018, 8, 1. [Google Scholar]
- Kozlova, A.A.; German, S.V.; Atkin, V.S.; Zyev, V.V.; Astle, M.A.; Bratashov, D.; Svenskaya, Y.; Gorin, D. Magnetic Composite Submicron Carriers with Structure-Dependent MRI Contrast. Inorganics 2020, 8, 11. [Google Scholar] [CrossRef]
- Rieter, W.J.; Kim, J.S.; Taylor, K.M.L.; An, H.; Lin, W.; Tarrant, T.; Lin, W. Hybrid silica nanoparticles for multimodal imaging. Angew. Chem. Int. Ed. 2007, 46, 3680. [Google Scholar] [CrossRef]
- Davis, J.J.; Huang, W.-Y.; Davies, G.-L. Location-tuned relaxivity in Gd-doped mesoporous silica nanoparticles. J. Mater. Chem. 2012, 22, 22848–22850. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Davies, G.-L.; Davis, J.J. High signal contrast gating with biomodified Gd doped mesoporous nanoparticles. Chem. Commun. 2012, 49, 60–62. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Huang, J.; Wang, L.; Shen, H.; Luo, Y.; Li, Z.; Zhang, H.; Deng, Z.; Zhang, Z. Ultrasmall graphene oxide based T1 MRI contrast agent for in vitro and in vivo labeling of human mesenchymal stem cells. Nanomed. Nanotechnol. Boil. Med. 2018, 14, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Panich, A.M.; Salti, M.; Goren, S.D.; Yudina, E.B.; Aleksenskii, A.E.; Vul, A.Y.; Shames, A.I. Gd(III)-Grafted Detonation Nanodiamonds for MRI Contrast Enhancement. J. Phys. Chem. C 2019, 123, 2627–2631. [Google Scholar] [CrossRef]
- Xu, L.; Hong, S.H.; Sun, Y.; Sun, Z.; Shou, K.; Cheng, K.; Chen, H.; Huang, D.; Xu, H.; Cheng, Z. Dual T 1 and T 2 weighted magnetic resonance imaging based on Gd 3+ loaded bioinspired melanin dots. Nanomed. Nanotechnol. Boil. Med. 2018, 14, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chen, D.; Zhang, Y.; Li, C.; Liu, L.; Shao, Y. MRI relaxivity enhancement of gadolinium oxide nanoshells with a controllable shell thickness. Phys. Chem. Chem. Phys. 2018, 20, 10038–10047. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, L.; Zhou, Z.; Wang, X.; Zhang, Y.; Wang, J.; Mi, P.; Liu, G.; Zhou, L. Gadolinium hybrid iron oxide nanocomposites for dual T1- and T2-weighted MR imaging of cell labeling. Biomater. Sci. 2017, 5, 50–56. [Google Scholar] [CrossRef]
- Jacques, V.; Dumas, S.; Sun, W.-C.; Troughton, J.S.; Greenfield, M.T.; Caravan, P. High-Relaxivity Magnetic Resonance Imaging Contrast Agents Part 2. Investig. Radiol. 2010, 45, 613–624. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, C.; Bao, J.; Yang, L.; Wei, R.; Cheng, J.; Lin, H.; Gao, J. Surface manganese substitution in magnetite nanocrystals enhances T1 contrast ability by increasing electron spin relaxation. J. Mater. Chem. B 2017, 6, 401–413. [Google Scholar] [CrossRef]
- Hequet, E.; Henoumont, C.; Muller, R.N.; Laurent, S. Fluorinated MRI contrast agents and their versatile applications in the biomedical field. Future Med. Chem. 2019, 11, 1157–1175. [Google Scholar] [CrossRef]
- Helm, L.; Morrow, J.R.; Bond, C.J.; Carniato, F.; Botta, M.; Braun, M.; Baranyai, Z.; Pujales-Paradela, R.; Regueiro-Figueroa, M.; Esteban-Gómez, D.; et al. Chapter 2. Gadolinium-based Contrast Agents. In New Developments in NMR; University of Debrecen, Hungary, Royal Society of Chemistry (RSC): London, UK, 2017; pp. 121–242. [Google Scholar]
- Peterson, K.L.; Srivastava, K.; Pierre, V.C. Fluorinated Paramagnetic Complexes: Sensitive and Responsive Probes for Magnetic Resonance Spectroscopy and Imaging. Front. Chem. 2018, 6, 1. [Google Scholar] [CrossRef]
- Liu, C.-L.; Peng, Y.-K.; Chou, S.-W.; Tseng, W.-H.; Tseng, Y.-J.; Chen, H.-C.; Hsiao, J.-K.; Chou, P.-T. One-Step, Room-Temperature Synthesis of Glutathione-Capped Iron-Oxide Nanoparticles and their Application in In Vivo T1-Weighted Magnetic Resonance Imaging. Small 2014, 10, 3962–3969. [Google Scholar] [CrossRef]
- Leung, K.C.-F.; Wang, Y.-X.; Wang, H.-H.; Chak, C.-P. Novel one-dimensional MnFe oxide nanowires for cell labeling and magnetic resonance imaging. In Proceedings of the 2008 International Conference on Technology and Applications in Biomedicine; Institute of Electrical and Electronics Engineers (IEEE): Shenzhen, China, 2008; pp. 193–195. [Google Scholar]
- Martínez-Banderas, A.I.; Aires, A.; Plaza-García, S.; Colás, L.; Moreno, J.A.; Ravasi, T.; Merzaban, J.S.; Ramos-Cabrer, P.; Cortajarena, A.L.; Kosel, J. Magnetic core–shell nanowires as MRI contrast agents for cell tracking. J. Nanobiotechnology 2020, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, J.; Mitra, A.; Tyagi, H.; Bahadur, D.; Aslam, M. Iron oxide nanorods as high-performance magnetic resonance imaging contrast agents. Nanoscale 2015, 7, 9174–9184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ju, Y.; Ali, Z.; Yin, H.; Sheng, F.; Lin, J.; Hai, J.; Hou, Y. Near-infrared light and tumor microenvironment dual responsive size-switchable nanocapsules for multimodal tumor theranostics. Nat. Commun. 2019, 10, 4412–4418. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, N.; Kim, H.; Kim, J.; Choi, S.H.; Kim, J.H.; Kim, T.; Song, I.C.; Park, S.P.; Moon, W.K.; et al. Uniform Mesoporous Dye-Doped Silica Nanoparticles Decorated with Multiple Magnetite Nanocrystals for Simultaneous Enhanced Magnetic Resonance Imaging, Fluorescence Imaging, and Drug Delivery. J. Am. Chem. Soc. 2010, 132, 552–557. [Google Scholar] [CrossRef]
- Piñeiro, Y.; Gómez, M.G.; Alves, L.D.C.; Prieto, A.A.; Acevedo, P.G.; Gudiña, R.S.; Puig, J.; Teijeiro, C.; Vilar, S.Y.; Rivas, J. Hybrid Nanostructured Magnetite Nanoparticles: From Bio-Detection and Theragnostics to Regenerative Medicine. Magnetochemistry 2020, 6, 4. [Google Scholar] [CrossRef]
- Efremova, M.V.; Naumenko, V.A.; Spasova, M.; Garanina, A.; Abakumov, M.A.; Blokhina, A.D.; Melnikov, P.A.; Prelovskaya, A.O.; Heidelmann, M.; Li, Z.-A.; et al. Magnetite-Gold nanohybrids as ideal all-in-one platforms for theranostics. Sci. Rep. 2018, 8, 11295. [Google Scholar] [CrossRef]
- Sun, L.; Joh, D.Y.; Al-Zaki, A.; Stangl, M.; Murty, S.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Kao, G.D.; Tsourkas, A.; et al. Theranostic Application of Mixed Gold and Superparamagnetic Iron Oxide Nanoparticle Micelles in Glioblastoma Multiforme. J. Biomed. Nanotechnol. 2016, 12, 347–356. [Google Scholar] [CrossRef]
- Sousa, C.T.; Leitão, L.; Ventura, J.; Tavares, P.; Araújo, J.P. A versatile synthesis method of dendrites-free segmented nanowires with a precise size control. Nanoscale Res. Lett. 2012, 7, 168. [Google Scholar] [CrossRef]
- Espinosa, A.; Kolosnjaj-Tabi, J.; Abou-Hassan, A.; Sangnier, A.P.; Curcio, A.; Silva, A.K.A.; Di Corato, R.; Neveu, S.; Pellegrino, T.; Liz-Marzán, L.M.; et al. Magnetic (Hyper)Thermia or Photothermia? Progressive Comparison of Iron Oxide and Gold Nanoparticles Heating in Water, in Cells, and In Vivo. Adv. Funct. Mater. 2018, 28, 1803660. [Google Scholar] [CrossRef]
- Carvalho, A.; Gallo, J.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Hilliou, L.; Ferreira, P.M.T.; Bañobre-López, M.; Martins, J.A. Magnetic Dehydrodipeptide-Based Self-Assembled Hydrogels for Theragnostic Applications. Nanomaterials 2019, 9, 541. [Google Scholar] [CrossRef]
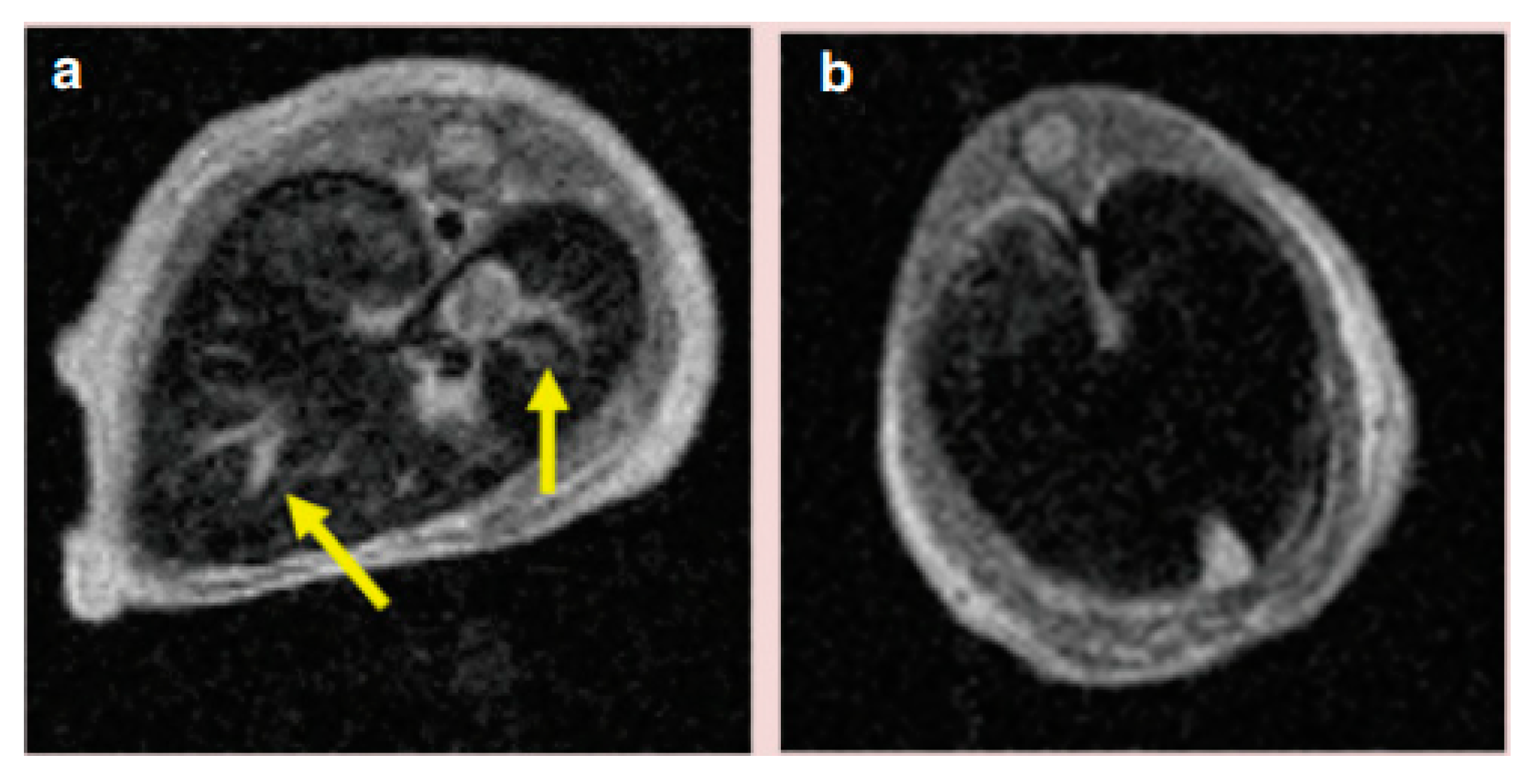
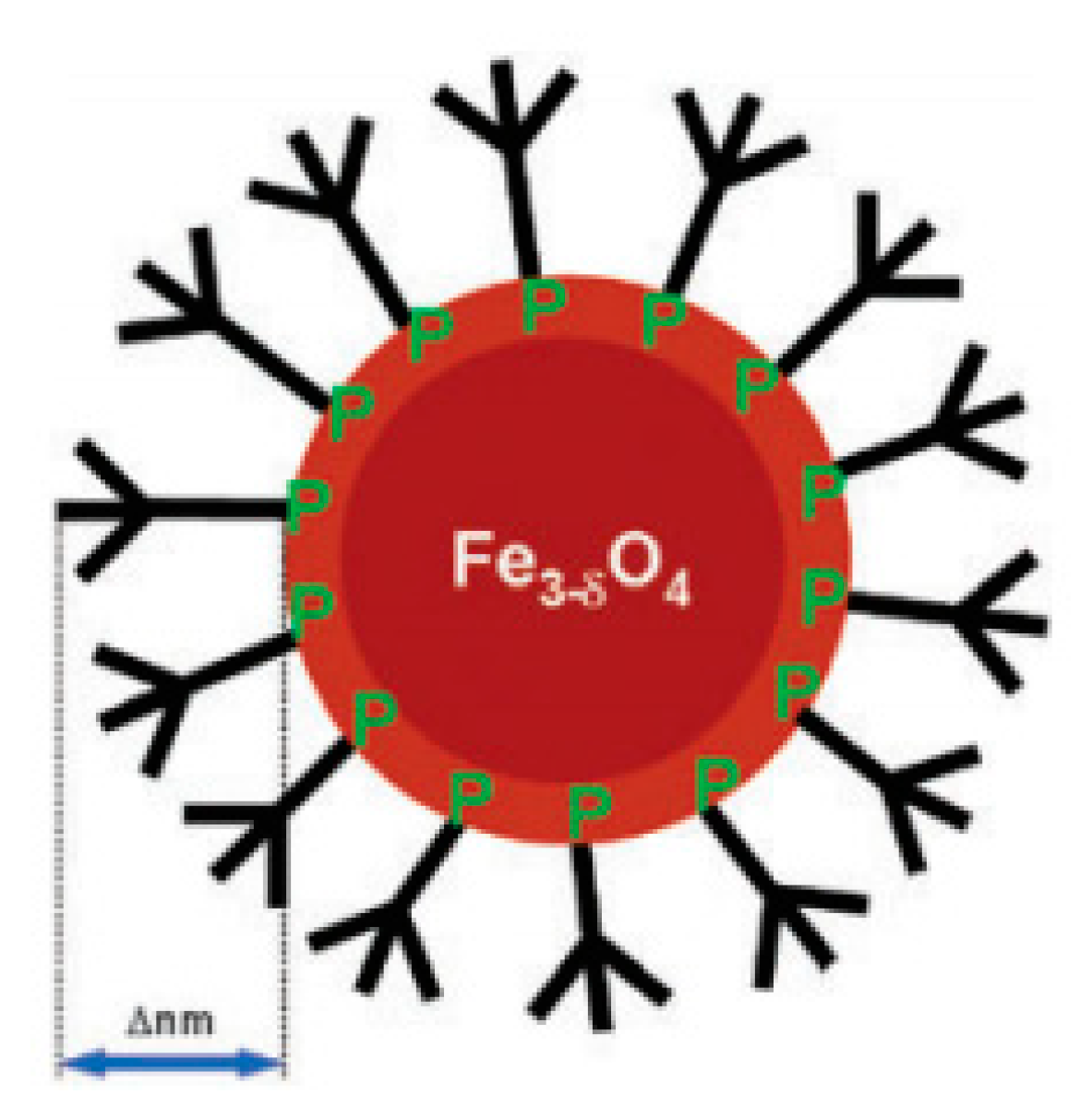
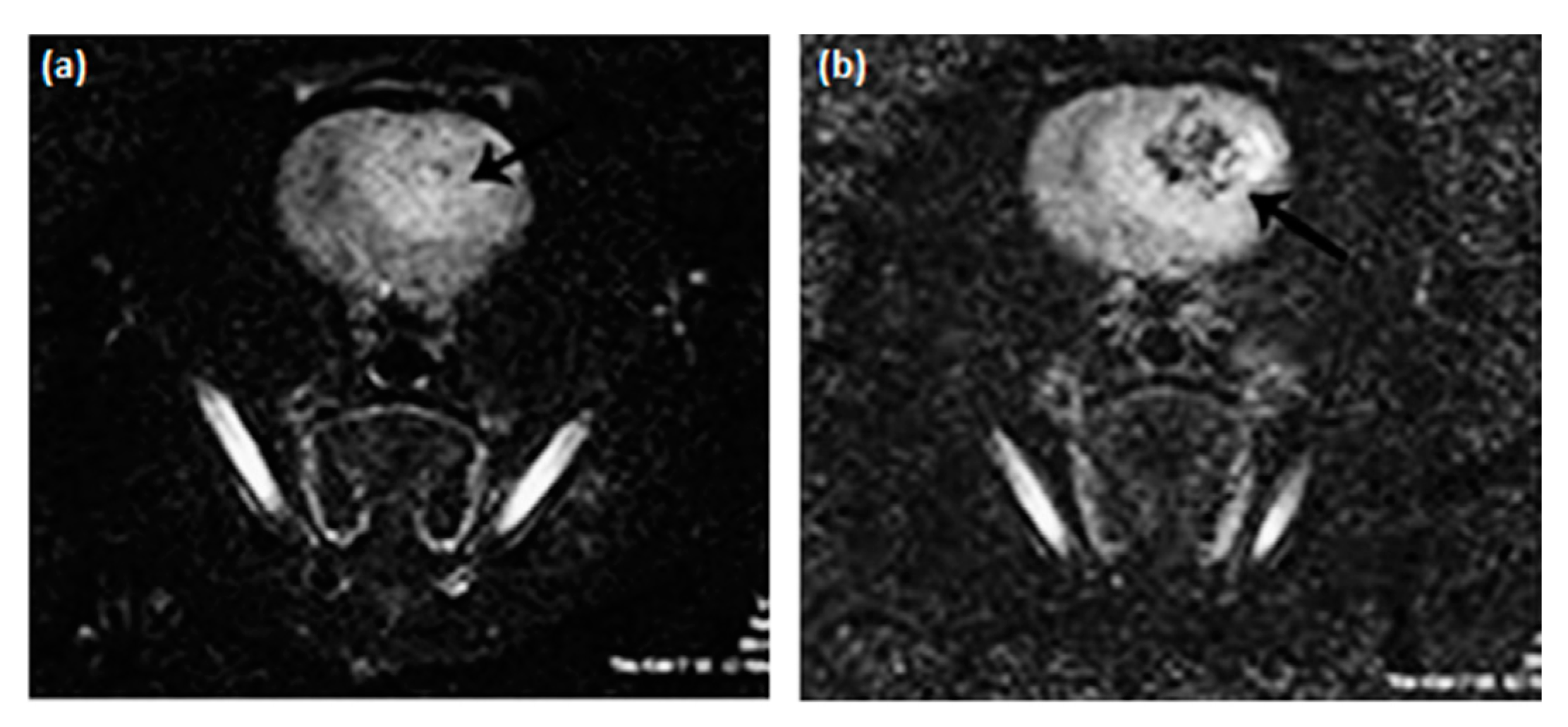
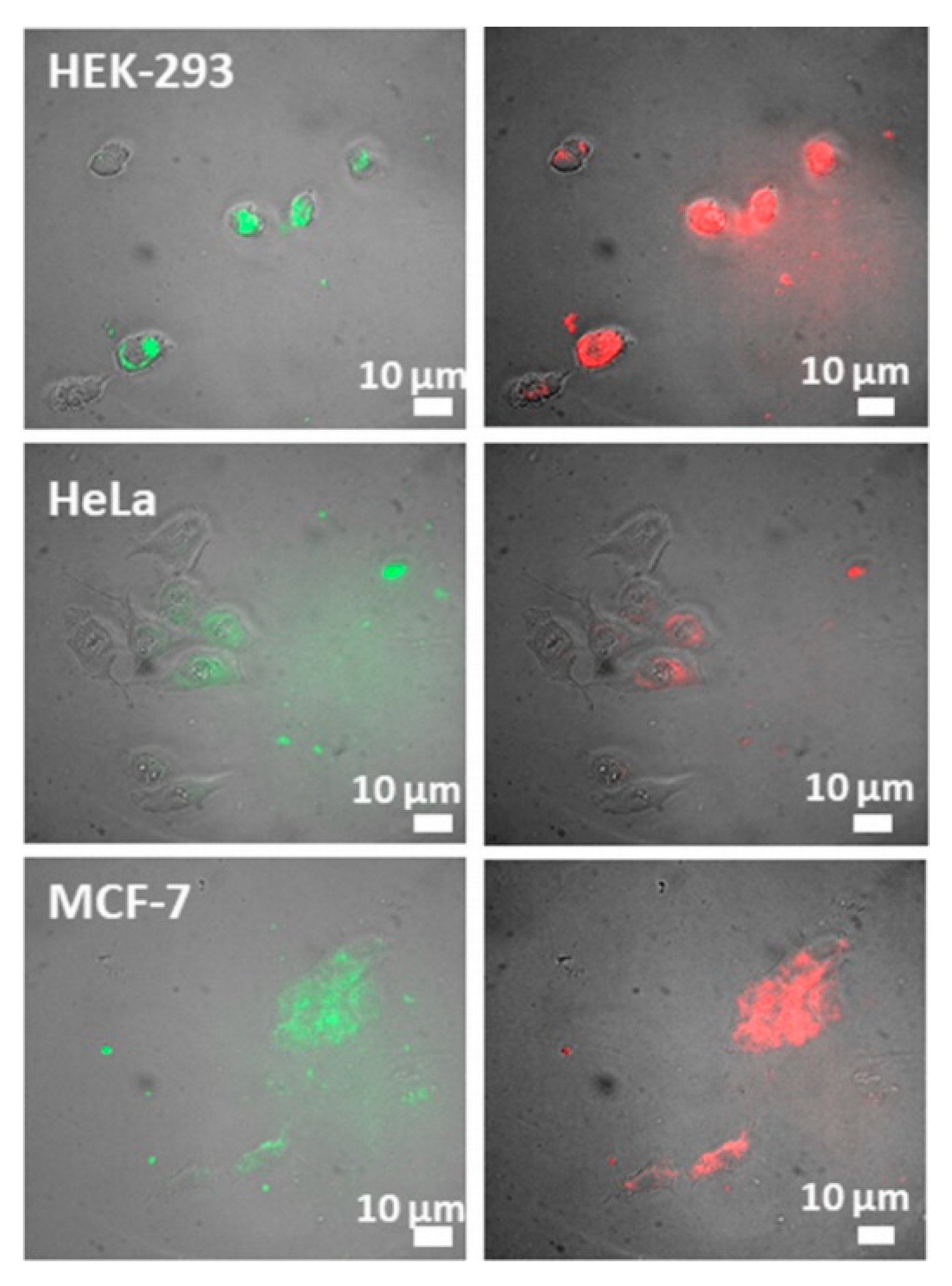
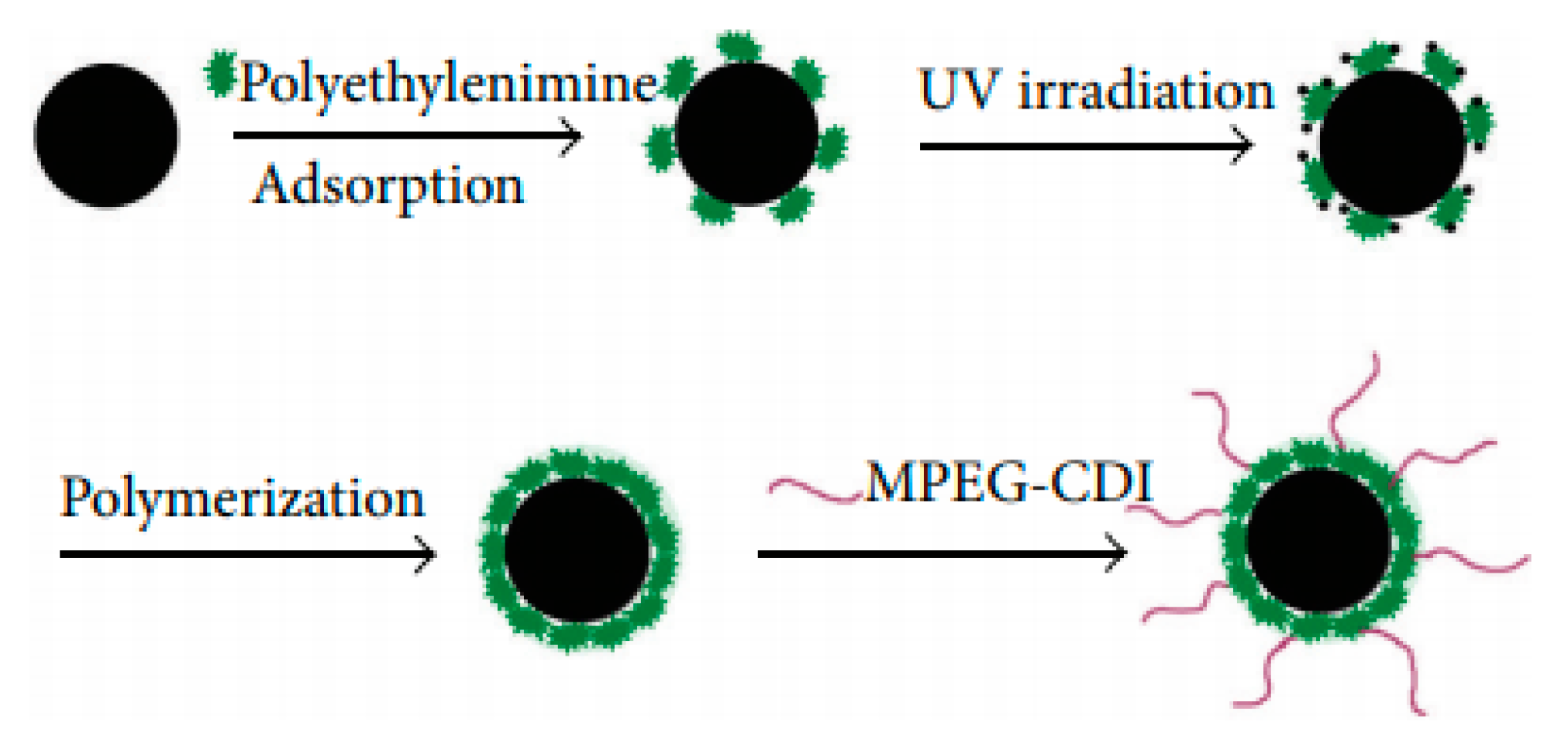
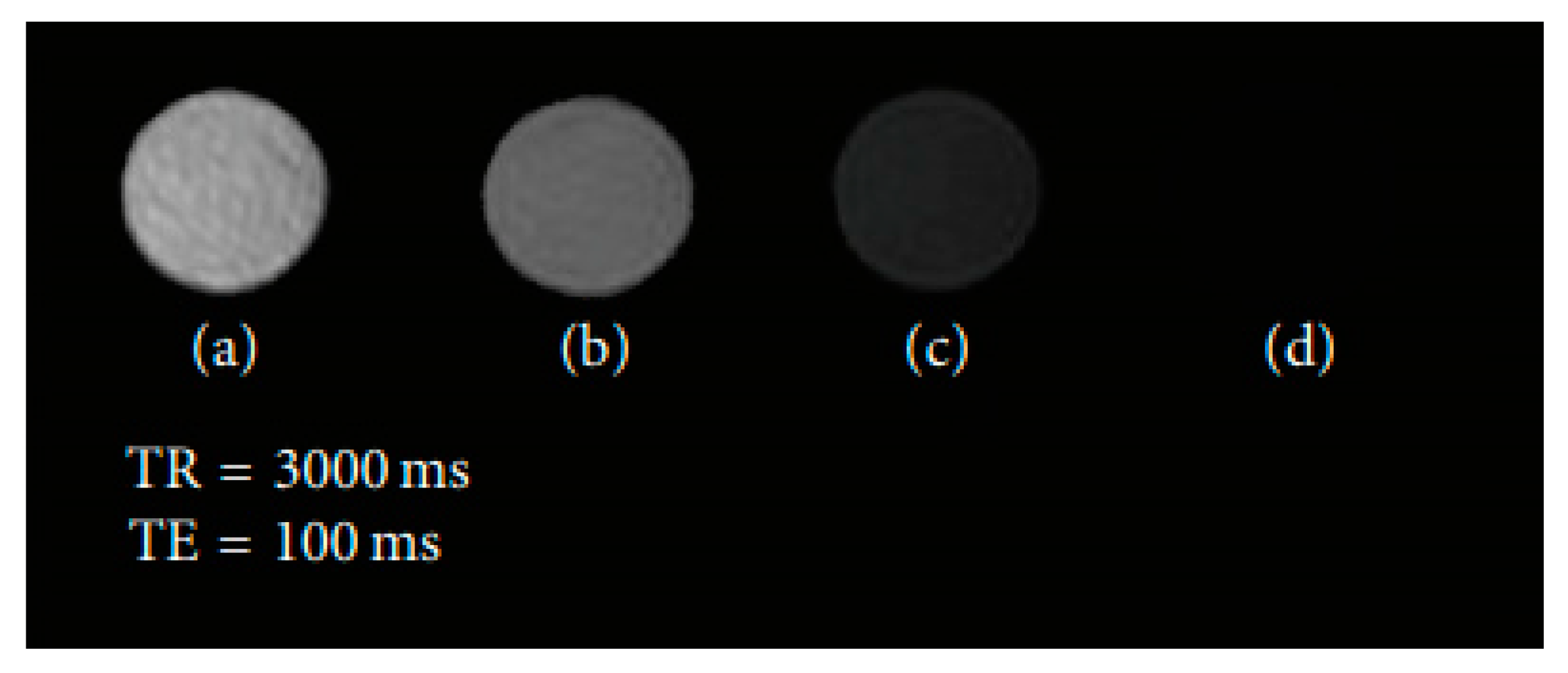
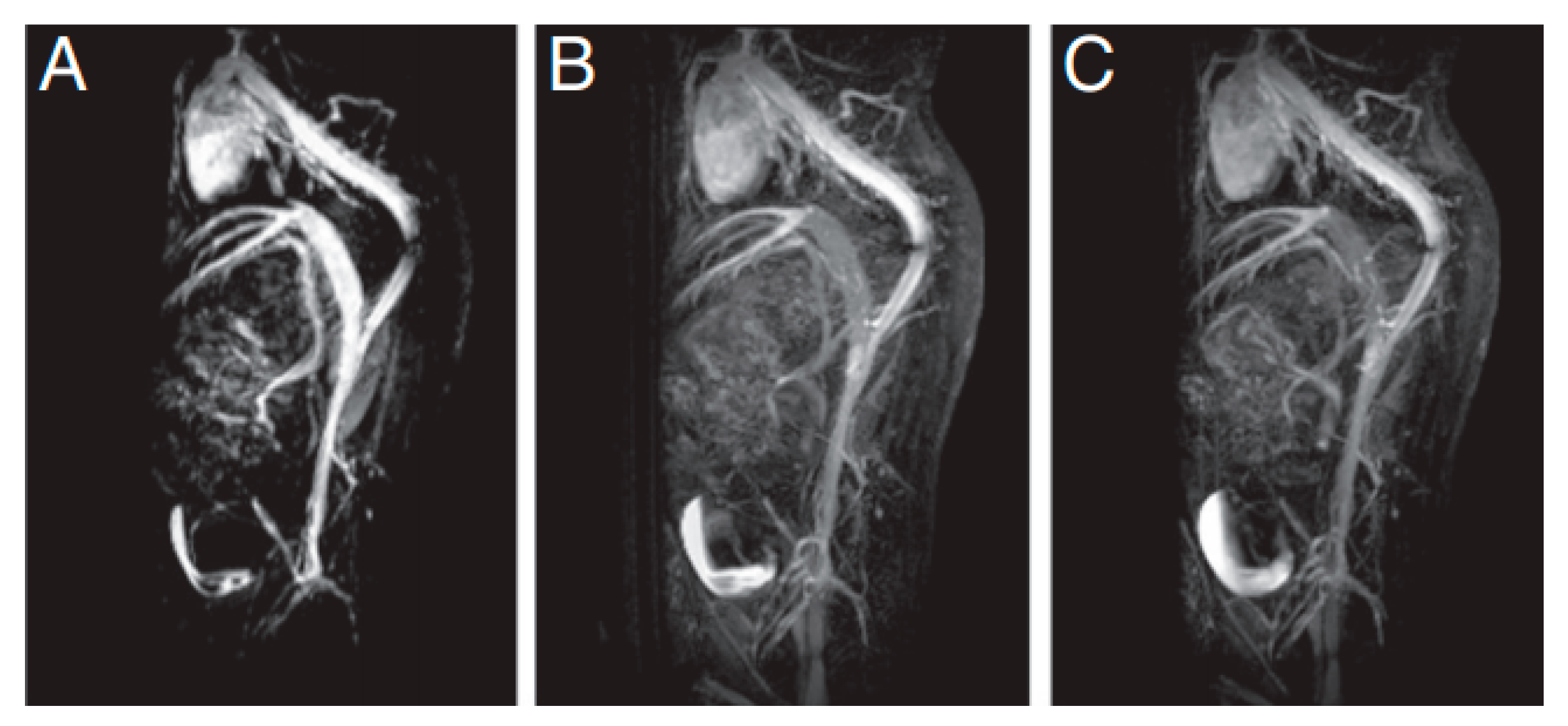
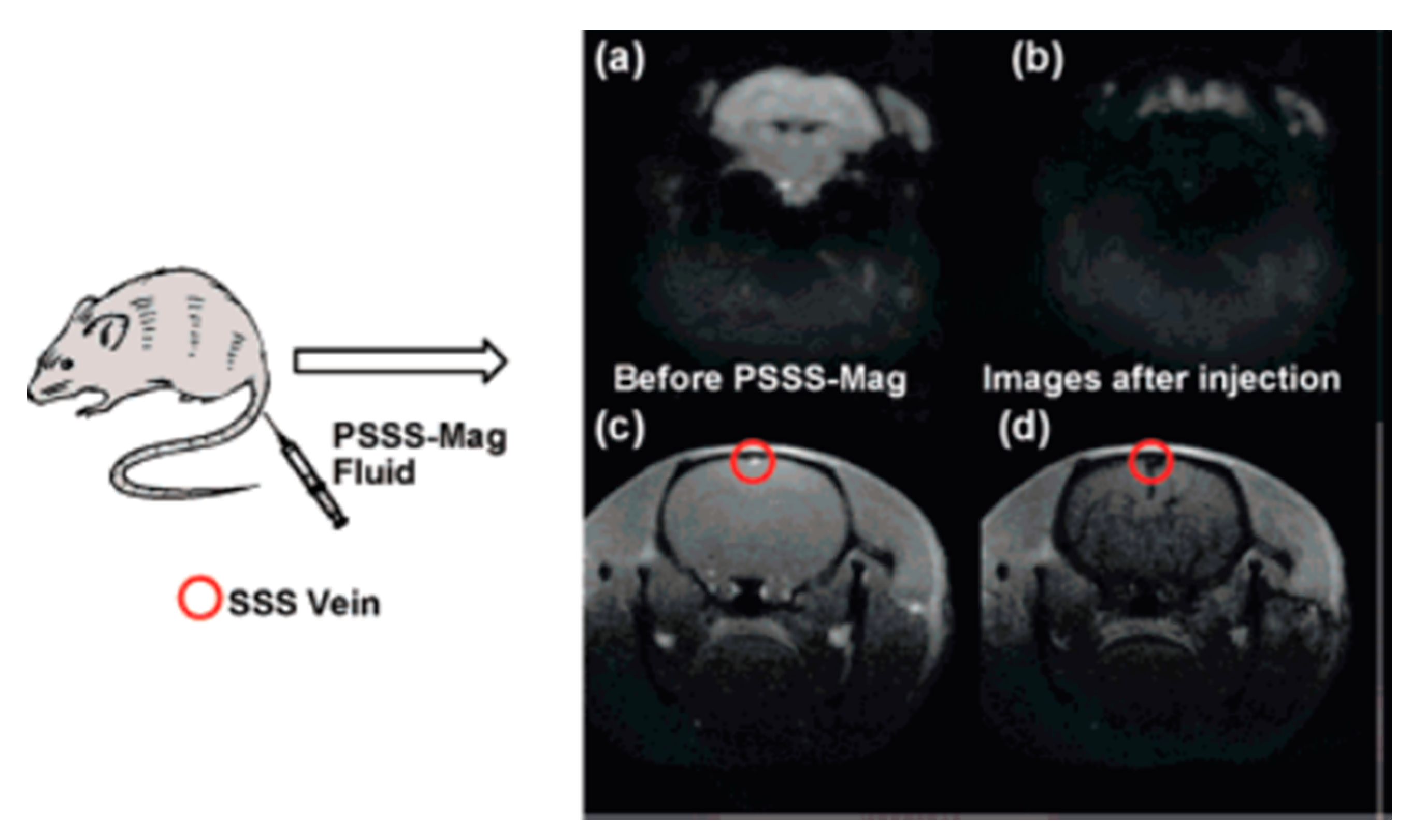

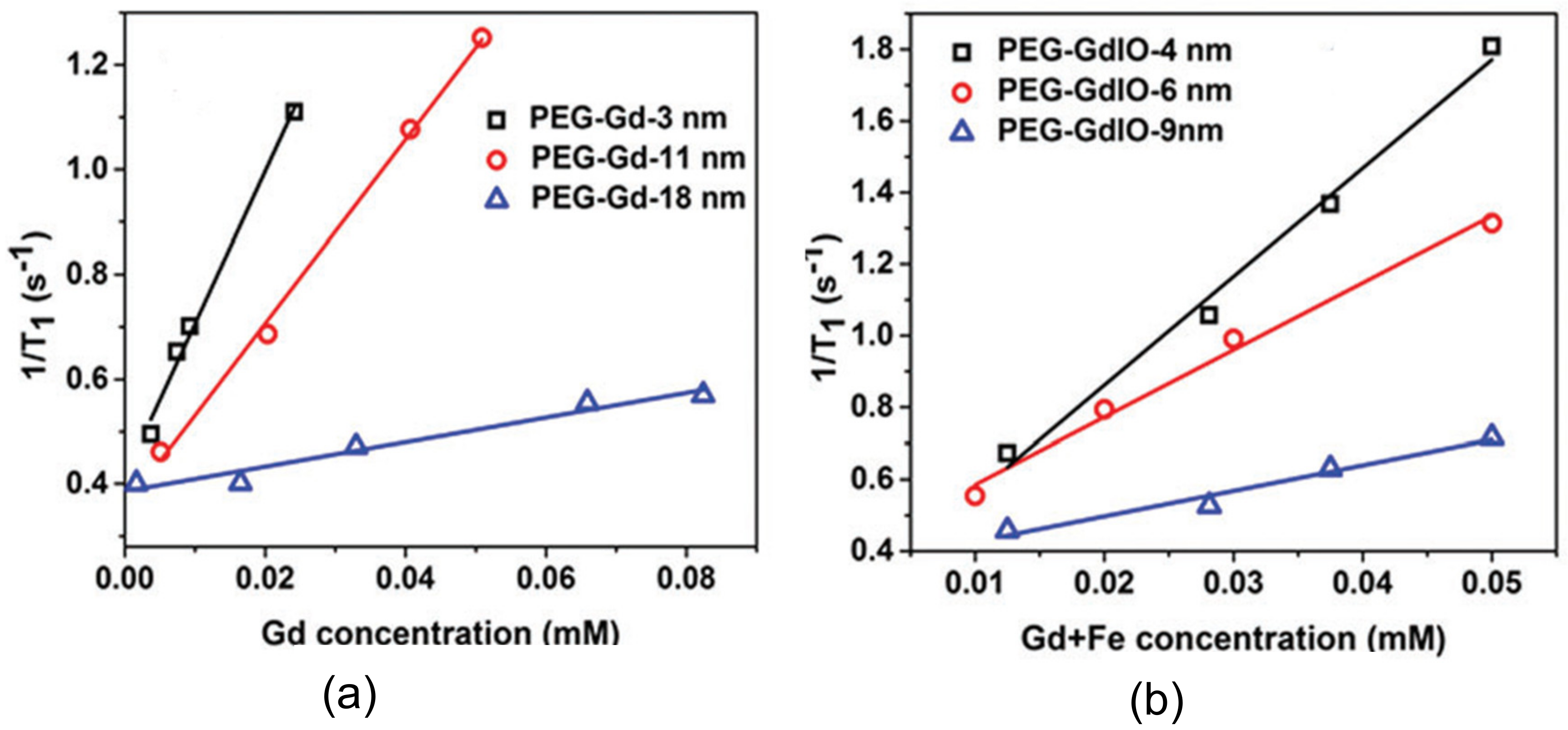
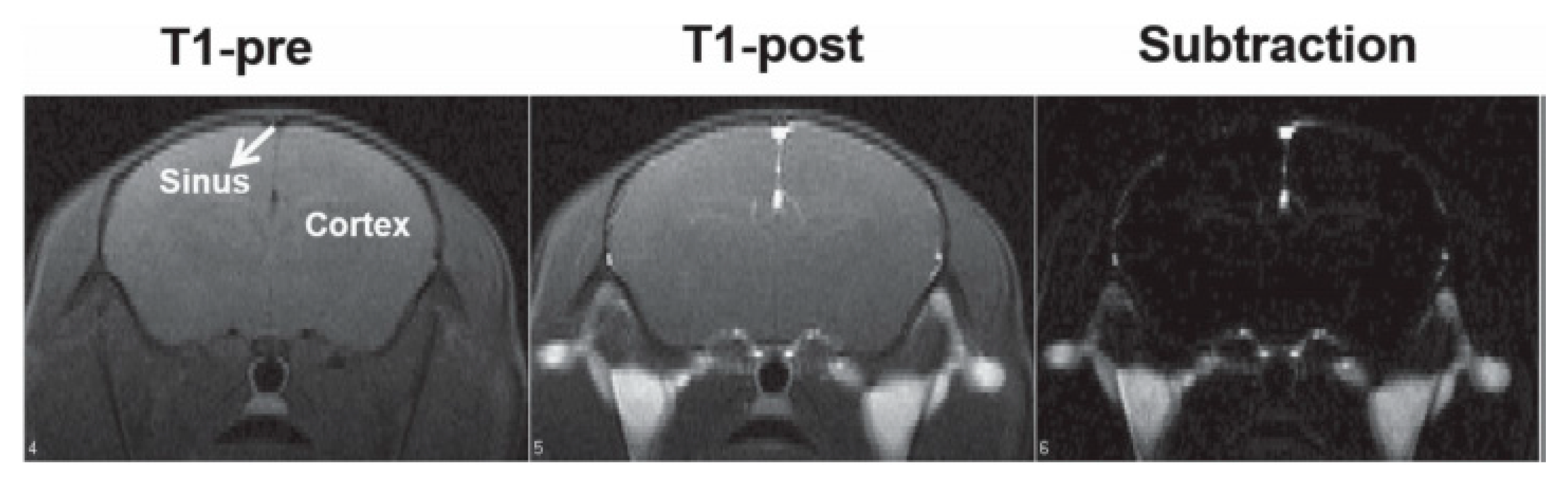
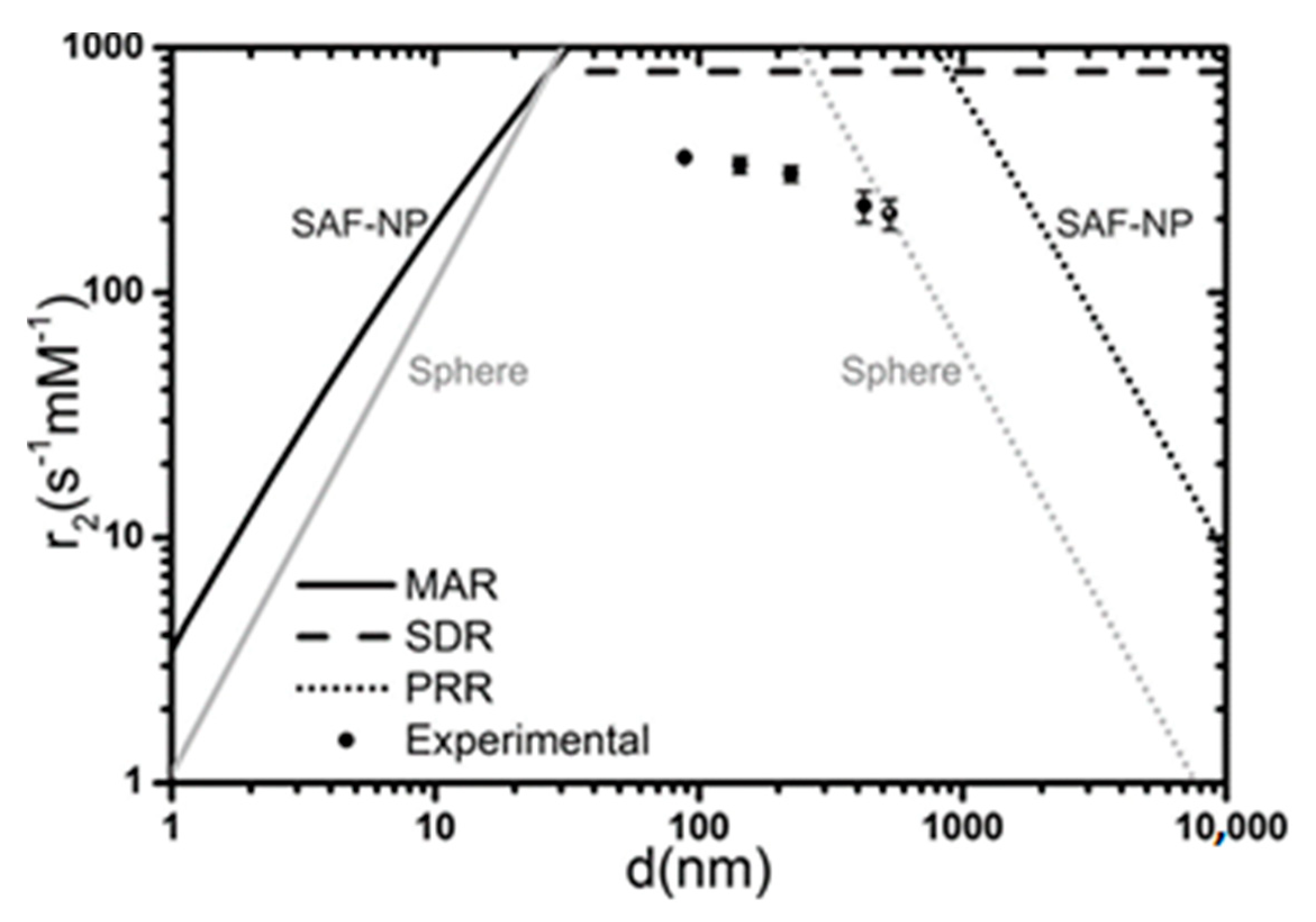

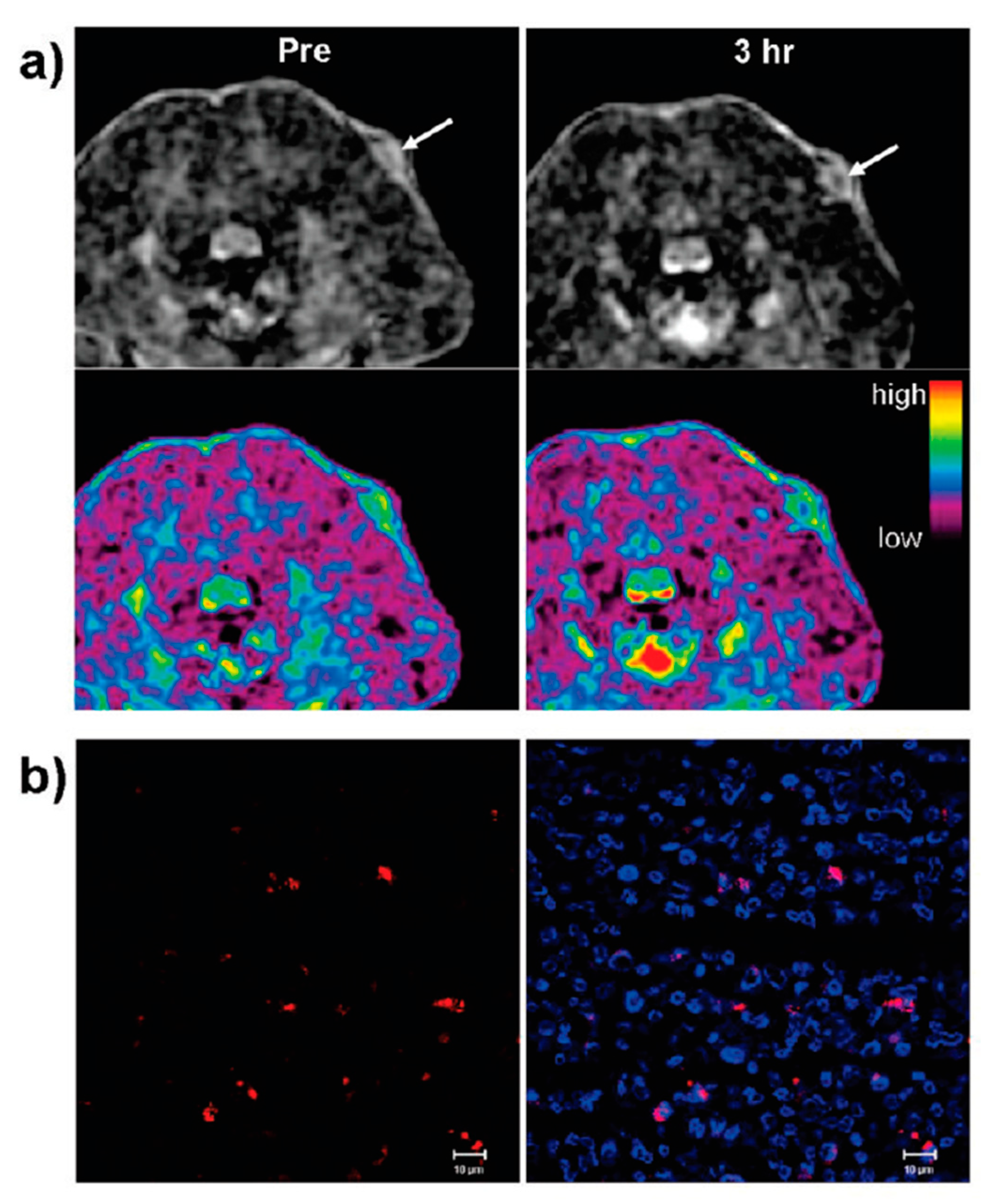
| Trade Name | Generic Name | Administration Route (Category) | Applications | Approval |
|---|---|---|---|---|
| Dotarem/ Clariscan | Gadoterate meglumine | Intravenous (ECF) | Multipurpose | 1989 EU 2013 US |
| Prohance | Gadoteridol | Intravenous (ECF) | Multipurpose | 1992 |
| Gadovist (EU) Gadavist (US) | Gadobutrol | Intravenous (ECF) | Multipurpose | 1998 EU 2011 US |
| Magnevist a | Gadopentetate dimeglumine | Intravenous (ECF) | Multipurpose | 1988 |
| Omniscan a | Gadodiamide | Intravenous (ECF) | Multipurpose | 1993 |
| Optimark a | Gadoversetamide | Intravenous (ECF) | Multipurpose | 1999 |
| Multihance | Gadobenate dimeglumine | Intravenous (ECF) | Liver, Multipurpose | 2004 |
| Gadomer | Gadomer-17 | Intravenous (ECF) | Multipurpose | - |
| Vistarem | Gadomelitol | Intravenous (Blood Pool) | Multipurpose | - |
| Clariscan | PEG-feron (USPIO) | Intravenous (Blood Pool) | Multipurpose | - |
| Sinerem/ Combidex | Ferumoxtran-10 (USPIO) | Intravenous (Blood Pool) | Multipurpose | - |
| Ablavar (Vasovist, Angiomark) b | Gadofosveset trisodium | Intravenous (Blood Pool) | Angiography | 2005 EU 2008 US |
| Primovist (EU) Eovist (US) | Disodium gadoxetic acid | Intravenous (organ-specific) | Liver | 2005 EU 2008 US |
| Teslascan | Mangafodipir trisodium | Intravenous (organ-specific) | Liver, myocardium | 1997 |
| Endorem (EU) Feridex (US) | Ferumoxides (SPIO) | Intravenous (organ-specific) | Liver | 1996 US |
| - | Sprodiamde | Intravenous (organ-specific) | myocardium, brain perfusions | - |
| Sinerem/ Combidex | Ferumoxtran-10 (USPIO) | Intravenous (organ-specific) | metastatic lymph nodes, macrophage imaging | - |
| Feraheme c | Ferumoxytol (USPIO) | Intravenous (organ-specific) | brain lesions, liver, lymph nodes, blood vessels | 2009 US 2013 UE |
| Lumirem/ GastroMARK | ferumoxsil (MPIO) | Oral | Gastrointestinal tract | 1996 |
| Ferriseltz | ferric ammonium citrate | Oral | Gastrointestinal tract | 1992 |
| Lumenhance | manganese chloride | Oral | Gastrointestinal tract | 1997 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. https://doi.org/10.3390/ma13112586
Caspani S, Magalhães R, Araújo JP, Sousa CT. Magnetic Nanomaterials as Contrast Agents for MRI. Materials. 2020; 13(11):2586. https://doi.org/10.3390/ma13112586
Chicago/Turabian StyleCaspani, Sofia, Ricardo Magalhães, João Pedro Araújo, and Célia Tavares Sousa. 2020. "Magnetic Nanomaterials as Contrast Agents for MRI" Materials 13, no. 11: 2586. https://doi.org/10.3390/ma13112586
APA StyleCaspani, S., Magalhães, R., Araújo, J. P., & Sousa, C. T. (2020). Magnetic Nanomaterials as Contrast Agents for MRI. Materials, 13(11), 2586. https://doi.org/10.3390/ma13112586






