Investigations on the Processing of Ceramic Filled Inks for 3D InkJet Printing
Abstract
1. Introduction
2. Materials and Methods
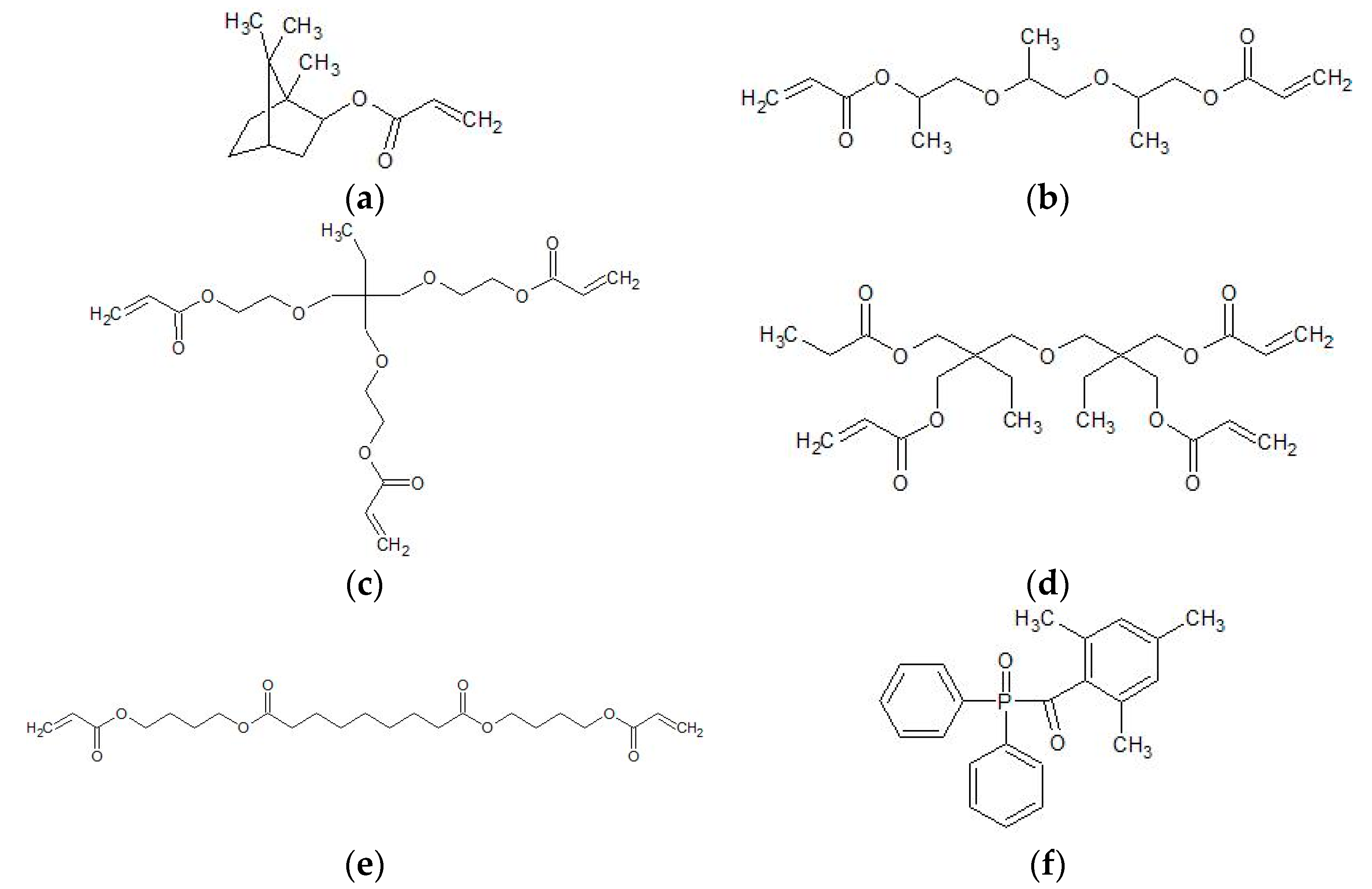
2.1. Materials
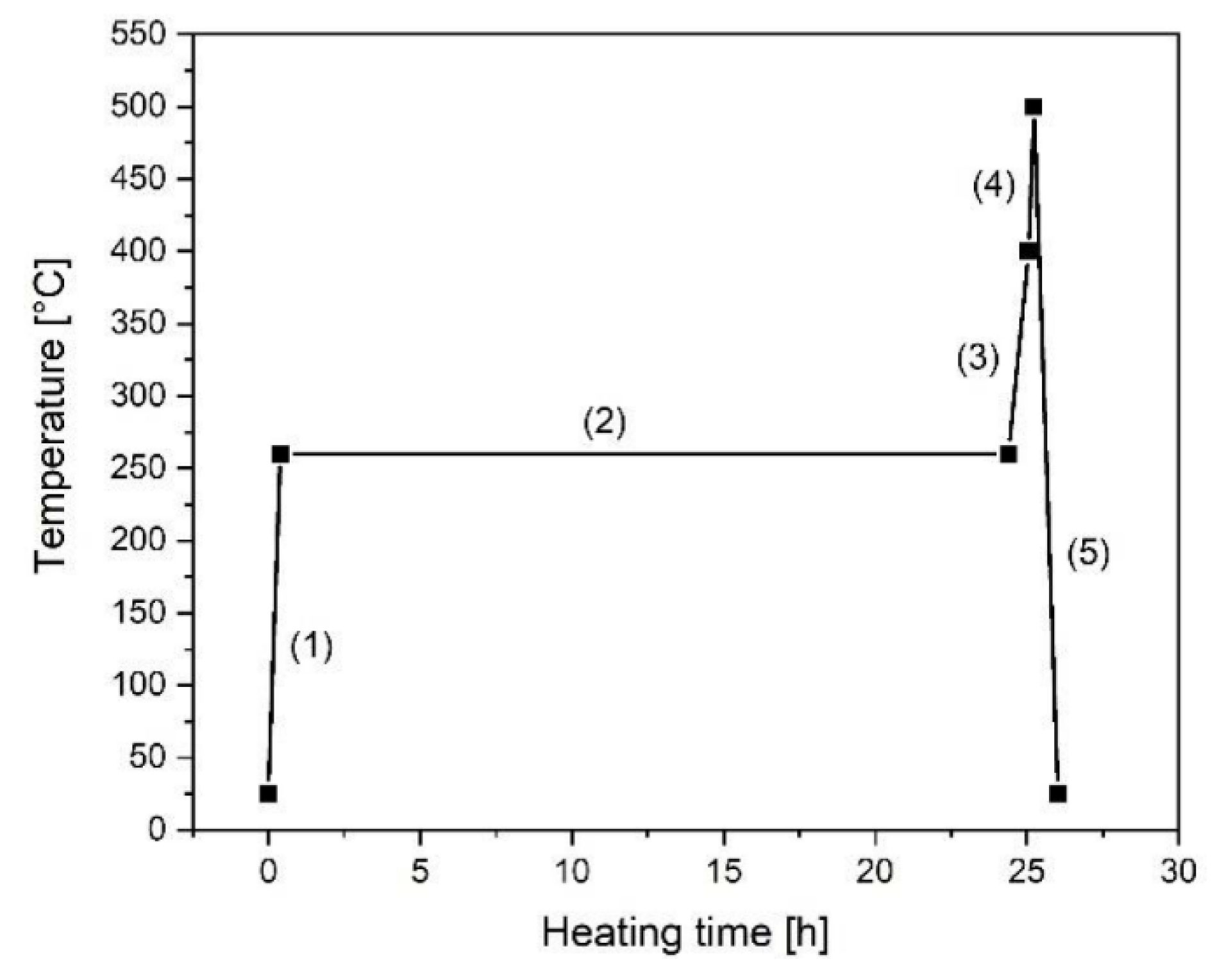
2.2. Filler Preparation and Charakterization
2.3. Ink Preparation and Characterization
2.4. Composite Preparation and Characterization
3. Results and Discussion
3.1. Filler Characterization
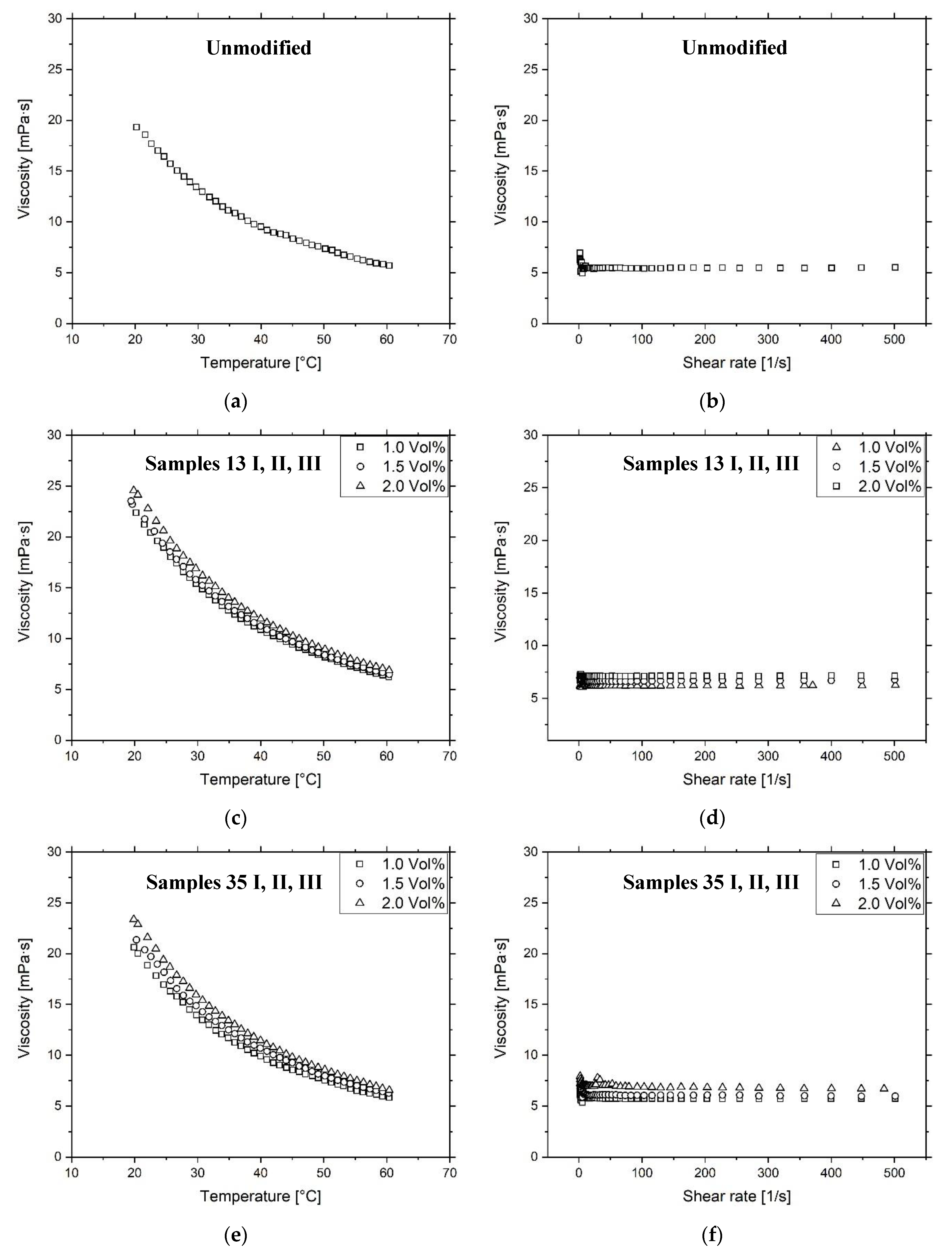
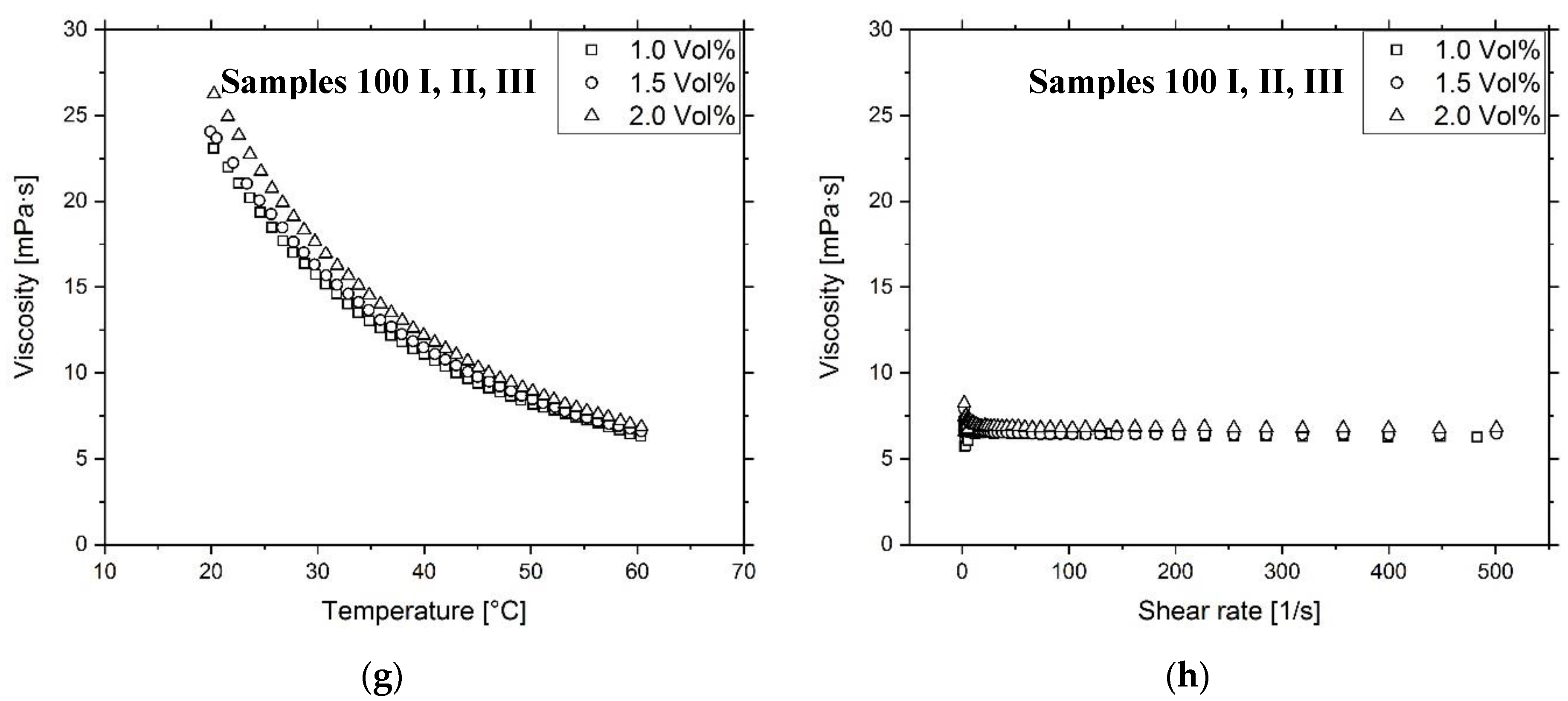
3.2. Ink Characterization
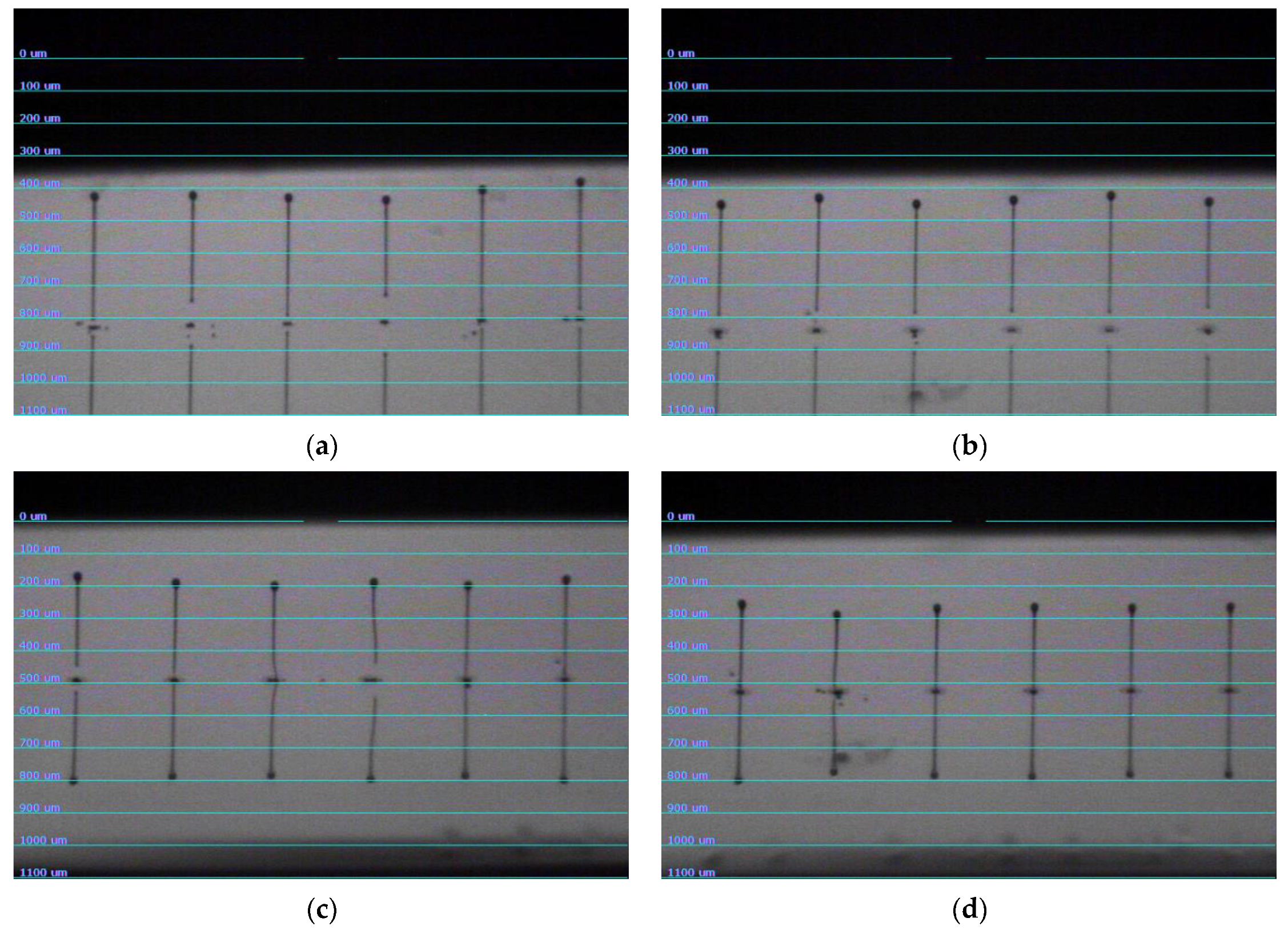
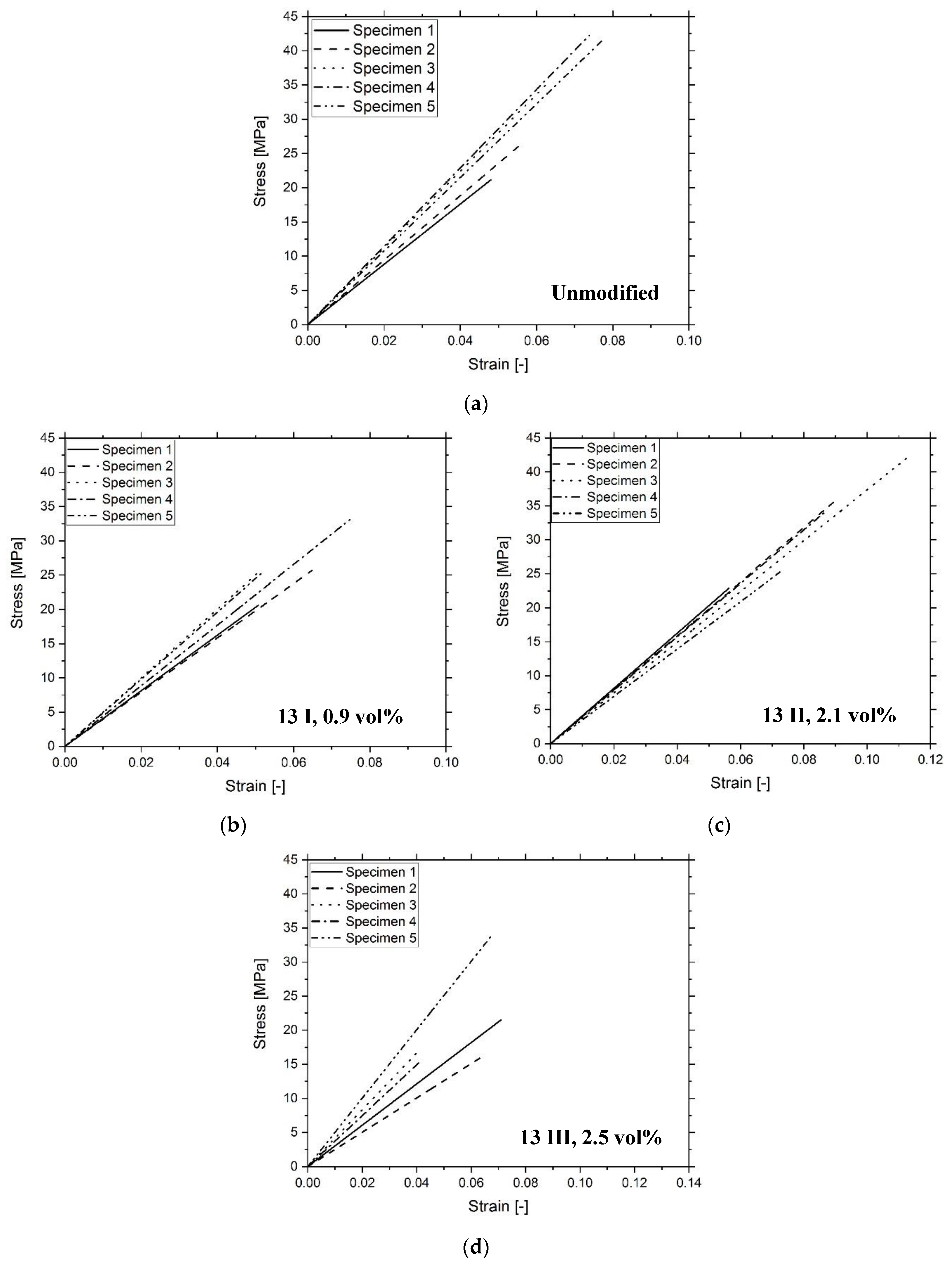
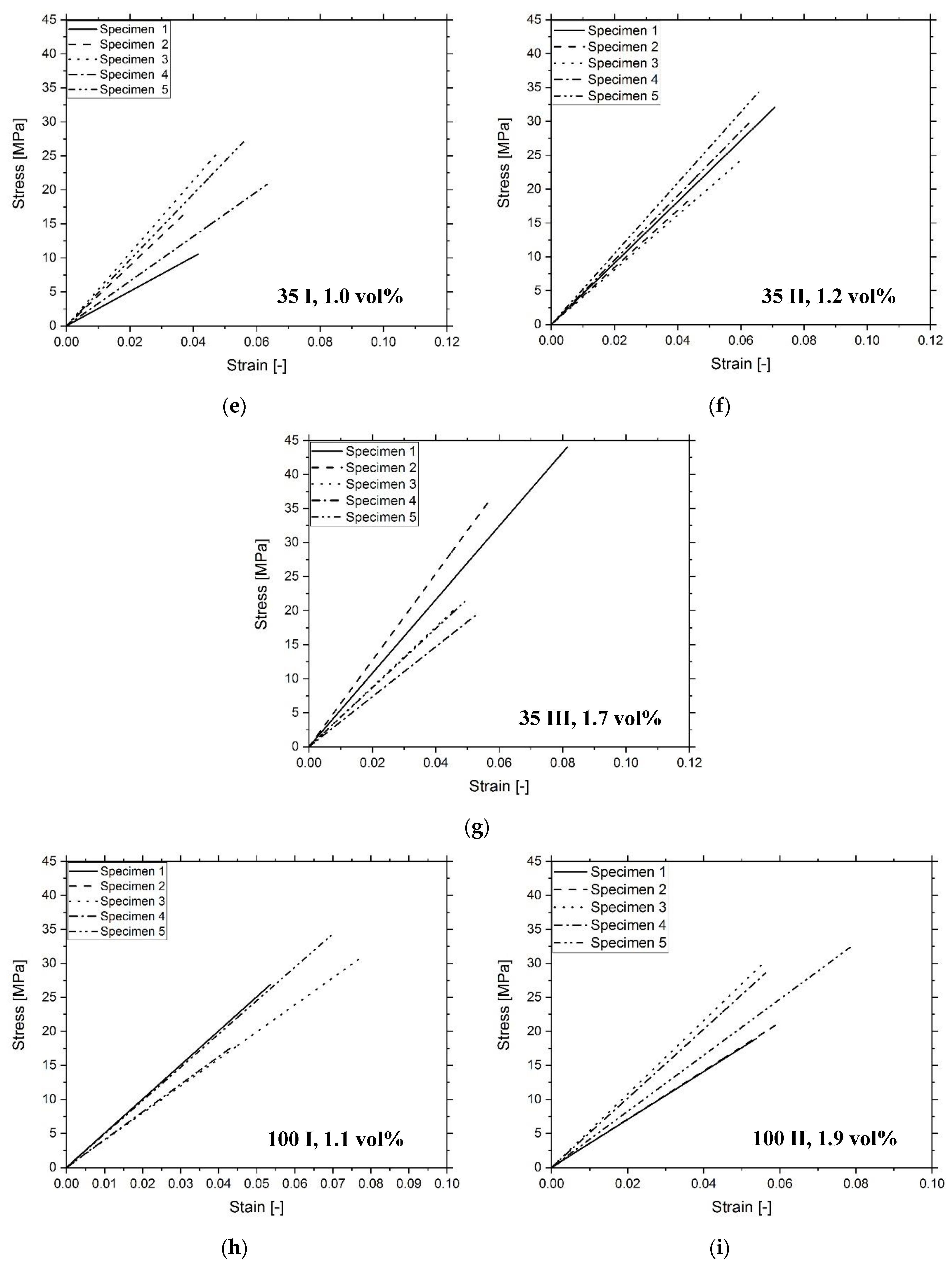
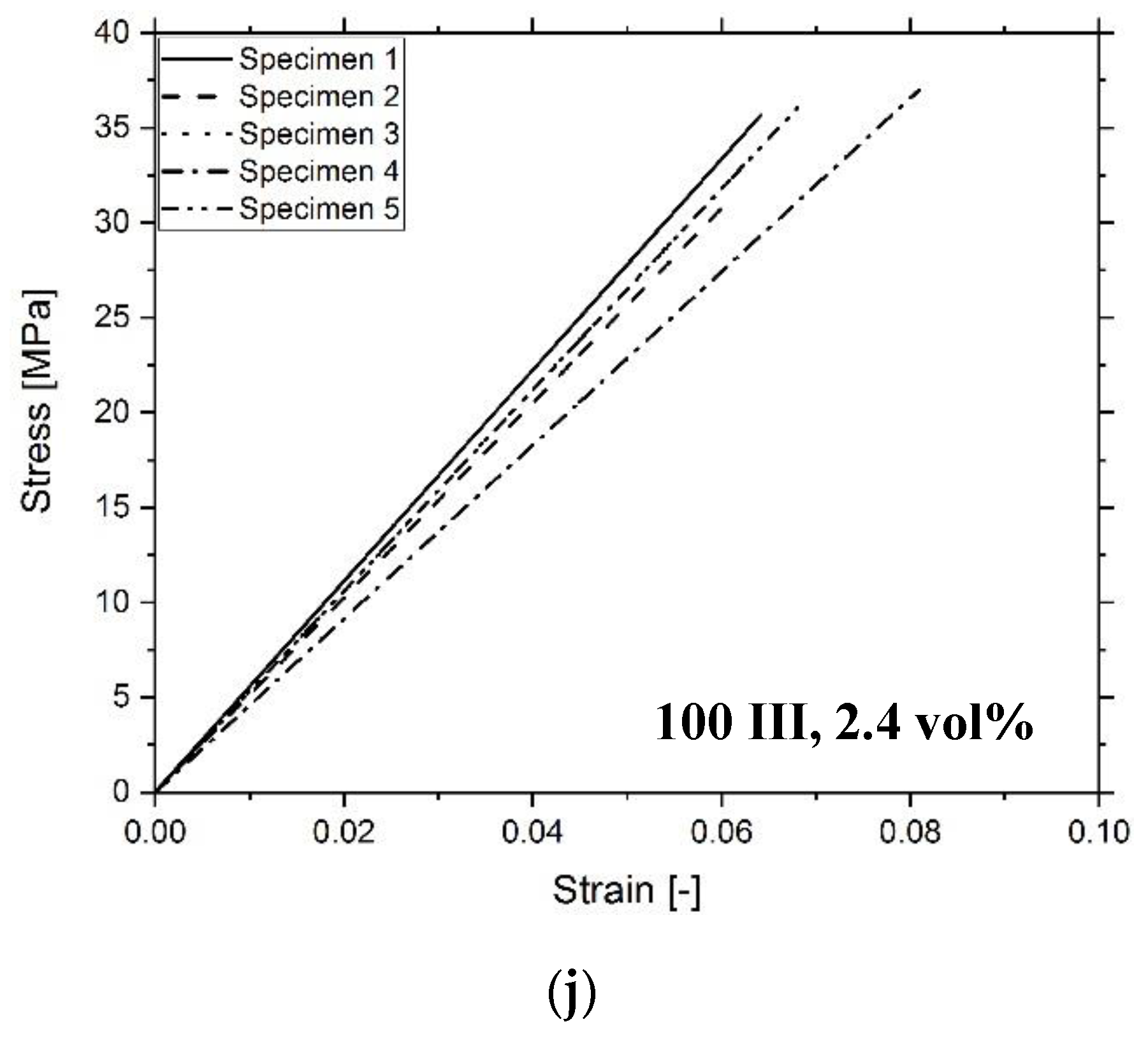
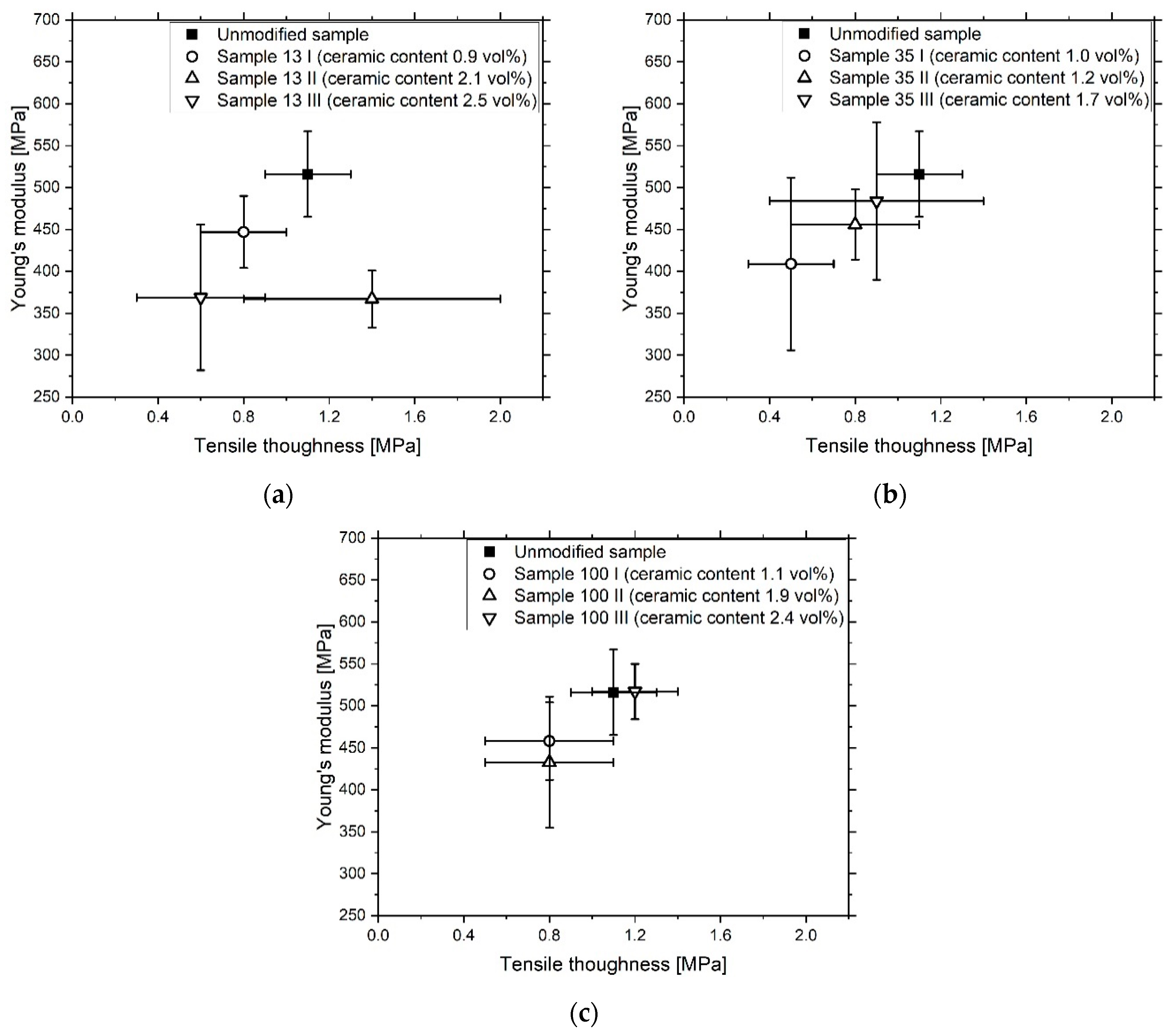
3.3. Composite Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | Viscosity (60 °C) (mPa∙s) | Surface Tension (mN/m) |
|---|---|---|
| Unmodified | 5.5 ± 0.2 | 27.3 ± 1.5 |
| 13 I | 6.2 ± 0.02 | 30.0 ± 2.4 |
| 13 II | 6.7 ± 0.08 | 32.5 ± 2.1 |
| 13 III | 7.1 ± 0.11 | 30.3 ± 2.4 |
| 35 I | 5.7 ± 0.19 | 27.2 ± 1.3 |
| 35 II | 5.9 ± 0.04 | 32.0 ± 2.4 |
| 35 III | 6.7 ± 0.13 | 29.7 ± 2.3 |
| 100 I | 6.3 ± 0.12 | 30.0 ± 2.9 |
| 100 II | 6.4 ± 0.04 | 30.4 ± 1.9 |
| 100 III | 6.8 ± 0.06 | 31.9 ± 1.2 |
| Sample | Tg (°C) | E (MPa) | σmax (MPa) | UT (MPa) |
|---|---|---|---|---|
| Unmodified | 66 ± 7 | 516 ± 51 | 33 ± 6 | 1.1 ± 0.2 |
| 13 I | 59 ± 4 | 447 ± 43 | 26 ± 4 | 0.8 ± 0.2 |
| 13 II | 58 ± 4 | 367 ± 34 | 32 ± 7 | 1.4 ± 0.6 |
| 13 III | 55 ± 5 | 369 ± 87 | 21 ± 7 | 0.6 ± 0.3 |
| 35 I | 64 ± 8 | 409 ± 103 | 20 ± 6 | 0.5 ± 0.2 |
| 35 II | 62 ± 1 | 456 ± 42 | 28 ± 6 | 0.8 ± 0.3 |
| 35 III | 63 ± 1 | 484 ± 94 | 28 ± 10 | 0.9 ± 0.5 |
| 100 I | 66 ± 5 | 458 ± 46 | 27 ± 6 | 0.8 ± 0.3 |
| 100 II | 64 ± 5 | 433 ± 78 | 29 ± 6 | 0.8 ± 0.3 |
| 100 III | 66 ± 3 | 517 ± 33 | 35 ± 2 | 1.2 ± 0.2 |
References
- Hutmacher, D.W.; Sittinger, M.; Risbud, M.V. Scaffold-based tissue engineering: Rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004, 22, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Beaman, J.J.; Barlow, J.W.; Bourell, D.L.; Crawford, R.H.; Marcus, H.L.; McAlea, K.P. Solid Freeform Fabrication: A New Direction in Manufacturing; Springer US: New York, NY, USA, 1997. [Google Scholar]
- Melchels, F.P.; Domingos, M.A.; Klein, T.; Malda, J.; Bartolo, P.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Kruth, J.-P.; Leu, M.; Nakagawa, T. Progress in Additive Manufacturing and Rapid Prototyping. CIRP Ann. 1998, 47, 525–540. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies; Springer US: New York, NY, USA, 2015; Available online: https://link.springer.com/content/pdf/10.1007/978-1-4939-2113-3.pdf (accessed on 2 March 2020).
- Huang, Y.; Leu, M.; Mazumder, J.; Donmez, A. Additive Manufacturing: Current State, Future Potential, Gaps and Needs, and Recommendations. J. Manuf. Sci. Eng. 2015, 137, 014001. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.L.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The status, challenges, and future of additive manufacturing in engineering. Comput. Des. 2015, 69, 65–89. [Google Scholar] [CrossRef]
- Thompson, M.K.; Moroni, G.; Vaneker, T.; Fadel, G.; Campbell, R.I.; Gibson, I.; Bernard, A.; Schulz, J.; Graf, P.; Ahuja, B.; et al. Design for Additive Manufacturing: Trends, opportunities, considerations, and constraints. CIRP Ann. 2016, 65, 737–760. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Galliker, P.; Schneider, J.; Eghlidi, H.; Kress, S.; Sandoghdar, V.; Poulikakos, D. Direct printing of nanostructures by electrostatic autofocussing of ink nanodroplets. Nat. Commun 2012, 3, 890. [Google Scholar] [CrossRef]
- Han, Y.; Wei, C.; Dong, J. Super-resolution electrohydrodynamic (EHD) 3D printing of micro-structures using phase-change inks. Manuf. Lett. 2014, 2, 96–99. [Google Scholar] [CrossRef]
- Rega, R.; Gennari, O.; Mecozzi, L.; Pagliarulo, V.; Bramanti, A.; Ferraro, P.; Grilli, S. Maskless Arrayed Nanofiber Mats by Bipolar Pyroelectrospinning. ACS Appl. Mater. Interfaces 2019, 11, 3382–3387. [Google Scholar] [CrossRef]
- Mecozzi, L.; Gennari, O.; Rega, R.; Grilli, S.; Bhowmick, S.; Gioffrè, M.A.; Coppola, G.; Ferraro, P. Spiral formation at the microscale by μ-pyro-electrospinning. Soft Matter 2016, 12, 5542–5550. [Google Scholar] [CrossRef] [PubMed]
- Dämmer, G.; Gablenz, S.; Hildebrandt, A.; Major, Z. Design of an Additively Manufacturable Multi-Material Light-Weight Gripper with integrated Bellows Actuators. Adv. Sci. Technol. Eng. Syst. J. 2019, 4, 23–33. [Google Scholar] [CrossRef]
- Dämmer, G.; Gablenz, S.; Hildebrandt, A.; Major, Z. PolyJet-Printed Bellows Actuators: Design, Structural Optimization, and Experimental Investigation. Front. Robot. AI 2019, 6. [Google Scholar] [CrossRef]
- Sochol, R.D.; Sweet, E.; Glick, C.C.; Venkatesh, S.; Avetisyan, A.; Ekman, K.F.; Raulinaitis, A.; Tsai, A.; Wienkers, A.; Korner, K.; et al. 3D printed microfluidic circuitry via multijet-based additive manufacturing. Lab Chip 2016, 16, 668–678. [Google Scholar] [CrossRef]
- Vaezi, M.; Seitz, H.; Yang, S. A review on 3D micro-additive manufacturing technologies. Int. J. Adv. Manuf. Technol. 2013, 67, 1721–1754. [Google Scholar] [CrossRef]
- Magdassi, S. The Chemistry of Inkjet Inks; World Scientific Publishing: Singapore, 2010. [Google Scholar]
- Tibbits, S. 4D Printing: Multi-Material Shape Change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Nalbant, B.; Egeli, T.; Ünek, T.; Altay, C.; Kavur, E.; Selver, A.; Obuz, F.; Ağalar, C.; Özbilgin, M.; Astarcıoğlu, I. Utilizing 3 Dimensional Print of the Liver in Living Donor Liver Transplantation for Preoperative Evaluation. J. Basic Clin. Health Sci. 2020. [Google Scholar] [CrossRef]
- Lee, H.-J.; Fehmer, V.; Kwon, K.-R.; Burkhardt, F.; Pae, A.; Sailer, I. Virtual diagnostics and guided tooth preparation for the minimally invasive rehabilitation of a patient with extensive tooth wear: A validation of a digital workflow. J. Prosthet. Dent. 2019, 123, 20–26. [Google Scholar] [CrossRef]
- Wei, X.; Thakare, K.; Zeng, L.; Pei, Z. Experimental Investigation of Stratasys J750 PolyJet Printer: Effects of Finish Type and Shore Hardness on Dimensional Accuracy; AMER SOC MECHANICAL ENGINEERS: Erie, PA, USA, 2019. [Google Scholar]
- Kamyshny, A.; Magdassi, S. Conductive Nanomaterials for Printed Electronics. Small 2014, 10, 3515–3535. [Google Scholar] [CrossRef]
- Ligon-Auer, S.C.; Gorsche, C.; Stampfl, J.; Schwentenwein, M.; Liska, R. Toughening of photo-curable polymer networks: A review. Polym. Chem. 2016, 7, 257–286. [Google Scholar] [CrossRef]
- Dorfinger, P.; Stampfl, J.; Liska, R. Toughening of Photopolymers for Stereolithography (SL). Mater. Sci. Forum 2015, 825, 53–59. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Wu, L.; Baghdachi, J. Functional Polymer Coatings—Principles, Methods, and Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Landry, V.; Riedl, B.; Blanchet, P. Alumina and zirconia acrylate nanocomposites coatings for wood flooring: Photocalorimetric characterization. Prog. Org. Coat. 2008, 61, 76–82. [Google Scholar] [CrossRef]
- Preinfalk, J.B.; Eiselt, T.; Wehlus, T.; Rohnacher, V.; Hanemann, T.; Gomard, G.; Lemmer, U. Large-Area Screen-Printed Internal Extraction Layers for Organic Light-Emitting Diodes. ACS Photonics 2017, 4, 928–933. [Google Scholar] [CrossRef]
- Sangermano, M.; Priola, A.; Kortaberria, G.; Jimeno, A.; Garcia, I.; Mondragon, I.; Rizza, G. Photopolymerization of Epoxy Coatings Containing Iron-Oxide Nanoparticles. Macromol. Mater. Eng. 2007, 292, 956–961. [Google Scholar] [CrossRef]
- Landry, V.; Riedl, B.; Blanchet, P. Nanoclay dispersion effects on UV coatings curing. Prog. Org. Coat. 2008, 62, 400–408. [Google Scholar] [CrossRef]
- Kang, D.J.; Han, D.-H.; Kang, D.P. Fabrication and characterization of photocurable inorganic–organic hybrid materials using organically modified colloidal-silica nanoparticles and acryl resin. J. Non. Cryst. Solids 2009, 355, 397–402. [Google Scholar] [CrossRef]
- Gläsel, H.-J.; Bauer, F.; Ernst, H.; Findeisen, M.; Hartmann, E.; Langguth, H.; Mehnert, R.; Schubert, R. Preparation of scratch and abrasion resistant polymeric nanocomposites by monomer grafting onto nanoparticles, 2 Characterization of radiation-cured polymeric nanocomposites. Macromol. Chem. Phys. 2000, 201, 2765–2770. [Google Scholar] [CrossRef]
- Soloukhin, V.A.; Posthumus, W.; Brokken-Zijp, J.; Loos, J.; De With, G. Mechanical properties of silica–(meth) acrylate hybrid coatings on polycarbonate substrate. Polymer 2002, 43, 6169–6181. [Google Scholar] [CrossRef]
- Bautista, Y.; González, J.; Gilabert, J.; Ibáñez, M.; Sanz, V. Correlation between the wear resistance, and the scratch resistance, for nanocomposite coatings. Prog. Org. Coat. 2011, 70, 178–185. [Google Scholar] [CrossRef]
- Goodrich, J.E. New raw materials for high-temperature UV ink jet. Pigm. Resin Technol. 2005, 11, 40–44. [Google Scholar]
- Di Gianni, A.; Amerio, E.; Monticelli, O.; Bongiovanni, R. Preparation of polymer/clay mineral nanocomposites via dispersion of silylated montmorillonite in a UV curable epoxy matrix. Appl. Clay Sci. 2008, 42, 116–124. [Google Scholar] [CrossRef]
- Mohamadpour, S.; Pourabbas, B.; Fabbri, P. Anti-scratch and adhesion properties of photo-curable polymer/clay nanocomposite coatings based on methacrylate monomers. Sci. Iran. 2011, 18, 765–771. [Google Scholar] [CrossRef]
- Corcione, C.E.; Frigione, M. UV-cured polymer-boehmite nanocomposite as protective coating for wood elements. Prog. Org. Coat. 2012, 74, 781–787. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Zeng, Z.; Huang, L.; Chen, Y.; Wang, H. Stabilized dispersions of titania nanoparticles via a sol–gel process and applications in UV-curable hybrid systems. Polym. Int. 2006, 55, 466–472. [Google Scholar] [CrossRef]
- Bauer, F.; Sauerland, V.; Glasel, H.-J.; Ernst, H.; Findeisen, M.; Hartmann, E.; Langguth, H.; Marquardt, B.; Mehnert, R. Preparation of Scratch and Abrasion Resistant Polymeric Nanocomposites by Monomer Grafting onto Nanoparticles, 3 Effect of Filler Particles and Grafting Agents. Macromol. Mater. Eng. 2002, 287, 546–552. [Google Scholar] [CrossRef]
- Ackler, H.D.; French, R.H.; Chiang, Y.-M. Comparisons of Hamaker Constants for Ceramic Systems with Intervening Vacuum or Water: From Force Laws and Physical Properties. J. Colloid Interface Sci. 1996, 179, 460–469. [Google Scholar] [CrossRef]
- Oberdisse, J. Aggregation of colloidal nanoparticles in polymer matrices. Soft Matter 2006, 2, 29–36. [Google Scholar] [CrossRef]
- De Nicola, A.; Avolio, R.; Della Monica, F.; Gentile, G.; Cocca, M.; Capacchione, C.; Errico, M.; Milano, G. Rational design of nanoparticle/monomer interfaces: A combined computational and experimental study of in situ polymerization of silica based nanocomposites. RSC Adv. 2015, 5, 71336–71340. [Google Scholar] [CrossRef]
- Rosen, M.J. Relationship of structure to properties in surfactants. III. Adsorption at the solid-liquid interface from aqueous solution. J. Am. Oil Chem. Soc. 1975, 52, 431–435. [Google Scholar] [CrossRef]
- Check, C.; Chartoff, R.; Chang, S. Inkjet printing of 3D nano-composites formed by photopolymerization of an acrylate monomer. React. Funct. Polym. 2015, 97, 116–122. [Google Scholar] [CrossRef]
- Seerden, K.A.M.; Reis, N.; Evans, J.R.G.; Grant, P.; Halloran, J.W.; Derby, B. Ink-Jet Printing of Wax-Based Alumina Suspensions. J. Am. Ceram. Soc. 2001, 84, 2514–2520. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet Printing for Materials and Devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Kim, C.; Ian, G.; Eric, B.; Mike, S.; Stuart, S. Direct legend printing (DLP) on printed circuit boards using piezoelectric inkjet technology. Circuit World 2002, 28, 24–31. [Google Scholar]
- Chen, P.-H.; Chen, W.-C.; Ding, P.-P.; Chang, S. Droplet formation of a thermal sideshooter inkjet printhead. Int. J. Heat Fluid Flow 1998, 19, 382–390. [Google Scholar] [CrossRef]
- Dong, H.; Carr, W.W.; Morris, J.F. An experimental study of drop-on-demand drop formation. Phys. Fluids 2006, 18, 72102. [Google Scholar] [CrossRef]
- Abbel, R.; Meinders, E.R. Printing Technologies for Nanomaterials. In Nanomaterials for 2D and 3D Printing; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–26. [Google Scholar]
- German Version EN ISO 527-2:2012. Plastics–Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics; Beuth Verlag GmbH: Berlin, Germany, 2012. [Google Scholar]
- Navrotsky, A. Energetics of nanoparticle oxides: Interplay between surface energy and polymorphism. Geochem. Trans. 2003, 4, 34. [Google Scholar] [CrossRef]
- McHale, J.M.; Auroux, A.; Perrotta, A.J.; Navrotsky, A. Surface Energies and Thermodynamic Phase Stability in Nanocrystalline Aluminas. Science 1997, 277, 788–791. [Google Scholar] [CrossRef]
- Koga, N.; Fukagawa, T.; Tanaka, H. Preparation and Thermal Decomposition of Synthetic Bayerite. J. Therm. Anal. Calorim. 2001, 64, 965–972. [Google Scholar] [CrossRef]
- Sato, T. Thermal decomposition of aluminium hydroxides to aluminas. Thermochim. Acta 1985, 88, 69–84. [Google Scholar] [CrossRef]
- Morterra, C.; Magnacca, G. A case study: Surface chemistry and surface structure of catalytic aluminas, as studied by vibrational spectroscopy of adsorbed species. Catal. Today 1996, 27, 497–532. [Google Scholar] [CrossRef]
- Lange, F.F.; Radford, K.C. Fracture energy of an epoxy composite system. J. Mater. Sci. 1971, 6, 1197–1203. [Google Scholar] [CrossRef]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.; Marchon, D.; Flatt, R.; Estrela-Lopis, I.; Llop, J.; Moya, S.E.; et al. Nanoparticle decoration with surfactants: Molecular interactions, assembly, and applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, Q. Concepts and conflicts in nanoparticles reinforcement to polymers beyond hydrodynamics. Prog. Mater. Sci. 2016, 84, 1–58. [Google Scholar] [CrossRef]
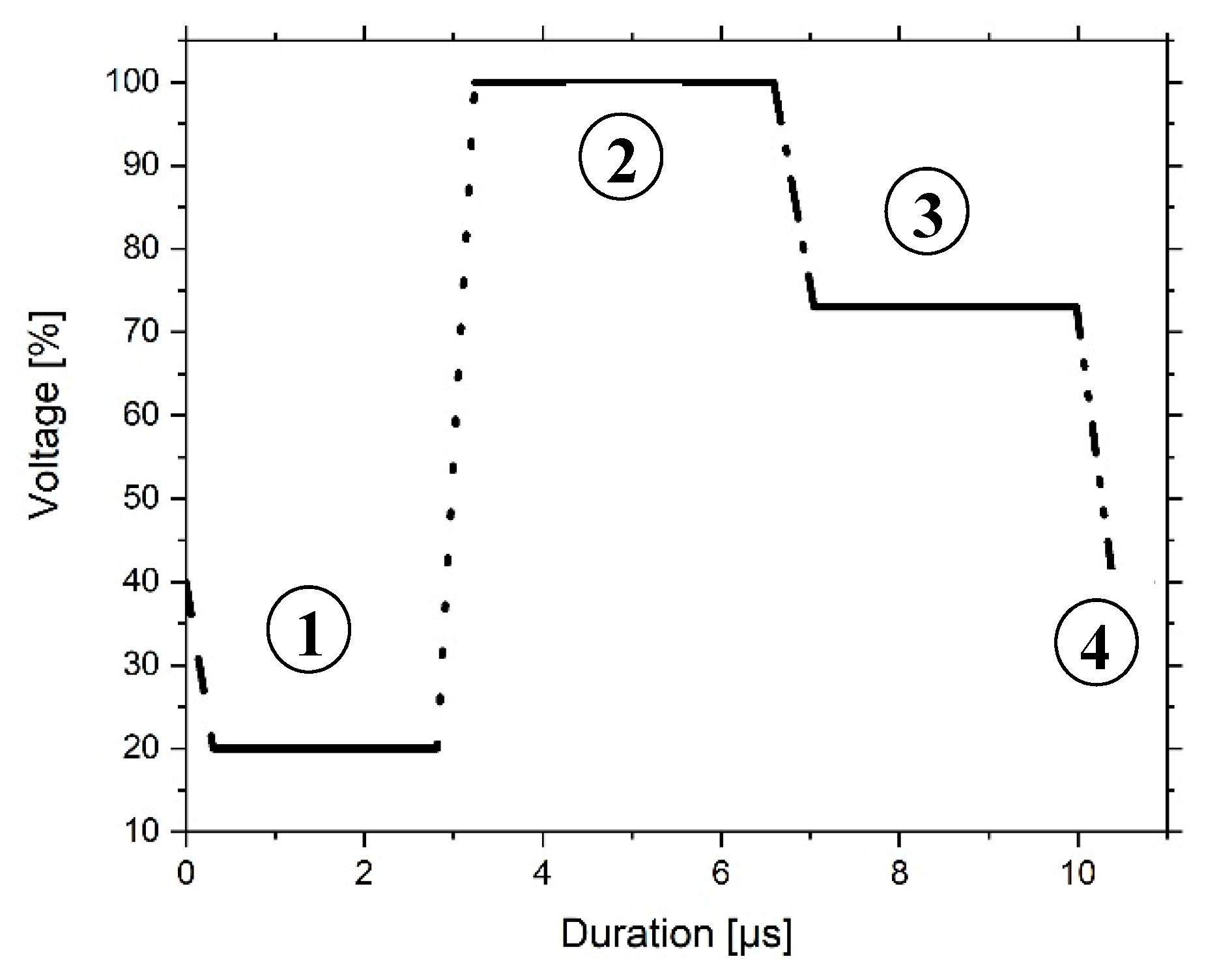

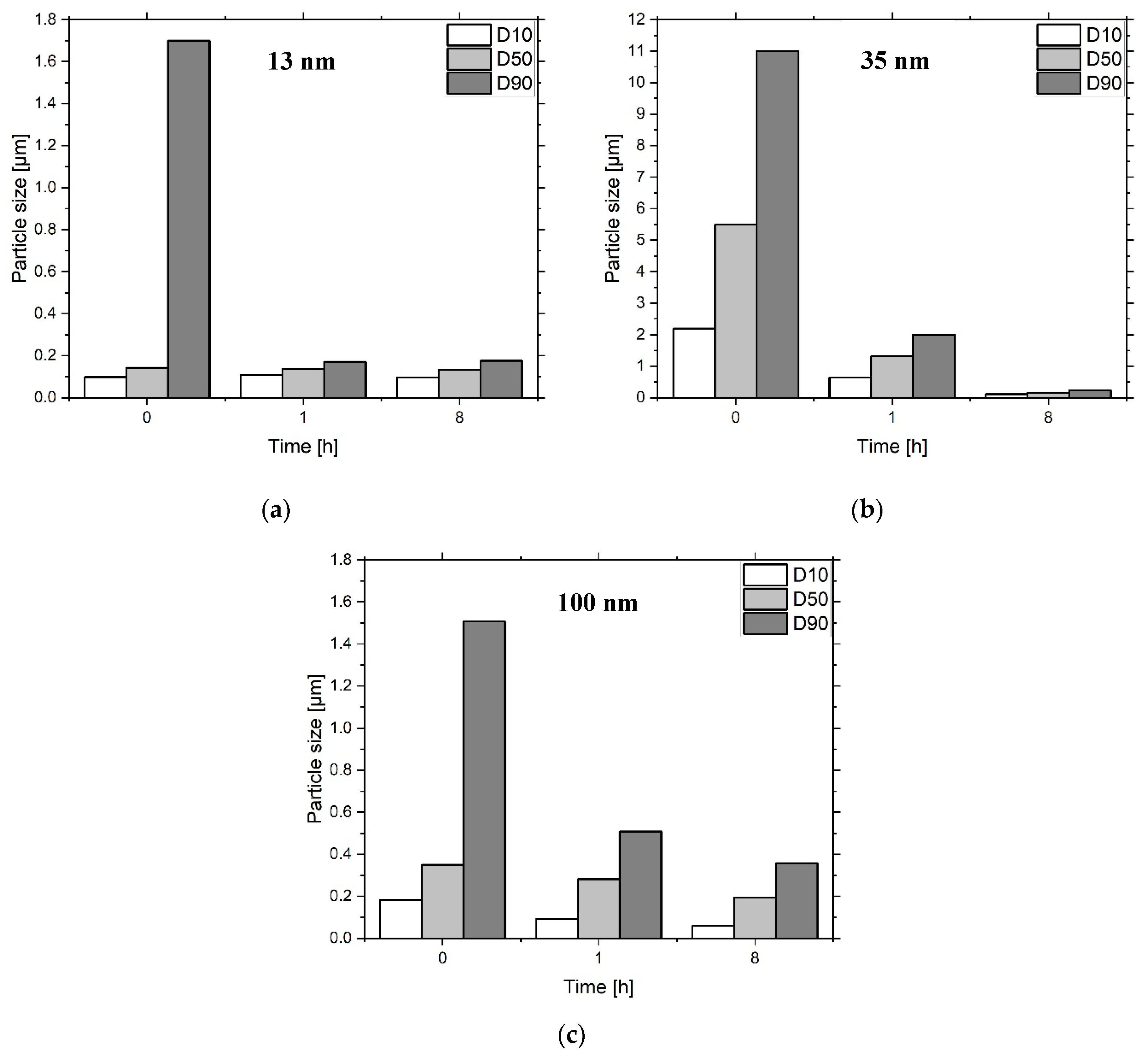
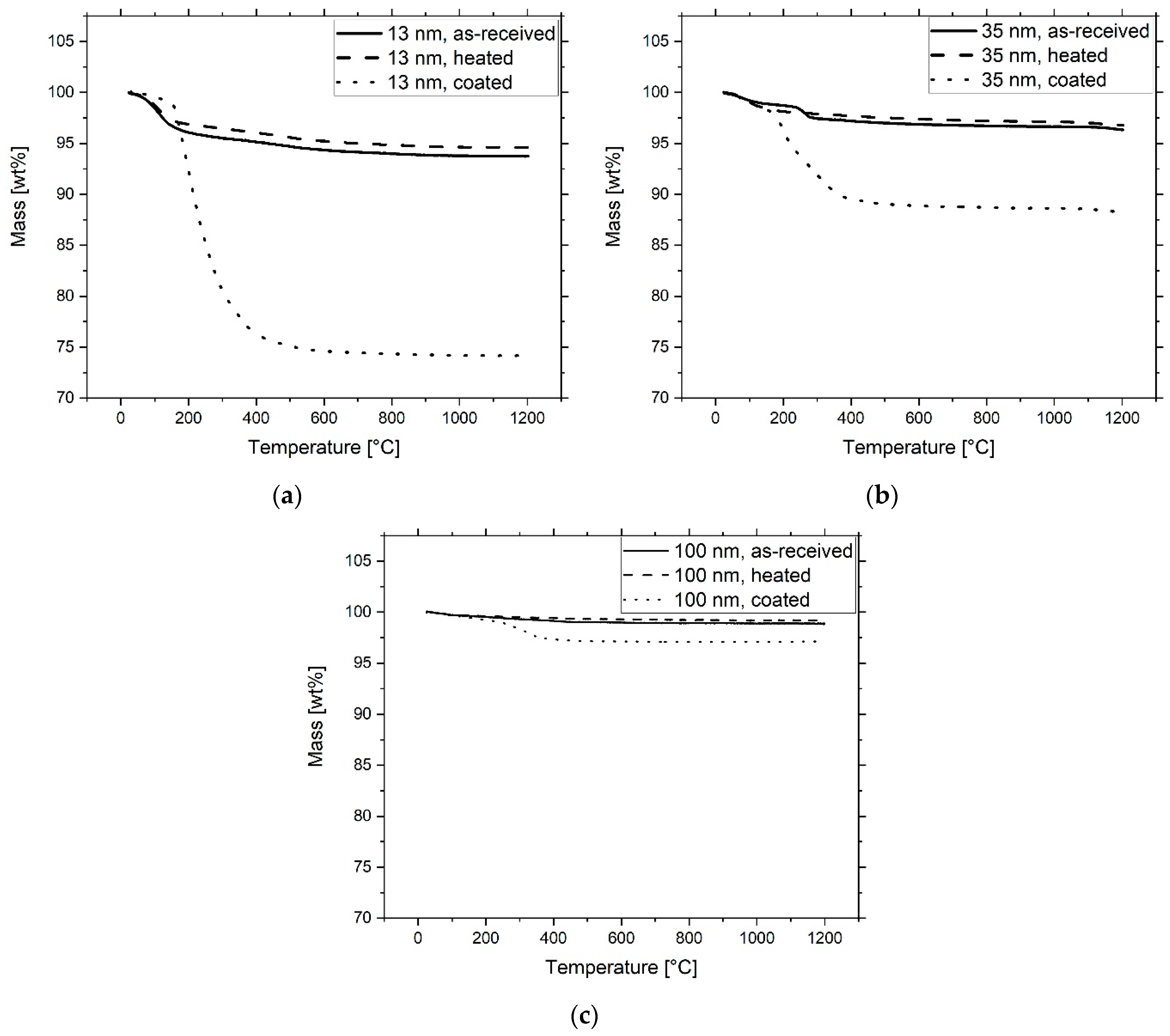

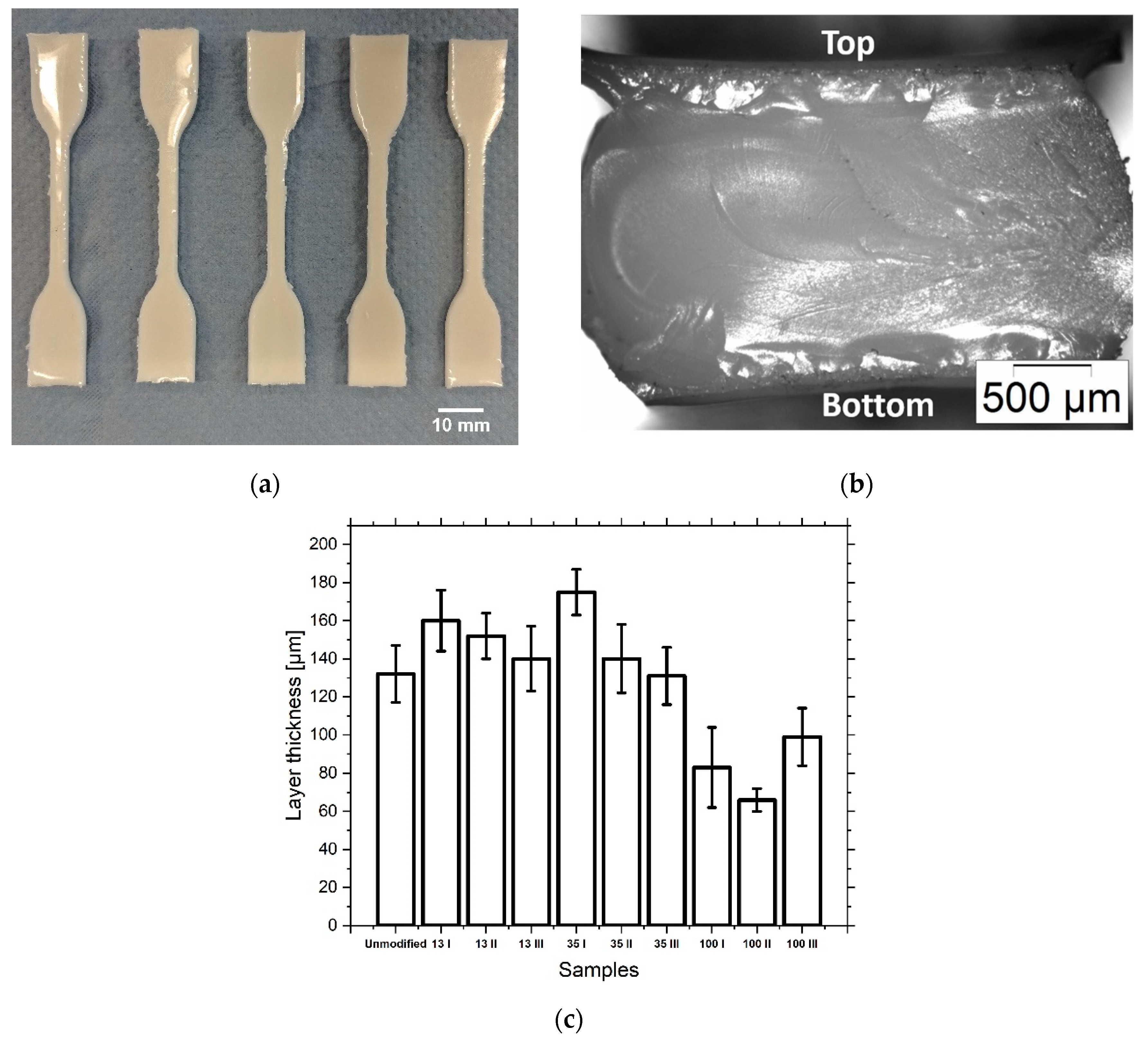
| Sample | Al2O3 Particles | Ceramic n. 1 (vol%) |
|---|---|---|
| Unmodified | - | 0.0 |
| 13 I | 13 nm coated | 1.0 |
| 13 II | 1.5 | |
| 13 III | 2.0 | |
| 35 I | 35 nm coated | 1.0 |
| 35 II | 1.5 | |
| 35 III | 2.0 | |
| 100 I | 100 nm coated | 1.0 |
| 100 II | 1.5 | |
| 100 III | 2.0 |
| Al2O3 Nanoparticles | Mass Loss (wt.%) | Weighted TODA (wt.%) | Measured TODA (wt.%) |
|---|---|---|---|
| 13 nm as received | 6.3 | - | - |
| 13 nm heated | 5.4 | - | - |
| 13 nm coated | 25.9 | 23.6 | 23.5 |
| 35 nm as received | 3.7 | - | - |
| 35 nm heated | 3.2 | - | - |
| 35 nm coated | 8.9 | 7.1 | 7.6 |
| 100 nm as received | 1.2 | - | - |
| 100 nm heated | 0.8 | - | - |
| 100 nm coated | 2.9 | 2.0 | 2.4 |
| Samples | Drop Velocity (m/s) | Drop Volume (pL) | Drop Weight (ng) | Jetting Time (s) |
|---|---|---|---|---|
| Unmodified | 11.5 | 7.8 | 8 | >600 |
| 13 III | 10 | 10 | 11 | >600 |
| 35 III | 9 | 8.2 | 9 | >600 |
| 100 III | 8 | 7.3 | 8 | >600 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graf, D.; Qazzazie, A.; Hanemann, T. Investigations on the Processing of Ceramic Filled Inks for 3D InkJet Printing. Materials 2020, 13, 2587. https://doi.org/10.3390/ma13112587
Graf D, Qazzazie A, Hanemann T. Investigations on the Processing of Ceramic Filled Inks for 3D InkJet Printing. Materials. 2020; 13(11):2587. https://doi.org/10.3390/ma13112587
Chicago/Turabian StyleGraf, Dennis, Afnan Qazzazie, and Thomas Hanemann. 2020. "Investigations on the Processing of Ceramic Filled Inks for 3D InkJet Printing" Materials 13, no. 11: 2587. https://doi.org/10.3390/ma13112587
APA StyleGraf, D., Qazzazie, A., & Hanemann, T. (2020). Investigations on the Processing of Ceramic Filled Inks for 3D InkJet Printing. Materials, 13(11), 2587. https://doi.org/10.3390/ma13112587





