Thermal and Morphological Properties of Poly(L-Lactic Acid)/Poly(D-Lactic Acid)-B-Polycaprolactone Diblock Copolymer Blends
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Blends of PLLA and PCL Oligomers
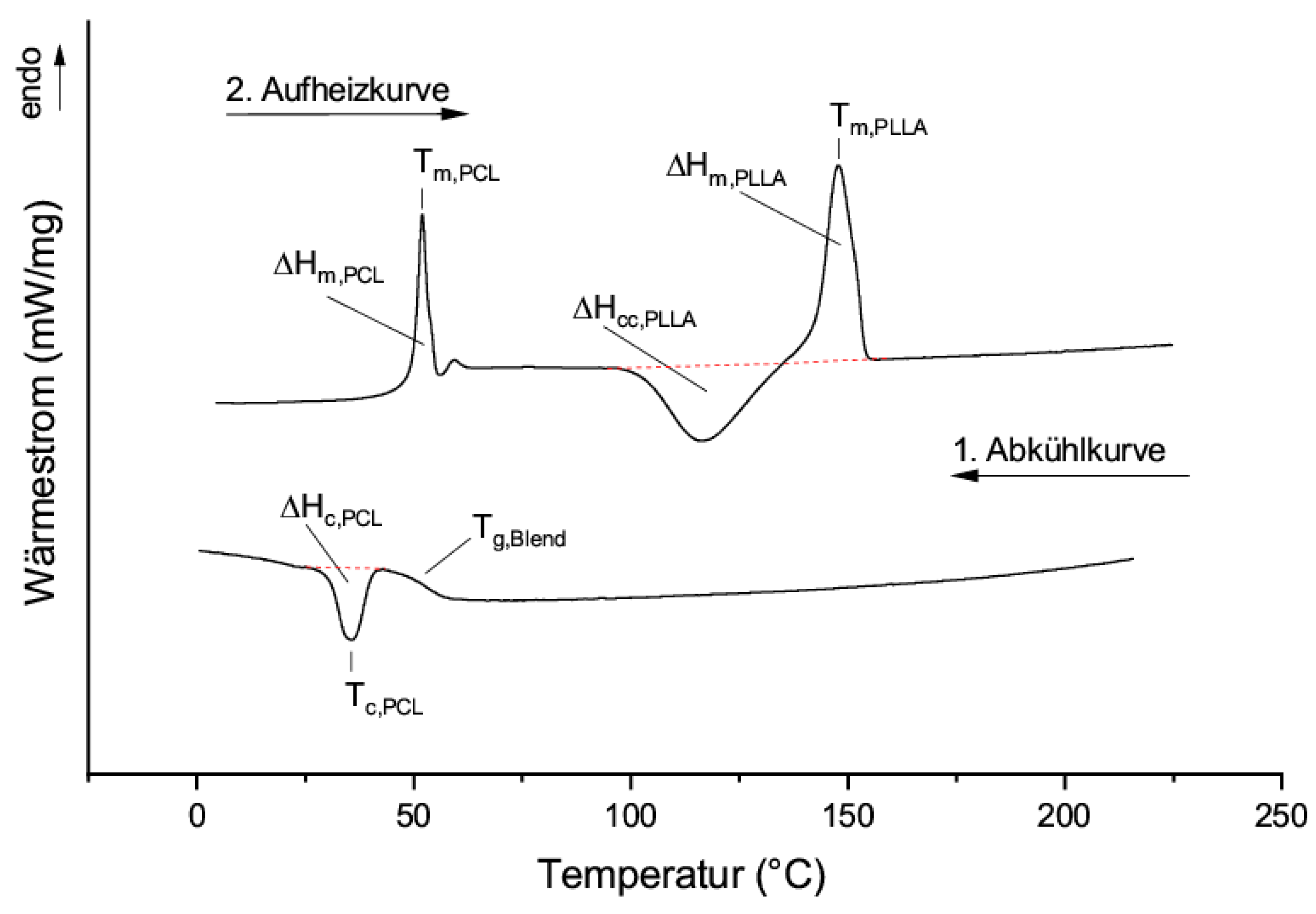
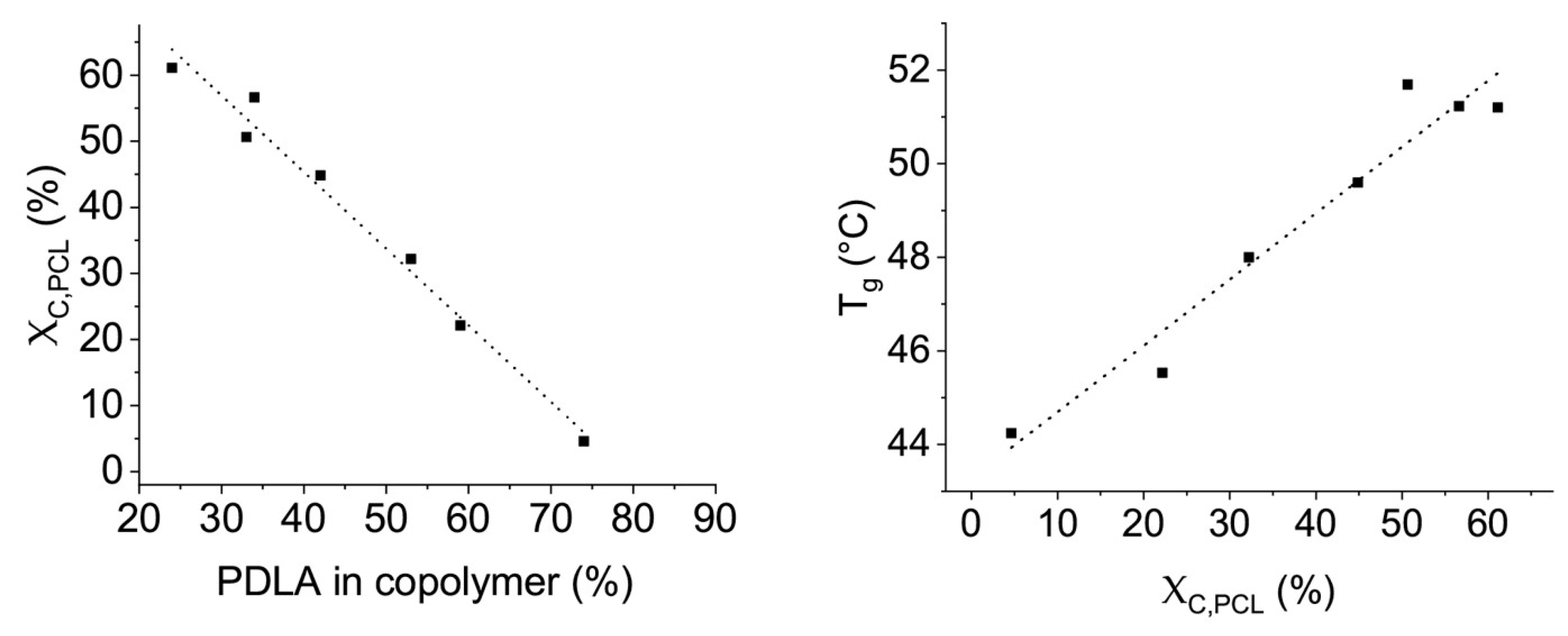
3.2. Thermal Properties of Blends of PLLA and PCL Oligomers
- The miscibility of the individual components
- The crystallinity of the individual components
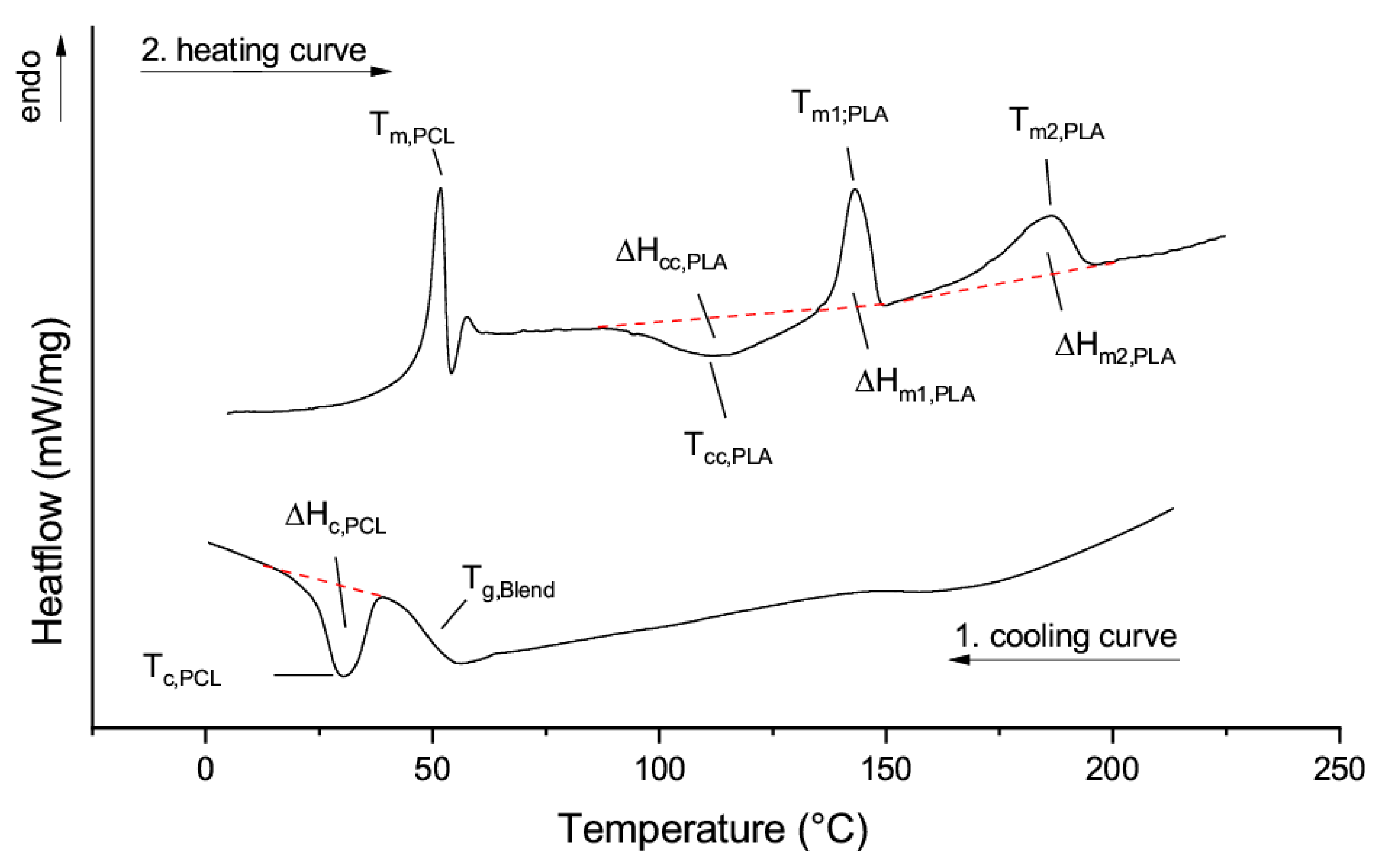
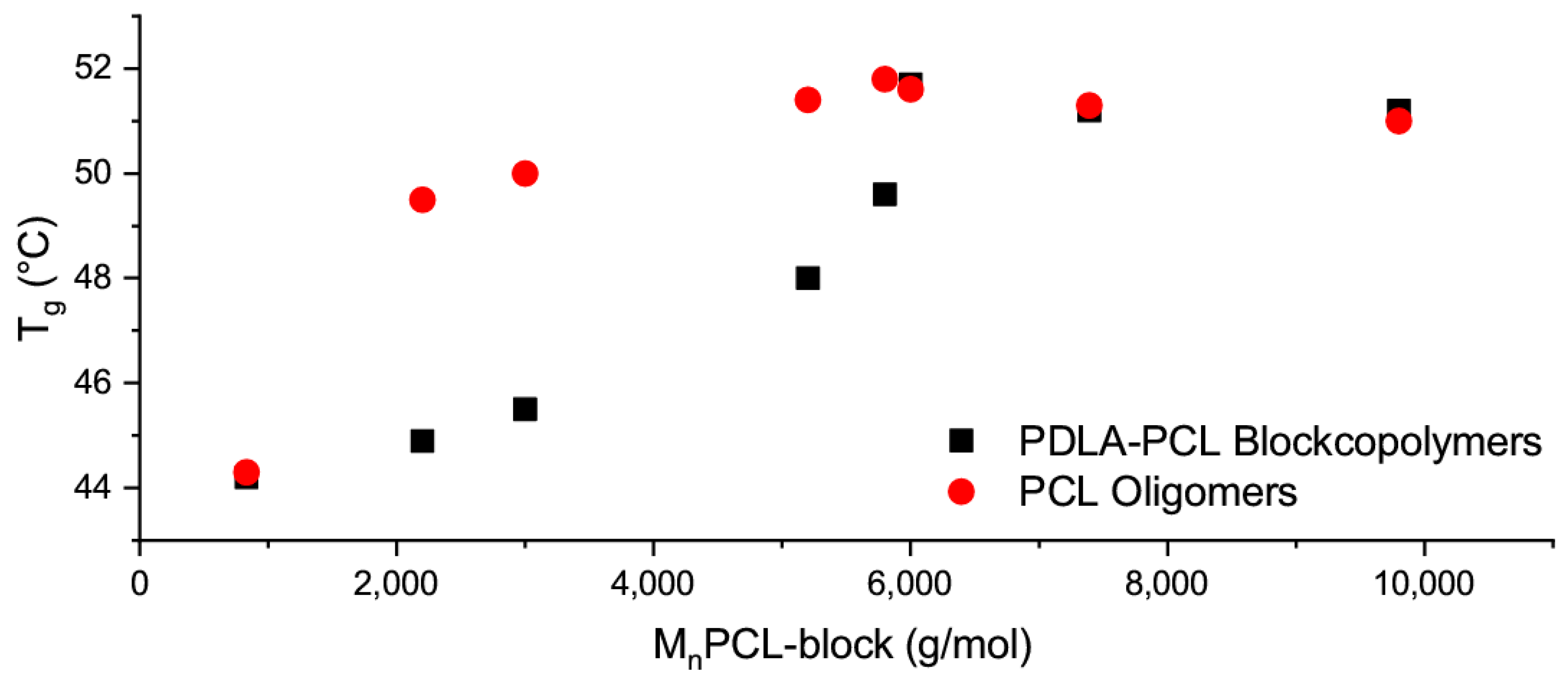
3.3. Thermal Properties of Blends of PLLA and PDLA-PCL Diblock Copolymers
3.4. Thermooptical Properties of Blends of PLLA and PDLA-b-PCL Diblock Copolymers
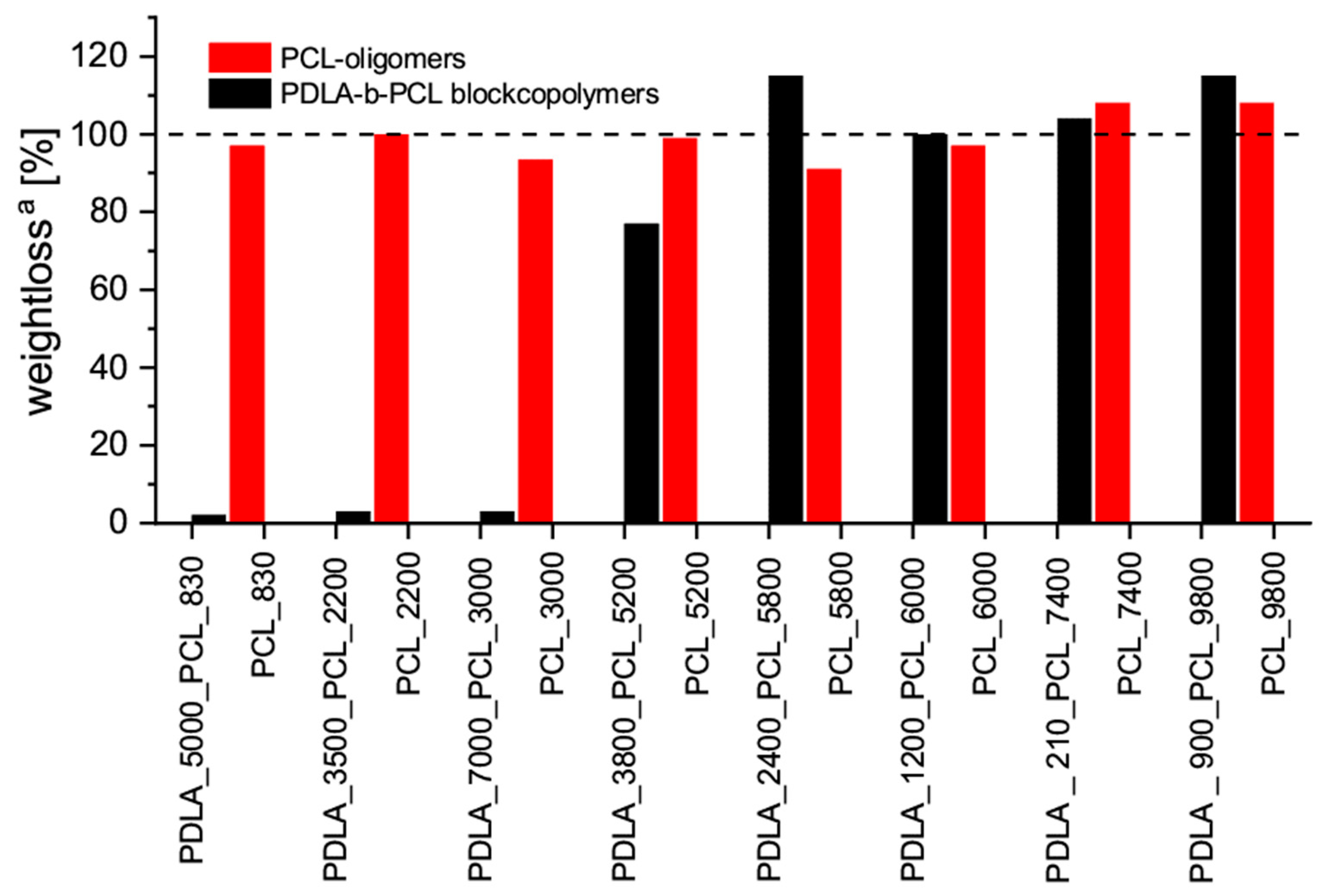
3.5. Migration of PCL Oligomers and PDLA-b-PCL Copolymers in Blends with PLLA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Labrecque, L.V.; Kumar, R.A.; Dave, R.; Gross, R.A.; McCarthy, S.P. Citrate esters as plasticizers for poly(lactic acid). J. Appl. Polym. Sci. 1997, 66, 1507–1513. [Google Scholar] [CrossRef]
- Ljungberg, N.; Wesslen, B. The effects of plasticizers on the dynamic mechanical and thermal properties of poly(lactic acid). J. Appl. Polym. Sci. 2002, 86, 1227–1234. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffiex, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly(L-lactic acid). J. Polym. Sci. A Polym. Chem. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- López-Rodríguez, N.; López-Arraiza, A.; Meaurio, E.; Sarasua, J.R. Crystallization, morphology, and mechanical behavior of polylactide/poly(ɛ-caprolactone) blends. Polym. Eng. Sci. 2006, 46, 1299–1308. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. [Google Scholar] [CrossRef]
- López-Rodríguez, N.; Martínez de Arenaza, I.; Meaurio, E.; Sarasua, J.R. Improvement of toughness by stereocomplex crystal formation in optically pure polylactides of high molecular weight. J. Mech. Behav. Biomed. Mater. 2014, 37, 219–225. [Google Scholar] [CrossRef]
- Stevels, W.M.; Ankone, M.J.K.; Dijksta, P.J.; Feijen, J. Stereocomplex formation in ABA triblock copolymers of poly(lactide) (A) and poly(ethylene glycol) (B). Macromol. Chem. Phys. 1995, 25, 3687–3694. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H.; Lin, T.; He, C. Recent advances in stereocomplexation of enantiomeric PLA-based copolymers and applications. Prog. Polym. Sci. 2016, 62, 22–72. [Google Scholar] [CrossRef]
- Jing, Z.; Shi, X.; Zhang, G.; Lei, R. Investigation of poly(lactide) stereocomplexation between linear poly(L -lactide) and PDLA-PEG-PDLA tri-block copolymer. Polym. Int. 2015, 64, 1399–1407. [Google Scholar] [CrossRef]
- Song, Y.; Wang, D.; Jiang, N.; Gan, Z. Role of PEG Segment in Stereocomplex Crystallization for PLLA/PDLA- b -PEG- b -PDLA Blends. ACS Sustain. Chem. Eng. 2015, 3, 1492–1500. [Google Scholar] [CrossRef]
- Rathi, S.; Chen, X.; Coughlin, E.B.; Hsu, S.L.; Golub, C.S.; Tzivanis, M.J. Toughening semicrystalline poly(lactic acid) by morphology alteration. Polymer 2011, 52, 4184–4188. [Google Scholar] [CrossRef]
- Rathi, S.; Coughlin, E.B.; Hsu, S.L.; Golub, C.S.; Ling, G.H.; Tzivanis, M.J. Effect of midblock on the morphology and properties of blends of ABA triblock copolymers of PDLA-mid-block-PDLA with PLLA. Polymer 2012, 53, 3008–3016. [Google Scholar] [CrossRef]
- Small, P.A. Some factors affecting the solubility of polymers. J. Appl. Chem. 1953, 3, 71–80. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Properties of Polymers. Their Correlation with Chemical Structure. In Their Numerial Estimation and Prediction from Additive Group Contributions, 3rd ed.; Elsevier: Oxford, UK, 1997; ISBN 9780444596123. [Google Scholar]
- Mark, J.E. Physical Properties of Polymers Handbook, 2nd ed.; Springer: New York, NY, USA, 2007; ISBN 978-0-387-31235-4. [Google Scholar]
- Flory, P.J. Thermodynamics of High Polymer Solutions. J. Chem. Phys. 1942, 10, 51–61. [Google Scholar] [CrossRef]
- Izuka, A.; Winter, H.H.; Hashimoto, T. Molecular weight dependence of viscoelasticity of polycaprolactone critical gels. Macromolecules 1992, 25, 2422–2428. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of diluent and copolymer composition on the glass transition temperature of a poly-mer system. Bull. Am. Phys. Soc. 1956, 1, 123. [Google Scholar]
- Vilay, V.; Mariatti, M.; Ahmad, Z.; Pasomsouk, K.; Todo, M. Characterization of the mechanical and thermal properties and morphological behavior of biodegradable poly(L-lactide)/poly(ε-caprolactone) and poly(L-lactide)/poly(butylene succinate- co -L-lactate) polymeric blends. J. Appl. Polym. Sci. 2009, 114, 1784–1792. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Hamada, H. The effect of crosslinking on the mechanical properties of polylactic acid/polycaprolactone blends. J. Appl. Polym. Sci. 2006, 101, 1816–1825. [Google Scholar] [CrossRef]
- Auras, R.; Lim, L.-T.; Selke, S.E.M.; Tsuji, H. Polylacticacid; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Rosa, D.S.; Guedes, C.G.F.; Bardi, M.A.G. Evaluation of thermal, mechanical and morphological properties of PCL/CA and PCL/CA/PE-g-GMA blends. Polym. Test. 2007, 26, 209–215. [Google Scholar] [CrossRef]
- Elias, H.G. Makromoleküle: Chemische Struktur und Synthesen; Wiley VCH: Weinheim, Germany, 1999. [Google Scholar]
- Purnama, P.; Kim, S.H. Stereocomplex Formation of High-Molecular-Weight Polylactide Using Supercritical Fluid. Macromolecules 2010, 43, 1137–1142. [Google Scholar] [CrossRef]



| δSmall | δHoy | δvanKrevelen | δØ | Tg1 | |
|---|---|---|---|---|---|
| PCL | 19.08 | 19.45 | 18.29 | 18.94 | −59.0 |
| PLLA | 19.86 | 20.55 | 18.86 | 19.75 | 58.0 |
(J/g) | Tcc PLLA a (°C) | (J/g) | Tm1, PLLAa (°C) | XC,PLLA a (%) | (J/g) | Tc, PCL b (°C) | XC,PCL b (%) | Tg, Blend b (°C) | Tg,PCL Oligomer c (°C) | |
|---|---|---|---|---|---|---|---|---|---|---|
| PLLA | 20.0 | 124.7 | 20.0 | 147.1 | 0 | - | - | - | 58,0 | - |
| PCL_830 | 25.7 | 105.6 | 25.8 | 139.8 | 0.1 | 0.2 | 18.0 | 1.4 | 44.3 | −59.0 |
| PCL_2200 | 1.3 | 120.6 | 2.1 | 145.0 | 0.8 | 6.6 | 12.3 | 47.3 | 50.4 | −58.7 |
| PCL_3000 | 10.1 | 118.0 | 11.7 | 144.3 | 1.5 | 6.7 | 23.4 | 48.0 | 50.9 | −58.5 |
| PCL_5200 | 21.0 | 116.4 | 21..5 | 147.8 | 0.5 | 6.8 | 35.7 | 48.7 | 51.4 | −59.6 |
| PCL_5800 | 20.1 | 115.9 | 21.0 | 147.5 | 0.9 | 6.7 | 34.5 | 48.0 | 51.8 | −59.0 |
| PCL_6000 | 3.8 | 118.1 | 4.8 | 144.1 | 1.0 | 6.9 | 35.2 | 49.5 | 51.6 | −59.3 |
| PCL_7400 | 1.1 | 121.4 | 2.0 | 145.1 | 0.9 | 7.2 | 33.3 | 51.6 | 51.9 | −59.8 |
| PCL_9800 | 8.4 | 117.5 | 10.2 | 144.4 | 1.7 | 7.4 | 22.3 | 53.0 | 51.3 | −59.4 |
| PLLA Blend with | PDLA, Copolymer (%) | PCL, Copolymer (%) | Copolymer, Blend (%) |
|---|---|---|---|
| PDLA5000_PCL830 | 72 | 28 | 31 |
| PDLA3500_PCL2200 | 55 | 45 | 24 |
| PDLA7000_PCL3000 | 59 | 41 | 25 |
| PDLA3800_PCL5200 | 47 | 53 | 19 |
| PDLA2400_PCL5800 | 41 | 59 | 15 |
| PDLA1200_PCL6000 | 18 | 82 | 12 |
| PDLA210_PCL7400 | 24 | 76 | 15 |
| PDLA900_PCL9800 | 20 | 80 | 13 |
| PLLA Blend with | ΔHcc,PLLA a (J/g) | ΔHm1, PLLA a (J/g) | Tm1,PLLA a (°C) | ΔHm2, PLAsc a (J/g) | Tm2,PLAsc a (°C) | ΔHm0, mix (J/g) | XC,PLA a (%) | ΔHm,PCL b (J/g) | XC,PCL b (%) | Tg,Blend b (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| PDLA5000_PCL830 | 7.0 | 0.5 | 142.4 | 17.4 | 193 | 105.8 | 10.3 | 0.2 | 1.4 | 44.2 |
| PDLA3500_ | 2.5 | 6.6 | 147.0 | 27.3 | 195 | 97.0 | 32.4 | 3.1 | 22.2 | 45.8 |
| PCL2200 | ||||||||||
| PDLA7000_ | 10.1 | 9.8 | 143.5 | 24.7 | 205 | 98.5 | 24.8 | 3.5 | 25.1 | 45.5 |
| PCL3000 | ||||||||||
| PDLA3800_ | 10.1 | 10.7 | 142.7 | 15.9 | 194 | 92.9 | 17.8 | 4.0 | 28.7 | 48.0 |
| PCL5200 | ||||||||||
| PDLA2400_ | 4.9 | 4.9 | 143.0 | 3.5 | 186 | 90.2 | 3.9 | 5.7 | 40.9 | 49.6 |
| PCL5800 | ||||||||||
| PDLA1200_ | - | 0.5 | 146.0 | - | - | 86.3 | 0.5 | 6.4 | 45.9 | 51.1 |
| PCL6000 | ||||||||||
| PDLA210_PCL7400 | - | 0.5 | 147.0 | - | - | 87.7 | 0.5 | 9.8 | 70.3 | 51.2 |
| PDLA900_PCL9800 | 3.7 | 3.8 | 147.0 | - | - | 86.8 | 0.1 | 7.1 | 50.9 | 51.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weidner, E.; Kabasci, S.; Kopitzky, R.; Mörbitz, P. Thermal and Morphological Properties of Poly(L-Lactic Acid)/Poly(D-Lactic Acid)-B-Polycaprolactone Diblock Copolymer Blends. Materials 2020, 13, 2550. https://doi.org/10.3390/ma13112550
Weidner E, Kabasci S, Kopitzky R, Mörbitz P. Thermal and Morphological Properties of Poly(L-Lactic Acid)/Poly(D-Lactic Acid)-B-Polycaprolactone Diblock Copolymer Blends. Materials. 2020; 13(11):2550. https://doi.org/10.3390/ma13112550
Chicago/Turabian StyleWeidner, Eckhard, Stephan Kabasci, Rodion Kopitzky, and Philip Mörbitz. 2020. "Thermal and Morphological Properties of Poly(L-Lactic Acid)/Poly(D-Lactic Acid)-B-Polycaprolactone Diblock Copolymer Blends" Materials 13, no. 11: 2550. https://doi.org/10.3390/ma13112550
APA StyleWeidner, E., Kabasci, S., Kopitzky, R., & Mörbitz, P. (2020). Thermal and Morphological Properties of Poly(L-Lactic Acid)/Poly(D-Lactic Acid)-B-Polycaprolactone Diblock Copolymer Blends. Materials, 13(11), 2550. https://doi.org/10.3390/ma13112550







