Abstract
Renewable vinyl compounds itaconic acid (IA) and its derivative 10-hydroxyhexylitaconic acid (10-HHIA) are naturally produced by fungi from biomass. This provides the opportunity to develop new biobased polyvinyls from IA and 10-HHIA monomers. In this study, we copolymerized these monomers at different ratios through free radical aqueous polymerization with potassium peroxodisulfate as an initiator, resulting in poly(IA-co-10-HHIA)s with different monomer compositions. We characterized the thermal properties of the polymers by thermogravimetric analysis (TGA) and Fourier-transform infrared spectroscopy (FT-IR). The nuclear magnetic resonance analysis and the gel permeation chromatography showed that the polymerization conversion, yield, and the molecular weights (weight-averaged Mw and number-averaged Mn) of the synthesized poly(IA-co-10-HHIA)s decreased with increasing 10-HHIA content. It is suggested that the hydroxyhexyl group of 10-HHIA inhibited the polymerization. The TGA results indicated that the poly(IA-co-10-HHIA)s continuously decomposed as temperature increased. The FT-IR analysis suggested that the formation of the hydrogen bonds between the carboxyl groups of IA and 10-HHIA in the polymer chains was promoted by heating and consequently the polymer dehydration occurred. To the best of our knowledge, this is the first time that biobased polyvinyls were synthesized using naturally occurring IA derivatives.
1. Introduction
Owing to the growing environmental interest, the development of renewable polymer materials derived from biomass is increasing in popularity [1,2,3,4]. To develop polymers with unique properties, novel monomer type building blocks are in demand because the monomer unit predominantly affects the polymer properties [5,6,7]. Some renewable monomers that are produced by microbes from biomass consist of larger carbon numbers (higher than C5) [8]. These monomers are barely synthesized from petroleum resources and possess unique structural characteristics increasing their potential to be applied as new monomers.
Itaconic acid (IA) is one of the most promising renewable vinyl compounds commercially produced using fungi, mostly Aspergillus terreus [9,10,11,12]. IA is a sustainable monomer because it is fermentatively produced from biomass. To date, many IA copolymers have been synthesized from IA by free radical copolymerization (e.g., with methyl methacrylate (MMA)) or polycondensation (e.g., with diols) [8,13,14,15,16,17,18,19,20]. For instance, Ranjha et al., have synthesized poly(MMA-co-IA) by free radical copolymerization using crosslinkers for development of controlled drug delivery [19]. On the other hand, Dai et al., have synthesized a series of polyesters by melt polycondensation of IA with diols and glycerol [20]. The resulting copolymers further copolymerized with acrylated epoxidized soybean oil showed great potential for applications as coatings, adhesives, and composites. Specifically, some papers reported that IA-based polyvinyls have cation-exchange properties [21,22,23]. Interestingly, naturally occurring IA derivatives possessing a vinyl group have been found as metabolic products from fungi and lichens. This suggests that it is possible to synthesize new biobased polymers from these IA derivatives. However, there is no report on the polymers synthesized using naturally occurring IA derivatives.
Recently, we have developed a new screening method for microbes from soil to produce IA [24,25,26]. In our screening study, we have also isolated a fungus Aspergillus niger S17-5 producing two IA derivatives from glucose, 9-hydroxyhexylitaconic acid (9-HHIA) and 10-hydroxyhexylitaconic acid (10-HHIA) [27]. These compounds possess an alkyl chain with a hydroxy group. Therefore, it might be possible to synthesize novel biobased polyvinyls excellent in impact resistance, tensile strength, and moldability, compared to IA homopolymers. The production titer of 10-HHIA is higher than that of 9-HHIA; therefore, 10-HHIA would be the preferred monomer for the polymer synthesis.
The present study reports on the free radical polymerization of poly(IA-co-10-HHIA)s with IA and 10-HHIA monomers in water using potassium peroxodisulfate (KPS) as initiator (Figure 1). The thermal properties of the polymers were characterized with thermogravimetric analysis (TGA) and Fourier-transform infrared spectroscopy (FT-IR).
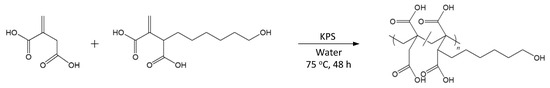
Figure 1.
Synthesis of poly(IA-co-10-HHIA)s.
2. Materials and Methods
2.1. Materials
IA (Molecular weight: 130.1) was purchased from Wako Pure Chemical Corp. (Osaka, Japan). KPS, sodium dihydrogenphosphate, disodium hydrogenphosphate, deuterium oxide, and sodium hydroxide were purchased from Nacalai Tesque Inc. (Kyoto, Japan). All reagents were used without further purification.
2.2. Preparation of 10-HHIA
The 10-HHIA (Molecular weight: 230.2 g mol−1) was obtained from the culture supernatant of A. niger S17-5 according to a method described elsewhere [27]. Briefly, A. niger S17-5 was cultured in 1 L of a GM2 liquid medium (per 1 L: 130 g glycerol, 0.154 g MgSO4·7H2O, 0.19 mg FeCl2·4H2O, 0.46 g NH4NO3, 15.4 mg KH2PO4, 96 mg CaCl2, 1.2 mg ZnSO4·7H2O, 2.3 mg CuSO4·5H2O) [28]. The culture was centrifuged to obtain the supernatant. The supernatant typically contained 10-HHIA at a concentration of 0.5 g L−1. The supernatant was purified using a preparative high-performance liquid chromatograph (HPLC, LaChrom Elite, Hitachi High-Technologies, Tokyo, Japan) equipped with a preparative HPLC Inertsil ODS 10 μm column (GL sciences, Tokyo, Japan). The 10-HHIA was eluted using a water/acetonitrile/trifluoro acetic acid solution (flow rate: 5 mL min−1). The eluate was monitored at an absorbance of 210 nm. The eluate was dried by freeze dehydration, resulting in the purified 10-HHIA (approx. 0.1 g).
2.3. Copolymerization
Different IA/10-HHIA monomer ratios were used (200/0, 160/40, 100/100, and 0/200 μmol in feed). The corresponding monomer ratio and 2 μmol KPS were added to 0.4 mL of water in a glass ampule. After the mixtures were degassed three times by freeze-thaw, the glass ampule was sealed and then placed in an oil bath at 75 °C for 48 h. Then, the reaction mixtures were dialyzed in water at 25 °C for 3 days with a Spectra/Por 7 dialysis membrane (Molecular weight cut-off: 1 kDa) (Spectrum Laboratories Inc., Rancho Dominguez, CA, USA). The dialyzed solutions were dried by freeze dehydration, resulting in the purified poly(IA-co-10-HHIA)s.
2.4. Measurements
The 1H NMR spectra were recorded on a Bruker AV-300 (Billerica, MA, USA). All samples were analyzed by 1H NMR with D2O after freeze dehydration. Especially, when samples of poly(IA-co-10-HHIA) with IA/10-HHIA monomer ratios of 100/100 and 0/200 were analyzed by NMR, an aliquot of 10 mM NaOH in D2O was used as a solvent to completely dissolve the synthesized polymers. The conversion of the synthesized copolymers was calculated as follows:
Conversion (%) = (1 – Am/(Am + Ap)) × 100
Am: the area of vinylidene proton signals at 6.5 ppm;
Ap: the area of copolymer proton signals.
The gel permeation chromatography (GPC) was conducted with a PU-2089 pump (JASCO, Tokyo, Japan), a CO-2065 column oven (JASCO), and an RI-2031 refractive index detector (JASCO). A Shodex OHpak SB-804 HQ (8.0 × 300 mm, Showa Denko K.K., Tokyo, Japan) column was used with 20 mM phosphate buffer (pH 7.0) as the eluent (flow rate: 0.5 mL min−1 at 30 °C). The molecular weights were calibrated against pullulan standards according to the method for GPC analysis of IA-derived polymers [16].
The TGA was carried out with a Discovery TGA (TA instruments, New Castle, DE, USA). The polymer samples were analyzed under a nitrogen gas purge of 10 mL/min at a heating rate of 10 °C min−1 and a temperature ranging from 25 to 700 °C.
The FT-IR was performed with a FT/IR 4600 spectrometer (JASCO). The polymer samples were heated at 200 °C for 18 h under a nitrogen gas atmosphere. After natural cooling, the spectra were recorded in transmittance mode within the range from 4000 cm−1 to 400 cm−1 with a resolution of 4 cm−1 and 16 scans were co-added.
3. Results and Discussion
3.1. Synthesis of Poly(IA-co-10-HHIA)s
According to reported homo-polymerizations of IA [29,30,31,32,33,34], the poly(IA-co-10-HHIA) copolymers were synthesized by the free radical polymerization of IA and 10-HHIA in water with KPS as an initiator. Figure 2 shows a representative 1H NMR spectrum of the poly(IA-co-10-HHIA) copolymer. The methylene signal at 2.7 ppm represents the polymer backbone. The signals at 2.2–2.3 ppm correspond to methine and methylene protons of IA and 10-HHIA. The signals at 1.3–1.6 and 3.4 ppm correspond to the methylene protons of the hexyl group of 10-HHIA. These results indicated that novel biobased polyvinyls could be synthesized using the naturally occurring IA derivative as a monomer. The reproducibility of the copolymer structure was confirmed because similar NMR spectra were obtained after the same experiment several times (data not shown). Figure 3 shows GPC curves of poly(IA-co-10-HHIA)s synthesized with different monomer feed ratios. The GPC curves show that all synthesized copolymers have a single peak, indicating that two distinct molecular weight species were absent in the samples analyzed.
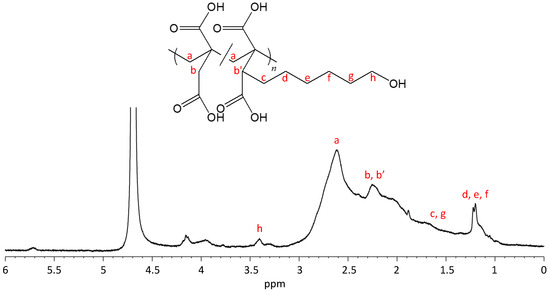
Figure 2.
1H NMR spectrum of poly(IA-co-10-HHIA) synthesized with an IA/10-HHIA monomer ratio of 100/100 μmol (code 3). The letters indicate the positions of protons and their corresponding signals.
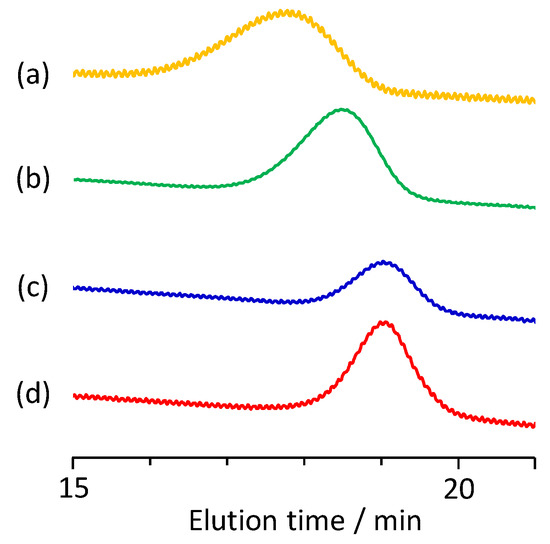
Figure 3.
Gel permeation chromatography (GPC) traces of poly(IA-co-10-HHIA)s. (a) Code 1, (b) code 2, (c) code 3, (d) code 4.
Table 1 summarizes the polymerization conversion, the monomer compositions, the copolymer yields, the number-average molecular weight (Mn), and the molecular weight distribution (MWD) of the molecular weight distribution of the copolymers with weight-average molecular weight (Mw). The polymerization conversion, yield, Mw, and Mn of the synthesized poly(IA-co-10-HHIA)s decreased by increasing the feed ratio of 10-HHIA. Interestingly, all obtained copolymers have, despite the free radical polymerization, relatively low MWDs (1.10–1.29). A related paper reported that the homo-polymerization of IA in water with KPS required a relatively long reacting period (48 h) and the resulting poly(IA) showed low MWDs (1.12–1.14) [34]. Our results with 10-HHIA are in good agreement with results reported so far.

Table 1.
Synthesis of poly(IA-co-10-HHIA)s with different monomer feed ratios.
In summary, we demonstrated that 10-HHIA can be polymerized by free radical polymerization in the same way as IA, resulting in novel renewable polyvinyls, but the degree of polymerization of 10-HHIA is lower than that of IA; this may be due to the steric effect of the hydroxyhexyl group of 10-HHIA.
3.2. Thermal Characterization of Poly(IA-co-10-HHIA)s
To characterize the thermal properties of poly(IA-co-10-HHIA)s, TGA and FT-IR were conducted. Figure 4 shows TGA thermograms of poly(IA-co-10-HHIA)s from 25 to 700 °C. The TGA of all poly(IA-co-10-HHIA)s indicated a similar profile and they continuously decomposed as the temperature increased. Previous papers on TGA of poly(acrylic acid) and poly(IA) described similar results [31,35,36,37,38]. Krušić et al., have reported that the derivative thermogravimetry curve of poly(IA) has three maxima, at 185 °C, 315 °C, and 388 °C, assigned to the elimination of water and the formation of polyanhydride, followed by decarboxylation of anhydride groups and the breaking of the main polymer backbone [32,39]. Kayaman et al., have reported that poly(IA) showed a small weight loss at 100 °C implying the loss of moisture, and that poly(IA) had a significant weight loss at around 165 °C when TGA thermal decomposition was analyzed [37]. In addition, Ha et al., have reported that the carboxyl groups in poly(acrylic acid) were crosslinked by dehydration at 160 °C [40]. This suggested that dehydration occurred by linking the carboxyl groups of IA and 10-HHIA units by heating. Interestingly, the TGA results indicated that the poly(IA-co-10-HHIA)s continuously decomposed as temperature increased. This may be because the hydroxyhexyl group of 10-HHIA inhibited the formation of the linkage between the carboxyl groups of IA and 10-HHIA units. As the 10-HHIA content increased, the weight loss rate of poly(IA-co-10-HHIA)s was reduced. This may be due to the weight contribution of the carboxyl group in the copolymers decreased.
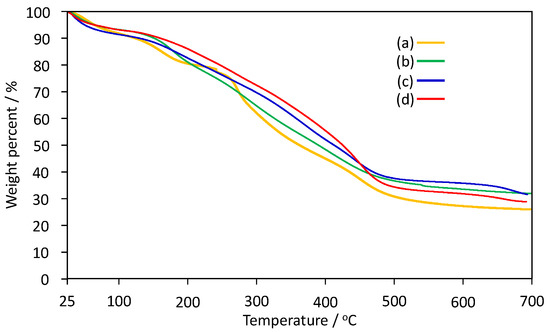
Figure 4.
TGA thermograms of poly(IA-co-10-HHIA)s. (a) Code 1, (b) code 2, (c) code 3, (d) code 4.
Figure 5 shows FT-IR spectra of poly(IA) and a representative poly(IA-co-10-HHIA) copolymer before and after heating at 200 °C for 18 h. The non-heated poly(IA) has the characteristic peaks at 2800–3400 cm−1, 1707 cm−1, 1639 cm−1, 1411 cm−1, 1193 cm−1, and 908 cm−1 assigned to a broad -OH stretching, the carboxylic acid (C=O) stretching, asymmetric C=O stretching of carboxylate anion, symmetric C=O stretching of carboxylate anion, C-O-H in-plane bending interactions, C-O stretching dimer, and O-H out-of-plane bending, respectively, according to the FT-IR spectrum of poly(IA) [37]. The non-heated poly(IA-co-10-HHIA) copolymer showed a similar profile. The poly(IA) and poly(IA-co-10-HHIA) copolymer heated at 200 °C for 18 h have a clear peak corresponding to the symmetric C=O stretching of carboxylate anion at 1610–1616 cm−1. This indicates that the heating promoted the formation of the hydrogen bonds between the carboxyl groups of IA and 10-HHIA. The intensity of the peak corresponding to the carboxylic acid (C=O) stretching at 1706–1715 cm−1 decreased after the heating. This suggests dissociation of the carboxyl groups of IA and 10-HHIA by the heating. It has been reported that heating poly(IA) results in the dehydration between carboxyl groups in the polymer and consequently the formation of poly(IA anhydride) [31,38]. These suggest that the formation of the hydrogen bonds between the carboxyl groups of IA and 10-HHIA in the polymer chains was promoted by heating and consequently the polymer dehydration occurred.
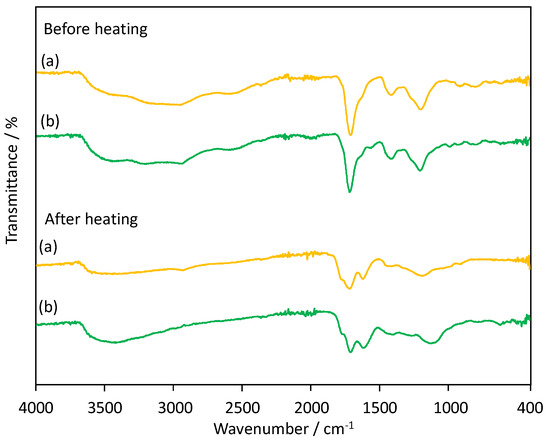
Figure 5.
FT-IR spectra of (a) poly(IA) and (b) poly(IA-co-10-HHIA) synthesized with an IA/10-HHIA monomer ratio of 160/40 μmol (code 2) before and after heating at 200 °C for 18 h.
4. Conclusions
The present study reports the synthesis and thermal characterization of poly(IA-co-10-HHIA)s. The poly(IA-co-10-HHIA)s were synthesized by free radical polymerization with different monomer feed ratios (IA/10-HHIA: 200/0–0/200 μmol). The resulting polymers with higher 10-HHIA content showed a lower polymerization conversion, yield, Mn, and Mw of the synthesized copolymers. The copolymer MWDs were also relatively low (1.10–1.29). We related this to the steric effect of the hydroxyhexyl group of 10-HHIA. Thermal analyses suggested that heating promoted the formation of the hydrogen bonds between the carboxyl groups of IA and 10-HHIA in the polymer chains and then the polymer dehydration occurred. To the best of our knowledge, this is the first study that reports on the biobased polyvinyl synthesis using naturally occurring IA derivatives.
Author Contributions
Y.A. designed the study and wrote the manuscript; M.S. and R.Y. performed the experiments; T.T. designed the study and reviewed the manuscript; T.A. contributed to analysis of the samples; H.O., T.K., K.M., and K.W. contributed to the discussion. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number 19K05767 and Grant-in-Aid for JSPS Fellows Number 18J13414.
Conflicts of Interest
Keiji Matsumoto and Kazuhito Wada are employees of Kaneka Corporation.
References
- Charlotte, K.W.; Marc, A.H. Polymers from renewable resources: A perspective for a special issue of polymer reviews. Polym. Rev. 2008, 48, 1–10. [Google Scholar]
- Ramesh, P.B.; Kevin, O.C.; Ramakrishna, S. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar]
- Yunqing, Z.; Charles, R.; Charlotte, K.W. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar]
- George, Z.P. Thinking green: Sustainable polymers from renewable resources. Polymers 2018, 10, 952. [Google Scholar]
- Achim, G.; Robert, S.L. The influence of microstructure and monomer properties on the erosion mechanism of a class of polyanhydrides. J. Pol. Sci. Part A Pol. Chem. 1993, 31, 2445–2458. [Google Scholar]
- Jewell, G.W. Latex properties: Effect of monomer composition. In Surface Coatings; Springer: Dordrecht, The Netherlands, 1993; pp. 303–319. [Google Scholar]
- Thiago, F.C.; Maria, I.F. The influence of rigid and flexible monomers on the physical-chemical properties of polyimides. J. Appl. Pol. Sci. 2014, 131, 40351. [Google Scholar]
- Aso, Y. Bio-acrylates. In Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Tate, B.E. Itaconic acid and derivatives. In Encyclopedia of Chemical Technology; Wiley: New York, NY, USA, 1981; pp. 865–873. [Google Scholar]
- Bonnarme, P.; Gillet, B.; Sepulchre, A.M.; Role, C.; Beloeil, J.C.; Ducrocq, C. Itaconate biosynthesis in Aspergillus Terreus. J. Bacteriol. 1995, 177, 3573–3578. [Google Scholar] [CrossRef]
- Willke, T.; Vorlop, K.-D. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef]
- Okabe, M.; Lies, D.; Kanamasa, S.; Park, E.Y. Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl. Microbiol. Biotechnol. 2009, 84, 597–606. [Google Scholar] [CrossRef]
- Marvel, C.S.; Shepherd, T. Polymerization reactions of itaconic acid and some of its derivatives. J. Org. Chem. 1959, 24, 599–605. [Google Scholar] [CrossRef]
- Tate, B.E. Polymerization of itaconic acid and derivatives. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1967; pp. 214–232. [Google Scholar]
- Okuda, T.; Ishimoto, K.; Ohara, H.; Kobayashi, S. Renewable biobased polymeric materials: Facile synthesis of itaconic anhydride-based copolymers with poly(L-lactic acid) grafts. Macromolecules 2012, 45, 4166–4174. [Google Scholar] [CrossRef]
- Ali, M.A.; Tateyama, S.; Oka, Y.; Kaneko, D.; Okajima, M.K.; Kaneko, T. Syntheses of high-performance biopolyamides derived from itaconic acid and their environmental corrosion. Macromolecules 2013, 46, 3719–3725. [Google Scholar] [CrossRef]
- Winkler, M.; Lacerda, T.M.; Mack, F.; Meier, M.A.R. Renewable polymers from itaconic acid by polycondensation and ring-opening-metathesis polymerization. Macromolecules 2015, 48, 1398–1403. [Google Scholar] [CrossRef]
- Tobias, R.; Stefan, F. Itaconic acid–a versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016, 18, 2922–2934. [Google Scholar]
- Ranjha, N.M.; Mudassir, J.; Akhtar, N. Methyl methacrylate-co-itaconic acid (MMA-co-IA) hydrogels for controlled drug delivery. J. Sol. Gel Sci. Technol. 2008, 47, 23–30. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Wu, Y.; Han, L.; Zhang, L.; Zhua, J.; Liu, X. Polyesters derived from itaconic acid for the properties and bio-based content enhancement of soybean oil-based thermosets. Green Chem. 2015, 17, 2383–2392. [Google Scholar] [CrossRef]
- Nagai, S.; Yoshida, K. The polymerization and polymers of itaconic acid derivatives. VIII. A cation exchange resin from itaconic anhydride. Bull. Chem. Soc. Jpn. 1965, 38, 1402–1403. [Google Scholar] [CrossRef]
- Katime, I.; Rodríguez, E. Absorption of metal ions and swelling properties of poly (acrylic acid-co-itaconic acid) hydrogels. J. Macromol. Sci. Part A 2001, 38, 543–558. [Google Scholar] [CrossRef]
- Bashir, W.; Tyrrell, E.; Feeney, O.; Paull, B. Retention of alkali, alkaline earth and transition metals on an itaconic acid cation-exchange column. Eluent pH, ionic strength and temperature effects upon selectivity. J. Chromatogr. A 2002, 964, 113–122. [Google Scholar] [CrossRef]
- Sano, M.; Chin, T.; Takahashi, T.; Ohara, H.; Aso, Y. A simple TLC-densitometric method for the quantification of acrylic acid in aqueous solutions. J. Planar Chromatogr. 2015, 28, 12–16. [Google Scholar] [CrossRef]
- Sano, M.; Kuroda, H.; Ohara, H.; Ando, H.; Matsumoto, K.; Aso, Y. A high-throughput screening method based on the Mizoroki-Heck reaction for isolating itaconic acid-producing fungi from soils. Heliyon 2019, 5, e02048. [Google Scholar] [CrossRef] [PubMed]
- Aso, Y.; Sano, M.; Kuroda, H.; Ohara, H.; Ando, H.; Matsumoto, K. DISCOVER: A facile structure-based screening method for vinyl compound producing microbes. Sci. Rep. 2019, 9, 16007. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Yada, R.; Nomura, Y.; Kusukawa, T.; Ando, H.; Matsumoto, K.; Wada, K.; Tanaka, T.; Ohara, H.; Aso, Y. Microbial screening based on the Mizoroki–Heck reaction permits exploration of hydroxyhexylitaconic-acid-producing fungi in soils. Microorganisms 2020, 8, 648. [Google Scholar] [CrossRef] [PubMed]
- Hevekerl, A.; Kuenz, A.; Vorlop, K.-D. Filamentous fungi in microtiter plates-an easy way to optimize itaconic acid production with Aspergillus terreus. Appl. Microbiol. Biotechnol. 2014, 98, 6983–6989. [Google Scholar] [CrossRef]
- Nagai, S.; Yoshida, K. Studies on polymerization and polymers of itaconic acid derivatives. Kobunshi Kagaku 1960, 17, 748–752. [Google Scholar] [CrossRef]
- Nagai, S. The polymerization and polymers of itaconic acid derivatives. V. The copolymerization reactivity of itaconic acid in an aqueous solution. Bull. Chem. Soc. Jpn. 1965, 38, 1459–1463. [Google Scholar] [CrossRef]
- Veličković, J.; Filipović, J.; Petrović-Djakov, D. The synthesis and characterization of poly(itaconic) acid. Polym. Bull. 1994, 32, 169–172. [Google Scholar] [CrossRef]
- Filipović, J.M.; Katsikas, L.; Popović, I.G.; Veličković, S.J.; Djakov, T.A.; Petrović-Djakov, D.M. The thermal degradation of some alkali metal salts of poly(itaconic acid). J. Therm. Anal. 1997, 49, 335–341. [Google Scholar] [CrossRef]
- Erbil, C.; Uyanık, N. Interactions between poly(acrylamide)–poly(itaconic acid) and cerium(IV)–nitrilotriacetic acid redox pair in the synthesis of acrylamide and itaconic acid homo- and copolymers. Polym. Int. 2001, 50, 792–795. [Google Scholar] [CrossRef]
- Lárez, C.V.; Canelón, F.; Millán, E.; Perdomo, G.; Katime, I. New results on the polymerisation of the itaconic acid in aqueous medium. Polym. Bull. 2002, 49, 119–126. [Google Scholar] [CrossRef]
- Tomić, S.L.; Filipović, J.M. Synthesis and characterization of complexes between poly(itaconic acid) and poly(ethylene glycol). Polym. Bull. 2004, 52, 355–364. [Google Scholar] [CrossRef]
- Moharram, M.A.; Allam, M.A. Study of the interaction of poly(acrylic acid) and poly(acrylic acid-poly acrylamide) complex with bone powders and hydroxyapatite by using TGA and DSC. J. Appl. Polym. Sci. 2007, 105, 3220–3227. [Google Scholar] [CrossRef]
- Kayaman, N.; Hamurcu, E.E.G.; Uyanik, N.; Baysal, B.M. Interpenetrating hydrogel networks based on polyacrylamide and poly(itaconic acid): Synthesis and characterization. Macromol. Chem. Phys. 1999, 200, 231–238. [Google Scholar] [CrossRef]
- Krušić, M.K.; Dzunuzovic, E.; Trifunovic, S.S.; Filipović, J. Polyacrylamide and poly(itaconic acid) complexes. Eur. Polym. J. 2004, 40, 793–798. [Google Scholar] [CrossRef]
- Krušić, M.K.; Dzunuzovic, E.; Trifunovic, S.S.; Filipović, J. Semi-IPNs based on polyacrylamide and poly(itaconic acid). Polym. Bull. 2003, 51, 159–166. [Google Scholar] [CrossRef]
- Ha, H.; Shanmuganathan, K.; Ellison, C.J. Mechanically stable thermally crosslinked poly(acrylic acid)/reduced graphene oxide aerogels. ACS Appl. Mater. Interfaces 2015, 25, 6220–6229. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).