Alkali-Activated Slag Paste with Different Mixing Water: A Comparison Study of Early-Age Paste Using Electrical Resistivity
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Sample Preparation
2.3. Test Methods
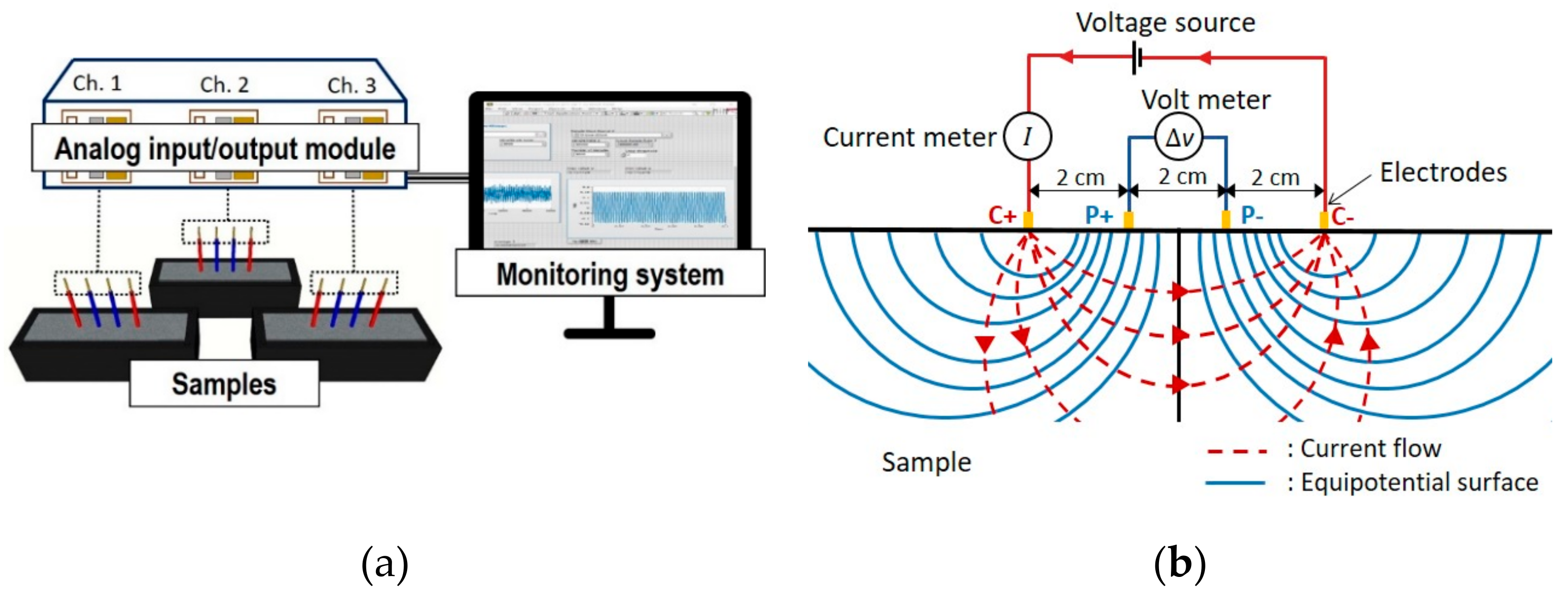
2.3.1. Electrical Resistivity Measurement
2.3.2. Compressive Strength Test
2.3.3. XRD
3. Results and Discussions
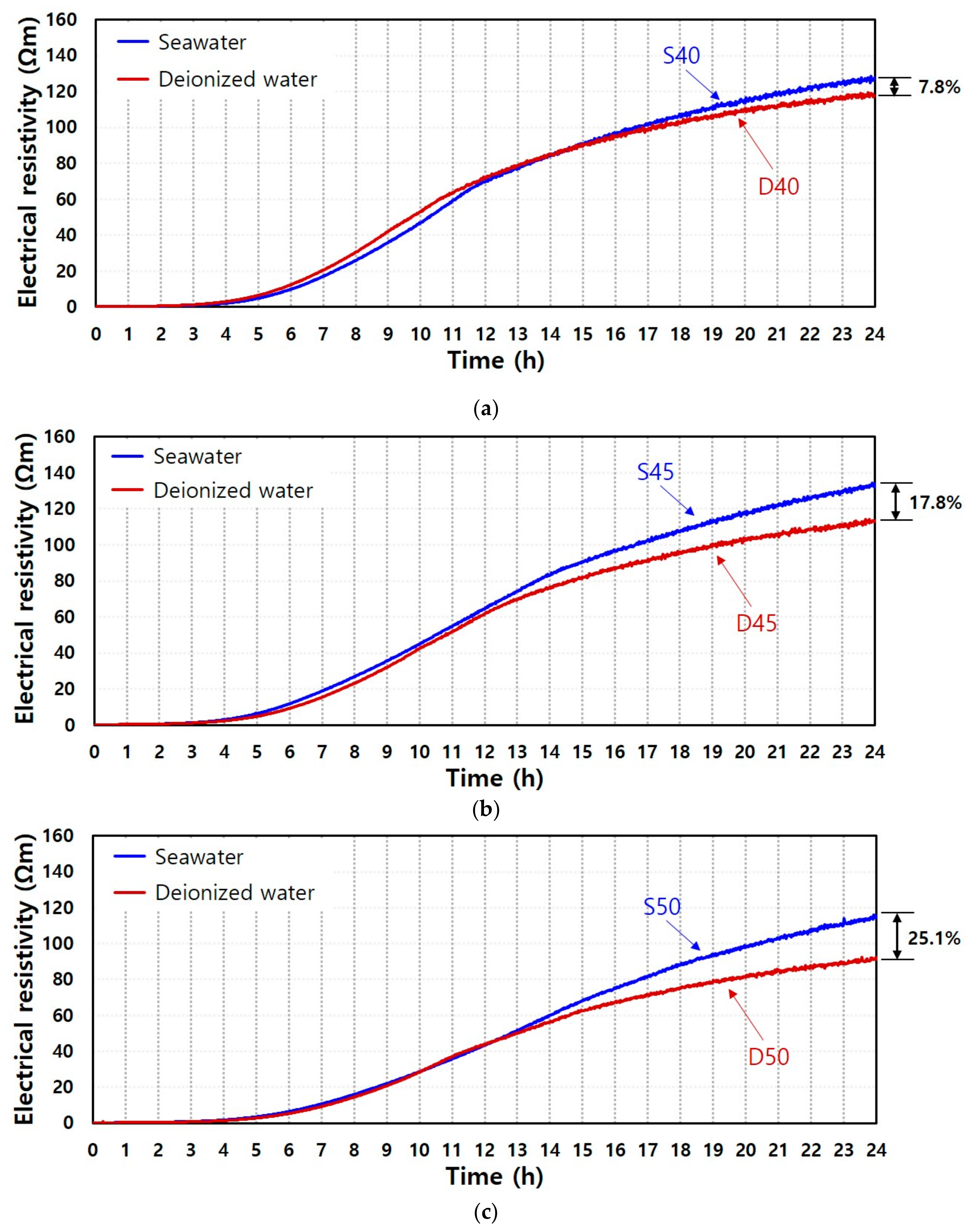
3.1. Electrical Resistivity
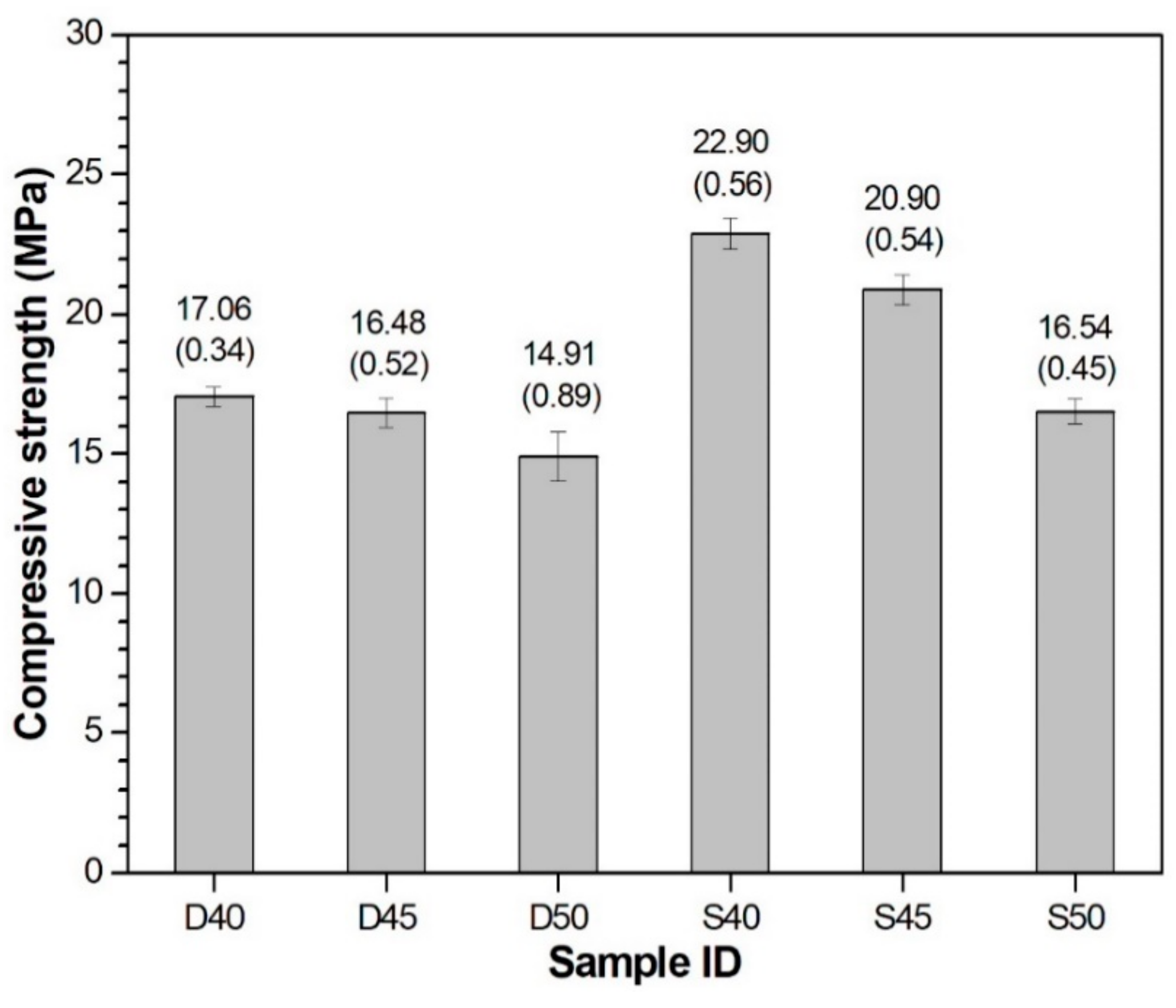
3.2. Compressive Strength
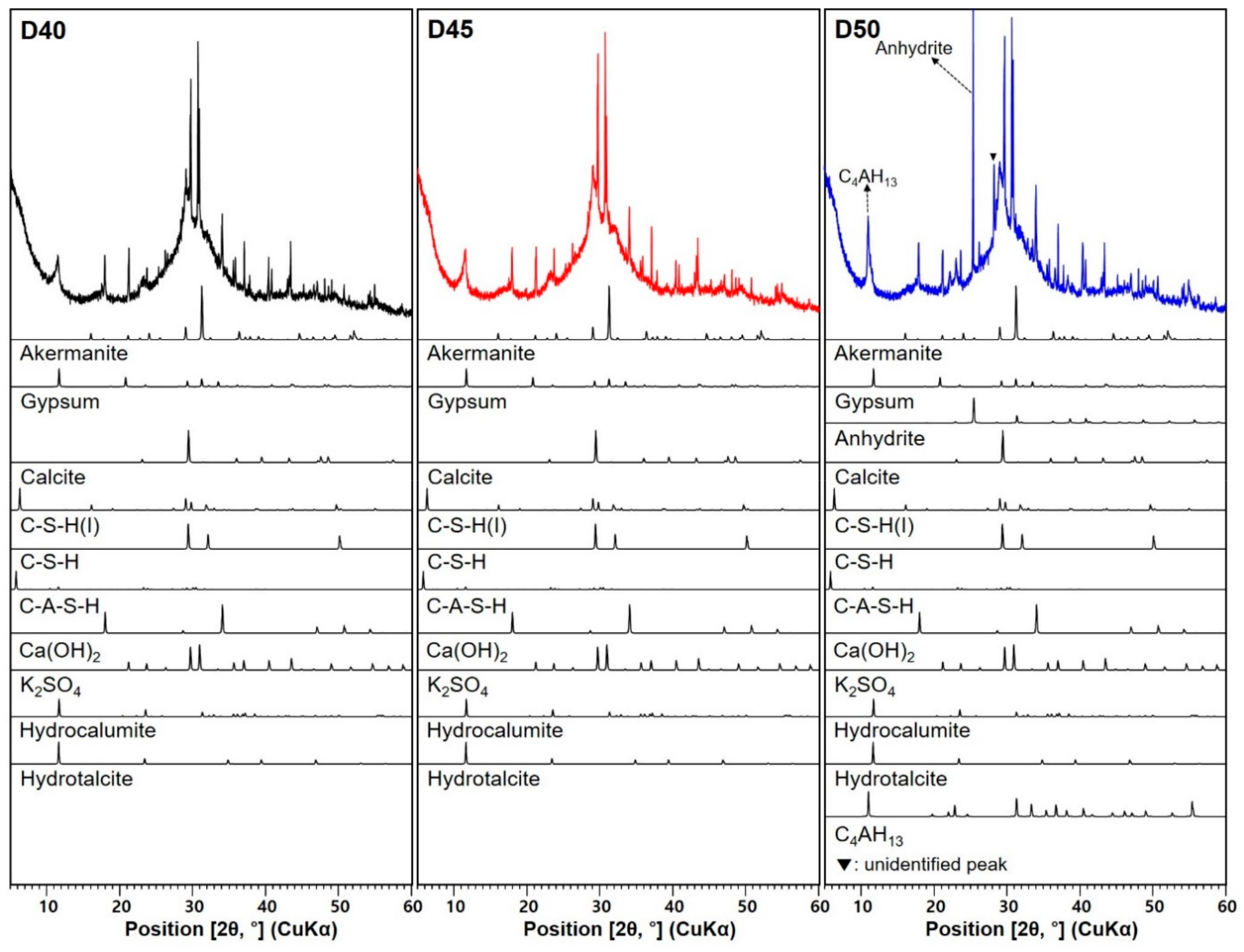
3.3. XRD Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Osborne, G.J. Durability of Portland blast-furnace slag cement concrete. Cem. Concr. Compos. 1999, 21, 11–21. [Google Scholar] [CrossRef]
- De Domenico, D.; Faleschini, F.; Pellegrino, C.; Ricciardi, G. Structural behavior of RC beams containing EAF slag as recycled aggregate: Numerical versus experimental results. Constr. Build. Mater. 2018, 171, 321–337. [Google Scholar] [CrossRef]
- Menéndez, G.; Bonavetti, V.; Irassar, E.F. Strength development of ternary blended cement with limestone filler and blast-furnace slag. Cem. Concr. Compos. 2003, 25, 61–67. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag concrete to carbonation. Cem. Concr. Res. 2001, 31, 1277–1283. [Google Scholar] [CrossRef]
- Criado, M.; Aperador, W.; Sobrados, I. Microstructural and Mechanical Properties of Alkali Activated Colombian Raw Materials. Materials 2016, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.M.; Jiang, W.; Silsbee, M.R. Chloride diffusion in ordinary, blended, and alkali-activated cement pastes and its relation to other properties. Cem. Concr. Res. 2000, 30, 1879–1884. [Google Scholar] [CrossRef]
- Levita, G.; Marchetti, A.; Gallone, G.; Princigallo, A.; Guerrini, G.L. Electrical properties of fluidified Portland cement mixes in the early stage of hydration. Cem. Concr. Res. 2000, 30, 923–930. [Google Scholar] [CrossRef]
- Xiao, L.; Li, Z. Early-age hydration of fresh concrete monitored by non-contact electrical resistivity measurement. Cem. Concr. Res. 2008, 38, 312–319. [Google Scholar] [CrossRef]
- Ylmén, R.; Jäglid, U.; Steenari, B.-M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Yim, H.J.; Lee, H.; Kim, J.H. Evaluation of mortar setting time by using electrical resistivity measurements. Constr. Build. Mater. 2017, 146, 679–686. [Google Scholar] [CrossRef]
- Yim, H.J.; An, Y.-K.; Kim, J.H. Water depercolation of setting cement paste evaluated by diffuse ultrasound. Cem. Concr. Compos. 2016, 71, 10–19. [Google Scholar] [CrossRef]
- Arya, C.; Buenfeld, N.R.; Newman, J.B. Factors influencing chloride-binding in concrete. Cem. Concr. Res. 1990, 20, 291–300. [Google Scholar] [CrossRef]
- Topçu, İ.B.; Uygunoğlu, T.; Hocaoğlu, İ. Electrical conductivity of setting cement paste with different mineral admixtures. Constr. Build. Mater. 2012, 28, 414–420. [Google Scholar] [CrossRef]
- Zuo, Y.; Zi, J.; Wei, X. Hydration of cement with retarder characterized via electrical resistivity measurements and computer simulation. Constr. Build. Mater. 2014, 53, 411–418. [Google Scholar] [CrossRef]
- Rajabipour, F.; Weiss, J. Electrical conductivity of drying cement paste. Mater. Struct. 2007, 40, 1143–1160. [Google Scholar] [CrossRef]
- McCarter, W.J.; Curran, P.N. The electrical response characteristics of setting cement paste. Mag. Concr. Res. 1984, 36, 42–49. [Google Scholar] [CrossRef]
- Xiao, L.-z.; Li, Z.-j.; Wei, X.-s. Selection of superplasticizer in concrete mix design by measuring the early electrical resistivities of pastes. Cem. Concr. Compos. 2007, 29, 350–356. [Google Scholar] [CrossRef]
- Heikal, M.; Morsy, M.S.; Aiad, I. Effect of treatment temperature on the early hydration characteristics of superplasticized silica fume blended cement pastes. Cem. Concr. Res. 2005, 35, 680–687. [Google Scholar] [CrossRef]
- Schwarz, N.; DuBois, M.; Neithalath, N. Electrical conductivity based characterization of plain and coarse glass powder modified cement pastes. Cem. Concr. Compos. 2007, 29, 656–666. [Google Scholar] [CrossRef]
- Koleva, D.A.; Copuroglu, O.; van Breugel, K.; Ye, G.; de Wit, J.H.W. Electrical resistivity and microstructural properties of concrete materials in conditions of current flow. Cem. Concr. Compos. 2008, 30, 731–744. [Google Scholar] [CrossRef]
- Humad, M.A.; Habermehl-Cwirzen, K.; Cwirzen, A. Effects of Fineness and Chemical Composition of Blast Furnace Slag on Properties of Alkali-Activated Binder. Materials 2019, 12, 3447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haha, M.B.; Saout, G.L.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Park, H.; Jeong, Y.; Jun, Y.; Jeong, J.-H.; Oh, J.E. Strength enhancement and pore-size refinement in clinker-free CaO-activated GGBFS systems through substitution with gypsum. Cem. Concr. Compos. 2016, 68, 57–65. [Google Scholar] [CrossRef]
- Ground Granulated Blast-Furnace Slag For Use in Concrete; KS F 2563:2009; Korean Standard Association: Seoul, Korea, 2009.
- Jun, Y.; Oh, J.E. Microstructural characterization of alkali-activation of six Korean Class F fly ashes with different geopolymeric reactivity and their zeolitic precursors with various mixture designs. KSCE J. Civ. Eng. 2015, 19, 1775–1786. [Google Scholar] [CrossRef]
- Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency; ASTM C305-14; ASTM International: West Conshohocken, PA, USA, 2014.
- Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens); ASTM C109/C109M-01; ASTM International: West Conshohocken, PA, USA, 2016.
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- Etxeberria, M.; Gonzalez-Corominas, A.; Pardo, P. Influence of seawater and blast furnace cement employment on recycled aggregate concretes’ properties. Constr. Build. Mater. 2016, 115, 496–505. [Google Scholar] [CrossRef]
- Allmann, R.; Hinek, R. The introduction of structrue types into the inorganic crystal structure database ICSD. Acta Crystallogr. Sect. A 2007, 63, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Belsky, A.; Hellenbrandt, M.; Karen, V.L.; Luksch, P. New developments in the Inorganic Crystal Structure Database (ICSD): Accessibility in support of materials research and design. Acta Crystallogr. Sect. B 2002, 58, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Altan, E.; Erdoğan, S.T. Alkali activation of a slag at ambient and elevated temperatures. Cem. Concr. Compos. 2012, 34, 131–139. [Google Scholar] [CrossRef]
- Wilding, C.R.; McHugh, G. Hydration of Blast Furnace Slag Cements; AEA Technology: Didcot, UK, 1987. [Google Scholar]
- Richardson, I.G.; Li, S. Composition and structure of an 18-year-old 5 M KOH-activated ground granulated blast-furnace slag paste. Constr. Build. Mater. 2018, 168, 404–411. [Google Scholar] [CrossRef]
- Song, S.; Jennings, H.M. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Schneider, J.; Cincotto, M.A.; Panepucci, H. 29Si and 27Al high-resolution NMR characterization of calcium silicate hydrate phases in activated blast-furnace slag pastes. Cem. Concr. Res. 2001, 31, 993–1001. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.; Peethamparan, S. Very early-age reaction kinetics and microstructural development in alkali-activated slag. Cem. Concr. Compos. 2015, 55, 91–102. [Google Scholar] [CrossRef]
- Jun, Y.; Yoon, S.; Oh, E.J. A Comparison Study for Chloride-Binding Capacity between Alkali-Activated Fly Ash and Slag in the Use of Seawater. Appl. Sci. 2017, 7, 971. [Google Scholar] [CrossRef] [Green Version]
- Jun, Y.; Oh, E.J. Use of Gypsum as a Preventive Measure for Strength Deterioration during Curing in Class F Fly Ash Geopolymer System. Materials 2015, 8, 3053–3067. [Google Scholar] [CrossRef] [Green Version]
- Grover, K.; Komarneni, S.; Katsuki, H. Synthetic hydrotalcite-type and hydrocalumite-type layered double hydroxides for arsenate uptake. Appl. Clay Sci. 2010, 48, 631–637. [Google Scholar] [CrossRef]
- El-Halwagi, M.M.; Linninger, A.A. Design for Energy and the Environment. Proceedings of the Senventh International Conference on the Foundations of Computer-Aided Process Design; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 223–231. [Google Scholar]
- Gu, J.-E.; Jun, B.-M.; Kwon, Y.-N. Effect of chlorination condition and permeability of chlorine species on the chlorination of a polyamide membrane. Water Res. 2012, 46, 5389–5400. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; de Gutierrez, R.M. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]



| CaO | SiO2 | Al2O3 | K2O | SO3 | Fe2O3 | MgO | Na2O | TiO2 | MnO | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| 43.02 | 32.20 | 13.59 | 0.61 | 4.77 | 0.51 | 3.68 | 0.28 | 0.70 | 0.46 | 0.19 |
| Ca2+ | K+ | Mg2+ | Na+ | Cl– | SO42− |
|---|---|---|---|---|---|
| 380 | 380 | 1200 | 10,000 | 17,000 | 1900 |
| Sample Label | a/b | Activator (g) | Binder (g) | |
|---|---|---|---|---|
| 4 M KOH in Deionized Water | 4 M KOH in Seawater | GGBFS | ||
| D40 | 0.4 | 1000 | - | 2500 |
| S40 | - | 1000 | 2500 | |
| D45 | 0.45 | 1125 | - | 2500 |
| S45 | - | 1125 | 2500 | |
| D50 | 0.5 | 1250 | - | 2500 |
| S50 | - | 1250 | 2500 | |
| Sample ID | Rising Time (h) | Increasing Ratio (Ωm/h) |
|---|---|---|
| D40 | 2.78 | 5.67 |
| D45 | 2.95 | 5.43 |
| D50 | 3.55 | 4.40 |
| S40 | 3.10 | 6.24 |
| S45 | 2.97 | 6.30 |
| S50 | 3.32 | 5.51 |
| Sample ID | D40 | D45 | D50 |
|---|---|---|---|
| Crystalline Phase | |||
| Akermanite * (PDF #35−0592) | O | ≈ | ≈ |
| Gypsum * (PDF #21−0816) | O | ↓ | ↓↓ |
| Anhydrite * (PDF #37−1496) | X | X | O |
| Calcite * (PDF #47−1743) | O | ≈ | ≈ |
| C–S–H(I) (PDF #29−0331) | O | ↓ | ↑(similar to D40) |
| C–S–H (PDF #33−0306) | O | ↑ | ≈ |
| C–A–S–H (PDF #46−1405) | O | ↓ | ↑(similar to D40) |
| Ca(OH)2 (PDF #44−1481) | O | ≈ | ↑ |
| K2SO4 (PDF #01−0939) | O | ↑ | ↑↑ |
| Hydrocalumite (PDF #14−0083) | O | ≈ | ↓ |
| Hydrotalcite (ICSD collection #6296) | O | ≈ | ↓ |
| C4AH13 (PDF #11−0203) | X | X | O |
| Sample ID | S40 | S45 | S50 |
|---|---|---|---|
| Crystalline Phase | |||
| Akermanite * (PDF #35−0592) | O | ≈ | ≈ |
| Gypsum * (PDF #21−0816) | O | ↓ | ↓↓ |
| Anhydrite * | X | X | X |
| Calcite * (PDF #47−1743) | O | ≈ | ≈ |
| C–S–H(I) (PDF #29−0331) | O | ↓ | ↓↓ |
| C–S–H (PDF #33−0306) | O | ↓ | ≈ |
| C–A–S–H (PDF #46−1405) | O | ≈ | ↓ |
| Ca(OH)2 (PDF #44−1481) | O | ↓ | ≈ |
| K2SO4 (PDF #01−0939) | O | ↑ | ↑↑ |
| Hydrocalumite (PDF #14−0083) | O | ↓ | ↓↓ |
| Hydrotalcite (ICSD collection #6296) | O | ↓ | ↓↓ |
| Cl-bearing hydrocalumite (ICSD collection #088617) | O | ↓ | ↓↓ |
| AlOCl (PDF #74−1864) | O | ↓ | ↓↓ |
| Aluminum chloride hydrate (ICSD collection #026139) | O | ↓ | ↓↓ |
| C4AH13 (PDF #11−0203) | X | O | X |
| Gismondine (PDF #81−1858) | X | X | O |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, Y.; Bae, Y.H.; Shin, T.Y.; Kim, J.H.; Yim, H.J. Alkali-Activated Slag Paste with Different Mixing Water: A Comparison Study of Early-Age Paste Using Electrical Resistivity. Materials 2020, 13, 2447. https://doi.org/10.3390/ma13112447
Jun Y, Bae YH, Shin TY, Kim JH, Yim HJ. Alkali-Activated Slag Paste with Different Mixing Water: A Comparison Study of Early-Age Paste Using Electrical Resistivity. Materials. 2020; 13(11):2447. https://doi.org/10.3390/ma13112447
Chicago/Turabian StyleJun, Yubin, Young Hwan Bae, Tae Yong Shin, Jae Hong Kim, and Hong Jae Yim. 2020. "Alkali-Activated Slag Paste with Different Mixing Water: A Comparison Study of Early-Age Paste Using Electrical Resistivity" Materials 13, no. 11: 2447. https://doi.org/10.3390/ma13112447
APA StyleJun, Y., Bae, Y. H., Shin, T. Y., Kim, J. H., & Yim, H. J. (2020). Alkali-Activated Slag Paste with Different Mixing Water: A Comparison Study of Early-Age Paste Using Electrical Resistivity. Materials, 13(11), 2447. https://doi.org/10.3390/ma13112447






