Abstract
This work concerns the hydration mechanism of calcium zirconium aluminate as a ternary compound appearing in the CaO-Al2O3-ZrO2 diagram besides the calcium aluminates commonly used as the main constitutes of calcium aluminate cements (CACs). Moreover, a state-of-the-art approach towards significant changes in hydraulic properties was implemented for the first time in this work, where the effect of structural modification on the hydration behavior of calcium zirconium aluminate was proved by XRD, 27Al MAS NMR and SEM-EDS. The substitution of Sr2+ for Ca2+ in the Ca7ZrAl6O18 lattice decreases the reactivity of Sr-substituted Ca7ZrAl6O18 in the presence of water. Since the original cement grains remain unhydrated up to 3 h (Ca7ZrAl6O18) or 72 h (Sr1.25Ca5.75ZrAl6O18) of curing period in the hardened cement paste structures, strontium can be considered as an inhibition agent for cement hydration. The complete conversion from anhydrous 27AlIV to hydrated 27AlVI species was achieved during the first 24 h (Ca7ZrAl6O18) or 7 d(Sr1.25Ca5.75ZrAl6O18) of hydration. Simultaneously, the chemical shift in the range of octahedral aluminum from ca. 4 ppm to ca. 6 ppm was attributed to the transformation of the hexagonal calcium aluminate hydrates and Sr-rich (Sr,C)3AH6 hydrate into the cubic phase Ca-rich (Sr,C)3AH6 or pure C3AH6 in the hardened Sr-doped cement paste at the age of 7 d. The same 27Al NMR chemical shift was detected at the age of 24 h for the reference hardened undoped Ca7ZrAl6O18 cement paste.
1. Introduction
Calcium aluminate cements (CACs) [1,2,3] and other related cementitious systems [4,5,6,7] are believed to play an important role in a wide range of specialist are as from some construction areas and civil engineering, to refractory materials industry, due to their ability to gain strength rapidly in the initial days after casting and to withstand aggressive environments and high temperatures. The temperature–time weight ratio of water-to-cement (the w/c ratio) dependencies of the cement hydration processes have been widely investigated so far and are presented elsewhere in detail [8,9,10]. It must be made clear at this stage that both temperature and a weight ratio of water-to-cement (the w/c ratio) affect the performances of calcium aluminate cements, especially at an early age. The phase composition, microstructure and other properties of the cement pastes can easily be designed by choosing appropriate curing parameters. Nevertheless, upon curing of CAC-based paste at not adequately controlled, ambient conditions, these materials are very sensitive to humidity, CO2, temperature and time. Due to the fact of not being able to predict their long-term behavior makes them practically not suitable for constructions but suitable for building chemistry. Nevertheless, the high-early-heat and high-early-strength gain makes CACs attractive, especially in the winter months and/or when rapid repairs are needed. The hydration product formation in CACs containing mainly monocalcium aluminate (CaAl2O4; CA) and monocalcium di aluminate (CaAl4O7; CA2) is characterized by the dependence of C−A−H* (C = CaO, A = Al2O3, H = H2O) phases on temperature. At a temperature below ca. 15 °C, calcium aluminates are hydrated to form metastable CAH10. At room temperature, the hydration process proceeds through the formation of other metastable phases C2AH8 and/or C4AH13–19 (hexagonal hydrates). At higher temperatures (above 28 °C), the metastable hydrates will spontaneously convert to the cubic hydrogarnet C3AH6. Furthermore, according to insights into hydration of CACs, the hydration products during long hydration shall be presented as follows: C3AH6 + AH3. The 10-year results, which have been added to existing results on the hydration of calcium aluminate compounds, are of interest as showing the CaO-Al2O3-ZrO2 [4,6,11] as an interesting alternative to CACs-based materials. This can be attributed to the fact, that this category of special cement which contains both hydraulic phases (CA, CA2, C12A7 and C7A3Z*), and Zr-bearing compounds (CZ and Z) [4] is synthesized by one environmentally friendly and effective technological process. This fact makes the attention-getter of a C-A-Z cements’ users more important than those of other CACs users and worth considering from the point of view of economics. The principal interest here is concerned with the C7A3Z area including crystal structure, hydration behavior of C7A3Z and its solid solutions with SrO and BaO, hydration products and carbonation processes in cement paste structures [12,13]. Special interest was given to strontium. Because strontium is chemically closely related to calcium, it is easily introduced as a natural substitute for calcium in aluminate phases. Strontium has been shown to affect the hydration behavior of calcium zirconium aluminate cement [5,13]. Furthermore, strontium-containing cements attract the attention of materials scientists due to their higher refractory properties, increased resistance to thermal shock, increased resistance to chemical aggressive solutions and possible applications as special binders for shielding constructions in nuclear power plants [14]. Although this approach is unique, there are other approaches to affecting the hydration behavior of aluminates-bearing CACs and related materials. Results from other studies indicate that the metal chloride and nitrate salts are known in CAC science as agents affecting the CAC hydration behavior [15,16]. The exact nature of this behavior is different for alkali metal salts (NaCl, KCl, RbCl, CsCl, LiCl), alkaline earth metal salts (MgCl2∙6H2O, CaCl2, SrCl2∙6H2O and BaCl2∙2H2O) and transition metal salts (MnCl2∙H2O, CoCl2∙6H2O, CuCl2∙2H2O and ZnCl2). Generally, the addition of alkali metal salts accelerates the setting time of CAC, whereas the transition metal salts can be widely used as retarders. The effect of alkaline earth metal salts on setting the behavior of CAC is determined by the amount of addition [15]. Another approach in the acceleration effect is the addition of chlorides and nitrates of Li(I), Cr(III), Zn(II) and Cr(VI) (chromate), whereas Pb(II) and Cu(II) retarded the hydration [16].
Many various techniques including XRD, FT-IR, Raman spectroscopy, DSC-TG-EGA(MS), SEM-EDS, isothermal calorimetry and EIS were being accepted in this research. It is also well known that solid-state nuclear magnetic resonance (ssNMR) spectroscopy is an effective tool for the characterization of cement paste at the atomic scale in different stages of its aging. The application of NMR to monitor the progress of CACs hydration has a long history and can be found elsewhere [17,18,19,20]. In cement chemistry, four-fold coordinated (tetrahedral)IVAl sites and six-fold coordinated (octahedral)VIAl sites typically resonate within the regions 50–80 ppm and 0–20 ppm, respectively. The resonances assigned to five-coordinated VAl and highly distorted tetrahedral Al environments have been observed in the region 20–50 ppm [18,21,22]. For example, the 27Al MAS NMR spectrum of CA shows a peak maximum at ca. 80 ppm and a shoulder at 76 ppm due to six crystal ographically different aluminum tetrahedral sites [19,23,24,25]. For another example, the 27Al MAS NMR spectrum of CA2 exhibits the peaks between 50 and 75 ppm due to the two distinct 27Al environments [18,25]. On the other hand, calcium aluminate hydrates (CAH10, C2AH8, C4AH13-19, C3AH6), originating from the hydration process of CAC clinker phases, are octahedrally coordinated Al(OH)6 and cause a 27Al MAS NMR signal at about 0 ppm. Hence, 27Al MAS NMR provides the relative changes of tetrahedral and octahedral Al sites of the hydrating cement paste at different stages of the curing. The resonance position from an octahedral 27Al NMR signal varies slightly depending on the types of calcium aluminate hydrates present [19]. The resonance at ca. 12.36 ppm is due to tricalcium aluminate hexahydrate C3AH6 [18,20], at ca. 10.2 ppm due to CAH10 and C4AH13 [18,19,20] and at ca. 10.3 ppm due to C2AH8 [17].
The aim of this work is to implement the NMR technique to monitor the progress of hydration of both undoped- and strontium-doped calcium zirconium aluminate cement. Hence, the influence of structural modification with SrO on the hydraulic activity of Ca7ZrAl6O18 phase has been demonstrated. This work fills in a significant gap in the literature on the exploiting ssNMR spectroscopy to probe the early stages of hydration of new types of cements belonging to the CaO-Al2O3-ZrO2 and CaO-SrO-Al2O3-ZrO2 systems.
2. Experimental Procedure
2.1. Synthesis and Phase Identification
Low-cost synthesis of Ca7ZrAl6O18, Sr1.25Ca5.75Al6O18 and SrAl2O4 cements via the solid-state reactive sintering technique was employed using the stoichiometric amount of the cationic ratios of Ca:Zr:Al = 7:1:6, Sr:Ca:Zr:Al = 1.25:5.75:1:6 and Sr:Al = 1:2, respectively. Strontium was incorporated into Ca7ZrAl6O18 by replacing 1.25 atoms of calcium, since the maximum level of doping was experimentally established. Both Ca7ZrAl6O18 and SrAl2O4 cements were synthesized as reference materials to obtain pure C-A-H and Sr-A-H phases. Starting raw materials were reagent-grade CaCO3 (99.9%, POCH), SrCO3 (98.0%, Merck), Al2O3 (99.0%, Acros Organics) and ZrO2 (98.5%, Acros Organics). The 100 g mixtures of substrates were homogenized for 2 h, pressed into cylindrical pellets at pressures of 50 MPa and calcined at 1000 °C for 10 h (Sr-free sample) or at 1300 °C for 10 h (Sr-containing samples) in air. Phase pure cement clinker minerals were made from mixtures of prereacted powders via grinding, pressing and sintering at 1420 °C (Ca7ZrAl6O18 and Sr1.25Ca5.75Al6O18) and 1550 °C (SrAl2O4) for 20 h in air.
Phase identification for the sintered samples was carried out using X-ray diffraction (XRD, PANalytical, Malvern PANalytical, Malvern, UK) on a ProPANalytical X’Pert X-ray diffractometer, with Cu Kα radiation (λ = 0.15418 nm), with 0.02° per step and 3s time per step (2theta range from 5° to 45°).
The NMR spectra were recordedat room temperature on Bruker Avance III 400WB (9.4T) spectrometer, (Bruker BioSpin, Rheinstetten, Germany) using 4 mm MAS (Magic Angle Spinning), dual-channel (1H/BB) probe-head, operating at a resonance frequency of 104.26 MHz for 27Al. The sample was spun at a MAS frequency of 8 kHz in the rotors made of zirconium dioxide (4 mm). 32 K data points and 1024 scans FIDs were accumulated with a Single Pulse Excitation (SPE) pulse sequence using the observed 90° pulse (27Al) set at 6.0 us with a relaxation delay of 200 ms. Note no proton decoupling was applied during the experiment. Prior to Fourier transformation, the data were zero-filled twice and 80 Hz apodization filter was applied. The 27Al chemical shifts were referenced using a sample of AlCl3·6H2O in 1M solution as an external reference (0 ppm).
The microstructures of fracture surfaces of hydrated cement pastes were investigated using a scanning electron microscope (SEM, FEI Nova Nano SEM 200, Kyoto, Japan.). The chemical compositions of the samples were determined with electron-probe microanalysis using an energy-dispersive X-ray spectrometer (EDAX, Sapphire Si(Li) EDS detector, Mahwah, NJ, USA).
2.2. Preparation and Treatment of Cement Paste
Comparison study of hydration characteristics between Ca7ZrAl6O18, Sr1.25Ca5.75Al6O18 and SrAl2O4 cements were determined for cement pastes prepared with water-to-cement (w/c) ratios of 1.0 or 0.5. The water-to-cement ratio of 0.5 was applied to achieve plastic properties without any undesirable sedimentation of neat SrAl2O4 paste. Whereas, both Ca7ZrAl6O18 and Sr1.25Ca5.75Al6O18 cements require w/c = 1.0 to obtain the well-homogenized cement pastes without any undesirable phenomena of sedimentation. The cement powders which were obtained by grinding the sintered pellets and necessary mass of water were mixed together in a glass beaker to obtain three homogeneous neat cement pastes. Each neat cement paste was then placed in a polyethylene bag and sealed until 14 d at 50 °C. According to Litwinek and Madej [5], the optimal synthesis temperature for C3AH6 from different precursors through hydration is suggested to be 50 °C. Moreover, this period for curing cement pastes was accepted to attain the maximum degree of hydration, as concluded from the previous studies [13]. Moreover, as it was previously mentioned by Garcés et al. [26] and Zhang et al. [27], at temperatures as high as 60 °C, only the cubic phase and the gibbsite appear in the calcium aluminates-based cement pastes. At 24 h and 7 d, the microstructure of cement pastes was investigated by SEM. Acetone quenching was used to stop hydration at 15 min, 0.5 h, 1 h, 2 h, 3 h, 24 h, 48 h, 72 h, 7 d and 14 d (Table 1). The use of cold acetone, aiming to withdraw free water and inhibit further reactions within cement paste is known from the Ref. [28]. Cold acetone is related to acetone stored under laboratory conditions. Quenched pastes were characterized by XRD and 27Al MAS NMR (Bruker BioSpin, Rheinstetten, Germany), according to the procedures presented in Section 2.1.

Table 1.
Cement paste formulations, curing conditions and details of sample designation.
3. Results and Discussion
3.1. X-ray Diffraction Analysis of Special Cements Hydration
According to X-ray diffraction analysis, the cement clinkers synthesized by the solid-state reactive sintering technique were all crystalline, single-phase aluminate phases Ca7ZrAl6O18, Sr-doped Ca7ZrAl6O18 and SrAl2O4. The positions of the characteristic diffraction peaks in the XRD patterns of the Ca7ZrAl6O18 (Sample C) and SrAl2O4 (Sample B) cement clinkers have a strong agreement with the standards JCPDS No. 98-018-2622 and JCPDS No. 98-016-0296, respectively. This confirms that the powders are mainly composed of Ca7ZrAl6O18 and SrAl2O4, respectively. As expected, the slight shift in diffraction peaks towards lower 2θ value in the XRD pattern of Sr1.25Ca5.75ZrAl6O18 cement clinker confirms an increase in lattice parameter of Sr-doped Ca7ZrAl6O18 solid solution. Since no secondary phases containing Sr were detected, Sr was recognized as fully incorporated into the Ca7ZrAl6O18 structure.
Compared with the XRD patterns of unhydrated phases, the decreasing intensity of peaks corresponding to Sr1.25Ca5.75ZrAl6O18 and Ca7ZrAl6O18, and new peaks of low intensity corresponding to hydration products formation were recorded using XRD (Figure 1, Figure 2 and Figure 3). A low degree of hydrates crystallinity was indicated by a poor XRD pattern, especially in the early stage of hydration. Moreover, the hydration processes result in a corresponding change in the XRD patterns of the initial cement clinker phases and formation of amorphous material, besides the crystalline hydrates, as it can be concluded from the severe intensity reduction and peaks broadening in the XRD pattern.
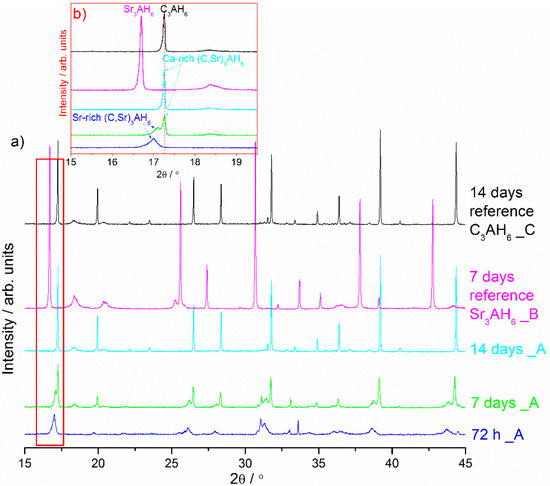
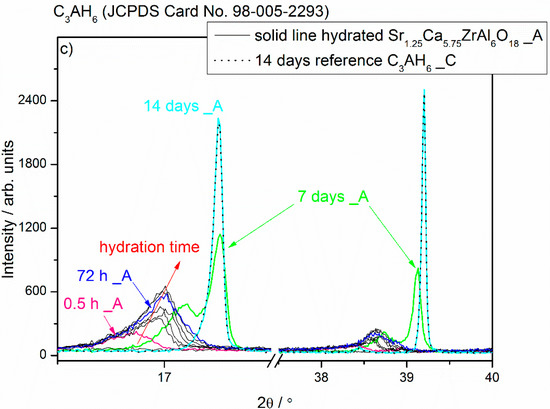
Figure 1.
X-ray diffraction patterns of the Sr1.25Ca5.75ZrAl6O18 cement paste (Sample A) at different curing periods. (a,b) contains lines of two reference materials, i.e., Sr3AH6 synthesized through hydration from SrAl2O4 cement (Sample B) and C3AH6 synthesized through hydration from Ca7ZrAl6O18 cement (Sample C). (c) contains a line (dot line) of the reference C3AH6 formed at 14 d (Sample C).
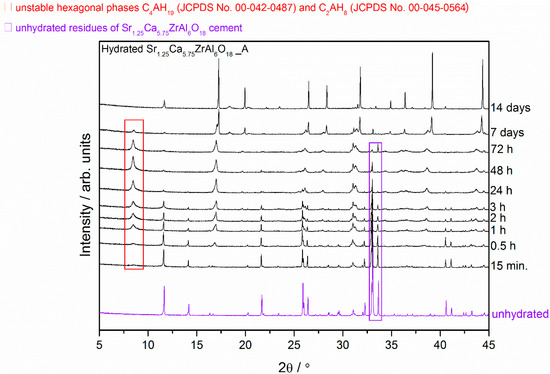
Figure 2.
X-ray diffraction patterns of the Sr1.25Ca5.75ZrAl6O18 cement paste (Sample A) at different curing periods from 15 min to 14 d compared with the XRD pattern of the unhydrated compound.
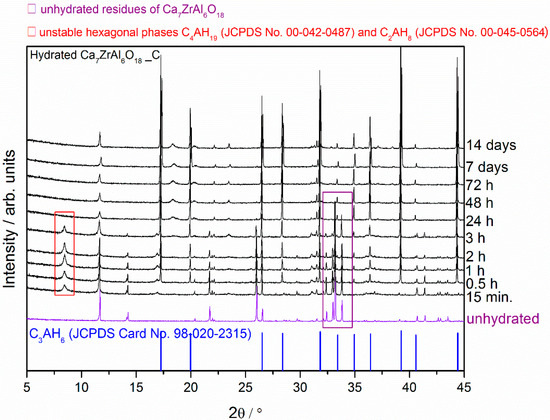
Figure 3.
X-ray diffraction patterns of the Ca7ZrAl6O18 cement paste (Sample C) at different curing periods from 15 min to 14 d compared with the XRD pattern of the unhydrated compound.
The results of powder X-ray diffraction patterns evaluation show the progress of Sr1.25Ca5.75ZrAl6O18 hydration in cement paste between 15 min and 14 d of curing which are more or less similar at an early stage of hydration (up to 72 h) Figure 1a–c. The XRD profiles of the cement pastes hydrated between 0.5 and 72 h demonstrate diffraction peak at ca. 17.00° 2θ which can now be interpreted as belonging to Sr-rich (Sr,C)3AH6 hydrate (Figure 1c). Hence, this XRD peak located at the position of 2θ = 17.00 which belongs to Sr-rich (Sr,C)3AH6 needs to be considered between the reference C3AH6 synthesized through Ca7ZrAl6O18 hydration and other reference sample Sr3AH6 synthesized from SrAl2O4 precursor through hydration. As is evident from this figure, the formation of the intermediate Sr-rich (Sr,C)3AH6 hydrate precedes the formation of the stable Ca-rich (Sr,C)3AH6 hydrate at 7 d of curing. In this sample, two isostructural compounds with a hydrogarnet type crystal lattice were present. The position of the lower-intensity XRD line at ca. 17.00° 2θ is situated between lines belonging to pure phases Sr3AH6 (Sample B) and C3AH6 (Sample C). The second position of the higher intensity XRD line at ca. 17.26° 2θ is similar to that found for reference C3AH6 (Figure 1a–c). In addition, it is worth noting that the Sr-rich (Sr,C)3AH6 exists in the hardened cement paste between 0.5 h and 7 d of curing. This phase disappeared after longer curing times and became replaced by Ca-rich (Sr,C)3AH6 or C3AH6. This work has successfully shown the existence of the solid solution of strontium in the tricalcium hydrate C3AH6 lattice by direct verification using XRD. By reason of structural modification of C3AH6 through ionic substitution, the lattice parameter of the cubic phase was increased and the slight shift in XRD peaks belonging to (Sr,C)3AH6 solid solution towards lower 2θ value was observed (Figure 1b). This increase in the lattice parameter was due to the size of the ionic radius of Sr2+ (132 pm) which is bigger than the ionic radius of Ca2+ (114 pm).
A brief summary of XRD results is given as Figure 2 and Figure 3. This overview XRD spectra recorded from the Sr1.25Ca5.75ZrAl6O18 cement paste (Sample A) at different curing periods from 15 min to 14 d showed a progressive reduction in the peaks associated with Sr1.25Ca5.75ZrAl6O18 due to its hydration process, which led to the formation of hydration products (Figure 2). The cement paste at the age of 15 min is a mixture of the unhydrated phase and amorphous or poorly crystalline hexagonal hydrates, whereas the cement paste at the age between 0.5 h and 72 h contained a mixture of the Sr-rich (Sr,C)3AH6 cubic phase, hexagonal hydrates and the still unhydrated residues of the Sr1.25Ca5.75ZrAl6O18 cement grains. It should be noted that the positions of the XRD peaks of C4AH19 (JCPDS No. 00-042-0487; h k l = 0 0 6, d = 10.64350 Å, 2θ = 8.301°, I = 100%) are coincident well with those belonging to C2AH8 (JCPDS No. 00-045-0564, h k l = 0 0 6, d = 10.81270 Å, 2θ = 8.170°, I = 100%). Therefore, it is often difficult to clearly differentiate between C4AH19 and C2AH8 in the XRD patterns, as is clearly demonstrated with a red rectangle (□) in Figure 2. However, at 7d second adjacent cubic phase, Ca-rich (Sr,C)3AH6 or pure C3AH6, exists together with the initially formed cubic phase Sr-rich (Sr,C)3AH6 and some residues of the hexagonal hydrates. As a general trend at the age of 14 d, XRD pattern of cement paste achieved profile similar to that of pure C3AH6 (Figure 1c and Figure 2) without any metastable hydrates and unhydrated cement residues, i.e., unhydrated cement clinker mineral Sr1.25Ca5.75ZrAl6O18.
The overview XRD spectra recorded from the reference Ca7ZrAl6O18 cement paste (Sample C) at different curing periods from 15 min to 14 d is shown in Figure 3. The hexagonal hydrates exist with the still unhydrated residues of the Ca7ZrAl6O18 in cement paste between 15 min and 3 h of curing period, whereas C3AH6 hydrated phase is formed at the curing age of 0.5 h. At the age of 24 h, the XRD pattern of the cement pastes exhibits profile similar to that of pure C3AH6 without any traces of unhydrated cement particles Ca7ZrAl6O18 and metastable hydrates.
From the X-ray diffraction results, it seems obvious that strontium doping affects the hydration behavior of the cement clinker mineral phase Ca7ZrAl6O18, and leads to changes in the hydration products properties. There is a relationship between the proportion of residual unhydrated cement particles and the properties of the particular cement clinker mineral phases involved. After 24 h of curing at 50 °C, where the hardened cement paste (Sample C) consists primarily of C3AH6, the original Ca7ZrAl6O18 cement particles are no longer evident. In the Sr-doping of Ca7ZrAl6O18 case, there is inhibition of hydration, and the Sr1.25Ca5.75ZrAl6O18 cementitious particles exist in the hardened cement paste up to 72 h (Sample A). This material would need to cure over 72 h to reach complete hydration. Hence, XRD data for 0.5 h–7 d materials containing strontium indicates the appearance of additional peaks adjacent to each of the reference C3AH6 lines caused by the presence of an additional Sr-rich cubic phase. These results confirmed the strontium retention by calcium zirconium aluminate cement paste through the chemical bonding to C-A-H in the hydrated phase. The presence of strontium in the C-A-H matrix is also known to delay the transformation of hexagonal hydrates into the cubic phases.
3.2. Ex-Situ 27Al NMR Study of the Hydration Reaction at 50 °C
Figure 4a,b presents the 27Al NMR spectra of synthesized Ca7ZrAl6O18 and Sr-doped Ca7ZrAl6O18 cements together with their products of hydration. The 27Al MASNMR spectra of the starting unhydrated samples are shown by the red lines. The intense and broad peak at ca. 50 ppm is due to Ca7ZrAl6O18, which consists of orientationally disordered six AlO4 tetrahedra linked together by sharing corners, to form [Al6O18] rings [29]. The 27Al MAS NMR spectra of the hydrated samples during the first 15 min all contain peaks near 4 ppm due to VIAl in the cement hydration reaction products (amorphous or poorly crystalline hexagonal hydrates C4AH19 or C2AH8) (Figure 4a,b).
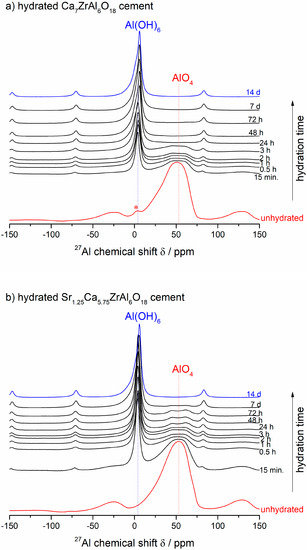
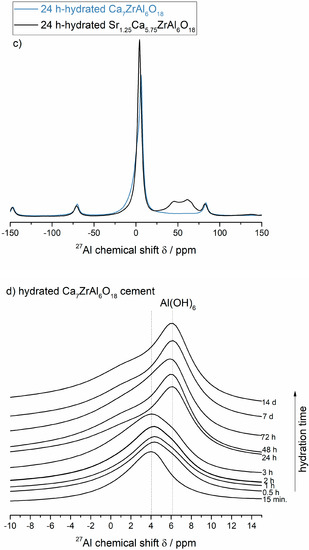
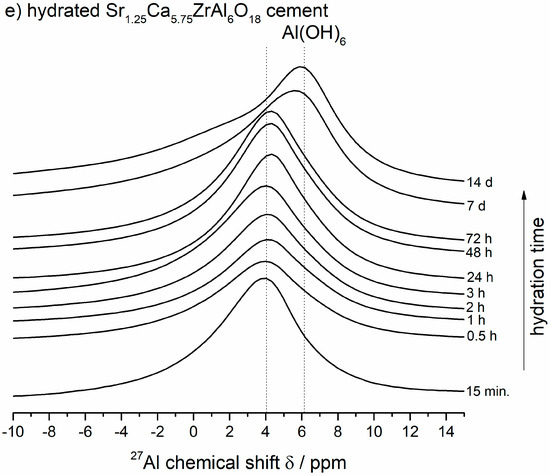
Figure 4.
(a,b) The room-temperature 27Al MASNMR spectra of unhydrated Ca7ZrAl6O18 (Sample C) and Sr1.25Ca5.75ZrAl6O18 (Sample A) cements, and their products of hydration formed between 15 min and 14 d at 50 °C. The arrow means the changes according to the direction of increasing hydration time. (c) A comparison of NMR spectra of both cements at 24 h of hydration. (d,e) The lines at ca. 4–6 ppm for octahedrally coordinated lattice Al3+ in calcium aluminate hydrates.
For the partially reacted samples, the signals are in the ca. 4 ppm and ca. 46–61 ppm ranges due to both hydrates and unhydrated reactants, respectively (Figure 4c). For the totally hydrated (and converted) samples, all of the signalsarein the ca. 6 ppm range, consistent with total conversion of Al from tetrahedral coordination in the unhydrated Ca7ZrAl6O18 and Sr1.25Ca5.75ZrAl6O18 cements to octahedral coordination [19] in the final hydrates formed at 24 h (undoped cement) or 7 d (Sr-doped cement), as expected from previous works on the calcium aluminate cement hydration processes investigated by solid-state27Al MAS NMR studies [19,25]. The maximum for the VIAl peak alters slightly depending on the calcium aluminate hydrates present [18,19]. For the undoped Ca7ZrAl6O18 cement hydrated between 15 min and 3 h, in which the detected crystalline hydrates are mainly hexagonal phases, the peak maximum is at ca. 4 ppm and shifts to 6 ppm for this cement hydrated between 24 h and 14 d (Figure 4d), which by XRD contain cubic C3AH6 as the predominant phase. This type of shift is delayed up to 7 d in the hydrated Sr-doped cement (Figure 4e), where Ca-rich (Sr,C)3AH6 or pure C3AH6 begin to form. Hence, it can be summarized that the chemical shift occurring at ca. 4 ppm was due to octahedrally coordinated framework aluminum atoms in Sr-rich (Sr,C)3AH6 (Sr1.25Ca5.75ZrAl6O18 cement paste), poorly crystalline C3AH6 (Ca7ZrAl6O18 cement paste) and hexagonal hydrates (both cement pastes) formed at an early stage of hydration. The chemical shift occurring at ca. 6 ppm was due to octahedrally coordinated framework aluminum atoms in Ca-rich (Sr,C)3AH6 or pure C3AH6 formed in the totally reacted Sr-doped sample, as it can be referenced in for pure C3AH6 formed in the reference fully hydrated and converted undoped cement paste. It is worth discussing that the maximum for the Al(6) peak belonging to C-A-H phases varies slightly from data presented in Ref. [18,19]. In those works, and many others, the peak maximum belonging to C3AH6 was located at about 12 ppm, whereas the peak maxima belonging to hexagonal hydrates were located at ca. 10–11 ppm.
3.3. Microstructural Studies on the Hydrated Sr1.25Ca5.75ZrAl6O18Cement Paste
The development of Sr1.25Ca5.75ZrAl6O18 cement paste microstructure in time can directly be linked to the evolution of phase composition presented in Section 3.1 and Section 3.2. Figure 5 shows the typical microstructure of this cement paste fragment after 24 h hydration at 50 °C. The presence of Sr in Sr1.25Ca5.75ZrAl6O18 clinker affects formation of Sr-rich (Sr,C)3AH6 (Figure 5a—point 1) with a cubic/isometric crystal form [30,31]. The EDS spectrum presenting intensity vs. energy of the detected X-ray clearly identifies the peaks of Sr, Ca, Al and O (Figure 5b). The EDS spectrum from the hexagonal irregular flakes is shown in Figure 5c. The Ca, Al and O peaks are mainly due to C-A-H phases. EDS intensity ratio of calcium and aluminum peaks indicates the presence of C4AH19 hydrate rather than C2AH8 hydrate.
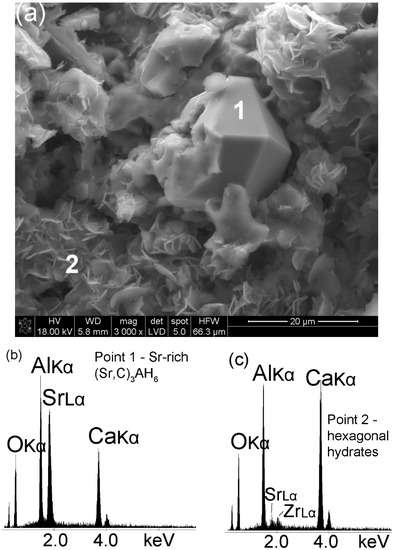
Figure 5.
SEM image of the hydrated Sr1.25Ca5.75ZrAl6O18 cement (Sample A) at the age of 24 h (a). Spot 1–2 EDS analysis; (b,c) EDS spectra of the sample in the microarea 1 (Sr-rich (Sr,C)3AH6) and 2 (hexagonal calcium aluminate hydrates), respectively.
Most of the C3AH6 or Ca-rich (Sr,C)3AH6 crystals formed after a hydration time of 7 d attain the shape of cubes, pyritohedra or other more complex forms of the isometric system, which are reinforced with Al(OH)3 crystals (Figure 6a,c). As observed before, those hydration products are strongly dependent on curing time and the Ca peak (Figure 6b) intensity increases relative to the Sr peak intensity (Figure 5b). Hence, as the curing time increases, the crystals belonging to a cubic or isometric system formed initially as a transient Sr-rich (Sr,C)3AH6 were replaced with Ca-rich (Sr,C)3AH6 or pure C3AH6. An unavoidable change of one form of calcium aluminate hydrate to another can be found elsewhere [32,33,34].
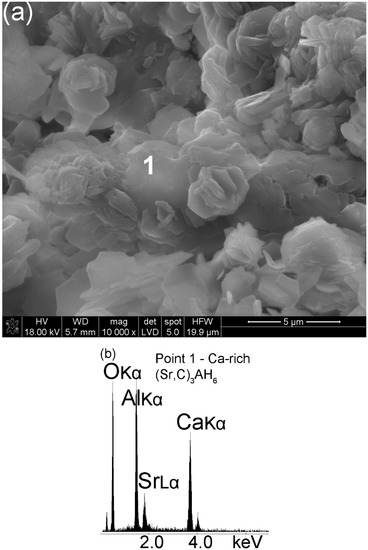

Figure 6.
SEM image of the hydrated Sr1.25Ca5.75ZrAl6O18 cement (Sample A) at the age of 7 d (a). Spot 1 EDS analysis; (b) EDS spectrum of the sample in the microarea 1—Ca-rich (Sr,C)3AH6; (c) overview of microstructure.
4. Conclusions
According to the current research, many conclusions can be drawn:
- (1)
- The Sr-doped cement is developed through structural substitution for Ca ions by Sr ions in the Ca7ZrAl6O18 clinker phase.
- (2)
- Strontium was used as a retarding agent to block this cement clinker phase hydration at a curing temperature of 50 °C. Hence, the residual unhydrated cement particles in the hardened Sr1.25Ca5.75ZrAl6O18 cement paste were present for much longer than for the undoped Ca7ZrAl6O18 clinker phase sample as it was observed by XRD.
- (3)
- The hydration of both Ca7ZrAl6O18 and Sr1.25Ca5.75ZrAl6O18 cements was also inspected using the 27Al MAS NMR technique. This hydration is accompanied by a change of Al-coordination from tetrahedral to octahedral. This complete conversion from anhydrous 27AlIV to hydrated 27AlVI species was achieved during the first 24 h of hydration at 50 °C for Ca7ZrAl6O18 and during 7 d of hydration at 50 °C for Sr1.25Ca5.75ZrAl6O18.
- (4)
- The hexagonal phases were formed starting in the very first minutes of hydration of these cements. For each cement type tested, these unstable hydrates consist mainly of C4AH19 and probably of C2AH8 as it was observed by XRD.
- (5)
- The formation of a thermodynamically stable phase pure C3AH6 or Ca-rich (Sr,C)3AH6 in the hardened Sr1.25Ca5.75ZrAl6O18 cement paste was preceded by that of a number of less stable phases, i.e.,Sr-rich (Sr,C)3AH6 hydrate and other hexagonal Ca-Al hydrates. The Sr-rich (Sr,C)3AH6 hydrate existing between 0.5 h and 7 d of curing was isostructural with the Ca-rich (Sr,C)3AH6 or pure C3AH6 formed at the age of 7 d.
- (6)
- The transformation of the hexagonal calcium aluminate hydrates and Sr-rich (Sr,C)3AH6 hydrate into the cubic phase Ca-rich (Sr,C)3AH6 or pure C3AH6 was expressed in terms of chemical shift from ca. 4 ppm to ca. 6 ppm in the hardened Sr1.25Ca5.75ZrAl6O18 cement paste at the age of 7 d. The same 27Al NMR chemical shift was detected at the age of 24 h for the reference hardened Ca7ZrAl6O18 cement paste.
Funding
This project was financed by the National Science Centre, Poland, project number 2017/26/D/ST8/00012 (Recipient: D. Madej). The sponsor had no role in the design, execution, interpretation, or writing of the study.
Conflicts of Interest
The author declares no conflicts of interest.
Nomenclature
| C | CaO |
| A | Al2O3 |
| Z | ZrO2 |
| Sr | SrO |
| H | H2O |
References
- Nowacka, M.; Pacewska, B. Effect of structurally different aluminosilicates on early-age hydration of calcium aluminate cement depending on temperature. Constr. Build. Mater. 2020, 235, 117404. [Google Scholar] [CrossRef]
- Cao, Y.-F.; Tao, Z.; Pan, Z.; Wuhrer, R. Effect of calcium aluminate cement on geopolymer concrete cured at ambient temperature. Constr. Build. Mater. 2018, 191, 242–252. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Lu, C.; Huang, Z. Effects of sodium gluconate on early hydration and mortar performance of Portland cement-calcium aluminate cement-anhydrite binder. Constr. Build. Mater. 2017, 157, 1065–1073. [Google Scholar] [CrossRef]
- Madej, D. Synthesis, formation mechanism and hydraulic activity of novel composite cements belonging to the system CaO-Al2O3-ZrO2. J. Therm. Anal. Calorim. 2017, 130, 1913–1924. [Google Scholar] [CrossRef]
- Litwinek, E.; Madej, D. Structure, microstructure and thermal stability characterizations of C3AH6 synthesized from different precursors through hydration. J. Therm. Anal. Calorim. 2019. [Google Scholar] [CrossRef]
- Madej, D.; Rajska, M.; Kruk, A. Synthesis and hydration behaviour of calcium zirconium aluminate powders by modifying co-precipitation method. Ceram. Int. 2020, 46, 2373–2383. [Google Scholar] [CrossRef]
- Madej, D. A new implementation of electrochemical impedance spectroscopy (EIS) and other methods to monitor the progress of hydration of strontium monoaluminate (SrAl2O4) cement. J. Therm. Anal. 2019, 139, 17–28. [Google Scholar] [CrossRef]
- Liu, K.; Chen, A.; Shang, X.; Chen, L.; Zheng, L.; Gao, S.; Zhou, Y.; Wang, Q.; Ye, G. The impact of mechanical grinding on calcium aluminate cement hydration at 30 °C. Ceram. Int. 2019, 45, 14121–14125. [Google Scholar] [CrossRef]
- Kerienė, J.; Antonovič, V.; Stonys, R.; Boris, R. The influence of the ageing of calcium aluminate cement on the properties of mortar. Constr. Build. Mater. 2019, 205, 387–397. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Irisawa, K.; Jin, F.; Meguro, Y.; Kinoshita, H. Reduction of water content in calcium aluminate cement with/out phosphate modification for alternative cementation technique. Cem. Concr. Res. 2018, 109, 243–253. [Google Scholar] [CrossRef]
- Madej, D.; Szczerba, J.; Nocuń-Wczelik, W.; Gajerski, R. Hydration of Ca7ZrAl6O18 phase. Ceram. Int. 2012, 38, 3821–3827. [Google Scholar] [CrossRef]
- Madej, D.; Boris, R. Synthesis, characterization and hydration analysis of Ba2+-, Cu2+- or Bi3+-doped CaO-Al2O3-ZrO2-based cements. J. Therm. Anal. Calorim. 2019, 138, 4331–4340. [Google Scholar] [CrossRef]
- Madej, D. Hydration, carbonation and thermal stability of hydrates in Ca7-xSrxZrAl6O18 cement. J. Therm. Anal. Calorim. 2018, 131, 2411–2420. [Google Scholar] [CrossRef]
- Pöllmann, H.; Kaden, R. Mono- (strontium-, calcium-) aluminate based cements. In Calcium Aluminates Proceedings of the International Conference; Building Research Establishment: Watford, UK, 2014; pp. 99–108. [Google Scholar]
- Ukrainczyk, N.; Vrbos, N.; Šipušić, J. Influence of metal chloride salts on calcium aluminate cement hydration. Adv. Cem. Res. 2012, 24, 249–262. [Google Scholar] [CrossRef]
- Duran, A.; Sirera, R.; Nicolas, M.P.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Study of the early hydration of calcium aluminates in the presence of different metallic salts. Cem. Concr. Res. 2016, 81, 1–15. [Google Scholar] [CrossRef]
- Faucon, P.; Charpentier, T.; Bertrandie, D.; Nonat, A.; Virlet, J.; Petit, J.C. Characterization of calcium aluminate hydrates and related hydrates of cement pastes by 27Al MQ-MAS NMR. Inorg. Chem. 1998, 37, 3726–3733. [Google Scholar] [CrossRef]
- Skibsted, J.; Henderson, E.; Jakobsen, H.J. Characterization of calcium aluminate phases in cements by 27Al MAS NMR spectroscopy. Inorg. Chem. 1993, 32, 1013–1027. [Google Scholar] [CrossRef]
- Cong, X.; Kirkpatrick, R.J. Hydration of calcium aluminate cements: A solid-state 27Al NMR study. J. Am. Ceram. Soc. 1991, 76, 409–416. [Google Scholar] [CrossRef]
- Mercury, J.M.R.; Pena, P.; de Aza, A.H.; Turrillas, X.; Sobrados, I.; Sanz, J. Solid-state 27Al and 29Si NMR investigations on Si-substituted hydrogarnets. Acta Mater. 2007, 55, 1183–1191. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; Gehman, J.D.; Van Deventer, J.S.J.; Provis, J. The role of Al in cross-linking of alkali-activated slag cements. J. Am. Ceram. Soc. 2014, 98, 996–1004. [Google Scholar] [CrossRef]
- Walkley, B.; Provis, J.L. Solid-state nuclear magnetic resonance spectroscopy of cements. Mater. Today Adv. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Müller, D.; Rettel, A.; Gessner, W.; Scheler, G. An application of solid state magic-angle spinning 27Al NMR to the study of cement hydration. J. Magn. Reson. 1984, 57, 152–156. [Google Scholar] [CrossRef]
- Muller, D.; Gessner, W.; Samoson, A.; Lippmaa, E.; Scheler, G. Solid state 27Al NMR studies of polycrystalline aluminates in the system CaO-Al2O3. Polyhedron 1986, 5, 779–785. [Google Scholar] [CrossRef]
- Hughes, C.E.; Walkley, B.; Gardner, L.; Walling, S.A.; Bernal, S.A.; Iuga, D.; Provis, J.; Harris, K.D.M. Exploiting in-situ solid-state NMR spectroscopy to probe the early stages of hydration of calcium aluminate cement. Solid State Nucl. Magn. Reson. 2019, 99, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Garces, P.; Alcocel, E.; Chinchon, S.; Andreu, C.; Alcaide, J. Alcaide, Effect of curing temperature in some hydration characteristics of calcium aluminate cement compared with those of portland cement. Cem. Concr. Res. 1997, 27, 1343–1355. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, G.; Gu, W.; Ding, D.; Chen, L.; Zhu, L. Conversion of calcium aluminate cement hydrates at 60 °C with and without water. J. Am. Ceram. Soc. 2018, 101, 2712–2717. [Google Scholar] [CrossRef]
- Luz, A.P.; Pandolfelli, V.C. Halting the calcium aluminate cement hydration process. Ceram. Int. 2011, 37, 3789–3793. [Google Scholar] [CrossRef]
- Fukuda, K.; Iwata, T.; Nishiyuki, K. Crystal structure, structural disorder, and hydration behavior of calcium zirconium aluminate, Ca7ZrAl6O18. Chem. Mater. 2007, 19, 3726–3731. [Google Scholar] [CrossRef]
- Das, S.K.; Kumar, S.K.; Das, P.K. Crystal morphology of calcium aluminates hydrated for 14 days. J. Mater. Sci. Lett. 1997, 16, 735–736. [Google Scholar] [CrossRef]
- Kumar, S.; Das, S.K.; Daspoddar, P.K. Microstructure of calcium aluminates and their mixes after 3 days of hydration. Trans. Indian Ceram. Soc. 1999, 58, 115–117. [Google Scholar] [CrossRef]
- Antonovič, V.; Keriené, J.; Boris, R.; Aleknevičius, M. The effect of temperature on the formation of the hydrated calcium aluminate cement structure. Procedia Eng. 2013, 57, 99–106. [Google Scholar] [CrossRef]
- López, A.H.; Calvo, J.L.G.; Olmo, J.G.; Petit, S.; Alonso, M.C. Microstructural evolution of calcium aluminate cements hydration with silica fume and fly ash additions by scanning electron microscopy, and mid and near-infrared spectroscopy. J. Am. Ceram. Soc. 2008, 91, 1258–1265. [Google Scholar] [CrossRef]
- Rashid, S.; Barnes, P.; Bensted, J.; Turrillas, X. Conversion of calcium aluminate cement hydrates re-examined with synchrotron energy-dispersive diffraction. J. Mater. Sci. Lett. 1994, 13, 1232–1234. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).