Preparation and Characterization of Paramagnetic Bis (8-Hydroxyquinoline) Manganese Crystals
Abstract
1. Introduction
2. Experimental Details
3. Computational Methods
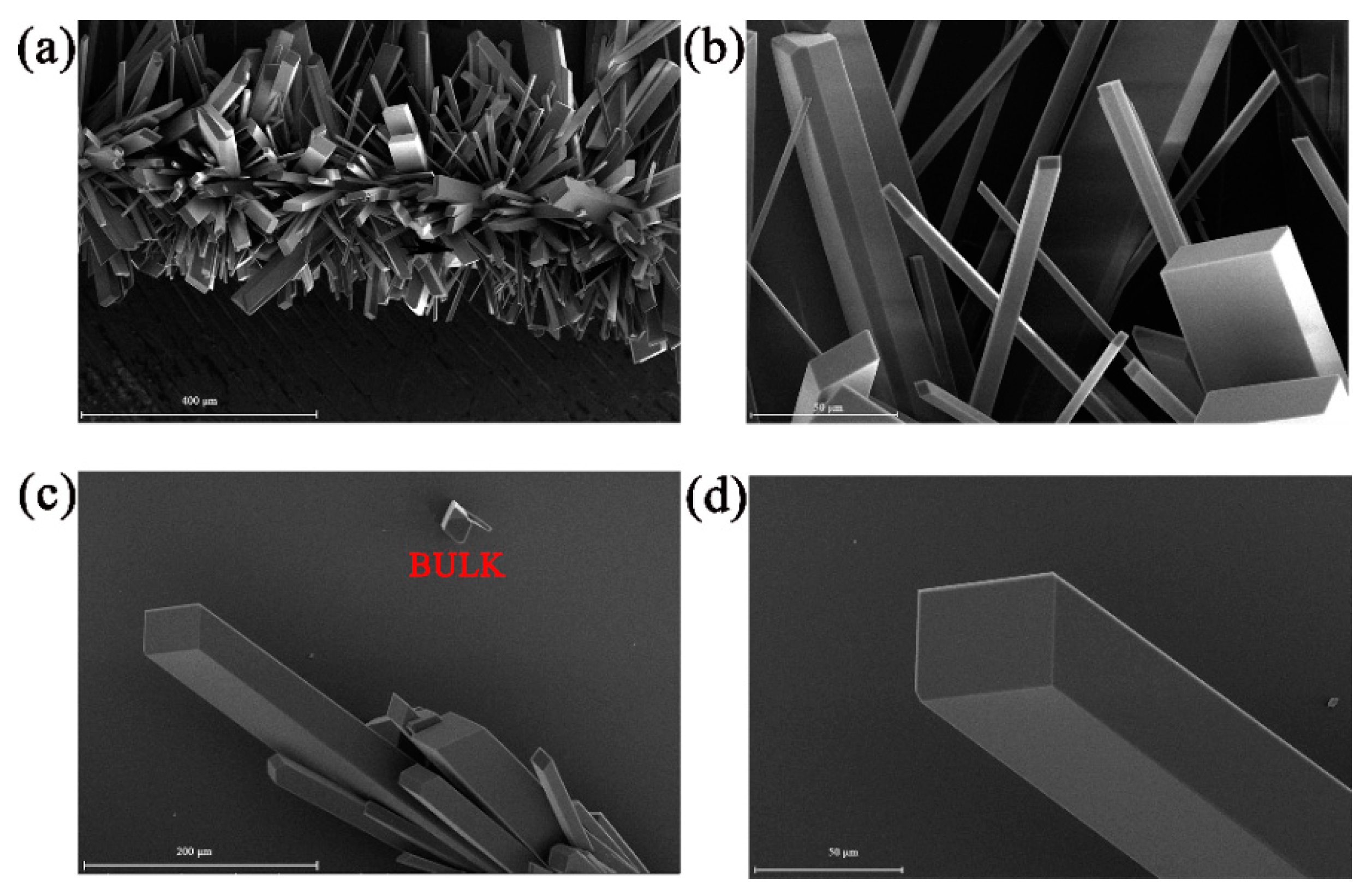
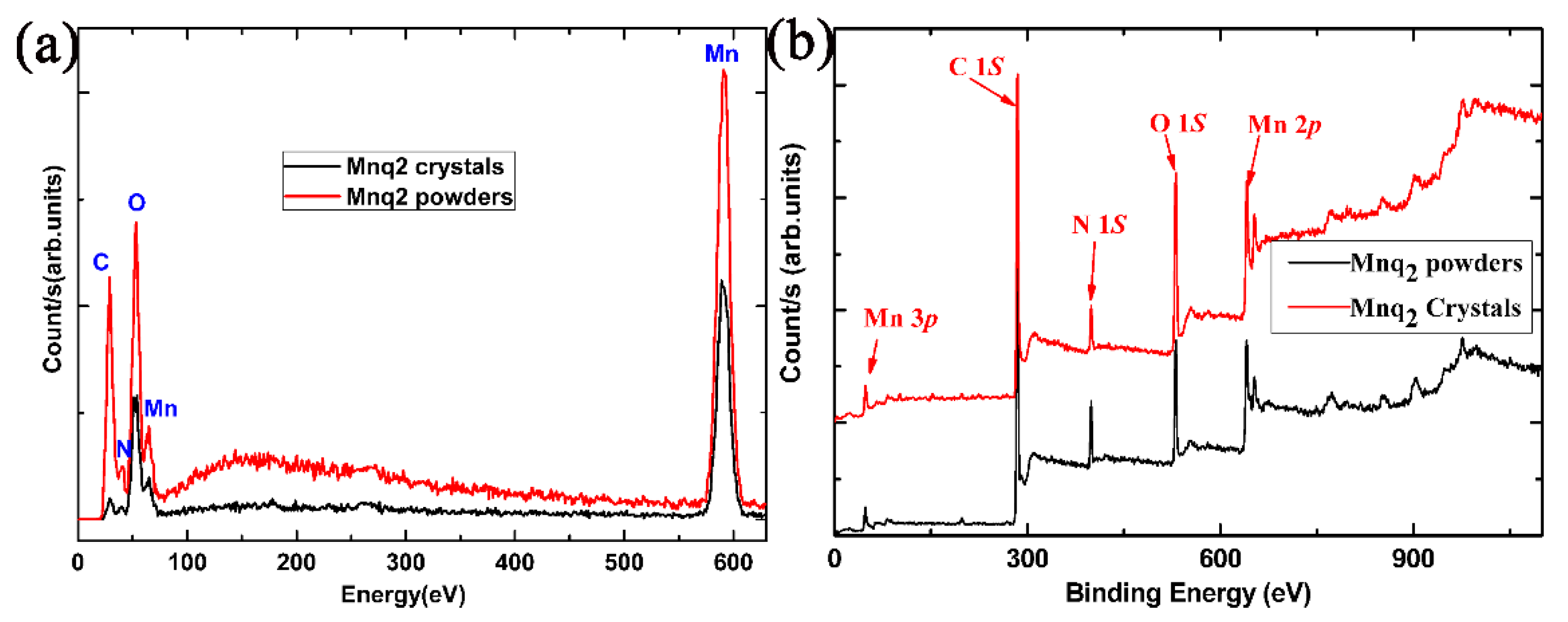
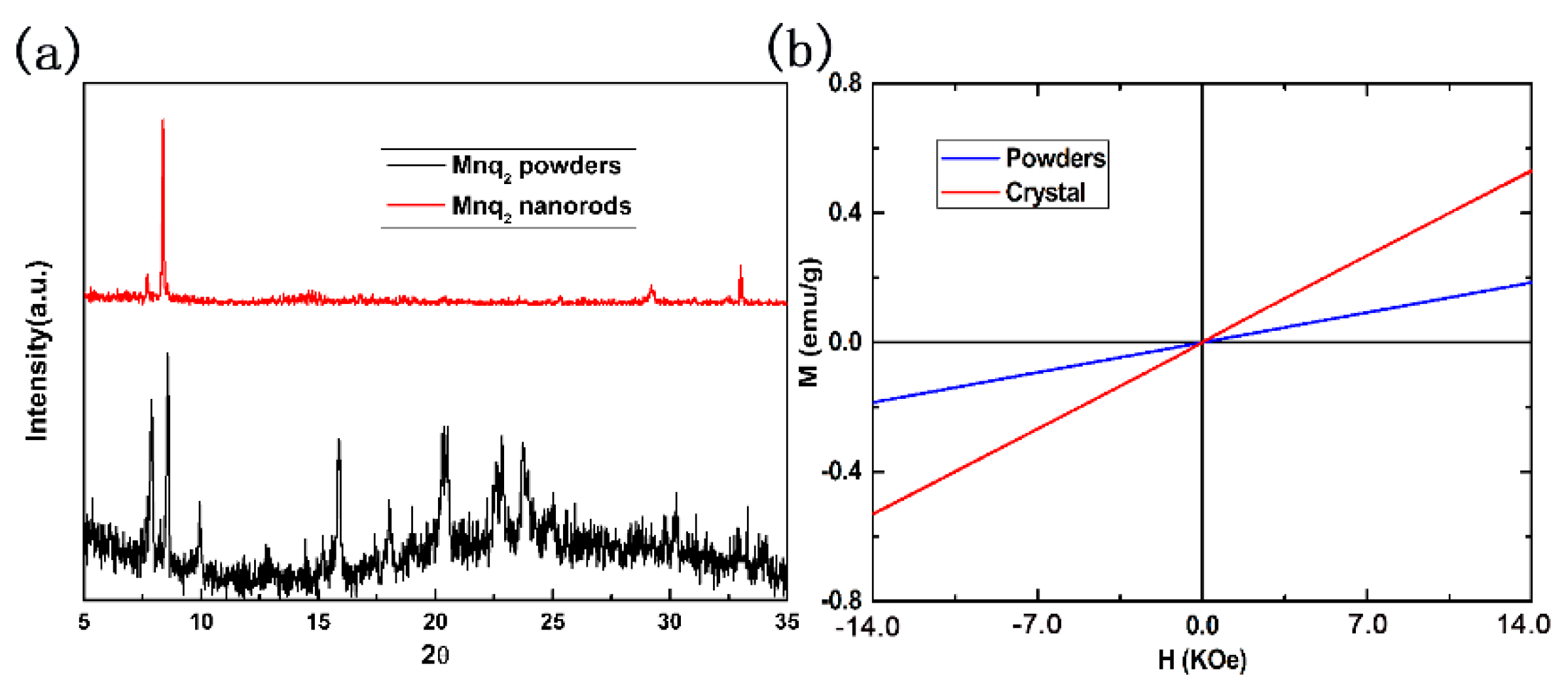
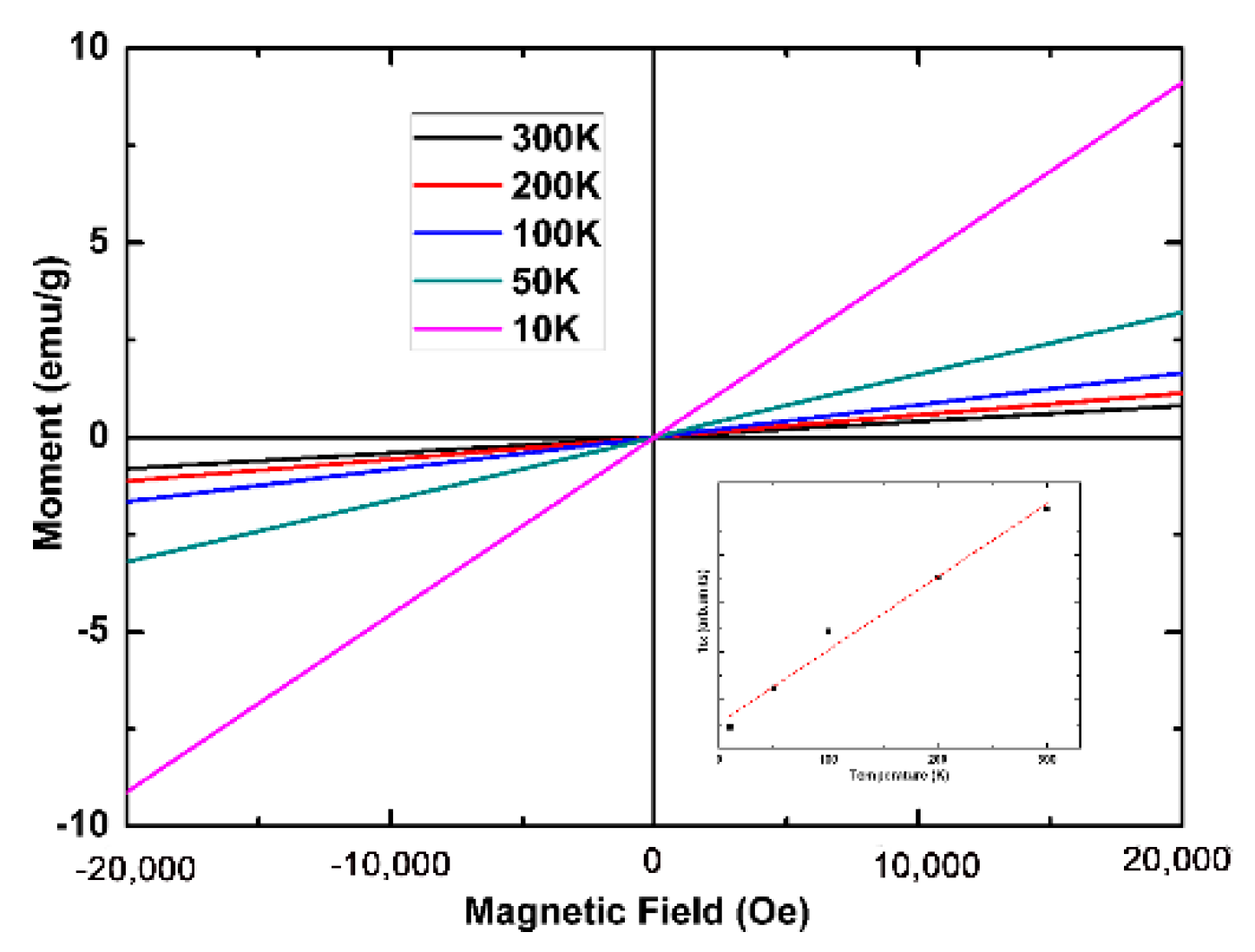
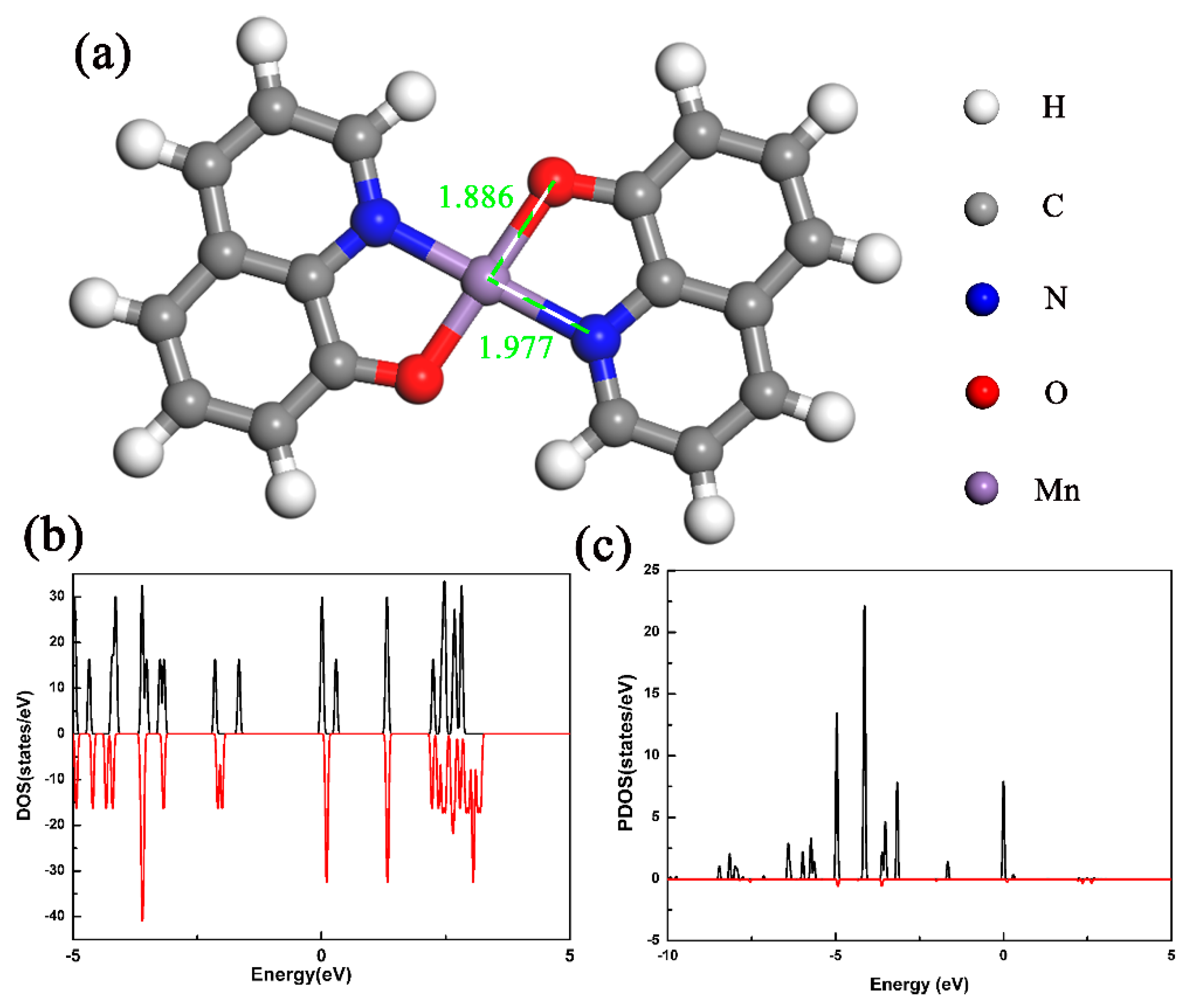
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Su, Z.S.; Li, W.L.; Chu, B.; Li, T.L.; Zhu, J.Z.; Zhang, G.; Yan, F.; Li, X.; Chen, Y.R.; Lee, C.S. High response organic ultraviolet photodetector based on blend of 4,4′,4″-tri-(2-methylphenyl phenylamino) triphenylaine and tris-(8-hydroxyquinoline) gallium. Appl. Phys. Lett. 2008, 93, 3. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, C.H.; Ha, C.S.; Lee, J.K. Organic solar cell devices based on PVK/porphyrin system. Synth. Met. 2001, 117, 289–291. [Google Scholar] [CrossRef]
- Cai, E.; Wang, S.; Wang, B.; Wang, S.; Zhao, E.; Cheng, J.; Chi, Z.; Xie, W. Facile solution synthesis of tris (8-hydroxyquinoline) gallium crystalline nanostructures and their optical properties. Opt. Mater. Express 2018, 8, 2670–2676. [Google Scholar] [CrossRef]
- Xiong, Z.H.; Wu, D.; Valy Vardeny, Z.; Shi, J. Giant magnetoresistance in organic spin-valves. Nature 2004, 427, 821–824. [Google Scholar] [CrossRef]
- Dediu, V.; Hueso, L.E.; Bergenti, I.; Riminucci, A.; Borgatti, F.; Graziosi, P.; Newby, C.; Casoli, F.; De Jong, M.P.; Taliani, C.; et al. Room-temperature spintronic effects in Alq3-based hybrid devices. Phys. Rev. B 2008, 78, 115203. [Google Scholar] [CrossRef]
- Majumdar, S.; Majumdar, H.S.; Laiho, R.; Osterbacka, R. Comparing small molecules and polymer for future organic spin-valves. J. Alloy. Compd. 2006, 423, 169–171. [Google Scholar] [CrossRef]
- Yoo, J.W.; Chen, C.Y.; Jang, H.W.; Bark, C.W.; Prigodin, V.N.; Eom, C.B.; Epstein, A.J. Spin injection/detection using an organic-based magnetic semiconductor. Nat. Mater. 2010, 9, 638–642. [Google Scholar] [CrossRef]
- Muhammad, F.F.; Abdul Hapip, A.I.; Sulaiman, K. Study of optoelectronic energy bands and molecular energy levels of tris (8-hydroxyquinolinate) gallium and aluminum organometallic materials from their spectroscopic and electrochemical analysis. J. Organomet. Chem. 2010, 695, 2526–2531. [Google Scholar] [CrossRef]
- Jiang, F.; Pang, Z.Y.; Yuan, H.M.; Wei, Z.X.; Xie, W.F.; Wu, Z.Y.; Han, S.H. Room temperature ferromagnetic properties of dysprosium-doped tris (8-hydroxyquinoline) aluminum: Experimental and theoretical investigation. RSC Adv. 2016, 6, 43780–43785. [Google Scholar] [CrossRef]
- Baik, J.M.; Shon, Y.; Lee, S.J.; Jeong, Y.H.; Kang, T.W.; Lee, J.L. Electronic Structure and Magnetism in Transition Metals Doped 8-Hydroxy-Quinoline Aluminum. J. Am. Chem. Soc. 2008, 130, 13522–13523. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wei, F.F.; Yuan, H.M.; Xie, W.F.; Pang, Z.Y.; Zhang, X.J. Room temperature ferromagnetic properties of Al-doped bis (8-hydroxyquinoline) cobalt (Coq(2)) molecules. J. Magn. Magn. Mater. 2015, 387, 155–158. [Google Scholar] [CrossRef]
- Jiang, F.; Yuan, H.M.; Xie, W.F.; Pang, Z.Y.; Han, S.H. Effect of oxygen on the magnetic property of Bis (8-hydroxyquinoline) copper (CuQ (2)): An experimental and theoretical study. Int. J. Mod. Phys. B 2015, 29, 6. [Google Scholar] [CrossRef]
- Monzon, L.M.A.; Burke, F.; Coey, J.M.D. Optical, Magnetic, Electrochemical, and Electrical Properties of 8-Hydroxyquinoline-Based Complexes with Al3+, Cr3+, Mn2+, Co2+, Ni2+, Cu2+, and Zn2+. J. Phys. Chem. C 2011, 115, 9182–9192. [Google Scholar] [CrossRef]
- Wang, Z.L. Characterizing the structure and properties of individual wire-like nanoentities. Adv. Mater. 2000, 12, 1295–1298. [Google Scholar] [CrossRef]
- Li, G.H.; Li, R.C.; Zhou, W.J. A Wire-Shaped Supercapacitor in Micrometer Size Based on Fe3O4 Nanosheet Arrays on Fe Wire. Nano Micro Lett. 2017, 9, 8. [Google Scholar] [CrossRef]
- Xu, P.; Wei, B.Q.; Cao, Z.Y.; Zheng, J.; Gong, K.; Li, F.X.; Yu, J.Y.; Li, Q.W.; Lu, W.B.; Byun, J.H.; et al. Stretchable Wire-Shaped Asymmetric Supercapacitors Based on Pristine and MnO2 Coated Carbon Nanotube Fibers. ACS Nano 2015, 9, 6088–6096. [Google Scholar] [CrossRef]
- Xie, W.; Pang, Z.; Zhao, Y.; Jiang, F.; Yuan, H.; Song, H.; Han, S. Structural and optical properties of ε-phase tris (8-hydroxyquinoline) aluminum crystals prepared by using physical vapor deposition method. J. Cryst. Growth 2014, 404, 164–167. [Google Scholar] [CrossRef]
- Kao, F.H.; Lin, K.Y.; Jan, D.J.; Tang, S.J.; Yang, C.C.; Lin, C.H.; Yeh, J.M.; Chiu, K.C. Re-condensation and decomposition of Tris (8-hydroxyquinoline)-aluminum in a vapor transport ampoule. J. Cryst. Growth 2012, 357, 9–14. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Petit, S.; Coquerel, G.; Perez, G.; Louer, D.; Louer, M. Synthesis, Characterization, and Ab-Initio Structure Determination from Powder Diffraction Data of a New X’ Form of Anhydrous Copper (Ii) 8-Hydroxyquinolinate Doped with Amine—Modeling of the Polymorphic Transformation to the Stable Anhydrous Beta “Form”. Chem. Mat. 1994, 6, 116–121. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Kloc, C.; Simpkins, P.G.; Siegrist, T.; Laudise, R.A. Physical vapor growth of centimeter-sized crystals of alpha-hexathiophene. J. Cryst. Growth 1997, 182, 416–427. [Google Scholar] [CrossRef]
- Brinkmann, M.; Gadret, G.; Muccini, M.; Taliani, C.; Masciocchi, N.; Sironi, A. Correlation between molecular packing and optical properties in different crystalline polymorphs and amorphous thin films of mer-tris (8-hydroxyquinoline) aluminum (III). J. Am. Chem. Soc. 2000, 122, 5147–5157. [Google Scholar] [CrossRef]
- Jiang, F.; Pang, Z.; Yuan, H.; Wei, Z.; Xie, W.; Han, S. Effect of Low-Temperature Annealing on Structure and Optical Properties of Amorphous Tris (8-Hydroxyquinoline) Aluminum Films. Surf. Rev. Lett. 2019, 26, 1850183. [Google Scholar] [CrossRef]
- Wang, F.; Pang, Z.; Lin, L.; Fang, S.; Dai, Y.; Han, S. Magnetism in Co-doped tris-8-hydroxyquinoline aluminum studied by first-principles calculations. Appl. Phys. Lett. 2010, 96, 053304. [Google Scholar] [CrossRef]
- Yuan, H.; Jiang, F.; Xie, W.; Zhang, X.; Pang, Z.; Han, S. Structural, electronic and magnetic properties of 8-hydroxyquinoline-based small molecules TMQx (TM = Cr, Mn, Fe, Co, Ni, Cu, Zn, and x = 2 or 3). Phys. E 2015, 70, 77–83. [Google Scholar] [CrossRef]
- Wang, F.; Pang, Z.; Lin, L.; Fang, S.; Dai, Y.; Han, S. Magnetism in undoped MgO studied by density functional theory. Phys. Rev. B 2009, 80, 144424. [Google Scholar] [CrossRef]

| Initial Direction | ∆EL (meV) | Magnetic Coupling | |
|---|---|---|---|
| Mn1 | Mn2 | ||
| ↑ | ↑ | 0 | FM |
| ↑ | ↓ | 0.1 | AFM |
| ↓ | ↑ | 0.1 | AFM |
| ↓ | ↓ | 0 | FM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Song, J.; Dong, M.; Wang, Y. Preparation and Characterization of Paramagnetic Bis (8-Hydroxyquinoline) Manganese Crystals. Materials 2020, 13, 2379. https://doi.org/10.3390/ma13102379
Jiang F, Song J, Dong M, Wang Y. Preparation and Characterization of Paramagnetic Bis (8-Hydroxyquinoline) Manganese Crystals. Materials. 2020; 13(10):2379. https://doi.org/10.3390/ma13102379
Chicago/Turabian StyleJiang, Feng, Jiawen Song, Mengqi Dong, and Yinong Wang. 2020. "Preparation and Characterization of Paramagnetic Bis (8-Hydroxyquinoline) Manganese Crystals" Materials 13, no. 10: 2379. https://doi.org/10.3390/ma13102379
APA StyleJiang, F., Song, J., Dong, M., & Wang, Y. (2020). Preparation and Characterization of Paramagnetic Bis (8-Hydroxyquinoline) Manganese Crystals. Materials, 13(10), 2379. https://doi.org/10.3390/ma13102379




