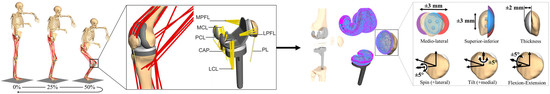
Musculoskeletal Multibody Simulation Analysis on the Impact of Patellar Component Design and Positioning on Joint Dynamics after Unconstrained Total Knee Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
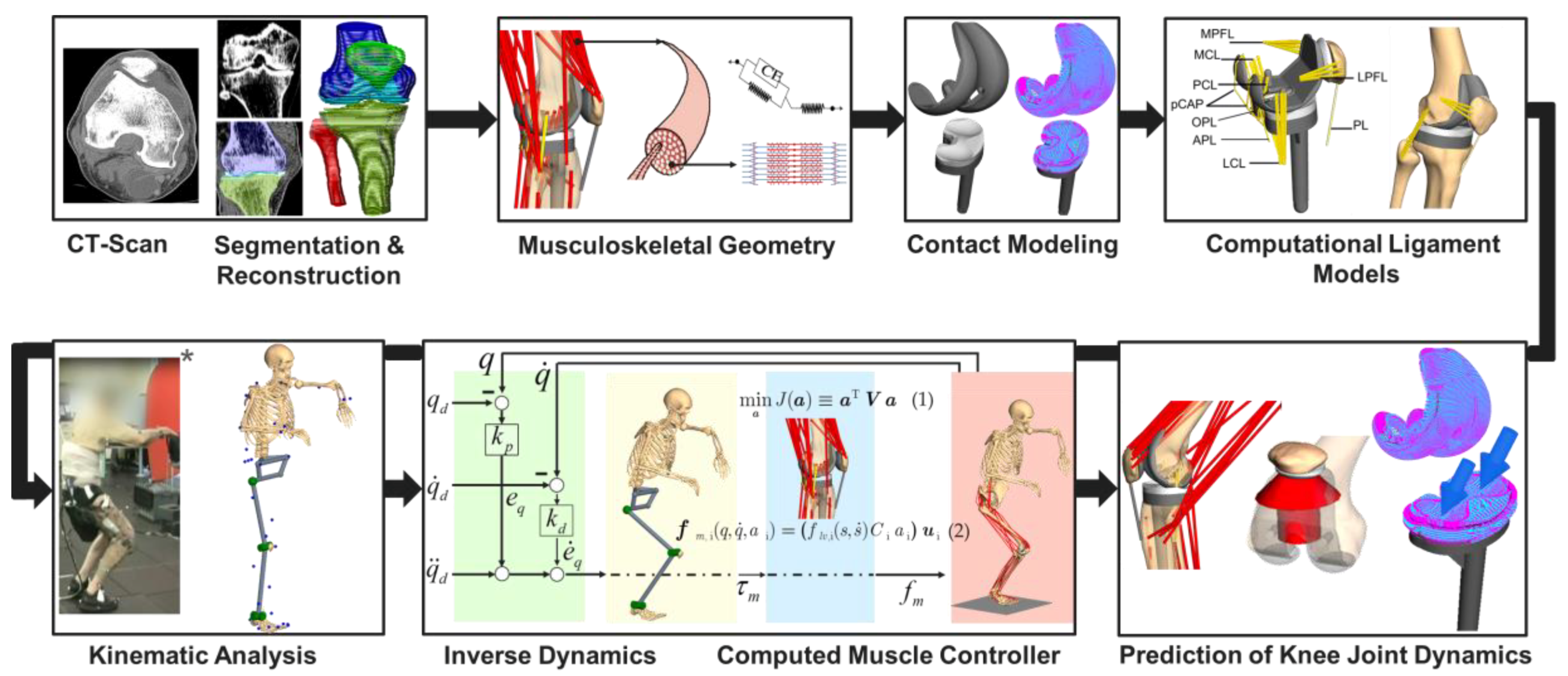
2.1. Overview of the Deployed Workflow of Musculoskeletal Multibody Simulation
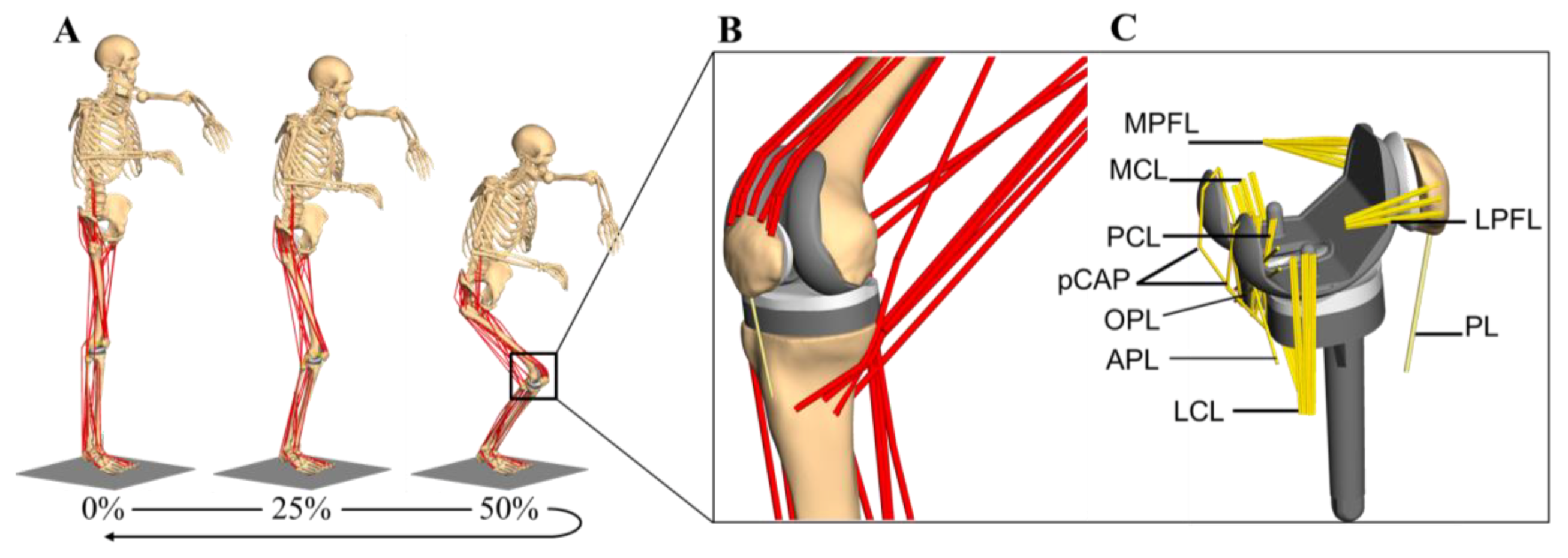
2.2. Musculoskeletal Multibody Simulation Model with a Cruciate-Retaining Total Knee Replacement
2.3. Kinematic Analysis
2.4. Forward Dynamic Musculoskeletal Multibody Simulation of a Squat Motion
2.5. Validation of the Musculoskeletal Multibody Simulation Model
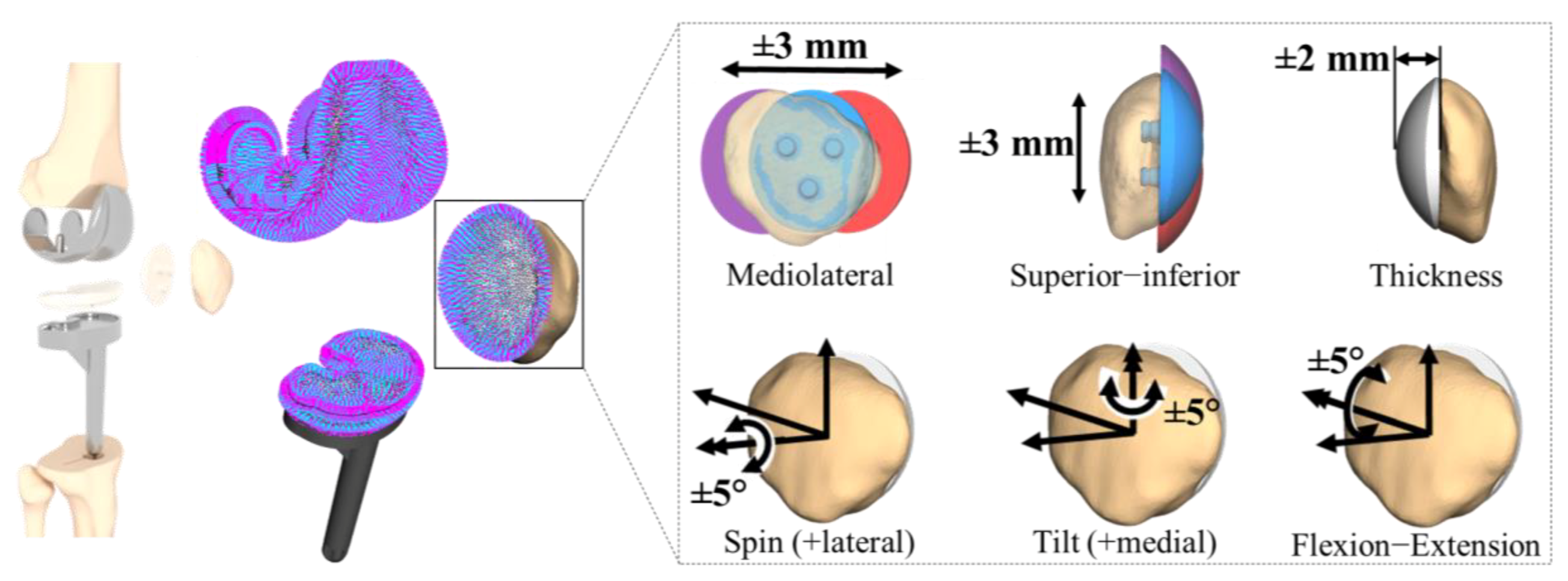
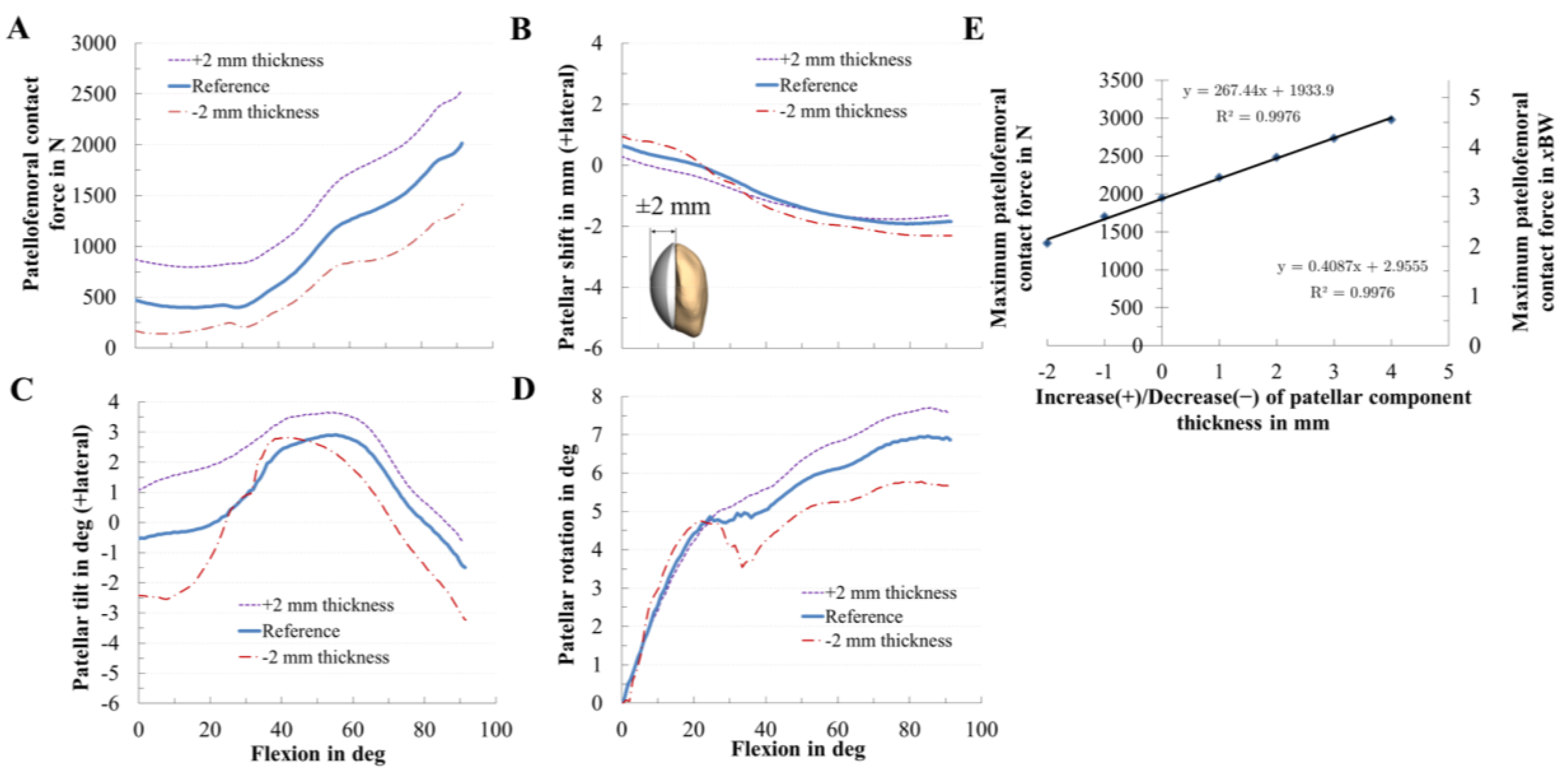
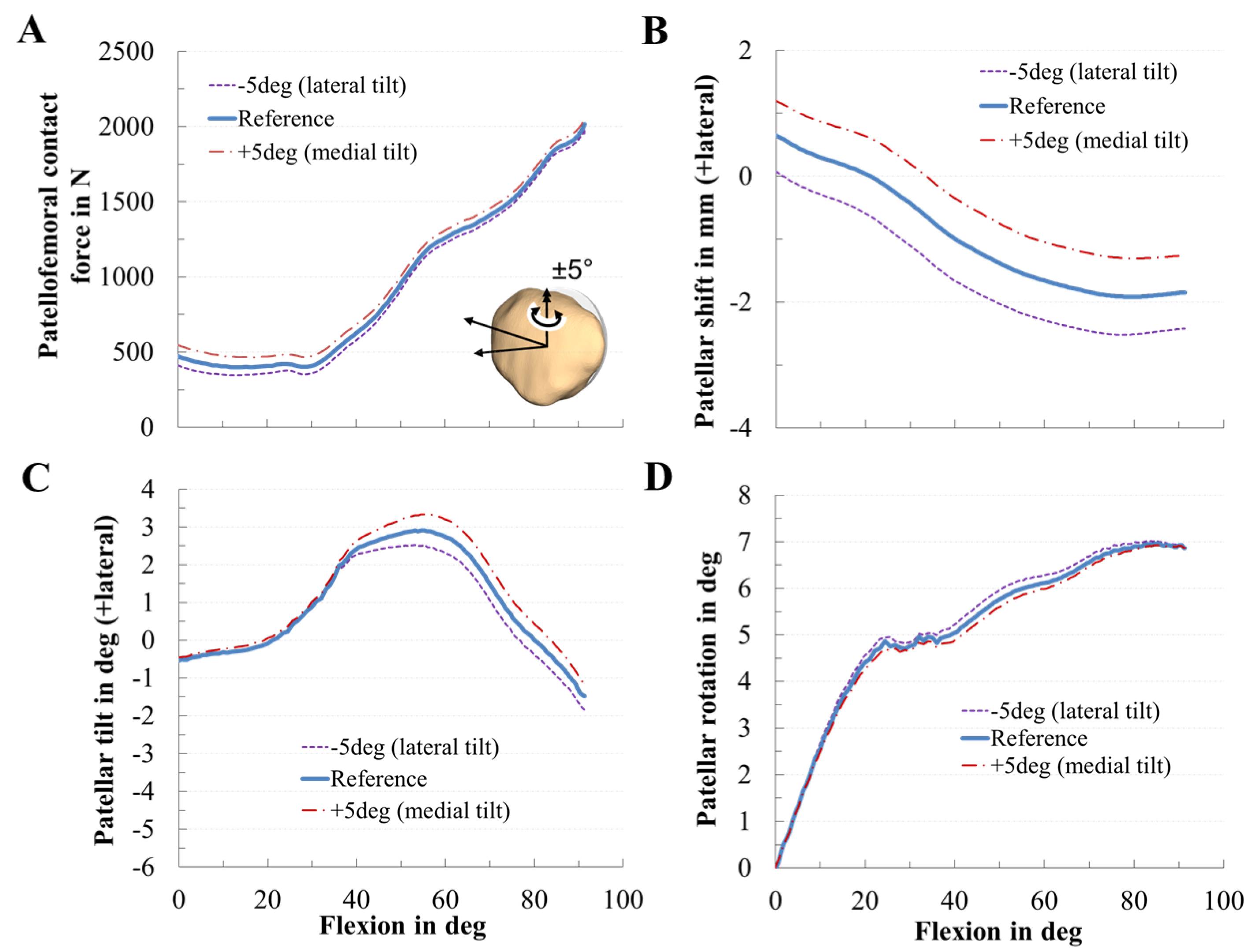
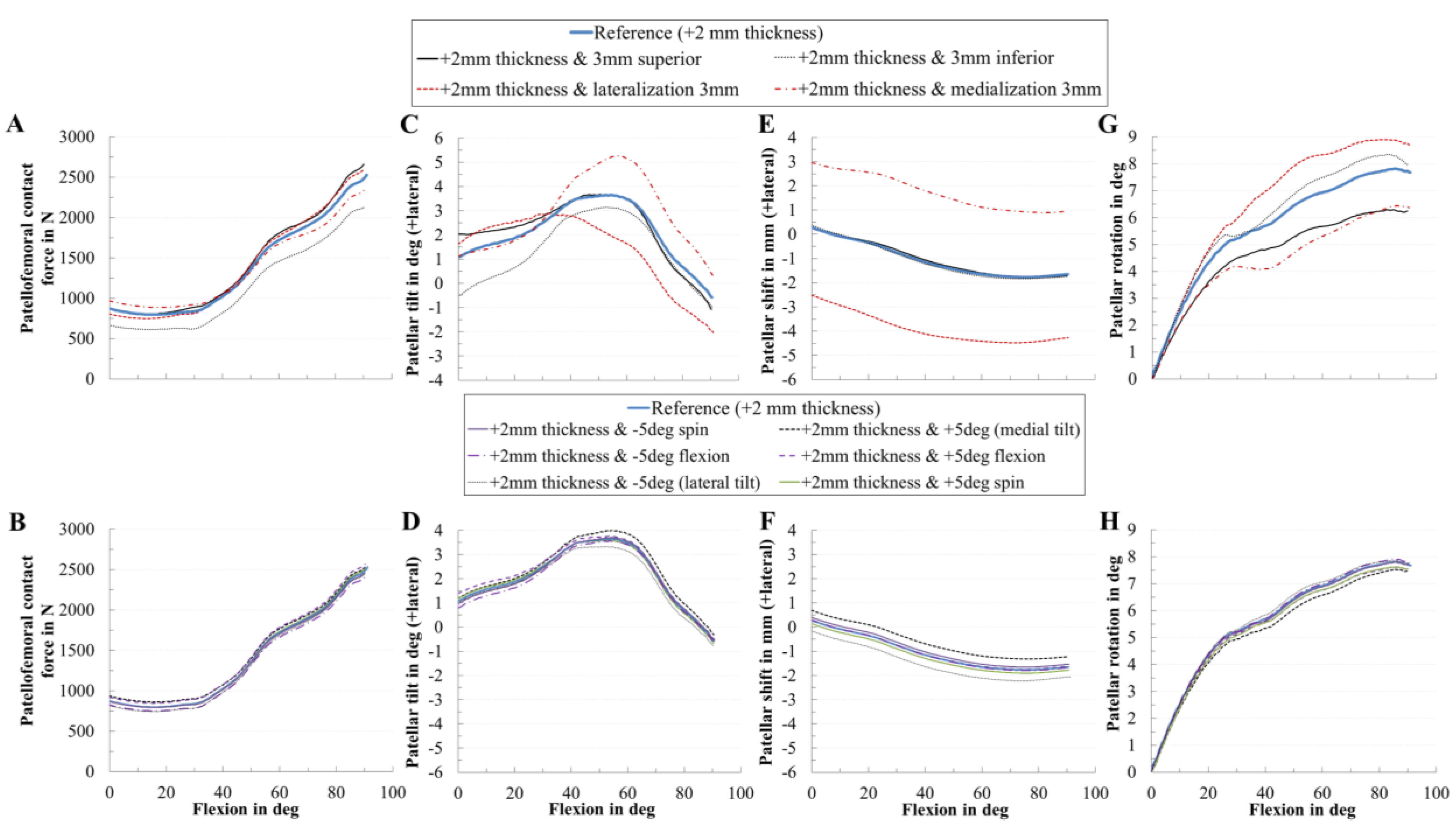
2.6. In Silico Study on the Effect of the Patellar Component Design and Positioning on Patellofemoral Joint Dynamics after TKR
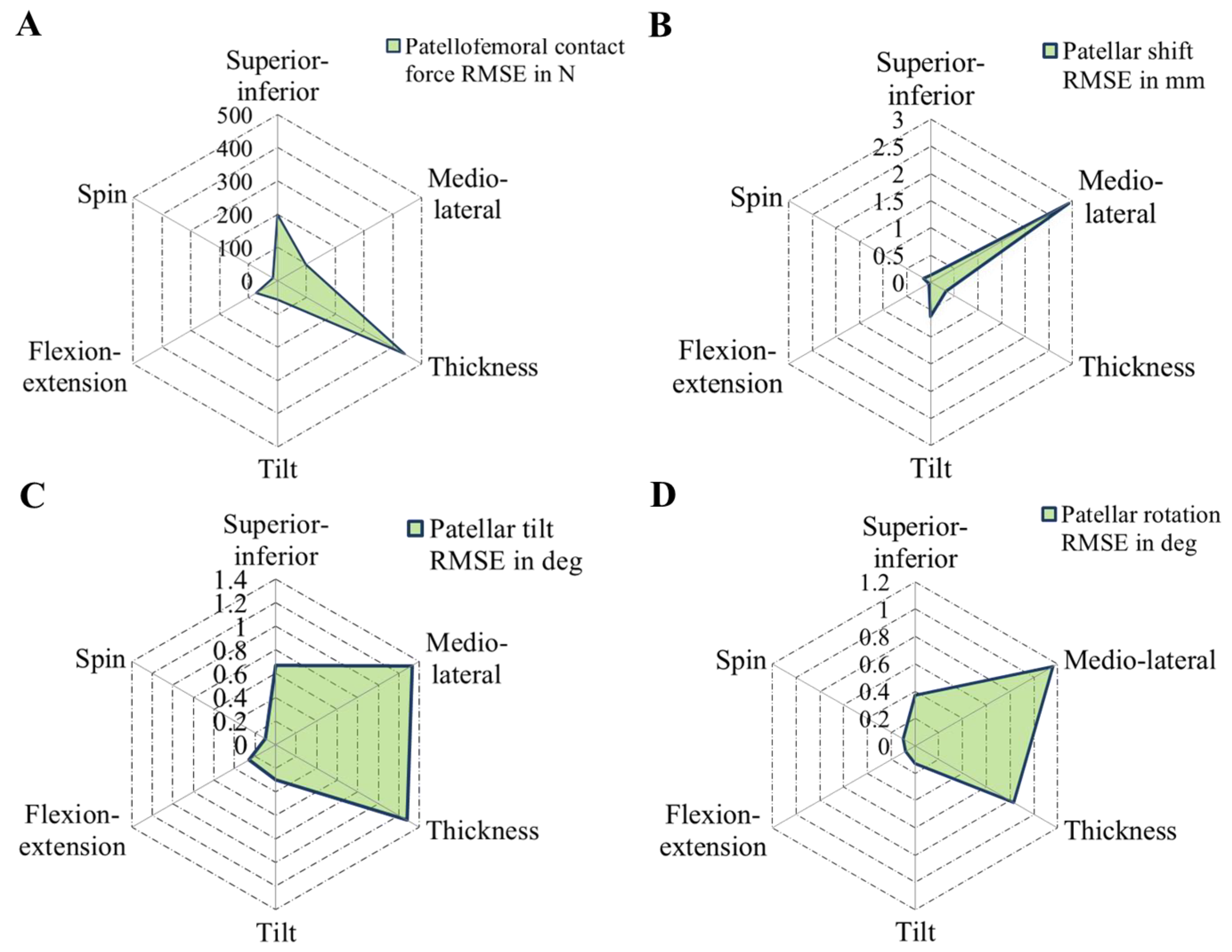
2.7. Statistical Metrics
3. Results
3.1. Validation of the Musculoskeletal Multibody Simulation Model
3.2. Effect of Patellar Component Design and Positioning on Patellofemoral Joint Dynamics after Unconstrained Total Knee Arthroplasty
3.3. Effect of Coupling Patellar Component Design and Positioning Parameters on Patellofemoral Joint Dynamics after Unconstrained Total Knee Arthroplasty
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Mechanical Material Properties of the Ligaments
| Tibiofemoral | Patellofemoral | |||||||
|---|---|---|---|---|---|---|---|---|
| Ligament | Ligament Bundle | Stiffness (N) | Ref. Strain | Ligament | Ligament Bundle | Stiffness (N) | Ref. Strain | |
| PCL | a | 3000 | −0.10 | MPFL | p | 2500 | 0.12 | |
| p | 1500 | −0.03 | c | 2500 | 0.08 | |||
| d | 2500 | 0.08 | ||||||
| MCL | a | 1500 | 0.04 | LPFL | p | 2000 | 0.06 | |
| c | 2000 | 0.06 | ||||||
| d | 2000 | 0.06 | ||||||
| c | 1500 | 0.04 | PL | − | − | |||
| p | 1500 | 0.02 | a | anterior | ||||
| LCL | a | 2250 | −0.25 | c | central | |||
| p | 2250 | 0.08 | d | distal | ||||
| OPL | p | 1250 | 0.06 | i | inferior | |||
| d | 1500 | 0.04 | l | lateral | ||||
| pCAP | l | 2500 | 0.05 | m | medial/middle | |||
| m | 2500 | 0.05 | p | posterior/proximal | ||||
| APL | 1500 | 0.04 | s | superior | ||||
Appendix B

Appendix C
Equations Used for Statistical Evaluation
References
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Bozic, K.J. Impact of the economic downturn on total joint replacement demand in the United States: Updated projections to 2021. J. Bone Jt. Surg. Am. 2014, 96, 624–630. [Google Scholar] [CrossRef]
- Price, A.J.; Alvand, A.; Troelsen, A.; Katz, J.N.; Hooper, G.; Gray, A.; Carr, A.; Beard, D. Knee replacement. Lancet 2018, 392, 1672–1682. [Google Scholar] [CrossRef]
- Bourne, R.B.; Chesworth, B.M.; Davis, A.M.; Mahomed, N.N.; Charron, K.D.J. Patient satisfaction after total knee arthroplasty: Who is satisfied and who is not? Clin. Orthop. Relat. Res. 2010, 468, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Pabinger, C.; Berghold, A.; Boehler, N.; Labek, G. Revision rates after knee replacement. Cumulative results from worldwide clinical studies versus joint registers. Osteoarthr. Cartil. 2013, 21, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Assi, C.; Kheir, N.; Samaha, C.; Chamoun, M.; Yammine, K. Novel anatomical-based surgical technique for positioning of the patellar component in total knee arthroplasty. SICOT J. 2017, 3, 67. [Google Scholar] [CrossRef][Green Version]
- Gasparini, G.; Familiari, F.; Ranuccio, F. Patellar malalignment treatment in total knee arthroplasty. Joints 2013, 1, 10–17. [Google Scholar]
- He, J.-Y.; Jiang, L.-S.; Dai, L.-Y. Is patellar resurfacing superior than nonresurfacing in total knee arthroplasty? A meta-analysis of randomized trials. Knee 2011, 18, 137–144. [Google Scholar] [CrossRef]
- Matz, J.; Lanting, B.A.; Howard, J.L. Understanding the patellofemoral joint in total knee arthroplasty. Can. J. Surg. 2019, 62, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.-B.; Qi, Y.-S.; Song, C.-H.; Zhang, J.-Y.; Yang, Y.-Q.; Yu, J.-K. Novel customized template designing for patellar resurfacing in total knee arthroplasty. J. Orthop. Res. 2016, 34, 1798–1803. [Google Scholar] [CrossRef]
- Shah, J.N.; Howard, J.S.; Flanigan, D.C.; Brophy, R.H.; Carey, J.L.; Lattermann, C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am. J. Sports Med. 2012, 40, 1916–1923. [Google Scholar] [CrossRef]
- Fraser, J.F.; Spangehl, M.J. International Rates of Patellar Resurfacing in Primary Total Knee Arthroplasty, 2004–2014. J. Arthroplast. 2017, 32, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Belvedere, C.; Ensini, A.; Tamarri, S.; d’Amato, M.; Barbadoro, P.; Feliciangeli, A.; Rao, A.; Leardini, A. Does navigated patellar resurfacing in total knee arthroplasty result in proper bone cut, motion and clinical outcomes? Clin. Biomech. (Bristol. Avon.) 2019, 69, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.K.; Wai, J.; Lee, E.; Hutchison, C.; Myden, C.; Batuyong, E.; Anglin, C. Computer-assisted patellar resection system: Development and insights. J. Orthop. Res. 2012, 30, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Narkbunnam, R.; Electricwala, A.J.; Huddleston, J.I.; Maloney, W.J.; Goodman, S.B.; Amanatullah, D.F. Suboptimal patellofemoral alignment is associated with poor clinical outcome scores after primary total knee arthroplasty. Arch. Orthop. Trauma Surg. 2019, 139, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Steinbrück, A.; Schröder, C.; Woiczinski, M.; Schmidutz, F.; Müller, P.E.; Jansson, V.; Fottner, A. Mediolateral femoral component position in TKA significantly alters patella shift and femoral roll-back. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3561–3568. [Google Scholar] [CrossRef]
- Steinbrück, A.; Fottner, A.; Schröder, C.; Woiczinski, M.; Schmitt-Sody, M.; Müller, T.; Müller, P.E.; Jansson, V. Influence of mediolateral tibial baseplate position in TKA on knee kinematics and retropatellar pressure. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2602–2608. [Google Scholar] [CrossRef]
- Petersen, W.; Rembitzki, I.V.; Brüggemann, G.-P.; Ellermann, A.; Best, R.; Koppenburg, A.G.; Liebau, C. Anterior knee pain after total knee arthroplasty: A narrative review. Int. Orthop. 2014, 38, 319–328. [Google Scholar] [CrossRef]
- Steinbrück, A.; Schröder, C.; Woiczinski, M.; Müller, T.; Müller, P.E.; Jansson, V.; Fottner, A. Influence of tibial rotation in total knee arthroplasty on knee kinematics and retropatellar pressure: An in vitro study. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2395–2401. [Google Scholar] [CrossRef]
- Clement, N.D.; MacDonald, D.; Patton, J.T.; Burnett, R. Post-operative Oxford knee score can be used to indicate whether patient expectations have been achieved after primary total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1578–1590. [Google Scholar] [CrossRef]
- Hofmann, A.A.; Tkach, T.K.; Evanich, C.J.; Camargo, M.P.; Zhang, Y. Patellar component medialization in total knee arthroplasty. J. Arthroplast. 1997, 12, 155–160. [Google Scholar] [CrossRef]
- Merican, A.M.; Ghosh, K.M.; Iranpour, F.; Deehan, D.J.; Amis, A.A. The effect of femoral component rotation on the kinematics of the tibiofemoral and patellofemoral joints after total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1479–1487. [Google Scholar] [CrossRef]
- Schroer, W.C.; Berend, K.R.; Lombardi, A.V.; Barnes, C.L.; Bolognesi, M.P.; Berend, M.E.; Ritter, M.A.; Nunley, R.M. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J. Arthroplast. 2013, 28, 116–119. [Google Scholar] [CrossRef]
- Wibeeg, G. Roentgenographs and Anatomic Studies on the Femoropatellar Joint: With Special Reference to Chondromalacia Patellae. Acta Orthop. Scand. 1941, 12, 319–410. [Google Scholar] [CrossRef]
- Mahfouz, M.; Abdel Fatah, E.E.; Bowers, L.S.; Scuderi, G. Three-dimensional morphology of the knee reveals ethnic differences. Clin. Orthop. Relat. Res. 2012, 470, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Phillips, M.; Bhandari, M.; Watson, J.; Malhotra, R. What Differences in Morphologic Features of the Knee Exist Among Patients of Various Races? A Systematic Review. Clin. Orthop. Relat. Res. 2017, 475, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Clary, C.W.; Smoger, L.M.; Dennis, D.A.; Fitzpatrick, C.K.; Rullkoetter, P.J.; Laz, P.J. Computational framework for population-based evaluation of TKR-implanted patellofemoral joint mechanics. Biomech. Model. Mechanobiol. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Fitzpatrick, C.K.; Baldwin, M.A.; Laz, P.J.; FitzPatrick, D.P.; Lerner, A.L.; Rullkoetter, P.J. Development of a statistical shape model of the patellofemoral joint for investigating relationships between shape and function. J. Biomech. 2011, 44, 2446–2452. [Google Scholar] [CrossRef]
- Fitzpatrick, C.K.; Baldwin, M.A.; Clary, C.W.; Wright, A.; Laz, P.J.; Rullkoetter, P.J. Identifying alignment parameters affecting implanted patellofemoral mechanics. J. Orthop. Res. 2012, 30, 1167–1175. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Liu, Y.; He, J.; Lian, Q.; Li, D.; Jin, Z. Effect of component mal-rotation on knee loading in total knee arthroplasty using multi-body dynamics modeling under a simulated walking gait. J. Orthop. Res. 2015, 33, 1287–1296. [Google Scholar] [CrossRef]
- Luyckx, T.; Didden, K.; Vandenneucker, H.; Labey, L.; Innocenti, B.; Bellemans, J. Is there a biomechanical explanation for anterior knee pain in patients with patella alta? Influence of patellar height on patellofemoral contact force, contact area and contact pressure. J. Bone Jt. Surg. Br. 2009, 91, 344–350. [Google Scholar] [CrossRef]
- Kebbach, M.; Grawe, R.; Geier, A.; Winter, E.; Bergschmidt, P.; Kluess, D.; D’Lima, D.; Woernle, C.; Bader, R. Effect of surgical parameters on the biomechanical behaviour of bicondylar total knee endoprostheses—A robot-assisted test method based on a musculoskeletal model. Sci. Rep. 2019, 9, 14504. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Q.; Budoff, J.E.; Glaser, F.E. Patellar component positioning in total knee arthroplasty. Clin. Orthop. Relat. Res. 1999, 366, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Tanaka, Y.; Kuriyama, S.; Nishitani, K.; Ito, H.; Furu, M.; Matsuda, S. Superior–inferior position of patellar component affects patellofemoral kinematics and contact forces in computer simulation. Clin. Biomech. (Bristol. Avon.) 2017, 45, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Rosso, I.; Surace, C.; Antonaci, P.; Surace, F.M.; Negretto, R.J. Influence of the patellar button thickness on the knee flexion after total knee arthroplasty. Acta Bioeng. Biomech. 2018, 20, 121–134. [Google Scholar]
- Bengs, B.C.; Scott, R.D. The effect of patellar thickness on intraoperative knee flexion and patellar tracking in total knee arthroplasty. J. Arthroplast. 2006, 21, 650–655. [Google Scholar] [CrossRef]
- Miller, M.C.; Zhang, A.X.; Petrella, A.J.; Berger, R.A.; Rubash, H.E. The effect of component placement on knee kinetics after arthroplasty with an unconstrained prosthesis. J. Orthop. Res. 2001, 19, 614–620. [Google Scholar] [CrossRef]
- Mochizuki, T.; Yano, K.; Ikari, K.; Hiroshima, R.; Okazaki, K. Effect on patellar kinematics of the different patellar component designs in total knee arthroplasty: Intraoperative measurement of dome type versus anatomic type. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 419–424. [Google Scholar] [CrossRef]
- Joseph, L.; Batailler, C.; Roger, J.; Swan, J.; Servien, E.; Lustig, S. Patellar component size effects patellar tilt in total knee arthroplasty with patellar resurfacing. Knee Surg. Sports Traumatol. Arthrosc. 2020. [Google Scholar] [CrossRef]
- Atzori, F.; Sabatini, L.; Deledda, D.; Schirò, M.; Lo Baido, R.; Baido, R.L.; Massè, A. Evaluation of anterior knee pain in a PS total knee arthroplasty: The role of patella-friendly femoral component and patellar size. Musculoskelet. Surg. 2015, 99, 75–83. [Google Scholar] [CrossRef]
- van de Groes, S.A.W.; Koëter, S.; Waal Malefijt, M.; de Verdonschot, N. Effect of medial–lateral malpositioning of the femoral component in total knee arthroplasty on anterior knee pain at greater than 8years of follow-up. Knee 2014, 21, 1258–1262. [Google Scholar] [CrossRef]
- Yoshii, I.; Whiteside, L.A.; Anouchi, Y.S. The effect of patellar button placement and femoral component design on patellar tracking in total knee arthroplasty. Clin. Orthop. Relat. Res. 1992, 275, 211–219. [Google Scholar] [CrossRef]
- Bracey, D.N.; Brown, M.L.; Beard, H.R.; Mannava, S.; Nazir, O.F.; Seyler, T.M.; Lang, J.E. Effects of patellofemoral overstuffing on knee flexion and patellar kinematics following total knee arthroplasty: A cadaveric study. Int. Orthop. 2015, 39, 1715–1722. [Google Scholar] [CrossRef]
- Abolghasemian, M.; Samiezadeh, S.; Sternheim, A.; Bougherara, H.; Barnes, C.L.; Backstein, D.J. Effect of patellar thickness on knee flexion in total knee arthroplasty: A biomechanical and experimental study. J. Arthroplast. 2014, 29, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-C.; Luo, Z.-P.; Rand, J.A.; An, K.-N. Influence of patellar thickness on patellar tracking and patellofemoral contact characteristics after total knee arthroplasty. J. Arthroplast. 1996, 11, 69–80. [Google Scholar] [CrossRef]
- Youm, Y.-S.; Cho, W.-S.; Woo, J.-H.; Kim, B.-K. The effect of patellar thickness changes on patellar tilt in total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 923–927. [Google Scholar] [CrossRef]
- Reuben, J.D.; McDonald, C.L.; Woodard, P.L.; Hennington, L.J. Effect of patella thickness on patella strain following total knee arthroplasty. J. Arthroplast. 1991, 6, 251–258. [Google Scholar] [CrossRef]
- Oishi, C.S.; Kaufman, K.R.; Irby, S.E.; Colwell, C.W. Effects of patellar thickness on compression and shear forces in total knee arthroplasty. Clin. Orthop. Relat. Res. 1996, 283–290. [Google Scholar] [CrossRef]
- Anglin, C.; Brimacombe, J.M.; Wilson, D.R.; Masri, B.A.; Greidanus, N.V.; Tonetti, J.; Hodgson, A.J. Biomechanical consequences of patellar component medialization in total knee arthroplasty. J. Arthroplast. 2010, 25, 793–802. [Google Scholar] [CrossRef]
- Donell, S. Patellar tracking in primary total knee arthroplasty. EFORT Open Rev. 2018, 3, 106–113. [Google Scholar] [CrossRef]
- Kawano, T.; Miura, H.; Nagamine, R.; Urabe, K.; Matsuda, S.; Mawatari, T.; Moro-Oka, T.; Iwamoto, Y. Factors affecting patellar tracking after total knee arthroplasty. J. Arthroplast. 2002, 17, 942–947. [Google Scholar] [CrossRef]
- Didden, K.; Luyckx, T.; Bellemans, J.; Labey, L.; Innocenti, B.; Vandenneucker, H. Anteroposterior positioning of the tibial component and its effect on the mechanics of patellofemoral contact. J. Bone Jt. Surg. Br. 2010, 92, 1466–1470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lewonowski, K.; Dorr, L.D.; McPherson, E.J.; Huber, G.; Wan, Z. Medialization of the patella in total knee arthroplasty. J. Arthroplast. 1997, 12, 161–167. [Google Scholar] [CrossRef]
- Kuriyama, S.; Ishikawa, M.; Furu, M.; Ito, H.; Matsuda, S. Malrotated tibial component increases medial collateral ligament tension in total knee arthroplasty. J. Orthop. Res. 2014, 32, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Tischer, T.; Geier, A.; Lenz, R.; Woernle, C.; Bader, R. Impact of the patella height on the strain pattern of the medial patellofemoral ligament after reconstruction: A computer model-based study. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3123–3133. [Google Scholar] [CrossRef]
- Geier, A.; Aschemann, H.; D Lima, D.; Woernle, C.; Bader, R. Force Closure Mechanism Modeling for Musculoskeletal Multibody Simulation. IEEE Trans. Biomed. Eng. 2018, 65, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Affatato, S.; Ruggiero, A. A Critical Analysis of TKR In Vitro Wear Tests Considering Predicted Knee Joint Loads. Materials 2019, 12, 1597. [Google Scholar] [CrossRef]
- Fregly, B.J.; Besier, T.F.; Lloyd, D.G.; Delp, S.L.; Banks, S.A.; Pandy, M.G.; D’Lima, D.D. Grand challenge competition to predict in vivo knee loads. J. Orthop. Res. 2012, 30, 503–513. [Google Scholar] [CrossRef]
- Spitzer, V.M.; Whitlock, D.G. The visible human dataset: The anatomical platform for human simulation. Anat. Rec. 1998, 253, 49–57. [Google Scholar] [CrossRef]
- Hippmann, G. An Algorithm for Compliant Contact Between Complexly Shaped Bodies. Multibody Syst. Dyn. 2004, 12, 345–362. [Google Scholar] [CrossRef]
- Wismans, J.; Veldpaus, F.; Janssen, J.; Huson, A.; Struben, P. A three-dimensional mathematical model of the knee-joint. J. Biomech. 1980, 13, 677–685. [Google Scholar] [CrossRef]
- Blankevoort, L.; Kuiper, J.H.; Huiskes, R.; Grootenboer, H.J. Articular contact in a three-dimensional model of the knee. J. Biomech. 1991, 24, 1019–1031. [Google Scholar] [CrossRef]
- Herrmann, S.; Kluess, D.; Kaehler, M.; Grawe, R.; Rachholz, R.; Souffrant, R.; Zierath, J.; Bader, R.; Woernle, C. A Novel Approach for Dynamic Testing of Total Hip Dislocation under Physiological Conditions. PLoS ONE 2015, 10, e0145798. [Google Scholar] [CrossRef] [PubMed]
- Leardini, A.; Chiari, L.; Della Croce, U.; Cappozzo, A. Human movement analysis using stereophotogrammetry. Part 3. Soft tissue artifact assessment and compensation. Gait Posture 2005, 21, 212–225. [Google Scholar] [CrossRef]
- Anderson, F.C.; Pandy, M.G. Static and dynamic optimization solutions for gait are practically equivalent. J. Biomech. 2001, 34, 153–161. [Google Scholar] [CrossRef]
- Geier, A.; Kebbach, M.; Soodmand, E.; Woernle, C.; Kluess, D.; Bader, R. Neuro-musculoskeletal flexible multibody simulation yields a framework for efficient bone failure risk assessment. Sci. Rep. 2019, 9, 6928. [Google Scholar] [CrossRef]
- Thelen, D.G.; Anderson, F.C.; Delp, S.L. Generating dynamic simulations of movement using computed muscle control. J. Biomech. 2003, 36, 321–328. [Google Scholar] [CrossRef]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; Wiley: Hoboken, NJ, USA, 2009; ISBN 9780470398180. [Google Scholar]
- Wu, G.; Siegler, S.; Allard, P.; Kirtley, C.; Leardini, A.; Rosenbaum, D.; Whittle, M.; D’Lima, D.D.; Cristofolini, L.; Witte, H.; et al. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion--part I: Ankle, hip, and spine. International Society of Biomechanics. J. Biomech. 2002, 35, 543–548. [Google Scholar] [CrossRef]
- Grood, E.S.; Suntay, W.J. A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. J. Biomech. Eng. 1983, 105, 136–144. [Google Scholar] [CrossRef]
- Bull, A.M.J.; Katchburian, M.V.; Shih, Y.-F.; Amis, A.A. Standardisation of the description of patellofemoral motion and comparison between different techniques. Knee Surg. Sports Traumatol. Arthrosc. 2002, 10, 184–193. [Google Scholar] [CrossRef]
- Carbone, V.; Fluit, R.; Pellikaan, P.; van der Krogt, M.M.; Janssen, D.; Damsgaard, M.; Vigneron, L.; Feilkas, T.; Koopman, H.F.J.M.; Verdonschot, N. TLEM 2.0—A comprehensive musculoskeletal geometry dataset for subject-specific modeling of lower extremity. J. Biomech. 2015, 48, 734–741. [Google Scholar] [CrossRef]
- An, K.N.; Kaufman, K.R.; Chao, E.Y. Physiological considerations of muscle force through the elbow joint. J. Biomech. 1989, 22, 1249–1256. [Google Scholar] [CrossRef]
- Charlton, I.W.; Johnson, G.R. Application of spherical and cylindrical wrapping algorithms in a musculoskeletal model of the upper limb. J. Biomech. 2001, 34, 1209–1216. [Google Scholar] [CrossRef]
- Smith, C.R.; Vignos, M.F.; Lenhart, R.L.; Kaiser, J.; Thelen, D.G. The Influence of Component Alignment and Ligament Properties on Tibiofemoral Contact Forces in Total Knee Replacement. J. Biomech. Eng. 2016, 138, 21017. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.A.; Vanheule, V.; Fluit, R.; Koopman, B.H.F.J.M.; Rasmussen, J.; Verdonschot, N.; Andersen, M.S. A subject-specific musculoskeletal modeling framework to predict in vivo mechanics of total knee arthroplasty. J. Biomech. Eng. 2015, 137, 20904. [Google Scholar] [CrossRef]
- Ortug, A.; Ormeci, T.; Yuzbasioglu, N.; Albay, S.; Seker, M. Evaluation of normal tibial tubercle to trochlear groove distance in adult Turkish population. Niger. J. Clin. Pract. 2018, 21, 1403–1407. [Google Scholar] [CrossRef]
- Schlenzka, D.; Schwesinger, G. The height of the patella: An anatomical study. Eur. J. Radiol. 1990, 11, 19–21. [Google Scholar] [CrossRef]
- Baldwin, J.L.; House, C.K. Anatomic dimensions of the patella measured during total knee arthroplasty. J. Arthroplast. 2005, 20, 250–257. [Google Scholar] [CrossRef]
- Herrmann, S.; Kähler, M.; Grawe, R.; Kluess, D.; Woernle, C.; Bader, R. Physiological-Like Testing of the Dislocation Stability of Artificial Hip Joints. In New Trends in Mechanism and Machine Science: From Fundamentals to Industrial Applications; Flores, P., Viadero, F., Eds.; Springer: Cham, Swizerland, 2015; pp. 659–667. ISBN 978-3-319-09410-6. [Google Scholar]
- Thelen, D.G.; Won Choi, K.; Schmitz, A.M. Co-simulation of neuromuscular dynamics and knee mechanics during human walking. J. Biomech. Eng. 2014, 136, 21033. [Google Scholar] [CrossRef]
- Wallace, D.A.; Salem, G.J.; Salinas, R.; Powers, C.M. Patellofemoral joint kinetics while squatting with and without an external load. J. Orthop. Sports Phys. Ther. 2002, 32, 141–148. [Google Scholar] [CrossRef]
- Innocenti, B.; Pianigiani, S.; Labey, L.; Victor, J.; Bellemans, J. Contact forces in several TKA designs during squatting: A numerical sensitivity analysis. J. Biomech. 2011, 44, 1573–1581. [Google Scholar] [CrossRef]
- Sharma, A.; Komistek, R.D.; Ranawat, C.S.; Dennis, D.A.; Mahfouz, M.R. In vivo contact pressures in total knee arthroplasty. J. Arthroplast. 2007, 22, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Kutzner, I.; Heinlein, B.; Graichen, F.; Bender, A.; Rohlmann, A.; Halder, A.; Beier, A.; Bergmann, G. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J. Biomech. 2010, 43, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Woiczinski, M.; Steinbrück, A.; Weber, P.; Müller, P.E.; Jansson, V.; Schröder, C. Development and validation of a weight-bearing finite element model for total knee replacement. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- McPherson, E.J. Patellar tracking in primary total knee arthroplasty. Instr. Course Lect. 2006, 55, 439–448. [Google Scholar]
- Schütz, P.; Postolka, B.; Gerber, H.; Ferguson, S.J.; Taylor, W.R.; List, R. Knee implant kinematics are task-dependent. J. R. Soc. Interface 2019, 16, 20180678. [Google Scholar] [CrossRef]
- Mulholland, S.J.; Wyss, U.P. Activities of daily living in non-Western cultures: Range of motion requirements for hip and knee joint implants. Int. J. Rehabil. Res. 2001, 24, 191–198. [Google Scholar] [CrossRef]
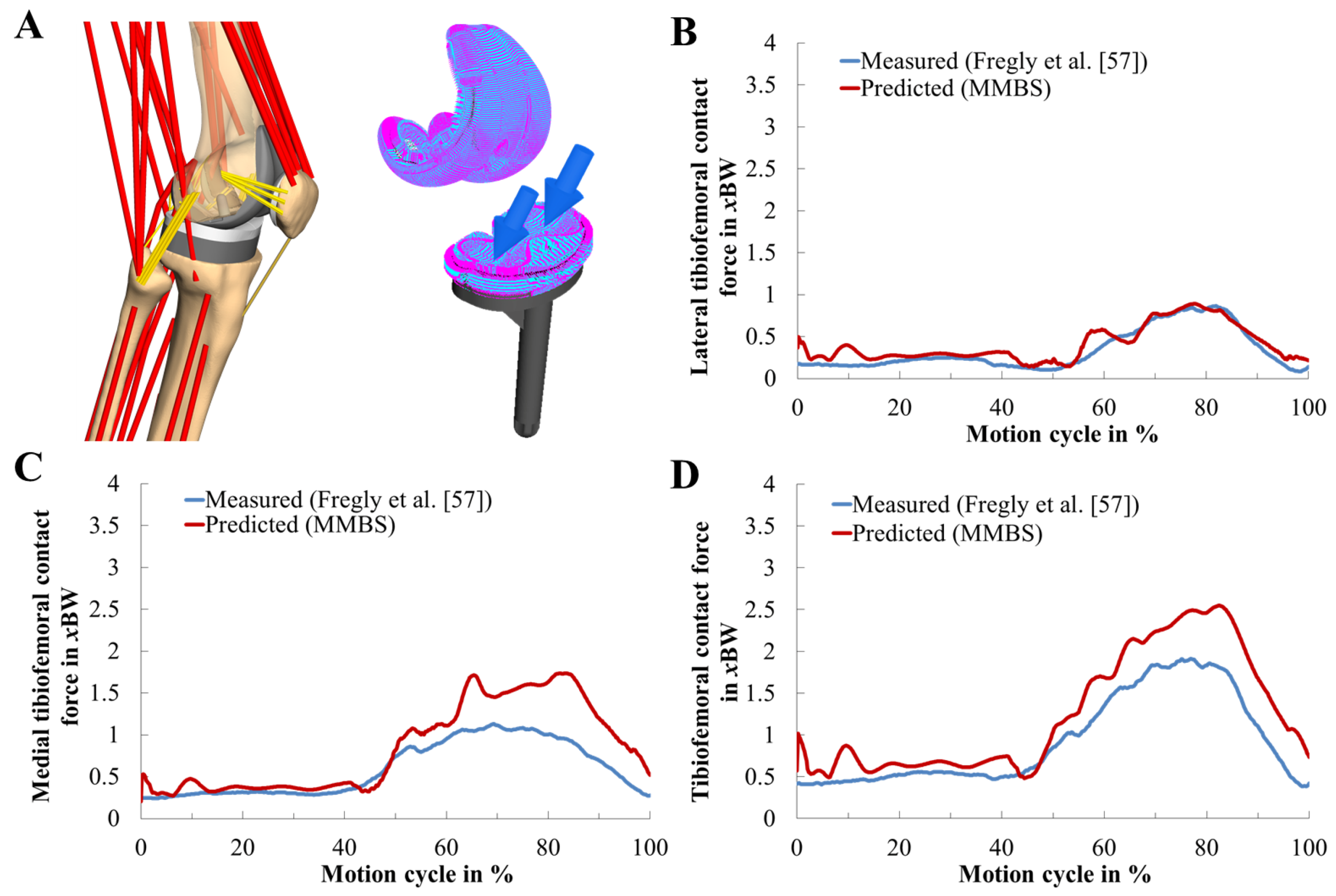
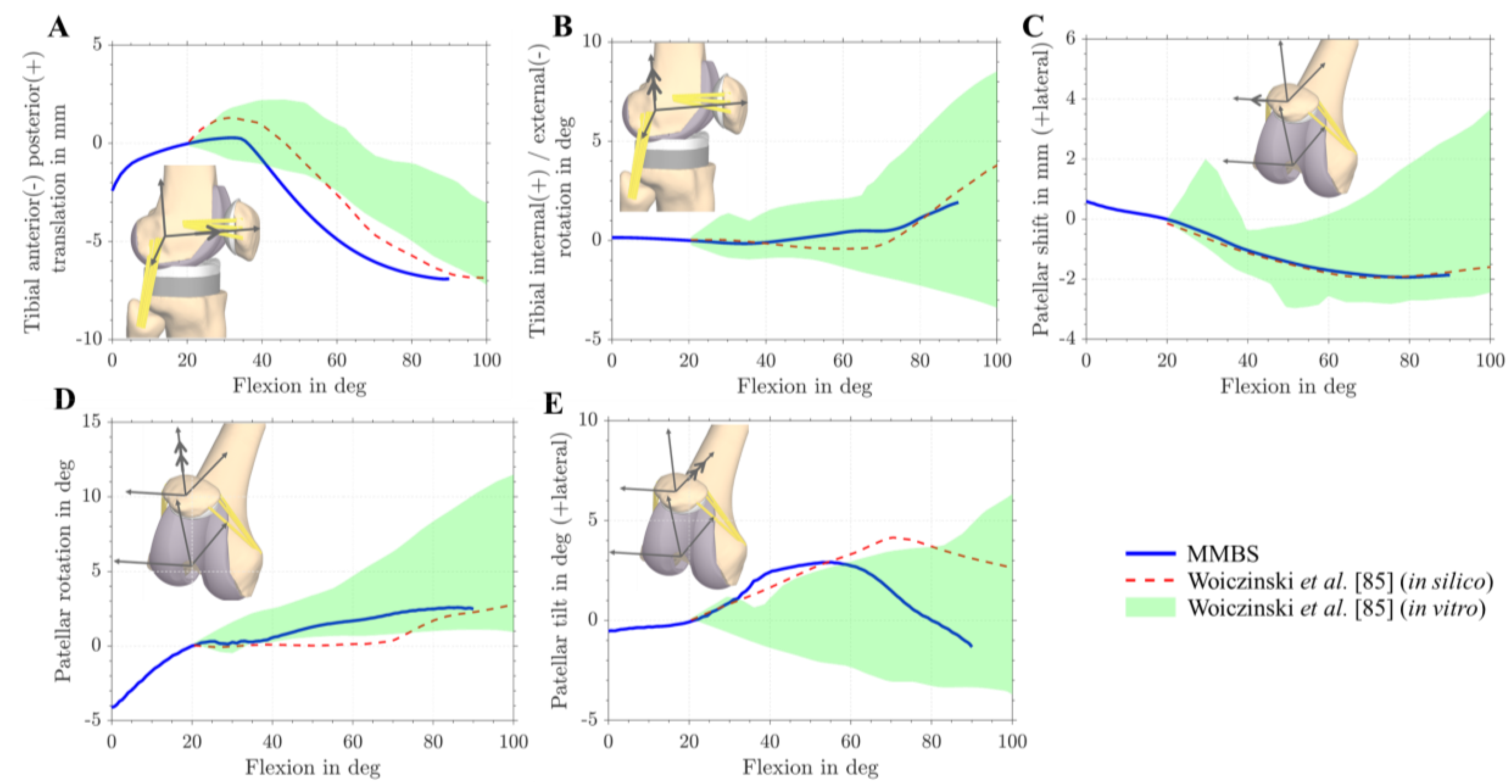
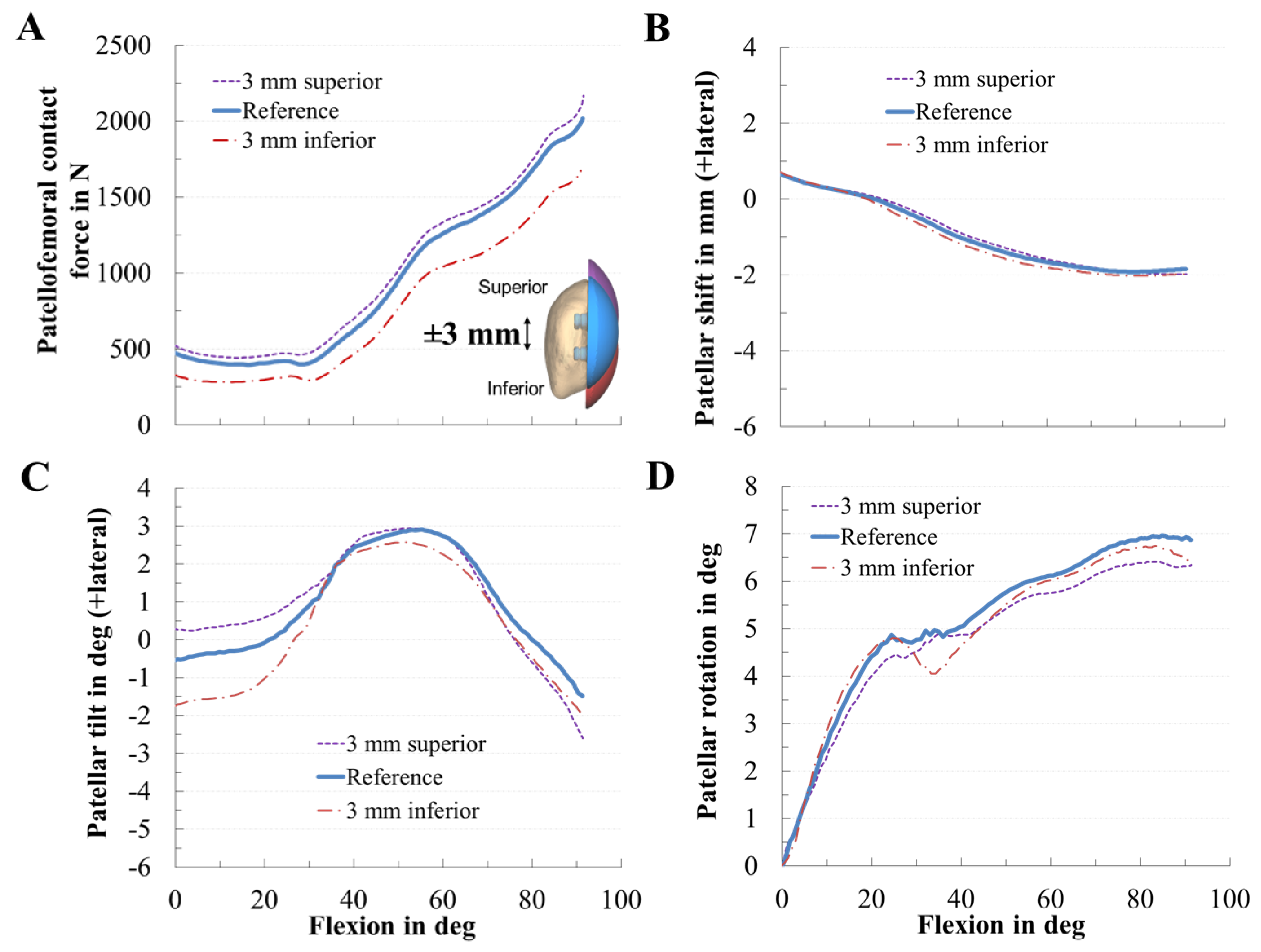
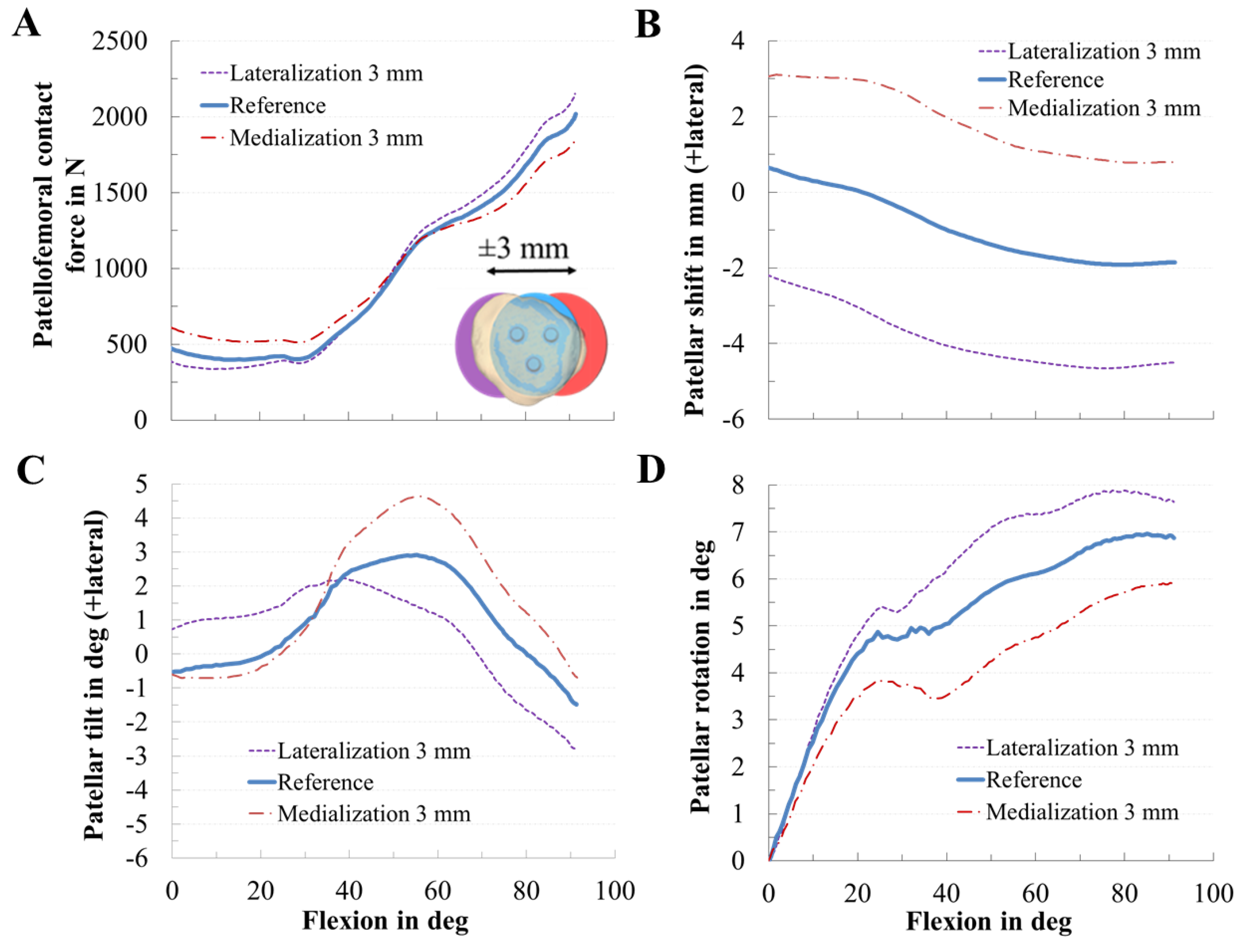
| Value | MAD [N] | MAD [xBW] | RMSE [N] | RMSE [xBW] | ||
|---|---|---|---|---|---|---|
| Total knee contact force | 209.68 | 0.32 | 255.19 | 0.39 | 0.97 | 0.94 |
| Medial contact force | 163.02 | 0.25 | 226.65 | 0.35 | 0.94 | 0.89 |
| Lateral contact force | 46.67 | 0.07 | 68.28 | 0.10 | 0.95 | 0.91 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kebbach, M.; Darowski, M.; Krueger, S.; Schilling, C.; Grupp, T.M.; Bader, R.; Geier, A. Musculoskeletal Multibody Simulation Analysis on the Impact of Patellar Component Design and Positioning on Joint Dynamics after Unconstrained Total Knee Arthroplasty. Materials 2020, 13, 2365. https://doi.org/10.3390/ma13102365
Kebbach M, Darowski M, Krueger S, Schilling C, Grupp TM, Bader R, Geier A. Musculoskeletal Multibody Simulation Analysis on the Impact of Patellar Component Design and Positioning on Joint Dynamics after Unconstrained Total Knee Arthroplasty. Materials. 2020; 13(10):2365. https://doi.org/10.3390/ma13102365
Chicago/Turabian StyleKebbach, Maeruan, Martin Darowski, Sven Krueger, Christoph Schilling, Thomas M. Grupp, Rainer Bader, and Andreas Geier. 2020. "Musculoskeletal Multibody Simulation Analysis on the Impact of Patellar Component Design and Positioning on Joint Dynamics after Unconstrained Total Knee Arthroplasty" Materials 13, no. 10: 2365. https://doi.org/10.3390/ma13102365
APA StyleKebbach, M., Darowski, M., Krueger, S., Schilling, C., Grupp, T. M., Bader, R., & Geier, A. (2020). Musculoskeletal Multibody Simulation Analysis on the Impact of Patellar Component Design and Positioning on Joint Dynamics after Unconstrained Total Knee Arthroplasty. Materials, 13(10), 2365. https://doi.org/10.3390/ma13102365