Effect of Pre-Wetted Zeolite Sands on the Autogenous Shrinkage and Strength of Ultra-High-Performance Concrete
Abstract
1. Introduction
2. Materials and Methods
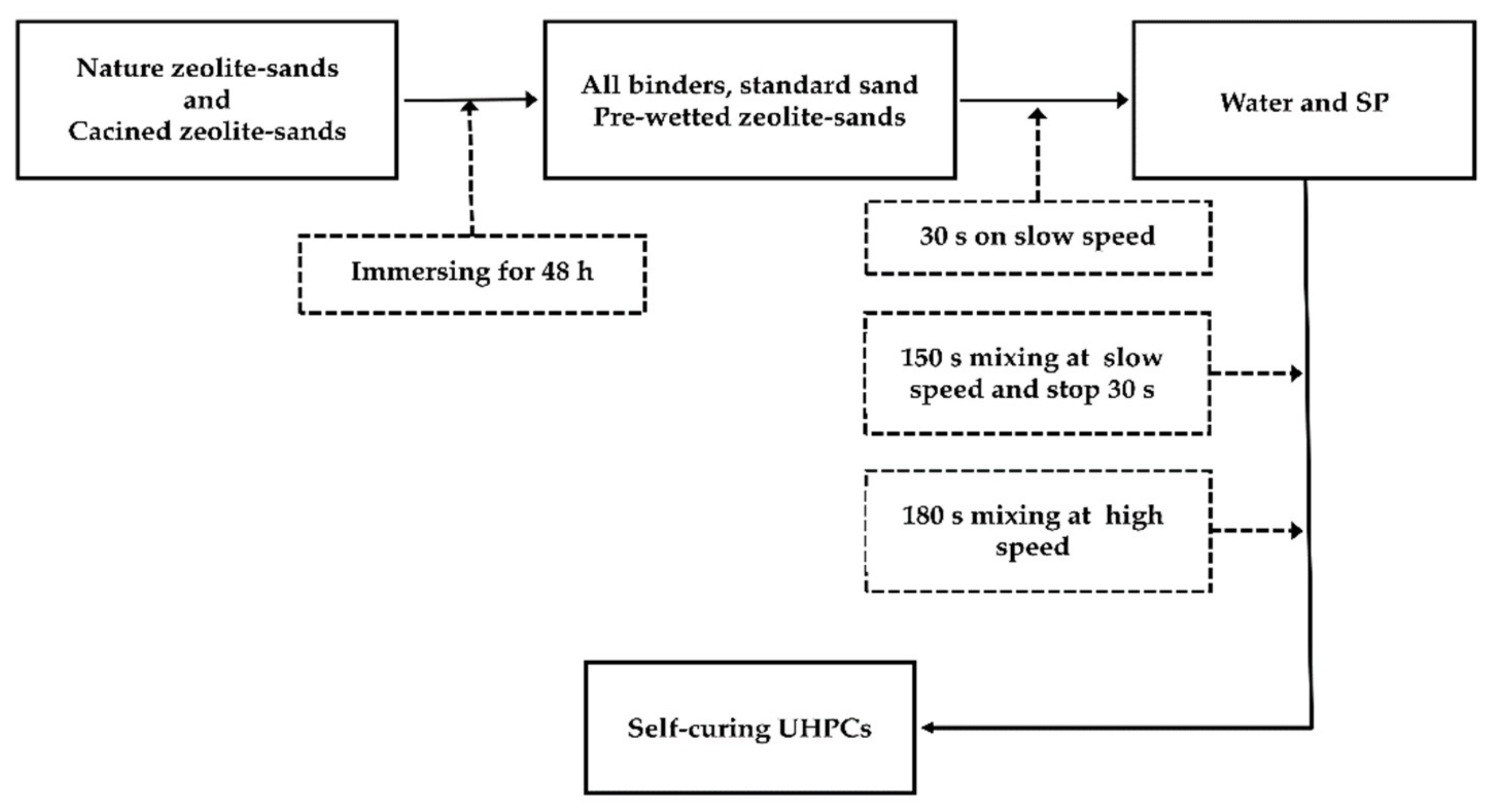
2.1. Materials and Sample Preparation
2.2. Test Methods
2.2.1. Water Absorption
2.2.2. Autogenous Shrinkage
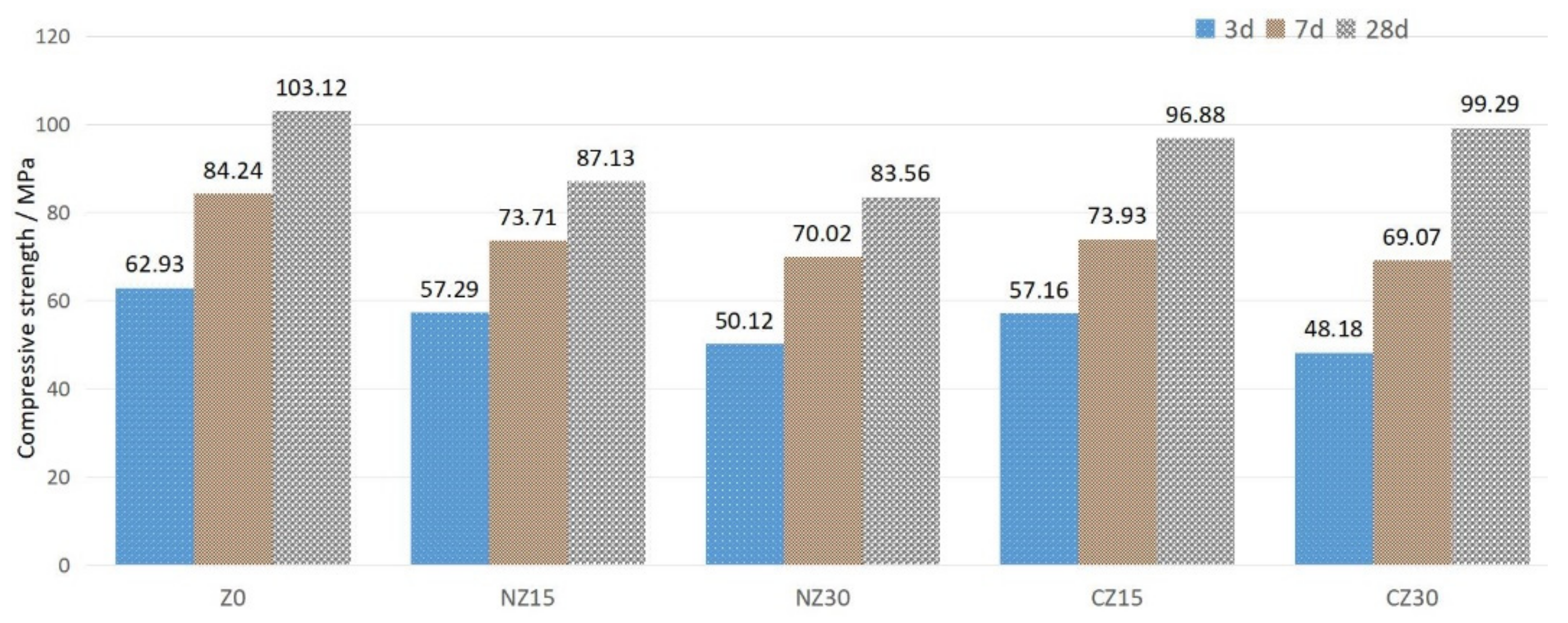
2.2.3. Compressive Strength Test
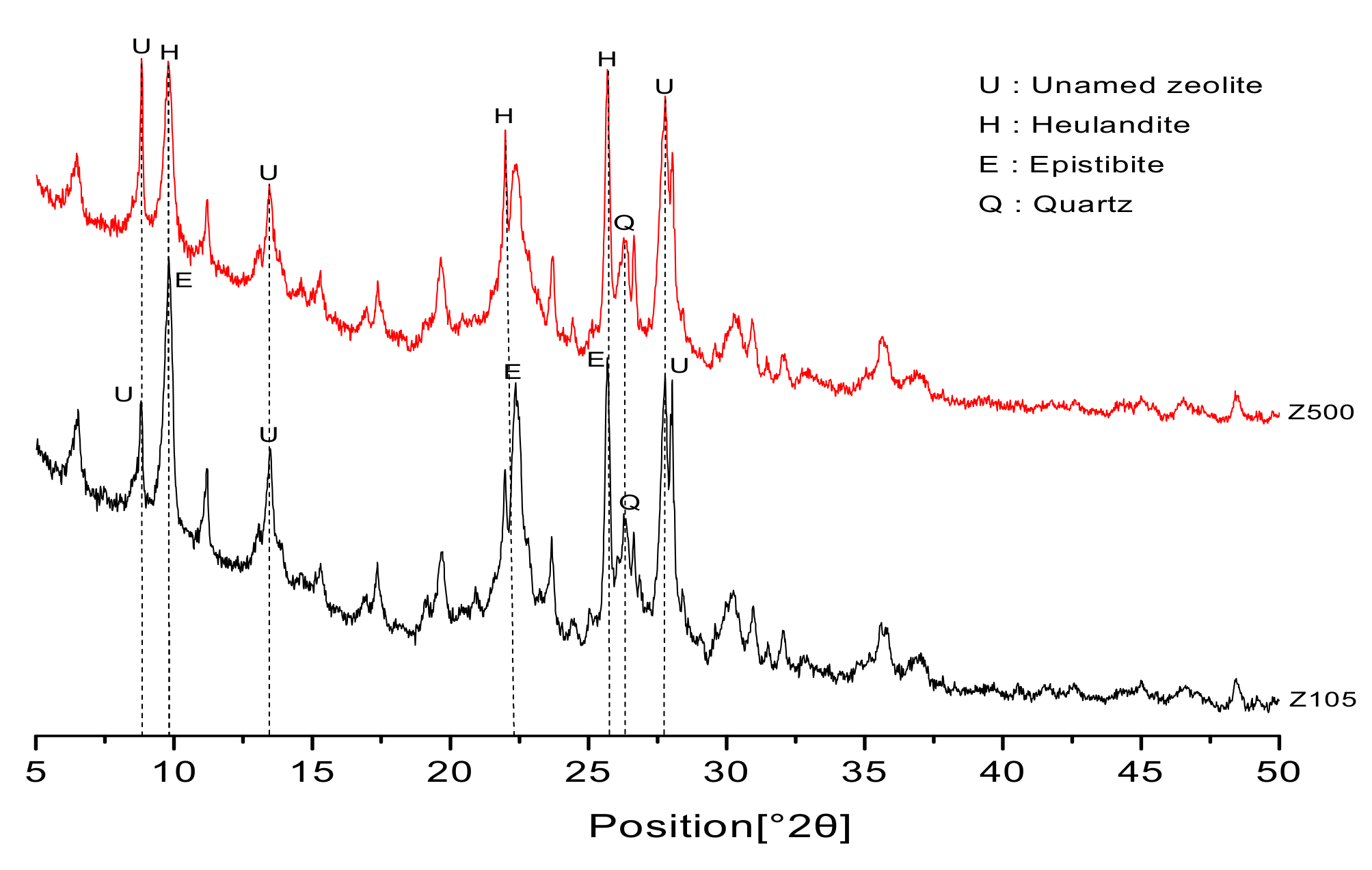
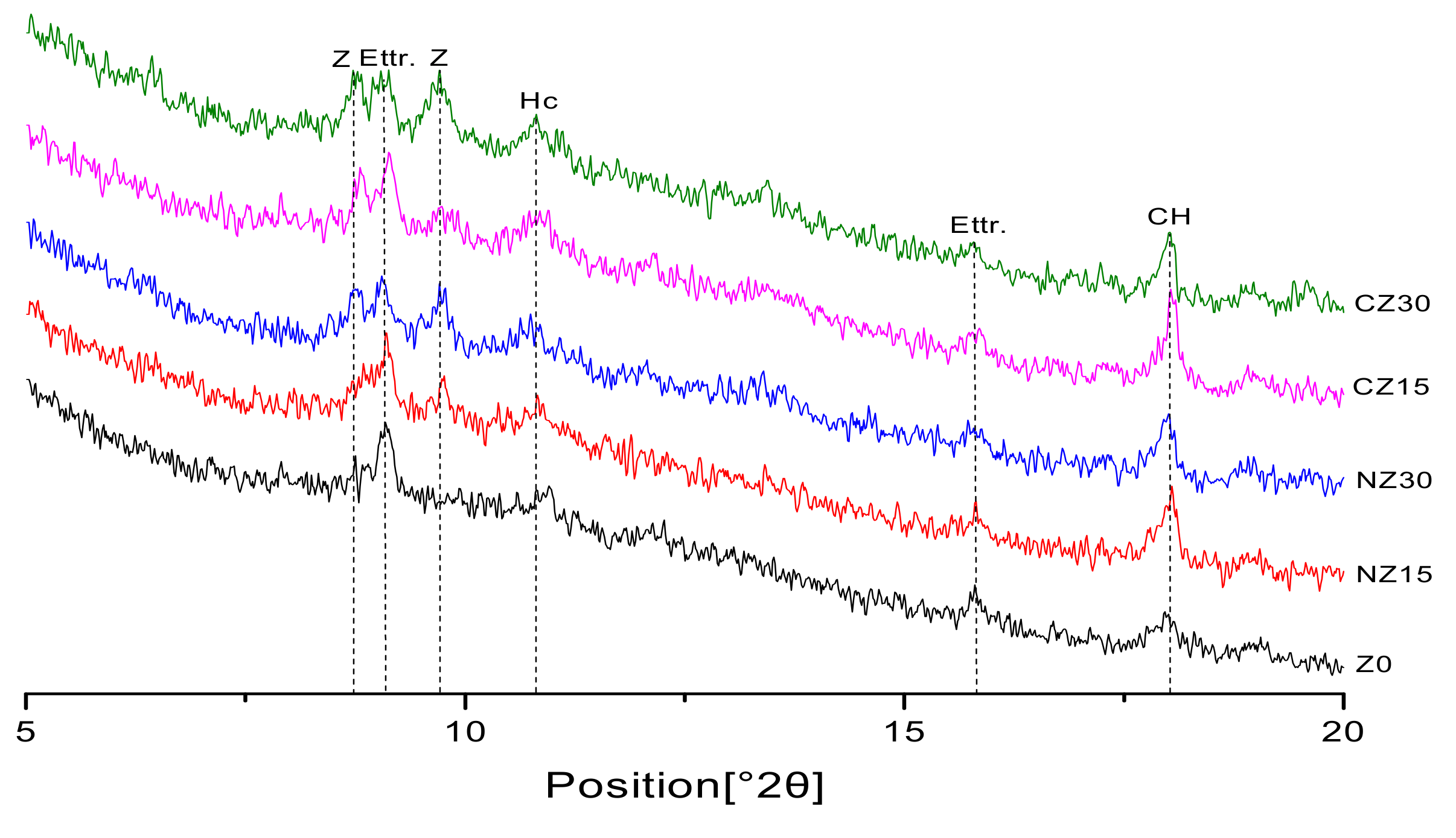
2.2.4. X-Ray Diffraction
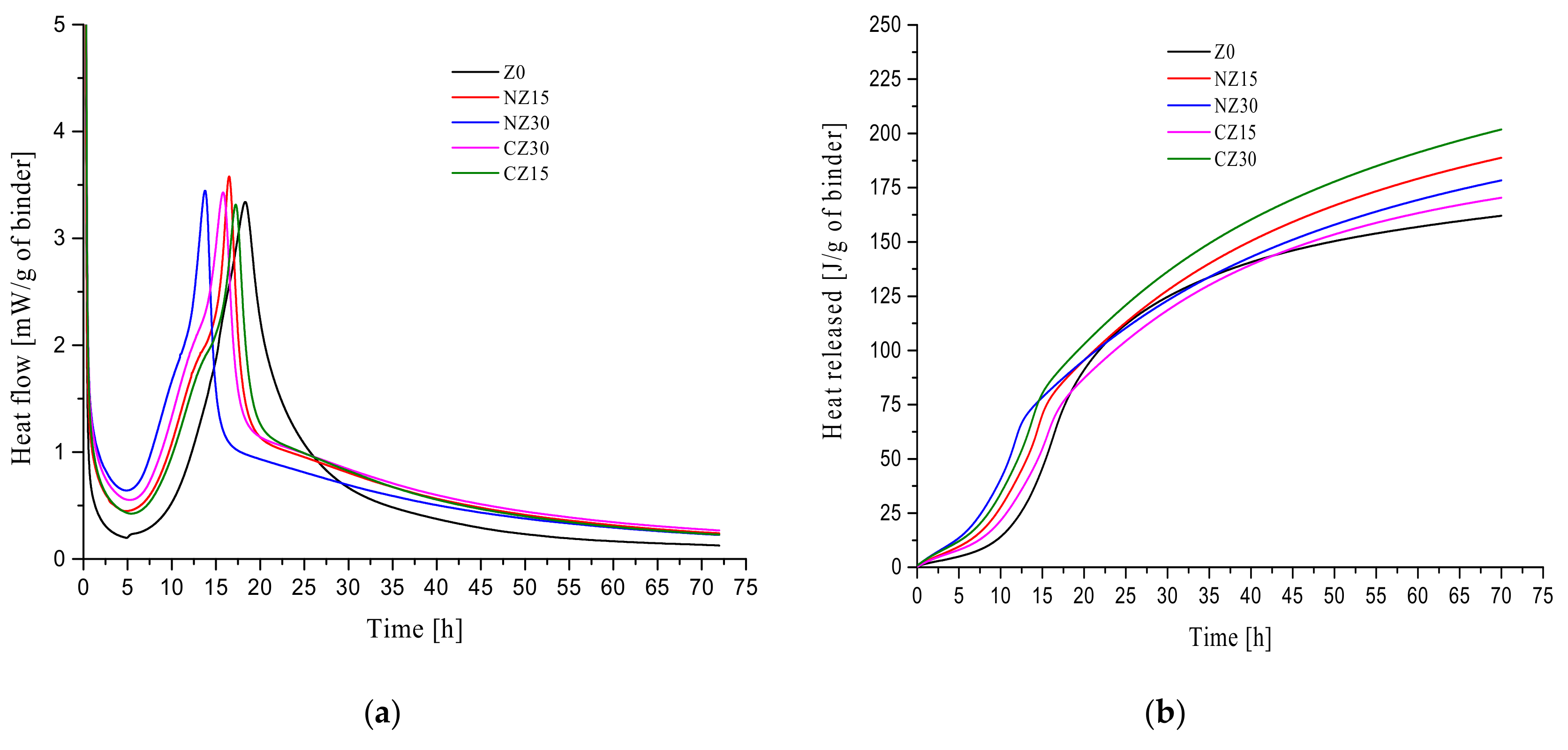
2.2.5. Isothermal Calorimetry
3. Results and Discussions
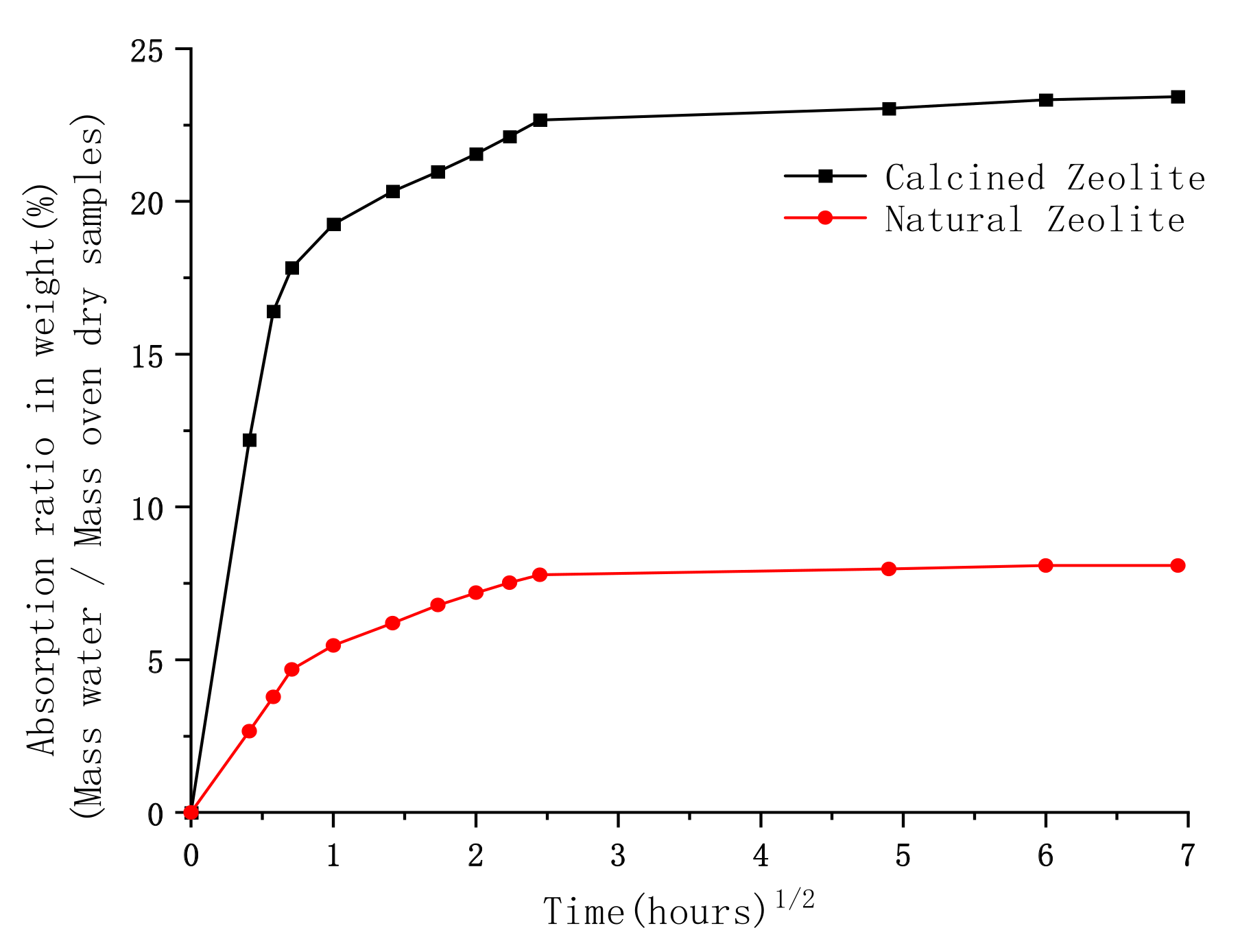
3.1. Water Absorption
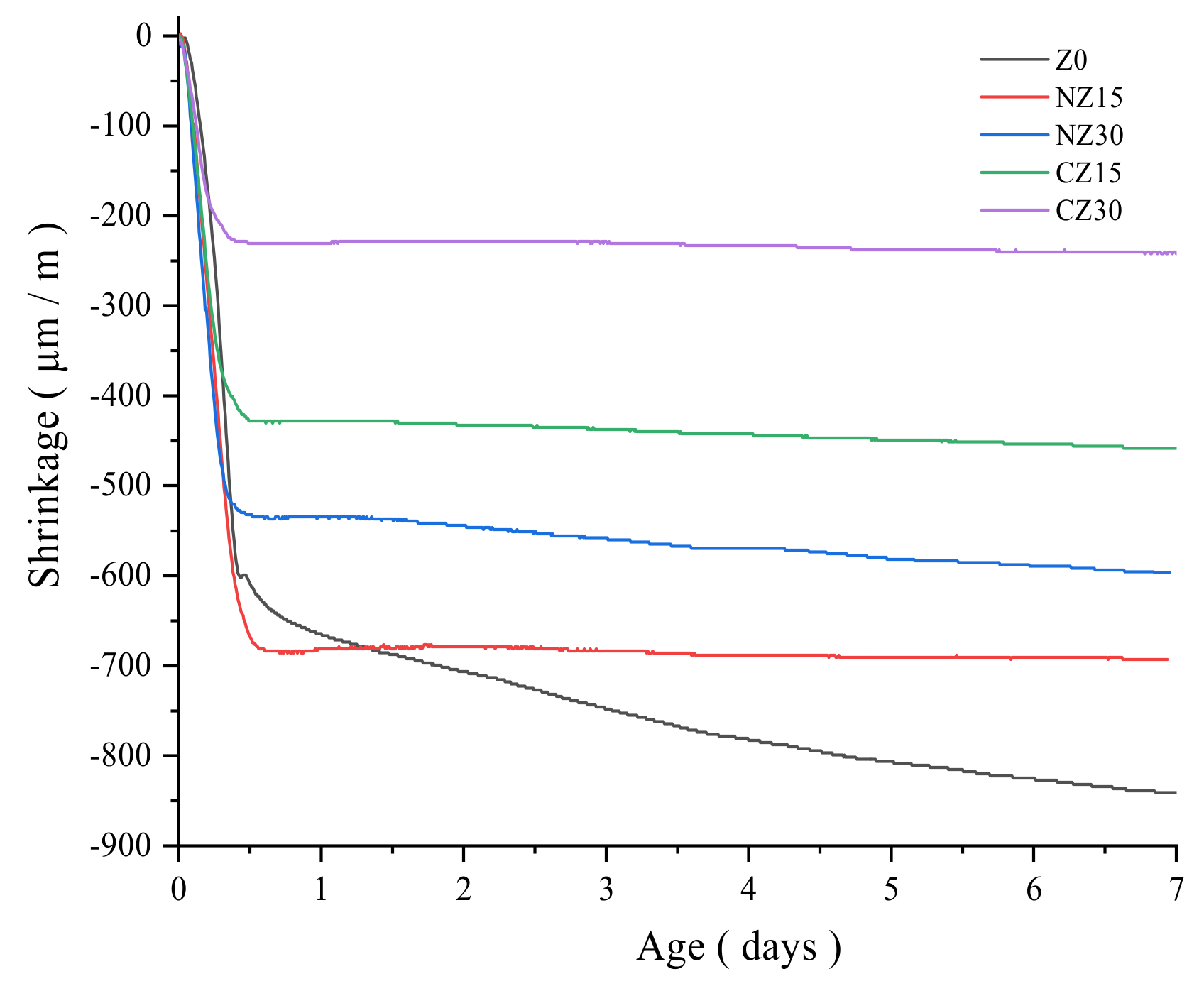
3.2. Autogenous Shrinkage
3.3. X-Ray Diffraction (XRD)
3.4. Isothermal Calorimetry
3.5. Compressive Strength
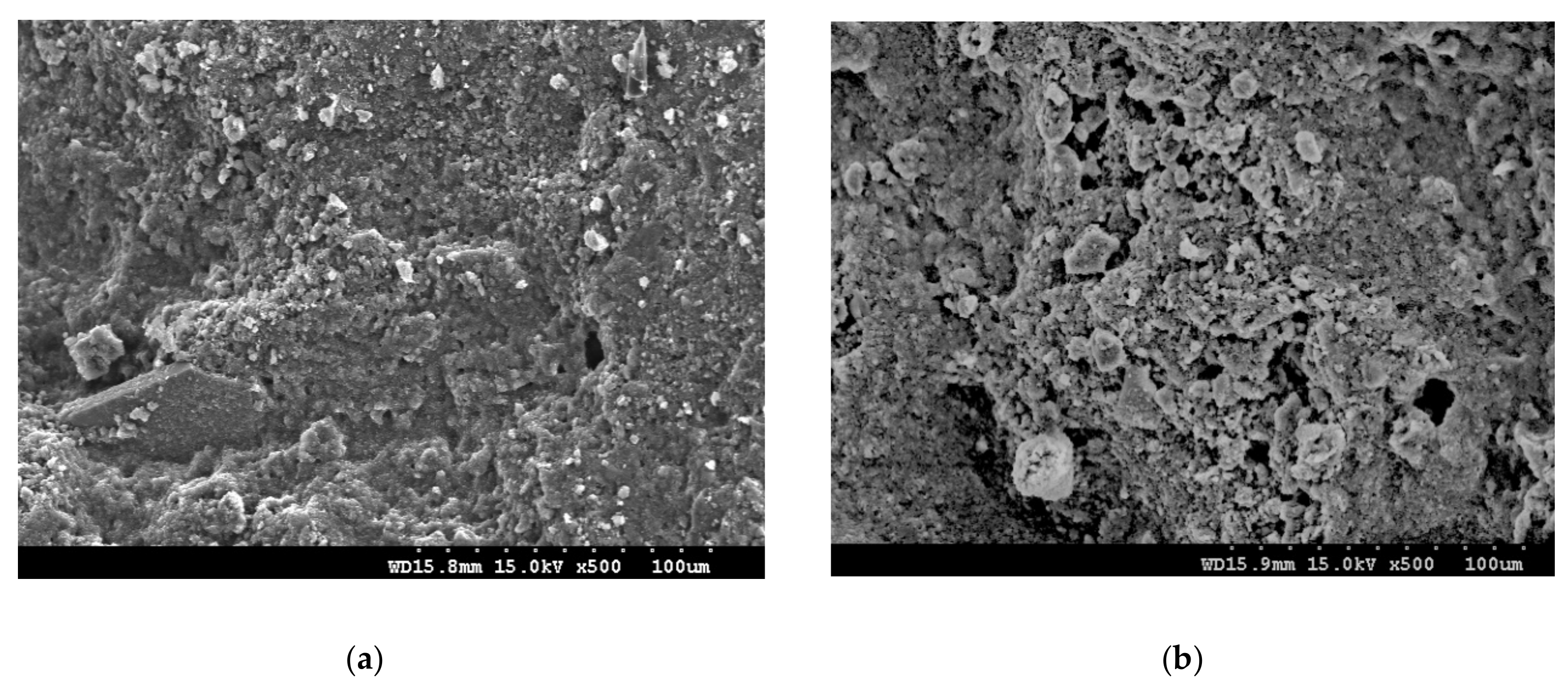
3.6. Discussion
4. Conclusions
- Compared with natural zeolite sand, the water absorption of calcined zeolite sand is obviously increased. This is due to the initial physical bonding water present in the natural zeolite sand being evaporated during heating, resulting in more pores becoming empty.
- The autogenous shrinkage of self-curing UHPC is significantly reduced due to the addition of zeolite sands. Among the resulting outcomes, the effect of adding calcined zeolite sand to UHPC is the most obvious. This is because calcined zeolite sand can absorb more internal curing water before mixing. With the occurrence of the hydration reaction, internal curing water is released to continue to participate in the hydration reaction, thereby reducing autogenous shrinkage.
- For specimens with zeolite sand additions, XRD analysis showed that there were no new hydration-product peaks, indicating that zeolite sand particles do not participate in binder hydration. The peak of calcium hydroxide increased significantly with the addition of the zeolite sands. This is because the specimens containing zeolite sands contain more internal curing water. With the occurrence of the cement hydration reaction, the water in the reservoir is released to further promote cement hydration, and more hydration products are obtained.
- It could be observed that the total heat released from self-curing UHPC is higher than that from the control group, Z0. In the initial period, the heat release rate of self-curing UHPC increased because of the addition of zeolite sand. As the content of calcined zeolite sand increased, the heat release rate of the mortars accelerated. In the acceleration period, the time at which heat flow peaks of each hydration heat level appeared is in the order of NZ30 > CZ30 > NZ15 > CZ15 > Z0. During the deceleration period, it could be observed that the slope of the heat release rate cures of CZ15 is the same as that of CZ30 with respect to the hydration reaction. The slope of the heat release rate cures of NZ15 is the same as that of NZ30 with respect to the hydration reaction.
- At the age of 3 days, UHPC has a negative effect due to the addition of zeolite sands with pores, which reduces the compressive strength. However, at the age of 28 days, UHPC with calcined zeolite sand showed a good compressive strength, compared with the control group. This is due to the fact that calcined zeolite sand absorbs more internal curing water, and the internal curing water is released to participate in binder hydration and contributes to the development of compressive strength.
Author Contributions
Funding
Conflicts of Interest
References
- Abdulkareem, O.M.; Fraj, A.B.; Bouasker, M.; Khelidj, A. Mixture design and early age investigations of more sustainable UHPC. Constr. Build. Mater. 2018, 163, 235–246. [Google Scholar] [CrossRef]
- Li, P.P.; Yu, Q.L.; Brouwers, H.J.H. Effect of PCE-type superplasticizer on early-age behaviour of ultra-high performance concrete (UHPC). Constr. Build. Mater. 2017, 153, 740–750. [Google Scholar] [CrossRef]
- Randl, N.; Steiner, T.; Ofner, S.; Baumgartner, E.; Mészöly, T. Development of UHPC mixtures from an ecological point of view. Constr. Build. Mater. 2014, 67, 373–378. [Google Scholar] [CrossRef]
- Ghourchian, S.; Wyrzykowski, M.; Lura, P.; Shekarchi, M.; Ahmadi, B. An investigation on the use of zeolite aggregates for internal curing of concrete. Constr. Build. Mater. 2013, 40, 135–144. [Google Scholar] [CrossRef]
- Buck, J.J.; McDowell, D.L.; Zhou, M. Effect of microstructure on load-carrying and energy-dissipation capacities of UHPC. Cem. Concr. Res. 2013, 43, 34–50. [Google Scholar] [CrossRef]
- Yoo, D.-Y.; Min, K.-H.; Lee, J.-H.; Yoon, Y.-S. Shrinkage and cracking of restrained ultra-high-performance fiber-reinforced concrete slabs at early age. Constr. Build. Mater. 2014, 73, 357–365. [Google Scholar] [CrossRef]
- Yang, L.; Shi, C.; Wu, Z. Mitigation techniques for autogenous shrinkage of ultra-high-performance concrete—A review. Compos. Part B Eng. 2019, 178, 107456. [Google Scholar] [CrossRef]
- Justs, J.; Wyrzykowski, M.; Bajare, D.; Lura, P. Internal curing by superabsorbent polymers in ultra-high performance concrete. Cem. Concr. Res. 2015, 76, 82–90. [Google Scholar] [CrossRef]
- Mo, J.; Ou, Z.; Zhao, X.; Liu, J.; Wang, Y. Influence of superabsorbent polymer on shrinkage properties of reactive powder concrete blended with granulated blast furnace slag. Constr. Build. Mater. 2017, 146, 283–296. [Google Scholar] [CrossRef]
- Liu, J.; Ou, Z.; Mo, J.; Chen, Y.; Guo, T.; Deng, W. Effectiveness of Saturated Coral Aggregate and Shrinkage Reducing Admixture on the Autogenous Shrinkage of Ultrahigh Performance Concrete. Adv. Mater. Sci. Eng. 2017, 2017, 2703264. [Google Scholar] [CrossRef]
- Meng, W.; Khayat, K. Effects of saturated lightweight sand content on key characteristics of ultra-high-performance concrete. Cem. Concr. Res. 2017, 101, 46–54. [Google Scholar] [CrossRef]
- Liu, J.; Farzadnia, N.; Shi, C. Effects of superabsorbent polymer on interfacial transition zone and mechanical properties of ultra-high performance concrete. Constr. Build. Mater. 2020, 231, 117142. [Google Scholar] [CrossRef]
- Sun, B.; Wu, H.; Song, W.; Li, Z.; Yu, J. Design methodology and mechanical properties of Superabsorbent Polymer (SAP) cement-based materials. Constr. Build. Mater. 2019, 204, 440–449. [Google Scholar] [CrossRef]
- Farzanian, K.; TeixeiraI, K.P.; Rocha, P.; Carneiro, L.D.S.; Ghahremaninezhad, A. The mechanical strength, degree of hydration, and electrical resistivity of cement pastes modified with superabsorbent polymers. Constr. Build. Mater. 2016, 109, 156–165. [Google Scholar] [CrossRef]
- Bentz, D.P.; Geiker, M.; Jensen, O.M. On the mitigation of early age cracking. Int. Semin. Self Desiccation III 2002, 3, 195–204. [Google Scholar]
- Woyciechowski, P.P.; Kalinowski, M. The Influence of Dosing Method and Material Characteristics of Superabsorbent Polymers (SAP) on the Effectiveness of the Concrete Internal Curing. Materials 2018, 11, 1600. [Google Scholar] [CrossRef]
- Liu, K.; Yu, R.; Shui, Z.; Li, X.; Guo, C.; Yu, B.; Wu, S. Optimization of autogenous shrinkage and microstructure for Ultra-High Performance Concrete (UHPC) based on appropriate application of porous pumice. Constr. Build. Mater. 2019, 214, 369–381. [Google Scholar] [CrossRef]
- Wang, X.F.; Fang, C.; Kuang, W.Q.; Lin, D.W.; Han, X.; Xing, F. Experimental investigation on the compressive strength and shrinkage of concrete with pre-wetted lightweight aggregates. Constr. Build. Mater. 2017, 155, 867–879. [Google Scholar] [CrossRef]
- Ackley, M.W.; Rege, S.U.; Saxena, H. Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 2003, 61, 25–42. [Google Scholar] [CrossRef]
- Klaysom, C.; Shahid, S. Chapter 6—Zeolite-based mixed matrix membranes for hazardous gas removal. Advanced Nanomaterials for Membrane Synthesis and Its Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 127–157. [Google Scholar]
- Ates, A.; Hardacre, C. The effect of various treatment conditions on natural zeolites: Ion exchange, acidic, thermal and steam treatments. J. Colloid Interface Sci. 2012, 372, 130–140. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Zorpas, A.A. Natural Zeolites Structure and Porosity; Bentham Books: Bacau, Romania, 2012; p. 14. ISBN 9781608054466. [Google Scholar]
- Zhang, J.; Wang, Q.; Ding, X.; Zheng, X. High-strength concrete mixture with calcined zeolite particles for shrinkage reduction. Mag. Concr. Res. 2019, 71, 690–699. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Zhang, J. Shrinkage of internal cured high strength engineered cementitious composite with pre-wetted sand-like zeolite. Constr. Build. Mater. 2017, 134, 664–672. [Google Scholar] [CrossRef]
- Pezeshkian, M.; Delnavaz, A.; Delnavaz, M. Effect of Natural Zeolite on Mechanical Properties and Autogenous Shrinkage of Ultrahigh-Performance Concrete. J. Mater. Civ. Eng. 2020, 32, 04020093. [Google Scholar] [CrossRef]
- Li, C.; Thomas, M.D.A.; Ideker, J.H. A mechanistic study on mitigation of alkali-silica reaction by fine lightweight aggregates. Cem. Concr. Res. 2018, 104, 13–24. [Google Scholar] [CrossRef]
- Suraneni, P.; Fu, T.; Azad, V.J.; Isgor, O.B.; Jason, W. Pozzolanicity of finely ground lightweight aggregates. Cem. Concr. Compos. 2018, 88, 115–120. [Google Scholar] [CrossRef]
- Lin, R.-S.; Park, K.-B.; Wang, X.-Y.; Zhang, G.-Y. Increasing the early strength of high-volume Hwangtoh–cement systems using bassanite. J. Build. Eng. 2020, 30, 101317. [Google Scholar] [CrossRef]
- Bentz, D.P.; Snyder, K.A. Protected paste volume in concrete: Extension to internal curing using saturated lightweight fine aggregate. Cem. Concr. Res. 1999, 29, 1863–1867. [Google Scholar] [CrossRef]
- Holm, T.A.; Ooi, O.-S.; Bremner, T.W. Moisture Dynamics in Lightweight Aggregate and Concrete. In Proceedings of the 6th International Conference on the Durability of Concrete, Thessaloniki, Greece, 1–7 June 2003. [Google Scholar]
- Castro, J.; Varga, I.D.L.; Weiss, J. Using Isothermal Calorimetry to Assess the Water Absorbed by Fine LWA during Mixing. J. Mater. Civ. Eng. 2012, 24, 996–1005. [Google Scholar] [CrossRef]
- Castro, J.; Keiser, L.; Golias, M.; Weiss, J. Absorption and desorption properties of fine lightweight aggregate for application to internally cured concrete mixtures. Cem. Concr. Compos. 2011, 33, 1001–1008. [Google Scholar] [CrossRef]
- ASTM C1698. Standard Test Method for Autogenous Strain of Cement Paste and Mortar; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM International. Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Snellings, R.; Chwast, J.; Cizer, Ö.; De Belie, N.; Dhandapani, Y.; Durdzinski, P.; Elsen, J.; Haufe, J.; Hooton, D.; Patapy, C.; et al. Report of TC 238-SCM: Hydration stoppage methods for phase assemblage studies of blended cements—Results of a round robin test. Mater. Struct. 2018, 51, 111. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Lin, R.-S.; Wang, X.-Y.; Lee, H.-S.; Cho, H.-K. Hydration and Microstructure of Cement Pastes with Calcined Hwangtoh Clay. Materials 2019, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-S.; Wang, X.-Y.; Zhang, G.-Y. Effects of Quartz Powder on the Microstructure and Key Properties of Cement Paste. Sustainability 2018, 10, 3369. [Google Scholar] [CrossRef]
- Lv, Y.; Ye, G.; De Schutter, G. Investigation on the potential utilization of zeolite as an internal curing agent for autogenous shrinkage mitigation and the effect of modification. Constr. Build. Mater. 2019, 198, 669–676. [Google Scholar] [CrossRef]
- Montanari, L.; Suraneni, P.; Weiss, W.J. Accounting for Water Stored in Superabsorbent Polymers in Increasing the Degree of Hydration and Reducing the Shrinkage of Internally Cured Cementitious Mixtures. Adv. Civ. Eng. Mater. 2017, 6, 583–599. [Google Scholar] [CrossRef]
- Liu, J.; Shi, C.; Farzadnia, N.; Ma, X. Effects of pretreated fine lightweight aggregate on shrinkage and pore structure of ultra-high strength concrete. Constr. Build. Mater. 2019, 204, 276–287. [Google Scholar] [CrossRef]
- Koizumi, M.; Roy, R. Zeolite Studies. I. Synthesis and Stability of the Calcium Zeolites. J. Geol. 1960, 68, 41–53. [Google Scholar] [CrossRef]
- Seraj, S.; Ferron, R.D.; Juenger, M.C.G. Calcining natural zeolites to improve their effect on cementitious mixture workability. Cem. Concr. Res. 2016, 85, 102–110. [Google Scholar] [CrossRef]
- Lura, P.; Jensen, O.M.; Breugel, K.V. Autogenous shrinkage in high-performance cement paste: An evaluationof basic mechanisms. Cem. Concr. Res. 2003, 33, 223–232. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics, reaction products and compressive strength of ternary activators activated slag designed by Taguchi method. Mater. Des. 2015, 86, 878–886. [Google Scholar] [CrossRef]
- Abbas, S.; Soliman, A.M.; Nehdi, M.L. Exploring mechanical and durability properties of ultra-high performance concrete incorporating various steel fiber lengths and dosages. Constr. Build. Mater. 2015, 75, 429–441. [Google Scholar] [CrossRef]
- Arora, A.; Aguayo, M.; Hansen, H.; Castro, C.; Federspiel, E.; Mobasher, B.; Neithalath, N. Microstructural packing- and rheology-based binder selection and characterization for Ultra-high Performance Concrete (UHPC). Cem. Concr. Res. 2018, 103, 179–190. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Hussein, H.H.; Liu, J.; Chen, G. Experimental study and theoretical prediction on shrinkage-induced restrained stresses in UHPC-RC composites under normal curing and steam curing. Cem. Concr. Compos. 2020, 110, 103602. [Google Scholar] [CrossRef]
- Garas, V.Y.; Kurtis, K.E.; Kahn, L.F. Creep of UHPC in tension and compression: Effect of thermal treatment. Cem. Concr. Compos. 2012, 34, 493–502. [Google Scholar] [CrossRef]
- Valipour, M.; Khayat, K.H. Coupled effect of shrinkage-mitigating admixtures and saturated lightweight sand on shrinkage of UHPC for overlay applications. Constr. Build. Mater. 2018, 184, 320–329. [Google Scholar] [CrossRef]

| Materials | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | ZnO | K2O | P2O5 | Loss |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement (%) | 21.65 | 5.57 | 2.45 | 62.68 | 2.60 | 2.34 | 0.11 | 1.08 | 0.10 | 0.46 |
| Silica fume (%) | 93.80 | 0.93 | 0.56 | 0.518 | 0.66 | 0.23 | 0.16 | 1.76 | 0.08 | 0.63 |
| Zeolite sand (%) | 65.35 | 13.58 | 1.61 | 1.95 | 1.35 | 15.8 |
| Number | Binder / % | Sand/ Binder | Water/ Binder | Zeolite Sand / Sand % | Superplasticizer/ Binder % | |
|---|---|---|---|---|---|---|
| Cement | Silica Fume | |||||
| Z0 | 85 | 15 | 1.5 | 0.2 | 0 | 3 |
| NZ15 | 85 | 15 | 1.5 | 0.2 | 15 | 3 |
| NZ30 | 85 | 15 | 1.5 | 0.2 | 30 | 3 |
| CZ15 | 85 | 15 | 1.5 | 0.2 | 15 | 3 |
| CZ30 | 85 | 15 | 1.5 | 0.2 | 30 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.-Z.; Wang, X.-Y. Effect of Pre-Wetted Zeolite Sands on the Autogenous Shrinkage and Strength of Ultra-High-Performance Concrete. Materials 2020, 13, 2356. https://doi.org/10.3390/ma13102356
Zhang G-Z, Wang X-Y. Effect of Pre-Wetted Zeolite Sands on the Autogenous Shrinkage and Strength of Ultra-High-Performance Concrete. Materials. 2020; 13(10):2356. https://doi.org/10.3390/ma13102356
Chicago/Turabian StyleZhang, Guang-Zhu, and Xiao-Yong Wang. 2020. "Effect of Pre-Wetted Zeolite Sands on the Autogenous Shrinkage and Strength of Ultra-High-Performance Concrete" Materials 13, no. 10: 2356. https://doi.org/10.3390/ma13102356
APA StyleZhang, G.-Z., & Wang, X.-Y. (2020). Effect of Pre-Wetted Zeolite Sands on the Autogenous Shrinkage and Strength of Ultra-High-Performance Concrete. Materials, 13(10), 2356. https://doi.org/10.3390/ma13102356






