Properties of Concrete Made with Low-Emission Cements CEM II/C-M and CEM VI
Abstract
:1. Introduction
- production of multicomponent cements CEM II-CEMV according to EN 197-1 [8] using significant quantities of main ingredients other than Portland clinker;
- modification of the production process of cement clinker by modification of the raw material set (belite clinkers, belite-sulphate-aluminate clinkers, etc.) and use of alternative (non-fossil) biomass-rich fuels.
2. Materials and Methods
2.1. Characteristics of Components and Composition of Tested Cements
2.2. Concrete Composition and Properties’ Test Methods
3. Results and Discussion
3.1. Properties of CEM II/C-M and CEM VI Cements
3.2. Concrete Properties with CEM II/C-M and CEM VI Low-Emission Cements
3.2.1. Properties of Concrete Mixture
3.2.2. Properties of Hardened Concrete
- Compressive strength
- Water absorption and water penetration under pressure
- Carbonation susceptibility
- Chloride ions permeation
- Freeze–thaw resistance
3.2.3. CO2 Emissions from Concrete Made of CEM II/C-M and CEM VI Cement
4. Conclusions
- The results of the research as well as ecological aspects (mainly reduction of CO2 emissions) confirmed the advisability of further development of the assortment of general-use cements with the following ternary cements: Portland multicomponent cement CEM II/C-M and multicomponent cement CEM VI. The properties of these cements and the concretes made with them are the result of the properties of the main components and the synergistic interaction of the composition: granulated blast furnace slag S-limestone LL, siliceous fly ash V-limestone LL, granulated blast furnace slag S-siliceous fly ash V.
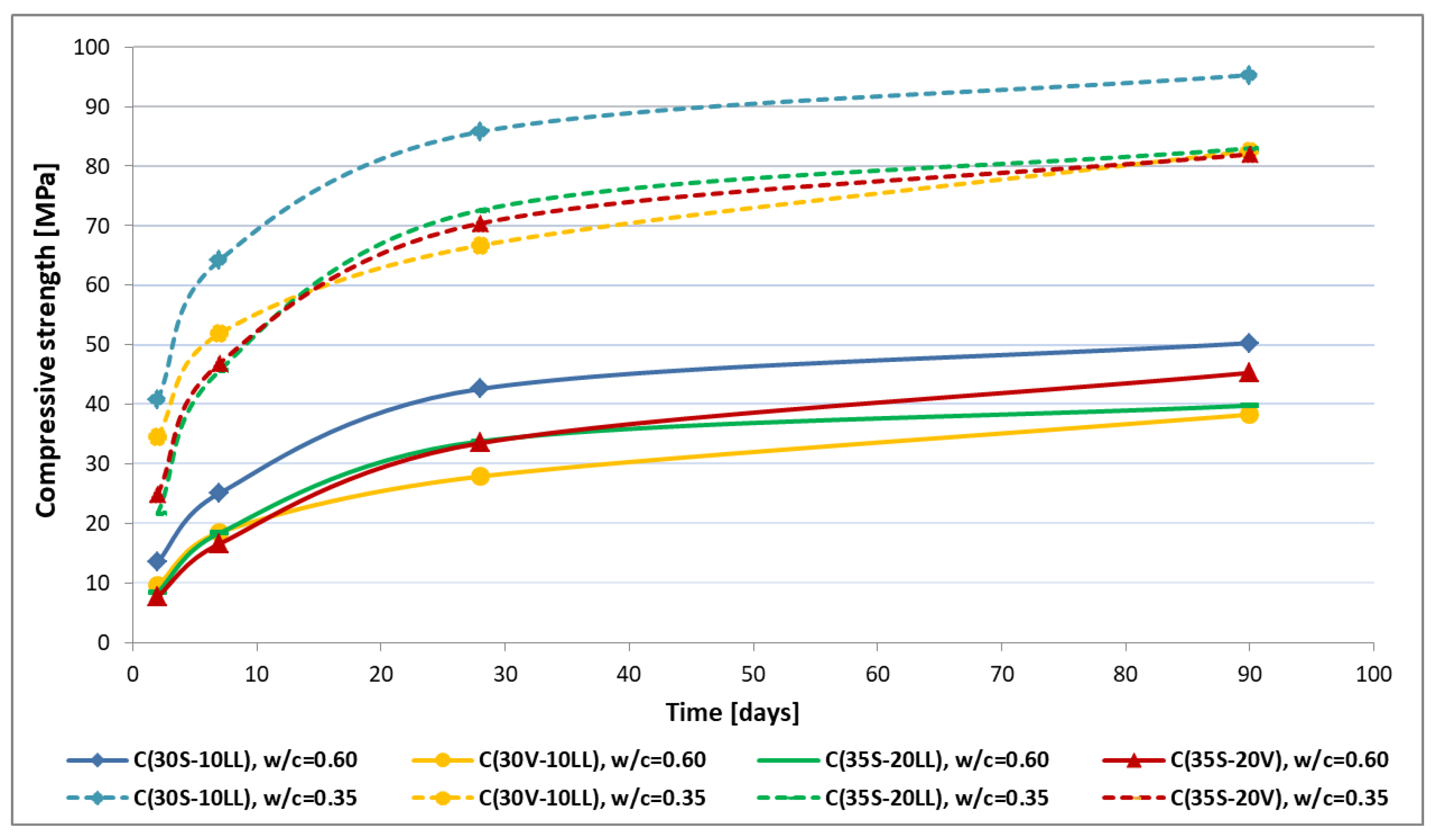
- When evaluating the synergy effect of additives in the composition of cements, it can be observed that much better strength effects are obtained in the composition of ground granulated blast furnace slag (S) with limestone (LL) than fly ash (V) with limestone (LL). This is due to the higher activity of the ground slag compared to fly ash (V). The results obtained also confirm a very good synergy effect, known from construction literature and practice, between slag (S) and fly ash (V). A characteristic feature of CEM II/C-M and CEM VI ternary cements is a low level of early compressive strength (up to 7 days) and a significant increase in compressive strength at later stages (especially between 28 and 90 days of hardening).
- A typical attribute of CEM II/C-M and CEM VI ternary cements is the low level of early compressive strength (up to 7 days) and a significant increase in compressive strength at a later date (especially between 28 and 90 days of hardening).
- The results obtained indicate the possibility of wider use of ground limestone LL in cement (concrete) composition. This is important in terms of the limited market availability of fly ash and granulated blast furnace slag.
- The results obtained from tests on concrete showed that a low w/c ratio is a prerequisite for obtaining high strength class and durability of concrete made of CEM II/C-M and CEM VI ternary cements. This can be achieved by using the latest generation of liquefying admixtures (superplasticizers).
- The durability characteristics of concrete (carbonation susceptibility, chloride ion permeation, frost resistance) from CEM II/C-M and CEM VI cements should be determined after 90 days of hardening. This period of curing better reflects the performance properties of the concrete.
- Portland multicomponent cements CEM II/C-M and CEM VI are low-emission binders with CO2 emissions ranging from 340 (CEM VI) kg to 453 (CEM II/C-M) kg per Mg of cement. Because of a high content of non-clinker main components, these cements characterize a much lower degree of hydration after 28 days of hardening than ordinary Portland cement CEM I. The presence of slag (S) and fly ash (V) results in a significant increase in the strength of low-emission cements between 28 and 90 days of hardening, and hence a better effect in terms of reducing emissions per 1 MPa is achieved. In some countries, Poland among others, for this reason the quality of concrete with low-emission cements is assessed after 90 days.
Author Contributions
Funding
Conflicts of Interest
References
- The European Cement Association. Activity Report, 1st ed.; CEMBUREAU: Brussels, Belgium, 2017; pp. 1–42. [Google Scholar]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 487–495. [Google Scholar] [CrossRef]
- World Business Council for Sustainable Development. Cement Sustainability Initiative: Getting the Numbers Right; Project Emissions Report; WBCSD: Geneva, Switzerland, 2016. [Google Scholar]
- Sanjuán, M.Á.; Andrade, C.; Mora, P.; Zaragoza, A. Carbon dioxide uptake by cement-based materials: A Spanish case study. Appl. Sci. 2020, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Bjegovic, D.; Strimer, N.; Serdar, M. Ecological aspects of concrete production. In Proceedings of the Second International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; pp. 1483–1492. [Google Scholar]
- World Business Council for Sustainable Development (WBCSD). Cement Industry Energy and CO2 Performance. Getting the Numbers Right (GNR) Project, 1st ed.; World Business Council for Sustainable Development: Geneva, Switzerland, 2018. [Google Scholar]
- Baran, T.; Garbacik, A.; Ostrowski, M.; Radelczuk, H. Methods of production of low emission Portland clinker. In Proceedings of the Dni Betonu Conference, Wisła, Poland, 1–2 July 2016; pp. 439–448. [Google Scholar]
- EN 197-1:2011. Cement-Part 1: Composition, Specifications and Conformity Criteria for Common Cements; British Standards Institution: London, UK, 2011. [Google Scholar]
- Król, A. The role of the granulated blast furnace slag in sustainable cement production and waste management. Econ. Environ. Stud. 2017, 17, 613–624. [Google Scholar] [CrossRef]
- Król, A. The role of the silica fly ash in sustainable waste management. In Proceedings of the E3S Web of Conferences, 1st International Conference on the Sustainable Energy and Environment Development (SEED), Cracow, Poland, 17–19 May 2016; Volume 10. [Google Scholar]
- Khan, M.I.; Lynsdale, C.J. Strength, permeation, and carbonation of high-performance concrete. Cem. Concr. Res. 2002, 32, 123–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X. Influences of superplasticizer, polymer latexes and asphalt emulsions on the pore structure and impermeation of hardened cementitious materials. Constr. Build. Mater. 2014, 53, 392–402. [Google Scholar] [CrossRef]
- Giergiczny, Z. Fly Ash in Cement and Concrete Composition; Silesian University of Technology Publishing House: Gliwice, Poland, 2013. [Google Scholar]
- Sathyan, D.; Anand, K.B. Influence of superplasticizer family on the durability characteristics of fly ash incorporated cement concrete. Constr. Build. Mater. 2019, 204, 864–874. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Gholampour, A.; Ozbakkaloglu, T. Performance of sustainable concretes containing very high volume class-F fly ash and ground granulated blast furnace slag. J. Clean. Prod. 2017, 162, 1401–1417. [Google Scholar] [CrossRef]
- Gursel, A.P.; Maryman, H.; Ostertag, C. A life-cycle approach to environmental, mechanical, and durability properties of “green” concrete mixes with rice husk ash. J. Clean. Prod. 2016, 112, 823–836. [Google Scholar] [CrossRef]
- Proske, T.; Rezvani, M.; Palm, S.; Muller, C.; Graubner, C.A. Concretes made of efficient multi-composite cements with slag and limestone. Cem. Concr. Compos. 2018, 89, 107–119. [Google Scholar] [CrossRef]
- Zajac, M.; Rossberg, A.; Le Saout, G.; Lothenbach, B. Influence of limestone and anhydrite on the hydration of Portland cements. Cem. Concr. Compos. 2014, 46, 99–108. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Hea, H.; Courardb, L.; Pirardc, E. Particle packing density and limestone fillers for more sustainable cement. Key Eng. Mater. 2012, 517, 331–337. [Google Scholar] [CrossRef]
- Githachuri, K.; Alexander, M.G. Durability performance potential and strength of blended Portland limestone cement concrete. Cem. Concr. Compos. 2013, 39, 115–121. [Google Scholar] [CrossRef]
- Chłądzyński, S.; Garbacik, A. Multicomponent Cements in Construction Industry; Association of Cement Producers: Cracow, Poland, 2008. [Google Scholar]
- Mirvalad, S.; Nokken, M. Minimum SCM requirements in mixtures containing limestone cement to control thaumasite sulfate attack. Constr. Build. Mater. 2015, 84, 19–29. [Google Scholar] [CrossRef]
- CEN/TC 51/WG prEN 197-5-Working Document. Available online: https://www.cen.eu/news/brochures/brochures/cen-cenelec_wp_2019.pdf (accessed on 1 April 2020).
- EN 196-3:2016-11. Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness; British Standards Institution: London, UK, 2016. [Google Scholar]
- EN 196-6:2011. Methods of Testing Cement—Part 6: Determination of Fineness; British Standards Institution: London, UK, 2011. [Google Scholar]
- EN 1097-7:2008. Tests for Mechanical and Physical Properties of Aggregates—Part 7: Determination of the Particle Density of Filler-Pyknometer Method; British Standards Institution: London, UK, 2008. [Google Scholar]
- EN 196-1:2016-05. Methods of Testing Cement—Part 1: Determination of Strength; British Standards Institution: London, UK, 2016. [Google Scholar]
- EN 12350-2:2011. Testing Fresh Concrete—Part 2: Slump-Test; British Standards Institution: London, UK, 2011. [Google Scholar]
- EN 12350-6:2011. Testing Fresh Concrete—Part 6: Density; British Standards Institution: London, UK, 2011. [Google Scholar]
- EN 12350-7:2011. Testing Fresh Concrete—Part 7: Air Content. Pressure Methods; British Standards Institution: London, UK, 2011. [Google Scholar]
- EN 12390-3:2011. Testing Hardened Concrete—Part 3: Compressive Strength of Test Specimens; British Standards Institution: London, UK, 2011. [Google Scholar]
- PN-EN 206-1:2003. Concrete. Part 1: Requirements, Properties, Production and Compliance; PN-B-06250:1988 standard withdrawn and replaced; British Standards Institution: London, UK, 2003. [Google Scholar]
- EN 12390-8:2011. Testing Hardened Concrete—Part 8: Depth of Penetration of Water Under Pressure; British Standards Institution: London, UK, 2011. [Google Scholar]
- CEN/TS 12390-12:2010. Testing Hardened Concrete—Part 12: Determination of The Potential Carbonation Resistance of Concrete: Accelerated Carbonation Method; British Standards Institution: London, UK, 2010. [Google Scholar]
- C 1202-05. Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration; American Society for Testing and Materials: West Conshohocken, PA, USA, 2012. [Google Scholar]
- WCA. Concrete-Requirements, Properties, Production and Conformity-National Supplementation PN-B-06265:2018-10; Association of Cement Producers: London, UK, 2018. [Google Scholar]
- PKN-CEN/TS 12390-9:2007. Testing Hardened Concrete—Part 9: Freeze-Thaw Resistance-Scaling; European Committee for Standardization: Brussels, Belgium, 2007. [Google Scholar]
- Giergiczny, Z.; Piechówka-Mielnik, M. Limestone-the main component of multi-component Portland cements CEM II A/B-M. Bud. Tech. Archit. 2011, 2, 72–76. [Google Scholar]
- De Weerdt, K.; Kjellsen, K.O.; Sellevold, E.; Justnes, H. Synergy between fly ash and limestone powder in ternary cements. Cem. Concr. Compos. 2011, 33, 30–38. [Google Scholar] [CrossRef]
- De Weerdt, K.; Ben Haha, M.; Le Saout, G.; Kjellsen, K.O.; Justnes, E.H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- EN 13877-2:2013-04. Concrete Pavements—Part 2: Functional Requirements for Concrete Pavements; British Standards Institution: London, UK, 2013. [Google Scholar]

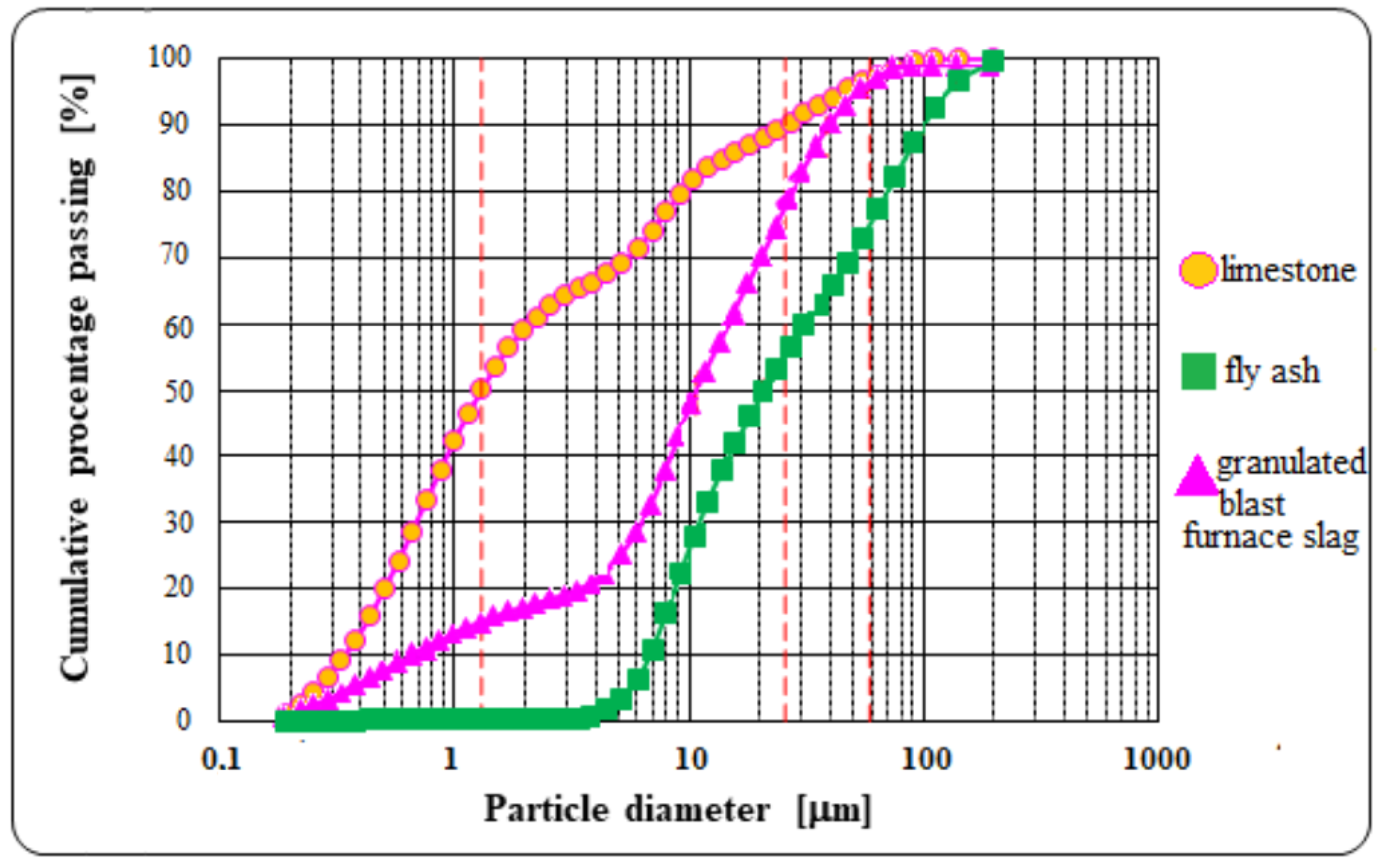


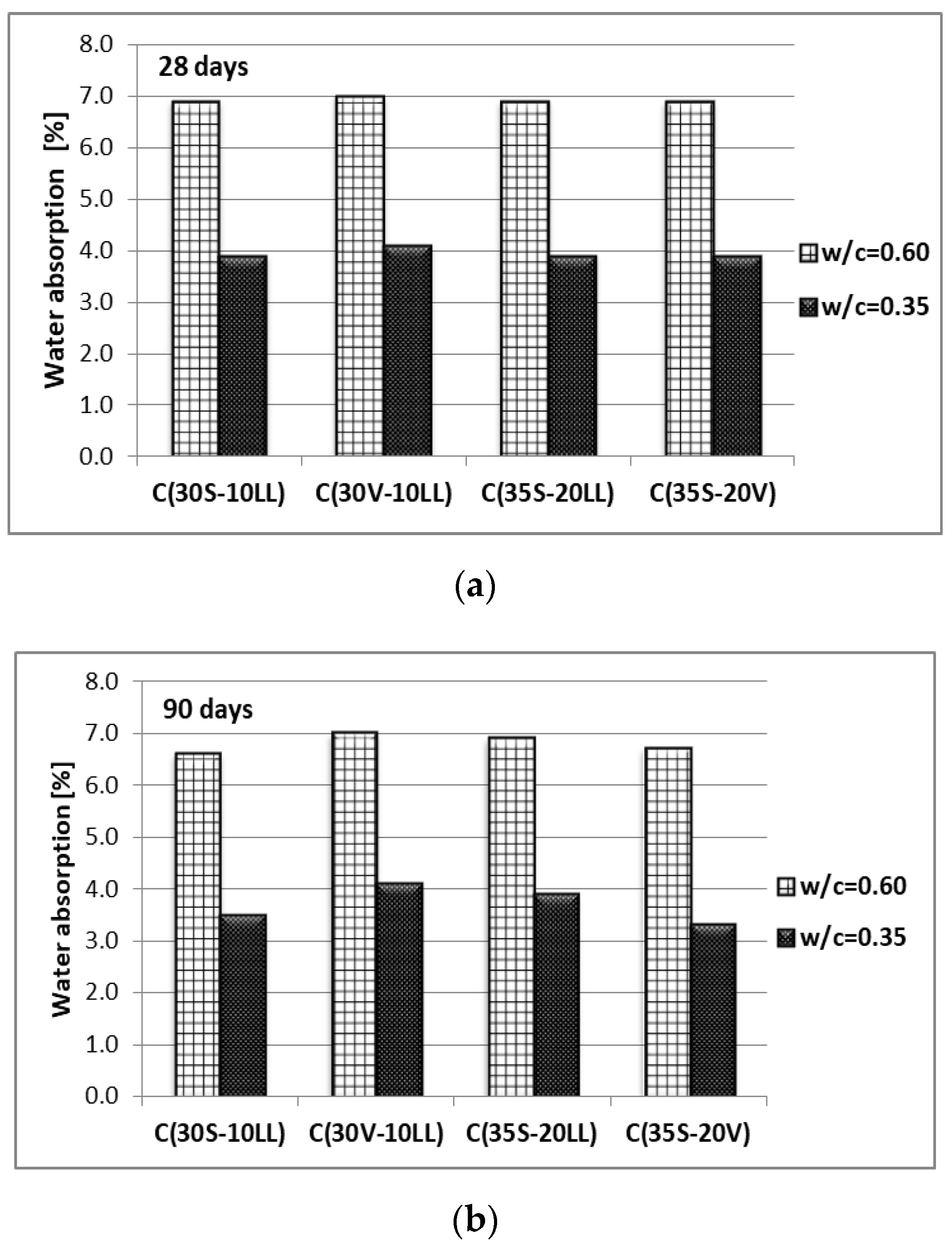

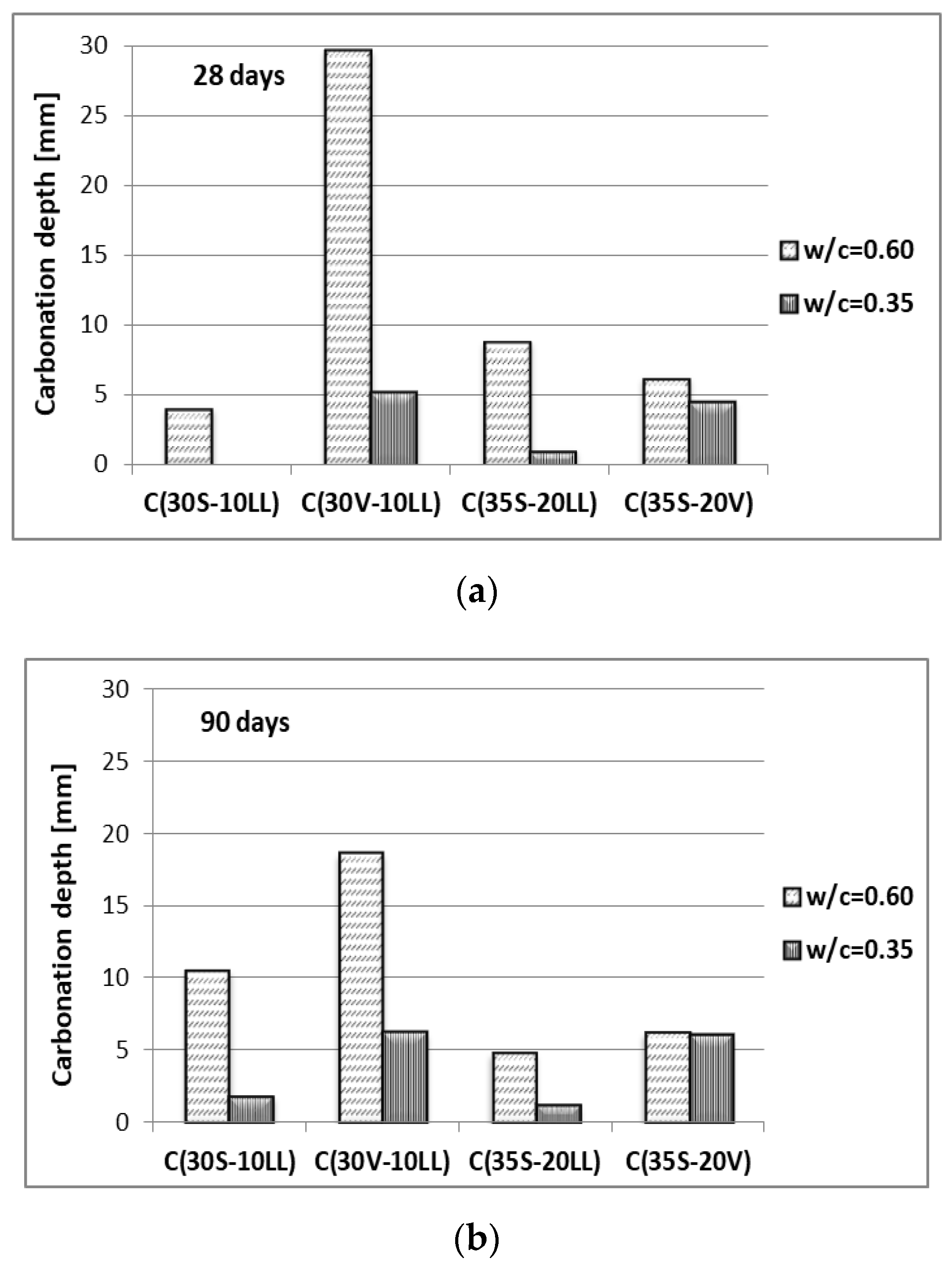

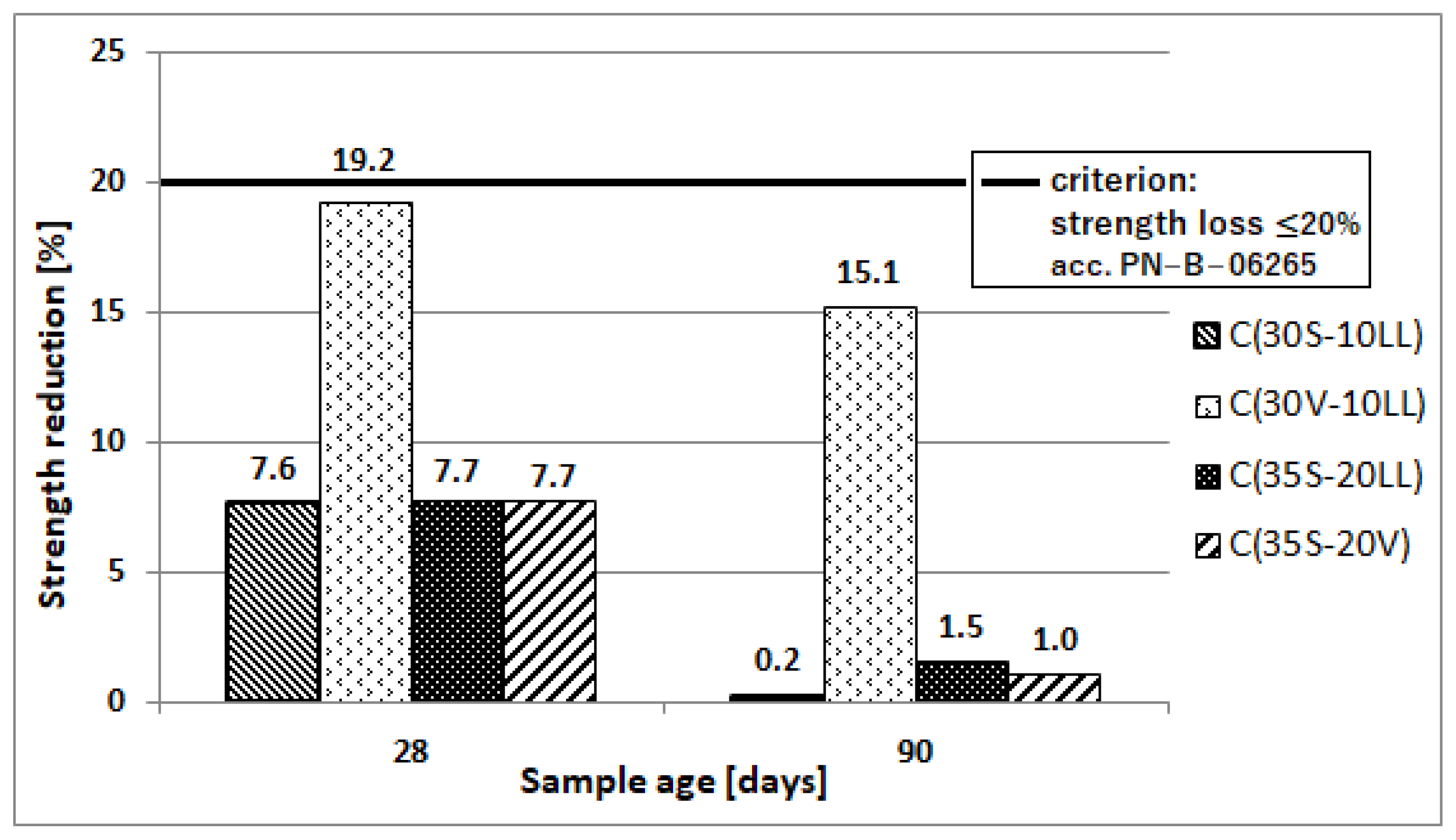
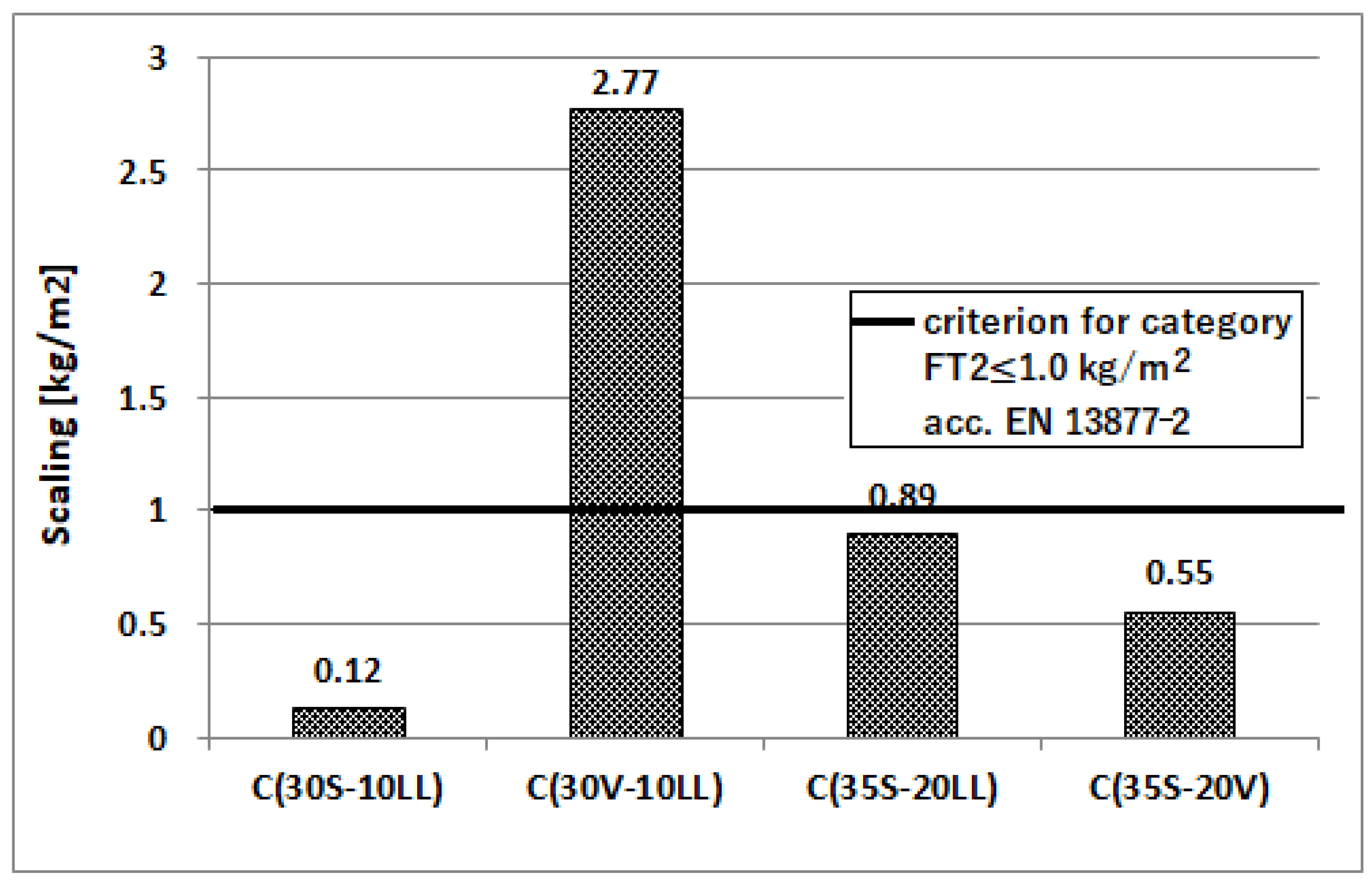
| Type of Raw Material | Content of Component, (wt. %) | Specific Gravity (g/cm3) | Specific Area ACC. Blaine (cm2/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | Cl− | |||
| Limestone (LL) (1) | 5.4 | 1.3 | 1.1 | 49.7 | 1.8 | 0.03 | <0.1 | <0.1 | 0.005 | 2.70 | 6150 |
| Granulated blast furnace slag (S) | 40.5 | 7.4 | 1.26 | 43.7 | 5.0 | 0.14 | 0.77 | 0.45 | 0.046 | 2.92 | 3800 |
| Siliceous fly ash (V) (2) | 52.3 | 27.5 | 5.80 | 3.6 | 2.6 | 0.29 | 0.94 | 3.15 | 0.008 | 2.14 | 2750 |
| Portland cement CEM I | 20.65 | 5.1 | 2.57 | 62.94 | 1.4 | 5.0 | 0.15 | 0.63 | 0.07 | 3.16 | 4500 |
| Cement Marking | Type of Cement | Content of Component, (wt. %) | CO2 Emission Level from Mg of Cement, (kg) | |||
|---|---|---|---|---|---|---|
| Cement CEM I | Slag S | Limestone LL | Fly Ash V | |||
| C(30S-10LL) | CEM II/C-M (30S-10LL) | 60 | 30 | 10 | - | 453.6 |
| C(30V-10LL) | CEM II/C-M (30V-10LL) | 60 | - | 10 | 30 | 453.6 |
| C(35S-20LL) | CEM VI (35S-20LL) | 45 | 35 | 20 | - | 340.2 |
| C(35S-20V) | CEM VI (35S-20V) | 45 | 35 | - | 20 | 340.2 |
| C(40V) (1) | CEM (40V) | 60 | - | - | 40 | 453.6 |
| C(55S) (1) | CEM (55S) | 45 | 55 | - | - | 340.2 |
| C(40LL) (1) | CEM (40LL) | 60 | - | 40 | - | 453.6 |
| Property | Standard Test Method |
|---|---|
| Constancy of volume | EN 196-3:2016-11 [26] |
| Initial setting time | EN 196-3:2016-11 [26] |
| Specific surface area | EN 196-6:2011 [27] |
| Density | EN 1097-7:2008 [28] |
| Compressive strength | EN 196-1:2016-05 [29] |
| Type | Concrete Designation | w/c | Cement Content (kg/m3) | Water (kg/m3) | PCE Admixture (kg/m3) | Coarse Aggregate (kg/m3) | Sand 0–2 mm (kg/m3) | |
|---|---|---|---|---|---|---|---|---|
| 8–16 mm | 2–8 mm | |||||||
| I | C(30S-10LL) | 0.60 | 300 | 180 | - | 680 | 530 | 680 |
| C(30V-10LL) | 300 | 180 | - | 680 | 530 | 680 | ||
| C(35S-20LL) | 300 | 180 | - | 680 | 530 | 680 | ||
| C(35S-20V) | 300 | 180 | - | 680 | 530 | 680 | ||
| II | C(30S-10LL) | 0.35 | 340 | 120 | 10.2 | 725 | 565 | 725 |
| C(30V-10LL) | 340 | 120 | 10.2 | 725 | 565 | 725 | ||
| C(35S-20LL) | 340 | 120 | 10.2 | 725 | 565 | 725 | ||
| C(35S-20V) | 340 | 120 | 10.2 | 725 | 565 | 725 | ||
| Property | Standard Test Method (Procedures) |
|---|---|
| Consistency (fall cone method) | EN 12350-2:2011 [30] |
| Density of concrete mixture | EN 12350-6 [29,31] |
| Air content | EN 12350-7:2011 [32] |
| Compressive strength | EN 12390-3:2011 [33] |
| Absorption | PN-B-06250:1988 [34] |
| Depth of water penetration under pressure | EN 12390-8:2011 [35] |
| Depth of carbonation | prCEN/TS 12390-12:2010 [36] |
| Permeation of chloride ions | ASTM C 1202-05 [37] |
| Ordinary frost resistance | PN-B-06265:2018-10 [38] |
| De-icing salts frost resistance (surface scaling) | CEN/TS 12390-9:2007 [39] |
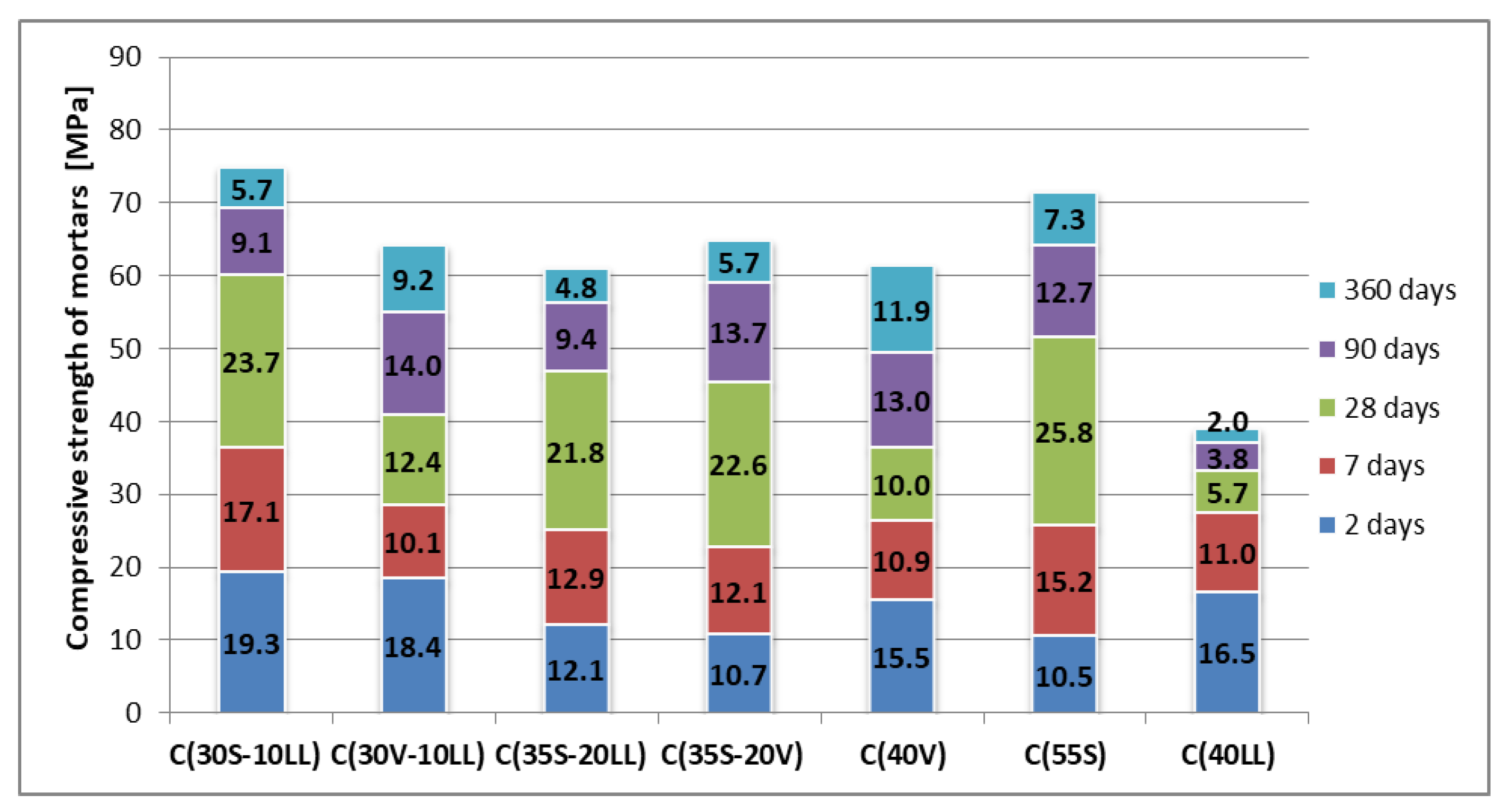
| No. | Cement Designation | Constancy of Volume (mm) | Density (g/cm3) | Initial Setting Time (h, min) | Specific Surface Area (cm2/g) | Compressive Strength (MPa) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 Days | 7 Days | 28 Days | 90 Days | 360 Days | ||||||
| 1. | C(30S-10LL) | 0.5 | 3.00 | 2:15 | 4750 | 19.3 | 36.4 | 60.1 | 69.2 | 74.9 |
| 2. | C(30V-10LL) | 0 | 2.70 | 2:40 | 4700 | 18.4 | 28.5 | 40.9 | 54.9 | 64.1 |
| 3. | C(35S-20LL) | 0 | 2.96 | 2:45 | 4600 | 12.1 | 25.0 | 46.8 | 56.2 | 61.0 |
| 4. | C(35S-20V) | 0 | 2.82 | 3:25 | 4350 | 10.7 | 22.8 | 45.4 | 59.1 | 64.8 |
| 5. | C(40V) | 0 | 2.64 | 3:00 | 4300 | 15.5 | 26.4 | 36.4 | 49.4 | 61.3 |
| 6. | C(55S) | 0 | 2.98 | 3:10 | 4330 | 10.5 | 25.7 | 51.5 | 64.2 | 71.5 |
| 7. | C(40LL) | 1 | 2.92 | 1:55 | 5765 | 16.5 | 27.5 | 33.2 | 37.0 | 39.0 |
| Property | Ratio w/c | Concrete Designation—Corresponding to the Composition of the Cement | |||
|---|---|---|---|---|---|
| C(30S-10LL) | C(30V-10LL) | C(35S-20LL) | C(35S-20V) | ||
| Consistency, (mm) | 0.60 | 110 | 60 | 50 | 50 |
| S3 * | S2 * | S2 * | S2 * | ||
| 0.35 | 30 | 20 | 30 | 30 | |
| S1 * | S1 * | S1 * | S1 * | ||
| Density, (kg/m3) | 0.60 | 2360 | 2350 | 2350 | 2350 |
| 0.35 | 2430 | 2420 | 2430 | 2420 | |
| Air content, (vol.%) | 0.60 | 0.8 | 0.8 | 1.0 | 0.6 |
| 0.35 | 1.6 | 1.8 | 2.0 | 2.0 | |
| Concrete Age (Days) | Tested Properties | Concrete Designation, w/c = 0.35 | |||
|---|---|---|---|---|---|
| C(30S-10LL) | C(30V-10LL) | C(35S-20LL) | C(35S-20V) | ||
| 28 | Strength of samples after freeze-thaw cycles, (MPa) | 86.7 | 63.4 | 77.2 | 76.4 |
| Strength of reference samples, (MPa) | 93.8 | 78.5 | 83.7 | 82.7 | |
| Decrease in strength, (%) | 7.6 | 19.2 | 7.7 | 7.7 | |
| Loss of sample weight after test, (wt. %) | 0.0 | 0.1 | 0.1 | 0.1 | |
| Scaling of the material after 56 freeze-thaw cycles in the presence of NaCl salt, (kg/m2) | 0.12 | 2.77 | 0.89 | 0.55 | |
| 90 | Strength of samples after freeze-thaw cycles, (MPa) | 94.8 | 71.6 | 84.6 | 85.7 |
| Strength of reference samples, (MPa) | 95.0 | 84.3 | 85.9 | 84.9 | |
| Decrease in strength, (%) | 0.2 | 15.1 | 1.5 | 1.0 | |
| Loss of sample weight after test, (wt. %) | 0.0 | 0.0 | 0.1 | 0.1 | |
| Concrete Designation | Cement Content (kg/m3 Concrete) | w/c Ratio | CO2 Emission (kg/m3 Concrete) | 28-Day Concrete Compressive Strength fcm,cube 28 (MPa) | CO2 Emission (kg) Converted into 1 MPa fcm,cube 28 | 90-Day Concrete Compressive Strength fcm,cube 90 (MPa) | CO2 Emission (kg) Converted into 1 MPa fcm,cube 90 |
|---|---|---|---|---|---|---|---|
| C(30S-10LL) | 300.0 | 0.60 | 136.1 | 42.6 | 3.2 | 50.3 | 2.7 |
| C(30V-10LL) | 300.0 | 136.1 | 27.9 | 4.9 | 38.3 | 3.6 | |
| C(35S-20LL) | 300.0 | 102.1 | 33.8 | 3.0 | 39.8 | 2.6 | |
| C(35S-20V) | 300.0 | 102.1 | 33.5 | 3.0 | 45.3 | 2.3 | |
| C(30S-10LL) | 340.0 | 0.35 | 154.2 | 85.8 | 1.8 | 95.3 | 1.6 |
| C(30V-10LL) | 340.0 | 154.2 | 66.7 | 2.3 | 82.6 | 1.9 | |
| C(35S-20LL) | 340.0 | 115.7 | 72.6 | 1.6 | 83.0 | 1.4 | |
| C(35S-20V) | 340.0 | 115.7 | 70.4 | 1.6 | 82.0 | 1.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, A.; Giergiczny, Z.; Kuterasińska-Warwas, J. Properties of Concrete Made with Low-Emission Cements CEM II/C-M and CEM VI. Materials 2020, 13, 2257. https://doi.org/10.3390/ma13102257
Król A, Giergiczny Z, Kuterasińska-Warwas J. Properties of Concrete Made with Low-Emission Cements CEM II/C-M and CEM VI. Materials. 2020; 13(10):2257. https://doi.org/10.3390/ma13102257
Chicago/Turabian StyleKról, Anna, Zbigniew Giergiczny, and Justyna Kuterasińska-Warwas. 2020. "Properties of Concrete Made with Low-Emission Cements CEM II/C-M and CEM VI" Materials 13, no. 10: 2257. https://doi.org/10.3390/ma13102257
APA StyleKról, A., Giergiczny, Z., & Kuterasińska-Warwas, J. (2020). Properties of Concrete Made with Low-Emission Cements CEM II/C-M and CEM VI. Materials, 13(10), 2257. https://doi.org/10.3390/ma13102257




