Dielectric Properties of Graphene/Titania/Polyvinylidene Fluoride (G/TiO2/PVDF) Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
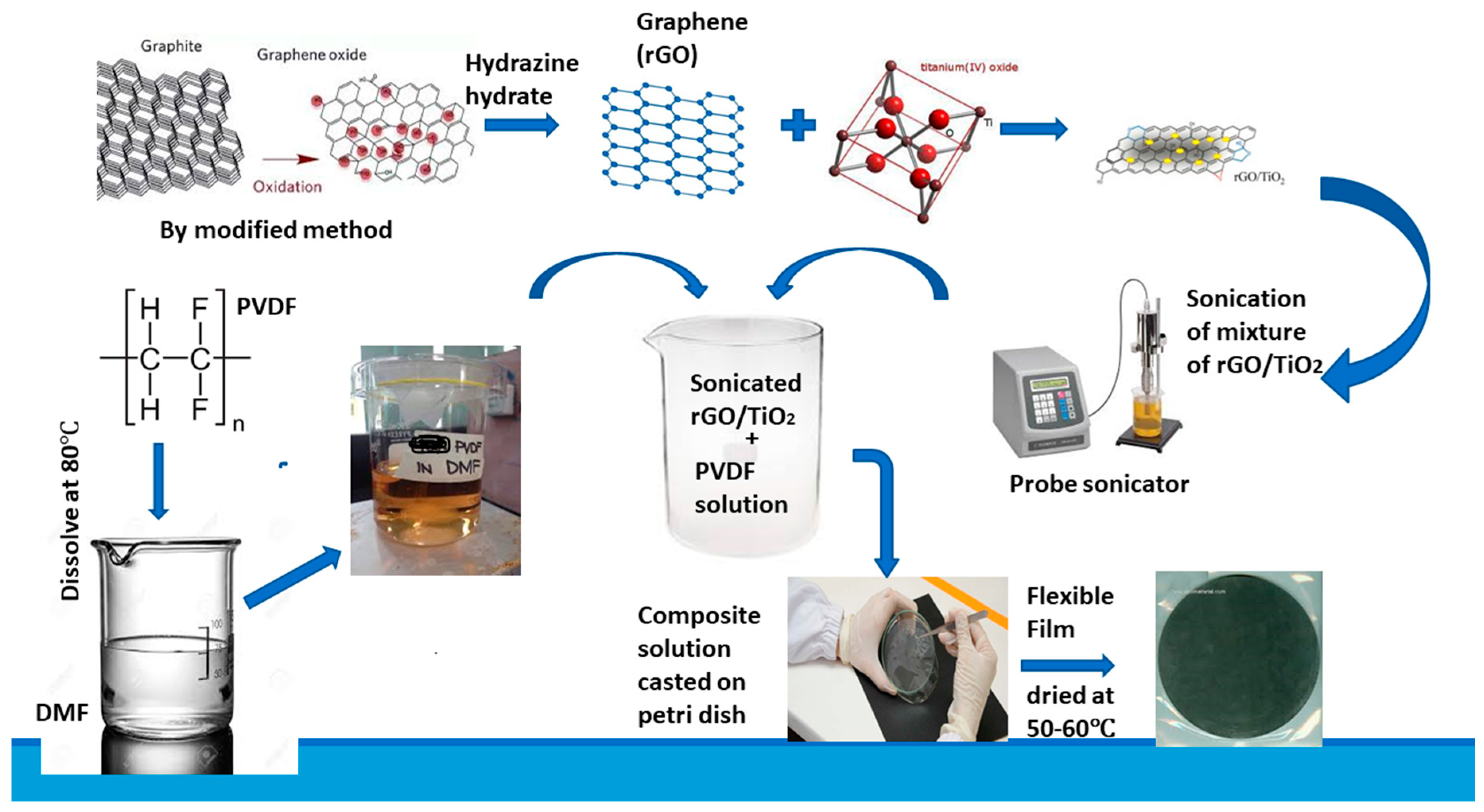
2.2. Preparation of Graphene/Titania/Polyvinylidene Fluoride Nanocomposite Films
2.3. Characterizations
3. Results and Discussion
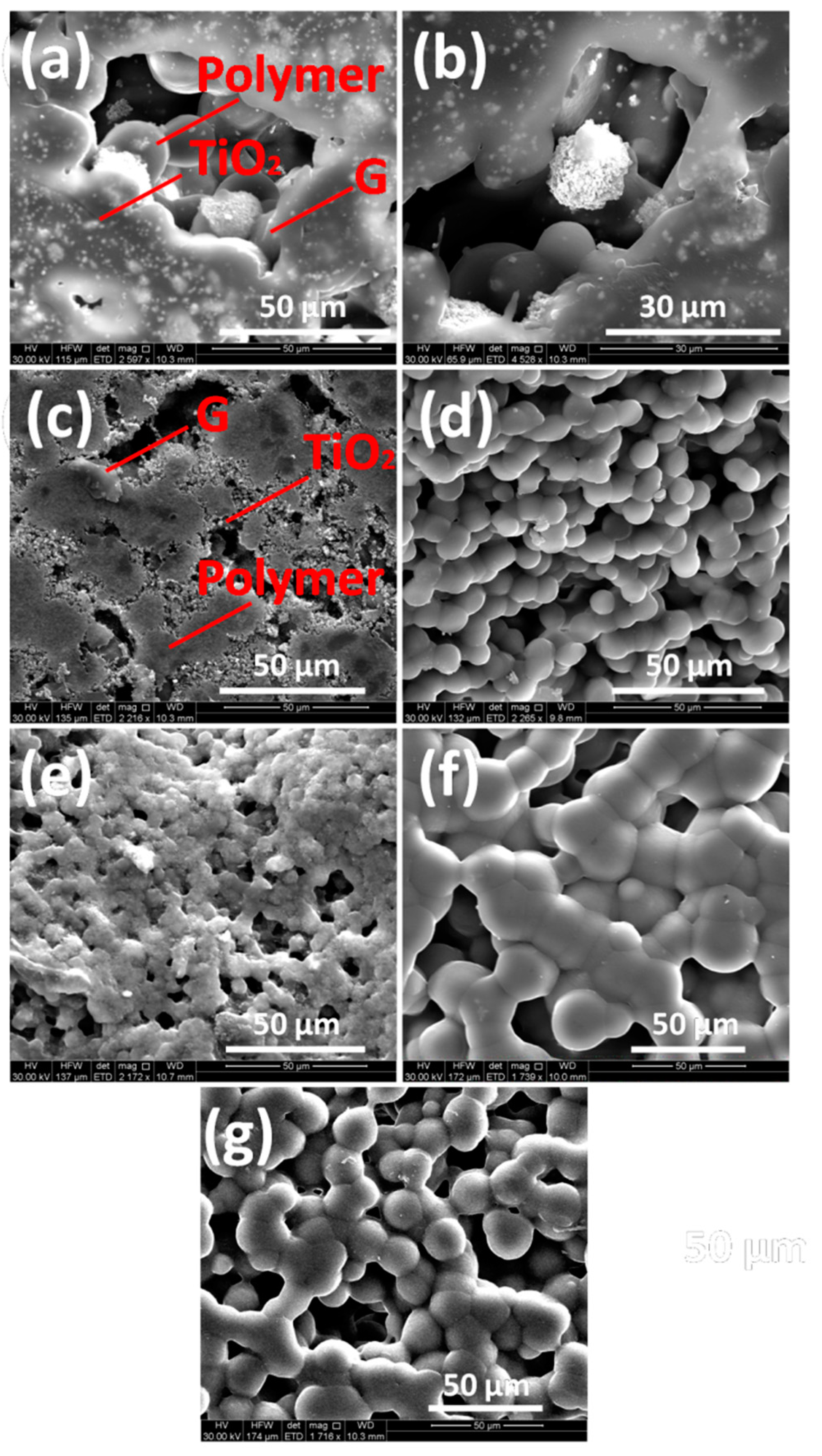
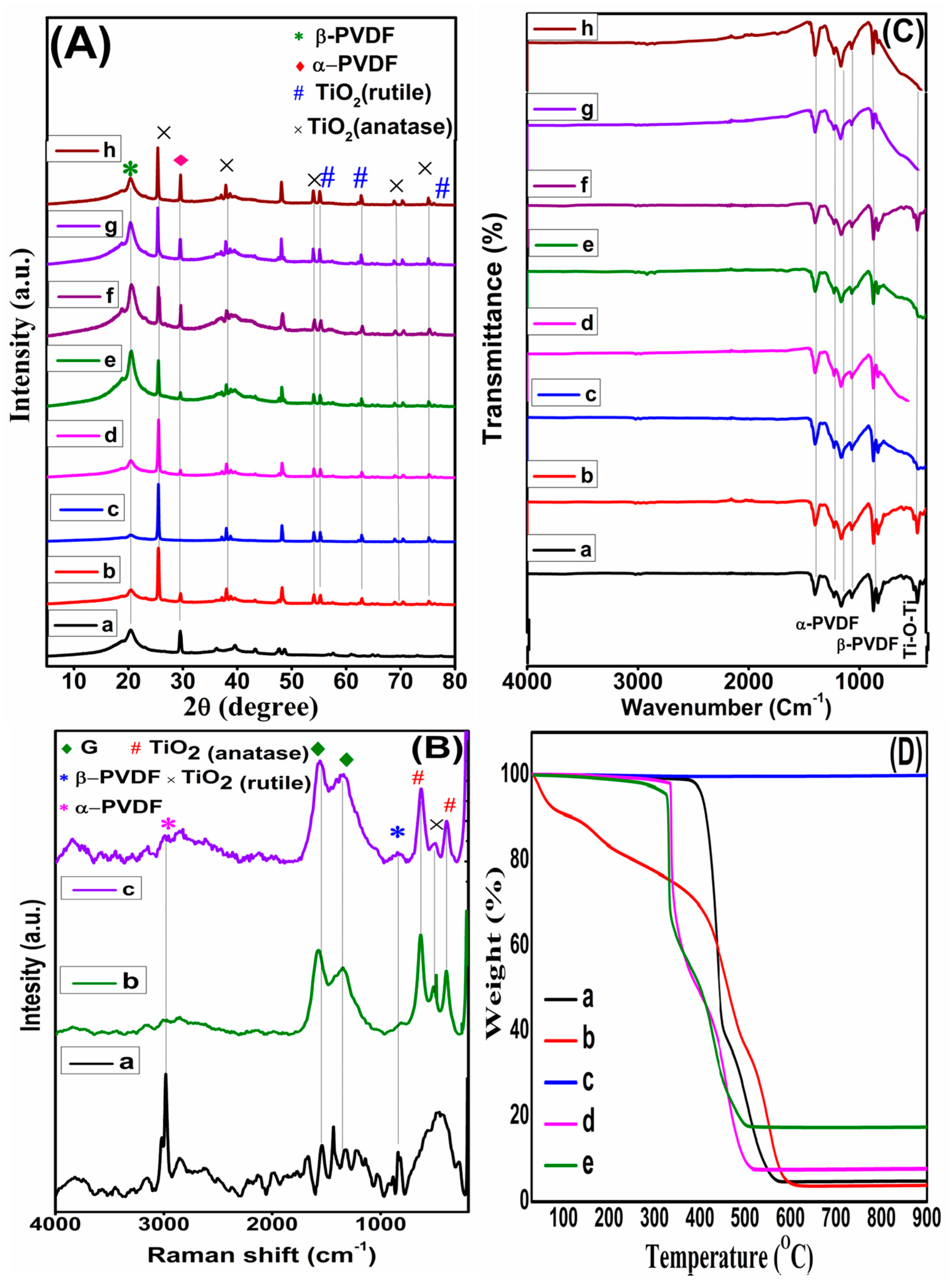
3.1. Characterization of Graphene/Titania/Polyvinylidene Fluoride Nanocomposite Films
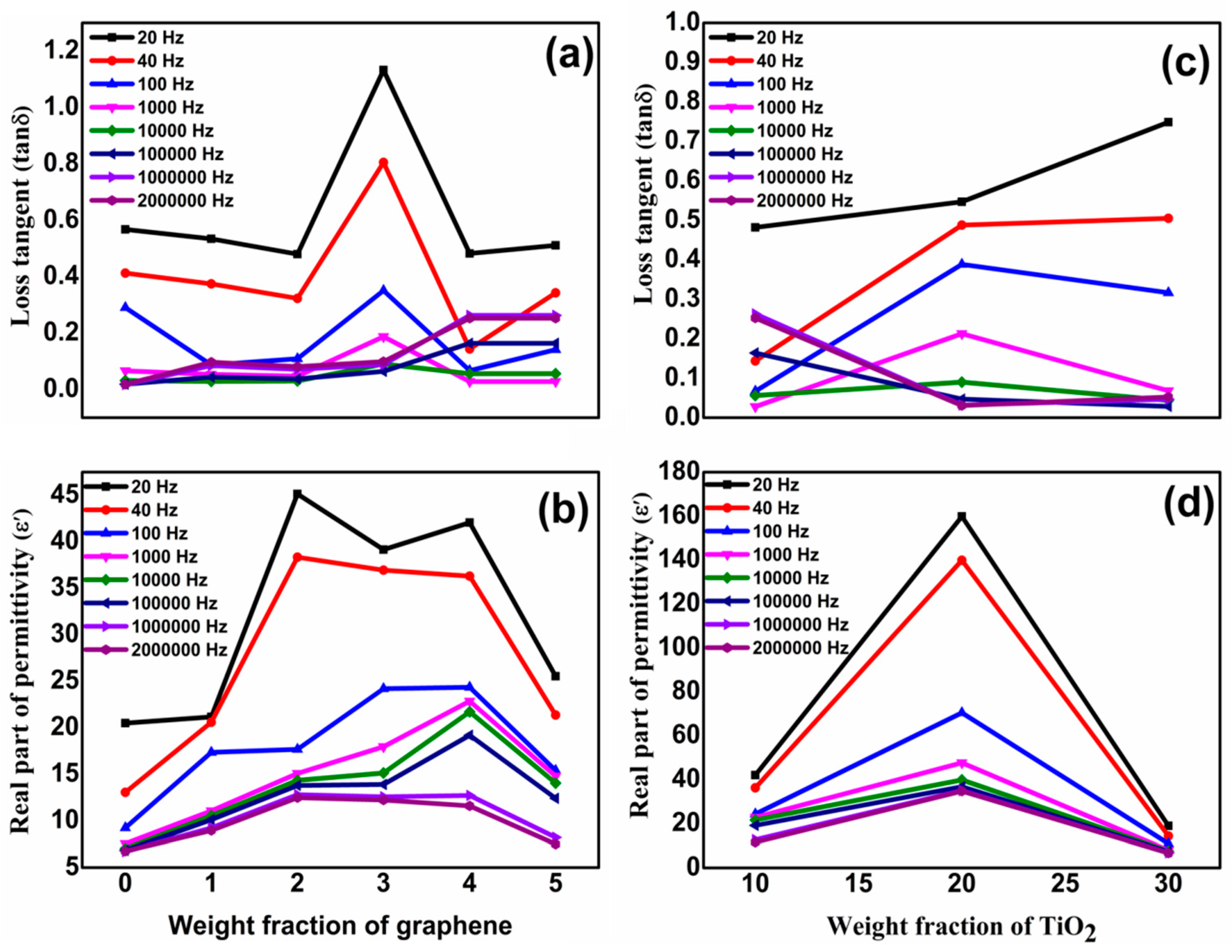
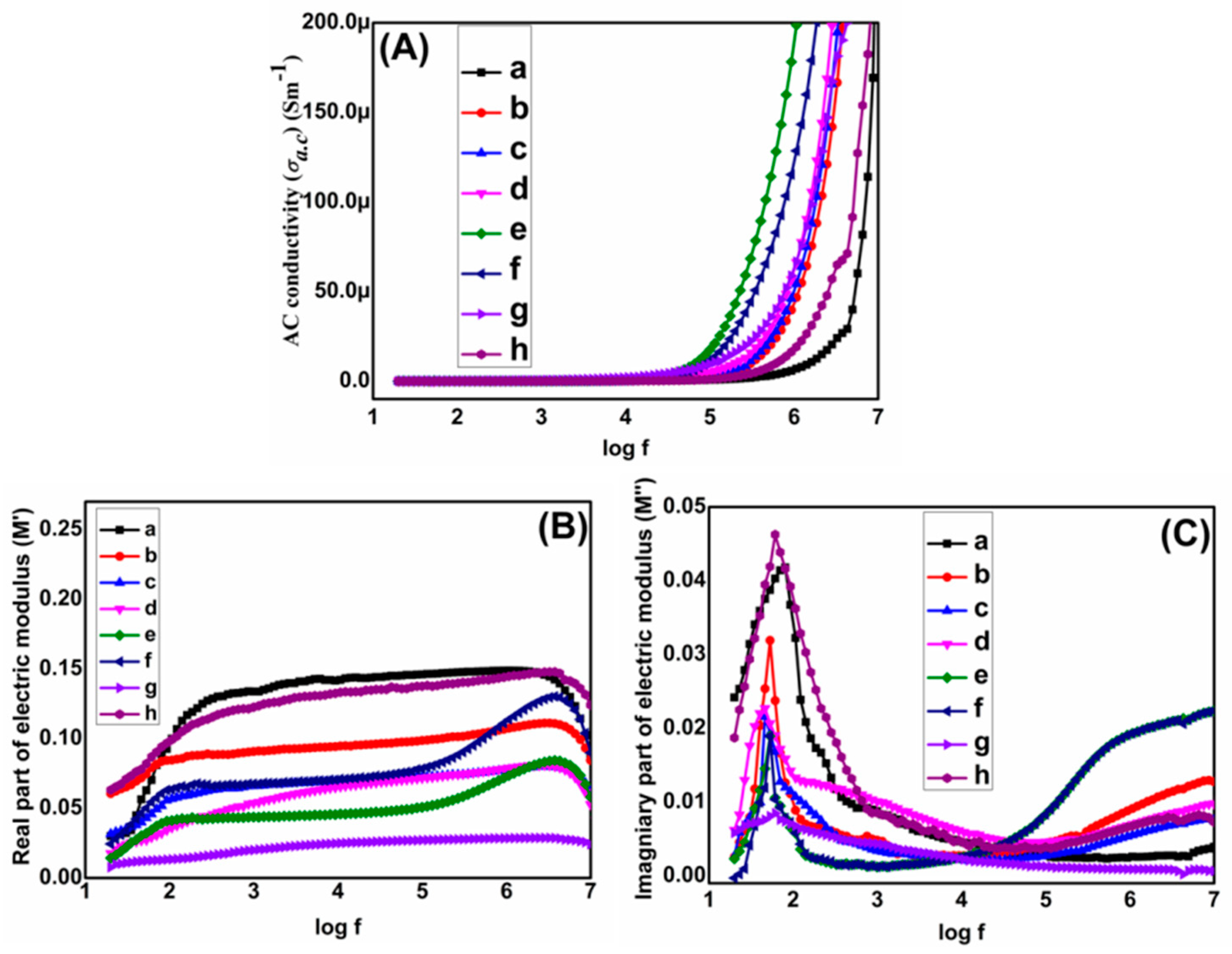
3.2. Dielectric Studies of Graphene/Titania/Polyvinylidene Fluoride Nanocomposite Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jung, S.; Albariqi, M.; Gruntz, G.; Al-Hathal, T.; Peinado, A.; Garcia-Caurel, E.; Nicolas, Y.; Toupance, T.; Bonnassieux, Y.; Horowitz, G. A tips-tpdo-tetracn-based n-type organic field-effect transistor with a cross-linked pmma polymer gate dielectric. ACS Appl. Mater. Interfaces 2016, 8, 14701–14708. [Google Scholar] [CrossRef] [Green Version]
- Rajib, M.; Shuvo, M.A.I.; Karim, H.; Delfin, D.; Afrin, S.; Lin, Y. Temperature influence on dielectric energy storage of nanocomposites. Ceram. Int. 2015, 41, 1807–1813. [Google Scholar] [CrossRef]
- Huang, B.; Li, M.; Mei, T.; McCoul, D.; Qin, S.; Zhao, Z.; Zhao, J. Wearable stretch sensors for motion measurement of the wrist joint based on dielectric elastomers. Sensors 2017, 17, 2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Z.; Song, Z.; Hao, H.; Yu, Z.; Cao, M.; Zhang, S.; Lanagan, M.T.; Liu, H. Homogeneous/inhomogeneous-structured dielectrics and their energy-storage performances. Adv. Mater. 2017, 29, 1601727. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, U.; Chung, G.-S. Effect of surface treated mwcnts and batio3 nanoparticles on the dielectric properties of a P(VDF-TrFe) matrix. J. Alloys Compd. 2017, 695, 1231–1236. [Google Scholar] [CrossRef]
- Ishaq, S.; Kanwal, F.; Atiq, S.; Moussa, M.; Losic, D. Synthesis of three phase graphene/titania/polydimethylsiloxane nanocomposite films and revealing their dielectric and impedance properties. Ceram. Int. 2019, 45, 8713–8720. [Google Scholar] [CrossRef]
- Zhang, L.; Shan, X.; Bass, P.; Tong, Y.; Rolin, T.D.; Hill, C.W.; Brewer, J.C.; Tucker, D.S.; Cheng, Z.-Y. Process and microstructure to achieve ultra-high dielectric constant in ceramic-polymer composites. Sci. Rep. 2016, 6, 35763. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Li, M.-L.; Li, W.-L.; Zhang, T.-D.; Zhao, Y.; Fei, W.-D. Polymer/metal multi-layers structured composites: A route to high dielectric constant and suppressed dielectric loss. Appl. Phys. Lett. 2018, 112, 022901. [Google Scholar] [CrossRef]
- Zhao, B.; Hamidinejad, M.; Zhao, C.; Li, R.; Wang, S.; Kazemi, Y.; Park, C.B. A versatile foaming platform to fabricate polymer/carbon composites with high dielectric permittivity and ultra-low dielectric loss. J. Mater. Chem. A 2019, 7, 133–140. [Google Scholar] [CrossRef]
- Pan, Z.; Yao, L.; Zhai, J.; Shen, B.; Wang, H. Significantly improved dielectric properties and energy density of polymer nanocomposites via small loaded of batio3 nanotubes. Compos. Sci. Technol. 2017, 147, 30–38. [Google Scholar] [CrossRef]
- Yang, W.; Yu, S.; Sun, R.; Du, R. Nano- and microsize effect of ccto fillers on the dielectric behavior of ccto/pvdf composites. Acta Mater. 2011, 59, 5593–5602. [Google Scholar] [CrossRef]
- Arbatti, M.; Shan, X.; Cheng, Z.Y. Ceramic–polymer composites with high dielectric constant. Adv. Mater. 2007, 19, 1369–1372. [Google Scholar] [CrossRef]
- Wang, S.; He, X.; Chen, Q.; Chen, Y.; He, W.; Zhou, G.; Zhang, H.; Jin, X.; Su, X. Graphene-coated copper calcium titanate to improve dielectric performance of ppo-based composite. Mater. Lett. 2018, 233, 355–358. [Google Scholar] [CrossRef]
- Qi, F.; Chen, N.; Wang, Q. Dielectric and piezoelectric properties in selective laser sintered polyamide11/batio3/cnt ternary nanocomposites. Mater. Des. 2018, 143, 72–80. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, T.; Zha, J.-W.; Zhao, J.; Shi, C.-Y.; Dang, Z.-M. Functionalized graphene–batio 3/ferroelectric polymer nanodielectric composites with high permittivity, low dielectric loss, and low percolation threshold. J. Mater. Chem. A 2013, 1, 6162–6168. [Google Scholar] [CrossRef]
- Chanmal, C.; Jog, J. Dielectric relaxations in pvdf/batio3 nanocomposites. Express Polym. Lett. 2008, 2, 294–301. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Xu, C.; Li, Y.; Gao, J.; Wang, W.; Liu, Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater. Chem. 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Grant, F. Properties of rutile (titanium dioxide). Rev. Mod. Phys. 1959, 31, 646. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite-and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Yaqoob, U.; Uddin, A.I.; Chung, G.-S. The effect of reduced graphene oxide on the dielectric and ferroelectric properties of pvdf–batio 3 nanocomposites. RSC Adv. 2016, 6, 30747–30754. [Google Scholar] [CrossRef]
- Masuda, Y.; Kato, K. Synthesis and phase transformation of TiO2 nano-crystals in aqueous solutions. J. Ceram. Soc. Jpn. 2009, 117, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; He, Y.; Zhang, M.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912. [Google Scholar] [CrossRef]
- Li, R.; Chen, C.; Li, J.; Xu, L.; Xiao, G.; Yan, D. A facile approach to superhydrophobic and superoleophilic graphene/polymer aerogels. J. Mater. Chem. A 2014, 2, 3057–3064. [Google Scholar] [CrossRef]
- Musić, S.; Gotić, M.; Ivanda, M.; Popović, S.; Turković, A.; Trojko, R.; Sekulić, A.; Furić, K. Chemical and micro structural properties of tio2 synthesized by sol-gel procedure. Mater. Sci. Eng. B 1997, 47, 33–40. [Google Scholar] [CrossRef]
- Tantis, I.; Psarras, G.; Tasis, D. Functionalized graphene–poly (vinyl alcohol) nanocomposites: Physical and dielectric properties. Express Polym. Lett. 2012, 6, 283–292. [Google Scholar] [CrossRef]
- He, F.; Lau, S.; Chan, H.L.; Fan, J. High dielectric permittivity and low percolation threshold in nanocomposites based on poly(vinylidene fluoride) and exfoliated graphite nanoplates. Adv. Mater. 2009, 21, 710–715. [Google Scholar] [CrossRef]
- Kalini, A.; Gatos, K.; Karahaliou, P.; Georga, S.; Krontiras, C.; Psarras, G. Probing the dielectric response of polyurethane/alumina nanocomposites. J. Polym. Sci. B Polym. Phys. 2010, 48, 2346–2354. [Google Scholar] [CrossRef]
- Ping, F.; Lei, W.; Jintao, Y.; Feng, C.; Mingqiang, Z. Graphene/poly(vinylidene fluoride) composites with high dielectric constant and low percolation threshold. Nanotechnology 2012, 23, 365702. [Google Scholar]
- Li, J.; Seok, S.I.; Chu, B.; Dogan, F.; Zhang, Q.; Wang, Q. Nanocomposites of ferroelectric polymers with tio2 nanoparticles exhibiting significantly enhanced electrical energy density. Adv. Mater. 2009, 21, 217–221. [Google Scholar] [CrossRef]
- Mo, T.-C.; Wang, H.-W.; Chen, S.-Y.; Yeh, Y.-C. Synthesis and dielectric properties of polyaniline/titanium dioxide nanocomposites. Ceram. Int. 2008, 34, 1767–1771. [Google Scholar] [CrossRef]
- Pradhan, D.; Samantaray, B.; Choudhary, R.; Thakur, A. Complex impedance analysis of layered perovskite structure electroceramics—NaDyTiO4. J. Mater. Sci. 2005, 40, 5419–5425. [Google Scholar] [CrossRef]
- Singh, G.; Tiwari, V. Effect of zr concentration on conductivity behavior of (1−x) pmn-xpz ceramic: An impedance spectroscopy analysis. J. Appl. Phys. 2009, 106, 124104. [Google Scholar] [CrossRef]
- Amin, M.; Rafique, H.M.; Yousaf, M.; Ramay, S.M.; Atiq, S. Structural and impedance spectroscopic analysis of sr/mn modified BiFeO3 multiferroics. J. Mater. Sci. Mater. Electron. 2016, 27, 11003–11011. [Google Scholar] [CrossRef]
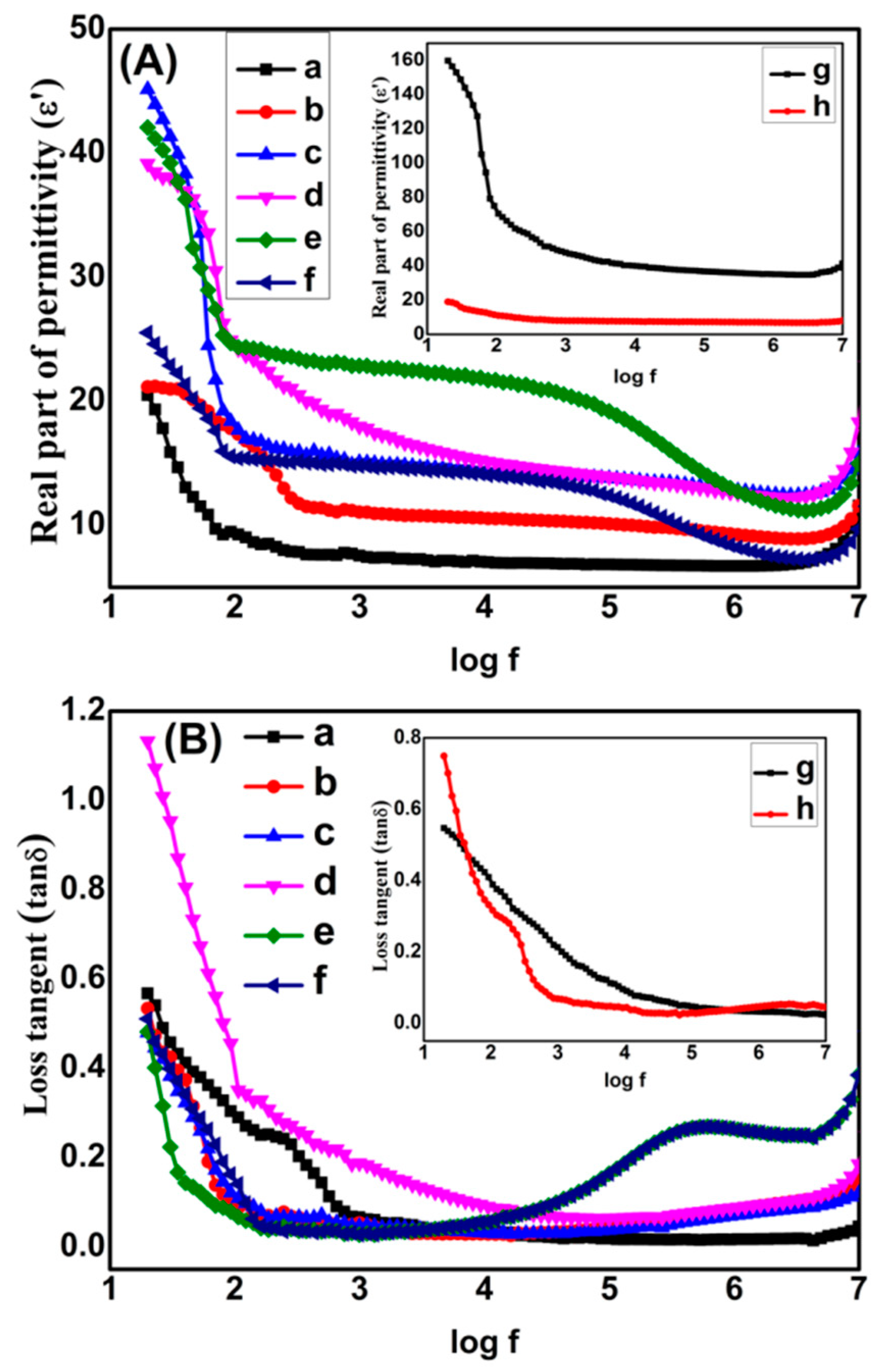
| Frequency (Hz) | Dielectric Permittivity (ε′) | Loss Tangent (tanδ) | AC Conductivity (σac) (Sm−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Neat PVDF | G4T10PVDF | G4T20PVDF | Neat PVDF | G4T10PVDF | G4T20PVDF | Neat PVDF | G4T10PVDF | G4T20PVDF | |
| 20 | 20.5 | 42.1 | 159.8 | 0.6 | 0.5 | 0.5 | 1.1 × 10−8 | 8.4 × 10−9 | 1.8 × 10−8 |
| 40 | 13.1 | 36.3 | 139.8 | 0.4 | 0.1 | 0.49 | 6.5 × 10−9 | 4.1 × 10−9 | 7.6 × 10−8 |
| 100 | 9.3 | 24.4 | 70.4 | 0.3 | 0.07 | 0.39 | 1.8 × 10−8 | 1.9 × 10−8 | 6.9 × 10−8 |
| 103 | 7.6 | 22.9 | 47.7 | 0.07 | 0.03 | 0.21 | 6.8 × 10−9 | 3.5 × 10−8 | 5.7 × 10−7 |
| 104 | 7.0 | 21.7 | 39.9 | 0.03 | 0.06 | 0.09 | 1.3 × 10−7 | 7.2 × 10−7 | 2.1 × 10−6 |
| 105 | 6.8 | 19.2 | 36.8 | 0.02 | 0.17 | 0.05 | 7.1 × 10−7 | 1.7 × 10−5 | 9.6 × 10−6 |
| 106 | 6.7 | 12.7 | 35.0 | 0.02 | 0.26 | 0.03 | 6.9 × 10−6 | 1.9 × 10−5 | 6.6 × 10−5 |
| 2 × 106 | 6.7 | 6.7 | 11.6 | 34.8 | 0.02 | 0.25 | 0.03 | 1.5 × 10−5 | 3.5 × 10−4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishaq, S.; Kanwal, F.; Atiq, S.; Moussa, M.; Azhar, U.; Losic, D. Dielectric Properties of Graphene/Titania/Polyvinylidene Fluoride (G/TiO2/PVDF) Nanocomposites. Materials 2020, 13, 205. https://doi.org/10.3390/ma13010205
Ishaq S, Kanwal F, Atiq S, Moussa M, Azhar U, Losic D. Dielectric Properties of Graphene/Titania/Polyvinylidene Fluoride (G/TiO2/PVDF) Nanocomposites. Materials. 2020; 13(1):205. https://doi.org/10.3390/ma13010205
Chicago/Turabian StyleIshaq, Saira, Farah Kanwal, Shahid Atiq, Mahmoud Moussa, Umar Azhar, and Dusan Losic. 2020. "Dielectric Properties of Graphene/Titania/Polyvinylidene Fluoride (G/TiO2/PVDF) Nanocomposites" Materials 13, no. 1: 205. https://doi.org/10.3390/ma13010205
APA StyleIshaq, S., Kanwal, F., Atiq, S., Moussa, M., Azhar, U., & Losic, D. (2020). Dielectric Properties of Graphene/Titania/Polyvinylidene Fluoride (G/TiO2/PVDF) Nanocomposites. Materials, 13(1), 205. https://doi.org/10.3390/ma13010205






