Pronounced Impact of p-Type Carriers and Reduction of Bandgap in Semiconducting ZnTe Thin Films by Cu Doping for Intermediate Buffer Layer in Heterojunction Solar Cells
Abstract
:1. Introduction
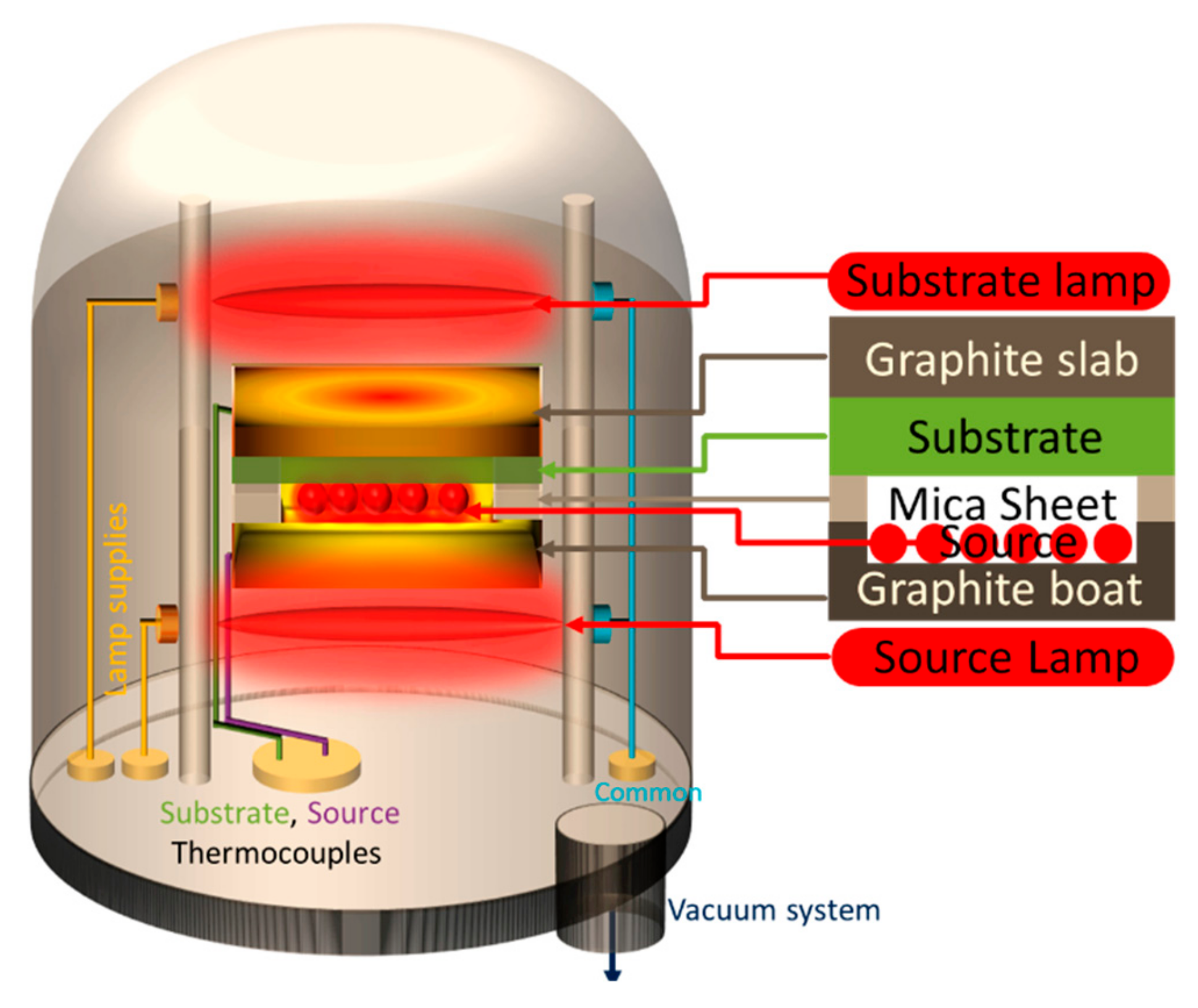
2. Experimental Procedure
3. Results and Discussion
3.1. X-rays Diffraction (XRD)
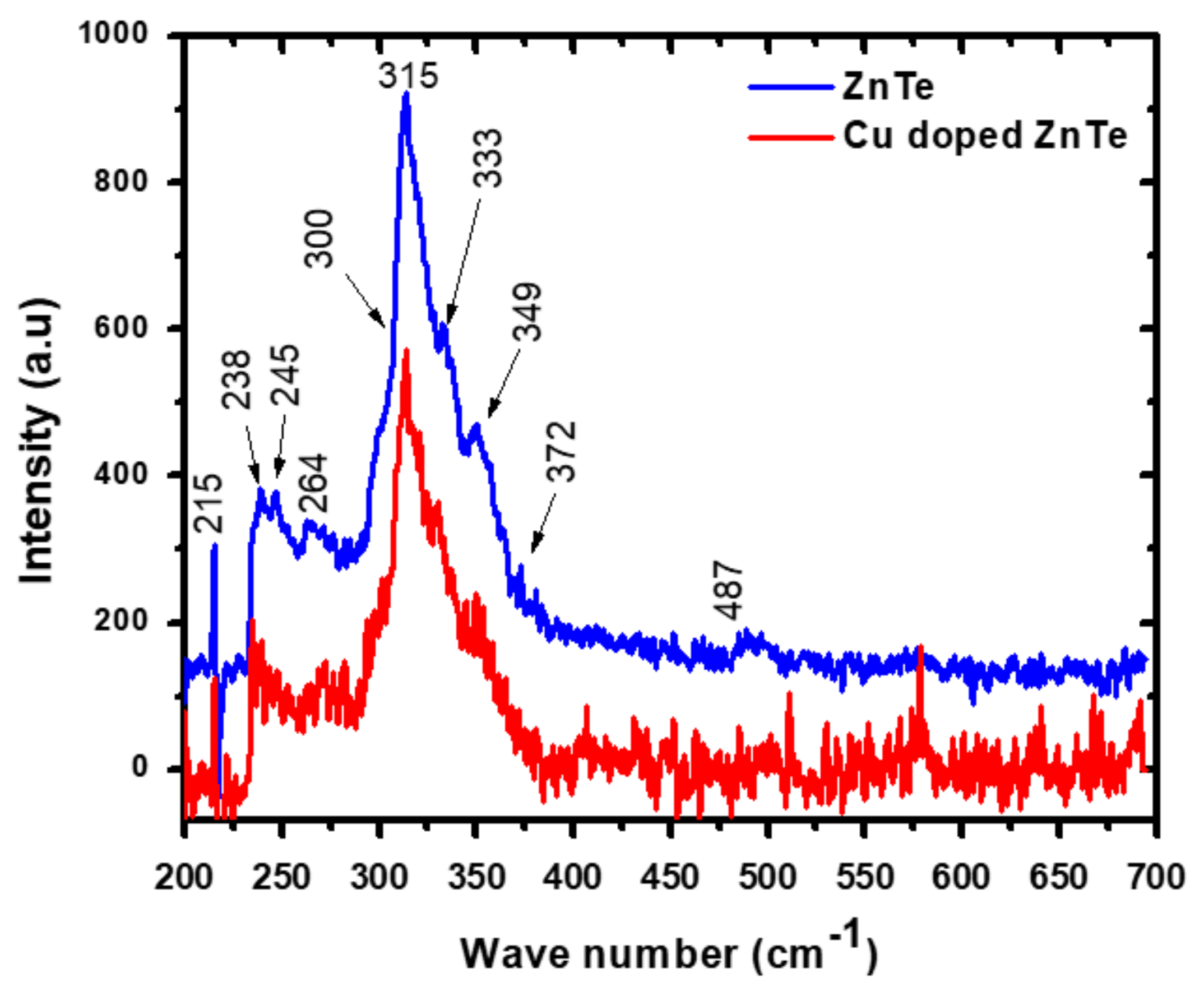
3.2. Raman Spectroscopy
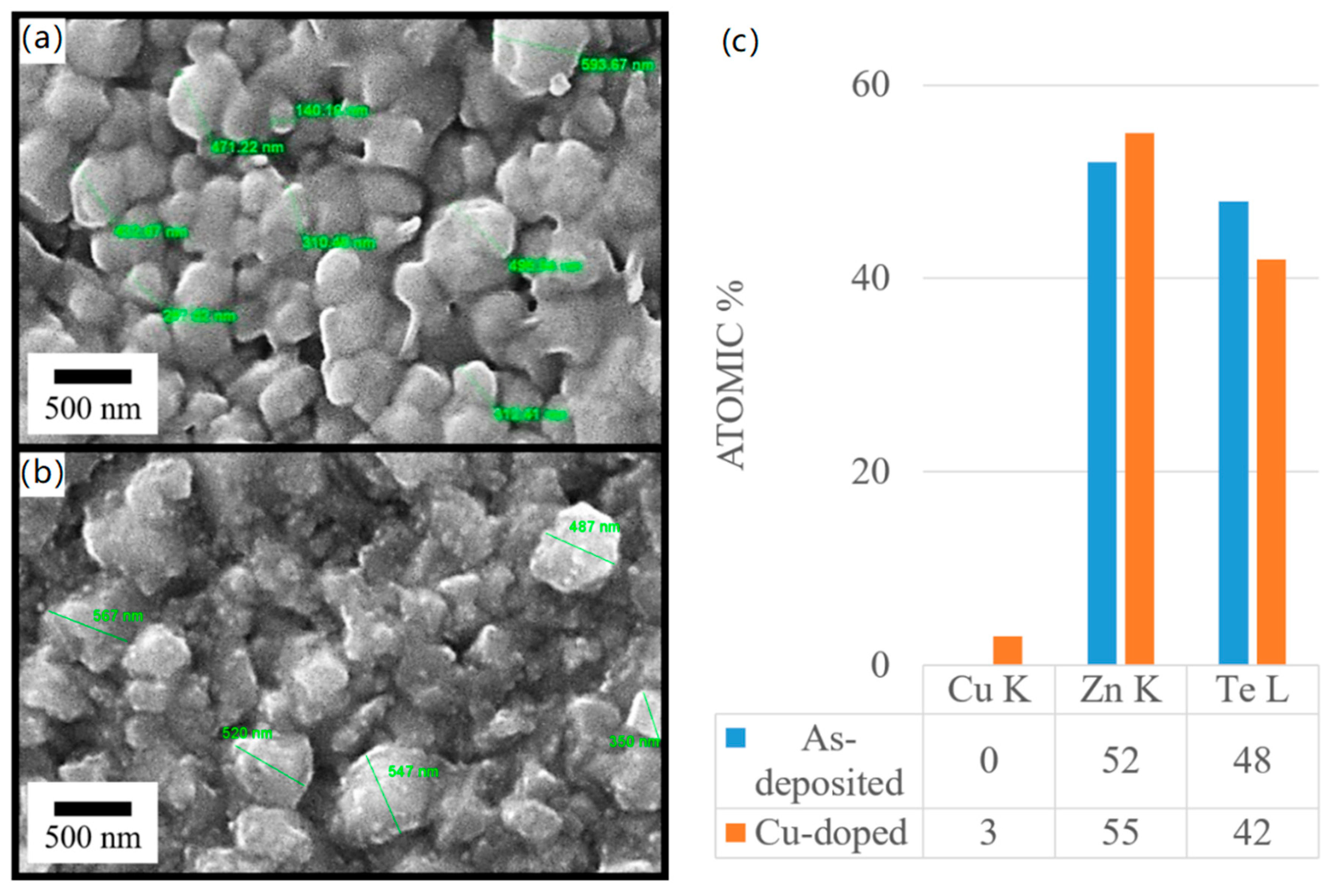
3.3. Microstructural Measurements
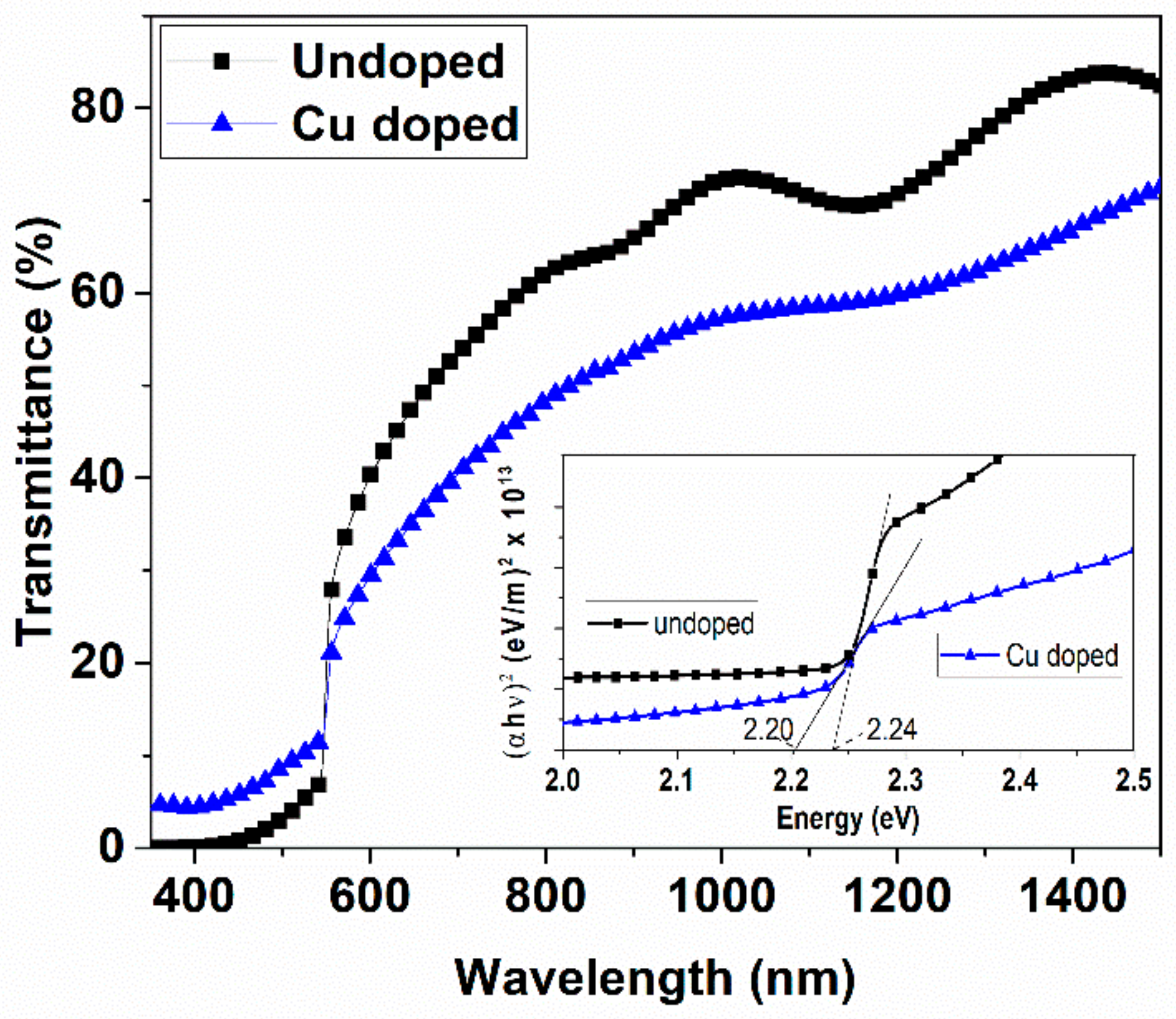
3.4. Optical Transmission Spectroscopy
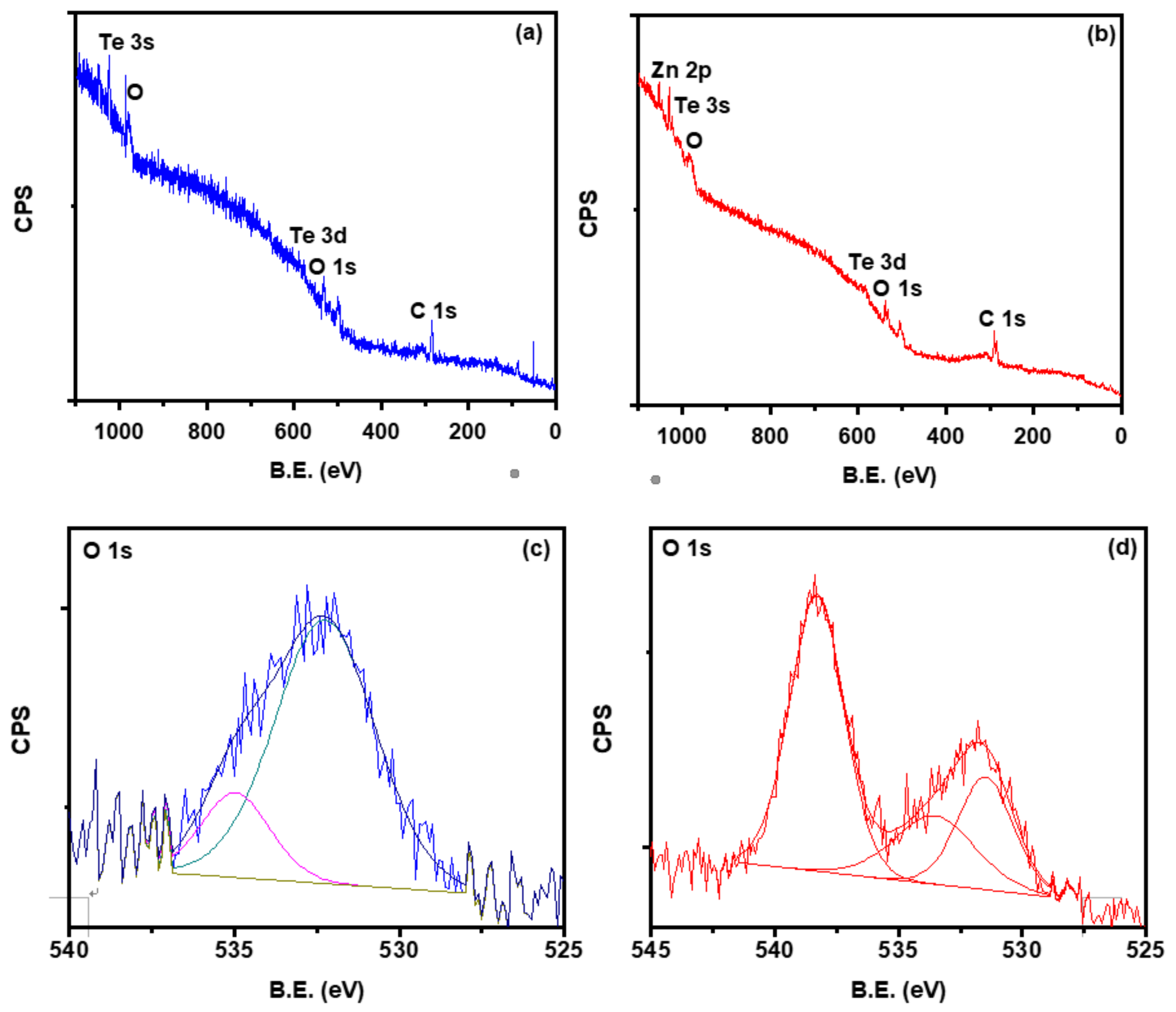
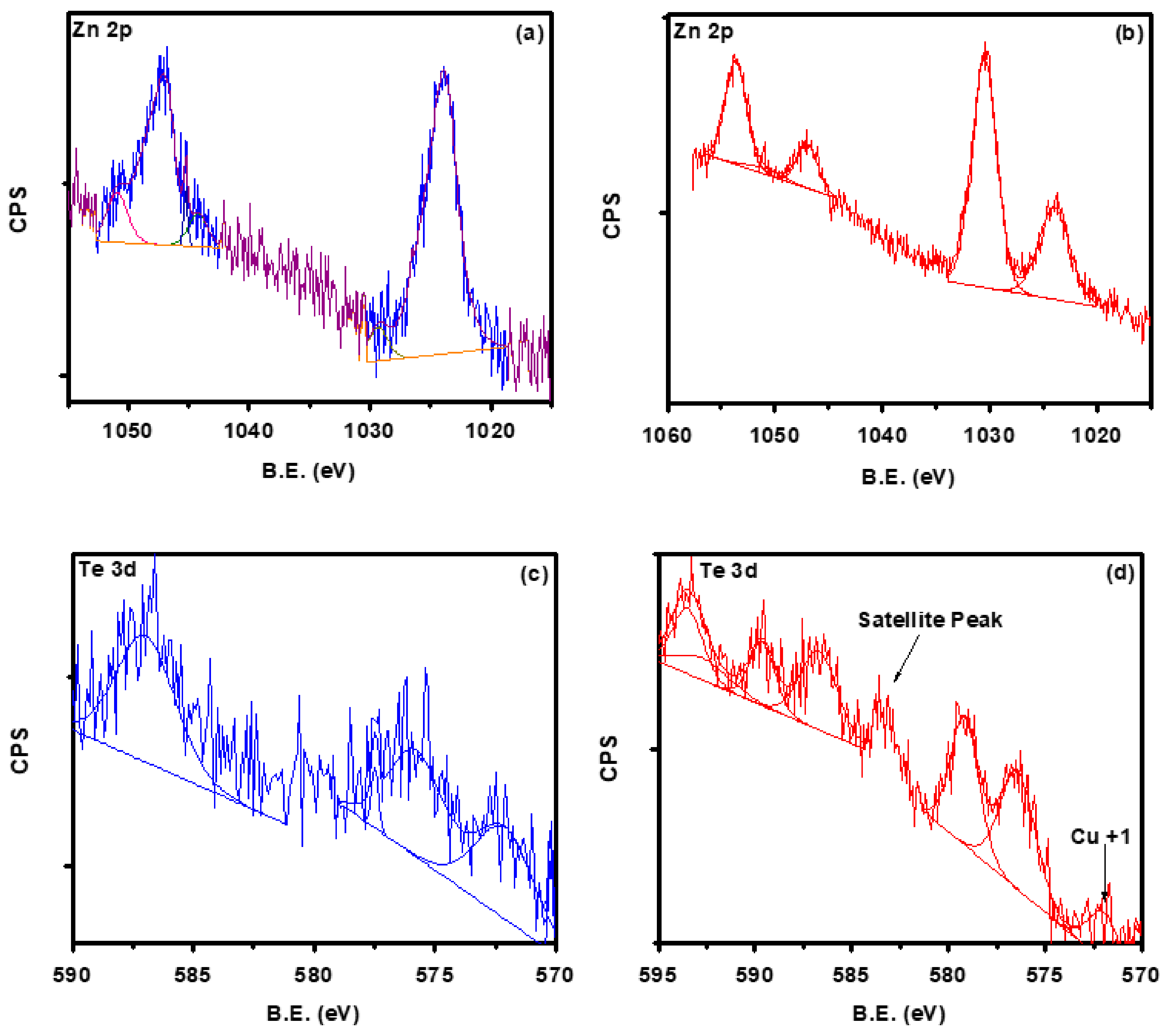
3.5. X-ray Photoelectron Spectroscopy (XPS)
3.6. Electrical Properties
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ho, S.M.; Olusola, O.I.; Sharma, D.C.; Mahmood, W. Zinc Telluride Thin Films: A Review. Asian J. Chem. 2018, 30, 469–473. [Google Scholar] [CrossRef]
- Wang, W.; Xia, G.; Zheng, J.; Feng, L.; Hao, R. Study of polycrystalline ZnTe (ZnTe:Cu) thin films for photovoltaic cells. J. Mater. Sci. Mater. Electron. 2007, 18, 427–431. [Google Scholar] [CrossRef]
- Ahamed, J.U.; Begum, N.P.; Khan, M.N.I. Property elucidation of vacuum-evaporated zinc telluride thin film towards optoelectronic devices. Sadhana-Acad. Proc. Eng. Sci. 2017, 42, 1773–1781. [Google Scholar] [CrossRef]
- Fields, J.D.; Pach, G.; Horowitz, K.A.W.; Stockert, T.R.; Woodhouse, M.; van Hest, M.F.A.M. Printed interconnects for photovoltaic modules. Sol. Energy Mater. Sol. Cells 2017, 159, 536–545. [Google Scholar] [CrossRef]
- Bosio, A.; Ciprian, R.; Lamperti, A.I.; Rago, I.; Ressel, B.; Rosa, G.; Stupar, M.; Weschke, E. Interface phenomena between CdTe and ZnTe:Cu back contact. Solar Energy 2018, 176, 186–193. [Google Scholar] [CrossRef]
- Gessert, T.A.; Colegrove, E.; Stafford, B.; Kodama, R.; Gao, W.; Moutinho, H.R.; Kuciauskas, D.; Reedy, R.C.; Barnes, T.M.; Sivananthan, S. II-VI Material Integration With Silicon for Detector and PV Applications. MRS Adv. 2016, 1, 3391–3402. [Google Scholar] [CrossRef]
- Potlog, T.; Duca, D.; Dobromir, M. Temperature-dependent growth and XPS of Ag-doped ZnTe thin films deposited by close space sublimation method. Appl. Surf. Sci. 2015, 352, 33–37. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Galkin, S.; Kraus, H.; Mokina, V.; Hrytsak, A.; Kapustianyk, V.; Panasiuk, M.; Rudko, M.; Rudyk, V. ZnTe cryogenic scintillator. J. Lumin. 2017, 188, 600–603. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, Q.; Ling, L.; Li, C.-X.; Chen, L.; Chen, S. Interfacial fabrication of single-crystalline ZnTe nanorods with high blue fluorescence. J. Am. Chem. Soc. 2013, 135, 10618–10621. [Google Scholar] [CrossRef]
- Khorsand Zak, A.; Abd Majid, W.H.; Abrishami, M.E.; Ramin Yousef, A. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Shaaban, E.R.; Kansal, I.; Mohamed, S.H.; Ferreira, J.M.F. Microstructural parameters and optical constants of ZnTe thin films with various thicknesses. Phys. B Condens. Matter 2009, 404, 3571–3576. [Google Scholar] [CrossRef]
- El Akkad, F.; Abdulraheem, Y. Morphology, electrical, and optical properties of heavily doped ZnTe:Cu thin films. J. Appl. Phys. 2013, 114, 183501. [Google Scholar] [CrossRef]
- Mahmood, W.; Shah, N.A. CdZnS thin films sublimated by closed space using mechanical mixing: A new approach. in Optical Mater. 2014, 36, 1449–1453. [Google Scholar] [CrossRef]
- Abken, A.E.; Halliday, D.P.; Durose, K. Photoluminescence study of polycrystalline photovoltaic CdS thin film layers grown by close-spaced sublimation and chemical bath deposition. J. Appl. Phys. 2009, 105, 64515. [Google Scholar] [CrossRef]
- Mahmood, W.; Shah, N.A.; Akram, S.; Mehboob, U. Investigation of substrate temperature effects on physical properties of ZnTe thin films by close spaced sublimation technique. Chalcogenide Lett. 2013, 10, 273–281. [Google Scholar]
- Mahmood, W.; Ali, J.; Zahid, I.; Thomas, A.; ul Haq, A. Optical and electrical studies of CdS thin films with thickness variation. Optik (Stuttg). 2018, 158, 1558–1566. [Google Scholar] [CrossRef]
- Mahmood, W.; Thomas, A.; Haq, A.; ul Shah, N.A.; Nasir, M.F. Reduced electrical performance of Zn enriched ZnTe nanoinclusion semiconductors thin films for buffer layer in solar cells. J. Phys. D. Appl. Phys. 2017, 50, 255503. [Google Scholar] [CrossRef]
- Mahmood, W.; Shah, N.A. Effects of metal doping on the physical properties of ZnTe thin films. Curr. Appl. Phys. 2014, 14, 282–286. [Google Scholar] [CrossRef]
- Mahmood, W.; Ali, J.; Thoma, A.; ullah Awan, S.; Jackman, M.; ul Haq, A.; Hassan, M.U.; Shah, N.A. Role of Ag+ substitutional defects on the electronic and optical properties of n-type CdS thin films semiconductor for sustainable and stable window layer in solar cells technology. Optical Mater. 2018, 85, 143–152. [Google Scholar] [CrossRef]
- Irwin, J.C.; LaCombe, J. Raman Scattering in ZnTe. J. Appl. Phys. 1970, 41, 1444–1450. [Google Scholar] [CrossRef]
- Cao, L.Z.; Cheng, B.L.; Wang, S.Y.; Fu, W. Influence of stress on Raman spectra in Ba1−xSrxTiO3 thin films. J. Phys. D. Appl. Phys. 2016, 39, 2819–2823. [Google Scholar] [CrossRef]
- Amirtharaj, P.M.; Pollak, F.H. Raman scattering study of the properties and removal of excess Te on CdTe surfaces. Appl. Phys. Lett. 1984, 45, 789–791. [Google Scholar] [CrossRef]
- Shah, N.A.; Mahmood, W. Physical properties of sublimated zinc telluride thin films for solar cell applications. Thin Solid Films 2013, 544, 307–312. [Google Scholar] [CrossRef]
- Tang, J.; Mao, D.; Trefny, J.U. Effect of Cu doping on the properties of ZnTe:Cu thin films and CdS/CdTe/ZnTe solar cells. AIP Conference Proc. 1997, 394, 639–646. [Google Scholar]
- Siddiqui, G.; Ali, J.; Doh, Y.-H.; Choi, K.H. Fabrication of zinc stannate based all-printed resistive switching device. Mater. Lett. 2016, 166, 311–316. [Google Scholar] [CrossRef]
- Swanepoel, R.; Swanepoel, R. Determination of the thickness and optical constants of amorphous silicon. J. Phys. E. 1983, 16, 1214–1222. [Google Scholar] [CrossRef]
- Ullah Awan, S.; Hasanain, S.K.; Bertino, M.F.; Hassnain Jaffari, G. Ferromagnetism in Li doped ZnO nanoparticles: The role of interstitial Li. J. Appl. Phys. 2012, 112, 103924. [Google Scholar] [CrossRef]
- Awan, S.U.; Hasanain, S.K.; Bertino, M.F.; Jaffari, G.H. Effects of substitutional Li on the ferromagnetic response of Li co-doped ZnO:Co nanoparticles. J. Phys. Condens. Matter 2013, 25, 156005. [Google Scholar] [CrossRef]
- Dabera, G.D.M.R.; Walker, M.; Sanchez, A.M.; Pereira, H.J.; Beanland, R.; Hatton, R.A. Retarding oxidation of copper nanoparticles without electrical isolation and the size dependence of work function. Nat. Commun. 2017, 8, 1894. [Google Scholar] [CrossRef]
- Kozak, D.S.; Sergiienko, R.A.; Shibata, E.; Iizuka, A.; Nakamura, T. Non-electrolytic synthesis of copper oxide/carbon nanocomposite by surface plasma in super-dehydrated ethanol. Sci. Rep. 2016, 6, 21178. [Google Scholar] [CrossRef]
- Hasanain, S.K.; Mehmood, Z.; Anjum, D.H.; Shah, S.A.; Aftab, M.; Abbas, T.A. Study of room temperature Raman scattering and XPS, high temperature electrical and low temperature magnetic properties of Zn1−yLiyO (0.00 ≤ y ≤ 0.10) nanoparticles. Smart Mater. Struct. 2015, 24, 115025. [Google Scholar]
- Mahmood, W. Fabrication and Characterization of II-VI Semiconductor Thin Films and the Study of Post Doping Effects. Ph.D. Thesis, COMSATS Institute of Information Technology, Islamabad, Pakistan, 2015; pp. 1–135. Available online: http://eprints.hec.gov.pk/id/eprint/6358 (accessed on 25 April 2019).
- Aqili, A.K.S.; Maqsood, A.; Ali, Z. Properties of copper-doped ZnTe thin films by immersion in Cu solution. Appl. Surf. Sci. 2001, 180, 73–80. [Google Scholar] [CrossRef]
- Teeter, G. X-ray and ultraviolet photoelectron spectroscopy measurements of Cu-doped CdTe(111)-B: Observation of temperature-reversible CuxTe precipitation and effect on ionization potential. J. Appl. Phys. 2007, 102, 34504. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, W.; Awan, S.U.; Ud Din, A.; Ali, J.; Nasir, M.F.; Ali, N.; ul Haq, A.; Kamran, M.; Parveen, B.; Rafiq, M.; et al. Pronounced Impact of p-Type Carriers and Reduction of Bandgap in Semiconducting ZnTe Thin Films by Cu Doping for Intermediate Buffer Layer in Heterojunction Solar Cells. Materials 2019, 12, 1359. https://doi.org/10.3390/ma12081359
Mahmood W, Awan SU, Ud Din A, Ali J, Nasir MF, Ali N, ul Haq A, Kamran M, Parveen B, Rafiq M, et al. Pronounced Impact of p-Type Carriers and Reduction of Bandgap in Semiconducting ZnTe Thin Films by Cu Doping for Intermediate Buffer Layer in Heterojunction Solar Cells. Materials. 2019; 12(8):1359. https://doi.org/10.3390/ma12081359
Chicago/Turabian StyleMahmood, Waqar, Saif Ullah Awan, Amad Ud Din, Junaid Ali, Muhammad Farooq Nasir, Nazakat Ali, Anwar ul Haq, Muhammad Kamran, Bushra Parveen, Muhammad Rafiq, and et al. 2019. "Pronounced Impact of p-Type Carriers and Reduction of Bandgap in Semiconducting ZnTe Thin Films by Cu Doping for Intermediate Buffer Layer in Heterojunction Solar Cells" Materials 12, no. 8: 1359. https://doi.org/10.3390/ma12081359
APA StyleMahmood, W., Awan, S. U., Ud Din, A., Ali, J., Nasir, M. F., Ali, N., ul Haq, A., Kamran, M., Parveen, B., Rafiq, M., & Abbas Shah, N. (2019). Pronounced Impact of p-Type Carriers and Reduction of Bandgap in Semiconducting ZnTe Thin Films by Cu Doping for Intermediate Buffer Layer in Heterojunction Solar Cells. Materials, 12(8), 1359. https://doi.org/10.3390/ma12081359




